Abstract
Aims:
Arrhythmogenic Cardiomyopathy (AC) is an inherited, frequently under diagnosed disorder, predisposing to sudden cardiac death. Rare, recessive forms of AC can be associated with woolly hair and palmoplantar keratoderma, but most autosomal dominant AC forms have been reported as cardiac specific. Causative mutations frequently occur in desmosomal genes including desmoplakin (DSP) In this study, we have systematically investigated the presence of a skin and hair phenotype in heterozygous desmoplakin (DSP) mutation carriers with AC.
Methods and Results:
6 AC pedigrees with 38 carriers of a dominant loss of function (nonsense or frameshift) mutation in DSP were evaluated by detailed clinical examination (cardiac, hair and skin) and molecular phenotyping. All carriers with mutations affecting both major DSP isoforms (DSP I and II) were observed to have curly or wavy hair in the pedigrees examined except for members of Family 6, where the position of the mutation only affected the cardiac-specific isoform, DSP I. A mild palmoplantar keratoderma was also present in many carriers. Sanger sequencing of cDNA from non-lesional carrier skin suggested degradation of the mutant allele. Immunohistochemistry of patient skin demonstrated mislocalisation of DSP and other junctional proteins (plakoglobin, connexin 43) in the basal epidermis. However, in Family 6, DSP localisation was comparable to control skin.
Conclusions
This study identifies a highly recognizable cutaneous phenotype associated with dominant, loss of function DSPI/II mutations underlying AC. Increased awareness of this phenotype amongst healthcare workers could facilitate a timely diagnosis of AC in the absence of overt cardiac features.
Keywords: keratoderma, curly hair, desmosomes, cardiocutaneous disease, arrhythmia
INTRODUCTION
Arrhythmogenic cardiomyopathy (AC) is an inherited cardiac disorder characterized by a high risk of arrhythmias and is a cause of sudden cardiac death in the young (<35 years old) 1,2. The foremost clinical challenge in AC is making an accurate and timely diagnosis. This is hampered by a prolonged, silent pre-clinical phase of the disease, lack of overt symptoms and variability of cardiac findings 2,3. The genetic basis of AC in the majority of cases is linked to a mutation in a component of the desmosome, a key electromechanical adhesion complex between cardiomyocytes. AC associated mutations have been reported to occur in five desmosomal genes: plakoglobin, desmoplakin, plakophilin 2, desmoglein 2 and desmocollin 24.
Desmosomes are also the major cell-cell adhesion structures contributing to mechanoresilience in the skin 4,5. As such, rare, predominantly recessively inherited mutations in desmosomal proteins can present with a unique “cardio-cutaneous” phenotype. Both Carvajal Disease (caused by desmoplakin mutations, DSP) and Naxos Disease (caused by plakoglobin mutations, JUP) characteristically present with a triad of “woolly” hair, markedly thickened skin of the palms and soles (palmoplantar keratoderma) from infancy and later, development of severe cardiomyopathy in childhood 6,7. Though rare autosomal dominant cardiocutaneous cases have been reported 8–12, there was confusion whether both the skin and the heart would be affected in heterozygous loss of function (nonsense or frameshift) mutation careers, which was often due to observational bias in these families as either purely cardiac 11, or only dermatological 10 examinations were carried out. On this basis, we have performed the first systematic analysis of a cohort of patients: we selected AC families with a well-defined subset of heterozygous loss of function DSP mutations, and investigated their putative cutaneous symptoms using deep clinical and molecular phenotyping.
METHODS
Patient cohort and clinical evaluation
The study involved 6 families with a history of AC, specifically focusing on a cohort harbouring heterozygous N-terminal loss of function (nonsense/frameshift) mutations of DSP. The patients were recruited at the Heart Hospital, University College London Hospitals NHS Trust (UCLH), London, UK. Patients were diagnosed with AC according to the revised Task Force Criteria1. Individuals were examined from cardiac and cutaneous perspectives. Systematic evaluation of first and second degree family members was performed in whom mutation-carrier status was verified. In total, personal history of cutaneous features was collected from 58 family members, 38 mutation carriers, including five deceased members, and 20 non-carrier healthy family members. All 33 living mutation carriers were recruited for detailed phenotyping, while 8 of them had 3mm punch biopsies of non-lesional skin of the lower forearm collected for molecular characterisation. Personal history of cutaneous features was collected, and detailed clinical examination of the skin and hair phenotype was carried out by a dermatologist (TM). Cardiac phenotyping was performed according to the protocol described in 30 family members (3 of the 33 carriers resided abroad and were therefore unavailable to participate in cardiac phenotyping) 13. Arrhythmic characterization included arrhythmia recorded during 24 hour ambulatory ECG monitoring and/or exercise testing, cardiac arrest (CA) and implantable cardioverter defibrillator (ICD) appropriate discharges. Patients with atypical chest pain, palpitations and/or syncope suggestive of vaso-vagal origin were considered asymptomatic. All patients provided written informed consent. The study protocol conforms to the ethical guidelines of the 1975 Declaration of Helsinki and was approved by the local ethics committees.
DNA Sequencing
10*10 µm skin cryosections were used for DNA and RNA extractions with the QIAamp DNA and RNeasy mini kits (Qiagen, Hilden, Germany), following the instructions of the manufacturer. RNA was reverse transcribed into cDNA using SuperScript II (Invitrogen, Carlsbad, CA, USA). Primer sequences and PCR conditions are available upon request.
Immunohistochemistry
5µm cryosections were prepared on microscopic slides. The sections were fixed in Methanol-Acetone (1:1) and blocked in 5% goat serum. The following antibodies and dilutions were used: DSP (11–5F, 1:50), Jup (OBT0641, 1:10, AbD Serotec, Bio-Rad, Hercules, CA, USA), Gja1 (Mab3067, 1:200, Millipore, Billerica, MA, USA), GFP-labelled goat anti-mouse (A11029, 1:500, Thermo Fisher, Waltham, MA, USA). The negative controls (no primary antibody) were performed on patient skin plus control breast reduction and tummy tuck derived skin. The mounted stainings were imaged on a Zeiss 710 confocal microscope. Maximum Intensity Projections were created and exported using ImageJ.
RESULTS
Cardiac phenotype in DSP-carriers
Among all patients, 12/30 (40%) fulfilled the 2010 Task Force Criteria for AC diagnosis. The fulfilment of the diagnostic criteria is presented in Table 1 and Figure 1. Ten patients (33%) had a history of arrhythmic symptoms, inverted T waves on electrocardiogram (ECG) were present in 9 (30%) patients whilst signal-averaged ECG (SA-ECG) was positive in 12 (40%) mutation carriers. Ejection fraction was impaired in 9 (30%) patients at echocardiogram. During 24 hour ambulatory ECG monitoring, >500 ventricular ectopics were noted in 13 (43%) mutation carriers, in particular, in 7 of these a non-sustained ventricular tachycardia (NSVT) was recorded. Cardiac Magnetic Resonance (CMR) showed fibrofatty infiltration in 10 patients (33%) and 11 (37%) patients had an ICD implanted, two of these for secondary prevention.
Table 1.
shows the overview of cardiac and cutaneous features observed in the index patients and their mutation carrier family members.
| Family Number |
Variant | Patient |
DSP Mutation carrier |
Age sex |
Major Criteria |
Minor Criteria |
Curly/ Wavy hair |
Skin phenotype |
|---|---|---|---|---|---|---|---|---|
| 1 | pN274Efs | |||||||
| I:2 | y | 71 F | f | a, d | yes | yes | ||
| II:2 | y | 53 F | c, e | a, d | yes | yes | ||
| II:3 | y | 54F | N/A | N/A | yes | no | ||
| II:5 | y | 46 M | f | d | yes | no | ||
| III:2 | y | 17 M | f | yes | yes | |||
| III:3 | y | 18F | N/A | N/A | yes | no | ||
| III:4 | y | 17M | N/A | N/A | yes | no | ||
| III:5 | y | 17 F | f | yes | no | |||
| III:1 | n | 26 M | no | no | ||||
| III:6 | n | 16 M | no | N/A | ||||
| 2 | pH586Tfs | |||||||
| II-1 | y | 55 M | * | yes | N/A | |||
| II-2 | y | 50 M | a, e, f | d | yes | yes | ||
| I-1 | y | 61 F | a, c, f | d, e | yes | yes | ||
| II-3 | y | 36 F | e, f | d | yes | yes | ||
| II-4 | y | 42 M | e, f | a | yes | yes | ||
| I-2 | y | 71 F | f | yes | N/A | |||
| I-3 | y | 76 F | e, f | d | yes | yes | ||
| III-1 | n | 20 F | no | no | ||||
| II-5 | n | 42 M | no | no | ||||
| II-6 | n | 39 F | no | N/A | ||||
| 3 | pT922Sfs | |||||||
| II:1 | y | 30 F | b, e | c, d | yes | yes | ||
| II:2 | n | 28 F | no | no | ||||
| 4 | pR1015Sfs | |||||||
| I-1 | y | F | * | yes | N/A | |||
| II:2 | y | 53 F | a, b, e, f | d | yes | yes | ||
| II:4 | y | 56 F | a, e, f | d | yes | yes | ||
| II:5 | y | 26 F | * | yes | N/A | |||
| II:10 | y | 61 M | c, e, f | a, d | yes | no | ||
| III:2 | y | 31 M | f | a | yes | yes | ||
| III:8 | y | 18 M | f | yes | no | |||
| III:1 | N | 30 F | no | No | ||||
| III:3 | N | 27 F | no | no | ||||
| III:4 | N | 16 F | no | No | ||||
| III:5 | N | 17 M | no | no | ||||
| III:6 | N | 33 M | no | N/A | ||||
| III:7 | N | 32 F | no | N/A | ||||
| III:12 | N | 64 F | no | N/A | ||||
| III:13 | N | 52 F | no | N/A | ||||
| 5 | pR1113X | |||||||
| I:1 | y | 84 M | f | d, e | yes | yes | ||
| II:2 | y | 30 F | *ʇ | yes | N/A | |||
| II:3 | y | 54 F | c, f | a, d | yes | yes | ||
| II:5 | y | 50 F | c, f | a, d | yes | yes | ||
| III:1 | y | 26 M | f | yes | yes | |||
| III:2 | y | 24 M | f | yes | yes | |||
| III:6 | y | 20 F | f | yes | yes | |||
| III:7 | y | 15 F | f | yes | no | |||
| III:3 | n | 22 F | no | no | ||||
| III:4 | n | 25 F | no | no | ||||
| III:5 | n | 23 M | no | No | ||||
| 6 | pE1493X | |||||||
| I:2 | y | 64 M | f | a, c, d | yes | yes | ||
| I:3 | y | 59 M | a, f | d | no | no | ||
| I:5 | y | 38 M | * ʇ | |||||
| II:3 | y | 36 F | f | d | no | no | ||
| II:4 | y | 34 M | f | d | no | no | ||
| II:6 | y | 32 F | f | a,d | no | yes | ||
| II:8 | y | 31 F | f | no | no | |||
| II:1 | n | M | no | N/A | ||||
| II:2 | n | F | no | N/A | ||||
| II:5 | N | M | No | N/A | ||||
Categories:
global or regional dysfunction and structural alterations
tissue characterization of wall
repolarization abnormalities
depolarization abnormalities
arrhythmias
family history. Abbreviations: F - female, M - male.
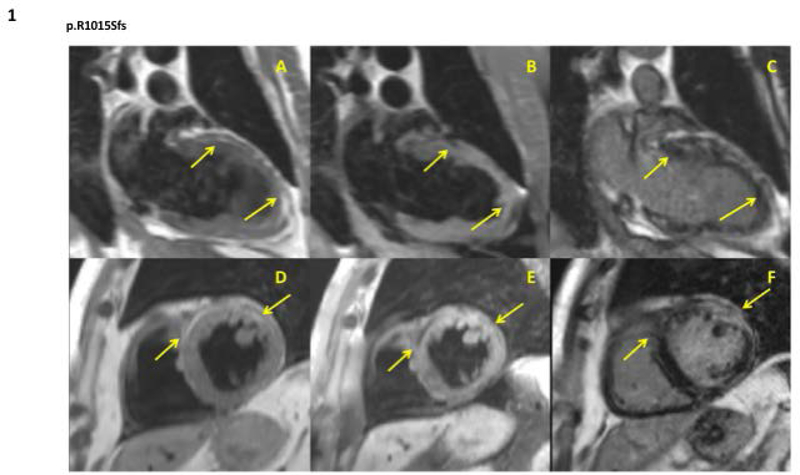
Figure 1. Cardiac phenotype in DSP mutation-carriers.
A representative cardiac MRI image from patient II:10 in family 4. It shows 2 chambers and short-axis LV turbo spin echo images with and without fat saturation and late gadolinium enhancement image on the same slice of the AC patient. Arrows in A and D indicate the white areas of fat infiltration that appear in black in B and E. Arrows in C and F indicate white areas of myocardial fibrosis.
Cutaneous phenotypic features in DSP-carriers
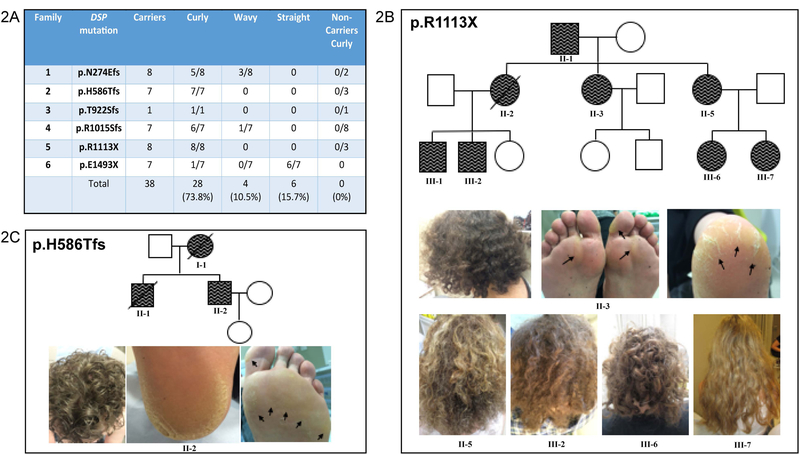
Detailed dermatological evaluation of scalp hair and palmoplantar skin was performed in 33 mutation-carriers (Families 1–6) and the presence of curly hair was found in all DSP carriers from families 1–5 (Fig. 2). Family 6 (pE1493X) was an exception where only the index patient had curly hair, and the 6 other mutation carriers within this family had straight hair (Figs 2A and4A). The presence of curly hair in mutation carriers was 5 times the incidence observed in the general Caucasian population (73.8% vs 15%) and this difference was highly significant (p=0.0012; unpaired t test)14. The hair was observed to be tightly curled with a coarse, wiry texture (Figs 2B and C). No features of defective hair growth or excessive breakage were noted. A key finding was the absence of curly hair in 20 non-carrier offspring within the same families, indicating a high level of specificity for this phenotype within the cohort of patients and their relatives. The presence of palmoplantar keratoderma (PPK) showed more variable penetrance between individuals from the same family and additional factors such as age and physical activity influenced their presence (Table 1). Carriers of mutation p.H586Tfs and p.R1113X showed classical band-like thickened hyperkeratosis affecting the plantar footpad (“striate” keratoderma) in addition to hyperkeratosis and marked fissuring of the heel region, resembling Carvajal Syndrome (Figs 2B and2C). Others (p.N274Efs) displayed focal thickening of the pressure bearing regions of the plantar skin and described callus formation on the palms following physical activity. Some subjects, for example carriers of p.R1015Sfs, also complained of xerosis (excessive drying of the skin) following exposure to water. A common complaint of mutation carriers was having painful rough, hard skin on their feet and the need to regularly file or cut or shave this off using a razor.
Figure 2. Cutaneous phenotype in DSP mutation-carriers.
(A) Table summarizing the presence of curly/wavy or straight hair in DSP mutation carriers and unaffected family members. (B) A representative pedigree and clinical photographs of family 5. Symbols: circle – female; square – male; dark – affected by AC, wavy line – curly hair; diagonal line – deceased. Co-segregation of the hair phenotype with the genotype can be observed in all members of Family 5. Black arrowheads mark plantar hyperkeratosis and fissuring. (C) A further representative family tree from Family 2 with clinical photographs demonstrating curly hair, black arrow heads mark palmoplantar keratoderma, fissuring and hyperkeratosis.
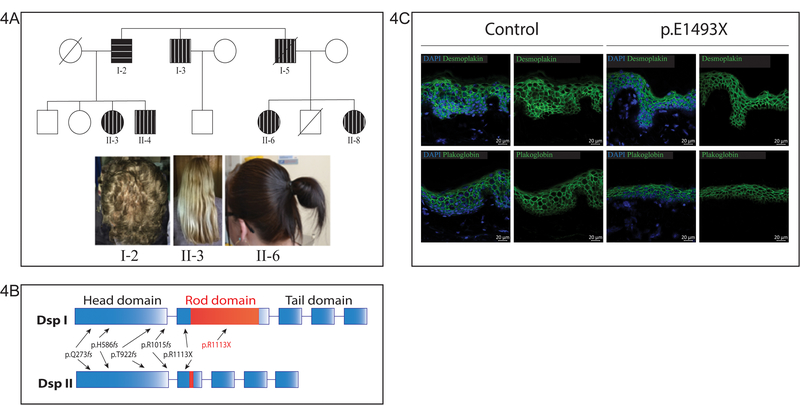
Figure 4. Family 6.
(A) Family tree of members of Family 6 p.E1493X. Symbols: circle = female, square = male, dark circle/square with wavy lines = mutation carrier with curly hair, dark circle/square with straight lines = mutation carrier with straight hair. 6/7 mutations carriers in this family had straight hair with only the index case having curly hair. (B) A Schematic representation of DSP transcripts I and II with the position of the 6 mutations indicated. The causative mutation in Family 6 lies in the DSP-I specific part of the protein and therefore does not affect DSP-II. (C) Immunohistochemistry of control skin and patient skin from a member of Family 6 (p.E1493X) showing comparable distribution of Plakoglobin and Desmoplakin throughout the epidermis. Scale bar: 20 µm.
Molecular characterisation
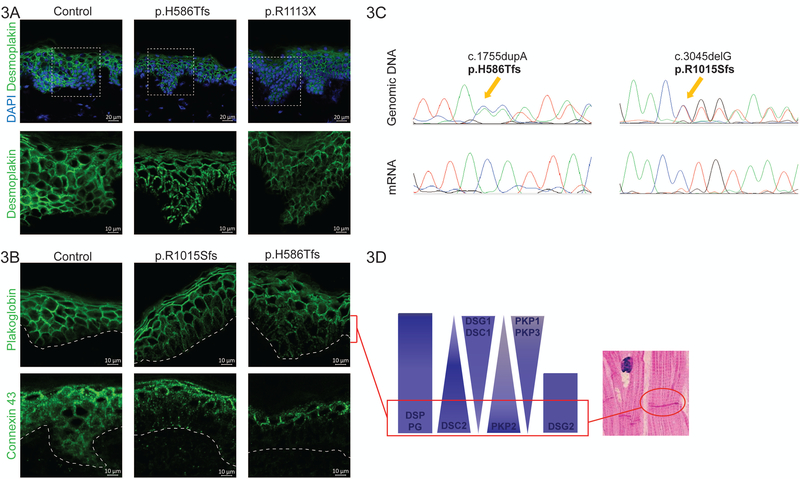
To determine the pathophysiology underlying the cutaneous phenotype, skin biopsies were obtained from non-lesional skin on the forearm from 8 mutation-carriers. Immunohistochemical staining was performed and compared with control skin from unaffected individuals. The immunoreactive signal for desmoplakin showed a granular and irregular localisation in the basal layers of the epidermis of patient skin, rather than confluent membranous distribution seen in normal skin (Fig. 3A). Similarly, albeit more subtle abnormalities in the expression pattern of plakoglobin were also observed (Fig. 3B), with granular deposition in the basal layers. The expression of connexin 43 (Cx43) appeared reduced in the basal epidermis from affected patient skin (Fig. 3B). This particular junctional component is critical for electrical conduction in the cardiac intercalate disc. These findings suggest that even in clinically “normal” appearing skin in mutation-carriers, there is abnormal localisation of key desmosomal proteins and reduced expression of Cx43, in the basal epidermis. To investigate the effect of these specific DSP mutations on mRNA expression, Sanger sequencing of skin biopsies from affected patients was performed. This confirmed the presence of the mutation in genomic DNA, but the truncated mRNA of the mutant allele was notably absent (Fig. 3C). This absence of the mutant allele in mRNA/cDNA of non-lesional patient skin indicates that nonsense-mediated decay (NMD) is underlying the disease.
Figure 3. Molecular results in DSP mutation-carriers.
(A) is a collection of representative confocal images of DSP immunohistochemistry on non-lesional patient skin. Desmoplakin forms the most prominent aggregates in the index patient of family 2 (p.H586Tfs). The white dashed squares indicate the zoomed area indicated below without DAPI. Scale bar: 10 or 20 µm. (B) is a collection of representative confocal images of immunohistochemistry on non-lesional patient skin. The perforated white line indicates the border between the epidermis and the dermis. The suprabasal half of the epidermis has weaker plakoglobin and connexin 43 signal, while connexin 43 also seems to be more speckled and overexpressed on the surface when compared to control skin. Scale bar: 10 or 20 µm. (C) Representative DNA and cDNA sequences of patient skin in families 2 and 4. The orange arrows indicate the site of the duplication/insertion and the start of the frameshift in the genomic DNA. At the same site, we can only find the wild type sequence on the mRNA/cDNA level; the mutant allele has been degraded. (D) The schematic diagram illustrates the normal distribution of desmosomal proteins throughout the epidermal layer of the skin. The molecular composition of desmosomes in the lower part of the epidermis is similar to that of the intercalated discs in the myocardium (shown on histological inset image).
DISCUSSION
We have identified the presence of a highly penetrant cutaneous phenotype of curly hair and to a lesser extent, palmoplantar keratoderma in DSP mutation-carriers, but not in unaffected family members. These readily recognisable non-cardiac features, could be used in conjunction with traditional diagnostic strategies to facilitate a timely diagnosis of AC. Desmoplakin is a key desmosomal protein associated with the predominantly left ventricular forms of AC. To date, there are only a limited number of genotype-phenotype studies in AC 15,16, or in particular in DSP mutation carriers 13,17–20.
Our cutaneous findings are not completely unexpected, as in Carvajal disease the very severe, often lethal pediatric cardiomyopathy is combined with the presence of woolly hair and striate palmoplantar keratoderma 6. However, in those families, the homozygous premature stop mutation is in the third (and last) globular unit of the C-terminal tail domain of DSP. Even though this mutation leads to a truncated protein product, and weakens the resilience of the interaction between DSP and the keratin/desmin intermediate filaments in the skin/heart of homozygous patients, their carrier parents showed no sign of the cardiocutaneous disease. Interestingly, other heterozygous cases, such as carriers of an N-terminal in-frame duplication mutation do exhibit the Carvajal-phenotype 8. The consistent presence of this milder cardiocutaneous phenotype in N-terminal head-domain mutations of our well-defined, relatively large, heterozygous cohort is hence intriguing.
Desmoplakin and desmosomal proteins also have a key role in hair follicle biology 4. Although no scalp biopsies were available for analysis, we hypothesise that abnormal distribution of desmosomal components in the follicle and consequent impairment of cell-cell adhesion could contribute to angulation of the follicle, which is known to cause curvature of the hair strand 14. In the skin, the expression of individual components of the desmosome vary from the basal layer to the superficial epidermis. However, in the heart, desmosomal components are uniform, and overlap with those expressed in the basal epidermis 21. Therefore, mislocalisation of desmosomal proteins in the basal skin could mirror what occurs at the intercalated disc in cardiomyocytes (Fig. 3D) 22, even if the biopsy is taken from non-lesional skin. In the skin this manifests clinically as impaired mechanoresilience in response to mechanical stress, with hyperkeratosis and fissuring at pressure-bearing sites on the soles. Whereas in the heart, abrogated cell-cell adhesion results in conduction defects, fibrofatty infiltration and cardiomyopathy 23. Abnormal localisation of key desmosomal proteins in the heart and skin and their deleterious effect on cell-cell adhesion therefore reconciles the presence of both cardiac and cutaneous phenotypes in these patients.
The curly hair phenotype shows 100% segregation with mutation-carrier status within families 1–5 in which the heterozygous nonsense or frameshift mutation falls to the N-terminal head domain of desmoplakin, or the N-terminal part of the rod domain that is present in both main isoforms. Curly hair is a highly sensitive and specific indicator of carriership status in the N-terminal mutation-carrier families, all of whom happen to be Caucasian, but with respect to the general population, it is not specific. For example, genome wide association studies have found a single nucleotide polymorphism in the TCHH gene, which encodes Trichohyalin, a component of the hair follicle inner root sheath. This gene accounts for approximately 6% of hair curliness in the Caucasian population 24. Some patients also developed a palmoplantar keratoderma with hyperkeratosis and fissuring at pressure-bearing sites, and the same patients seemed to have more severe cardiac symptoms as well, potentially indicating higher amount of environmental stress in those individuals. On the molecular level, we observed abnormal localisation of desmosomal proteins and connexin 43 in the skin, which reflects some observations reported in heart tissue of patients with AC 23. Our data supports haploinsufficiency due to NMD of the mutant allele as the molecular mechanism and that DSP dosage, particularly of DSPII, is critical to form stable junctions in proliferative (basal) /stressed keratinocytes or cardiomyocytes.
In Family 6, only 1 of the 7 mutation-carriers demonstrated a curly hair/cutaneous phenotype (Fig. 4A). Their cardiac symptoms were also somewhat less severe: no patient in the family has ICD implanted, and many of them were borderline cases (not yet fulfilling all Task Force Criteria) with normal echo and T-wave inversion (Table 1). The mutation p.E1493X is localised in the middle of the DSP rod domain, in the fragment (between coordinates c.3585–5379, or p.1195–1793) that is included only in isoform 1 of desmoplakin (DSPI) while it is spliced out in isoform 2 (DSPII) (Fig. 4B). We have previously shown that DSPII is the major isoform regulating keratinocyte adhesion 25. Consistent with this, immunohistochemical staining of skin from these patients demonstrated a distribution of Plakoglobin and Desmoplakin comparable to control skin (Fig. 4C).
In clinical practice, the presence of curly hair and keratoderma is likely to be a useful additional clinical identifier of AC patients. When incorporated into family screening for AC, it could be particularly useful in persuading individuals to partake in genetic screening programmes and identify high risk family members who warrant further diagnostic tests, lifestyle changes and participating in regular follow-up. This study did not include pedigrees with missense mutations in DSP, nor did it examine carriers of other desmosomal AC gene mutations. All pedigrees included in this study happened to be of Caucasian origin, however it would be important to consider the significance of the cutaneous phenotype in other populations including those in which curly hair is known to be more highly prevalent. Although our study includes the largest number of DSP mutation carriers reported, and the first systematic approach of a well-defined mutation subtype cohort, larger-scale genotype-phenotype correlation studies are necessary to confirm these dermatological findings.
What’s already known about this topic?
The diagnosis of the early phases of Arrhythmogenic Cardiomyopathy (AC) remains challenging.
AC is linked to mutations in desmosomal genes including desmoplakin (DSP).
Desmosomes have key functions in the heart, skin and hair highlighted by rare, predominantly recessively inherited DSP mutations being linked to “woolly” hair, keratoderma and severe cardiomyopathy in childhood.
• What does this study add?
The presence of curly hair and keratoderma is likely to be a useful additional clinical identifier of autosomal dominant AC patients associated with DSPI/II mutations.
Haploinsufficiency is the underlying genetic mechanism
Immunohistochemistry of patient skin demonstrated mislocalisation of DSP and other junctional proteins (plakoglobin, connexin 43) in the basal epidermis.
What is the translational message?
Here, we show that dominant AC associated loss-of-function DSP mutations can also be linked to curly hair and mild palmoplantar keratoderma.
These cutaneous features could therefore provide clinical clues enabling early diagnosis and directed family screening, especially in the subtle, early AC phases.
ACKNOWLEDGEMENTS
This study was supported by a British Heart Foundation grant (RG/13/19/30568) to DPK. TM was supported by a MRC Clinical Research Training fellowship (MR/K002740/1). SC was funded by the European Society of Cardiology Research Grant and by the Italian Society of Cardiology with a grant by the MSD Italia-Merck Sharp & Dohme Corporation. WJM is a UK NIHR Senior Investigator and work by WJM and PS is supported by a Fondation Leducq Transatlantic Networks of Excellence Program grant (no 14 CVD03). PDL is supported by UCLH Biomedicine NIHR funding, and the Stephen Lyness Memorial Fund. The National Institutes of Health grant R37AR43380 and the J.L. Mayberry Endowment (to KJG). The work was facilitated by the NIHR Biomedical Research Centre at Barts.
Footnotes
Conflict of Interest: none declared.
REFERENCES
- 1.Marcus FI, McKenna WJ, Sherrill D et al. Diagnosis of arrhythmogenic right ventricular cardiomyopathy/dysplasia: proposed modification of the Task Force Criteria. European heart journal 2010; 31: 806–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Corrado D, Basso C, Judge DP. Arrhythmogenic Cardiomyopathy. Circ Res 2017; 121: 784–802. [DOI] [PubMed] [Google Scholar]
- 3.Sen-Chowdhry S, Syrris P, McKenna WJ. Role of genetic analysis in the management of patients with arrhythmogenic right ventricular dysplasia/cardiomyopathy. J Am Coll Cardiol 2007; 50: 1813–21. [DOI] [PubMed] [Google Scholar]
- 4.Brooke MA, Nitoiu D, Kelsell DP. Cell-cell connectivity: desmosomes and disease. The Journal of pathology 2012; 226: 158–71. [DOI] [PubMed] [Google Scholar]
- 5.Broussard JA, Getsios S, Green KJ. Desmosome regulation and signaling in disease. Cell Tissue Res 2015; 360: 501–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Norgett EE, Hatsell SJ, Carvajal-Huerta L et al. Recessive mutation in desmoplakin disrupts desmoplakin-intermediate filament interactions and causes dilated cardiomyopathy, woolly hair and keratoderma. Hum Mol Genet 2000; 9: 2761–6. [DOI] [PubMed] [Google Scholar]
- 7.McKoy G, Protonotarios N, Crosby A et al. Identification of a deletion in plakoglobin in arrhythmogenic right ventricular cardiomyopathy with palmoplantar keratoderma and woolly hair (Naxos disease). Lancet 2000; 355: 2119–24. [DOI] [PubMed] [Google Scholar]
- 8.Norgett EE, Lucke TW, Bowers B et al. Early death from cardiomyopathy in a family with autosomal dominant striate palmoplantar keratoderma and woolly hair associated with a novel insertion mutation in desmoplakin. J Invest Dermatol 2006; 126: 1651–4. [DOI] [PubMed] [Google Scholar]
- 9.Pigors M, Schwieger-Briel A, Cosgarea R et al. Desmoplakin mutations with palmoplantar keratoderma, woolly hair and cardiomyopathy. Acta Derm Venereol 2015; 95: 337–40. [DOI] [PubMed] [Google Scholar]
- 10.Armstrong DK, McKenna KE, Purkis PE et al. Haploinsufficiency of desmoplakin causes a striate subtype of palmoplantar keratoderma. Hum Mol Genet 1999; 8: 143–8. [DOI] [PubMed] [Google Scholar]
- 11.Norman M, Simpson M, Mogensen J et al. Novel mutation in desmoplakin causes arrhythmogenic left ventricular cardiomyopathy. Circulation 2005; 112: 636–42. [DOI] [PubMed] [Google Scholar]
- 12.Rasmussen TB, Hansen J, Nissen PH et al. Protein expression studies of desmoplakin mutations in cardiomyopathy patients reveal different molecular disease mechanisms. Clin Genet 2013; 84: 20–30. [DOI] [PubMed] [Google Scholar]
- 13.Castelletti S, Vischer AS, Syrris P et al. Desmoplakin missense and non-missense mutations in arrhythmogenic right ventricular cardiomyopathy: Genotype-phenotype correlation. Int J Cardiol 2017; 249: 268–73. [DOI] [PubMed] [Google Scholar]
- 14.Loussouarn G, Garcel AL, Lozano I et al. Worldwide diversity of hair curliness: a new method of assessment. Int J Dermatol 2007; 46 Suppl 1: 2–6. [DOI] [PubMed] [Google Scholar]
- 15.Bhonsale A, Groeneweg JA, James CA et al. Impact of genotype on clinical course in arrhythmogenic right ventricular dysplasia/cardiomyopathy-associated mutation carriers. Eur Heart J 2015; 36: 847–55. [DOI] [PubMed] [Google Scholar]
- 16.Kapplinger JD, Landstrom AP, Salisbury BA et al. Distinguishing arrhythmogenic right ventricular cardiomyopathy/dysplasia-associated mutations from background genetic noise. J Am Coll Cardiol 2011; 57: 2317–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rampazzo A, Nava A, Malacrida S et al. Mutation in human desmoplakin domain binding to plakoglobin causes a dominant form of arrhythmogenic right ventricular cardiomyopathy. Am J Hum Genet 2002; 71: 1200–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bauce B, Basso C, Rampazzo A et al. Clinical profile of four families with arrhythmogenic right ventricular cardiomyopathy caused by dominant desmoplakin mutations. European heart journal 2005; 26: 1666–75. [DOI] [PubMed] [Google Scholar]
- 19.Sen-Chowdhry S, Syrris P, McKenna WJ. Desmoplakin disease in arrhythmogenic right ventricular cardiomyopathy: early genotype-phenotype studies. Eur Heart J 2005; 26: 1582–4. [DOI] [PubMed] [Google Scholar]
- 20.Coats CJ, Quarta G, Flett AS et al. Arrhythmogenic left ventricular cardiomyopathy. Circulation 2009; 120: 2613–4. [DOI] [PubMed] [Google Scholar]
- 21.Bolling MC, Jonkman MF. Skin and heart: une liaison dangereuse. Exp Dermatol 2009; 18: 658–68. [DOI] [PubMed] [Google Scholar]
- 22.Rasmussen TB, Nissen PH, Palmfeldt J et al. Truncating plakophilin-2 mutations in arrhythmogenic cardiomyopathy are associated with protein haploinsufficiency in both myocardium and epidermis. Circ Cardiovasc Genet 2014; 7: 230–40. [DOI] [PubMed] [Google Scholar]
- 23.Gomes J, Finlay M, Ahmed AK et al. Electrophysiological abnormalities precede overt structural changes in arrhythmogenic right ventricular cardiomyopathy due to mutations in desmoplakin-A combined murine and human study. Eur Heart J 2012; 33: 1942–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Medland SE, Nyholt DR, Painter JN et al. Common variants in the trichohyalin gene are associated with straight hair in Europeans. Am J Hum Genet 2009; 85: 750–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cabral RM, Tattersall D, Patel V et al. The DSPII splice variant is crucial for desmosome-mediated adhesion in HaCaT keratinocytes. J Cell Sci 2012; 125: 2853–61. [DOI] [PubMed] [Google Scholar]