Abstract
While androgen deprivation therapy (ADT) is an effective treatment for metastatic prostate cancer (mPCa), incurable castration-resistant prostate cancer (CRPC) inevitably develops. Importantly, androgen receptor (AR) continues to be critical for PCa growth and progression after ADT. One of the underlying molecular mechanisms is derepression of AR-repressed genes involved in cell cycle and proliferation after ADT. Here, the data demonstrate that C-X-C chemokine receptor type 7 (CXCR7), a seven-transmembrane G-protein-coupled chemokine receptor, is an AR-repressed gene and is upregulated after ADT. AR directly regulates CXCR7 using Clustered Regularly Interspaced Short Palindromic Repeats/CRISPR associated protein 9 (CRISPR/Cas9) gene editing. Macrophage migration inhibitory factor (MIF) was identified as a ligand for CXCR7, which induces expression of cell cycle genes through activating AKT signaling pathway. Previous studies have been focused on chemokine CXCL12 and its receptor CXCR4 in mediating metastasis of various cancer types, including PCa. The critical roles of CXCL12/CXCR4 axis in the interaction between cancer cells and their microenvironment render it a promising therapeutic target in cancer treatment. The data suggest that the MIF/CXCR7/AKT pathway drives CRPC growth and metastasis independent of the CXCL12/CXCR4 axis. Furthermore, CXCR7 blockade in combination with anti-androgen enzalutamide inhibits CRPC tumor growth and potentially prevents metastasis. Notably, both MIF and CXCR7 are overexpressed in CRPC patient specimens and therefore are attractive therapeutic targets for these patients.
Implication:
This work suggests that CXCR7 plays more important roles than CXCR4 in CRPC progression; thus, targeting CXCR7 in combination with anti-androgen is a promising therapeutic approach for metastatic CRPC.
Keywords: CXCR7, MIF, prostate cancer, castration resistance, androgen receptor
Introduction
Prostate cancer (PCa) remains the second leading cause of cancer-related death among American men behind only lung cancer (1). Androgen deprivation therapy (ADT) has been considered the standard of care for metastatic PCa patients since 1941 when Huggins and Hodges demonstrated significant remissions in PCa after castration (2). While ADT is initially effective, PCa cells tend to survive and proliferate under androgen-deprived conditions and castration-resistant prostate cancer (CRPC) inevitably develops. Despite the development of next-generation anti-androgen therapies, including enzalutamide and abiraterone, acquired resistance to these drugs is nearly universal over time. Metastatic CRPC remains a lethal disease and further treatments are palliative. Castration resistance is often mediated by restoration of androgen receptor (AR) activity through AR mutations, AR variants, AR amplification and overexpression. Interestingly, recent studies revealed a subset of genes that are normally suppressed by AR are upregulated in CRPC patients (3,4). It has been suggested that one of the molecular mechanisms for PCa cells to survive ADT is derepression of AR-repressed genes. However, it is unclear which and how AR-repressed genes contribute to PCa growth and progression after ADT.
It is well known that chemokines and their receptors not only are key mediators of inflammation, but also play a crucial role in tumor growth and more importantly metastasis since tumor cell migration share many similarities with leukocyte trafficking. CXCR4 is one of the most studied chemokine receptors in a variety of cancers, including PCa. CXCR4 and its respective ligand CXCL12 have been increasingly demonstrated to regulate tumor progression. This is achieved by metastatic spread of CXCR4-positive tumor cells to organs (such as bone) expressing high levels of CXCL12. CXCR4 and CXCL12 are the key factors in the link between cancer cells and their microenvironment. The critical role of CXCR4/CXCL12 interaction in determining the metastatic destination was initially discovered in breast cancer (5). Further studies have shown that CXCR4 is overexpressed in at least 20 different cancers, including PCa (6). CXCL12 functions as a chemotactic factor involved in PCa cell migration through activation of the CXCR4 (7). For a long time, CXCR4 was thought to be the only receptor for CXCL12, with CXCL12 being its only ligand. However, interaction between chemokines and their receptors is promiscuous. CXCR7 (also known as atypical chemokine receptor 3, ACKR3) was identified as an alternative receptor for CXCL12 based on structural similarity and experimental evidence (8). Studies have shown that upregulation of CXCR7 is associated with invasive activities and growth of PCa cells (9). CXCR4 and CXCR7 may form homo- and heterodimers and mediate G-protein signaling, contributing to PCa progression (10). Thus, CXCR4 and CXCR7 are considered as emerging targets for PCa.
CXCR7 was recently identified as an AR-repressed gene (11), implying its unique role in PCa, although it remains unclear whether AR directly regulates CXCR7 expression. The importance of CXCL12/CXCR4/CXCR7 signaling in PCa progression has also been elucidated (12). Targeting CXCR7 in combination with anti-androgen treatment inhibits tumor growth and angiogenesis in pre-clinical androgen-dependent PCa models (13). However, it is unclear whether and how CXCR7 plays a role in metastatic CRPC progression. In the present study, we identified CXCR7 as one of the top AR-repressed genes at a genomic level and demonstrated that AR directly regulates CXCR7 using a CRISPR/Cas9 gene editing approach. We further explored the molecular mechanism by which CXCR7 mediates CRPC progression. We identified MIF is a ligand for CXCR7 in CRPC cells. The MIF/CXCR7 pathway appears to play a crucial role in CRPC growth and progression by regulating gene expression closely associated with cell cycle through AKT activation. Finally, combination treatment of CXCR7 inhibitor with enzalutamide not only inhibits or delays CRPC tumor growth, but also prevents the development of metastases, which may potentially result in a more profound, long-lasting remission in CRPC patients.
Materials and Methods
Cell lines and materials
LNCaP, PC-3, DU145 (from ATCC, Manassas, VA), and C4–2B (from ViroMed Laboratories, Minneapolis, MN) cells were maintained in RPMI 1640 (Gibco) medium supplemented with 5% fetal bovine serum (FBS) as previously described (14). The AR-expressing PC-3 stable cell line was a gift from Dr. Baruch Frenkel (15). All cell lines were authenticated using high resolution small tandem repeats (STR) profiling at Dana-Farber Cancer Institute (DFCI) Molecular Diagnostics Core Laboratory. Cells were grown for 20 passages and then replaced with fresh stocks. Cells are free of mycoplasma examined by MycoSensor PCR Assay Kit (Cat # 302108; Agilent Technologies). Human Recombinant MIF (rMIF) and CXCL12 (rCXCL12) were purchased from PeproTech (Hamburg, Germany). The CXCR7 small molecule inhibitor, CCX771, was a gift from ChemoCentryx (Mountain View, CA) (16). The CXCR4 inhibitor, AMD3100, was obtained from Sigma-Aldrich.
Chromatin immunoprecipitation (ChIP)
LNCaP and C4–2B cells were grown in RMPI 1640 with 5% charcoal stripped fetal bovine serum (CSS) for 3 days followed by DHT (10 nM) treatment for 4 hours. AR ChIP was performed as previously described (17). ChIP DNA was quantified by quantitative PCR (qPCR). The primer sequences are listed in Supplementary Table S1.
Identification of AR-repressed genes
To assess the AR-repressed genes, we performed Binding and Expression Target Analysis (BETA) with default parameters by integrating androgen-regulated gene expression microarray dataset (GSE7868) with six AR ChIP-seq datasets (GSM759658, GSM980657, GSM980662, GSM699631, GSM969565, and GSM696842) respectively (18–23). Rank products (p-value) from individual dataset were calculated by BETA, and were combined with Fisher’s method to rank the gene list. Genes that were downregulated by androgen [with log2 (fold change) < −0.58 and p-value < 0.01] and with an AR binding site nearby (combined rank product < 0.01) were defined as AR-repressed genes.
Western blot analysis
Whole cell lysates were prepared from PCa cells as indicated. Western blot was performed as previously described (14). Antibodies are: anti-ERK (#4695), anti-p-ERK (#4370), anti-AKT (#4691), anti-p-AKT (#4051), anti-JNK (#9252), anti-p-JNK (#4668), anti-p38 (#8690), and anti-p-p38 (#4511) from Cell Signaling Technology; anti-β-Actin (sc-47778) and anti-β-Tubulin (sc-80011) from Santa Cruz Technology; anti-MIF (AF-289-PB) from R&D Systems.
Cell viability assay
PCa cells were seeded in 96-well plates (1×104 cells/well) and treated as indicated. The viability of cells was measured using Alamar Blue assay kit (Thermo Fisher Scientific) according to the manufacturer’s instructions.
Transwell migration assay
C4–2B and PC-3 cells were grown in RMPI 1640 with 5% CSS and 5% FBS respectively, and pretreated with or without CCX771 (5 μM) for 24 hrs. C4–2B (2×105 cells/well) and PC-3 (1×105 cells/well) cells were then suspended in 200 μl RPMI 1640 with 0.5% CSS (or FBS) and added into 8.0 μm pore-sized transwell inserts (Cat# 353097, BD Falcon). The inserts were subsequently placed in a 24-well plate containing 600 μl RPMI 1640 with 15% CSS (or FBS), or 0.5% CSS with or without rMIF (10 ng/ml). After 18 hours of incubation, the cells that had migrated through the membrane were stained with crystal violate. The cell images were captured at ×20 magnification and counted in 4 representative fields. Quantification of migrated cells was performed using ImageJ software.
Flow Cytometry
Cells were treated as indicated. Approximately 1×106 cells/ml were washed with ice-cold phosphate-buffered saline (PBS) containing 0.5% BSA and 1% sodium azide. Cells were stained with 10 μg/mL phycoerythrin (PE)-conjugated anti-CXCR7 (Cat #331103, Biolegend) on ice for 40 minutes in the dark. Non-specific isotype-matched IgG was used as a control. The stained cells were washed 3 times with PBS, resuspended and fixed in 1% (w/v) paraformaldehyde for analysis. Ten thousand cells from each sample were evaluated using FACSCanto II (BD Biosciences) or EPICS ALTRA (Beckman Coulter). The fluorescence intensity was analyzed with FlowJo software (FlowJo LLC, Ashland, OR, USA) and was presented in arbitrary units.
Human serum specimens
Clinical serum samples were obtained from the Authur and Linda Gelb Center for Translational Research at DFCI. The study protocol was approved by the institute. Localized PCa patients (n = 20) had been treated with radical prostatectomy at the time of blood collection. Metastatic hormone-naïve PCa patients (n = 20) had not been treated with ADT at the time of blood collection. Metastatic CRPC patients (n = 20) had been treated with ADT at the time of blood collection and had evidence of progression of disease with either rising PSA or evidence of radiographic progression. Normal individuals (n = 20) without cancer were used as controls.
ELISA
Serum MIF levels were determined with the MIF ELISA kit (R&D Systems) according to the manufacturer’s instructions. To measure secreted MIF from cell culture, conditioned medium was collected and measured with ELISA. Values were normalized to protein concentration from whole cell lysates.
RNA interference
Cells were transfected with CXCR7 siRNA, MIF siRNA, or non-specific siRNA at a final concentration of 20 nM using Lipofectamine RNAiMAX transfection reagent (Life Technologies) according to the manufacturer’s protocol. The siRNA information is listed in supplementary Table S1.
Reverse transcription qPCR (RT-qPCR)
Total RNA was extracted from cells treated as indicated using TRIzol reagent (Invitrogen). cDNA synthesis was performed using the iScript™ cDNA synthesis kit (Bio-Rad). RT-qPCR reactions were performed using SYBR Green PCR master mix reagents (Bio-Rad). Each measurement was performed in triplicate and the mRNA levels of each gene were normalized to GAPDH levels. The primer sequences are listed in Supplementary Table S1.
Generation of knockout (KO) cell line with clustered regularly-interspaced short palindromic repeats/CRISPR associated protein 9 (CRISPR/Cas9)
To perform genome editing via CRISPR/Cas9 approach, specific guide RNAs (gRNAs) targeting CXCR7 AR binding site (ARBS) or CXCR7 exon were designed using http://crispr.mit.edu/ and http://crispr.dfci.harvard.edu/SSC/ (24,25). gRNAs with a 20 nucleotides spacer were cloned into lentiGuide-Puro vector (#52963, Addgene). The vector containing gRNA targeting GFP was obtained from Dr. William C. Hahn (Dana-Farber Cancer Institute, Boston) as a gift, and used as a control in parallel. The lentiCas9-Blast vector that expresses Cas9 was obtained from Addgene (# 52962). Lentiviruses that carry each gRNA or Cas9 were packaged in 293T cells. All gRNA sequences are listed in Supplementary Table S1.
To generate CXCR7 ARBS KO cell lines, C4–2B cells were transected with lentiCas9-Blast and dual gRNAs targeting CXCR7 ARBS (A1/B1 or A2/B2). Transfected cells were selected using 2 μg/ml Puromycin and 10 μg/ml Blasticidin for 3 days before plated at clonal density, and single-cell-derived colonies were generated by picking clones into 96-well plate. For genotyping, genomic DNA was extracted using QuickExtract™ (Lucigen), and PCR was performed with screening primers (listed in Supplementary Table S1). Selected KO clones were then expanded and further characterized.
To generate CXCR7 gene KO cell lines, C4–2B were transfected with lentiCas9-Blast and 4 pooled gRNAs (CXCR7 KO A/B/C/D) that targeting CXCR7 second exon. Single-cell-derived colonies were generated as above and validated by flow cytometry for CXCR7 protein expression.
To search for potential off-target sites of gRNAs in KO cell lines, we used the CRISPR/Cas9 target online predictor (CCTop) with default settings (26). The genomic regions of on-target and top two off-target sites for each gRNA were PCR-amplified and analyzed by Sanger sequencing (Supplementary Table S2). The sequence traces were analyzed and the frequency of mutations generated by CRISPR/Cas9 (or gene editing efficacy) was determined by TIDE (27).
RNA-seq analysis
RNA-seq was performed as previously described with modifications (17). Briefly, RNA-seq libraries were prepared using NEBNext Ultra RNA Library Prep Kit (New England Biolabs). The libraries were sequenced in the NextSeq 550 system (Illumina) according to the manufacturer’s instructions. RNA-seq reads were mapped to the human genome GRCh37, using Tophat2 v2.0.14 and raw read counts were generated with Htseq (0.6.1) using the union method (28,29). Genes that did not have at least 3 raw mapped counts in the sum of all six samples were filtered prior to differential testing. Differentially expressed genes (p < 0.01, FDR < 0.01, and fold of change > 2) were identified using EdgeR (3.12.0) (30). Gene ontology analysis was performed by David online analysis tools using all genes identified by our RNA-seq as a background (31). Gene expression is reported in counts per million.
Animal studies
The animal protocol was approved by the institutional Anima Care and Use Committee (IACUC). C4–2B cells (1×106 cells/site mixed with Matrigel at a 1:1 ratio, v/v) were injected subcutaneously into 6-week-old male ICR-SCID intact mice (Taconic Biosciences). After tumor formation (approximately 100 mm3), mice were randomized into four groups (9 mice/group) and treated with vehicle, enzalutamide (25mg/kg, orally), CCX771 (30mg/kg, subcutaneously), or enzalutamide + CCX771 in combination daily for 5 weeks. DMSO was used as the vehicle for enzalutamide. A special vehicle for CCX771 was provided by ChemoCentryx (Mountain View, CA). The tumor growth was monitored bi-weekly using caliper measurement. Tumor volume was compared between the groups. The expression of CXCR7 mRNA in tumor tissues was analyzed using RT-qPCR. To detect metastasis, genomic DNA was isolated from bone marrow and liver tissues using Puregene DNA purification system (Qiagen), and the presence of tumor cells was analyzed by quantification of human Alu sequence as previously described (32,33). Human Alu-specific TaqMan qPCR was performed using the primers and probe listed in Supplementary Table S1.
Clinical expression data analysis
Two gene expression microarray datasets from primary and metastatic tumors (GSE21034 and GSE32269) were acquired from Gene Set Omnibus (GEO) using GEO2R (34,35). The expression levels of CXCR7 and CXCR4 were isolated for each patient using “NM_020311 /212977_at” or “NM_003467 /211919_s_at” respectively. To study the association between the expression levels of CXCR7 and CXCR4 and the disease-free time of PCa patients, expression data (Z-scores) for CXCR7 and CXCR4 were downloaded from The Cancer Genome Atlas (TCGA) dataset through cBioPortal (36). Patients were then split into two groups with high (> medium) and low (≤medium) expression of CXCR7 and CXCR4 respectively. The Kaplan-Meier plots of biochemical relapse-free survival proportion were generated, and the statistical analysis was performed using log-rank (Mantel-Cox) test.
Statistical methods
All the experiments were performed at least three times. Values are shown as mean ± SD of three replicates from one representative experiment. All statistical testing was done using two-tailed t-test. p-value < 0.05 was considered as statistically significant.
Results
CXCR7 is a direct AR-repressed gene.
Emerging evidence has suggested that AR-mediated gene repression contributes to the development of CRPC (3,4). To elucidate the molecular mechanisms of AR-repressed genes in promoting PCa growth and progression after ADT, we first defined such genes by analyzing AR cistrome in PCa cell lines using BETA (18). We integrated a gene expression microarray dataset from LNCaP cells with six AR ChIP-seq datasets from three different PCa cell lines (three from LNCaP, two from VCaP and one from LNCaP-Abl cells). We identified 88 AR-repressed genes (Supplementary Fig. S1 and Table S3), including well-known AR-repressed genes, OPRK1 and AMIGO2 (3,4). These genes were suppressed by androgen treatment and had AR binding sites nearby. Gene ontology analysis revealed that AR-repressed genes were enriched in cell cycle regulation (Supplementary Fig. S2), suggesting their important roles in PCa cell growth.
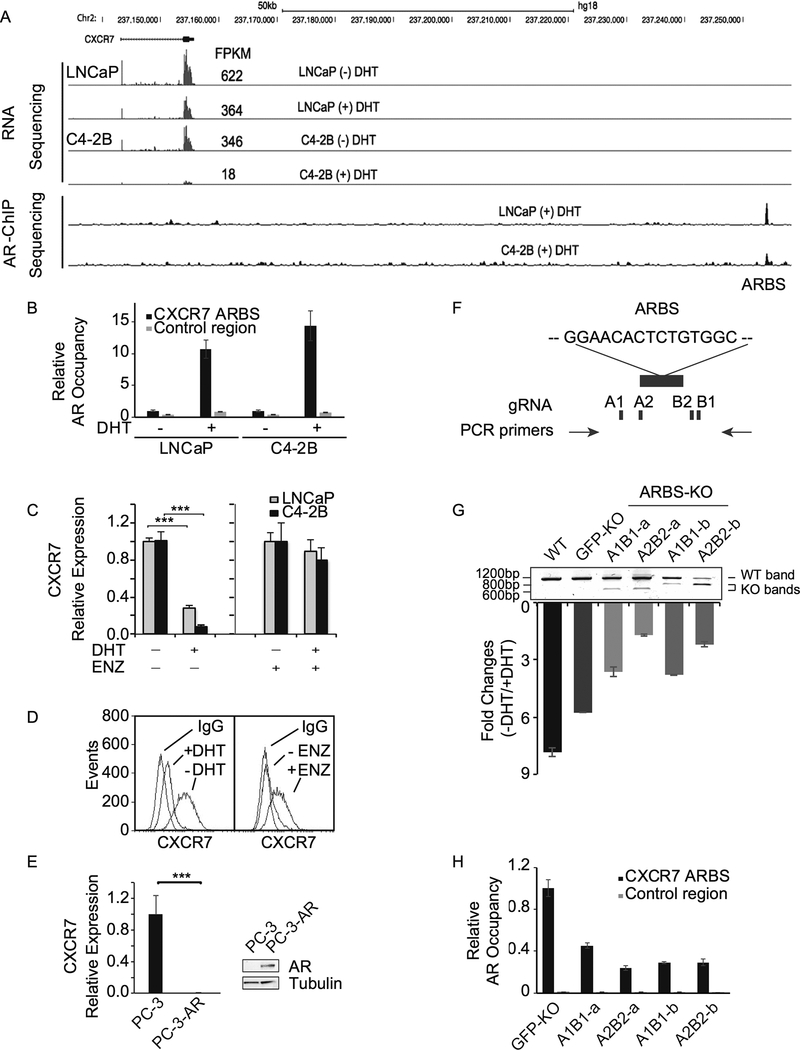
Among these genes, we discovered CXCR7 as one of the top AR-repressed genes. More importantly, a small molecule CXCR7 antagonist, CCX771, became available for in vitro and in vivo studies (37,38). Therefore, we decided to select CXCR7 for a further investigation. We next examined our previously published RNA-seq and ChIP-seq data in LNCaP (androgen-dependent) and C4–2B (LNCaP-derived CRPC) cells (17), and found that CXCR7 expression was inhibited upon dihydrotestosterone (DHT) treatment in both cells, but more so in C4–2B cells (Fig. 1A). Notably, androgen withdrawal dramatically elevated CXCR7 mRNA levels (about 20-fold) in CRPC C4–2B cells. Furthermore, our ChIP-seq analysis detected a strong ARBS about 100 kb downstream of the CXCR7 transcription start site. There are no annotated genes between the body of CXCR7 gene and the ARBS that contains an androgen response element (GGAACACTCTGTGGC), suggesting a AR cis-regulatory element. We validated DHT-induced AR occupancy at the ARBS in both LNCaP and C4–2B cells using site-specific ChIP-qPCR (Fig. 1B). We further validated RNA-seq results by RT–qPCR (Fig. 1C). Notably, DHT-induced CXCR7 repression was completely abolished by AR antagonist, enzalutamide. In line with mRNA expression, flow cytometry analysis showed that CXCR7 protein levels on C4–2B cell surface were inhibited by DHT but enhanced by enzalutamide (Fig. 1D). In addition, we examined CXCR7 mRNA levels in AR-negative CRPC PC-3 cells and AR-expressing PC-3 cells (Fig. 1E). Overexpression of AR almost completely abolished CXCR7 expression in PC-3 cells.
Figure 1.
CXCR7 is an AR-repressed gene. A, Genome browser view of RNA-seq and AR ChIP-seq results at the CXCR7 locus in the presence or absence of 10 nM dihydrotestosterone (DHT) in LNCaP and C4–2B cells. Fragment Per Kilobase of transcript per Million (FPKM) was used as RNA-seq expression unit. B, AR occupancy at the AR binding site (ARBS) was examined by ChIP-qPCR in the presence and absence of DHT (10 nM) in both LNCaP and C4–2B cells. C, CXCR7 mRNA levels were measured by RT-qPCR in LNCaP and C4–2B cells after DHT (10 nM) or vehicle treatment in the presence or absence of 10 μM enzalutamide (ENZ). The values were normalized to GAPDH levels. D, Flow cytometry analysis of CXCR7 expression on C4–2B cell surface after treatment with DHT (10 nM) or ENZ (10 μM). E, CXCR7 mRNA levels were measured by RT-qPCR in AR-negative PC-3 and AR-expressing PC-3 (PC-3-AR) cells. F, The ARBS at the CXCR7 locus was knocked out using 2 sets of gRNAs (A1/B1 and A2/B2) as indicated. G, Four ARBS knockout (KO) cell lines were validated by PCR using genomic DNA from each cell line. Wild-type (WT) and KO bands were observed indicating partial ARBS KO. GFP knockout (GFP-KO) is the C4–2B control line using a gRNA against GFP. Parental C4–2B cells are also examined in parallel. CXCR7 mRNA expression levels were measured by RT-qPCR after treatment with or without DHT (10 nM) and normalized to GAPDH levels. Fold changes of CXCR7 expression (-DHT/+DHT) are represented. H, AR occupancy at the ARBS was examined in GFP-KO and ARBS-KO cells in the presence of DHT (10 nM). Because the ARBS was partially deleted, the control region was used as input normalization for the ARBS.
Studies have shown that AR primarily binds distal enhancers that can be several kb to over 100 kb away from the transcription start site of its regulated genes (18–23). Interestingly, a previous study has reported an ARBS at the CXCR7 promoter (11). This ARBS defined by ChIP-qPCR is likely acquired from interaction between the distal AR-bound enhancer and the CXCR7 promoter through chromatin looping. To demonstrate whether CXCR7 transcription is directly regulated by the ARBS identified through our ChIP-seq result, we knocked out the ARBS in C4–2B cells using a CRISPR/Cas9 dual-guide approach. We used two independent sets of gRNAs to rule out the off-target effect (Fig. 1F). Two single-cell-derived knockout colons from each gRNA set were used to assess the regulatory effect of the ARBS on CXCR7 expression. As shown in the PCR-based genotyping results (Fig. 1G), we were not able to generate a complete knockout clone. However, partially knocking out the ARBS, which presumably disrupt AR binding, significantly attenuated the androgen-mediated CXCR7 repression. This was confirmed by decreased AR binding at the ARBS (Fig. 1H) suggesting AR directly regulates CXCR7 expression by binding to the cis-regulatory element. We further analyzed potential off-target sites that might be recognized by the gRNAs used for ARBS KO. We found very minimum mutations at the putative off-target sites (Supplementary Fig. S3).
CXCR7 is overexpressed in metastatic PCa patients and associated with high risk of recurrence.
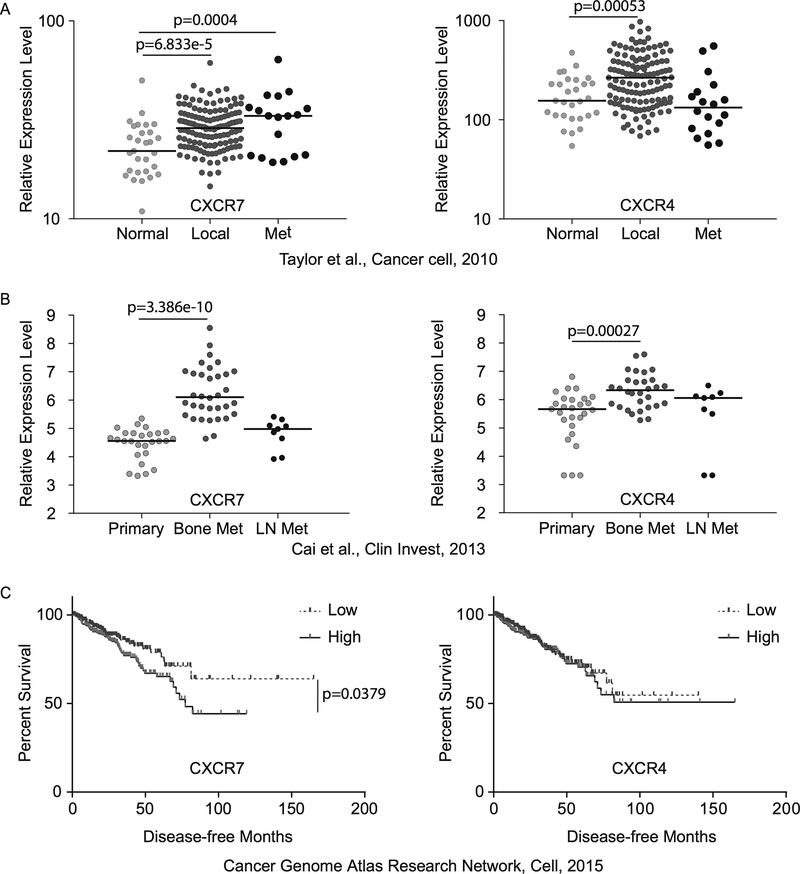
Next, we examined CXCR7 mRNA expression levels in clinical PCa tissues using two publicly available microarray datasets (34,35). Because CXCR4 is a well-studied chemokine receptor in PCa and shares the same chemokine ligand with CXCR7, we analyzed CXCR4 expression in parallel. Meta-analysis showed that both CXCR4 and CXCR7 were upregulated in localized tumors compared to normal prostate tissues and CXCR7 levels were further elevated in metastatic tumors (Fig. 2A). The metastatic tumors used in this dataset were collected from different metastatic sites, including lymph node, brain, bone, lung, neck, testes, or bladder. Interestingly, in an independent dataset, both CXCR4 and CXCR7 expression levels were significantly increased in metastatic bone samples but not in lymph node samples, indicating their implication in bone metastasis (Fig. 2B). Furthermore, we plotted Kaplan-Meier curves between high and low CXCR7 expression with disease-free time using clinical follow-up data from 492 patients in TCGA dataset (36) (Fig. 2C). Patients with low CXCR7 expression had significantly longer disease-free time compared with patients with high CXCR7 expression (p < 0.0379). In contrast, CXCR4 expression was not associated with biochemical recurrence. These results suggest that CXCR7 may play more important roles in CRPC progression than CXCR4.
Figure 2.
A and B, Meta-analyses of two publicly available microarray datasets showing CXCR7 and CXCR4 mRNA expression levels in normal prostate tissues vs. localized and metastatic (Met) tumors. LN = lymph node. C, Kaplan-Meier survival curves of biochemical recurrence-free survival were plotted between high and low CXCR4 and CXCR7 expression.
CXCR7 is required for CRPC growth and migration in vitro.
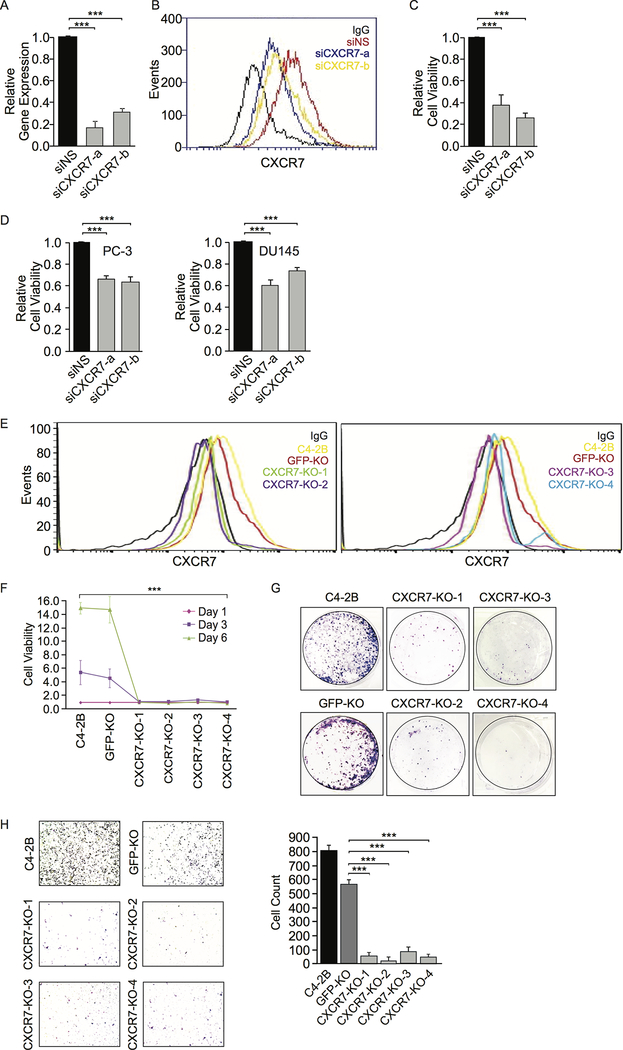
To determine whether CXCR7 is required for CRPC cell growth, we examined the growth of several CRPC cell lines after inhibition of CXCR7 through genetic and pharmacological means. We initially knocked down CXCR7 in C4–2B cells using RNA interference (RNAi). CXCR7 knockdown efficiency was confirmed at mRNA levels using RT–qPCR (Fig. 3A) and at protein levels using flow cytometry (Fig. 3B). We observed a significant decrease of C4–2B cell viability after CXCR7 knockdown (Fig. 3C). The same inhibitory effect was observed in two other CRPC cell lines, PC-3 and DU145 (Fig. 3D).
Figure 3.
CXCR7 knockdown (KD) or knockout (KO) decreases viability and migration in CRPC cells. A, CXCR7 mRNA expression levels in C4–2B cells were measured by RT-qPCR after CXCR7 siRNA KD. Gene expression with non-specific (NS) siRNA is defined as 1. B, CXCR7 protein expression on C4–2B cell surface was measured by flow cytometry after siRNA KD. C, C4–2B cell viability was measured by Alamar Blue assay after CXCR7 siRNA KD. D, PC-3 and DU145 cell viability was measured by Alamar Blue assay after CXCR7 siRNA KD. E, CXCR7 KO C4–2B cells were established using a CRISPR/Cas9 approach. Four cell clones (CXCR7-KO-1 and −2 on the left and CXCR7-KO-3 and −4 on the right) were selected for validation based on CXCR7 protein expression using flow cytometry. Non-specific IgG staining was used as a negative control. Parental C4–2B and GFP-KO C4–2B cells were used as positive controls. F, Cell viability of CXCR7 KO C4–2B cell lines was measured by Alamar Blue compared to control cell lines. G, Representative images of colony formation assays on CXCR7 KO C4–2B cells compared to control cell lines. H, Representative images of transwell migration assays in CXCR7 KO C4–2B cell lines compared to control cell lines. The images were quantified using ImageJ software. Data are representative of three independent experiments. Mean ± SD is plotted. P-value was determined by two-tailed Student’s t-test. *** p<0.0001.
Next, we generated CXCR7 gene KO C4–2B cell lines using CRISPR/Cas9 gene editing. Four knockout cell clones were selected and confirmed by flow cytometry (Fig. 3E), which showed abolished CXCR7 protein expression on cell surface. Analysis of mutations generated by CRISPR/Cas9 at on-target and putative off-target sites further confirmed CXCR7 gene knockout (Supplementary Fig. S4), although the gene editing efficacy at on-target site is around 40–70% estimated by sequence trace decomposition (27). Knockout of CXCR7 in C4–2B cells dramatically attenuated cell proliferation and colony formation compared to parental or GFP-KO C4–2B cells (Fig. 3F and 3G). Using transwell migration assay, we found that knockout of CXCR7 abolished C4–2B cell migration (Fig. 3H). Since the migration assay was performed within 18 hours, the reduction in cell migration was unlikely attributed to the reduced cells growth.
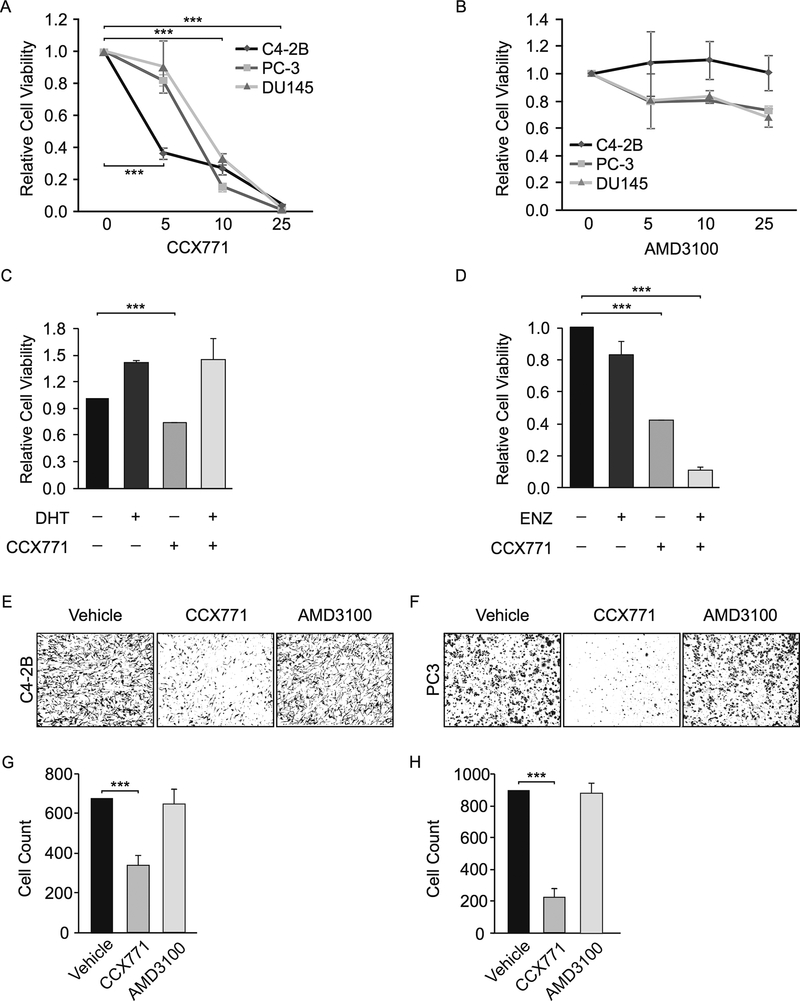
CCX771 is a small molecule CXCR7 antagonist and has been previously used in vitro and in vivo studies (37,38). We treated three CRPC cell lines with CCX771 and observed its inhibitory effect on cell growth in a dose-dependent manner (Fig. 4A). In contrast, treatment with CXCR4 inhibitor, AMD3100, rendered minor effect on CRPC cell growth under the same concentration (Fig. 4B). In addition, we found that CCX771 enhanced the inhibitory effects of enzalutamide and androgen withdrawal on C4–2B cell growth. (Fig. 4C and D). This was likely due to elevated CXCR7 expression after AR suppression in these cells, indicating its critical role in promoting CRPC growth after anti-androgen treatment. Furthermore, CCX771 abolished C4–2B and PC-3 cell migration in transwell migration assays (Fig. 4E and F). We tested AMD3100 in parallel and observed little effect on cell migration even when we increased the concentration to 50 μM (Supplementary Fig. S5). To determine the efficacy of AMD3100, we treated colorectal cancer HCT116 cells, which possess high CXCL12/CXCR4 activity (39), under the same concentration and observed reduced cell migration. In addition, we analyzed an independent RNA-seq dataset to compare CXCR4 and CXCR7 expression in 4 PCa cell lines (LNCaP, C4–2B, PC-3, and DU145), which we used in the present study (40). By plotting RNA-seq reads directly to the genome browser, we found very high CXCR7 levels in LNCaP, C4–2B, and PC-3 cells and a low CXCR7 level in DU145 cells (Supplementary Fig. S6). In contrast, CXCR4 expression levels were either low in C4–2B and DU145 cells or undetectable in LNCaP and PC-3 cells. Taken together, our results are consistent with high CXCR7 expression in clinical metastatic tumors, which is associated with biochemical recurrence.
Figure 4.
CXCR7 antagonist, CCX771, inhibits CRPC cell proliferation and migration. A, Cell viability of C4–2B, PC-3, and DU145 cells was measured by Alamar Blue 5 days after treatment with different concentration of CCX771. All cells were grown in the RPMI 1640 with 5% FBS. B, Same experiments were performed after treatment with AMD3100. C, C4–2B cells were grown in the RPMI 1640 with 5% CSS. Cell viability was measured by Alamar Blue 5 days after treatment with CCX771 in the presence or absence of DHT (10 nM) D, C4–2B cells were grown in the RPMI 1640 media with 5% FBS. Cell viability was measured in the presence or absence of 10 μM enzalutamide (ENZ). E, Representative images of transwell migration assays in C4–2B cells after treatment with CCX771 (5 μM) or AMD3100 (10 μM). The images were quantified using ImageJ software. F, Same experiments were performed in PC-3 cells. Data are representative of three independent experiments. Mean ± SD is plotted. P-value was determined by two-tailed Student’s t-test. *** p < 0.0001.
CXCR7 enhances CRPC growth and metastasis in vivo.
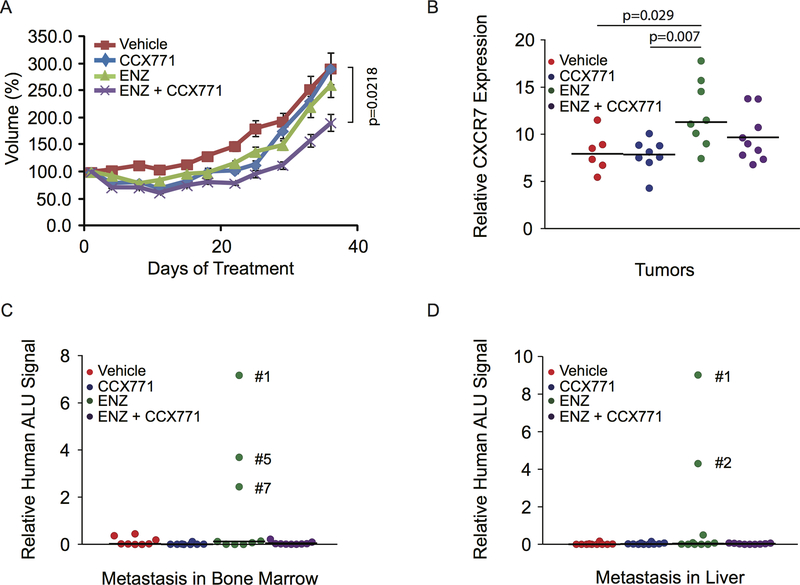
To examine the role of CXCR7 in CRPC growth and progression in vivo, we implanted C4–2B cells subcutaneously into severe immunodeficient mice. Mice bearing established tumors were randomly assigned into 4 groups (9 mice/group) and treated with vehicle, enzalutamide, CCX771, or enzalutamide + CCX771 in combination for 5 weeks. Tumor volume was measured and compared between groups. While enzalutamide and CCX771 initially showed marginal inhibitory effect on tumor growth, overall C4–2B tumors did not respond to enzalutamide or CCX771 treatment as a single agent (Fig. 5A). In contrast, the combination treatment significantly inhibited C4–2B tumor growth. Analysis of CXCR7 expression in tumor tissues revealed significantly elevated CXCR7 mRNA levels after enzalutamide treatment (Fig. 5B), which likely contributed to tumor growth and progression. Interestingly, CCX771 appeared to inhibit enzalutamide-induced CXCR7 expression with combination treatment although statistical significance was not reached. To investigate whether enzalutamide-induced CXCR7 expression enhances C4–2B metastasis, we harvested bone marrows from mouse tibia and liver tissues. Because we didn’t visually observe any metastases, we examined human Alu DNA sequences using TaqMan qPCR to determine whether there are micrometastases in collected samples (32,33). As shown in Fig. 5C and D, 4 out of 9 samples were found to contain high levels of human Alu DNA sequences in either bone marrow and/or liver tissues from the enzalutamide-treated group. In contrast, little Alu DNA signal was detected in other groups. Our data are consistent with the results from previous studies showing enzalutamide-enhanced metastasis in pre-clinical models (32,41). In line with our in vitro data, these results indicate that enzalutamide-induced CXCR7 expression may promote the development of metastatic CRPC, which can be potentially delayed or prevented by CXCR7 blockade.
Figure 5.
Combination treatment with CCX771 and enzalutamide (ENZ) inhibits C4–2B tumor growth and metastasis in vivo. A, C4–2B cells (1×106 cells/site) were injected subcutaneously into ICR-SCID male mice. Mice were assigned into 4 groups (9 mice/group) and treated with vehicle, CCX771 (30 mg/kg), ENZ (25 mg/kg), or CCX771 + ENZ for 5 weeks. The tumor growth was monitored using caliper measurement. Values of tumor volume are mean ± SE. P-value was calculated using two-tailed Student’s t-test between groups. B, CXCR7 mRNA expression levels in tumor tissues were measured by RT-qPCR. P-value was calculated using two-tailed Student’s t-test between groups. C and D, C4–2B tumor metastases were assessed by quantification of human-specific Alu sequences in DNAs extracted from bone marrow from tibia and liver tissues.
CXCR7 inhibition impacts M-phase cell cycle gene expression and AKT signaling
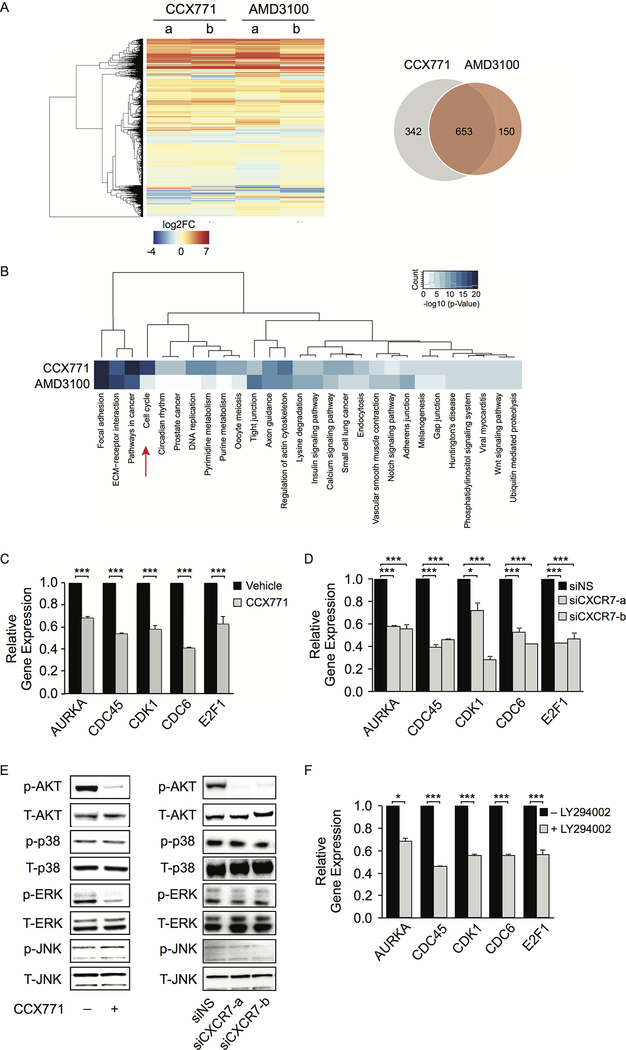
To determine the impact of CXCR7 on global gene expression, we performed RNA-seq in C4–2B cells upon CCX771 treatment in the absence of androgen. AMD3100 treatment was performed in parallel. We identified 995 and 803 genes with altered expression levels in CCX771- and AMD3100-treated cells respectively (Fig. 6A and Supplementary Table S4). There are 653 common genes shared by both treatments. Gene ontology analyses revealed that genes involved in focal adhesion, extracellular matrix (ECM)-receptor interaction, and pathways in cancer are enriched in both treatments (Fig. 6B). Importantly, we discovered that blocking CXCR7 with CCX771 resulted in downregulation of cell cycle genes including many critical M-phase cell cycle genes, such as AURKA, CDC6, CDC45, CDK1, and E2F1. We examined expression levels of these 5 genes using RT-qPCR and confirmed the RNA-seq results (Fig. 6C). We further validated this result by knocking down CXCR7 using two independent siRNAs (Fig. 6D). This alteration is unique to CXCR7 inhibition, indicating the critical role of CXCR7 in promoting CRPC cell proliferation through upregulation of cell cycle genes.
Figure 6.
CXCR7 induces M-phase cell cycle gene expression through AKT signal transduction pathway. A, Hierarchical clustering of gene expression alteration in C4–2B cells after treatment with CCX771 (5 μM) or AMD3100 (10 μM) for 16 hours in the absence of androgen. Venn diagram shows differentially expressed genes between the two groups. B, Heatmap showing the unsupervised clustering of the Gene Ontology (GO) terms enriched in CCX771- and AMD3100-altered genes. GO terms with -log10 (p-value) greater than 5 in either gene list were selected for analysis. C, Gene expression levels of top five altered cell cycle genes were examined using RT-qPCR after CCX771 (5 μM) treatment. D, Downregulation of five cell cycle gene expression was validated using RT-qPCR after CXCR7 siRNA knockdown (KD). E, Western blot showing phosphorylation of AKT, p-38, ERK, and JNK after CCX771 (5 μM) treatment for 24 hours or CXCR7 siRNA KD in C4–2B cells for 2 days. F, Expression levels of five cell cycle genes after treatment with LY294002 (10 μM) were measured by RT-qPCR. Data are representative of three independent experiments. Mean ± SD is plotted. p-value was determined by two-tailed Student’s t-test. *** p<0.0001.
To characterize the CXCR7-mediated signaling pathways involved in gene expression alteration, we analyzed the impact of CXCR7 on several mitogenic signaling pathways. We examined AKT, ERK, p38, and JNK activation using Western blot analysis in C4–2B cells after CCX771 treatment or CXCR7 siRNA knockdown in the absence of androgen (Fig. 6E). It was evident that AKT phosphorylation was significantly inhibited after targeting CXCR7 with small molecule inhibitor or RNAi. This result was consistent with a previous report, showing CXCR7 activates AKT signaling (42). ERK phosphorylation was also inhibited by CCX771, but was not confirmed by RNAi. Phosphorylation of JNK or p38 remained unchanged. We then treated C4–2B cells with the PI3K inhibitor, LY294002, and observed downregulation of AURKA, CDC6, CDC45, CDK1, and E2F1 expression (Fig. 6F). Our data suggest that CXCR7 affects cell cycle gene expression at least partially through a PI3K/AKT-dependent mechanism.
MIF promotes CRPC growth and migration through CXCR7.
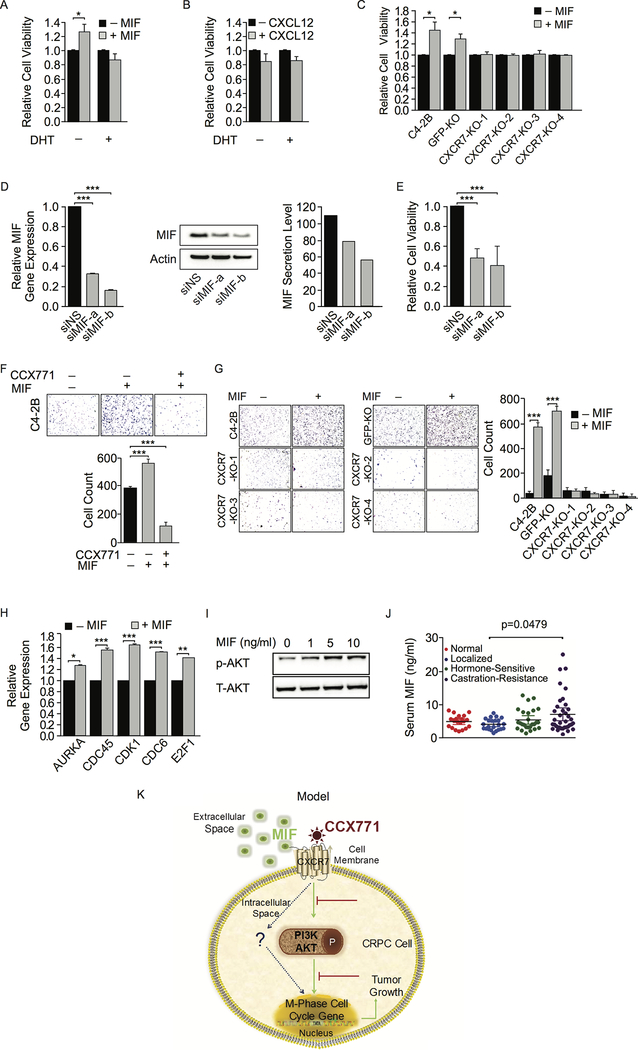
Previously studies have demonstrated both CXCL12 and MIF are ligands for CXCR7 (43,44). Both CXCL12/CXCR7 and MIF/CXCR7 physical interactions have been established. Here, we co-cultured C4–2B cells with mouse bone marrow stroma ST2 cells in the presence or absence of androgen and measured secreted chemokine and cytokine concentrations in the medium. Surprisingly, we found that MIF had the highest protein concentration in the co-culture medium. (Supplementary Fig. S7). CXCL12 was also detectable at a relatively lower level. Androgen treatment had no effect on MIF and CXCL12 levels. Using human- and mouse-specific ELISA, we found that MIF was secreted from both cancer cells and stroma cells, and that stroma cells promoted MIF secretion from cancer cells when they were co-cultured (Fig. S8). This indicated that tumor microenvironment may contribute to MIF-induced effects. We then examined C4–2B cell growth after treatment with human rMIF and rCXCL12. We found that rMIF significantly enhanced cell growth only in the absence of androgen in consistent with upregulation of CXCR7 after androgen deprivation (Fig. 7A). On the other hand, rCXCL12 had little effect on cell growth even when we increased its concentration to 100 ng/ml (Fig. 7B). In addition, we found that rMIF-induced C4–2B cell growth was abolished when CXCR7 was knocked out (Fig. 7C). We then knocked down endogenous MIF in C4–2B cells using RNAi. MIF mRNA and protein levels were significantly reduced after MIF siRNA knockdown (Fig 7D), which attenuated C4–2B cell growth (Fig. 7E). rMIF also promoted C4–2B cell migration in a transwell assay, which was inhibited by CXCR7 blockade with CCX771 (Fig. 7F). We did not observe increased cell migration after rCXCL12 stimulation (data not shown), indicating CXCR12 played only a minimal role in the C4–2B CRPC model. We further examined the migratory capacity of CXCR7 KO cell lines after rMIF stimulation. Cell migration was enhanced by rMIF in parental and GFP-KO C4–2B cell lines (Fig. 7G). In contrast, rMIF had no effect on CXCR7 KO cell migration in transwell assays. Our results support the notion that MIF but not CXCL12 is the ligand for CXCR7 in CRPC cells and that MIF promotes CRPC cell growth and migration through CXCR7.
Figure 7.
MIF enhances CRPC cell growth and migration through CXCR7. A and B, C4–2B cell viability was measured by Alamar Blue assay 5 days after treatment with rMIF (10 ng/ml) or rCXCL12 (100 ng/ml) in the presence or absence of DHT (10 nM). C, Viability of CXCR7 knockout (KO) cells was measured after MIF (10 ng/ml) stimulation compared to parental C4–2B and GFP-KO C4–2B cells. D, MIF siRNA knockdown (KD) efficiency was determined by mRNA levels using RT-qPCR and protein levels using Western blot and ELISA. E, C4–2B viability was measured after MIF siRNA KD. F, Representative images of transwell migration assays in rMIF-treated C4–2B cells in the presence or absence of CCX771 (5 μM). Migration images were quantified using ImageJ software. G, Representative images of transwell migration assays in rMIF-treated CXCR7 KO cell lines compared to control lines. Migration images were quantified using ImageJ software. H, Expression levels of five cell cycle gene were examined using RT-qPCR after treatment with rMIF (10 ng/ml) for 16 hours. I, AKT phosphorylation was examined by Western blot after different concentration of rMIF stimulation for 6 hours. J, MIF levels in patient serum samples were determined by ELISA. P-value was determined by two-tailed Student’s t-test. K, Schematic overview of a model depicting MIF/CXCR7/AKT signaling in CRPC cells leading to expression of cell cycle genes and CRPC cell growth.
To further address the biological function of MIF/CXCR7 axis in CRPC cells, we examined MIF-induced gene expression. We found that rMIF treatment significantly upregulated the expression levels of AURKA, CDC6, CDC45, CDK1, and E2F1 genes (Fig. 7H). Western blot analyses showed MIF-induced AKT phosphorylation in a dose-dependent manner (Fig. 7I). To determine whether CRPC patients have high levels of secreted MIF, we measured MIF protein levels in serum samples from normal individuals, localized PCa, hormone-sensitive PCa, and metastatic CRPC patients using ELISA. As shown in Figure 7J, normal individuals and localized PCa patients had low levels of MIF in serum. A slight increase was observed in hormone-sensitive patients. In contrast, a significantly higher MIF level was detected in a subset of CRPC patients compared to localized patients. Taken together, our results suggest that elevated MIF may stimulate CXCR7 in CRPC cells, leading to activation of PI3K/AKT signaling, upregulation of cell cycle gene expression, and CRPC cell growth and progression.
Discussion
Despite the development of next-generation anti-androgens, resistance to these drugs inevitably develops and renders patients largely incurable. To prevent or overcome anti-androgen resistance, a combination therapy with drugs targeting alternative survival signaling pathways may be necessary. Toward this end, we have discovered a MIF/CXCR7-mediated signaling pathway by which CRPC cells continue to grow and metastasize after anti-androgen treatment. MIF/CXCR7 promotes CRPC progression by inducing M-phase cell cycle gene expression at least partially through PI3K/AKT signal transduction pathway (Fig 7K). Targeting CXCR7 by a small molecule inhibitor, CCX771, in combination with AR antagonist, enzalutamide, inhibits CRPC tumor growth and potentially prevents metastasis.
Chemokines and their receptors play a crucial role in cancer metastasis. Much attention has been focused on CXCR4. Interaction between CXCR4 and its ligand CXCL12 and their biological activities in PCa has been extensively studied (7). The CXCL12/CXCR4 signaling plays a key role in homing of PCa cells to the bone microenvironment. CXCR4 has been targeted by small molecule inhibitors in clinical trials, which are analogues to the amino-terminal region of the ligand, CXCR12. While previous studies have showed that CXCL12 stimulates gene expression that leads to a more proliferative and invasive phenotype in PCa, we did not observe any biological effect induced by CXCR12 in our CRPC cell models. Inhibition of CXCR4 with AMD3100 also showed little effect on CRPC cell proliferation and migration. Lack of active CXCL12/CXCR4 signaling is likely due to lack of CXCR4 expression and activities in our cell models. Our data suggest that CXCR4 and CXCR7 may act independently in different cancer cell types. MIF/CXCR7 signaling is highly active in a subset of CRPC patients independent of CXCL12/CXCR4 signaling. Analyses of CXCR4 and CXCR7 expression in publicly available datasets further indicate that CXCR7 may play more important roles in PCa metastasis.
Previous studies have indicated that increased CXCR7 expression is associated with aggressiveness in a variety of cancers, including breast, prostate, lung, and pancreatic cancer, by enhancing cell survival, proliferation, migration, invasion and angiogenesis (6). In PCa, it was reported that pro-inflammatory chemokine IL-8 upregulates CXCR7, which in turn promotes PCa cell proliferation in a ligand-independent manner (45). Depletion of CXCR7 suppresses prostate tumor growth through cell cycle arrest. More recently, It was demonstrated AR inhibition increases CXCR7 expression through transcriptional regulation, which enhances PCa survival and proliferation under androgen-deprived conditions (11). Growth promoting is accompanied by enhanced epidermal growth factor receptor (EGFR)-mediated mitogenic signaling. In line with these results, we have discovered a novel MIF/CXCR7/AKT pathway, which enhances CRPC growth and metastasis after anti-androgen treatment. Studies on different aspects of CXCR7 in PCa have shown the complex processes of CXCR7-mediated oncogenic signaling. These results, however, have led to the conclusion that targeting CXCR7 in PCa is a promising therapeutic approach. It should be noted that previous studies have shown the benefit of combination treatment with CCX771 and enzalutamide in pre-clinical PCa models (13). The inhibitory effect on androgen-dependent VCaP and MDA 133–4 tumor growth are more dramatic compared to the C4–2B model used in our studies. This is likely because the VCaP and MDA 133–4 tumors are responsive to enzalutamide treatment as a single agent, whereas CRPC C4–2B cells derived from bone metastasis are more aggressive and enzalutamide-resistant. Our results support the notion of targeting CXCR7 in enzalutamide- and abiraterone-resistant patients.
MIF is a pro-inflammatory cytokine with chemokine-like activities. The release of MIF from cancer or stroma cells in response to various deleterious stimuli, such as hypoxia, creates a microenvironment favorable to the development of tumor. As an autocrine/paracrine factor, MIF enhances tumor cell proliferation, migration, and tumor-induced angiogenesis. Overexpression of MIF has been observed in many different types of cancer including PCa and associated with tumor aggressiveness (46). Early studies have showed elevated MIF levels in advanced PCa, which correlates with cancer progression (47). Targeting MIF with neutralizing antibody inhibits PCa growth in pre-clinical studies (48). CD74, CXCR2, and CXCR4 are three receptors for MIF that mediate its intracellular functions. More recently, MIF was demonstrated as an alternative ligand for CXCR7 with a functional role in lymphocyte migration (44). MIF induces cancer cell proliferation via sustained activation of several pathways such as MAPK and PI3K/AKT. In line with these results, we have provided evidence that CXCR7 is required for MIF-induced proliferation and migration in CRPC cells. We revealed upregulation of MIF and CXCR7 in CRPC patients. Further analyses suggest MIF/CXCR7 axis promotes CRPC cell growth and progression likely through AKT activation. These results support the notion that CXCL12 and MIF may interact with their receptors, CXCR4 and CXCR7, independently under different cellular contexts.
PI3K/AKT signaling pathway is a pro-survival pathway and therapeutic target for many cancers including PCa. A reciprocal feedback activation loop is produced as the result of inhibition of AR or AKT (49). Therefore, co-targeting AR and PI3K/AKT may restore CRPC sensitivity to anti-androgen therapy and prolong disease stabilization. In the present study, we demonstrate increased CXCR7 expression after ADT induces AKT activation, leading to alteration of gene expression involved in M-phase cell cycle progression, which provides a mechanistic rationale for dual inhibition of AR and CXCR7. It remains unclear whether inhibition of AKT is equivalent to inhibition of CXCR7 as CXCR7 may activate other signaling transduction pathways. Further studies are needed to understand how CXCR7 activates AKT phosphorylation upon ligand binding because CXCR7 activation does not commonly lead to canonical signaling through heterotrimeric G-proteins. Instead, CXCR7 may activate signaling through recruitment of β–arrestins as an accessory protein/adapter molecule in a ligand-dependent manner (10,50).
In conclusion, our results suggest that upregulation of CXCR7 after ADT is one of the underlying mechanisms for CRPC cell survival, growth and metastasis. Inhibition of CXCR7 may afford therapeutic benefits in certain clinical settings. Similar to targeting CXCR4, inhibition of CXCR7 may remove PCa cells from bone marrow niche so as to expose and sensitize them to chemotherapy. This is particularly important when PCa cells have low CXCR4 expression but high CXCR7 expression as we have observed in clinical metastatic tumors. These patients may not be responsive to CXCR4 blockade. More importantly, targeting CXCR7 in combination with anti-androgen treatment may not only inhibit CRPC tumor growth but also have the potential to prevent or delay metastasis in these patients. Our results set the stage for a further investigation on the benefits of combination therapy for metastatic CRPC.
Supplementary Material
Acknowledgement
This work was supported by grants from Developmental Research Award, NCI/NIH Dana Farber-Harvard Cancer Center SPORE in Prostate Cancer (P50CA090381–11A1) to L Jia, Research Scholar Award, American Cancer Society (RSG-16–113-01-TBE) to L Jia. We thank Quang-De Nguyen, Kristen L. Jones, Rebecca J. Modiste, Halle B Hall, and Michaela Bowden for their technical support in this study.
Footnotes
Conflicts of Interest: The authors declare no potential conflicts of interest
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin 2018;68:7–30 [DOI] [PubMed] [Google Scholar]
- 2.Tilki D, Schaeffer EM, Evans CP. Understanding Mechanisms of Resistance in Metastatic Castration-resistant Prostate Cancer: The Role of the Androgen Receptor. Eur Urol Focus 2016;2:499–505 [DOI] [PubMed] [Google Scholar]
- 3.Cai C, He HH, Chen S, Coleman I, Wang H, Fang Z, et al. Androgen receptor gene expression in prostate cancer is directly suppressed by the androgen receptor through recruitment of lysine-specific demethylase 1. Cancer cell 2011;20:457–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhao JC, Yu J, Runkle C, Wu L, Hu M, Wu D, et al. Cooperation between Polycomb and androgen receptor during oncogenic transformation. Genome research 2012;22:322–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Muller A, Homey B, Soto H, Ge N, Catron D, Buchanan ME, et al. Involvement of chemokine receptors in breast cancer metastasis. Nature 2001;410:50–6 [DOI] [PubMed] [Google Scholar]
- 6.Sun X, Cheng G, Hao M, Zheng J, Zhou X, Zhang J, et al. CXCL12 / CXCR4 / CXCR7 chemokine axis and cancer progression. Cancer Metastasis Rev 2010;29:709–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Taichman RS, Cooper C, Keller ET, Pienta KJ, Taichman NS, McCauley LK. Use of the stromal cell-derived factor-1/CXCR4 pathway in prostate cancer metastasis to bone. Cancer Res 2002;62:1832–7 [PubMed] [Google Scholar]
- 8.Hattermann K, Mentlein R. An infernal trio: the chemokine CXCL12 and its receptors CXCR4 and CXCR7 in tumor biology. Annals of anatomy = Anatomischer Anzeiger : official organ of the Anatomische Gesellschaft 2013;195:103–10 [DOI] [PubMed] [Google Scholar]
- 9.Wang J, Shiozawa Y, Wang J, Wang Y, Jung Y, Pienta KJ, et al. The role of CXCR7/RDC1 as a chemokine receptor for CXCL12/SDF-1 in prostate cancer. J Biol Chem 2008;283:4283–94 [DOI] [PubMed] [Google Scholar]
- 10.Decaillot FM, Kazmi MA, Lin Y, Ray-Saha S, Sakmar TP, Sachdev P. CXCR7/CXCR4 heterodimer constitutively recruits beta-arrestin to enhance cell migration. J Biol Chem 2011;286:32188–97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hoy JJ, Kallifatidis G, Smith DK, Lokeshwar BL. Inhibition of androgen receptor promotes CXC-chemokine receptor 7-mediated prostate cancer cell survival. Sci Rep 2017;7:3058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Saha A, Ahn S, Blando J, Su F, Kolonin MG, DiGiovanni J. Proinflammatory CXCL12-CXCR4/CXCR7 Signaling Axis Drives Myc-Induced Prostate Cancer in Obese Mice. Cancer Res 2017;77:5158–68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Luo Y, Azad AK, Karanika S, Basourakos SP, Zuo X, Wang J, et al. Enzalutamide and CXCR7 inhibitor combination treatment suppresses cell growth and angiogenic signaling in castration-resistant prostate cancer models. Int J Cancer 2018;142:2163–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zheng D, Gui B, Gray KP, Tinay I, Rafiei S, Huang Q, et al. Secretory leukocyte protease inhibitor is a survival and proliferation factor for castration-resistant prostate cancer. Oncogene 2016;35:4807–15 [DOI] [PubMed] [Google Scholar]
- 15.Baniwal SK, Khalid O, Sir D, Buchanan G, Coetzee GA, Frenkel B. Repression of Runx2 by androgen receptor (AR) in osteoblasts and prostate cancer cells: AR binds Runx2 and abrogates its recruitment to DNA. Mol Endocrinol 2009;23:1203–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zabel BA, Wang Y, Lewen S, Berahovich RD, Penfold ME, Zhang P, et al. Elucidation of CXCR7-mediated signaling events and inhibition of CXCR4-mediated tumor cell transendothelial migration by CXCR7 ligands. J Immunol 2009;183:3204–11 [DOI] [PubMed] [Google Scholar]
- 17.Decker KF, Zheng D, He Y, Bowman T, Edwards JR, Jia L. Persistent androgen receptor-mediated transcription in castration-resistant prostate cancer under androgen-deprived conditions. Nucleic Acids Res 2012;40:10765–79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang S, Sun H, Ma J, Zang C, Wang C, Wang J, et al. Target analysis by integration of transcriptome and ChIP-seq data with BETA. Nat Protoc 2013;8:2502–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang Q, Li W, Liu XS, Carroll JS, Janne OA, Keeton EK, et al. A hierarchical network of transcription factors governs androgen receptor-dependent prostate cancer growth. Mol Cell 2007;27:380–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sahu B, Laakso M, Pihlajamaa P, Ovaska K, Sinielnikov I, Hautaniemi S, et al. FoxA1 specifies unique androgen and glucocorticoid receptor binding events in prostate cancer cells. Cancer Res 2013;73:1570–80 [DOI] [PubMed] [Google Scholar]
- 21.Tan PY, Chang CW, Chng KR, Wansa KD, Sung WK, Cheung E. Integration of regulatory networks by NKX3–1 promotes androgen-dependent prostate cancer survival. Mol Cell Biol 2012;32:399–414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xu K, Wu ZJ, Groner AC, He HH, Cai C, Lis RT, et al. EZH2 oncogenic activity in castration-resistant prostate cancer cells is Polycomb-independent. Science 2012;338:1465–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Massie CE, Lynch A, Ramos-Montoya A, Boren J, Stark R, Fazli L, et al. The androgen receptor fuels prostate cancer by regulating central metabolism and biosynthesis. EMBO J 2011;30:2719–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hsu PD, Scott DA, Weinstein JA, Ran FA, Konermann S, Agarwala V, et al. DNA targeting specificity of RNA-guided Cas9 nucleases. Nat Biotechnol 2013;31:827–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xu H, Xiao T, Chen CH, Li W, Meyer CA, Wu Q, et al. Sequence determinants of improved CRISPR sgRNA design. Genome Res 2015;25:1147–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stemmer M, Thumberger T, Del Sol Keyer M, Wittbrodt J, Mateo JL. CCTop: An Intuitive, Flexible and Reliable CRISPR/Cas9 Target Prediction Tool. PLoS One 2015;10:e0124633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brinkman EK, Chen T, Amendola M, van Steensel B. Easy quantitative assessment of genome editing by sequence trace decomposition. Nucleic Acids Res 2014;42:e168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Trapnell C, Pachter L, Salzberg SL. TopHat: discovering splice junctions with RNA-Seq. Bioinformatics 2009;25:1105–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Anders S, Pyl PT, Huber W. HTSeq--a Python framework to work with high-throughput sequencing data. Bioinformatics 2015;31:166–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Robinson MD, McCarthy DJ, Smyth GK. edgeR: a Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 2010;26:139–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Huang da W, Sherman BT, Lempicki RA. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat Protoc 2009;4:44–57 [DOI] [PubMed] [Google Scholar]
- 32.Asangani IA, Dommeti VL, Wang X, Malik R, Cieslik M, Yang R, et al. Therapeutic targeting of BET bromodomain proteins in castration-resistant prostate cancer. Nature 2014;510:278–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.van der Horst EH, Leupold JH, Schubbert R, Ullrich A, Allgayer H. TaqMan-based quantification of invasive cells in the chick embryo metastasis assay. Biotechniques 2004;37:940–2, 4, 6 [DOI] [PubMed] [Google Scholar]
- 34.Taylor BS, Schultz N, Hieronymus H, Gopalan A, Xiao Y, Carver BS, et al. Integrative genomic profiling of human prostate cancer. Cancer cell 2010;18:11–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cai C, Wang H, He HH, Chen S, He L, Ma F, et al. ERG induces androgen receptor-mediated regulation of SOX9 in prostate cancer. J Clin Invest 2013;123:1109–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cancer Genome Atlas Research N. The Molecular Taxonomy of Primary Prostate Cancer. Cell 2015;163:1011–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Williams JL, Patel JR, Daniels BP, Klein RS. Targeting CXCR7/ACKR3 as a therapeutic strategy to promote remyelination in the adult central nervous system. J Exp Med 2014;211:791–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ierano C, Santagata S, Napolitano M, Guardia F, Grimaldi A, Antignani E, et al. CXCR4 and CXCR7 transduce through mTOR in human renal cancer cells. Cell Death Dis 2014;5:e1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang D, Jiao C, Zhu Y, Liang D, Zao M, Meng X, et al. Activation of CXCL12/CXCR4 renders colorectal cancer cells less sensitive to radiotherapy via up-regulating the expression of survivin. Exp Biol Med (Maywood) 2017;242:429–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Prensner JR, Iyer MK, Balbin OA, Dhanasekaran SM, Cao Q, Brenner JC, et al. Transcriptome sequencing across a prostate cancer cohort identifies PCAT-1, an unannotated lincRNA implicated in disease progression. Nat Biotechnol 2011;29:742–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lin TH, Lee SO, Niu Y, Xu D, Liang L, Li L, et al. Differential androgen deprivation therapies with anti-androgens casodex/bicalutamide or MDV3100/Enzalutamide versus anti-androgen receptor ASC-J9(R) Lead to promotion versus suppression of prostate cancer metastasis. J Biol Chem 2013;288:19359–69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang J, Shiozawa Y, Wang Y, Jung Y, Pienta KJ, Mehra R, et al. The role of CXCR7/RDC1 as a chemokine receptor for CXCL12/SDF-1 in prostate cancer. The Journal of biological chemistry 2008;283:4283–94 [DOI] [PubMed] [Google Scholar]
- 43.Tarnowski M, Grymula K, Liu R, Tarnowska J, Drukala J, Ratajczak J, et al. Macrophage migration inhibitory factor is secreted by rhabdomyosarcoma cells, modulates tumor metastasis by binding to CXCR4 and CXCR7 receptors and inhibits recruitment of cancer-associated fibroblasts. Mol Cancer Res 2010;8:1328–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Alampour-Rajabi S, El Bounkari O, Rot A, Muller-Newen G, Bachelerie F, Gawaz M, et al. MIF interacts with CXCR7 to promote receptor internalization, ERK1/2 and ZAP-70 signaling, and lymphocyte chemotaxis. FASEB J 2015;29:4497–511 [DOI] [PubMed] [Google Scholar]
- 45.Singh RK, Lokeshwar BL. The IL-8-regulated chemokine receptor CXCR7 stimulates EGFR signaling to promote prostate cancer growth. Cancer Res 2011;71:3268–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kindt N, Journe F, Laurent G, Saussez S. Involvement of macrophage migration inhibitory factor in cancer and novel therapeutic targets. Oncol Lett 2016;12:2247–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Muramaki M, Miyake H, Yamada Y, Hara I. Clinical utility of serum macrophage migration inhibitory factor in men with prostate cancer as a novel biomarker of detection and disease progression. Oncol Rep 2006;15:253–7 [PubMed] [Google Scholar]
- 48.Hussain F, Freissmuth M, Volkel D, Thiele M, Douillard P, Antoine G, et al. Human anti-macrophage migration inhibitory factor antibodies inhibit growth of human prostate cancer cells in vitro and in vivo. Mol Cancer Ther 2013;12:1223–34 [DOI] [PubMed] [Google Scholar]
- 49.Carver BS, Chapinski C, Wongvipat J, Hieronymus H, Chen Y, Chandarlapaty S, et al. Reciprocal Feedback Regulation of PI3K and Androgen Receptor Signaling in PTEN-Deficient Prostate Cancer. Cancer Cell 2011;19:575–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rajagopal S, Kim J, Ahn S, Craig S, Lam CM, Gerard NP, et al. Beta-arrestin- but not G protein-mediated signaling by the “decoy” receptor CXCR7. Proc Natl Acad Sci U S A 2010;107:628–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.