Abstract
Background/Objectives
Energy expenditure measured under sedentary conditions predicts weight change but evidence that directly measured VO2max is associated with weight change is lacking. The aim of this study was to determine the associations of VO2max with measures of predominantly sedentary 24-h thermogenesis, and subsequent weight change.
Subjects/Methods
Three hundred fifty-seven individuals (162 females; 27 Blacks, 72 Caucasians, and 258 American Indians) had measures of body composition, resting metabolic rate (RMR), and intermittent treadmill run test for assessment of VO2max. On a separate day, 24-h energy expenditure (EE), diet-induced thermogenesis (DIT) expressed as “awake and fed” thermogenesis (AFT), sleeping metabolic rate (SMR), and spontaneous physical activity (SPA) were measured in a whole-room indirect calorimeter. Follow-up weight for 217 individuals was available (median follow-up time, 9.5 y; mean weight change, 12.4±14.9 kg).
Results
After adjustment for fat free mass, fat mass, age, sex, and race, a higher VO2max was associated with a higher RMR (β=68.2 kcal/day per L/min, P<0.01) and 24-h EE (β=62.2 kcal/day per L/min, P<0.05) and including additional adjustment for energy intake higher AFT (β=66.1 kcal/day per L/min, P=0.01). Neither SMR (P>0.2) nor SPA (P>0.8) were associated with VO2max. VO2max at baseline did not predict follow-up weight after adjustment for baseline weight, follow-up time, sex, and race (P>0.4).
Conclusion
VO2max is associated with measures of EE including 24hEE, RMR and DIT implying a common mechanism regulating the energetics of skeletal muscle during exercise and thermogenesis. However, this did not translate to VO2max as a predictor of weight change.
Keywords: VO2max, whole-room colorimeter, weight change, diet-induced thermogenesis, resting metabolic rate, 24-h energy expenditure
Introduction
VO2max, the gold standard measurement of cardiorespiratory fitness (CRF), is mainly influenced by everyday physical activity (PA) [1] and heritability[2], and predicts both morbidity and mortality [3, 4]. Several studies also have shown that lower CRF, regardless of PA levels, is associated with weight gain [5, 6].This implies that VO2max may have an independent effect on energy balance apart from the energy expenditure (EE) of PA, possibly via an effect on or through a common mechanism associated with resting metabolic rate (RMR) or the diet-induced thermogenesis (DIT).
Previous evidence for an effect of VO2max on 24-h thermogenesis independent of PA is mixed finding no[7–10] or an inverse [11] association between VO2max and 24-h EE. Studies using a metabolic cart have produced equally mixed results with some demonstrating a positive association of VO2max with RMR [10, 12–18], sleeping metabolic rate (SMR) [10], and DIT [10, 14, 19–23], some showing no association with RMR [8, 22, 24–34], SMR [35, 36], or DIT [7, 9, 17, 32, 37, 38] and some studies have even shown an inverse association with these EE measures[23, 28, 39–41]. However, the sample size in all these studies have been relatively small.
While many previous longitudinal studies have reported an association between CRF and weight change[5, 42–45], most of these studies[5, 42, 43, 45] did not measure VO2max by respiratory gas exchange as a measure of CRF. In addition, those studies did not fully control for the effect of baseline weight or adiposity on CRF and weight change, despite a known effect of adiposity itself on both the relationship between running performance and CRF[46, 47] and on weight change[48]. When baseline adiposity is adequately controlled for, CRF is not associated with weight change[49]. To further address these questions, in a cohort of Native Americans of southwestern heritage with high prevalence of obesity and measures of VO2max and EE, we investigated the associations between VO2max and specific measures of EE, such as RMR, SMR, DIT, and 24-h EE. We also investigated whether VO2max predicted free-living weight change in this population over a long follow-up period.
Subjects and Methods
Subjects
Adults aged 18 to 45 years participated in a longitudinal inpatient study of risk factors for diabetes and obesity (NCT00340132). All subjects gave written informed consent before participation and any testing. Only participants who were not on medications and free of chronic or acute medical conditions with the exception of obesity and prediabetes were eligible. Eligibility was determined prior to admission to the clinical research unit, by detailed history and physical, ECG, and comprehensive blood and urine tests. After 3 days of admission, all participants underwent a 75-gram oral glucose tolerance test to determine glucose regulation status. Individuals diagnosed with diabetes[50] were excluded from this analysis. Baseline studies were conducted between 1982 and 1992 at National Institute of Diabetes and Digestive and Kidney Diseases in Phoenix, Arizona, USA. This study was approved by the Institutional Review Board of the National Institute of Diabetes and Digestive and Kidney Diseases.
Study protocol
Following admission to the clinical research ward, subjects were fed a weight-maintaining diet (50% carbohydrate, 30% fat, and 20% protein) based on equations for energy requirements[51]. On day 2, participant’s body composition was assessed by hydrostatic underwater weighing with correction for the simultaneously measured residual lung volume[52]. After 4 days on the weight maintaining diet and following an overnight fast, RMR was measured upon awakening and then a graded exercise test for assessing VO2max was performed. On a separate day, participants also spent 24 hours in a whole room calorimeter for measurement of 24-h EE.
VO2max test
A graded exercise test with intermittent (discontinuous) work bouts was performed on a treadmill. Each test was started at 0 degrees elevation and involved walking at 1.5 mph. After an initial 4 min warm-up period, the elevation and speed of the treadmill were gradually increased. After 4 min at each work bout, the subject sat down until the heart rate was < 120 beats/min and they felt subjectively recovered. The test continued until the patient was subjectively exhausted, the heart rate reached 200 beats/min, or there was no further increase in the oxygen uptake. The oxygen uptake was measured during the last 30 s of each work bout. VO2max was defined as the highest VO2 recorded during the test. Details on expired gas collection and the analysis system used were previously described[53].
Indirect calorimetry assessment for resting metabolic rate and 24-h EE
The details of the measurements of EE and substrate oxidation by ventilated hood system [53] and in whole-body room calorimeter have been previously described [54]. Briefly, RMR was measured by ventilated hood system. After 1.5 h in supine position, a ventilated hood was placed over the subjects’ head, and then expired gas was recorded continuously for an hour. For the measurement of 24-h EE, the subject entered the metabolic chamber in the morning at ~0800h after an overnight fast and stayed until the following morning. Meals were provided at 0800h, 1130h, and 1700h and an evening snack at 2000h. EE and substrate oxidation were measured continuously and calculated every 15-minute interval. EE was calculated using the formula of Lusk[55]. Spontaneous physical activity (SPA) was measured using microwave sensors. SMR was calculated averaging the EE during the nightly hours between 2330h and 0500h when SPA was <1.5%, and then extrapolated to 24 hours. The methods for the calculation of “awake and fed thermogenesis” (AFT) which includes both the DIT and the cost of arousal have been previously published[56]. Briefly, DIT is calculated as follows: 1) daytime EE is plotted versus SPA and the intercept of the regression line is calculated (EE at zero activity); 2) SMR is then subtracted from EE at zero activity to calculate AFT. Our group has previously found that AFT predicts weight change in Native American subjects with a body mass index (BMI)>29 kg/m2 [56].
Longitudinal analyses for weight change and body composition.
Follow-up data on body composition was obtained from participants who returned for repeated admissions in the above described study. Body composition at follow-up was determined by underwater weighing or dual energy x-ray absorptiometry with percent fat regressed to original hydrostatic weight values [57].
For follow-up analyses of body weight, Native American participants of southwestern heritage also participated in a longitudinal study of health between 1965–2007 (NCT00339482). In this study, participants were invited for outpatient visits approximately every 2 years regardless of health status. At these visits participants came in fasting, underwent 75-gram oral glucose tolerance test and had measures of weight and height. Follow-up weights were collected at the last available follow-up visit or last visit prior to development of diabetes.
Statistical analyses
Prior to analyses, all variables were tested for normal distribution by sex based on kurtosis and skewness. Non-normally distributed variables were log transformed. The unpaired T-test was used to test for differences in variables by sex. Associations of VO2max with measures of body composition and age were analyzed using general linear models. General linear models were also used to investigate the associations of VO2max with EE measures, or follow-up weight, with adjustments for covariates. For all models, inclusion of variables was based on previous knowledge of these variables as confounders. Pearson’s correlation coefficient was also used to investigate the associations of VO2max with rate of weight change and for the associations between changes in VO2max and changes in body composition. An interaction term for sex*VO2max was tested in models of follow-up weight and weight change. When the interaction term was significant, analyses was performed separately by sex. All statistical analyses were performed by SAS Enterprise Guide 7.1 (SAS Institute Inc., Cary, NC, USA). P value was considered significant if < 0.05. Data are presented as mean ± SD.
Results
Subject characteristics
Baselines measures of body composition and EE are shown in Table 1. Three hundred fifty-seven individuals without diabetes had measures of anthropometry tests and VO2max. Of the 357 individuals, 207 individuals had measures of 24-h EE and 162 individuals of these 207 individuals had measures of RMR. As expected, BMI, fat mass (FM), and %FM were significantly higher in females than in males. By contrast, body weight, FFM, VO2max, RMR, SMR, and 24-h EE were significantly higher in males.
Table 1.
Subjects characteristics.
| Variables | Mean ± SD | Range (min-max) |
|---|---|---|
| N (women, %) | 357 (162,45.4%) | |
| Race (number) | Black = 27, Caucasian = 72, American Indian = 258 (Full heritage American Indians of southwestern heritage = 249) | |
| Age (years) | 26.8 ± 6.1 | 18.1–44.0 |
| Body mass index (kg/m2) | 33.74 ± 8.23 | 16.34–63.06 |
| Weight (kg) | 94.3 ± 24.4 | 41.2–181.1 |
| Fat free mass (kg) | 63.0 ± 13.1 | 33.9–115.4 |
| Fat mass (kg) | 31.3 ± 15.2 | 4.0–84.5 |
| % fat mass (%) | 31.7 ± 9.9 | 6.5–51.3 |
| VO2max (L/min) | 2.927 ± 0.783 | 1.263–5.381 |
| Residual VO2max (mL/min)* | 4.414 ×10−13 ±406.7 | -1176–1491 |
| Resting metabolic rate (kcal/day, N = 162) | 1815 ± 297 | 1097 – 2641 |
| Sleep metabolic rate (kcal/day, N = 202) | 1665 ± 293 | 994–2484 |
| Awake and fed thermogenesis (kcal/day, N = 165) | 424 ± 168 | 88–926 |
| Spontaneous physical activity (%, N = 173) | 9.5 ± 3.3 | 3.1–22.8 |
| 24-h energy expenditure (kcal, N = 207) | 2330 ± 406 | 1412–3651 |
| 24-h RQ(N = 207) | 0.852 ± 0.027 | 0.776–0.929 |
Residual VO2max was obtained by a model including fat-free mass, fat mass, age, and sex as independent variables.
Associations of VO2max with body composition and age
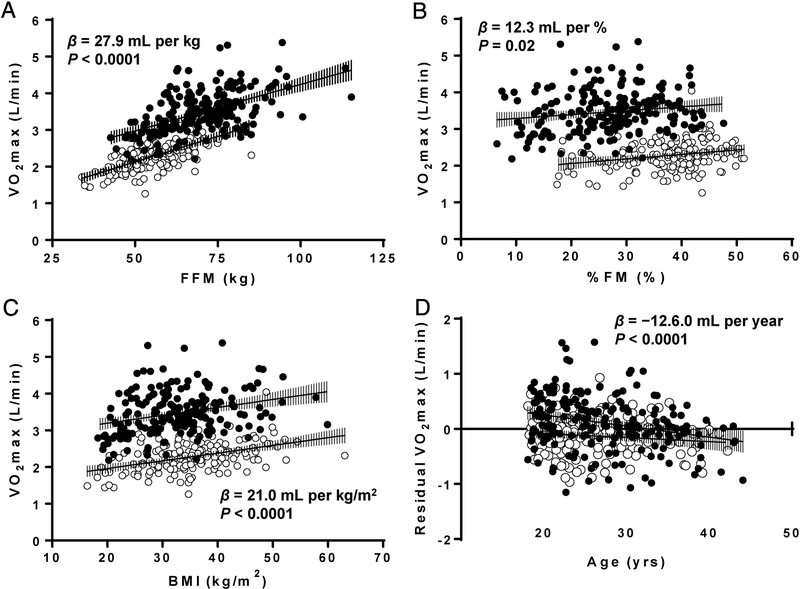
In linear models adjusting for sex, VO2max was positively correlated with body weight (β = 10.0 mL per kg, P < 0.0001), FFM (β = 27.9 mL per kg, P < 0.001; Figure1A), FM (β = 12.1 mL per kg, P < 0.0001), %FM (β = 12.3 mL per %, P = 0.02; Figure1B), and BMI (β = 21.0 mL per kg/m2, P < 0.0001; Figure1C). Sex remained a significant predictor of VO2max in the above models. There was no interaction by sex and above variables on VO2max. In models adjusted for sex, FFM, and FM, VO2max was negatively associated with age (β = −12.6 mL per year, P < 0.001; Figure1D) but there was no interaction between sex and age on VO2max (P > 0.1). In models adjusted for age, sex, FFM, and FM there was no difference in VO2max by race (P > 0.3). The model prediction for VO2max based on FFM, FM, age, and sex was as follows (R2 = 0.730, adjusted R2 = 0.727):
Figure 1-.
Association of VO2max with measures of body compositions and age in females (open circles) and males (closed circles). (A) VO2max versus fat free mass. (B) VO2max versus % fat. (C) VO2max versus body mass index. (D) VO2max, adjusted for fat free mass and fat mass versus age. BMI, body mass index; FFM, fat free mass; FM, fat mass. Error bars show 95% confidence intervals.
VO2max and EE measures
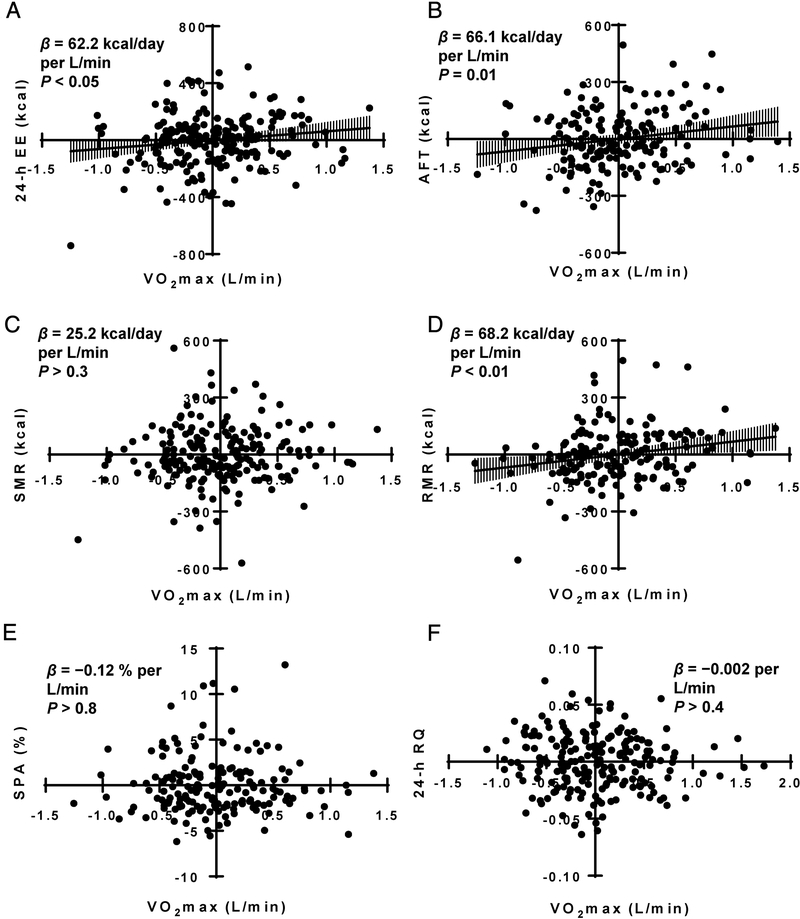
Figure 2 and Table 2 show the associations between adjusted VO2max and adjusted EE measures. VO2max and all EE measures were adjusted for FFM, FM, age, sex, and race while 24-h RQ was adjusted for %FM, age, sex, energy balance, and race. VO2max was positively associated with RMR (Standardized β = 0.214, P < 0.01; Figure 2D), AFT (Standardized β = 0.194, P = 0.015; Figure 2B), and 24-h EE (Standardized β = 0.147, P < 0.05; Figure 2A), but not with SMR (P > 0.2; Figure 2C), SPA (P > 0.8; Figure 2E), or 24-h RQ (P > 0.4; Figure 2F). Time from admission to EE assessments was not associated with residuals of RMR, AFT, and 24-h EE in any of the adjusted models (All P > 0.1).
Figure 2-.
Associations of VO2max with measures of EE. (A) Residuals for VO2max versus residuals 24-h energy expenditure (B) Residuals for VO2max versus residuals awake and fed thermogenesis (C) Residuals for VO2max versus residuals for sleeping metabolic rate (D) Residuals for VO2max versus residuals for resting metabolic rate. (E) Residuals for VO2max versus residuals for spontaneous physical activity (F) Residuals for VO2max versus residuals 24-h RQ. VO2max, 24-h EE, AFT, SMR, RMR and SPA were all adjusted for age, sex, race, fat free mass, fat mass. AFT additionally adjusted for energy balance. RQ adjusted for age, sex race, %fat and energy balance. AFT, awake and fed thermogenesis; EE, energy expenditure; RMR, resting metabolic rate; RQ, respiratory quotient; SMR, sleeping metabolic rate; SPA, spontaneous physical activity. Error bars show 95% confidence intervals.
Table 2.
Results for the associations between VO2max and EE measures using general linear models.
| Dependent variables | β | Standardized β | P- value | 95% confidence limits | R2 for model |
|---|---|---|---|---|---|
| Resting metabolic rate (kcal/day, N=162) | 68.2 ± 25.0 | 0.214 | 0.007 | 18.8–117.7 | 0.780 |
| Sleep metabolic rate (kcal/day, N=202) | 27.4 ± 24.3 | 0.081 | >0.2 | −20.6–75.3 | 0.753 |
| Awake and fed thermogenesis (kcal, N=165) | 66.1 ± 26.9 | 0.194 | 0.015 | 13.0–119.2 | 0.214 |
| Spontaneous physical activity (%, N=173) | −0.11± 0.55 | −0.016 | >0.8 | −1.2–1.0 | 0.127 |
| 24-h energy expenditure (kcal, N=207) | 62.2 ± 29.6 | 0.147 | <0.05 | 3.9–120.5 | 0.807 |
| 24-h RQ (N=207) | −0.003 ± 0.003 | −0.055 | >0.4 | −0.009–0.004 | 0.225 |
B, Standardized β, P-value, and 95% confidence limits represent the results for VO2max as independent variable of general linear models. Other covariates as independent variables for all EE measures were fat-free mass, fat mass, age, sex, and race, and for 24-hRQ were %fat mass, energy balance, age, sex, and race.
VO2max and subsequent body composition and weight change
Participants with baseline and follow-up data for both VO2max and body composition (n =135, 55 females) are shown in (Table 3). The average weight change was 6.1 ± 11.2 kg over a median follow-up of 4.4 years (IQR: 2.6–6.5 years). In this group, baseline VO2max did not predict follow-up FFM (P > 0.3) or FM (P > 0.3) when adjusted for baseline FFM, FM, age, sex, and follow-up time. Changes in VO2max were not correlated with changes in weight (P > 0.9), FFM (P > 0.3), or FM (P > 0.5).
Table 3.
Subject characteristics for follow-up analyses.
| Variables | Females |
Males |
P value for sex difference | P value for sex interaction | ||||
|---|---|---|---|---|---|---|---|---|
| Baseline | Follow up | Δ (P value) | Baseline | Follow up | Δ (P value) | |||
| Study 1: delta VO2max vs. delta body composition | ||||||||
| N (number) | 55 | 80 | ||||||
| Race (number) | Full heritage American Indians of southwestern heritage = 55 | Full heritage American Indians of southwestern heritage = 77, other Indians = 3 | > 0.1 | |||||
| Age (years) | 25.7 ± 5.7 | 29.7 ± 6.3 | 4.0 ± 2.3 | 25.9 ± 5.7 | 30.7 ± 5.6 | 4.8 ± 2.2 | 0.043 | 0.094 |
| Body mass index (kg/m2) | 36.60 ± 6.52 | 38.24 ± 7.08 | 1.64 ± 3.35 (< 0.001) | 33.81 ± 8.30 | 36.35 ± 9.50 | 2.54 ± 4.17 (< 0.0001) | > 0.6 | > 0.4 |
| Body weight (kg) | 92.9 ± 18.6 | 97.1 ± 20.0 | 4.2 ± 8.4 (< 0.001) | 99.0 ± 27.2 | 106.4 ± 31.5 | 7.4 ± 12.7 (< 0.0001) | > 0.5 | > 0.3 |
| Fat free mass (kg) | 55.1 ± 8.6 | 57.0 ± 8.9 | 1.9 ± 3.9 (< 0.001) | 68.5 ± 12.5 | 72.0 ± 13.8 | 3.5 ± 6.1 (< 0.0001) | > 0.6 | > 0.4 |
| Fat mass (kg) | 37.8 ± 11.6 | 40.0 ± 12.0 | 2.3 ± 6.8 (= 0.016) | 30.5 ± 16.0 | 34.6 ± 18.5 | 4.1 ± 8.7 (< 0.001) | > 0.3 | > 0.1 |
| % fat mass (%) | 39.9 ± 5.9 | 40.5 ± 4.6 | 0.7 ± 4.3 (> 0.2) | 28.7 ± 9.0 | 30.5 ± 7.8 | 1.8 ± 5.2 (< 0.01) | 0.030 | > 0.1 |
| VO2max (mL/min) | 2280.9 ± 357.1 | 2332.2 ± 443.0 | 51.3 ± 338.7 (> 0.2) | 3421.4 ± 525.4 | 3462.6 ± 696.3 | 41.2 ± 562.2 (> 0.5) | > 0.6 | > 0.9 |
| Study 2: VO2max vs. weight change | ||||||||
| N (number) | 97 | 120 | ||||||
| Race (number) | Full heritage American Indians of southwestern heritage = 97 | Full heritage American Indians of southwestern heritage = 114, other Indians = 6 | 0.026 | |||||
| Age (years) | 25.1 ± 5.5 | 36.4 ± 8.1 | 11.3 ± 6.8 | 26.2 ± 5.9 | 37.3 ± 7.9 | 11.1 ± 6.9 | > 0.5 | > 0.5 |
| Body mass index (kg/m2) | 35.17 ± 7.49 | 39.75 ± 8.59 | 4.58 ± 5.32 (< 0.0001) | 32.69 ± 8.19 | 36.79 ± 10.44 | 4.10 ± 5.28 (< 0.0001) | 0.028 | 0.033 |
| Body weight (kg) | 89.2 ± 20.4 | 101.5 ± 22.9 | 12.3 ± 13.6 (< 0.0001) | 95.6 ± 26.4 | 108.1 ± 33.3 | 12.5 ± 15.9 (< 0.0001) | 0.025 | 0.021 |
The differences between baseline and follow up were analyzed by pared t-test. A probability of sex difference in the frequency of Full heritage American Indians of southwestern heritage was analyzed by Chi-Square test. A probability of sex difference or sex interaction was analyzed by general linear model.
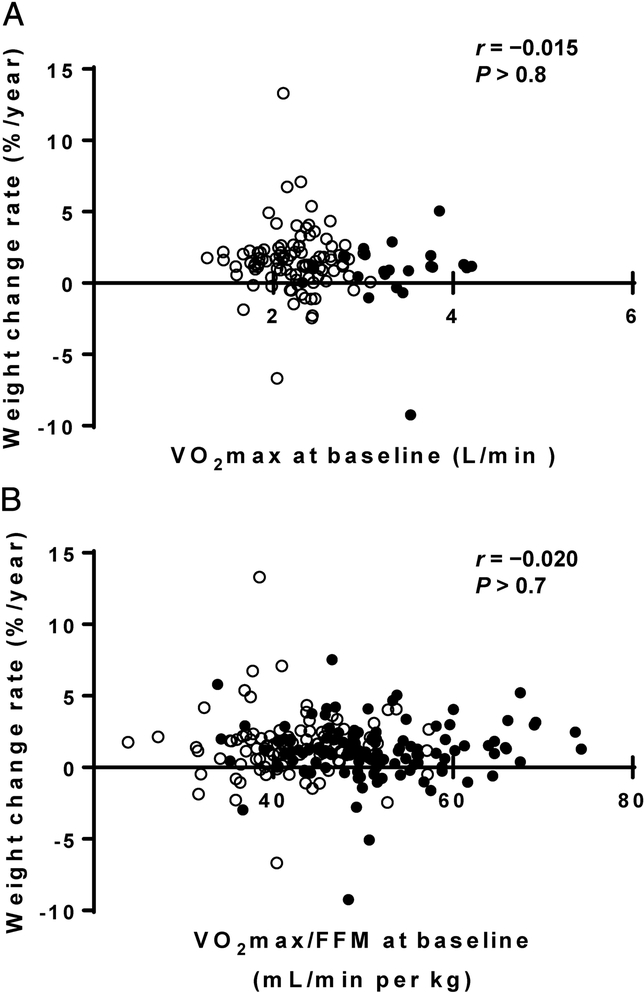
Of the 357 individuals, 217 (97 females) had available follow-up weight data (Table 3). The baseline characteristics of these 217 individuals did not differ from entire cohort. Mean weight change was 12.4 ± 14.9 kg over a median follow-up time of 9.5 years (IQR: 5.5–17.0 years). Nether VO2max (P > 0.8, Figure 3A) nor VO2max divided by baseline FFM (P > 0.7, Figure 3B) were associated with the rate of weight change (%/year). VO2max did not predict follow-up weight after adjustment for baseline weight, age, sex, and follow-up time as covariates (P > 0.4) or change in weight after adjustment for sex, age, and follow-up time (P > 0.1). Similar results were found when VO2max was divided by baseline FFM (P > 0.5). On the other hand, RMR inversely predicted follow-up weight (Standardized β = −0.175, P = 0.013, N = 206) after adjustment for baseline weight, sex, age, and follow-up time and change in weight (Standardized β = −0.159, P = 0.023, N = 206) after adjustment for sex, age, and follow-up time.
Figure 3-.
Associations between VO2max versus weight change rate. (A) Baseline VO2max versus weight change rate. (B) Baseline VO2max/FFM versus weight change rate. Open circles, females; closed circles, males.
Discussion
In a large cohort including Native Americans of southwestern heritage in whom measures of EE including RMR, 24-h EE and AFT predict weight change, we found that VO2max is associated with RMR, 24-h EE and AFT. However, this did not translate into an effect of VO2max on subsequent weight or body composition change, even though in this cohort we found that lower RMR was associated with higher weight gain in Native Americans as previously reported [58].
Previous studies have examined whether VO2max is associated with EE and/or weight change. These studies have shown mixed results. In terms of the association of VO2max with EE, most of the previous studies did not have complete or gold standard measures of EE, as we did in this study. In fact, we had measures of EE on 2 separate occasions (24-h EE as measured in our whole room calorimeter and RMR measured on a separate day using a ventilated hood system), and we demonstrated positive associations of VO2max with EE for both measures. Moreover, our measure of DIT (which we term AFT), but not sleeping EE, was also associated with VO2max.The only previous study with a relatively large sample size (n=78) and similar measures did not find significant associations between VO2max and 24-h EE, RMR, or DIT [7].
Despite these associations with EE, VO2max did not predict free-living weight change in this Native American population. Many previous longitudinal studies have demonstrated a protective effect of CRF on weight management[5, 42–45]. However, CRF was assessed differently across studies, and none has used indirect calorimetry during exercise along with appropriate measures of body composition to adjust for confounders. Because our study measured actual VO2max by expired gas during exercise at maximum effort, we were able to run the longitudinal analyses for weight change based on VO2max and body composition independently. To the extent that VO2max represents PA and CRF, our result is consistent with the conclusion of a recent systematic review which reported that PA does not strongly affect weight or fat gain[59].
For every 1 L of VO2max increase, there was an associated increase in daily EE by approximately 60 kcal. This increased EE is comparatively small when compared with total absolute EE (≈ < 3% of 24-h EE). However, the interindividual variability in VO2max after adjusting body composition, sex, age, and race still ranged from –1.1–1.4L; this translates to a 156 kcal difference in EE (2.7×62.2 kcal/day) between individuals with relatively higher and lower VO2max. This is on par with the EE difference which in our previous studies has accounted for weight gain in this population (a 100 kcal decrease in EE was associated with a 0.2kg/y weight gain)[60]. In addition, as it has been suggested by Hill et al.[61, 62], that a daily EE difference of less than 1% is sufficient to predispose to weight gain. Our data indicates that variability in VO2max however is not a determinant of weight change.
Whether a common mechanisms might underlie associations between VO2max and measures of EE, especially DIT, remains unclear. Skeletal muscle mitochondrial content and function share common associations between VO2max and EE. Consistent with our findings, in humans, mitochondrial density and respiration in the vastus lateralis correlates with VO2max, RMR, and glucose and insulin-induced thermogenesis during insulin clamp[63]. Adrenergic tone affects blood volume distribution and delivery in upright versus supine positions which might explain the association between VO2max and 24hEE but the lack of association with SMR [64]. Several lines of evidence indicate a common mechanism between VO2max and DIT. Exercising and sedentary adults have differences in the β-adrenergic thermic response which contributes to the variation in DIT between these individuals[38]. Postprandial gut hormone responses may also link VO2max and DIT. Regular exercise training leads to higher post-meal GLP-1 and PYY concentrations and higher GLP and PYY response to meal is associated with higher postprandial thermogenesis albeit following Roux-en-Y gastric bypass or vertical banded gastroplasty[65]. VO2max is also correlated with short-chain fatty acids production via fermentation[66]. Short-chain fatty acids act on colonic enteroendocrine L cells and promote GLP-1 and PYY secretion[67]. Thus, there is evidence for a possible common mechanism explaining these associations.
One possible explanation why VO2max was not a predictor of weight change despite its association with EE may be the mediating effect of exercise. A review including several studies using doubly labeled water for assessment of EE concluded that exercise-induced increases in EE resulted in increased food intake[68]. Thus, greater fitness, as influenced by regular PA and resulting in higher VO2max, may increase energy intake opposing any favorable effect on daily EE. However, even if regular PA does not result in weight loss or maintaining weight, it should be noted that regular PA has several other benefits for health. For example, a recent exercise intervention study showed that 4-weeks of exercise training did not lead to weight loss but lowered intrahepatic lipid content [69].
Our study has several strengths. We had measures of body composition, allowing appropriate adjustment for confounders for both EE and VO2max. Also, we had two separate measures of EE. One assessed using the gold standard method over 24 h in a whole room calorimeter and RMR using a metabolic cart performed on a separate day. We also had a large sample size with follow-up of both weight and body composition. In terms of confounding factors, most previous studies divide VO2max by FFM. As shown in Figure 1, the intercept of the association between VO2max and FFM was different from zero, indicating the need to use multiple regression analysis, as we did, for the proper adjustment of VO2max and for comparing the interindividual difference among individuals of varying body size.
We acknowledge our study has also some limitations. First, we were unable to explore potential physiological mechanisms underlying the association between the VO2max and EE measures. Second, those with follow-up measures of weight and body composition were fewer than the overall cohort and were only Native American. This, somewhat limits the overall power and the generalizability of our findings.
In conclusion, higher VO2max was associated with greater 24-h EE, greater AFT and higher RMR. Although every 1 L of VO2max was associated on average with increased daily EE by 60 kcal, we did not see an effect of VO2max on long-term weight change. Thus, VO2max and 24-h EE and DIT may share common mechanisms, however any protective effect of higher VO2max on weight gain appears to be limited. Thus, training to improve VO2max as a method to prevent weight gain or promote weight loss may not be an effective weight management strategy.
Highlights:
Higher VO2max was associated with greater 24-h energy expenditure (EE).
Every 1 L of VO2max was associated with increased daily EE by 60 kcal.
VO2max does not predict weight change in American Indians of southwestern heritage.
Acknowledgements:
Our heartfelt thanks are due to all staffs, researchers, and subjects who participated in this study.
Funding: Funded by intramural research program of the National Institutes of Diabetes, Digestive and Kidney Diseases.
Abbreviations:
- AFT
awake and fed thermogenesis
- BMI
body mass index
- CRF
cardiorespiratory fitness
- DIT
diet-induced thermogenesis
- EE
energy expenditure
- FFM
fat free mass
- FM
fat mass
- PA
physical activity
- RMR
resting metabolic rate
- RQ
respiratory quotient
- SMR
sleeping metabolic rate
- SPA
spontaneous physical activity
Footnotes
Competing Interests: None of eclare.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Berthouze SE, Minaire PM, Castells J, Busso T, Vico L, Lacour JR. Relationship between mean habitual daily energy expenditure and maximal oxygen uptake. Med Sci Sports Exerc. 1995;27:1170–9. [PubMed] [Google Scholar]
- [2].Williams CJ, Williams MG, Eynon N, Ashton KJ, Little JP, Wisloff U, et al. Genes to predict VO2max trainability: a systematic review. BMC Genomics. 2017;18:831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Kodama S, Saito K, Tanaka S, Maki M, Yachi Y, Asumi M, et al. Cardiorespiratory fitness as a quantitative predictor of all-cause mortality and cardiovascular events in healthy men and women: a meta-analysis. JAMA. 2009;301:2024–35. [DOI] [PubMed] [Google Scholar]
- [4].Harber MP, Kaminsky LA, Arena R, Blair SN, Franklin BA, Myers J, et al. Impact of Cardiorespiratory Fitness on All-Cause and Disease-Specific Mortality: Advances Since 2009. Prog Cardiovasc Dis. 2017;60:11–20. [DOI] [PubMed] [Google Scholar]
- [5].Brien SE, Katzmarzyk PT, Craig CL, Gauvin L. Physical activity, cardiorespiratory fitness and body mass index as predictors of substantial weight gain and obesity: the Canadian physical activity longitudinal study. Can J Public Health. 2007;98:121–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Johnson MS, Figueroa-Colon R, Herd SL, Fields DA, Sun M, Hunter GR, et al. Aerobic fitness, not energy expenditure, influences subsequent increase in adiposity in black and white children. Pediatrics. 2000;106:E50. [DOI] [PubMed] [Google Scholar]
- [7].Sharp TA, Reed GW, Sun M, Abumrad NN, Hill JO. Relationship between aerobic fitness level and daily energy expenditure in weight-stable humans. Am J Physiol. 1992;263:E121–8. [DOI] [PubMed] [Google Scholar]
- [8].Kriketos AD, Sharp TA, Seagle HM, Peters JC, Hill JO. Effects of aerobic fitness on fat oxidation and body fatness. Med Sci Sports Exerc. 2000;32:805–11. [DOI] [PubMed] [Google Scholar]
- [9].Schulz LO, Nyomba BL, Alger S, Anderson TE, Ravussin E. Effect of endurance training on sedentary energy expenditure measured in a respiratory chamber. Am J Physiol. 1991;260:E257–61. [DOI] [PubMed] [Google Scholar]
- [10].Morio B, Montaurier C, Pickering G, Ritz P, Fellmann N, Coudert J, et al. Effects of 14 weeks of progressive endurance training on energy expenditure in elderly people. Br J Nutr. 1998;80:511–9. [DOI] [PubMed] [Google Scholar]
- [11].Roy HJ, Lovejoy JC, Keenan MJ, Bray GA, Windhauser MM, Wilson JK. Substrate oxidation and energy expenditure in athletes and nonathletes consuming isoenergetic high- and low-fat diets. Am J Clin Nutr. 1998;67:405–11. [DOI] [PubMed] [Google Scholar]
- [12].Lee MG, Sedlock DA, Flynn MG, Kamimori GH. Resting metabolic rate after endurance exercise training. Med Sci Sports Exerc. 2009;41:1444–51. [DOI] [PubMed] [Google Scholar]
- [13].Gilliat-Wimberly M, Manore MM, Woolf K, Swan PD, Carroll SS. Effects of habitual physical activity on the resting metabolic rates and body compositions of women aged 35 to 50 years. J Am Diet Assoc. 2001;101:1181–8. [DOI] [PubMed] [Google Scholar]
- [14].Sullo A, Cardinale P, Brizzi G, Fabbri B, Maffulli N. Resting metabolic rate and post-prandial thermogenesis by level of aerobic power in older athletes. Clin Exp Pharmacol Physiol. 2004;31:202–6. [DOI] [PubMed] [Google Scholar]
- [15].Potteiger JA, Kirk EP, Jacobsen DJ, Donnelly JE. Changes in resting metabolic rate and substrate oxidation after 16 months of exercise training in overweight adults. Int J Sport Nutr Exerc Metab. 2008;18:79–95. [DOI] [PubMed] [Google Scholar]
- [16].Sjodin AM, Forslund AH, Westerterp KR, Andersson AB, Forslund JM, Hambraeus LM. The influence of physical activity on BMR. Med Sci Sports Exerc. 1996;28:85–91. [DOI] [PubMed] [Google Scholar]
- [17].Thorne A, Wahren J. Diet-induced thermogenesis in well-trained subjects. Clin Physiol. 1989;9:295–305. [DOI] [PubMed] [Google Scholar]
- [18].Miller WM, Spring TJ, Zalesin KC, Kaeding KR, Janosz KEN, McCullough PA, et al. Lower than predicted resting metabolic rate is associated with severely impaired cardiorespiratory fitness in obese individuals. Obesity. 2012;20:505–11. [DOI] [PubMed] [Google Scholar]
- [19].Davis JR, Tagliaferro AR, Kertzer R, Gerardo T, Nichols J, Wheeler J. Variations of dietary-induced thermogenesis and body fatness with aerobic capacity. Eur J Appl Physiol Occup Physiol. 1983;50:319–29. [DOI] [PubMed] [Google Scholar]
- [20].Tagliaferro AR, Kertzer R, Davis JR, Janson C, Tse SK. Effects of exercise-training on the thermic effect of food and body fatness of adult women. Physiol Behav. 1986;38:703–10. [DOI] [PubMed] [Google Scholar]
- [21].Hill JO, Heymsfield SB, McMannus C, 3rd DiGirolamo M Meal size and thermic response to food in male subjects as a function of maximum aerobic capacity. Metabolism. 1984;33:743–9. [DOI] [PubMed] [Google Scholar]
- [22].Segal KR, Blando L, Ginsberg-Fellner F, Edano A. Postprandial thermogenesis at rest and postexercise before and after physical training in lean, obese, and mildly diabetic men. Metabolism. 1992;41:868–78. [DOI] [PubMed] [Google Scholar]
- [23].Burkhard-Jagodzinska K, Nazar K, Ladyga M, Starczewska-Czapowska J, Borkowski L. Resting metabolic rate and thermogenic effect of glucose in trained and untrained girls age 11–15 years. Int J Sport Nutr. 1999;9:378–90. [DOI] [PubMed] [Google Scholar]
- [24].Byrne HK, Wilmore JH. The relationship of mode and intensity of training on resting metabolic rate in women. Int J Sport Nutr Exerc Metab. 2001;11:1–14. [DOI] [PubMed] [Google Scholar]
- [25].Broeder CE, Burrhus KA, Svanevik LS, Wilmore JH. The effects of either high-intensity resistance or endurance training on resting metabolic rate. Am J Clin Nutr. 1992;55:802–10. [DOI] [PubMed] [Google Scholar]
- [26].Broeder CE, Burrhus KA, Svanevik LS, Wilmore JH. The effects of aerobic fitness on resting metabolic rate. Am J Clin Nutr. 1992;55:795–801. [DOI] [PubMed] [Google Scholar]
- [27].Smith DA, Dollman J, Withers RT, Brinkman M, Keeves JP, Clark DG. Relationship between maximum aerobic power and resting metabolic rate in young adult women. J Appl Physiol (1985) 1997;82:156–63. [DOI] [PubMed] [Google Scholar]
- [28].Santa-Clara H, Szymanski L, Ordille T, Fernhall B. Effects of exercise training on resting metabolic rate in postmenopausal African American and Caucasian women. Metabolism. 2006;55:1358–64. [DOI] [PubMed] [Google Scholar]
- [29].Wilmore JH, Stanforth PR, Hudspeth LA, Gagnon J, Daw EW, Leon AS, et al. Alterations in resting metabolic rate as a consequence of 20 wk of endurance training: the HERITAGE Family Study. Am J Clin Nutr. 1998;68:66–71. [DOI] [PubMed] [Google Scholar]
- [30].Scharhag-Rosenberger F, Meyer T, Walitzek S, Kindermann W. Effects of one year aerobic endurance training on resting metabolic rate and exercise fat oxidation in previously untrained men and women. Metabolic endurance training adaptations. Int J Sports Med. 2010;31:498–504. [DOI] [PubMed] [Google Scholar]
- [31].Karstoft K, Brinklov CF, Thorsen IK, Nielsen JS, Ried-Larsen M. Resting Metabolic Rate Does Not Change in Response to Different Types of Training in Subjects with Type 2 Diabetes. Front Endocrinol (Lausanne). 2017;8:132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Ratcliff L, Gropper SS, White BD, Shannon DM, Huggins KW. The influence of habitual exercise training and meal form on diet-induced thermogenesis in college-age men. Int J Sport Nutr Exerc Metab. 2011;21:11–8. [DOI] [PubMed] [Google Scholar]
- [33].Ravussin E, Bogardus C. Relationship of genetics, age, and physical fitness to daily energy expenditure and fuel utilization. Am J Clin Nutr. 1989;49:968–75. [DOI] [PubMed] [Google Scholar]
- [34].Tanskanen M, Uusitalo AL, Hakkinen K, Nissila J, Santtila M, Westerterp KR, et al. Aerobic fitness, energy balance, and body mass index are associated with training load assessed by activity energy expenditure. Scand J Med Sci Sports. 2009;19:871–8. [DOI] [PubMed] [Google Scholar]
- [35].Midorikawa T, Tanaka S, Ando T, Tanaka C, Masayuki K, Ohta M, et al. Is There a Chronic Elevation in Organ-Tissue Sleeping Metabolic Rate in Very Fit Runners? Nutrients. 2016;8:196. [Google Scholar]
- [36].Meijer GA, Westerterp KR, Seyts GH, Janssen GM, Saris WH, ten Hoor F. Body composition and sleeping metabolic rate in response to a 5-month endurance-training programme in adults. Eur J Appl Physiol Occup Physiol. 1991;62:18–21. [DOI] [PubMed] [Google Scholar]
- [37].Burke CM, Bullough RC, Melby CL. Resting metabolic rate and postprandial thermogenesis by level of aerobic fitness in young women. Eur J Clin Nutr. 1993;47:575–85. [PubMed] [Google Scholar]
- [38].Stob NR, Bell C, van Baak MA, Seals DR. Thermic effect of food and beta-adrenergic thermogenic responsiveness in habitually exercising and sedentary healthy adult humans. J Appl Physiol (1985). 2007;103:616–22. [DOI] [PubMed] [Google Scholar]
- [39].Westerterp KR, Meijer GA, Schoffelen P, Janssen EM. Body mass, body composition and sleeping metabolic rate before, during and after endurance training. Eur J Appl Physiol Occup Physiol. 1994;69:203–8. [DOI] [PubMed] [Google Scholar]
- [40].Tremblay A, Cote J, LeBlanc J. Diminished dietary thermogenesis in exercise-trained human subjects. Eur J Appl Physiol Occup Physiol. 1983;52:1–4. [DOI] [PubMed] [Google Scholar]
- [41].LeBlanc J, Mercier P, Samson P. Diet-induced thermogenesis with relation to training state in female subjects. Can J Physiol Pharmacol. 1984;62:334–7. [DOI] [PubMed] [Google Scholar]
- [42].Lewis CE, Smith DE, Wallace DD, Williams OD, Bild DE, Jacobs DR Jr. Seven-year trends in body weight and associations with lifestyle and behavioral characteristics in black and white young adults: the CARDIA study. Am J Public Health. 1997;87:635–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].DiPietro L, Kohl HW 3rd, Barlow CE, Blair SN Improvements in cardiorespiratory fitness attenuate age-related weight gain in healthy men and women: the Aerobics Center Longitudinal Study. Int J Obes Relat Metab Disord. 1998;22:55–62. [DOI] [PubMed] [Google Scholar]
- [44].Larew K, Hunter GR, Larson-Meyer DE, Newcomer BR, McCarthy JP, Weinsier RL. Muscle metabolic function, exercise performance, and weight gain. Med Sci Sports Exerc. 2003;35:230–6. [DOI] [PubMed] [Google Scholar]
- [45].Tucker L, Peterson T. Fitness level and risk of weight gain in middle-age women: a prospective cohort study. J Phys Act Health. 2010;7:308–15. [DOI] [PubMed] [Google Scholar]
- [46].Goran M, Fields DA, Hunter GR, Herd SL, Weinsier RL. Total body fat does not influence maximal aerobic capacity. Int J Obes Relat Metab Disord. 2000;24:841–8. [DOI] [PubMed] [Google Scholar]
- [47].Maciejczyk M, Wiecek M, Szymura J, Szygula Z, Wiecha S, Cempla J. The influence of increased body fat or lean body mass on aerobic performance. PLoS One. 2014;9:e95797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Williamson DF, Kahn HS, Remington PL, Anda RF. The 10-year incidence of overweight and major weight gain in US adults. Arch Intern Med. 1990;150:665–72. [PubMed] [Google Scholar]
- [49].Fortier MD, Katzmarzyk PT, Bouchard C. Physical activity, aerobic fitness, and seven-year changes in adiposity in the Canadian population. Can J Appl Physiol. 2002;27:449–62. [DOI] [PubMed] [Google Scholar]
- [50].Genuth S, Alberti KG, Bennett P, Buse J, Defronzo R, Kahn R, et al. Follow-up report on the diagnosis of diabetes mellitus. Diabetes Care. 2003;26:3160–7. [DOI] [PubMed] [Google Scholar]
- [51].Pannacciulli N, Salbe AD, Ortega E, Venti CA, Bogardus C, Krakoff J. The 24-h carbohydrate oxidation rate in a human respiratory chamber predicts ad libitum food intake. Am J Clin Nutr. 2007;86:625–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Siri W Body composition from fluid spaces and desity: analysis of methods Techniques for Mensuring Body Composition Washington, DC: National Academia of Science and National Research Council; 1961:233–44. [Google Scholar]
- [53].Bogardus C, Lillioja S, Mott D, Reaven GR, Kashiwagi A, Foley JE. Relationship between obesity and maximal insulin-stimulated glucose uptake in vivo and in vitro in Pima Indians. J Clin Invest. 1984;73:800–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Ravussin E, Lillioja S, Anderson TE, Christin L, Bogardus C. Determinants of 24-hour energy expenditure in man. Methods and results using a respiratory chamber. J Clin Invest. 1986;78:1568–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Lusk G Animal calorimetry twenty-fourth paper. Analysis of the oxidation of mixtures of carbohydrate and fat. Journal of Biological Chemistry. 1924;59:41–2. [Google Scholar]
- [56].Piaggi P, Krakoff J, Bogardus C, Thearle MS. Lower “awake and fed thermogenesis” predicts future weight gain in subjects with abdominal adiposity. Diabetes. 2013;62:4043–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Tataranni PA, Ravussin E. Use of dual-energy X-ray absorptiometry in obese individuals. The American journal of clinical nutrition. 1995;62:730–4. [DOI] [PubMed] [Google Scholar]
- [58].Ravussin E, Lillioja S, Knowler WC, Christin L, Freymond D, Abbott WG, et al. Reduced rate of energy expenditure as a risk factor for body-weight gain. N Engl J Med. 1988;318:467–72. [DOI] [PubMed] [Google Scholar]
- [59].Wilks DC, Besson H, Lindroos AK, Ekelund U. Objectively measured physical activity and obesity prevention in children, adolescents and adults: a systematic review of prospective studies. Obes Rev. 2011;12:e119–29. [DOI] [PubMed] [Google Scholar]
- [60].Piaggi P, Thearle MS, Bogardus C, Krakoff J. Lower energy expenditure predicts long-term increases in weight and fat mass. J Clin Endocrinol Metab. 2013;98:E703–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Hill JO, Wyatt HR, Reed GW, Peters JC. Obesity and the environment: where do we go from here? Science. 2003;299:853–5. [DOI] [PubMed] [Google Scholar]
- [62].Hill JO. Can a small-changes approach help address the obesity epidemic? A report of the Joint Task Force of the American Society for Nutrition, Institute of Food Technologists, and International Food Information Council. Am J Clin Nutr. 2009;89:477–84. [DOI] [PubMed] [Google Scholar]
- [63].Toledo FGS, Dube JJ, Goodpaster BH, Stefanovic-Racic M, Coen PM, DeLany JP. Mitochondrial Respiration is Associated with Lower Energy Expenditure and Lower Aerobic Capacity in African American Women. Obesity (Silver Spring). 2018;26:903–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Warburton DE, Gledhill N, Quinney HA. Blood volume, aerobic power, and endurance performance: potential ergogenic effect of volume loading. Clin J Sport Med. 2000;10:59–66. [DOI] [PubMed] [Google Scholar]
- [65].Werling M, Olbers T, Fandriks L, Bueter M, Lonroth H, Stenlof K, et al. Increased postprandial energy expenditure may explain superior long term weight loss after Roux-en-Y gastric bypass compared to vertical banded gastroplasty. PLoS One. 2013;8:e60280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Estaki M, Pither J, Baumeister P, Little JP, Gill SK, Ghosh S, et al. Cardiorespiratory fitness as a predictor of intestinal microbial diversity and distinct metagenomic functions. Microbiome. 2016;4:42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Psichas A, Sleeth M, Murphy K, Brooks L, Bewick G, Hanyaloglu A, et al. The short chain fatty acid propionate stimulates GLP-1 and PYY secretion via free fatty acid receptor 2 in rodents. International journal of obesity. 2015;39:424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Westerterp KR. Physical activity, food intake, and body weight regulation: insights from doubly labeled water studies. Nutr Rev. 2010;68:148–54. [DOI] [PubMed] [Google Scholar]
- [69].Winn NC, Liu Y, Rector RS, Parks EJ, Ibdah JA, Kanaley JA. Energy-matched moderate and high intensity exercise training improves nonalcoholic fatty liver disease risk independent of changes in body mass or abdominal adiposity—A randomized trial. Metabolism. 2018;78:128–40. [DOI] [PubMed] [Google Scholar]