Kidney disease affects drug pharmacokinetics and pharmacodynamics, and the level of kidney function is a key consideration in the use of drugs. Carboplatin, a widely used cancer chemotherapeutic agent is excreted mainly by glomerular filtration. Maintaining efficacy and avoiding toxicity requires determination of glomerular filtration rate (GFR) for accurate dosing1. In a recent study published in the Journal of Clinical Oncology, Janowitz et al.2 evaluated the accuracy of GFR estimating equations in patients being treated with carboplatin and developed a new equation for the purpose of calculating a patient’s carboplatin dose to achieve a target exposure.
Carboplatin is approved by the FDA only for the treatment of ovarian cancer; however it is used in the treatment of many cancers, including small cell and non-small cell lung cancers, mesothelioma, seminoma, bladder, ovarian, cervical, and head and neck cancers, and cancers of unknown primary3. The side effect profile of carboplatin is dominated by myelotoxicity, in particular thrombocytopenia. The exposure-response relationship of carboplatin has clearly been documented, both for efficacy and toxicity, with exposure expressed as area-under-the-concentration-versus-time profile (AUC) of ultrafilterable carboplatin in plasma. In a landmark study of approximately 1000 ovarian cancer patients, a target AUC of 4 to 6 (mg/mL)•min appeared to be most appropriate in previously treated patients, as increasing AUC above 5 to 7 (mg/mL)•min did not improve the likelihood of response but did increase myelotoxicity4. In seminoma patients, a carboplatin dose reduction of as little as 10% was suggested to result in a doubling of 5-year relapse rate5. Both studies highlight the importance of accurate dosing.
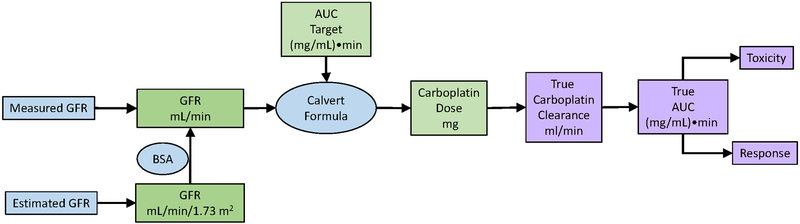
Because carboplatin clearance depends largely on GFR, the dose of carboplatin needed to achieve a target AUC has historically been calculated starting with an assessment of GFR (Figure), using the Calvert formula: Dose (mg) = target AUC ((mg/mL)•min) × (GFR + 25) (mL/min)6, where the constant of 25 mL/min represents the non-GFR clearance of carboplatin. Measured GFR (mGFR) has not been widely adopted in oncology practice. Instead, estimated GFR (eGFR) from serum creatinine is widely used, but serum creatinine is also influenced by its non-GFR determinants, especially its generation by muscle and diet, as well as by GFR7. eGFR estimation formulas using creatinine incorporate easily measured surrogates of muscle mass (age, sex, race and body size). Traditionally, the Cockcroft-Gault formula has been used to estimate creatinine clearance, which overestimates GFR due to tubular creatinine secretion. Newer formulas, such as the Modification of Diet in Renal Disease (MDRD) Study and Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equations, estimate GFR and have more accurate and precise performance characteristics7. Now, eGFR is reported routinely when serum creatinine is measured, and the CKD-EPI equation is recommended for use in routine clinical practice by the international guideline group, Kidney Disease: Improving Global Outcomes (KDIGO)8.
Figure 1: Determinants of carboplatin exposure, toxicity and response in an individual patient.
Carboplatin dose is determined using the Calvert formula which requires the clinician to determine the target AUC and GFR. The target AUC is determined by tumor type and patient characteristics. GFR can be determined using measured or estimated GFR.
Blue shaded cells indicate quantities determined by equations; green shaded cells indicate quantities decided by clinicians; purple shaded cells indicate true quantities and subsequent events.
What does this important study show?
The study by Janowitz et al2 tests the diagnostic accuracy of GFR estimating equations in cancer patients, a group in which creatinine production may be reduced due to the malignancy. The development and internal validation population included 2,471 adults (43% men, all white) with histologically confirmed cancer diagnoses at the Cambridge University Hospital NHS Trust between August 2006 and January 2013. The validation population included 111 men with stage 1 seminoma at Beatson West of Scotland Cancer Centre, Glasgow. The reference test was mGFR by plasma clearance of 51Cr-EDTA. GFR was expressed without indexing for body surface area (BSA). Median (interquartile range) mGFR in the two populations was 81 (63–103) and 113 (101–131) mL/min, respectively. The index tests included seven existing creatinine-based eGFR equations and one new equation derived in the development dataset, with and without indexing for BSA. The serum creatinine concentration was measured using the kinetic Jaffe method within a month of the mGFR determination. The existing equations included the MDRD, CKD-EPI, Martin, Wright, and Mayo equations that estimate eGFR, and the Cockcroft-Gault and the Jelliffe equations that estimate creatinine clearance. The new equation was developed using linear regression. Performance of estimating equations was assessed in the development, internal validation and external validation datasets by comparing eGFR vs. mGFR and comparing carboplatin dose for an AUC of 5 (mg/mL)•min computed from the Calvert equation using eGFR vs. mGFR.
Within the development and internal validation dataset, the CKD-EPI equation without indexing for BSA had the best performance compared to mGFR. In comparison to the Cockcroft Gault equation, it had lower root mean square error (RMSE) (17 vs. 23 mL/min), median residual closer to zero (0.5 vs. −1.9 mL/min), smaller interquartile range for the residuals (19 vs. 24 mL/min), median percent error closer to zero (−0.8 vs. −2.4%), smaller median absolute percent error (12 vs. 15%), and smaller percent of carboplatin doses with an absolute percent error of 20% or more (17 vs. 24%). The new equation is a 3rd order polynomial for creatinine with additional coefficients for age, sex and BSA to estimate the square root of GFR. In the external validation dataset, the CKD-EPI equation without indexing for BSA again had the best performance of the existing equations, but the new equation performed even better. Compared to the CKD-EPI equation, it had a lower RMSE (19 vs. 21 mL/min), median residual closer to zero (1.6 vs. −5.3 mL/min), smaller interquartile range for the residuals (23 vs. 28 mL/min), median percent error closer to zero (1.4 vs. −4.4%), smaller median absolute percent error (11 vs. 13%), and smaller percent of carboplatin doses with an absolute percent error of 20% or more (12 vs. 19%). The authors concluded that the CKD-EPI equation without indexing for BSA is the most accurate published model to estimate measured GFR, and that their new equation improves this estimation and may present a new standard of care.
Strengths of the study by Janowitz et al. include the large sample size for comparison of existing equations and derivation of a new equation, evaluation of the most commonly used eGFR equations by comparison to an accepted reference method, and evaluation of the new equation in an external validation population. There are many limitations however. The development and internal validation population is all-white and from a single institution, and the authors provided few clinical details, making it difficult to assess its representativeness. The external validation population is small and non-representative. The predominance of high GFR in both populations makes it difficult to extrapolate to patients with low GFR. The method for mGFR determination is not detailed9. The authors did not comment on whether the serum creatinine assays were traceable to reference methods. The new equation does not include a term for race, and the coefficients for creatinine and other variables may not generalize well to other settings. Tests of statistical significance for the comparison of equation performance were not provided. Clinical outcomes (efficacy and toxicity) were not assessed.
How does this study compare with prior studies?
The study by Janowitz et al. confirms in a cancer population the superior performance of the CKD-EPI equation compared to other creatinine based equations as previously demonstrated in other populations8. Previous, similar work10–12 in cancer populations cannot be compared, because serum creatinine assays were not traceable to reference standards and are not relevant to current clinical practice. A previous study comparing drug dosing recommendations based on the CKD-EPI, MDRD Study and Cockcroft Gault equations in a non-cancer population reached similar conclusions as the study by Janowitz et al8, 13. Another study in intensive care unit patients demonstrated substantially more frequent achievement of therapeutic vancomycin levels with eGFR computed using the CKD-EPI equation for creatinine and cystatin C rather than the estimated creatinine clearance from the Cockcroft-Gault equation14.
What are the implications for nephrologists?
Clearly, there is a strong relationship between the accuracy of eGFR vs. mGFR and the accuracy of drug dosing recommendations based on eGFR vs. mGFR. In addition, there is a strong relationship between carboplatin exposure and outcome. However, we should be mindful that many of the links between GFR, drug dosing, drug exposure and clinical outcomes of effectiveness and toxicity are based on estimates (Figure). Neither mGFR nor eGFR are without error15. In the landmark study defining the target carboplatin AUC, AUC was not measured, but estimated using the Calvert formula often with estimated creatinine clearance4. The Calvert formula was derived by measuring carboplatin AUC and GFR in 40 courses in 31 patients, with an R2 of 0.76 for carboplatin clearance vs GFR6. The DuBois and DuBois equation for BSA estimation was originally derived in 1916, using 10 subjects16.
We concur with the authors’ conclusion that the CKD-EPI equation likely enables more accurate carboplatin dosing than the other GFR estimating equations, and nephrologists should recommend that their oncology colleagues adopt it as a more accurate alternative to the older equations, such as the Cockcroft-Gault equation. Of note, the CKD-EPI equation estimates GFR indexed for BSA. Use in carboplatin dosing requires removing the BSA-indexing as follows: eGFR (ml/min) = eGFR (ml/min/1.73 m2) × BSA (m2)/1.73. In our view, the advantage of the authors’ new formula over the CKD-EPI equation requires further evaluation. Ultimately, even the best estimator of GFR does not directly address the overarching goal of improving patient outcomes. Therefore, future efforts to improve carboplatin dosing algorithms should not only measure GFR, but also carboplatin exposure and patient outcomes, to better understand when estimation of GFR is appropriate and when GFR should be measured, despite practical hurdles.
Acknowledgments
Support: Grants UM1-CA186690 (NCI-CTEP), P30-CA47904 (NCI), and R01DK087961 (NIDDK).
Footnotes
Authors’ disclosures of potential conflicts of interest:
Employment or Leadership Position: None; Consultant or Advisory Role: Tricidea (LAI) ; Stock Ownership: None; Honoraria: None; Research Funding: None; Expert Testimony: None; Other Remuneration: None: Other: Drs. Levey and Inker led the development of the CKD-EPI equation. Drs. Levey and Inker have a provisional patent filed 8/15/2014 –”Precise estimation of glomerular filtration rate from multiple biomarkers” PCT/US2015/044567.
REFERENCES
- 1.Levey AS, Inker LA. Assessment of Glomerular Filtration Rate in Health and Disease: A State of the Art Review. Clinical pharmacology and therapeutics. 2017;102:405–419. [DOI] [PubMed] [Google Scholar]
- 2.Janowitz T, Williams EH, Marshall A, et al. New Model for Estimating Glomerular Filtration Rate in Patients With Cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2017:JCO2017727578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ho GY, Woodward N, Coward JI. Cisplatin versus carboplatin: comparative review of therapeutic management in solid malignancies. Critical reviews in oncology/hematology. 2016;102:37–46. [DOI] [PubMed] [Google Scholar]
- 4.Jodrell DI, Egorin MJ, Canetta RM, et al. Relationships between carboplatin exposure and tumor response and toxicity in patients with ovarian cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 1992;10:520–528. [DOI] [PubMed] [Google Scholar]
- 5.Oliver RT, Mead GM, Rustin GJ, et al. Randomized trial of carboplatin versus radiotherapy for stage I seminoma: mature results on relapse and contralateral testis cancer rates in MRC TE19/EORTC 30982 study (ISRCTN27163214). Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2011;29:957–962. [DOI] [PubMed] [Google Scholar]
- 6.Calvert AH, Newell DR, Gumbrell LA, et al. Carboplatin dosage: prospective evaluation of a simple formula based on renal function. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 1989;7:1748–1756. [DOI] [PubMed] [Google Scholar]
- 7.Levey AS, Inker LA, Coresh J. GFR estimation: from physiology to public health. American journal of kidney diseases : the official journal of the National Kidney Foundation. 2014;63:820–834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Group KDIGOKCW. KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney international. Supplement 2013;3:1–150. [DOI] [PubMed] [Google Scholar]
- 9.Delanaye P, Ebert N, Melsom T, et al. Iohexol plasma clearance for measuring glomerular filtration rate in clinical practice and research: a review. Part 1: How to measure glomerular filtration rate with iohexol? Clinical kidney journal. 2016;9:682–699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cathomas R, Klingbiel D, Geldart TR, et al. Relevant risk of carboplatin underdosing in cancer patients with normal renal function using estimated GFR: lessons from a stage I seminoma cohort. Annals of oncology : official journal of the European Society for Medical Oncology / ESMO. 2014. [DOI] [PubMed] [Google Scholar]
- 11.Dooley MJ, Poole SG, Rischin D. Dosing of cytotoxic chemotherapy: impact of renal function estimates on dose. Annals of oncology : official journal of the European Society for Medical Oncology / ESMO. 2013;24:2746–2752. [DOI] [PubMed] [Google Scholar]
- 12.Ainsworth NL, Marshall A, Hatcher H, Whitehead L, Whitfield GA, Earl HM. Evaluation of glomerular filtration rate estimation by Cockcroft-Gault, Jelliffe, Wright and Modification of Diet in Renal Disease (MDRD) formulae in oncology patients. Annals of oncology : official journal of the European Society for Medical Oncology / ESMO. 2012;23:1845–1853. [DOI] [PubMed] [Google Scholar]
- 13.Stevens LA, Nolin TD, Richardson MM, et al. Comparison of drug dosing recommendations based on measured GFR and kidney function estimating equations. American journal of kidney diseases : the official journal of the National Kidney Foundation. 2009;54:33–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Frazee E, Rule AD, Lieske JC, et al. Cystatin C-Guided Vancomycin Dosing in Critically Ill Patients: A Quality Improvement Project. American journal of kidney diseases : the official journal of the National Kidney Foundation. 2017;69:658–666. [DOI] [PubMed] [Google Scholar]
- 15.Levey AS, Inker LA. GFR as the “Gold Standard”: Estimated, Measured, and True. American journal of kidney diseases : the official journal of the National Kidney Foundation. 2016;67:9–12. [DOI] [PubMed] [Google Scholar]
- 16.Du Bois D, Du Bois EF. A formula to estimate the approximate surface area if height and weight be known. 1916. Nutrition. 1989;5:303–311; discussion 312–303. [PubMed] [Google Scholar]