Abstract
Background:
Several single-site alcohol treatment clinical trials have demonstrated efficacy for immediate-release (IR) gabapentin in reducing drinking outcomes among individuals with alcohol dependence. The purpose of this study was to conduct a large, multisite clinical trial of gabapentin enacarbil extended-release (GE-XR) (HORIZANT®), a gabapentin prodrug formulation, to determine its safety and efficacy in treating alcohol use disorder (AUD).
Methods:
Men and women (n= 346) who met DSM–5 criteria for at least moderate AUD were recruited across 10 US clinical sites. Participants received double-blind GE-XR (600 mg twice a day [BID]) or placebo and a computerized behavioral intervention (Take Control) for 6 months. Efficacy analyses were pre-specified for the last 4 weeks of the treatment period.
Results:
The GE-XR and placebo groups did not differ significantly on the primary outcome measure, percentage of subjects with no heavy drinking days (28.3 vs 21.5, respectively, p=0.157). Similarly, no clinical benefit was found for other drinking measures (percent subjects abstinent, percent days abstinent, percent heavy drinking days, drinks per week, drinks per drinking day), alcohol craving, alcohol-related consequences, sleep problems, smoking, and depression/anxiety symptoms. Common side-effects were fatigue, dizziness, and somnolence.
A population pharmacokinetics analysis revealed that patients had lower gabapentin exposure levels compared with those in other studies using a similar dose but for other indications.
Conclusion:
Overall, GE-XR at 600 mg BID did not reduce alcohol consumption or craving in individuals with AUD. It is possible that, unlike the IR formulation of gabapentin, which showed efficacy in smaller Phase 2 trials at a higher dose, GE-XR is not effective in treating AUD, at least not at doses approved by the FDA for treating other medical conditions.
Keywords: alcohol use disorder, gabapentin enacarbil extended-release, Horizant, randomized placebo-controlled clinical trial
INTRODUCTION
Alcohol Use Disorder (AUD) is a highly prevalent, highly comorbid disorder, affecting more than 15 million adults in the US. (https://pubs.niaaa.nih.gov/publications/AlcoholFacts&Stats/AlcoholFacts&Stats.htm). Advances have been made in medications to treat AUD, highlighted by U.S. Food and Drug Administration (FDA) approval of three medications to treat alcohol dependence, specifically disulfiram, oral and long-acting injectable naltrexone, and acamprosate. Nonetheless, many people do not respond to these medications. Thus, efforts have been made to develop and evaluate new medications (Litten et al., 2015).
One promising agent being investigated for AUD treatment is gabapentin immediate-release (G-IR). G-IR currently is approved by the FDA for the treatment of epileptic seizures, neuropathic pain, and restless leg syndrome (http://www.caremark.com/portal/asset/FEP_Rationale_Gabapentin.pdf). The mechanism of action of gabapentin is unclear, though it appears to have multiple cellular effects, including selectively blocking voltage-gated calcium channels with the α2δ−1 subunit, enhancement of voltage-gated potassium channels, and modulation of GABA activity (Sills, 2006).
The rationale underlying gabapentin as a treatment for AUD is founded on preclinical evidence that G-IR reduced alcohol intake in alcohol-dependent rats and normalized stress-induced GABA activation in the extended amygdala (Roberto et al., 2008), a stress-related brain region activated during early abstinence in alcohol dependence (Koob, 2008). Clinically, G-IR reduced symptoms of acute alcohol withdrawal (Myrick et al., 2009) and improved alcohol-induced sleep disruption in a polysomnography study of normal participants (Bazil et al., 2005). In human laboratory studies, 1200 mg/d of G-IR diminished symptoms of protracted abstinence, including craving and sleep disturbance (Mason et al., 2009), which have been identified as risk factors for relapse (Lowman et al., 1996; Brower et al., 1998; Foster and Peters, 1999) (see also review Mason et al., 2018).
Several single-site, placebo-controlled randomized clinical trials (RCTs) have evaluated the efficacy of G-IR in alcohol dependent individuals. Mason et al. (2014) conducted a 12-week RCT of G-IR (900 mg/d and 1800 mg/d) in 150 men and women diagnosed with alcohol dependence. Compared with placebo, G-IR significantly increased rates of abstinence and percentage of subjects with no heavy drinking days in a dose dependent fashion. In addition, G-IR improved measures of mood and sleep and reduced alcohol craving. There were no serious adverse effects, with the most common side-effects being fatigue, insomnia, and headaches. Furieri and Nakamura-Palacios (2007) conducted a 4-week RCT of G-IR (600 mg/d) in 60 alcohol dependent men and, compared with placebo, found improvement in the number of drinks per day, percentage of heavy drinking days, and percentage of days abstinent. In another RCT, Brower et al. (2008) found that G-IR (titrated up to 1500 mg/d) significantly delayed the onset to heavy drinking in 21 individuals with alcohol dependence and comorbid insomnia. In a small study, Anton et al. (2009) found that G-IR (up to 1200 mg/d for 39 days) combined with flumazenil, a benzodiazepine receptor antagonist (20 mg/d for the first 2 days), was associated with an increase in the percentage of days abstinent and a longer delay to heavy drinking in a subgroup of alcohol dependent individuals (n=16) who had relatively high pre-treatment alcohol withdrawal symptoms. In another RCT, Anton et al. (2011) found that alcohol dependent individuals (n=150) treated with G-IR (1200 mg/d) combined with oral naltrexone (50 mg/d) experienced better outcomes on several measures of drinking, craving, and sleep than the group taking naltrexone alone or those receiving the placebo over the first six weeks.
The present study focuses on gabapentin enacarbil extended-release (GE-XR) (Horizant®, Arbor Pharmaceuticals, LLC, Atlanta, GA), a relatively new, extended-release, prodrug formulation of gabapentin approved by the FDA for the treatment of post-herpetic neuralgia (PHN) and restless legs syndrome (FDA, 2013). This prodrug formulation is actively absorbed by the high capacity nutrient transporters, monocarboxylate transporters Type 1 and sodium-dependent multivitamin transporters, located throughout the intestinal tract (Cundy et al., 2008). After absorption, conversion to gabapentin takes place by nonspecific esterases, primarily in enterocytes. One advantage of the prodrug, compared with G-IR, is a reduction in inter-patient variability in the blood levels and increased bioavailability (Cundy et al., 2008). Furthermore, whereas G-IR is taken 3 times per day, GE-XR only needs to be taken 2 times per day, which may result in better treatment adherence, an important aspect to consider when developing medications for addiction (Weiss, 2004).
The purpose of this study is to provide the first RCT evaluation of the efficacy and safety of GE-XR as a treatment for AUD. This was also the first 6-month, multi-site, double-blind, placebo-controlled RCT of a gabapentin formulation that adhered to FDA guidelines for pivotal alcohol pharmacotherapy trials (FDA, 2015).
MATERIALS AND METHODS
Study Population
Randomized participants (n= 346) were diagnosed with at least moderate AUD (i.e., 4 or more criteria) in the past year according to the Diagnostic and Statistical Manual, 5th edition (DSM–5) (American Psychiatric Association, 2013). Participants were eligible if they were at least 21 years of age; reported drinking an average of at least 21 standard drinks per week for women or 28 standard drinks per week for men and had at least one heavy drinking day per week during the 28-day period before consent; and at least 3 consecutive days of abstinence prior to randomization. Participants had not been diagnosed with a current substance use disorder (other than alcohol or nicotine) or major psychiatric disorder (psychotic, bipolar, and eating disorders; major depressive episode). They did not have underlying medical conditions for which gabapentin might be contraindicated or that could be exacerbated during trial participation. Use of most psychiatric medications was exclusionary except for the stable use of antidepressants (see Supplementary Appendices 1 and 2 for the full inclusion/exclusion criteria and assessment schedule, respectively).
Study Design
The study was a pivotal, randomized, double-blind, placebo controlled, parallel-group, 26-week treatment clinical trial. Candidates were treatment-seeking volunteers who responded by telephone to advertisements from 10 academic sites in the US between June 2015 and February 2017. The study (Protocol # NCIG 006) was approved by the local Institutional Review Board at each participating clinical site; all participants in the study provided their voluntary, written informed consent before initiation of any study procedures and were compensated for time and travel. See Supplementary Appendix 3 for details on clinical sites and study oversight.
Participants completed a screening and baseline visit, during which eligibility was established, as well as 11 in-clinic visits and 17 telephone visits during non-visit weeks. A follow-up telephone interview was conducted during weeks 28–29 (approximately 1–2 weeks after the last in-clinic study visit) to assess safety and changes in drinking. Participants were required to have a breath alcohol concentration ≤ 0.02% to complete the in-clinic assessments.
Participants were randomly assigned, in a 1:1 ratio, to receive either GE-XR or matched placebo using a permuted block randomization procedure stratified by clinical site. Clinical site was chosen as the stratification variable because both local study populations and the investigative staff influence on the subject’s drinking behaviors may differentially influence endpoints. Randomization was implemented via a centralized, interactive web-based response system (IWRS). See Supplementary Appendix 4 for additional details on randomization and blinding.
Investigational Product
GE-XR (manufactured by Arbor Pharmaceuticals, LLC) was dispensed during in-clinic visits for 26 weeks using a double-blind method. GE-XR was supplied in 600 mg tablets with identical matching placebo tablets. A 600 mg tablet of GE-XR contains 313 mg equivalents of gabapentin. For both the GE-XR and placebo groups, the daily dose was titrated from 1 tablet (600 mg or placebo) on days 1–3, to a target dose of 2 tablets (600 mg or placebo twice a day, for 1200mg total) on days 4–7 and weeks 2–25, followed by a taper to 1 tablet (600 mg or placebo) during week 26. GE-XR was selected over other oral gabapentin products because it confers more uniform and increased bioavailability, faster titration time to full therapeutic dose, and less fluctuating gabapentin blood levels with twice daily administration (Cundy et al., 2008). This dose (600 mg twice a day) was selected because it is the highest approved dose of GE-XR for an FDA-approved indication (PHN), and it achieves a similar level of efficacy as higher doses of GE-XR (2400 mg or 3600 mg) on pain outcomes while maintaining a more favorable adverse event profile (Zhang et al., 2013).
Participants who could not tolerate the target dose were permitted to taper their dose to 600 mg once daily. If 600 mg daily was not tolerated, medication was discontinued but those participants were encouraged to remain in the study, participate in study assessments, and continue to receive the behavioral platform (for details, see below). Dosage compliance was verified by comparing the participants’ self-report to pill count. Medication compliance was calculated as the total amount of medication taken, divided by the total amount prescribed during the maintenance phase of the study (weeks 2–25). To validate adherence and conduct a population pharmacokinetic (Pop PK) analysis, gabapentin plasma levels were determined from blood samples collected at weeks 12, 20, and 24 (pre-dose, 8 and 12 hours post dose) that were analyzed using a liquid chromatography–mass spectrometry/mass spectrometry (LC–MS/MS) method validated for gabapentin in plasma over the range 80–10,000 ng/mL Estimated population Pop PK parameters were used to compare drug exposure with prior studies (FDA, 2013) and to evaluate a dose-response relationship between gabapentin systemic exposure and drinking.
Behavioral Platform
Participants viewed Take Control modules, a computerized bibliotherapy platform (Devine et al., 2016), at each in-clinic visit.
Measures of Efficacy
Alcohol consumption was captured via the Time-Line Follow-Back and Form 90 interview methodology and procedures (Sobell and Sobell, 1992; Miller, 1992). Drinks were converted into standard drink units (1 standard drink = 0.6 oz of pure alcohol) for all subsequent analyses. The a priori primary efficacy endpoint was percentage of subjects with no heavy drinking days (PSNHDD) (Falk et al., 2010) during the last 4 weeks of the maintenance phase of the study (weeks 22–25). A ‘heavy drinking day’ was defined as four or more drinks (women) or five or more drinks (men) per drinking day.
A priori secondary efficacy end points (weeks 22–25) included other drinking measures (percentage of heavy drinking days, percentage of days abstinent, drinks per week, drinks per drinking day, percentage of subjects abstinent, and percentage of subjects with a reduction of at least 1- or 2-levels in World Health Organization drinking risk categories) (Hasin, 2017) as well as severity of alcohol craving (Alcohol Craving Scale–Short Form [ACQ-SF-R], Singleton et al., 2000); number of alcohol-related consequences (ImBIBe, a revised and abbreviated form of the Drinker Inventory of Consequences; Litten et al., 2013; Miller, 1995; Werner et al., 2008); Pittsburg Sleep Quality Index (PSQI) (Buysse et al., 1989) score; mood, as assessed by the Beck Anxiety Inventory (BAI) (Beck et al., 1988), Beck Depression Inventory Scale–II (BDI-II) (Beck et al., 1996), and the Profile of Moods States (POMS) (McNair et al., 1992); and the number of cigarettes smoked per week among smokers. Exploratory endpoints included the percentage of subjects with a negative blood phosphatidylethanol (PEth) (United States Drug Testing Laboratories, Inc., Des Plaines, IL), an objective biomarker used to confirm self-reported alcohol consumption endpoints); the number of AUD criteria endorsed, an indicator of AUD severity; and the percentage of subjects abstinent from smoking among smokers.
Prior research shows that drinking during the first several months of treatment is relatively unstable and not highly predictive of long-term outcomes (Kline-Simon et al., 2014). Thus, a 5-month grace period was granted for all efficacy endpoints. A grace period is an early period in a trial where outcome is not considered in the final analysis because the measured treatment effect is not thought to represent the full potential of the drug (Falk et al., 2010). Based on FDA guidance, a grace period is permitted for Phase 3 clinical trials (FDA, 2015). Sensitivity analyses examined other grace periods, as well as the full maintenance period (weeks 2–25).
Safety Assessments
Safety was assessed via vital signs; blood chemistry tests; urine tests for illicit drug use; blood alcohol concentration, as measured by breathalyzer; adverse events; concomitant medication use; cardiac conduction, measured by electrocardiogram; alcohol withdrawal, measured by the Clinical Institute Withdrawal Assessment for Alcohol–revised (CIWA-Ar) [Sullivan et al., 1989); and suicidal ideation, measured by the Columbia Suicide Severity Rating Scale (Posner et al., 2011). Adverse events were assessed in the clinic and during telephone interviews using the open-ended question: “How have you been feeling since your last visit?”
Statistical Analysis
Statistical analyses were similar to our previous trial (Ryan et al., 2017). Baseline safety and efficacy analyses (except for the prespecified models examining smoking among smokers) were analyzed on a modified intention-to-treat (mITT) population that included all randomized participants who received at least one dose of investigational product (n=338; GE-XR=170, placebo=168). The smoking efficacy models included only participants who were smokers (i.e., smoked at least one cigarette in the past week at baseline) (n=105; GE-XR=50, placebo=55). As a sensitivity analysis, efficacy analyses were also analyzed on an evaluable population of participants randomized to the study who took at least 80% of the per-protocol prescribed dose (269 tablets) during the maintenance period (weeks 2–25) and who did not have a major protocol violation (n=232; GE-XR=115, placebo=117).
Continuous outcomes were measured at multiple time points and analyzed using a repeated-measures mixed-effects model. Least-square means (LSMEANs), standard errors (SEs), and 95% confidence intervals (CIs) are presented for each treatment group and were derived from fully adjusted models on untransformed outcomes (to facilitate clinical interpretation), averaged across the last 4 weeks of the maintenance period (weeks 22–25). Cohen’s d and p-values were based on the fully adjusted models with the appropriately transformed outcome variables (if skewed).
For dichotomous outcomes, unadjusted prevalence rates were determined during the last 4 weeks of the maintenance period. Odds ratios (ORs) and p-values were derived from fully adjusted logistic regression models; the number of covariates were limited by the number of events for each dichotomous outcome (Peduzzi et al., 1996).
Except for the primary outcome, no imputation was performed for missing data in the tabled model results. However, as a sensitivity analysis, models were re-estimated with imputation for missing data. For dichotomous outcomes (besides the WHO outcomes), participants with any missing outcome data were imputed as treatment failures. For percentage of heavy drinking days and percentage of days abstinent, days with missing drinking data were imputed as heavy drinking days and drinking days, respectively. For other continuous outcomes, and WHO outcomes, missing data were handled by multiple imputation.
Exploratory moderator analyses were conducted on the imputed primary efficacy outcome, percentage of heavy drinking days (weeks 22–25), to evaluate whether a differential treatment effect existed as a function of 26 patient characteristics of theoretical and scientific interest. These characteristics included patient demographics; baseline measures of alcohol consumption, smoking, alcohol-related severity, mood, sleep, and impulsivity; and medication exposure. A model similar to the primary efficacy model was used for each moderator tested and included moderator and treatment-by-moderator interaction terms.
To evaluate the possibility that alcohol consumption affected the bioavailability of GE-XR (Bode and Bode, 2003; Cundy et al., 2008; Elamin et al., 2013; FDA, 2013), a post-hoc analysis compared the alcohol consumption in the 2 days prior to blood measurement among those with low versus high systemic exposure to gabapentin (AUC24,ss).
For all statistical tests, p<0.05 (two-tailed) was considered statistically significant. No adjustment was made for multiple inferential tests. For the primary outcome, an estimated sample size of 346 participants yielded 91% power to detect a treatment effect comparable to that obtained by Mason et al. (2014) (OR=2.5; GE-XR=27% and placebo=13%), given a two-tailed 0.05 significance level and assuming a 15% dropout rate where dropouts were imputed as treatment failures. Data were analyzed with SAS version 9.4 (SAS Institute, Cary, NC). See Supplementary Appendix 5 for additional details regarding the statistical analysis.
RESULTS
Study Sample
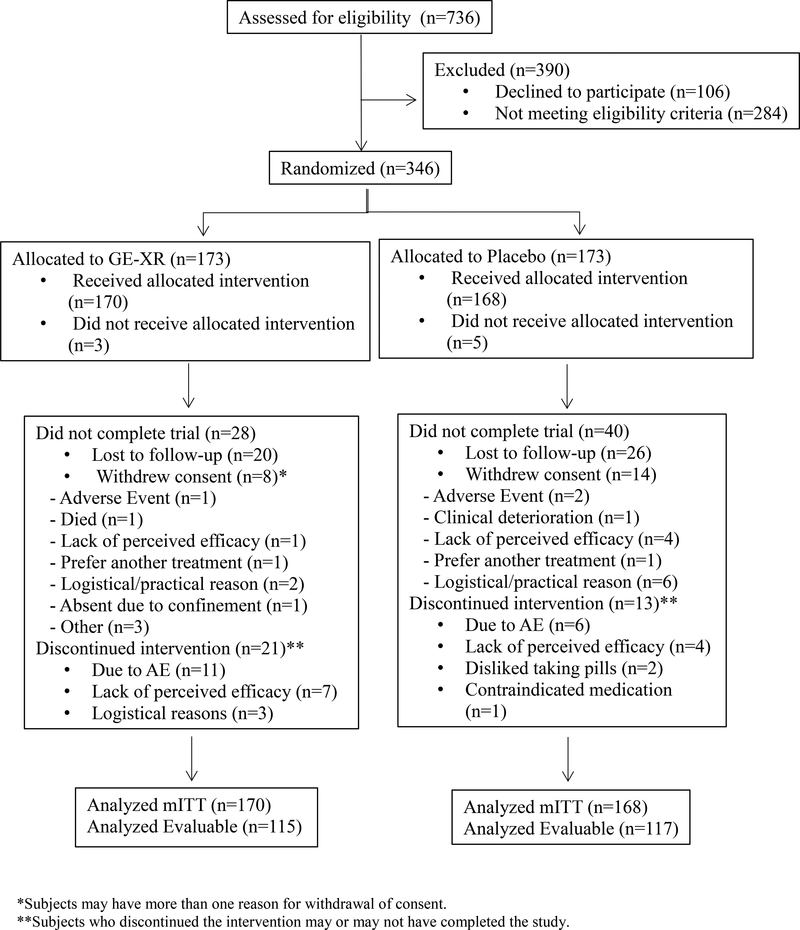
Of the 736 participants consented for the study, 346 were eligible and therefore randomized to receive GE-XR or placebo (n=173 per group); 390 were excluded because they did not meet eligibility criteria or they chose not to participate (see Fig 1). The top reasons for exclusion included positive urine toxicology drug screen (20.8%), not meeting drinking criteria (14.9%), unable to participate in clinic/phone visits (9.0%), and having a clinically-relevant complicating medical condition (7.7%). The mITT population excluded 8 randomized participants who never received investigational product resulting in GE-XR (n=170) and placebo (n=168). In the mITT population, fewer participants in the GE-XR group withdrew early from the study than in the placebo group (n=28 [16.5%] vs. n=40 [23.8%], respectively; p=0.092); however, more participants in the GE-XR group discontinued medication than in the placebo group (n=21 [12.4%] vs. n=13 [7.7], respectively; p=0.158].
Fig 1. Study Profile (CONSORT).
*Subjects may have more than one reason for withdrawal of consent.
**Subjects who discontinued the intervention may or may not have completed the study.
Participants in the GE-XR and placebo groups were not statistically different on any baseline characteristic except gender and the Barratt Impulsivity Scale – second order Attention factor (BIS-Attention; Table 1) (Patton et al., 1995). Randomized mITT participants were mostly male, white, employed, and middle-aged, with approximately 15 years of education. On average participants drank heavily, consuming ~ 56 drinks per week, and met or exceeded four drinks (women) or five drinks (men) per drinking day on ~ 77% of the days. With respect to treatment drinking goals, ~ 9% desired permanent abstinence, whereas the majority sought to drink in a limited manner. About one-third (31%) smoked at least one cigarette in the week before the screening visit, averaging 77.7 cigarettes per week. On average, participants had very low levels of alcohol withdrawal (CIWA-Ar=1.5); non-elevated levels of anxiety, depression, and mood disturbance (BAI=7.3, BDI-II=10.5, POMS Total Mood Disturbance=4.9); and were just above the cutoff for poor sleep quality (PSQI=6.7).
Table 1.
Baseline Characteristics of Patients (mITT Population)
| Placebo (n=168) | GE-XR (n=170) | p-valuea | |||||
|---|---|---|---|---|---|---|---|
| n | Mean or % | SD | n | Mean or % | SD | ||
| Demographics | |||||||
| Age | 49.4 | 11.4 | 50.7 | 10.3 | 0.268 | ||
| Gender | |||||||
| Male | 101 | 60.1% | 122 | 71.8% | 0.029 | ||
| Female | 67 | 39.9% | 48 | 28.2% | |||
| Employed | 122 | 73.1% | 133 | 78.7% | 0.252 | ||
| Married | 168 | 48.2% | 80 | 47.3% | 0.913 | ||
| Education (years) | 15.2 | 2.8 | 15.3 | 2.5 | |||
| Race/Ethnicity | |||||||
| White | 116 | 70.7% | 111 | 67.7% | 0.959 | ||
| Black | 26 | 15.9% | 33 | 20.1% | |||
| Hispanic | 18 | 11.0% | 14 | 8.5% | |||
| Other | 4 | 2.4% | 6 | 3.7% | |||
| Self-Reported Alcohol Consumptionb | |||||||
| Drinks per week | 56.3 | 29.4 | 56.8 | 28.0 | 0.878 | ||
| Drinks per drinking day | 9.3 | 4.5 | 9.3 | 4.5 | 0.979 | ||
| Percent days abstinent | 12.9 | 16.2 | 12.0 | 14.5 | 0.566 | ||
| Percent heavy drinking days | 76.6 | 23.3 | 78.2 | 20.9 | 0.499 | ||
| World Health Organization (WHO) risk level (drinks per day) | |||||||
| Medium (men: 2.9–4.3; women: 1.4–2.9) | 10 | 6.0% | 8 | 4.7% | 0.314 | ||
| High (men: 4.3–7.1; women: 2.9–4.3) | 42 | 25.0% | 55 | 32.4% | |||
| Very High (men: 7.1+; women: 4.3+) | 116 | 69.0% | 107 | 62.9% | |||
| Other Substance-Related Indicators | |||||||
| Alcohol Craving Questionnaire (ACQ-SF-R) | 3.6 | 1.2 | 3.6 | 1.2 | 0.891 | ||
| Alcohol-related consequences (ImBIBe) | 20.4 | 9.7 | 19.5 | 10.4 | 0.373 | ||
| Age onset of regular drinking | 20.2 | 7.0 | 19.7 | 7.0 | 0.490 | ||
| Alcohol use disorder symptoms (MINI) | 7.4 | 2.0 | 7.4 | 2.2 | 0.975 | ||
| Alcohol use disorder severity (MINI) | |||||||
| Moderate (4–5 symptoms) | 40 | 23.8% | 45 | 26.5% | 0.617 | ||
| Severe (6+ symptoms) | 128 | 76.2% | 125 | 73.5% | |||
| Abstinence alcohol-related treatment goalc | 13 | 7.7% | 18 | 10.6% | 0.452 | ||
| Motivation to reach goal | 8.8 | 1.4 | 8.9 | 1.5 | 0.501 | ||
| Confidence to reach goal | 6.6 | 2.4 | 6.9 | 2.2 | 0.121 | ||
| Parental history of alcohol-related problems | 85 | 50.9% | 89 | 53.3% | 0.743 | ||
| Current smoker (any vs. none) | 55 | 32.7% | 50 | 29.4% | 0.557 | ||
| Cigarettes (past-week) among smokers | 88.4 | 112 | 65.8 | 62.9 | 0.212 | ||
| Marijuana used | 17 | 10.1% | 14 | 8.2% | 0.577 | ||
| Psychiatric Characteristics | |||||||
| Pittsburgh Sleep Quality Index (PSQI) | 6.6 | 3.8 | 6.7 | 3.8 | 0.845 | ||
| Barratt Impulsivity Scale (BIS) | |||||||
| Attention - second order factor | 17.2 | 3.2 | 16.6 | 2.6 | 0.037 | ||
| Motor - second order factor | 22.4 | 4.2 | 21.7 | 4.0 | 0.124 | ||
| Non-Planning - second order factor | 28.7 | 4.6 | 28.8 | 4.5 | 0.855 | ||
| Beck Anxiety Inventory (BAI) | 7.2 | 7.7 | 7.3 | 7.2 | 0.964 | ||
| Beck Depression Inventory (BDI-II) | 10.8 | 9.1 | 10.2 | 8.3 | 0.492 | ||
| Profile of Mood States (POMS) - Total Mood Disturbance | 6.5 | 25.6 | 3.3 | 21.9 | 0.217 | ||
| Clinical Institute Withdrawal Assessment of Alcohol - Revised (CIWA-Ar) | 1.4 | 2.1 | 1.6 | 2.8 | 0.301 | ||
mITT = modified Intention-to-Treat (took at least one dose of investigational product).
Group mean differences were tested for significance via t-tests of independent samples for normally-distributed variables or Wilcoxon rank-sum tests for skewed variables. Group prevalence rate differences were tested for significance via chi-square or Fisher’s exact tests.
Reflects mean values during the 28-day period before screening.
Abstinence defined as permanent abstinence vs. other. Motivation and confidence are single likert scales (1–10).
Marijuana use based on positive urine drug screen.
Note: scale, number of questions (range), and interpretive values are as follows:
ACQ-SF-R: 12 questions (1–7)
ImBIBe: 15 questions (0–60)
PSQI: 19 questions (0–21); ≥6 indicative of "poor" sleep quality
BIS: Attention 8 questions (8–32); Motor 11 questions (11–44); Non-Planning 11 questions (11–44)
BAI: 21 questions (0–63); ≥10 indicative of at least "mild" anxiety
BDI-II: 21 questions (0–63); ≥14 indicative of at least "mild" depression
POMS: 65 questions (-32–200); ≥18 greater than "normal" in general population
CIWA-Ar: 10 questions (0–67); ≥10 indicative of alcohol withdrawal symptoms that may require treatment
Variable (n) missing data: race/ethnicity (GE-XR=6, placebo=4), marital status (GE-XR=1), employment status (GE-XR=1, placebo=1), POMS-TMD (placebo=1), Parental History (GE-XR=4, placebo=1), Motivation & Confidence (GE-XR=1)
Medication Compliance and Participation
Overall, medication compliance during the maintenance phase was 92.3% and was similar for both treatment groups (92.6% and 92.0% for GE-XR and placebo groups, respectively; p=0.699). The median number of pills taken during the maintenance phase was nearly identical in both groups: 318.5 pills in GE-XR group and 320 in the placebo (or ~95% of the possible 336 pills) (p=0.956). Analyte levels of gabapentin were largely consistent with patient self-reports of medication consumption (concordance rates: 86.8%–89.0% during weeks 12, 20, and 24). The estimated average peak concentration (Cmax) and 24-hour AUC at steady-state (AUC24,ss) obtained from a Pop PK analysis of GE-XR were 4.21 μg/mL and 83.1 μg.hr/mL, respectively. Overall, 83.4% of mITT participants had complete drinking data during the maintenance phase, with the GE-XR group being slightly higher than the placebo group (86.5% vs. 80.4%, respectively), which was not statistically significant (p= 0.145).
Primary Efficacy Outcome
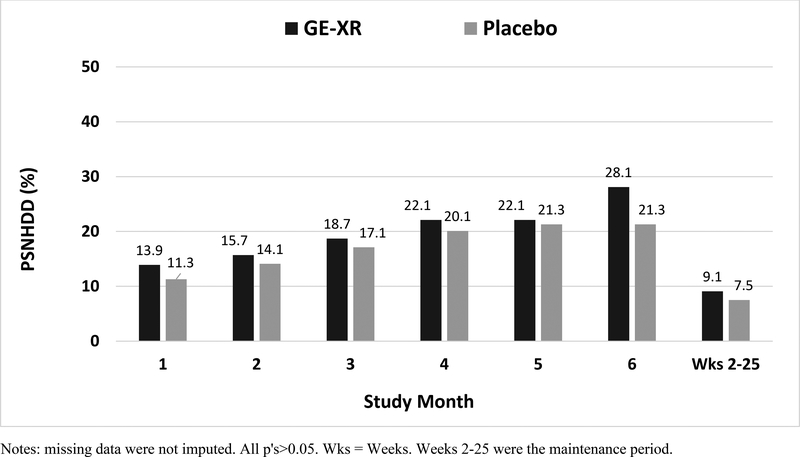
Averaged across the last 4 weeks of the maintenance period (weeks 22–25), the GE-XR group had somewhat higher levels of the primary outcome, percentage of subjects with no heavy drinking days (PSNHDD), than the placebo group (28.3 vs. 21.5, respectively); adjusted odds ratio (aOR)=1.53 (95% CI=0.85–2.75), although this small effect was not statistically significant (p=0.157) (Table 2). The treatment effect was similar, and also not statistically significant, when participants with missing drinking data were imputed as treatment failures (GE-XR=24.1 vs. placebo=17.3, respectively); aOR=1.50 (95% CI=0.86–2.62; p= 0.157) and also for the evaluable subpopulation (GE-XR =28.6% vs. placebo =19.8%, respectively); aOR=1.62 (95% CI = 0.84–3.14; p = 0.153). Treatment effects were small and nonsignificant for each month of the trial and across the entire maintenance period (all p’s>0.05) (Figure 2).
Table 2.
Treatment Outcomes: Differences Between Placebo and GE-XR during Last Treatment Month (Weeks 22–25)
| Placebo (n=168) | GE-XR (n=170) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Drinking Outcomes | LSMEANb | SE | 95% CI | LSMEAN | SE | 95% CI | LSMEAN Δ | SE | d | p-value |
| Percent heavy drinking days | ||||||||||
| No imputation | 32.6 | 3.8 | 25.1 – 40.0 | 31.8 | 3.7 | 24.4 – 39.1 | −0.8 | 3.6 | -0.03 | 0.826 |
| Missing imputed as heavy drinking days | 46.5 | 4.2 | 38.2 – 54.9 | 43.1 | 4.1 | 35.0 – 51.1 | −3.4 | 4.1 | -0.09 | 0.397 |
| Percent days abstinent | 49.0 | 3.9 | 41.3 – 56.7 | 49.3 | 3.9 | 41.7 – 56.9 | 0.3 | 3.8 | 0.09 | 0.371 |
| Drinks per week | 21.4 | 2.4 | 16.8– 26.0 | 23.1 | 2.3 | 18.5 – 27.7 | 1.7 | 2.3 | 0.07 | 0.545 |
| Drinks per drinking day | 3.9 | 0.4 | 3.5 – 5.0 | 4.1 | 0.4 | 3.3 – 4.8 | 0.2 | 0.4 | 0.05 | 0.641 |
| % | n | denom | % | n | denom | % Δ | aOR (95% CI)c | p-value | ||
| Percent subjects with no heavy drinking days* | ||||||||||
| No imputation | 21.5 | 29 | 135 | 28.3 | 41 | 145 | 6.8 | 1.53 (0.85–2.75) | 0.157 | |
| Missing imputed as heavy drinker | 17.3 | 29 | 168 | 24.1 | 41 | 170 | 6.8 | 1.50 (0.86–2.62) | 0.157 | |
| Percent subjects abstinent | 11.8 | 16 | 136 | 11.6 | 17 | 146 | −0.2 | 0.86 (0.38–1.95) | 0.717 | |
| WHO 1-shift reduction | 79.9 | 107 | 134 | 78.8 | 115 | 146 | −1.1 | 0.87 (0.48–1.58) | 0.642 | |
| WHO 2-shift reduction | 51.5 | 69 | 134 | 54.8 | 80 | 146 | 3.3 | 1.11 (0.69–1.80) | 0.674 | |
| Percent subjects negative blood PEth | 3.4 | 4 | 116 | 6.1 | 8 | 132 | 2.7 | 1.35 (0.37–4.96) | 0.653 | |
| Non-Drinking Outcomes | ||||||||||
| Percent subjects abstinent from smokinga | 17.1 | 7 | 41 | 7.1 | 3 | 42 | −10.0 | 0.37 (0.09–1.56) | 0.178 | |
| LSMEAN | SE | 95% CI | LSMEAN | SE | 95% CI | LSMEAN Δ | SE | d | p-value | |
| Cigarettes per weeka | 61.4 | 8.3 | 44.9 – 77.9 | 71.0 | 8.3 | 54.4 – 87.6 | 9.6 | 11.6 | 0.31 | 0.162 |
| Alcohol Craving Questionnaire (ACQ-SF-R) score | 2.5 | 0.1 | 2.4 – 2.7 | 2.5 | 0.1 | 2.3 – 2.6 | −0.1 | 0.1 | −0.06 | 0.623 |
| Alcohol-related consequences (ImBIBe) scoreg | 8.3 | 0.7 | 6.9 – 9.7 | 9.6 | 0.7 | 8.3 – 10.9 | 1.3 | 1.0 | 0.13 | 0.239 |
| Alcohol use disorder DSM-5 criteria (#) | 2.8 | 0.2 | 2.4 – 3.2 | 3.4 | 0.2 | 3.0 – 3.8 | 0.6 | 0.3 | 0.24 | 0.046 |
| Pittsburg Sleep Quality Inventory (PSQI) score | 4.4 | 0.2 | 4.0– 4.9 | 4.9 | 0.2 | 4.4 – 5.3 | 0.4 | 0.3 | 0.16 | 0.152 |
| Beck Depression Inventory (BDI-II) score | 5.2 | 0.5 | 4.1 – 6.2 | 6.5 | 0.5 | 5.5 – 7.5 | 1.3 | 0.7 | 0.23 | 0.046 |
| Beck Anxiety Inventory score | 3.3 | 0.5 | 2.4 4.2 | 4.6 | 0.4 | 3.7 5.4 | 1.3 | 0.6 | 0.14 | 0.208 |
| Profile of Mood Scale (POMS) - Total Mood | ||||||||||
| Disturbance score | 0.3 | 1.8 | −3.3 – 3.8 | 3.9 | 1.7 | 0.5 – 7.2 | 3.6 | 2.4 | 0.17 | 0.139 |
GE-XR = gabapentin enacarbil, LSMEANS = least squared means, SE = standard error, CI = confidence interval, Δ = GE-XR - placebo difference, denom = denominator, d= Cohen’s d (GE-XR-placebo), aOR = adjusted odds ratio, * = a priori primary endpoint.
Notes: Models were based on a mITT population that included subjects who received at least one dose of medication. No imputation was used for missing outcome data, unless otherwise specified. For continuous outcomes, LSMEANS were estimated from fully adjusted models on untransformed outcomes (for interpretive purposes); corresponding Cohen's d and p-values were based on the same model but with the appropriately transformed outcome.
Models for smoking outcomes included only participants who were smokers at baseline (i.e., smoked at least one cigarette per day in the past week) (GE-XR n=50, placebo n=55).
Fig 2. Percentage of Subjects No Heavy Drinking Days (Primary Outcome) across the Treatment Period (mITT).
Notes: missing data were not imputed. All p’s>0.05. Wks = Weeks. Weeks 2–25 were the maintenance period.
Of the 26 moderators evaluated, 2 were statistically significant: treatment drinking goal (p=0.016) and BIS-Attention (p=0.044). Specifically, compared with placebo, the GE-XR group had a significantly higher PSNHDD among the subset of participants whose goal was non-permanent abstinence (aOR=2.68, 95% CI=1.26–5.67, p=0.010), yet had non-significantly lower PSNHDD among the subset of participants who sought permanent abstinence (aOR=0.61, 95% CI=0.24–1.55, p=0.298). In addition, compared with placebo, the GE-XR group had a significantly higher PSNHDD among participants with low BIS-Attention scores (aOR=2.61, 95% CI=1.17–5.81, p=0.019) and non-significantly lower PSNHDD among participants with higher BIS-Attention scores (aOR=0.80, 95% CI=0.35–1.82, p=0.599). Although the treatment-by-moderator interactions were not statistically significant for the other moderators, GE-XR was significantly more efficacious than placebo in 2 subgroups (elevated PSQI [aOR=2.24, 95% CI=1.03–4.88, p=0.042]; and non-smokers [aOR=2.08, 95% CI=1.03–4.22, p=0.043]; non-statistical trends (p’s<0.10) were observed for 6 other subgroups (low alcohol consumption, low POMS Vigor-Activity, high alcohol craving, high reduction in alcohol consumption prior to randomization, no history of alcohol withdrawal, and women). A sensitivity analysis using another outcome, percentage of heavy drinking days, revealed no statistically significant moderator interactions or subgroups (data not shown). See Supplementary Appendix 6 for further moderator analysis results.
In the Pop PK analysis, participants with higher AUC24,ss (≥ 83 μg.hr/mL, [n=74]) were significantly more likely to be classified as a non-heavy drinker than participants with lower AUC24,ss (< 83 μg.hr/mL, [n=73]) (31.1% vs 17.8%, aOR=2.53, 95% CI-1.06–6.04, p=0.036), indicating that higher exposure resulted in less alcohol consumption (similar results were obtained for Cmax, data not shown).
Secondary Efficacy Outcomes
Averaged across the last 4 weeks of the maintenance period, the GE-XR and placebo groups were statistically similar on all secondary measures of alcohol consumption, alcohol craving, alcohol-related consequences, cigarette smoking, sleep quality, and anxiety (Table 2). The average number of DSM–5 AUD criteria was significantly higher in the GE-XR group than the placebo group (3.4 vs. 2.8, respectively; p=0.046; d= 0.24) as was the level of depression symptoms (BDI-II: 6.5 vs. 5.2, respectively; p=0.046; d=0.23). Results were similar when missing data were imputed or for the evaluable subpopulation (data not shown). Moreover, compared with placebo, GE-XR did not show a benefit on any secondary outcome for any of the times evaluated during the entire maintenance period (all treatment x time interactions p’s>0.05) (see Supplementary Appendix 7 for percentage of heavy drinking days outcome across maintenance period).
Analysis of alcohol affecting bioavailability of GE-XR revealed that, among participants with high GE-XR compliance (269+ pills), those with a relatively low blood levels of GE-XR (AUC24,ss below the median) drank more alcohol in the 2 days prior to blood measurement than those with relatively high bloods levels of GE-XR (4.5 vs. 3.0 drinks per day, p=0.010), despite having taken nearly an identical number of pills (321 vs. 323 pills, p=0.548).
Safety
Among participants who took at least one dose of study medication, 28 types of adverse effects (AEs) were reported in at least 5% of participants from either treatment group (Table 3). Of these, compared with the placebo group, the GE-XR group reported significantly greater rates of fatigue (25.9% vs. 15.5%; p= 0.022), somnolence (17.6% vs. 9.5%; p= 0.038), and tremor (5.9% vs. 0.6%; p= 0.010); a numerical, though not statistically significant, increase was found for dizziness (21.2% vs. 13.7%; p=0.085). Among GE-XR participants who reported at least 1 of these 4 AEs, the majority rated the AE as “mild” (65.0%), relative to “moderate” (33.3%) or “severe” symptoms (1.7%). Significantly lower rates of arthralgia and rash occurred in the GE-XR group compared with the placebo group; pruritus and depressed mood were statistical trends. Regarding AEs that occurred in fewer than 5% of participants, GE-XR produced a numerical, though not statistically significant, increase in suicidal ideation compared with placebo (n=7 [4.1%] vs. n=1 [0.6%]; p=0.067).
Table 3.
Number and Percentage of Participants with Adverse Events Occurring in at Least 5% of mITT Population
| MedDRA SOC/Preferred Term | Placebo (n=168) | GE-XR (n=170) | p-value1 |
|---|---|---|---|
| Blood pressure diastolic increased | 42 (25.0%) | 43 (25.3%) | 1.000 |
| Headache | 47 (28.0%) | 38 (22.4%) | 0.260 |
| Fatigue | 26 (15.5%) | 44 (25.9%) | 0.022 |
| Blood pressure systolic increased | 32 (19.0%) | 33 (19.4%) | 1.000 |
| Dizziness | 23 (13.7%) | 36 (21.2%) | 0.085 |
| Aspartate aminotransferase increased | 26 (15.5%) | 24 (14.1%) | 0.761 |
| Gamma-glutamyltransferase increased | 19 (11.3%) | 30 (17.6%) | 0.122 |
| Somnolence | 16 (9.5%) | 30 (17.6%) | 0.038 |
| Nasopharyngitis | 21 (12.5%) | 19 (11.2%) | 0.739 |
| Nausea | 23 (13.7%) | 17 (10.0%) | 0.316 |
| Upper respiratory tract infection | 17 (10.1%) | 22 (12.9%) | 0.497 |
| Insomnia | 17 (10.1%) | 18 (10.6%) | 1.000 |
| Alanine aminotransferase increased | 19 (11.3%) | 14 (8.2%) | 0.365 |
| Back pain | 19 (11.3%) | 11 (6.5%) | 0.130 |
| Vomiting | 8 (4.8%) | 15 (8.8%) | 0.194 |
| Blood creatinine increased | 8 (4.8%) | 14 (8.2%) | 0.270 |
| Anxiety | 7 (4.2%) | 14 (8.2%) | 0.175 |
| Diarrhoea | 10 (6.0%) | 11 (6.5%) | 1.000 |
| Arthralgia | 14 (8.3%) | 5 (2.9%) | 0.035 |
| Blood bilirubin increased | 9 (5.4%) | 10 (5.9%) | 1.000 |
| Cough | 6 (3.6%) | 13 (7.6%) | 0.155 |
| Paraesthesia | 6 (3.6%) | 11 (6.5%) | 0.320 |
| Abnormal dreams | 9 (5.4%) | 6 (3.5%) | 0.442 |
| Rash | 13 (7.7%) | 2 (1.2%) | 0.003 |
| Pruritus | 10 (6.0%) | 3 (1.8%) | 0.052 |
| Agitation | 3 (1.8%) | 9 (5.3%) | 0.139 |
| Depressed mood | 9 (5.4%) | 3 (1.8%) | 0.085 |
| Tremor | 1 (0.6%) | 10 (5.9%) | 0.010 |
Fisher‘s Exact test
Note: multiple occurrences of a specific adverse event for a subject were counted once in the frequency for the adverse event. Adverse events with at least 5% of participants occurring in either arm were included in the table, sorted by total prevalence.
As shown in Figure 1, only 1 patient in the GE-XR group withdrew from the study because of AEs (dizziness, headache, somnolence, feeling abnormal) vs. 2 in the placebo group (paranoia, suicidal ideation). However, more participants discontinued investigational product because of AEs in the GE-XR group than in the placebo group (n=11 [6.5%] vs. n=6 [3.6%], respectively).
Among participants reporting dizziness or somnolence, those in the GE-XR group had greater odds of experiencing dizziness and somnolence on drinking than non-drinking days (dizziness: OR=2.75, 95% CI =0.79–9.78, p=0.094; somnolence: OR=1.65, 95% CI=0.38–6.03, p=0.452) compared with the placebo group, suggesting that GE-XR may synergistically interact with alcohol to cause dizziness and somnolence; these associations were not statistically significant because of the small AE sample sizes.
Eight participants taking GE-XR experienced 11 events during the treatment phase that were rated as serious adverse events (SAEs): pneumonia (n=3), alcohol withdrawal syndrome (n=3), migraine headache (n=1), back pain (n=1), orbital fracture (n=1), orbital infection (n=1), and acute intoxication resulting in death (n=1). All SAEs were considered unlikely or unrelated to study medication by the Medical Monitor. Six participants taking placebo experienced 6 SAEs: bradycardia (n=1), suicidal ideation (n=1), paranoia (n=1), gastric ulcer (n=1), alcoholism (increasing alcohol consumption) (n=1), and humerus fracture (n=1). No additional differences between the GE-XR and placebo groups were rated as being clinically meaningful for any other safety measures.
DISCUSSION
This was the first multisite pivotal trial to evaluate the efficacy and safety of gabapentin enacarbil extended-release (GE-XR) in individuals with moderate-to-severe AUD. GE-XR was not effective in reducing any of the a priori-defined alcohol consumption or non-consumption outcomes. In fact, at the end of treatment, the placebo group reported significantly fewer AUD DSM–5 symptoms and lower depression scores than the GE-XR group (Table 2). The results were unexpected because several prior RCTs reported G-IR improved drinking and non-drinking outcomes in AUD patients (Anton et al., 2009; Anton et al., 2011; Brower et al., 1998; Furieri and Nakamura-Palacios, 2007; Mason et al., 2009; Mason et al., 2014).
There are several possible explanations for the lack of efficacy of GE-XR in this trial. First, because this was a new formulation of gabapentin, never studied for the treatment of AUD, it may not have been the optimal dose for showing efficacy in reducing drinking in AUD individuals. The dose used in this study was 1200 mg per day (600 mg twice a day), which is the dose approved by the FDA to treat PHN. For PHN, higher doses of 2400 and 3600 mg per day did not increase efficacy but did increase side-effects (Zhang et al., 2013). The FDA-approved dose of GE-XR for the treatment of restless legs syndrome is even lower, at 600 mg per day (FDA, 2013). In addition, our study target dose was selected because: 1) It was as efficacious on pain outcomes as the maximum approved daily dose of G-IR for treating PHN (1800 mg; 600 mg three times per day) (Rice and Maton, 2001; Zhang et al., 2013); and 2) given doses of G-IR ranging from 600 mg to 1800 mg per day have demonstrated efficacy in alcohol pharmacotherapy trials, the target dose for this study (1200 mg GE-XR) produces an intermediate systemic exposure (steady state AUC24,ss) between 900 mg and 1800 mg G-IR—approximately 40% lower systemic exposure than 1800 mg G-IR and 34% higher systemic exposure than 900 mg G-IR (Backonja et al., 2011; Bockbrader, 1995; FDA, 2012). Thus, the dose selected for this study was within the efficacious range for AUD in the literature. Yet it is possible that a higher dose may have been necessary to achieve efficacy for this indication as: 1) 1800 mg G-IR showed greater efficacy than 900 mg G-IR in a similarly-designed clinical trial (Mason et al., 2014) and 2) our Pop PK analysis indicated that higher exposure to gabapentin was associated with lower alcohol consumption. However, with regard to the latter, the possibility of reverse causation cannot be ruled out, that is, for the reasons discussed below, lower alcohol consumption could have resulted in higher exposure to gabapentin.
Second, alcohol may have reduced the bioavailability of gabapentin. It is possible that taking GE-XR in proximity to alcohol consumption may have degraded the extended-release properties, rendering it to be a mixture of extended (GE-XR) and immediate-release gabapentin enacarbil (GE-IR), which could have lowered the estimated AUC (Cundy et al., 2008). In vitro studies of GE-XR have demonstrated that alcohol accelerates the release of gabapentin enacarbil (between 43%–65% of gabapentin enacarbil is released within 1 hour when alcohol is present in concentrations ranging from 5%−40%) (FDA, 2013). In addition, because this prodrug formulation is actively absorbed by several transporters located throughout the gut (Cundy et al., 2008), it is possible that these transporters may have been negatively altered by alcohol. Alcohol is known to affect the integrity of the gut wall (Bode and Bode, 2003; Elamin et al., 2013), which could have diminished the medication’s bioavailability. Interestingly a post hoc analysis revealed that participants with relatively low blood levels of GE-XR drank more alcohol in the 2 days prior to blood measurement than those with relatively high bloods levels of GE-XR. Thus, consuming alcohol during the trial may have reduced the bioavailability of gabapentin.
Third, given the literature showing that bioavailability of GE-XR is greater in the fed than fasted state (particularly with high fat meals) (Cundy 2008; FDA 2012), and that individuals with high alcohol consumption often have poor dietary habits (Breslow et al., 2006), we conducted a post-hoc analysis of the PK data to explore whether bioavailability of gabapentin may have been impacted by food intake (or lack thereof) in the present study. Although diet was not explicitly studied, consistent with the PK literature, we found that AUC (and other PK parameters) was greater among patients whose PK samples were all taken in the fed state (87.5; 32% of patients) or a mixture of fed and fasted states (88.7; 55% of patients) than in patients whose samples were all taken in the fasted state (68.2; 13% of patients). Because the majority patients (87%) took GE-XR with food (on at least some days), the bioavailability of gabapentin could be considered maximized to some extent. Importantly, however, as we did not assess dietary fat content, it is unknown the degree to which this factor may have impacted gabapentin bioavailability.
Fourth, it is possible that, given the heterogeneity of the AUD population (Litten et al., 2015), average treatment effects do not sufficiently describe the efficacy of GE-XR and that more nuanced moderator analyses are necessary to show efficacy among only certain participant subgroups. However, despite an extensive analysis of 26 participant attributes, we were able to identify only 2 characteristics—treatment drinking goal and attentional impulsiveness—that were statistically significant, independent moderators of the treatment effect (i.e., greater treatment effects among participants with a treatment goal of non-permanent abstinence and low attention problems). Furthermore, only 2 additional subgroups (non-moderators) were statistically significant (i.e., those with elevated sleep problems and nonsmokers). Given the variety of these characteristics and the possibility of spurious findings given numerous statistical tests (2 of 26 moderators could be expected to be significant by chance alone), it is not possible to identify a cohesive participant responder profile. Because gabapentin is thought to reduce drinking by relieving aversive symptoms related to protracted withdrawal (Roberto et al., 2008; Mason et al., 2009; Mason et al., 2014), we hypothesized that gabapentin might have greater efficacy among subgroups with a history of withdrawal (endorsement of the MINI withdrawal symptom); relatively elevated sleep problems, anger/hostility, fatigue, tension/anxiety, mood disturbance, and depression; and relatively lower vigor-activity. However, GE-XR did not show a consistent pattern of efficacy across these characteristics (except for elevated sleep problems and lower vigor-activity). Similarly, GE-XR did not show differential efficacy as a function of AUD severity (AUD symptoms, alcohol consumption, craving, and alcohol-related consequences). Because GE-XR did not improve outcomes related to protracted withdrawal, it is perhaps not surprising that GE-XR generally had little effect on participants experiencing these symptoms at baseline.
GE-XR was well-tolerated in this study with no serious adverse effects related to the medication. Compared to placebo, medication adherence was similar, and study dropout was relatively lower, suggesting relatively low patient burden for GE-XR and good engagement, although somewhat more GE-XR patients discontinued medication. The most commonly reported side-effects were fatigue, dizziness, and somnolence, consistent with those of G-IR (FDA, 2013). Although there is some evidence from human laboratory studies that G-IR does not interact with alcohol (Bisaga and Evans, 2006; Myrick et al., 2007), consistent with the label for gabapentin, there was some evidence that GE-XR interacted with alcohol to increase rates of dizziness and somnolence, although the small numbers of participants experiencing these AEs preclude definitive conclusions. Additionally, while infrequent (<5% of participants), GE-XR was associated with higher rates of treatment-emergent suicidal ideation than placebo, which is consistent with the increased ideation rates reported for anti-epileptic medications (like gabapentin) as indicated in the GE-XR label (FDA, 2013). Thus, suicidal ideation should be monitored in patients taking GE-XR. Gabapentinoids, such as gabapentin and pregabalin have misuse potential, and there have also been reports that gabapentin is misused, especially among participants with opioid use disorder with some gabapentin-related deaths associated with other substances (Mersfelder and Nichols, 2016; Smith et al., 2016). Although not directly assessed, research staff did not voice any concerns that participants in this study were misusing study tablets.
Study strengths included a relatively large sample size, long treatment period (6 months vs. the 3 months typically used for Phase II trials within the alcohol field), high treatment retention, low rate of missing data, Pop PK evaluation, use of a standardized behavioral platform, and an extensive and rigorous evaluation of possible outcomes and moderators of treatment effect. Moreover, the study benefitted from a multisite design which increased the generalizability of results, though presumably at the expense of added site variability which may account for the observation that Phase 2 single-site trials are often not replicated in larger Phase 3 multisite trials (FDA, 2017). Study limitations included the lack of additional treatment arms to evaluate the efficacy and safety of higher doses of GE-XR in an AUD population and limited power to detect moderator effects. Also, like most AUD pharmacotherapy trials, the study excluded patients with significant psychiatric comorbidities and alcohol withdrawal which may limit generalizability to the subpopulation of severe patients seen in certain specialty treatment settings where these features are more prevalent.
In summary, although previous single-site studies have reported G-IR reduced drinking in patients with AUD, this multisite clinical trial did not observe any benefit of the GE-XR formulation on a variety of alcohol consumption and non-consumption outcomes in participants with moderate-to-severe AUD. It is possible the target dose was not adequate for this AUD population and/or that the heterogeneity of the population obscured a potential treatment effect. GE-XR was well-tolerated in trial participants. Additional studies may be needed to examine GE-XR at higher dosages, compare side-by-side GE-XR versus G-IR within the same RCT, and evaluate the effect of alcohol on the mechanism of action of the prodrug formulation as well as identifying subtypes of patients who might be more likely to benefit from this medication. Given the null efficacy results of the present study, weighed against the potential interaction of GE-XR with alcohol and the potential for misuse of gabapentinoids, GE-XR, at least at the dose tested in this present study, cannot be recommended for the treatment of AUD
Supplementary Material
Acknowledgments
The authors thank Barbara Vann of CSR, Incorporated for her excellent editorial comments.
Funding:
Supported by the National Institute on Alcohol Abuse and Alcoholism (NIAAA) (Contract HHSN275201400001l); ClinicalTrials.gov NCT 01613014.
Sponsor:
NIAAA
Other Support:
Medication and matched placebo were provided by Arbor Pharmaceuticals, LLC (previously known as Xenoport, Inc).
Footnotes
Conflicts of Interest
Daniel Falk, Megan Ryan, Joanne Fertig, Raye Litten, Mary Brunette, Kelly Dunn, Nassima Ait-Daoud Tiouririne, Catherine Brooks, Lara Ray, Steven Shoptaw, Eric Devine, Lorenzo Leggio, Barbara Mason, and Ricardo Cruz declare no conflicts of interest. Eric Strain consulted for or conducted research supported by Alkermes, Inc.; Indivior, PLC; Orphomed; and the World Health Organization; and served on Advisory Boards for Pinney Associates, Caron, Otsuka Pharmaceutical Development and Commercialization, Inc., and The Oak Group. Sherwood Brown received compensation from Otsuka pharmaceuticals (grant) and Genentech (honorarium). Ihsan Salloum received research support from Janssen Pharmaceutica, AstraZeneca, Abbott Laboratories, Ortho McNeil Pharm, Drug Abuse Sciences, Inc., Alkermes, Inc., Oy Contral Pharma, and Lipha Pharma; received unrestricted Educational Grants from AstraZeneca; served as consultant to Abbott Laboratories, Forest laboratories, Cephalon AstraZeneca, Eli Lilly, eTect, Otsuka Pharm, Takeda Pharm, and Orexigen; and served on the speakers’ bureaus for Abbott Laboratories and Sanofi-Aventis. D. Jeffrey Newport received research support from Eli Lilly, Glaxo SmithKline, Janssen, the National Alliance for Research on Schizophrenia and Depression, Takeda Pharmaceuticals, and Wyeth; served on speakers’ bureaus and/or received honoraria from Astra-Zeneca, Eli Lilly, Glaxo SmithKline, Pfizer, and Wyeth; and served on advisory boards for Glaxo SmithKline, Janssen, and Sage Therapeutics. Steve Caras is an employee of Arbor Pharmaceuticals LLC, the makers of Horizant® (the medication evaluated in this study). Alan Green received research support from Novartis and Janssen and has a U.S. patent regarding the treatment of substance misuse. Erik Gunderson received research support from Orexo, Inc and is on the scientific advisory boards for BDSI, Inc., Orexo, Inc, and Alkermes, Inc. Kyle Kampman received grant funding from Alkermes, Inc., Indivior, PLC Opiant, and Braeburn and is a consultant for Alkermes, Inc., and Opiant. Richard Rosenthal currently receives research funding from Braeburn pharmaceuticals and funding from Indivior, PLC and Alkermes, Inc., for CME activities.
REFERENCES
- American Psychiatric Association (2013) Diagnostic and statistical manual of mental disorders (5th ed.). Arlington, VA: Author. [Google Scholar]
- Anton RF, Myrick H, Baros AM, Latham PK, Randall PK, Wright TM, Stewart SH, Waid R, Malcolm R (2009) Efficacy of a combination of flumazenil and gabapentin in the treatment of alcohol dependence: relationship to alcohol withdrawal symptoms. J Clin Psychopharmacol 29:334–342. [DOI] [PubMed] [Google Scholar]
- Anton RF, Myrick H, Wright TM, Latham PK, Baros Am, Waid LR, Randall PK (2011) Gabapentin combined with naltrexone for the treatment of alcohol dependence. Am J Psychiatry 168:709–717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Backonja MM, Canafax DM, Cundy KC (2011) Efficacy of gabapentin enacarbil vs placebo in patients with postherpetic neuralgia and a pharmacokinetic comparison with oral gabapentin. Pain Med 12(7):1098–108. [DOI] [PubMed] [Google Scholar]
- Bazil CW, Battista J, Basner RC (2005) Gabapentin improves sleep in the presence of alcohol. J Clin Sleep Med 1(3):284–287. [PubMed] [Google Scholar]
- Beck AT, Epstein N, Brown G, Steer RA (1988) An inventory for measuring clinical anxiety: psychometric properties. J Consult Clinical Psych 56:893–897. [DOI] [PubMed] [Google Scholar]
- Beck AT, Steer RA, Ball R, Ranieri W (1996) Comparison of Beck Depression Inventories -IA and -II in psychiatric outpatients. J Personality Assess 67:588–597. [DOI] [PubMed] [Google Scholar]
- Bisaga A, Evans SM (2006) The acute effects of gabapentin in combination with alcohol in heavy drinkers. Drug Alcohol Depend 83(1):25–32. [DOI] [PubMed] [Google Scholar]
- Bode C, Bode JC (2003) Effect of alcohol consumption on the gut. Best Pract Res Clin Gastroenterol 17(4):575–592. [DOI] [PubMed] [Google Scholar]
- Bockbrader HN (1995) Clinical pharmacokinetics of gabapentin. Drugs of Today 31:613–619. [Google Scholar]
- Breslow RA, Guenther PM, Juan W, Graubard BI (2010) Alcoholic beverage consumption, nutrient intakes, and diet quality in the US adult population, 1999–2006. J Am Diet Assoc 110(4): 551–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brower KJ, Aldrich MS, Hall JM (1998) Polysomnographic and subjective sleep predictors of alcoholic relapse. Alcohol Clin Exp Res 22(8):1864–1871. [PubMed] [Google Scholar]
- Brower KJ, Myra Kim H, Strobbe S, Karam-Hage MA, Consens F, Zucker RA (2008) A randomized double-blind pilot trial of gabapentin versus placebo to treat alcohol dependence and comorbid insomnia. Alcohol Clin Exp Res 32(8):1429–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buysse DJ, Reynolds CF 3rd, Monk TH, Berman SR, Kupfer DJ (1989) The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res 28:193–213. [DOI] [PubMed] [Google Scholar]
- Cundy KC, Sastry S, Luo W, Zou J, Moors TL, Canafax DM (2008) Clinical pharmacokinetics of XP13512, a novel transported prodrug of gabapentin. J Clin Pharmacol 48:1378–1388. [DOI] [PubMed] [Google Scholar]
- Devine EG, Ryan ML, Falk DE, Fertig JB, Litten RZ (2016) An exploratory evaluation of Take Control: a novel computer-delivered behavioral platform for placebo-controlled pharmacotherapy trials for alcohol use disorder. Contemp Clin Trials 50:178–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elamin EE, Masclee AA, Dekker J, Jonkers DM (2013) Ethanol metabolism and its effects on the intestinal epithelial barrier. Nutr Rev 71(7):483–99. [DOI] [PubMed] [Google Scholar]
- Falk D, Wang XQ, Liu L, Fertig J, Mattson M, Ryan M, Johnson B, Stout R, Litten RZ (2010) Percentage of subjects with no heavy drinking days: evaluation as an efficacy endpoint for alcohol clinical trials. Alcohol Clin Exp Res 34(12):2022–2034. [DOI] [PubMed] [Google Scholar]
- Foster JH, Peters TJ (1999) Impaired sleep in alcohol misusers and dependent alcoholics and the impact upon outcome. Alcohol Clin Exp Res 23(6):1044–1051. [PubMed] [Google Scholar]
- Furieri FA, Nakamura-Palacios EM (2007) Gabapentin reduces alcohol consumption and craving: a randomized, double-blind, placebo-controlled trial. J Clin Psychiatry 68(11):1691–1700. [DOI] [PubMed] [Google Scholar]
- Hasin DS, Wall M, Witkiewitz K, Kranzler HR, Falk D, Litten R, Mann K, O’Malley SS, Scodes J, Robinson RL, Anton R; Alcohol Clinical Trials Initiative (ACTIVE) Workgroup (2017) Change in non-abstinent WHO drinking risk levels and alcohol dependence: a 3 year follow-up study in the US general population. Lancet Psychiatry 4(6):469–476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kline-Simon AH, Weisner CM, Parthasarathy S, Falk DE, Litten RZ, Mertens JR (2014) Five-year healthcare utilization and costs among lower-risk drinkers following alcohol treatment. Alcohol Clin Exp Res 38:579–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF (2008) A role for brain stress systems in addiction. Neuron 59:11–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litten RZ, Falk D, Ryan M, Fertig J (2015) Heterogeneity of alcohol use disorder: understanding mechanisms to advance personalized treatment. Alcohol Clin Exp Res 39:579–584. [DOI] [PubMed] [Google Scholar]
- Litten RZ, Ryan ML, Fertig JB, Falk DE, Johnson B, Dunn KE, Green AI, Pettinati HM, Ciraulo DA, Sarid-Segal O, Kampman K, Brunette MF, Strain EC, Tiouririne NA, Ransom J, Scott C, Stout R (2013) A double-blind, placebo-controlled trial assessing the efficacy of varenicline tartrate for alcohol dependence. J Addict Med 7:277–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowman C, Allen J, Stout RL (1996) Replication and extension of Marlatt’s taxonomy of relapse precipitants: overview of procedures and results. The Relapse Research Group. Addiction 91 Suppl:S51–S71. [PubMed] [Google Scholar]
- Mason BJ, Quello S, Shadan F (2018) Gabapentin for the treatment of alcohol use disorder. Expert Opin Investig Drugs 27:113–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason BJ, Quello S, Goodell V, Shadan F, Kyle M, Begovic A (2014) Gabapentin treatment for alcohol dependence: a randomized clinical trial. JAMA Intern Med 174:70–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason BJ, Light JM, Williams LD, Drobes DJ (2009) Proof-of-concept human laboratory study for protracted abstinence in alcohol dependence: Effects of gabapentin. Addict Biol 14(1):73–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNair DM, Lorr M, Droppleman LF (1992) EdITs Manual for the Profile of Mood States. EdITs/Educational and Industrial Testing Service: San Diego, CA. [Google Scholar]
- Mersfelder TL, Nichols WH (2016) Gabapentin: abuse, dependence, and withdrawal. Ann Pharmacother 50(3):229–233. [DOI] [PubMed] [Google Scholar]
- Miller W (1992) Form 90: A structured assessment interview for drinking and related behaviors (Test Manual). National Institute on Alcohol Abuse and Alcoholism: Bethesda, MD: (NIH publication no. 96–4004). [Google Scholar]
- Miller W (1995) The Drinker Inventory of Consequences (DrInC): an instrument for assessing adverse consequences of alcohol abuse, in NIAAA Project MATCH Monograph Series (Vol. 4) (Mattson M, Marshall LA eds), pp.1–94. National Institute on Alcohol Abuse and Alcoholism: Bethesda, MD. [Google Scholar]
- Myrick H, Malcolm R, Randall PK, Boyle E, Anton RF, Becker HC, Randall CL (2009) A double-blind trial of gabapentin versus lorazepam in the treatment of alcohol withdrawal. Alcohol Clin Exp Res 33:1582–1588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myrick H, Anton R, Voronin K, Wang W, Henderson S (2007) A double-blind evaluation of gabapentin on alcohol effects and drinking in a clinical laboratory paradigm. Alcoholism Clin Exp Res 31(2):221–227. [DOI] [PubMed] [Google Scholar]
- Patton JH, Stanford MS, Barratt ES (1995) Factor structure of the Barratt Impulsiveness Scale. J Clin Psych 51:768–774. [DOI] [PubMed] [Google Scholar]
- Peduzzi P, Concato J, Kemper E, Holford TR, Feinstein AR (1996) A simulation study of the number of events per variable in logistic regression analysis. J Clin Epidemiol 49:1373–1379. [DOI] [PubMed] [Google Scholar]
- Posner K, Brown GK, Stanley B, Brent DA, Yershova KV, Oquendo MA, Currier GW, Melvin GA, Greenhill L, Shen S, Mann JJ (2011) The Columbia-Suicide Severity Rating Scale: initial validity and internal consistency findings from three multisite studies with adolescents and adults. Am J Psychiatry 168(12):1266–1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice AS, Maton S, for the Postherpetic Neuralgia Study Group (2001). Gabapentin in postherpetic neuralgia: A randomised, double blind, placebo controlled study. Pain 94:215–224. [DOI] [PubMed] [Google Scholar]
- Roberto M, Gilpin NW, O’Dell LE, Cruz MT, Morse AC, Siggins GR, Koob GF (2008) Cellular and behavioral interactions of gabapentin with alcohol dependence. J Neurosci. 28(22): 5762–5771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan ML, Falk DE, Fertig JB, Rendenbach-Mueller B, Katz DA, Tracy KA, Strain EC, Dunn KE, Kampman K4 Mahoney E, Ciraulo DA, Sickles-Colaneri L, Ait-Daoud N, Johnson BA, Ransom J, Scott C, Koob GF, Litten RZ (2017) A Phase 2, Double-Blind, Placebo-Controlled Randomized Trial Assessing the Efficacy of ABT-436, a Novel V1b Receptor Antagonist, for Alcohol Dependence. Neuropsychopharmacology. 42(5):1012–1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sills GJ (2006) The mechanisms of action of gabapentin and pregabalin. Cur Opin Pharmacol 6:108–113. [DOI] [PubMed] [Google Scholar]
- Singleton EG (2000) A brief version of the Alcohol Craving Questionnaire (ACQ-NOW)? Proceedings of the 60th Annual Meeting, The College on Problems of Drug Dependence, Inc. Volume II: Abstracts. NIDA Research Monograph 180. Rockville, Maryland: National Institute on Drug Abuse, p. 304. [Google Scholar]
- Smith RV, Havens JR, Walsh S (2016) Gabapentin misuse, abuse and diversion: a systematic review. Addiction 111:1160–1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobell L, Sobell M (1992) Timeline follow-back: a technique for assessing self-reported alcohol consumption, in Measuring Alcohol Consumption: Psychosocial and Biochemical Methods (Litten R, Allen J, eds), pp. 41–72. Humana Press: Totowa, New Jersey. [Google Scholar]
- Sullivan JT, Sykora K, Schneiderman J, Naranjo CA, Sellers EM (1989) Assessment of alcohol withdrawal: the revised clinical institute withdrawal assessment for alcohol scale (CIWA-Ar). Br J Addict 84:1353–1357. [DOI] [PubMed] [Google Scholar]
- U.S. Food and Drug Administration (FDA) (2012). Review for NDA 022399 SUPPL-3. Available at: https://www.accessdata.fda.gov/drugsatfda_docs/nda/2012/022399Orig1s003.pdf. Accessed June 13, 2018.
- U.S. Food and Drug Administration (FDA) (2013) HORIZANT (gabapentin enacarbil) Extended-Release Tablets for oral use label. Revised April 2013. Available at: https://www.accessdata.fda.gov/drugsatfda_docs/label/2012/022399s006,s007lbl.pdf. Accessed May 16, 2018.
- U.S. Food and Drug Administration (2015) Alcoholism: developing drugs for treatment, Guidance for Industry (draft guidance). Available at: https://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM433618.pdf. Accessed May 16, 2018.
- U.S. Food and Drug Administration (2015) 22 case studies where phase 2 and phase 3 trials had divergent results. Available at: https://www.fda.gov/downloads/aboutfda/reportsmanualsforms/reports/ucm535780.pdf. Accessed September 21, 2018.
- Weiss RD (2004) Adherence to pharmacotherapy in patients with alcohol and opioid dependence. Addiction 99:1382–92. [DOI] [PubMed] [Google Scholar]
- Werner M, Rentz A, Frank L, Bowman L, Duhig A, Moss H (2008) Participant consequence measures. Presented at the annual meeting of the Research Society on Alcoholism, Washington, DC. [Google Scholar]
- Zhang L, Rainka M, Freeman R, Harden RN, Bell CF, Chen C, Graff O, Harding K, Hunter S, Kavanagh S, Laurijssens B, Schwartzbach C, Warren S, McClung C (2013). A randomized, double-blind, placebo-controlled trial to assess the efficacy and safety of gabapentin enacarbil in subjects with neuropathic pain associated with postherpetic neuralgia (PXN110748). J Pain 14(6):590–603. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.