Abstract
PTC299 was identified as an inhibitor of VEGFA mRNA translation in a phenotypic screen and evaluated in the clinic for treatment of solid tumors. To guide precision cancer treatment, we performed extensive biological characterization of the activity of PTC299 and demonstrated that inhibition of VEGF production and cell proliferation by PTC299 is linked to a decrease in uridine nucleotides by targeting dihydroorotate dehydrogenase (DHODH), a rate limiting enzyme for de novo pyrimidine nucleotide synthesis. Unlike previously reported DHODH inhibitors that were identified using in vitro enzyme assays, PTC299 is a more potent inhibitor of DHODH in isolated mitochondria suggesting that mitochondrial membrane lipid engagement in the DHODH conformation in situ is required for its optimal activity. PTC299 has broad and potent activity against hematological cancer cells in preclinical models, reflecting a reduced pyrimidine nucleotide salvage pathway in leukemia cells. Archived serum samples from patients treated with PTC299 demonstrated increased levels of dihydroorotate, the substrate of DHODH, indicating target engagement in patients. PTC299 has advantages over previously reported DHODH inhibitors, including greater potency, good oral bioavailability and lack of off-target kinase inhibition and myelosuppression, and thus may be useful for the targeted treatment of hematologic malignancies.
Keywords: Dihydroorotate dehydrogenase (DHODH), Vascular endothelial growth factor A (VEGFA), Hematologic Malignancies, Drug mechanism, Cancer therapy
INTRODUCTION
De novo pyrimidine nucleotide biosynthesis is activated in proliferating cancer cells in response to an increased demand for synthesis of DNA, RNA and phospholipids by activation of oncogenic pathways such as Myc, PI3K, PTEN and mTOR(1–5). DHODH, located on the surface of the inner mitochondrial membrane, catalyzes the fourth enzymatic step, the ubiquinone-mediated oxidation of dihydroorotate to orotate in the de novo pyrimidine synthesis pathway(6). DHODH is a validated therapeutic target for the treatment of autoimmune diseases such as rheumatoid arthritis(7, 8). Inhibition of DHODH was shown in preclinical studies to prevent growth of many types of cancers (9–13). Moreover, it was demonstrated that inhibition of DHODH induces differentiation of acute myeloid leukemia cells(14–16). Pharmacologic inhibition of de novo pyrimidine synthesis sensitizes triple negative breast cancer cells to genotoxic chemotherapy agents and reduces chemotherapy resistance(2). DHODH inhibitors such as teriflunomide, brequinar and vidofludimus, have been considered for use in oncology(17–20), but their use may be limited due to a narrow therapeutic window as a result of lesser potency and/or off-target activities such as their inhibition of kinases(21, 22).
There is great interest in the discovery and development of new DHODH inhibitors for therapeutic uses (23, 24). These efforts have mainly relied on in vitro enzyme assays to define and optimize activity, in which the purified enzyme conformation may differ from that found in the cells due to lack of membrane lipids in the assay(25). Recently, it was demonstrated that DHODH attaches to the inner mitochondrial membrane by binding charged phospholipids which stabilize the flexible substrate- and drug-binding site, suggesting that the design and development of novel inhibitors that additionally engage in lipid interactions may represent a promising approach to targeting DHODH in vivo(26).
PTC299 was identified as an inhibitor of the translation of VEGFA mRNA using PTC Therapeutics’ proprietary GEMS™ technology phenotypic screening platform(27). PTC299 was in clinical trials for the treatment of solid tumors (28, 29) and is currently being reevaluated for use in hematologic cancers. To guide safe and effective precision cancer treatment, we performed extensive biochemical characterization of PTC299 and demonstrated that it interacts with and inhibits dihydroorotate dehydrogenase (DHODH), a rate limiting enzyme in the de novo biosynthesis of pyrimidine nucleotides(5). Inhibition of VEGFA production by PTC299 is a downstream effect of inhibiting de novo pyrimidine synthesis as it can be completely rescued by exogenously added uridine but not by other nucleosides. Collectively, PTC299 represents a novel class of potent DHODH inhibitors and our data demonstrate the potential of PTC299 as a treatment option to address unmet needs in cancers, such as leukemia and lymphoma, that have lower levels of uridine salvage activity and thus rely on the de novo biosynthesis of pyrimidine nucleotides for survival and rapid proliferation.
METHODS
General methods.
DNA constructs were generated using standard procedures as reported previously (27). VEGFA 5’- and 3’- UTRs were amplified from human genomic DNA (Clontech) while the VEGFA165 open reading frame from RNA derived from HeLa cells incubated under hypoxia (1% oxygen) was amplified by RT-PCR. DNAs generated with PCR were confirmed by DNA sequencing.
PTC299, PTC-371, GSK983 and vidofludimus (4SC-101) were synthesized at PTC Therapeutics; for details, see the Supplementary Materials and Methods in the Supplementary Data file. Brequinar (SML0113) and teriflunomide (SML0936) were purchased from Sigma, while pyrazofurin-MP (P61-B01A) was purchased from TriLink Biotechnologies.
Cell culture
Tumor cell lines, except for MOLM-13 which were obtained from Dr. Kensuke Kojima in July 2015 (Saga University, Japan) and Huh-7 which were obtained in February 2001 from the Stuart Peltz laboratory at University of Medicine and Dentistry of New Jersey (now Rutgers University, New Jersey), were purchased from ATCC and were maintained in DMEM (adherent cells) or RPMI-1640 (suspension cells) supplemented with 10% fetal bovine serum (FBS), penicillin (50 IU/mL), and streptomycin (50 μg/mL). Culture media and supplement agents were purchased from (Gibco BRL, Invitrogen). Cells bought from ATCC were expanded and frozen at early passages. Cells were typically used for studies within two to five weeks after thawed from frozen stocks. Cells that were cultured for more than two months (about 20 passages) were discarded. Patient derived AML cells, purchased from AllCells (January 2017), Inc and Champions Oncology, Inc (April 2017), were cultured in StemSpan medium (Stemcell Technologies, Vancouver). Cell lines in culture were routinely verified once per month to be mycoplasma free assayed by using the MycoAlert™ Mycoplasma Detection Kit (Cat#: LT07–318, Lonza, USA). The 240 cell lines used for screening of PTC299 activity were authenticated with STR profiling and mycoplasma tested by the CRO (Crown Bioscience, Shanghai, China), otherwise we did not perform any additional cell line authentication in house. The information on the source of cells and when they were obtained can be found in Supplementary Table S1.
Stable cell lines were generated as reported previously (27). Briefly, plasmid DNA was transfected into HT1080 cells using Fugene-6 transfection reagent (Roche Diagnostics GmbH), followed by selection with 200 μg/mL hygromycin for luciferase stable cell lines or 200 μg/mL Zeocin (Invitrogen) for V5 tag stable cells in the culture medium.
Western blot analysis.
Western blot was performed as reported previously(27). Antibody (C1) specific for VEGFA was purchased from Santa Cruz Biotechnologies, CA (1:200 dilution); Antibody specific for human β-actin was purchased from Abcam (1:10000 dilution); Antibody specific for V5-tag was purchased from Invitrogen (1:5000 dilution). Antibody specific for DHODH was purchased from Proteintech (Cat#: 14877–1-AP, 1:500 dilution); and antibody specific for human prohibitin was purchased from Thermo Scientific (Cat#: PA5–12274 and PA5–14133, 1:1000 dilution). Immunodetection was done using the corresponding secondary antibodies conjugated with infrared dyes or horseradish peroxide. The expression levels of proteins were detected with Odyssey (LI-COR) or using enhanced chemiluminescence (Pierce, Rockford, IL).
Determination of ELISA EC50 and cytotoxicity CC50 values:
All ELISAs were performed using commercially available ELISA kits (R&D Systems) according to the manufacturer’s instructions. The screen of 240 cell lines was performed at Crown Bioscience (Shanghai, China). Cells were treated with PTC299 at a series of doses for 72 hours and the inhibition of cell proliferation was determined using a standard assay, CellTiter-Glo® Luminescent Cell Viability Assay (Promega, Madison, WI) that measures total cellular adenosine triphosphate (ATP) concentrations as an indicator of cell viability. Data generated from ELISA or cytotoxicity studies were plotted with Prism software. A sigmoidal dose response with a variable slope regression curve was generated for each compound. Maximal and minimal inhibition were set at 100% and 0%, respectively, and CC50 values were calculated after curve fitting using the Prism software.
35S-labeling and Immunoprecipitation.
HT1080 cells harboring either the VEGFA 5’-UTR-VEGFA165-V5 construct or the control VEGFA165-V5 were treated for 8 hours with vehicle alone (0.5% DMSO) or vehicle with 100 nM PTC299, then incubated with 35S-labeled methionine and cysteine for approximately 4 hours in the presence or absence of the protein transporter inhibitor brefeldin A or the proteasome inhibitor MG-132. Equal amounts of supernatant or the cell extracts were immunoprecipitated with the anti-V5 antibody (Invitrogen). The immunoprecipitated products were resolved by 4–20% gradient SDS-PAGE and exposed to Kodak Biomax MR film.
Human tumor xenograft studies.
All studies involving animals were performed in accordance with guidelines promulgated by the American Association for Accreditation of Laboratory Animal Care with the oversight of the animal use and care committees at Rutgers University. Subcutaneous tumor xenograft studies were performed as reported previously(27). For systemic leukemia lethality model, male NOD-SCID mice were inoculated with MOLT-4 human ALL tumor cells (1 × 107 cells in 200 μL of PBS) by intravenous (IV) injection. Eight days after tumor inoculation, mice were randomized into two groups (10 mice per groups) and treated with compounds as indicated in the results. The mice were observed until moribund, at which time they were euthanized. At 2- and 4-weeks post-inoculation, whole blood was obtained from 5 mice per group by retro-orbital bleeding, stained with an antibody against human CD45, and the number of CD45-positive cells was determined by FACs analysis.
Immunohistochemistry.
Tumor samples were cut into pieces of less than 3 mm in thickness and placed in a zinc fixative for 24 hours. Paraffin sections were prepared by American HistoLab, Inc. using standard procedures. Mouse endothelial cells were stained with rat anti-mouse CD31 monoclonal antibody MEC13.3 (BD Pharmingen, CA) and detected with a rabbit anti-rat IgG HRP detection kit according to the manufacturer’s instructions (BD Pharmingen, CA).
Transcriptome Profiling with Microarrays.
PTC299-resistant and parent HT1080 cells were seeded in 6-well plates (5 × 105 cells/well) and incubated overnight. Four replicates of cells for each cell type were performed. Total RNA was purified with RNeasy Mini Kit (Qiagen) according to the manufacturer’s instructions. cRNA was prepared according to the standard Affymetrix protocol. cRNA was hybridized at 45oC for 16 h on a GeneChip Human Genome U133 plus 2.0 Array. GeneChips were analyzed using the Affymetrix scanner. Data analysis was performed using Transcriptome Analysis Console (TAC) Software (Thermo Fisher Scientific). The MAS5 normalization method was used. ANOVA was used for statistical evaluation.
Quantification of pyrimidine nucleotides in PTC299 treated cells
Cells in log phase growth were cultured in 10 cm dishes (4 × 106 cells/dish) with glutamine free DMEM containing 1g/L glucose, 10% FBS, penicillin (50 IU/mL), streptomycin (50 μg/mL) and 1 mM 15N-glutamine (Sigma, Cat#: 490024). After culturing for the indicated time in the presence of compounds or vehicle control (0.5% DMSO), the cells were washed once with 5 mL cold PBS, harvested and lysed in 0.5 mL cold distilled H2O plus 1 mL −80oC methanol using a plastic cell scraper. The cell lysates were then centrifuged at 10,000 x g for 15 minutes at 4oC, and the supernatant was collected for LC-MS/MS analysis of the 15N-labelled de novo pyrimidine nucleotide metabolites.
LC-MS/MS detection of metabolites
Quantification of 15N-labelled de novo synthesized pyrimidine nucleotides was carried out on an Accela pump and a PAL auto-sampler coupled to a TSQ Quantum Ultra mass spectrometer. The mass spectrometer was equipped with a heated electrospray ionization source operated in negative-ion mode (Thermo Fisher Scientific, Waltham, MA). The ion spray voltage was set at 2500 V, capillary temperature at 350oC, vaporizer temperature at 300oC; sheath gas pressure at 50 units and auxiliary gas pressure at 5 units. A Thermo Fisher Scientific Hypercab column (3 μm, 50 × 2.1 mm) was used and maintained at 50°C for metabolite separation. Mobile phase A was 10 mM NH4HCO3 in water, pH 9.4, and mobile phase B was 10 mM NH4HCO3 in ACN-water (9:1). The flow rate was set at 0.50 mL/min. Ion transitions monitored were at m/z 156.0 → m/z 112.0 for 15N-orotic acid, m/z 158.0 → m/z 114.0 for 15N-dihydrorotic acid, m/z 324.0 → m/z 79.0 for 15N- monophosphate uridine (UMP).
Conjugation of PTC299 or PTC-371 to Sephorose-6B beads
PTC299 or its inactive enantiomer PTC-371, was conjugated to the epoxy-activated Sepharose 6B according to the manufacturer’s instructions (GE healthcare cat. # 17–0480-01). Briefly, 1 gram of epoxy-activated Sepharose 6B was stirred in water (20 mL) for 2h at RT, then filtered. The wet Sepharose 6B was diluted with pH 11 phosphate/NaOH buffer (5 mL). To a solution of 1 mM 4-chlorophenyl (S)-6-chloro-1-(4-hydroxyphenyl)-1,3,4,9-tetrahydro-2H-pyrido[3,4-b] indole-2-carboxylate in DMF (12 mL) at 40oC was added the Sepharose 6B in phosphate/NaOH buffer. The reaction mixture was stirred further for 24 hours at 40oC. The Sepharose 6B-PTC299 product was filtered and washed with DMF (100 mL), water (100 mL), pH 4 buffer (100 mL), pH 11 buffer (100 mL), water (100 mL), and 0.5M NaCl/water. Sepharose 6B-PTC-371 was prepared with 4-chlorophenyl (R)-6-chloro-1-(4-hydroxyphenyl)-1,3,4,9-tetrahydro-2H-pyrido-[3,4-b] indole-2-carboxylate using above reaction procedures.
Pull-down of proteins that are specifically bound to PTC299-conjugated beads
Cells in log phase growth were washed with cold PBS once and then collected by trypsin digestion. After centrifugation at 1200 rpm for 5 minutes, the cells were re-suspended in lysis buffer (PBS + 0.5% Triton X100 + 1x proteinase inhibitors (Halt, Roche, Cat#: 78440) and incubated on ice for 10 minutes; supernatants were collected after passing cells through a 22-gauge needle 10 times and centrifuging at 13,000 rpm for 15 min to remove cell debris. Subsequently, 1 mL of cell lysate solution (2 mg/mL protein) was added to each reaction tube containing 25 μl (packed volume) PTC299- or the R-enantiomer control (PTC-371) beads and incubated for 2 hours at 4oC with gentle agitation on a rotator; after three washes with 1 mL washing Buffer, 5 min/each, the protein bound with beads were eluted with indicated buffer as shown in the Results section. Proteins in the eluted samples were verified by western blot analysis.
Mitochondria isolated from cultured K562 cells as source of DHODH for in vitro enzyme inhibition studies
Mitochondria were isolated from K562 cells in log phase growth using a Dounce homogenizer as reported (30). The isolated mitochondria in the supernatant were pelleted at 12,000g for 15 min and re-suspended in 1 mL 1x MS homogenization buffer for analysis of downstream DHODH activity as described below.
In vitro DHODH activity assays
The chromogen reduction assay was carried out as previously reported(31). DHODH activity was determined in the presence of compounds or vehicle control using the standard colorimetric DHODH continuous assay in which the oxidation of dihydroorotic acid (DHO) and the subsequent coupled reduction of ubiquinone is measured by monitoring the reduction of 2,6-dichlorophenolindophenol (DCIP) on a BioTek Power Wave XS2. The velocity for each reaction was derived using the GEN5 software.
Docking analysis
Software Sybyl X 2.1.1 was used to prepare ligand structures. Ligands used in docking procedures were imported as SDF 3D format which were processed using Pipeline Pilot. Protein crystal structures were downloaded from the Protein Data Bank (PDB; http://www.rcsb.org/). Protein structure preparations were performed using the standard protocol of Sybyl X 2.1.1, and the resulting structures were saved as SYBYL ‘‘.mol2’’ files. The pockets Protocols were generated using standard fully automated Surflex-Dock procedures(32). Sybyl provides a docking score for each docked conformation, which includes two parts: an affinity score (-logKd) and a crash score (also pKd units). The crash score is the degree of inappropriate penetration into the protein by the ligand as well as the degree of internal self-clashing that the ligand is experiencing. The top docking scores for each ligand were used for comparison.
The Docking Mode was set as Surflex_Dock GeomX(SFXC). Input options were set to allow 3D Coordinate Generation, if necessary. Protein movement was set to allow for the flexibility of protein atoms whose van der Waals surface distances from ligand atoms are < 4 Å and for the adaptation of the active site conformation to the docked ligand. The covalent force field weighting of the ligand was set to 1.0, while the covalent force field weighting of the protein was set to 0.6 to allow heavy atoms to move. The maximum number of poses was set to 10 to optimize and rescore each ligand after a docking run that included protein movement. Additional Starting Conformations per Molecule were set to 6 (GeomX). The maximum number of conformations per fragment to be submitted to the docking procedure was set to 20. The Maximum Number of Poses per Ligand was set to 20. The minimum RMSD (Root-Mean-Square Distance) between Final Poses was set to 0.50. The Sybyl version of the AMBER7 FF99 Force Field was used for energy calculations(33).
Statistical analysis.
Data are the mean ± SD or SEM as indicated for quantitative experiments. For statistical analysis, p-values were derived using unpaired Student’s t-tests for any study with only two groups presented. Otherwise, comparisons of groups were performed on log-transformed data using a one-way ANOVA test. All analyses were made using GraphPad Prism Software.
Data availability.
All data generated or analyzed during this study are included in this article and its supplementary information files. Additional information is available from the corresponding author upon request.
RESULTS
PTC299 was identified from a phenotypic screen and chemical optimization as an inhibitor of VEGFA protein synthesis
Using the contextual regulation of the production of VEGFA in the tumor cell imparted by critical elements in the 5’- and 3’-UTRs of the VEGFA mRNA, we initiated a drug discovery program using PTC Therapeutics’ proprietary GEMS™ technology phenotypic screening platform(27). Subsequently, structure-activity-relationship (SAR) studies led to the identification of PTC299 (Fig. 1A) as a clinical development candidate for cancer therapy. PTC299 is the pharmacologically active S-enantiomer of 4-chlorophenyl (1S)-6-chloro-1-(4-methoxyphenyl)-1,3,4,9-tetrahydro-2H-beta-carboline-2-carboxylate (synthesis of PTC299 (5) and intermediates 1–4 shown in Supplementary Fig. S1A). Similar to the compounds identified in a high throughput screen as previously described(27), biological characterization suggested that PTC299 inhibited VEGFA-5’UTR-mediated mRNA translation rather than transcription (Supplementary Fig. S1B, C&D).
Figure 1. Structure, biological activity and stereoselectivity of PTC299.
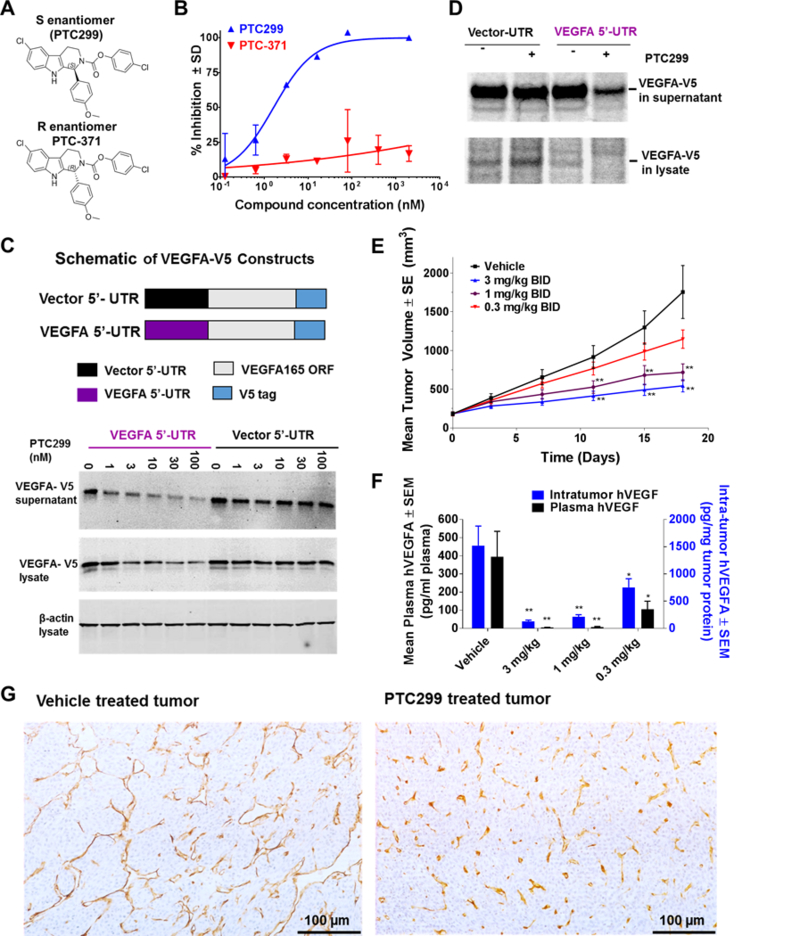
(A) Chemical structure of PTC299, the 1S configuration and the inactive R enantiomer, PTC-371. (B) PTC299 inhibits hypoxia-induced VEGFA expression in HeLa cells. Results are expressed as the percent inhibition of hypoxia induced VEGFA production relative to vehicle-treated controls. Hypoxia (1% oxygen) induced VEGFA production is measured by subtracting the VEGFA produced under normoxic conditions (21% oxygen) from the levels of VEGFA produced under hypoxia. Data are mean ± SD from a representative study (n = 3). (C) The VEGFA 5’-UTR is required for PTC299 inhibition of a V5-tagged VEGFA165 protein production. HT1080 cells were stably transfected with the constructs shown in the diagrams on the top of the graph. The culture supernatant and cell lysates were collected for western blot after treatment with PTC299 or 0.5% DMSO control for 36 hours. (D) PTC299 selectively inhibits production of pulse 35S labeled VEGFA. The VEGFA-V5 stable cell lines were treated with PTC299 (100 nM) for 8 hours and then pulsed/chased with 35S-radiolabled methionine and cysteine for 4 hours prior to immunoprecipitation with V5 antibody. (E) PTC299 dose-dependently inhibits HT1080 tumor growth in mice. Values represent the average ± SEM of 10 mice per group. Compound or vehicle was orally administered twice daily (BID) as indicated in the graph. ** p < 0.001, One-way ANOVA test. (F) PTC299 reduces intra-tumor and serum levels of human VEGFA. Values represent the average ± SEM of 10 mice. One-way ANOVA test compared to vehicle, * indicates p < 0.05, ** p < 0.001. (G) PTC299 inhibits tumor angiogenesis, tumor sections were stained with anti-CD31 antibody.
In a dose-dependent and stereo-selective manner, PTC299 inhibited hypoxia-induced VEGFA protein production in HeLa cells with an EC50 of 1.64 ± 0.83 nM (Fig. 1B). Similar activity was also observed in HT1080 cells which constitutively produce high levels of VEGFA (Supplementary Fig. S1E). The effect of PTC299 is not limited to secreted isoforms of the protein as the levels of high molecular weight matrix-bound variants of VEGFA189/206 were similarly decreased (Supplementary Fig. S1F). PTC-371, the R-enantiomer of PTC299 was inactive in inhibiting VEGFA synthesis.
To verify the role of the VEGFA 5’-UTR for PTC299 activity, a V5 epitope tagged plasmid containing the VEGFA165 open reading frame was constructed(Fig. 1C, top panel). PTC299 dose-dependently inhibited VEGF 5’-UTR-mediated production of the VEGFA165-V5 protein as determined by western blot analysis (Fig. 1C) and by ELISA (Supplementary Fig. S1G). Pulse-chase experiments using 35S-radiolabled methionine and cysteine demonstrated that PTC299 decreased the levels of newly synthesized VEGFA protein (Fig. 1D) while not decreasing total protein synthesis (Supplementary Fig. S1H). This effect could not be reversed by treatment with the protein transport blocker brefeldin A (BFA) or with the proteasome inhibitor MG132 (Supplementary Fig. S1I & 1J). These results suggest that PTC299 treatment results in the inhibition of VEGFA translation rather than the inhibition of VEGFA secretion or promotion of VEGFA protein degradation and support the conclusion that the VEGFA 5’-UTR is required for the suppression of VEGFA mRNA translation by PTC299.
In a mouse tumor xenograft model, PTC299 dose-dependently inhibited the growth of HT1080 fibrosarcoma (Fig. 1E), without overt toxicity or significant body weight changes at any dose when compared to treatment with the vehicle alone (Supplementary Fig. S1K). In parallel, treatment of mice with PTC299 resulted in a dose-dependent reduction in the concentrations of intratumor and circulating plasma human VEGFA relative to those in control mice (Fig. 1F) but had no significant impact on the levels of other cytokines such as PDGF and FGF-2 in tumors as assessed in a separate study (Supplementary Fig. S1L). At the suboptimal dose of 0.3 mg/kg, treatment of mice with PTC299 resulted in partial reductions in the levels of both tumor and plasma human VEGFA, indicating that inhibition of intra-tumor human VEGFA correlates with tumor growth control.
When stained with anti-murine CD31 antibody, the diameters of tumor vessels were visibly reduced and their distribution was more uniform in tumors from PTC299-treated mice than in vehicle treated mice (Fig. 1G), consistent with results in preclinical models using other VEGF-targeted therapies(34, 35).
Inhibition of VEGFA production by PTC299 is a downstream effect of inhibiting de novo pyrimidine synthesis
To gain insight into the mechanism of action of PTC299, we generated drug-resistant cells by culturing HT1080 cells in escalating concentrations (1 nM to 1000 nM) of PTC299, doubling the PTC299 concentration weekly. PTC299-resistant HT1080 cells proliferate at a rate similar to that of wild-type HT1080 cells (Supplementary Fig. S2A), but the IC50 for the inhibition of VEGFA production by PTC299 in resistant cells is shifted dramatically, from low nM to single digit μM values (Supplementary Fig. S2B). Transcriptome profiling with microarrays to compare the gene expression in PTC299-resistant and in parent HT1080 cells demonstrated that the gene with the greatest increase in expression in PTC299-resistant cells is cytidine deaminase (CDA) while the gene with greatest decrease is Eukaryotic Translation Initiation Factor 1A, Y-Linked (EIF1AY) (Figure 2A, the microarray data can be found in Supplementary Excel file-1 or downloaded from GEO database (Accession number: GSE121266)). These changes in expression were verified by quantitative PCR as shown in Supplementary Fig S2C. Since CDA is an enzyme in the uridine salvage pathway, we evaluated the effect of PTC299 treatment on intracellular nucleotide levels. PTC299 selectively decreased the total levels of the deoxypyrimidine nucleotides dCTP and dTTP, but not those of the deoxypurine nucleotides dATP and dGTP (Fig. 2B). Metabolic labeling studies using 15N-glutamine demonstrated that PTC299 inhibited de novo pyrimidine synthesis while its inactive R-enantiomer PTC-371 had no effect (Fig. 2C). Further studies demonstrated that inhibition was dose-dependent and measurable after only 30 minutes of treatment (Supplementary Fig. S3 A&B). The inhibition profile of PTC299 in these assays is similar to that of the known DHODH inhibitor brequinar rather than that of the UMP synthase (UMPS) inhibitor pyrazofurin-monophosphate (18, 36) (Supplementary Table S2). As shown in Fig. 2D, treatment with pyrazofurin-monophosphate resulted in an over 40-fold increase of 15N-orotate, the substrate of UMPS. However, co-treatment with PTC299 blocked the pyrazofurin-MP-induced increase of 15N-orotate, consistent with inhibition of DHODH that acts upstream of UMPS.
Figure 2. Inhibition of VEGFA translation by PTC299 is coupled with inhibition of de novo pyrimidine synthesis.
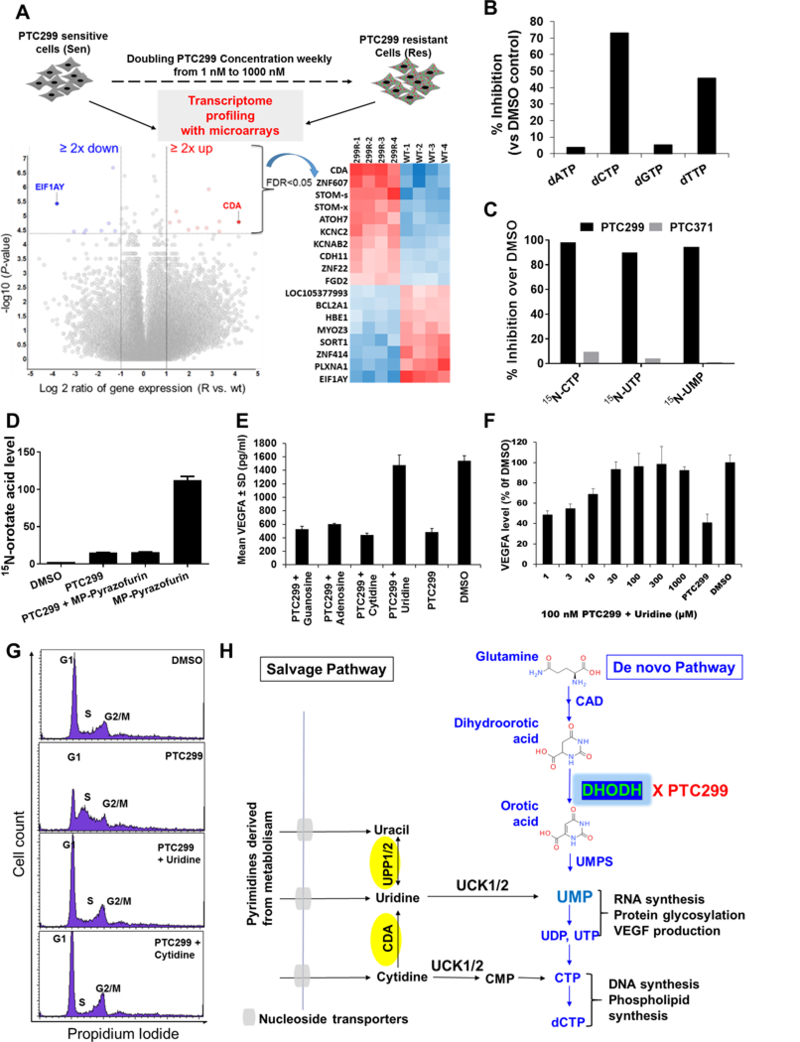
(A) Transcriptome profiling of PTC299-resistant HT1080 cells using microarrays. Highlighted on the left volcano plot are those genes with greater than 2-fold changes in expression with a false discovery rate (FDR) less than 0.05; the heat maps of the expression of these genes are shown on the right. (B) PTC299 selectively decreases pyrimidine nucleotides in HT1080 cells. HT1080 cells were treated with PTC299 (100 nM) or 0.5% DMSO control for 8 hours. Data are representative of two independent studies. (C) PTC299 inhibits de novo pyrimidine nucleotide synthesis. HT1080 cells in log phase growth were treated with compounds (100 nM) or vehicle control (0.5% DMSO) in the presence of 1 mM 15N-glutamine for 8 hours. 15N-labelled CTP, UTP and UMP were quantified with LC/MS. Data are representative of two independent studies. (D) PTC299 acts upstream of UMPS. Cells were treated with 1 μM PTC299 or MP-pyrazofurin or combination of the two for 8 hours. Peak area ratio of orotic acid (normalized to an internal control) for each treatment was determined and average ± SD was shown in the picture (n = 2). MP-pyrazofurin is a UMPS inhibitor; (E) Inhibition of VEGFA production in HT1080 cells by PTC299 is completely blocked by addition of exogenous uridine into the cell culture. Cells were treated with PTC299 (100 nM) or vehicle (0.5% DMSO) in the presence of 100 μM various nucleosides (indicated in the figure) for 48 hours. Data are mean ± SD, n = 2. (F) Inhibition of VEGFA production in HT1080 cells by PTC299 is dose-dependently blocked by addition of exogenous uridine. Assays were done in triplicate, and data represent average inhibition against DMSO control after VEGFA levels normalized to viable cell number measured by Celltiter-Glo. Data represent the mean ± SD, n = 2. (G) Cell cycle arrest in S-phase induced by PTC299 is completely blocked by addition of exogenous uridine or cytidine into the cell culture. HT1080 Cells were treated with PTC299 (100 nM) or vehicle (0.5% DMSO) in the presence of 100 μM of the indicated nucleosides for 24 hours. (H) A scheme showing de novo and salvage pathways of pyrimidine nucleotide synthesis. CDA: cytidine deaminase; UPP: Uridine phosphorylase; UCK: uridine cytidine kinase; CAD: Carbamoyl-phosphate synthetase 2, aspartate transcarbamylase, and dihydroorotase; UMPS: UMP synthase.
We performed nucleoside rescue studies and demonstrated that the PTC299-mediated inhibition of VEGFA protein synthesis was dose-dependently blocked by the addition of uridine, but not by the addition of adenosine, cytidine, or guanosine, to the culture medium (Fig. 2E&F). As a consequence of DHODH inhibition and subsequent pyrimidine nucleotide depletion, PTC299 also induced an S phase cell cycle arrest in HT1080 cells (Fig. 2G, 2nd panel). This cell cycle arrest was prevented by the addition of the pyrimidine nucleosides uridine or cytidine (Fig. 2G, bottom two panels), but not the purine nucleosides adenosine or guanosine (L. Cao, unpublished observations). These data suggest that inhibition of VEGFA translation and cell cycle arrest by PTC299 are two separate downstream effects of inhibiting de novo pyrimidine nucleotide synthesis (Fig. 2H).
PTC299 interacts with and inhibits DHODH activity
Extensive SAR studies demonstrated that a methoxy group on PTC299 can tolerate modification and is not critical for the biological activity of PTC299 (Y.C. Moon, unpublished observations). By utilizing this methoxy group, we linked either PTC299, or its inactive R-enantiomer PTC-371, to epoxy-activated Sepharose 6B (Fig. 3A). In pilot studies, we focused on those highly enriched proteins in PTC299 conjugated bead pull down samples by cutting out the most enriched protein bands for the proteomic studies and demonstrated that these proteins are Prohibitin-1/2 and voltage-dependent anion channel (VDAC). After demonstrating that PTC299 inhibits de novo pyrimidine synthesis and has an activity profile similar to that of the DHODH inhibitor brequinar (Fig. 2 and Supplementary Table S2), we performed another pull down study and demonstrated that PTC299-beads not only enriched for prohibitin-1/2 but also for DHODH in the pull-down samples (Fig. 3B). To determine if binding to these proteins was PTC299 specific, we first performed “compete-on” studies and demonstrated that pre-incubation with 100 μM PTC299 selectively blocked the binding of DHODH to the PTC299 conjugated beads while it had no effect on the binding of prohibitin-1/2 (Fig. 3C). The inactive enantiomer PTC-371 had no effect on the binding of either DHODH or Prohibitin 1/2. Similar results were observed when isolated mitochondrial lysates were used as the protein source (Supplementary Fig. S3C). We next demonstrated that both free PTC299 and brequinar effectively blocked the binding of DHODH to the PTC299 conjugated beads while having no effect on prohibitin binding (Fig. 3D), suggesting that PTC299 and brequinar bind to the same site in DHODH. Subsequently, we performed a “competing off” study and demonstrated both PTC299 and brequinar effectively eluted out the DHODH from the PTC299 conjugated beads while the inactive isomer PTC-371 did not (Fig. 3E), further supporting the notion that PTC299 and brequinar bind to the same pocket in DHODH. Proteomic study of those elution samples verified that one of the most selectively enriched proteins was DHODH (Supplementary Table S3). Since PTC299 is species selective and does not have activity in mouse and rat cell lines tested in vitro (Supplementary Table S4.), we performed a side by side pull-down study with human and rat cell lysates. As shown in Fig. 3F, PTC299 conjugated beads enriched for both human DHODH and prohibitin1/2. However, these beads only bound to rat prohibitin rather than rat DHODH when compared with the blank beads or the PTC-371 conjugated beads. These data suggest that PTC299 specifically binds to human DHODH, and that other enriched proteins such as prohibitin-1/2 are PTC299-bead selective rather than PTC299 specific.
Figure 3. PTC299 binds to and inhibits the activity of DHODH.
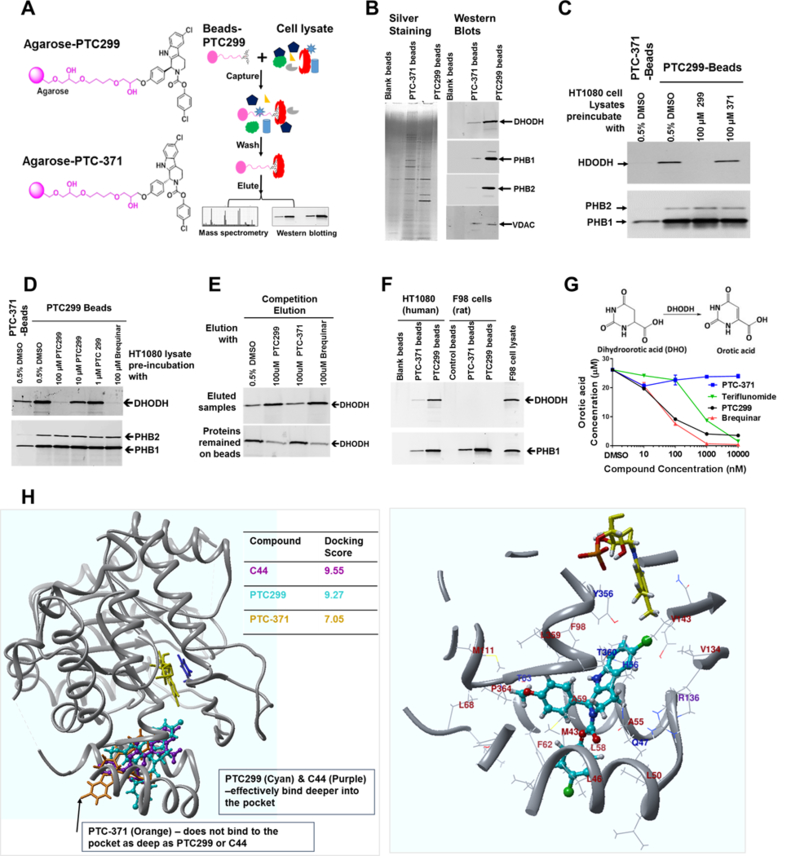
(A) Schematic drawing of PTC299, the inactive isomer linked to the agarose beads and the outline of chemical pulldown study; (B) PTC299 beads bind to DHODH and prohibitin-1/2. Pull-down samples from Blank, PTC-371 and PTC299 conjugated beads were resolved on 4–20% SDS-PAGE. Left panel shows the silver staining, while the right panel shows westerns blotted with antibodies against human DHODH, PHB1 (prohibitin-1), PHB2 (prohibitin-2) and VDAC (voltage-dependent anion channel) respectively; (C) PTC299 selectively inhibits DHODH binding to the PTC299-beads. HT1080 cell lysates were preincubated with 100 μM free PTC299 or PTC-371 as indicated in the figure for 2 hours at 4oC prior to the pull-down study described in the methods; (D) Western blot analysis demonstrated that free PTC299 and brequinar competes DHODH binding to the PTC299-beads. The experiment was done in the same way as for Fig.3C. (E) Western blot analysis demonstrated that free PTC299 selectively elutes DHODH from the PTC299-beads; (F) PTC299 conjugated beads selective bind to human rather than rat DHODH; (G) PTC299 inhibits DHODH activity in isolated mitochondria, data are representative of three independent mitochondria preparations, orotate production was quantified by LC-MS/MS analysis of the end product after 30 minutes enzyme reaction. (H) Computer modelling using crystal structures of the human DHODH protein. Both the brequinar analog C44 (Purple) and PTC299 (cyan) bind deep in the pocket, but the inactive enantiomer PTC-371 (orange) does not, which is reflected by the docking scores on the right. The cofactor flavin mononucleotide (FMN) and the product orotate are shown in yellow and blue respectively. Right panel shows a localized view that PTC299 (cyan) binds to the pocket of human DHODH crystal at 4IGH. Amino acids within 5 angstroms of PTC299 are shown as a ribbon. Amino acid residues within 3 angstroms were labeled (hydrophobic, brown; charged, purple; polar, blue). Relevant side chains that make up the pocket are displayed explicitly.
Further studies demonstrated PTC299 inhibited the activity of recombinant DHODH using in vitro enzyme assays, though not as potently as do brequinar or teriflunomide (Supplementary Fig. S3D). However, when purified mitochondria were used as the source of DHODH, PTC299 inhibited DHODH activity more potently than did teriflunomide and with potency similar to that of brequinar (Fig. 3G and Supplementary Fig. 3E). Along with the result that uridine can completely block the inhibition of VEGFA production and cell cycle arrest by PTC299, these data indicate that DHODH is the primary target responsible for the observed activities of PTC299.
Computer modelling shows that PTC299 binds to the same site in DHODH as does brequinar and with similar strength.
Docking studies on available human DHODH protein (PDB ID: 4IGH, http://www.rcsb.org/) revealed that PTC299 and the potent brequinar analog C44 bind to the same site with similar strength (Fig. 3H), consistent with the pull-down results that demonstrated brequinar competes with DHODH binding to PTC299 beads. The Sybyl Surflex docking score, calculated based on the binding affinity and crash score(37), is 9.55 for C44 vs 9.27 for PTC299. The interaction of PTC299 with the binding pocket is mainly a “hand-in-glove” type non-polar hydrophobic interaction. Unlike with C44, there are no H-bond interactions between PTC299 and Arg136 in DHODH. As anticipated from the pull-down studies, the inactive enantiomer, PTC-371, bound less efficiently in the pocket with a final docking score of 7.05, probably because of the unique non-symmetrical shape of the cavity. While PTC299 is a less potent inhibitor of rhDHODH in vitro, it more potently inhibits DHODH from isolated mitochondria (Fig. 3G and supplementary Fig. S3E). Since PTC299 is lipophilic, its entry into the binding pocket of the enzyme is likely facilitated when the enzyme is embedded in the mitochondrial membrane with engagement of membrane phospholipids. In studies using purified recombinant protein, there is no membrane or lipid layer that may hinder the entry of PTC299 into the binding pocket. Efforts to co-crystallize PTC299 and purified recombinant DHODH have not been successful to date (Nonato MC, Universidade de São Paulo, Brazil; personal communication), possibly because of the requirement for the lipid engagement with the enzyme(26). We are currently working to optimize crystallographic conditions to determine the structure of lipid-engaged DHODH interaction with PTC299.
PTC299 inhibits DHODH activity in patients with neurofibromatosis type 2 (NF2)
Comprehensive preclinical IND-enabling safety pharmacology, toxicology and genotoxicity studies (Supplementary Materials and Methods in the Supplementary Data file) demonstrated that PTC299 is safe and well tolerated in pre-clinical studies (Supplementary Table S5). Subsequently, several clinical trials in healthy volunteers and in patients with solid tumors were conducted to assess PTC299(28, 29), including a prospective Phase 2a study in 2009–2010 in patients with neurofibromatosis type 2, an autosomal dominant tumor suppressor syndrome characterized by bilateral vestibular Schwannomas. Eleven patients were enrolled including 4 males and 7 females with a median age of 25 (range 19–44) at the time of enrollment. The demographic information on these patients is summarized in Supplementary Table S6. After 8 weeks of PTC299 treatment, levels of serum VEGFA was significantly decreased (Fig. 4A, p<0.004, paired Student’s t-test). Baseline levels in healthy volunteers were reported to be approximately 140 pg/mL(38), indicating that serum VEGFA levels are elevated in NF2 patients and were reduced by treatment with PTC299 to levels found in healthy volunteers. Circulating VEGFA was decreased in 11 out of 11 patients as shown in Fig. 4B.
Figure 4. PTC299 inhibits DHODH activity and reduces elevated VEGFA levels in patients with neurofibromatosis type 2.
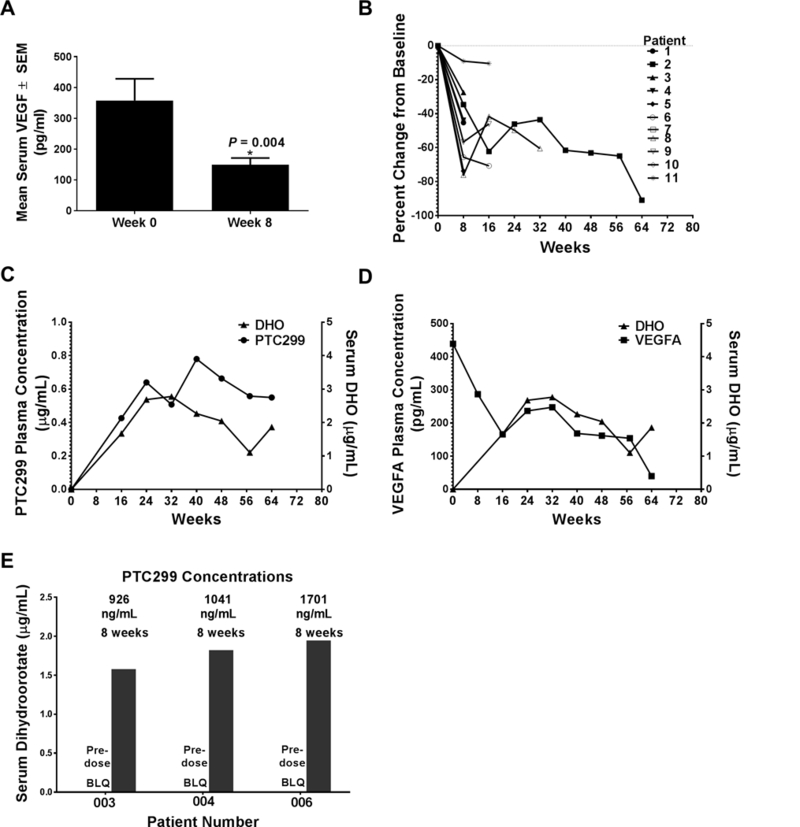
(A) Reduction in mean levels of serum VEGFA after 8 weeks of PTC299 treatment; Data are represented as mean ± SEM, n = 11. (B) VEGFA reduction for individual patients over the course of the PTC299 treatment; (C) Serum levels of DHO are correlated with PTC299 concentration in patient-002; (D) Serum levels of DHO are inversely correlated with serum VEGFA levels in patient-002. (E) PTC299 increases serum DHO in three additional patients whose serum samples were still available at the time of analysis. BLQ: below the level of quantification.
We next examined archived serum samples from some patients in the NF2 clinical study for DHO levels, hypothesizing that inhibition of DHODH would result in higher levels of this substrate as seen in patients with genetic deficiency in the DHODH enzyme (39). As shown in Fig. 4C&D, serum DHO levels in Patient 010–002 who had been on study for 64 weeks, were initially below the lower limit of quantification. After treatment with PTC299, plasma concentrations of PTC299 and serum levels of DHO were significantly increased (Fig. 4C) while serum VEGFA protein levels were continuously decreased (Fig. 4D). For another three patients whose sera were also available at the time the analyses were performed, DHO levels were also below the lower limit of quantification prior to treatment but were significantly increased with PTC299 treatment (Fig. 4E). The increased levels of DHO in these samples indicate that PTC299 inhibited DHODH in the previous clinical studies.
PTC299 demonstrates broad and potent inhibition of hematologic cancer cell proliferation.
PTC299 inhibits de novo pyrimidine nucleotide synthesis in all cancer cells analyzed whether the cells are responsive to inhibition of VEGFA production or not (Supplementary Fig. S4). Since pyrimidine nucleotides can be synthesized by the de novo or the salvage pathway(40), some cancer cells may be insensitive to PTC299 due to increased pyrimidine nucleotide salvage activity. Thus, we determined the IC50 (concentration to inhibit cell proliferation by 50%) of PTC299 against a panel of 240 tumor cell lines. We classified 68 cells lines as responders and 172 cells lines as non-responders based on an arbitrary IC50 cut-off of 1 μM. Based on these results, sensitivity to PTC299 was observed across different tumor types (Supplementary Fig. S5A). When grouped as hematopoietic vs solid tumor cells, 57% (32/56) of hematopoietic lines were sensitive to PTC299, whereas only 18% (33/184) in the solid tumor lines were sensitive to PTC299.
Gene expression of pyrimidine nucleotide synthesis enzymes was analyzed using published microarray data in the Cancer Cell Line Encyclopedia (CCLE) database(41). Of 240 cell lines tested, 232 were found in the CCLE database including 54 hematopoietic and 178 solid tumor cell lines. Two salvage enzymes, cytidine deaminase (CDA) and uridine phosphorylase 1 (UPP1) that convert cytidine and uracil, respectively, to uridine, were significantly less expressed (3.2-fold for CDA and 4.6-fold for UPP1) in the hematopoietic tumor lines than in the solid tumor lines (Fig. 5A, and Supplementary Table S7). We then performed LC-MS/MS analysis of UMP pools from salvage and de novo pathways in a panel of 8 tumor cell lines and demonstrated that PTC299 sensitive cells in general have less salvage UMP production relative to de novo UMP production when compared to the insensitive tumor cells (Fig. 5B). These data suggest that cells with reduced uridine salvage activity are more sensitive to PTC299.
Figure 5. PTC299 demonstrates broad activity and potent inhibition of leukemia cell proliferation in vitro and in animal models.
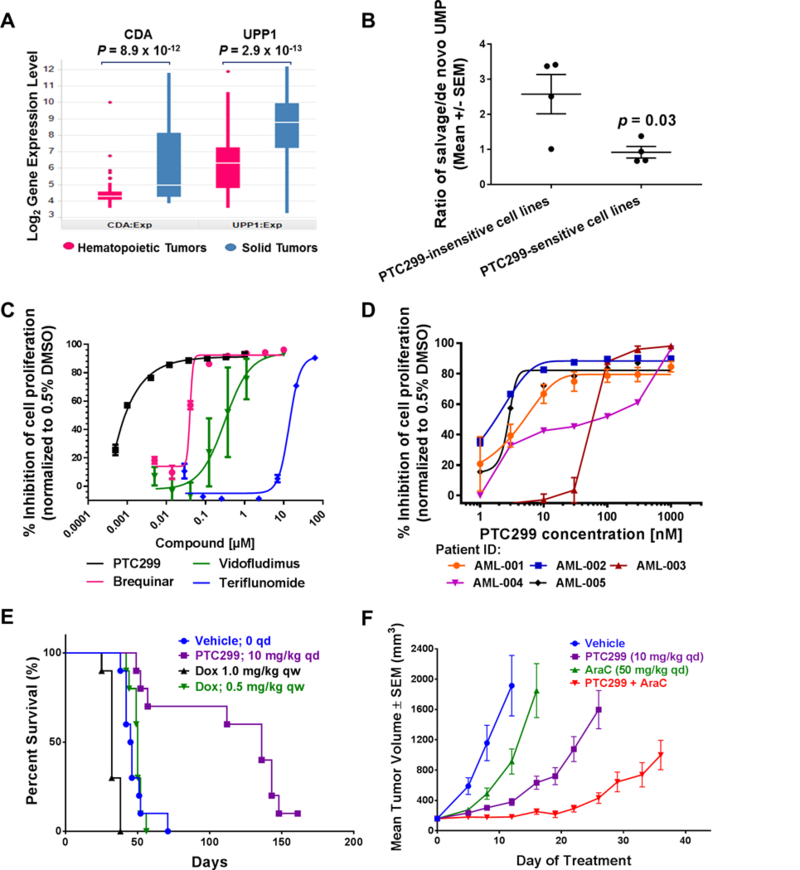
(A) Box-plot of log2 expression level (microarray) for CDA and UPP1, two pyrimidine salvage enzymes for converting cytidine and uracil, respectively, to uridine. The box represents the lower and upper quartile of the data. The white line in the box represents the median value. The whiskers represent the extension of data up to 1.5x of interquartile range (IQR) below the lower quartile or above the upper quartile. P-values are calculated with Software R (https://www.r-project.org/, version 3.3.1) based on t-test comparing hematopoietic tumors (n = 54) and solid tumors (n = 178); (B) PTC299 sensitive cells in general have less salvage UMP production. Four sensitive (HeLa, HT1080, A549 and K562) and four insensitive lines (U87MG, MCF7, PC3 and Huh7) were cultured in the presence of 15N-Glutamine for 8 hours, then the unlabeled and 15N-labeled UMP in the cell lysates were measured by LC-MS/MS analysis. Data are mean ± SEM, n = 4, p value from t-test. (C) PTC299 inhibits the proliferation of MOLM13 AML cells more potently than do other known DHODH inhibitors. Cells were treated with indicated compounds for 72 hours and cell proliferation was measured Celltiter Glo (Promega). % inhibition was calculated against the vehicle (0.5% DMSO) treatment. Data are mean ± SD, n = 2. (D) PTC299 is active in all five AML patient-derived cells in vitro. Patient-derived AML cells were treated with PTC299 for 72 hours and then cell proliferation was assessed using Celltiter Glo (Promega); Assays were done in duplicate (AML-002,004 and 005) or triplicate (AML-001 and 003). (E) PTC299 prolongs survival of mice bearing human acute lymphoid leukemia (ALL) after IV inoculation of NOD/SCID mice with MOLT-4 cells (10 mice/group), Treatment with PTC299 (10 mg/kg, qd), or with doxorubicin (0.5 mg/kg or 1 mg/kg IP once per week), was initiated 7 days after tumor inoculation; (F) PTC299 inhibits MOLM-13 acute myeloid leukemia xenograft tumor growth in nude mice. Values shown represent the mean of 8 mice per group and error bars show the standard error of the mean.
We then compared the potency of PTC299 in leukemia cells with that of several well-known DHODH inhibitors(17–19). PTC299 was the most potent inhibitor with an IC50 of about 1 nM, over 10 to 1000-fold more potent than brequinar, vidofludimus or teriflunomide (Fig. 5C), consistent with its superior potency in the inhibition of VEGFA production in tumor cells (Supplementary Fig. S6). We also tested PTC299 against acute myeloid leukemia (AML) patient-derived cells. As shown in Fig. 5D, PTC299 inhibited the proliferation of all five AML patient derived cell lines tested with IC50 values ranging from 2 nM to 60 nM. In addition, we have shown that PTC299 induced differentiation in AML MOLM-13 cells (Supplementary Fig S5B), consistent with the activity of various DHODH inhibitors as recently reported(14).
After confirming in vitro activity in leukemia cells, we assessed the efficacy of PTC299 in two mouse models of leukemia. As shown in Fig. 1E, the maximal effect in control of tumor growth and VEGFA production was achieved at a dose of 3 mg/kg twice daily (BID). Later studies found that once daily (QD) dosing 10 mg/kg was equivalently effective (M. Weetall, unpublished observation) and thus this experimentally more feasible dosing regimen was used in the leukemia animal models described below.
In the first model, MOLT-4 human acute lymphoblastic leukemia (ALL) cells were injected intravenously (IV) into NOD-SCID mice which were then treated with PTC299 or with doxorubicin at the indicated doses. Doxorubicin was tested at the highest dose tolerated by NOD-SCID mice. As shown in Fig. 5E, vehicle-dosed mice had a median survival time (MST) of 46 days and did not survive beyond 71 days. Doxorubicin did not prolong survival time. The MST for the mice dosed with PTC299 was 136 days (p<0.05, one-way ANOVA, multiple comparisons vs vehicle). The number of circulating MOLT-4 cells was significantly reduced in mice treated with PTC299 but not in mice treated with doxorubicin (Supplementary Fig. S5C).
In a second study, MOLM-13 AML cells were injected into the flank of nude mice to generate a solid tumor. Cytarabine (AraC) is a deoxycytidine analog used as a standard chemotherapeutic agent to treat AML(42). As shown in Fig. 5F, PTC299 treatment alone resulted in a significant delay of tumor growth when compared with vehicle or AraC treatment; the median time to reach a tumor volume of 1000 mm3 was 23 days for PTC299 vs 7 and 13 days for vehicle and AraC treated mice, respectively. The combination of PTC299 with AraC further delayed tumor growth for 39 days. The increased activity of the combination of AraC with PTC299 may reflect a more efficient incorporation of AraC into DNA due to a reduction in the levels of competing endogenous pyrimidine nucleotides.
DISCUSSION
Tumor cells require the upregulation and integration of multiple biosynthetic processes including synthesis of nucleotides and proteins to drive uncontrolled cell proliferation(43). Here, we demonstrate that decreased translation of VEGFA mRNA by PTC299 is linked to the inhibition of de novo pyrimidine nucleotide synthesis as exogenously added uridine, but not other nucleosides, can completely block the inhibition of VEGFA production by PTC299. Unlike uridine, cytidine only rescued the cell cycle arrest in S-phase but not the inhibition of VEGFA production. This discrepancy may be due to the lower levels of CDA expression (Fig. 2A and Supplementary Fig. S2C) and cytidine could not be efficiently converted to uridine in the HT1080 cells. Moreover, exogenously added cytidine in cells expressing low levels of CDA could be quickly converted to CTP/dCTP which in turn might further inhibit the CDA and other salvage enzymes (44, 45).
Extensive biological characterization demonstrated that DHODH is the primary target of PTC299 as all the observed activities of PTC299 can be completely rescued by addition of exogenous uridine rather than other nucleosides. The proteomic study showed other potential targets may exist, and the selectivity and significance of those proteins identified in the proteomic study warrant further investigation.
Similar to PTC299, other DHODH inhibitors such as brequinar also inhibit VEGFA production albeit less potently (Supplementary Fig. S6B&C). The link between the inhibition of VEGFA mRNA translation and de novo pyrimidine synthesis was unanticipated but may be critical for cancer cells to synchronize rapid proliferation and angiogenesis for tumor progression. Inhibition of VEGFA mRNA translation by PTC299 is mediated by the 5’-UTR of the mRNA prompting multiple hypotheses for this link: 1) Depletion of uridine nucleotide pools may affect certain translation initiation factor(s) required for the non-canonical protein synthesis of stress-regulated mRNAs such as the VEGFA mRNA. We observed that eIF1AY gene expression decreased over 90% in PTC299-resistant HT1080 cells, when compared with wild type HT1080 cells (Fig. 2A and Supplementary Fig. 2). eIF1A controls translation start codon recognition and has been reported to affect IRES-mediated translation(46); 2) Low levels of uridine nucleotides may also affect the mRNA substrate directly. For example, depletion of pyrimidine nucleotides in cancer cells can cause a starvation-like stress response which may lead to changes in pseudouridylation state of mRNA(47). Reduction of dyskerin expression, a component of pseudouridine synthase has been shown to selectively increase VEGFA-IRES mediated translation(48). As uridines in RNAs are substrates for dyskerin, depletion of uridine nucleotides by PTC299 may reduce free substrate competition and thus activate dyskerin to inhibit VEGFA mRNA translation; and 3) A recent study has demonstrated that the activity of mTOR, which controls synthesis of proteins and nucleotides(5), can be regulated by nucleotide pools(49). Thus, it is also possible that inhibition of the translation of regulated mRNAs, such as the VEGFA mRNA, is mediated via disrupting the production of pyrimidine nucleotides causing imbalances in nucleotide pools. Elucidating the molecular mechanisms linking inhibition of DHODH and VEGFA mRNA translation requires further investigation.
Two inhibitors of human DHODH have been approved for use in autoimmune diseases, leflunomide for rheumatoid arthritis and its active metabolite, teriflunomide for multiple sclerosis(50). Brequinar, another potent inhibitor of human DHODH was tested in the 1990s in clinical trials for solid tumors but was associated with myeloid suppression and had a limited therapeutic window (18, 22). These DHODH inhibitors are associated with side-effects believed to be due to their off-target activities such as inhibition of kinases (21, 22). Recently, the inhibition of DHODH by GSK983, which is structurally related to PTC299, was reported (51). However, we determined that this compound has a considerably lower bioavailability in mice than does PTC299 (Supplementary Fig. S7).
PTC299 represents a novel class of DHODH inhibitors with favorable biochemical and pharmacological properties that may distinguish it from previously published DHODH inhibitors: 1) Unlike the well-known DHODH inhibitors teriflunomide and brequinar, PTC299 did not show off-target effects including any overt effects on 205 kinases and 62 Novascreen pharmacological targets tested (Supplementary Table S5) and did not result in myeloid suppression in patients with solid tumors(28, 29); 2) PTC299 has been extensively tested and well-tolerated in preclinical studies. PTC299 did not inhibit the proliferation of normal primary cells including hematopoietic stem cells at concentrations 1000-fold greater than its IC50 in tumor cells (Supplementary Table S5); 3) DHODH inhibitors were identified by others using in vitro enzymatic assay in which the purified enzyme conformation may differ from that found in the cells due to lack of lipids in the assay(25, 26). The potent activity of PTC299 in mitochondrial preparations is likely due to the presence of the lipid bilayer when the enzyme is in the mitochondrial membrane that may facilitate the entry and/or docking of the lipophilic PTC299 into the binding pocket of the DHODH enzyme. This is consistent with the recent reports that interactions with the small lipophilic pocket in DHODH are critical for potent inhibition of its enzymatic activity(23, 26); 4) PTC299-resistant HT1080 cells are not cross-resistant to DHODH inhibitors such as brequinar or teriflunomide (Supplementary Fig. S6), indicating that the mode of action for PTC299 is different from that of other DHODH inhibitors even though they target the same docking site; 5) It was demonstrated that inhibition of DHODH activates p53 expression resulting in inhibition of cancer cell proliferation and apoptosis (11). However, we analyzed the p53 expression in the CCLE database and found no correlation of p53 expression with the activity of PTC299 (IC50) in those cell lines. Dr. Kensuke Kojima (Kyoto University, Japan) tested PTC299 in a panel of leukemia cell lines and also found that PTC299 activity does not correlate with the expression of p53 (personal communication); and finally, 6) a docking model shows that PTC299 binds DHODH in the same pocket and with similar strength as does brequinar analog C44. However, the interaction of PTC299 with the binding pocket is mainly a “hand-in-glove”, non-polar lipophilic interaction (Fig. 3H). These findings demonstrate that PTC299 is structurally and functionally different from previously published DHODH inhibitors, thus it may be a useful new tool for preclinical and clinical investigation of targeting DHODH for therapeutic uses in treatment of cancer and immune diseases.
The elucidation of the mechanism of action of PTC299 will facilitate the optimization of biomarker identification, patient selection and design of combination therapies with other drug(s) for clinical development. Here our data together point to the potential clinical utility of PTC299 for the treatment of cancers such as leukemia and lymphoma which in general have lower pyrimidine salvage activity and thus depend on de novo pyrimidine synthesis for uncontrolled proliferation. PTC299 was placed on clinical hold following the occurrence of severe hepatotoxicity in two patients out of 279 individuals administered with PTC299(28, 29). Based on recent preclinical assessments demonstrating antitumor activity in AML models at low doses of PTC299, we are reevaluating the potential use of PTC299 in leukemia and lymphoma, anticipating that efficacious concentration of PTC299 will be lower than those achieved in previous clinical studies in solid tumors.
Conclusion
PTC299 is a novel potent inhibitor of DHODH that is differentiated from and has advantages over other DHODH inhibitors. Studies demonstrated the target engagement of PTC299 in patients, thus supporting its potential for treatment of hematologic malignancies which rely on de novo pyrimidine nucleotide synthesis for survival and proliferation.
Supplementary Material
Acknowledgments
We thank all the members of the Discovery group at PTC for the thoughtful discussion and constructive advises throughout the PTC299 program. We thank Jana Narasimhan for a critical reading and discussion of this manuscript. This program was partially supported by funding from National Cancer Institute (NCI) to J.M. Colacino and L. Cao (2 R44 CA108330–02).
Footnotes
Conflict of interest statement: The authors are or were employed by PTC Therapeutic and have received salary compensation for time, effort, and hold or held financial interest in the company. Travel expenses related to attendance at scientific conferences to present data were reimbursed by PTC Therapeutics, Inc. All patents related to the work presented here are held by PTC Therapeutics, Inc.
Reference List
- (1).Martinez-Outschoorn UE, Peiris-Pages M, Pestell RG, Sotgia F, Lisanti MP. Cancer metabolism: a therapeutic perspective. Nat Rev Clin Oncol 2017;14:113. [DOI] [PubMed] [Google Scholar]
- (2).Brown KK, Spinelli JB, Asara JM, Toker A. Adaptive Reprogramming of De Novo Pyrimidine Synthesis Is a Metabolic Vulnerability in Triple-Negative Breast Cancer. Cancer Discov 2017;7:391–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (3).McMahon SB. Control of nucleotide biosynthesis by the MYC oncoprotein. Cell Cycle 2008;7:2275–6. [PMC free article] [PubMed] [Google Scholar]
- (4).Mathur D, Stratikopoulos E, Ozturk S, Steinbach N, Pegno S, Schoenfeld S, et al. PTEN Regulates Glutamine Flux to Pyrimidine Synthesis and Sensitivity to Dihydroorotate Dehydrogenase Inhibition. Cancer Discov 2017;7:380–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (5).Robitaille AM, Christen S, Shimobayashi M, Cornu M, Fava LL, Moes S, et al. Quantitative phosphoproteomics reveal mTORC1 activates de novo pyrimidine synthesis. Science 2013;339:1320–3. [DOI] [PubMed] [Google Scholar]
- (6).Rawls J, Knecht W, Diekert K, Lill R, Loffler M. Requirements for the mitochondrial import and localization of dihydroorotate dehydrogenase. Eur J Biochem 2000;267:2079–87. [DOI] [PubMed] [Google Scholar]
- (7).Vyas VK, Ghate M. Recent developments in the medicinal chemistry and therapeutic potential of dihydroorotate dehydrogenase (DHODH) inhibitors. Mini Rev Med Chem 2011;11:1039–55. [DOI] [PubMed] [Google Scholar]
- (8).Hoffmann HH, Kunz A, Simon VA, Palese P, Shaw ML. Broad-spectrum antiviral that interferes with de novo pyrimidine biosynthesis. Proc Natl Acad Sci U S A 2011;108:5777–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (9).Koundinya M, Sudhalter J, Courjaud A, Lionne B, Touyer G, Bonnet L, et al. Dependence on the Pyrimidine Biosynthetic Enzyme DHODH Is a Synthetic Lethal Vulnerability in Mutant KRAS-Driven Cancers. Cell Chem Biol 2018. [DOI] [PubMed] [Google Scholar]
- (10).Mohamad Fairus AK, Choudhary B, Hosahalli S, Kavitha N, Shatrah O. Dihydroorotate dehydrogenase (DHODH) inhibitors affect ATP depletion, endogenous ROS and mediate S-phase arrest in breast cancer cells. Biochimie 2017;135:154–63. [DOI] [PubMed] [Google Scholar]
- (11).Ladds MJGW, van Leeuwen IMM, Drummond CJ, Chu S, Healy AR, Popova G, et al. A DHODH inhibitor increases p53 synthesis and enhances tumor cell killing by p53 degradation blockage. Nat Commun 2018;9:1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (12).Dorasamy MS, Choudhary B, Nellore K, Subramanya H, Wong PF. Dihydroorotate dehydrogenase Inhibitors Target c-Myc and Arrest Melanoma, Myeloma and Lymphoma cells at S-phase. J Cancer 2017;8:3086–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (13).Zhang X, Yang J, Chen M, Li L, Huan F, Li A, et al. Metabolomics profiles delineate uridine deficiency contributes to mitochondria-mediated apoptosis induced by celastrol in human acute promyelocytic leukemia cells. Oncotarget 2016;7:46557–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (14).Sykes DB, Kfoury YS, Mercier FE, Wawer MJ, Law JM, Haynes MK, et al. Inhibition of Dihydroorotate Dehydrogenase Overcomes Differentiation Blockade in Acute Myeloid Leukemia. Cell 2016;167:171–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (15).Lewis TA, Sykes DB, Law JM, Munoz B, Rustiguel JK, Nonato MC, et al. Development of ML390: A Human DHODH Inhibitor That Induces Differentiation in Acute Myeloid Leukemia. ACS Med Chem Lett 2016;7:1112–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (16).Wu D, Wang W, Chen W, Lian F, Lang L, Huang Y, et al. Pharmacologic inhibition of dihydroorotate dehydrogenase induces apoptosis and differentiation in acute myeloid leukemia cells. Haematologica 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (17).Baumann P, Mandl-Weber S, Volkl A, Adam C, Bumeder I, Oduncu F, et al. Dihydroorotate dehydrogenase inhibitor A771726 (leflunomide) induces apoptosis and diminishes proliferation of multiple myeloma cells. Mol Cancer Ther 2009;8:366–75. [DOI] [PubMed] [Google Scholar]
- (18).Cody R, Stewart D, DeForni M, Moore M, Dallaire B, Azarnia N, et al. Multicenter phase II study of brequinar sodium in patients with advanced breast cancer. Am J Clin Oncol 1993;16:526–8. [DOI] [PubMed] [Google Scholar]
- (19).Fitzpatrick LR, Small JS, Doblhofer R, Ammendola A. Vidofludimus inhibits colonic interleukin-17 and improves hapten-induced colitis in rats by a unique dual mode of action. J Pharmacol Exp Ther 2012;342:850–60. [DOI] [PubMed] [Google Scholar]
- (20).Arteaga CL, Brown TD, Kuhn JG, Shen HS, O’Rourke TJ, Beougher K, et al. Phase I clinical and pharmacokinetic trial of Brequinar sodium (DuP 785; NSC 368390). Cancer Res 1989;49:4648–53. [PubMed] [Google Scholar]
- (21).Xu X, Williams JW, Gong H, Finnegan A, Chong AS. Two activities of the immunosuppressive metabolite of leflunomide, A77 1726. Inhibition of pyrimidine nucleotide synthesis and protein tyrosine phosphorylation. Biochem Pharmacol 1996;52:527–34. [DOI] [PubMed] [Google Scholar]
- (22).Xu X, Williams JW, Shen J, Gong H, Yin DP, Blinder L, et al. In vitro and in vivo mechanisms of action of the antiproliferative and immunosuppressive agent, brequinar sodium. J Immunol 1998;160:846–53. [PubMed] [Google Scholar]
- (23).Sainas S, Pippione AC, Giorgis M, Lupino E, Goyal P, Ramondetti C, et al. Design, synthesis, biological evaluation and X-ray structural studies of potent human dihydroorotate dehydrogenase inhibitors based on hydroxylated azole scaffolds. Eur J Med Chem 2017;129:287–302. [DOI] [PubMed] [Google Scholar]
- (24).Lucas-Hourani M, Munier-Lehmann H, El MF, Malmquist NA, Harpon J, Coutant EP, et al. Original 2-(3-Alkoxy-1H-pyrazol-1-yl)azines Inhibitors of Human Dihydroorotate Dehydrogenase (DHODH). J Med Chem 2015;58:5579–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (25).Basso LGM, Mendes LFS, Costa-Filho AJ. The two sides of a lipid-protein story. Biophys Rev 2016;8:179–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (26).Costeira-Paulo J, Gault J, Popova G, Ladds MJGW, van Leeuwen IMM, Sarr M, et al. Lipids Shape the Electron Acceptor-Binding Site of the Peripheral Membrane Protein Dihydroorotate Dehydrogenase. Cell Chem Biol 2018;25:309–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (27).Cao L, Weetall M, Bombard J, Qi H, Arasu T, Lennox W, et al. Discovery of Novel Small Molecule Inhibitors of Expression in Tumor Cells Using a Cell-Based High Throughput Screening Platform. PLoS One 2016;11:e0168366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (28).Weetall M, Davis T, Elfring G, Northcutt V, Cao L, Moon YC, et al. Phase 1 Study of Safety, Tolerability, and Pharmacokinetics of PTC299, an Inhibitor of Stress-Regulated Protein Translation. Clin Pharmacol Drug Dev 2016;5:296–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (29).Packer RJ, Rood BR, Turner DC, Stewart CF, Fisher M, Smith C, et al. Phase I and pharmacokinetic trial of PTC299 in pediatric patients with refractory or recurrent central nervous system tumors: a PBTC study. J Neurooncol 2015;121:217–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (30).Clayton DA, Shadel GS. Isolation of mitochondria from cells and tissues. Cold Spring Harb Protoc 2014;2014:db. [DOI] [PubMed] [Google Scholar]
- (31).Chen SF, Perrella FW, Behrens DL, Papp LM. Inhibition of dihydroorotate dehydrogenase activity by brequinar sodium. Cancer Res 1992;52:3521–7. [PubMed] [Google Scholar]
- (32).Ruppert J, Welch W, Jain AN. Automatic identification and representation of protein binding sites for molecular docking. Protein Sci 1997;6:524–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (33).Cheatham TE III, Cieplak P, Kollman PA. A modified version of the Cornell et al. force field with improved sugar pucker phases and helical repeat. J Biomol Struct Dyn 1999;16:845–62. [DOI] [PubMed] [Google Scholar]
- (34).Prewett M, Huber J, Li Y, Santiago A, O’Connor W, King K, et al. Antivascular endothelial growth factor receptor (fetal liver kinase 1) monoclonal antibody inhibits tumor angiogenesis and growth of several mouse and human tumors. Cancer Res 1999;59:5209–18. [PubMed] [Google Scholar]
- (35).Lichtenbeld HC, Ferarra N, Jain RK, Munn LL. Effect of local anti-VEGF antibody treatment on tumor microvessel permeability. Microvasc Res 1999;57:357–62. [DOI] [PubMed] [Google Scholar]
- (36).Ohnuma T, Roboz J, Shapiro ML, Holland JF. Pharmacological and biochemical effects of pyrazofurin in humans. Cancer Res 1977;37:2043–9. [PubMed] [Google Scholar]
- (37).Jain AN. Effects of protein conformation in docking: improved pose prediction through protein pocket adaptation. J Comput Aided Mol Des 2009;23:355–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (38).Di RF, Azzaro MP, Palumbo GA, Bagnato S, Stagno F, Giustolisi GM, et al. Elevated vascular endothelial growth factor (VEGF) serum levels in idiopathic myelofibrosis. Leukemia 2001;15:976–80. [DOI] [PubMed] [Google Scholar]
- (39).Duley JA, Henman MG, Carpenter KH, Bamshad MJ, Marshall GA, Ooi CY, et al. Elevated plasma dihydroorotate in Miller syndrome: Biochemical, diagnostic and clinical implications, and treatment with uridine. Mol Genet Metab 2016;119:83–90. [DOI] [PubMed] [Google Scholar]
- (40).Lane AN, Fan TW. Regulation of mammalian nucleotide metabolism and biosynthesis. Nucleic Acids Res 2015;43:2466–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (41).Barretina J, Caponigro G, Stransky N, Venkatesan K, Margolin AA, Kim S, et al. The Cancer Cell Line Encyclopedia enables predictive modelling of anticancer drug sensitivity. Nature 2012;483:603–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (42).Wang X, Harrison JS, Studzinski GP. Enhancement of arabinocytosine (AraC) toxicity to AML cells by a differentiation agent combination. J Steroid Biochem Mol Biol 2016;164:72–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (43).Cunningham JT, Moreno MV, Lodi A, Ronen SM, Ruggero D. Protein and nucleotide biosynthesis are coupled by a single rate-limiting enzyme, PRPS2, to drive cancer. Cell 2014;157:1088–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (44).Ipata PL, Falcone G, Serra MC. Some regulatory properties of cytidine deaminase and uridine phosphorylase of Bacillus cereus. FEBS Lett 1970;10:67–70. [DOI] [PubMed] [Google Scholar]
- (45).Gemble S, Ahuja A, Buhagiar-Labarchede G, Onclercq-Delic R, Dairou J, Biard DS, et al. Pyrimidine Pool Disequilibrium Induced by a Cytidine Deaminase Deficiency Inhibits PARP-1 Activity, Leading to the Under Replication of DNA. PLoS Genet 2015;11:e1005384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (46).Jaafar ZA, Oguro A, Nakamura Y, Kieft JS. Translation initiation by the hepatitis C virus IRES requires eIF1A and ribosomal complex remodeling. Elife 2016;5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (47).Wu G, Radwan MK, Xiao M, Adachi H, Fan J, Yu YT. The TOR signaling pathway regulates starvation-induced pseudouridylation of yeast U2 snRNA. RNA 2016;22:1146–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (48).Rocchi L, Pacilli A, Sethi R, Penzo M, Schneider RJ, Trere D, et al. Dyskerin depletion increases VEGF mRNA internal ribosome entry site-mediated translation. Nucleic Acids Res 2013;41:8308–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (49).Emmanuel N, Ragunathan S, Shan Q, Wang F, Giannakou A, Huser N, et al. Purine Nucleotide Availability Regulates mTORC1 Activity through the Rheb GTPase. Cell Rep 2017;19:2665–80. [DOI] [PubMed] [Google Scholar]
- (50).Schiff MH, Strand V, Oed C, Loew-Friedrich I. Leflunomide: efficacy and safety in clinical trials for the treatment of rheumatoid arthritis. Drugs Today (Barc ) 2000;36:383–94. [DOI] [PubMed] [Google Scholar]
- (51).Deans RM, Morgens DW, Okesli A, Pillay S, Horlbeck MA, Kampmann M, et al. Parallel shRNA and CRISPR-Cas9 screens enable antiviral drug target identification. Nat Chem Biol 2016;12:361–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analyzed during this study are included in this article and its supplementary information files. Additional information is available from the corresponding author upon request.


