Abstract
INTRODUCTION:
Mutations in the Cystic Fibrosis Transmembrane Conductance Regulator (CFTR) gene result in defective Cl− transport and cause chronic bacterial infections in the upper and lower airways of cystic fibrosis (CF) patients. Ivacaftor is a CFTR potentiator that improves Cl− transport in CF patients with at least one copy of the G551D mutation. Resveratrol is also a potent CFTR potentiator that increases determinants of mucociliary transport. The objective of this study is to evaluate whether resveratrol and ivacaftor improve Cl− secretion in G551D CFTR over either agent alone.
METHODS:
Fisher rat thyroid cells (FRT) transfected with G551D CFTR and human sinonasal epithelial cells (HSNE) containing the CFTR G551D mutation were subjected to pharmacologic manipulation of transepithelial ion transport in Ussing chambers. Activity was further evaluated using whole cell patch clamp methods in G551D FRT cells.
RESULTS:
In FRT-G551D cells, resveratrol (100 μM) and ivacaftor (10 μM) significantly increased Cl− transport (change in short-circuit current, ΔISC=μA/cm2) compared to single agent and dimethyl sulfoxide vehicle controls (resveratrol+ivacaftor, 4.97+/−0.57 vs. ivacaftor, 0.74+/−0.12 vs. resveratrol, 2.96+/−0.52 vs. control, 0.74+/−0.12;p<0.001). Maximal Cl− secretion (20 μM forskolin) was also significantly enhanced (p<0.0001). Activity was confirmed in G551D HSNE (resveratrol+ivacaftor,4.48+/−0.39 vs. ivacaftor, 1.05+/−0.11 vs. resveratrol, 0.84+/−0.3 vs. control, 0.0+/−0.02;p<0.001), and whole cell patch clamp analysis in G551D FRT cells (resveratrol+ivacaftor; −2535+/−179.3 pA vs. ivacaftor; −1408.9+/−101.3 pA vs. resveratrol; −766.2+/−71.2 pA; p<0.0001).
CONCLUSION:
Additive improvement in G551D CFTR-mediated Cl− secretion suggests that resveratrol could enhance ivacaftor therapy in these patients and improve CF-related rhinosinusitis.
Keywords: cystic fibrosis, CFTR, mucociliary clearance, mucociliary transport, sinusitis, chronic sinusitis, chronic rhinosinusitis, G551D, CFTR potentiator, resveratrol, ivacaftor
INTRODUCTION
Cystic Fibrosis (CF) is among the most common lethal genetic disorders, affecting approximately 1 in 2500 Caucasians annually.1 This multi-system disease caused by mutations in Cystic Fibrosis Transmembrane Conductance Regulator (CFTR) gene is characterized by defective transport of the anions chloride (Cl−)and bicarbonate across secretory epithelia, which leads to abnormally viscous secretions in the airways and other organ systems.1 Secretions in the airways normally function to trap and clear foreign materials via mucociliary clearance (MCC). This mechanism is widely considered the airway’s primary innate defense against bacterial infections. The most common genetic mutation is F508del CFTR, where improper protein folding and processing within the epithelial cells leads to ineffective anion secretion. In G551D CFTR, normal quantities of CFTR channels are present in the plasma membrane, but the open probability (Po) of channel gating is severely affected. Individuals with inspissated mucus are at an increased risk of MCC disruption and chronic, recurrent bacterial infections in the upper and lower airways, including severe chronic rhinosinusitis (CRS).2
Ivacaftor (Vertex phamaceuticals, Boston, Ma.) is a CFTR channel potentiator approved for treatment of CF patients with at least one copy of the G551D mutation and now expanded for use in patients with other Class III (problems with channel gating) and residual function mutations.2,3 Mechanistically, ivacaftor is a non-selective potentiator since it increases channel Po in wild type CFTR and various forms of mutated CFTR (e.g. G551D, F508del) in the presence of CFTR Regulatory domain (R-D) phosphorylation.3 This is best demonstrated in vitro following activation of cAMP/protein kinase A (PKA)-dependent pathways by drugs such as forskolin that phosphorylate the R-D.4 Ivacaftor has been shown to augment endpoints predictive of benefit in G551D CF patients including MCC, sweat Cl−, and lung function.2 While the drug does not normalize the underlying pathophysiology, the partial function attainable is reflective of in vivo studies correlating CFTR activity in CF patients to disease severity. Individuals exhibiting 10–25% of the CFTR activity observed in non-CF individuals have less severe disease according to age of diagnosis, pulmonary function, and pancreatic function than those with no detectable CFTR activity.5–7 Thus, increasing the activity of G551D CFTR by combining ivacaftor with other potentiators should impart further benefit with greater normalization of the underlying electrolyte imbalance responsible for the clinical disease phenotype. Importantly, the same strategy applied to activate Cl− secretion in the lower airways of CF patients is also applicable to sinus and nasal mucus clearance in CF-associated CRS, which is nearly universal in this population.8
One candidate molecule for further potentiation of G551D CFTR is resveratrol, which has also been identified as a powerful potentiator of wild type CFTR and improves determinants of mucociliary transport.4 Resveratrol is similar in structure to flavonoid molecules, such as genistein, which exhibit the capacity to activate CFTR Po,9–11 and may directly bind to CFTR, possibly at the interface between the 2 nucleotide binding domains (NBDs).12,13 A number CFTR potentiators probably share a common mechanism of action or binding site because no additive effect is observed when they are combined for measurement of CFTR function in vitro (e.g genistein and NS004).14,15 However, several agents clearly exhibit an additive effect (e.g. UCCF-029 and UCCF-853,15 genistein and curcumin16). Whether resveratrol mediates CFTR channel potentiation according to an identical mechanism of action as ivacaftor is currently unknown.
The objective of this study is to evaluate whether the combined application of resveratrol and ivacaftor improves G551D CFTR-mediated Cl− secretion compared to either agent alone.
MATERIALS AND METHODS
Cell culture
Institutional Review Board approval was obtained from the University of Alabama at Birmingham prior to the initiation of the study. Human sinonasal tissue was harvested from a single CF patient with G551D/F508del genotype undergoing sinus surgery. Cells were dissociated and grown on 6.5-mm-diameter permeable filter supports submerged in culture medium as previously described.17–24 The media was removed from the monolayers on day 4 after reaching confluence and the cells fed via the basal chamber. Cultures were utilized when fully differentiated with widespread ciliogenesis and transepithelial resistances > 300 Ω·cm.
FRT (Fisher Rat Thyroid) cells expressing human G551D CFTR were provided by the Gregory Fleming James Cystic Fibrosis Research Center. cDNAs carrying human G551D CFTR were introduced into FRT cells using the Flp-in system (Invitrogen, Grand Island, NY). FRT cells were grown in media containing Coon’s modified Ham’s F-12 with 5% FBS and 100μg/ml hygromycin. Cell media were replaced every 48 hours. For Ussing chamber studies, 5×104 FRT cells were grown on 6.5mm Transwell (0.4μm pore membrane) inserts for 5 days. Apical and basolateral media were replaced every 48 hours. For patch clamp studies, FRT cells were seeded on glass coverslips at a very low density and used the next day.
Ussing chamber measurements
Transwell inserts were placed in Ussing chambers for monitoring of ion transport and pharmacologic blockade as previously described.24 Apical monolayers were analyzed under short-circuit conditions following compensation of fluid resistance with automatic VCC 600 voltage clamps (Physiologic Instruments, San Diego, CA, USA). Inserts were mounted in a bath solution at 37°C consisting of (mM): 120 NaCl, 25 NaHCO3, 3.3 KH2PO4, 0.8 K2HPO4, 1.2 MgCl2, 1.2 CaCl2, and 10 glucose, with a pH of 7.3-7.4 in a gassed mixture of 95% O2:5% CO2. Chemicals were procured from Sigma Aldrich (Carlsbad, Ca.). All studies were conducted in low Cl− (6 mM) mucosal baths. Short-circuit current (ISC) measurements were then recorded at 1 sample/second and, by convention, positive deflections indicated net anion movement from the serosal to mucosal surface. Sequential pharmacologic administration consisted of amiloride (inhibits sodium channels) (100 μM), CFTR potentiator [ivacaftor (10 μM), Resveratrol (100 μM), or Ivacaftor 10 μM ) + resveratrol (100 μM)], forskolin (20 μM, phosphorylates CFTR regulatory domain via cAMP-dependent mechanisms) and CFTRinh-172 (10 μM, specific CFTR inhibitor).
Whole cell patch clamp
Stable G551D-CFTR transfected FRT cells on cover slips were transferred to an experimental chamber mounted on the microscope stage (Olympus). G551D-CFTR currents were recorded in whole cell mode of the patch clamp technique using an Axopatch 2000B amplifier interfaced to a computer through DIGITA 1440A. Data was recorded and analyzed using Pclamp software (Molecular Devices). During the experiments, cells were perfused with an external solution of the following ionic composition (in mM): 145 CsCl, 2 MgCl2, 2 Cacl2, 5.5 Glucose, 10 HEPES pH 7.4 (1 N NaOH). The pipette resistance used for whole recording ranged from 3 to 5 GΩ when filled with the following intrapipette solution (in mM): 135 CsCl, 10 KCl, 2 MgCl2, 0.1 EGTA, 5.5 Glucose, 10 HEPES, pH 7.2 (1N KOH). All experiments were performed at room temperature. Cells were continuously perfused with the external solution until development of the recording whole cell configuration. Forskolin, ivacaftor, resveratrol, and ivacaftor + resveratrol were applied through the same perfusion system. The rate of perfusion was adjusted to 1 ml/min.
Statistical Analysis
Statistical analyses usedtwo way ANOVA with Tukey-Kramer post hoc analysis. All values are reported as the mean ± standard error of the mean [SEM]. A p-value<0.05 was considered statistically significant.
RESULTS
Resveratrol and ivacaftor improve CFTR-mediated Cl− secretion in G551D FRT and G551D/F508del HSNE cells
The primary method to evaluate anion transport involves pharmacological manipulation of cell cultures mounted in Ussing chambers followed by measurements of short-circuit current (ΔISC= μA/cm2). Because HSNE primary cells are directly seeded onto filters following dissociation from the tissue to optimize the ion transport phenotype and differentiation, limited cells are available for testing. Thus, Ussing chamber analysis was performed using G551D-FRT cells first to optimize dosing for HSNE experiments. Resveratrol starts to impact maximal Cl-secretory capability in the presence of forskolin at doses above 100 μM (data not shown), and therefore this was considered the optimal dose. Ivacaftor also starts to impede maximal Cl-secretory capability at doses above 10 μM and this concentration has been considered appropriate in previous in vitro studies.3 FRT cells lack epithelial sodium channels (ENaC) and this was confirmed with absence of amiloride-sensitive ISC. Although ΔISC was overall quite low as a percentage of maximally activated ISC with forskolin, resveratrol and ivacaftor increased Cl− transport over resveratrol or ivacaftor alone and vehicle controls (n≥8 per condition; resveratrol+ivacaftor, 4.97+/−0.57 vs. ivacaftor, 0.74+/−0.12 vs. resveratrol, 2.96+/−0.52 vs. control, 0.74+/−0.12; p<0.001). More importantly, the maximum capability for vectorial Cl− transport through CFTR channels was significantly improved with both agents when forskolin was applied (resveratrol+ivacaftor, 254.5+/−7.53 vs. ivacaftor, 217.61+/−8.27 vs. resveratrol, 148.2+/−8.28 vs. DMSO, 92.93+/−4.22; p<0.0001). The forskolin-stimulated ΔISC of G551D FRT cells in the presence of the 2 potentiators was approximately 60% of forskolin-stimulated ΔISC observed in wild type CFTR FRT cells (data not shown). Representative tracings and summary data is presented in Figure 1.
Figure 1.
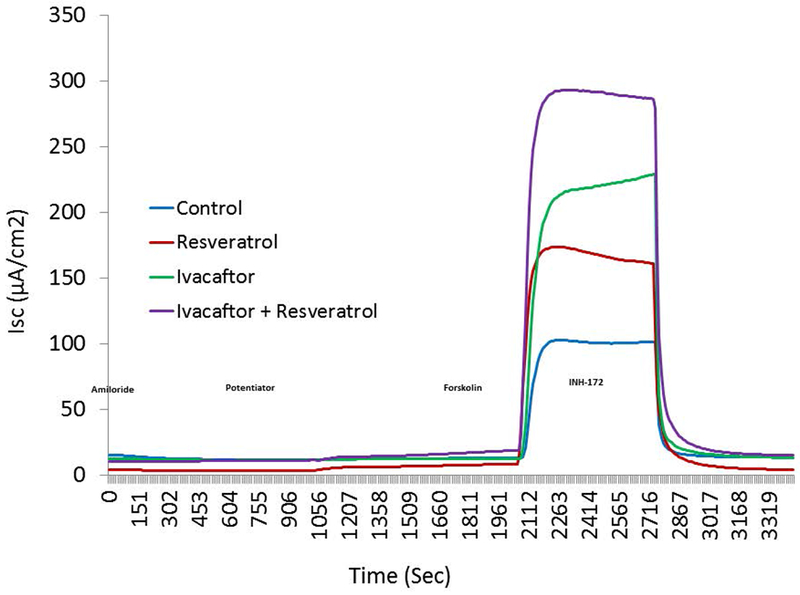
(A) Representative Ussing chamber current tracings in G551D FRT cells following sequential administration of amiloride, potentiator(s), and INH-172. (B) Summary of current measurements. Error bars represent SEM. All values within groups are significantly different from each other (potentiator, p<0.001; potentiator + forskolin, p<0.0001).
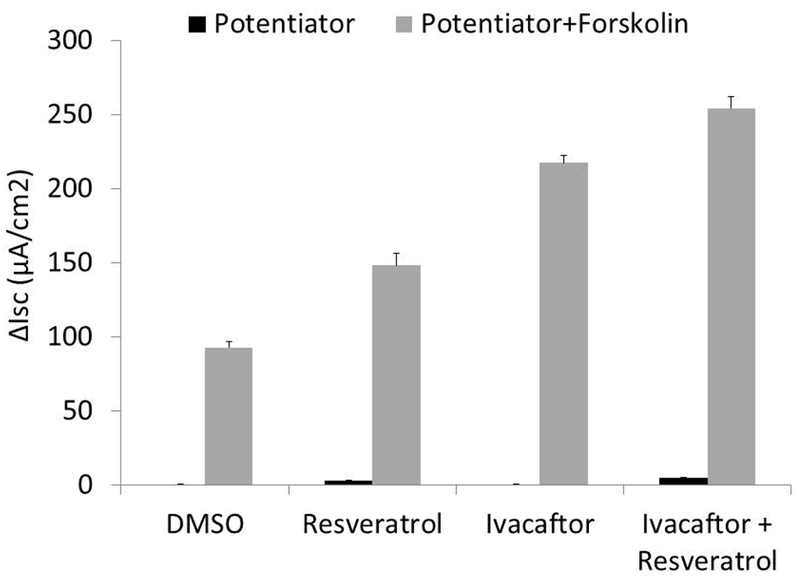
A similar pattern was observed with primary G551D HSNE cells (Figure 2) showing significant improvement with the 2 potentiators (n≥5 per condition; resveratrol+ivacaftor, 4.48+/−0.39 vs. ivacaftor, 1.05+/−0.11 vs. resveratrol, 0.84+/−0.3 vs. DMSO, 0.0+/−0.02; p<0.001). Stimulation of CFTR-mediated ISC with CFTR potentiators was typically better with primary HSNE as a percentage of overall forskolin-stimulated ISC.25 Maximal Cl− secretion was augmented with both agents (resveratrol+ivacaftor, 32.2+/−1.17 vs. resveratrol, 10.73+/−0.84 vs. ivacaftor, 21.29+/−0.85 vs. DMSO, 3.88+/−0.85; p<0.001) to levels consistent with what is detected in non-CF HSNE.26–28
Figure 2.

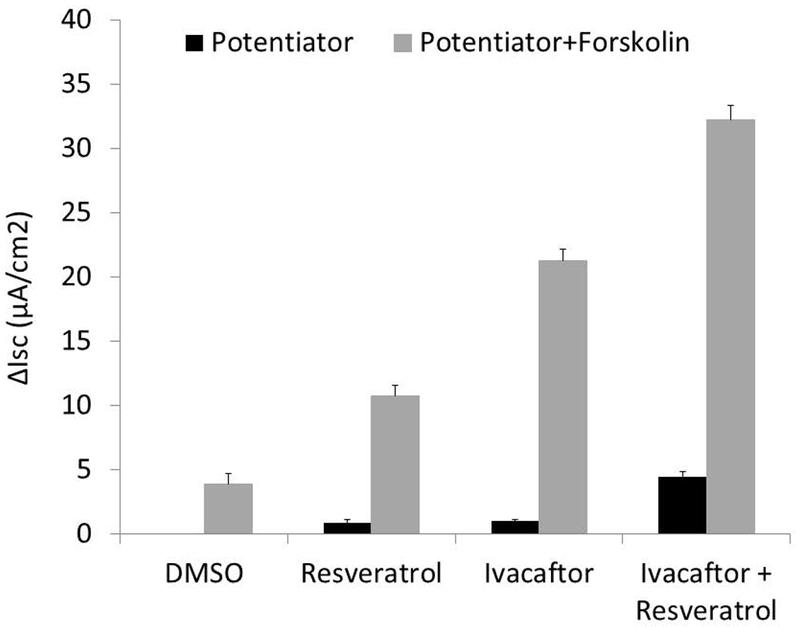
(A) Representative Ussing chamber current tracings in G551D/F508del HSNE cells following sequential administration of amiloride, potentiator(s), and INH-172. (B) Summary of current measurements. Error bars represent SEM. Significant findings are bracketed with an asterisk. All values within groups (potentiator or potentiator + forskolin) are significantly different from each other (p<0.001).
Combined potentiators improve G551D CFTR-mediated Cl− secretion as judged by whole cell patch clamp analysis
Whole-cell patch clamp currents confirmed that CFTR activity is markedly enhanced with combined application of resveratrol and ivacaftor. The potentiators did not activate CFTR currents when forskolin was not present. This was consistent with previous studies using patch clamp methods for CFTR potentiators, which require at least some baseline R-D phosphorylation.3 CFTR-mediated currents were markedly improved with co-administration of resveratrol and ivacaftor (−2535+/−179.3 pA) over ivacaftor (−1408.9+/−101.3 pA) and resveratrol (−766.2+/−71.2 pA) alone (Figure 3; p<0.0001). Patch clamp technique controls the membrane potential of the cell better and did show that the current related to combined application (in presence of forskolin) was slightly greater than the sum of the individual currents from either agent alone. .
Figure 3.
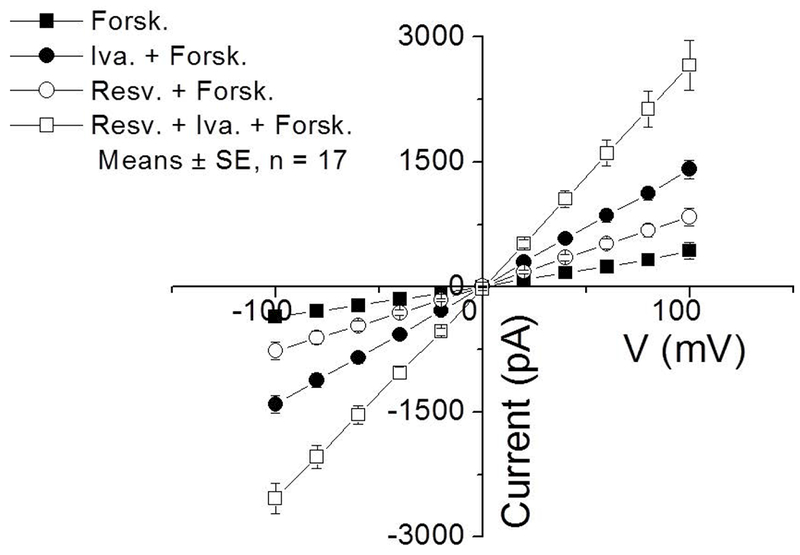
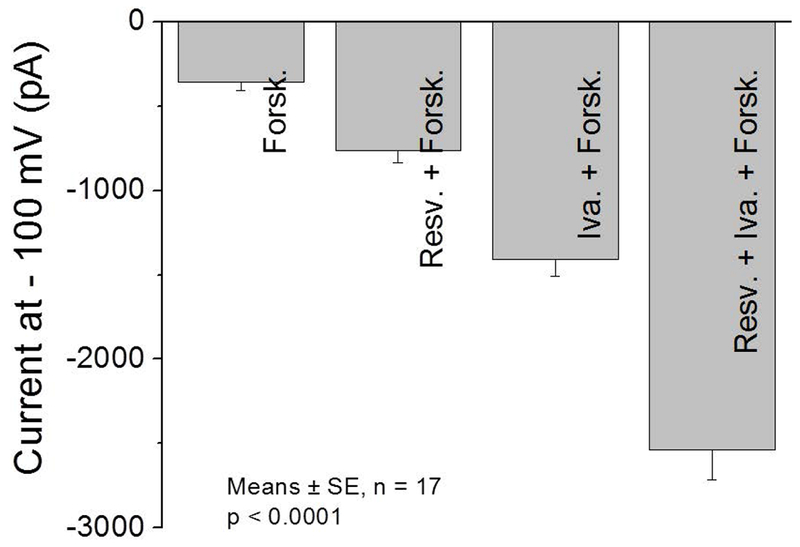
(A) IV curves are illustrated during the activation of the Cl− current with forskolin and forskolin + potentiators when voltage steps were varied from −100 mV to 100 mV in 20 mV increments for 500 ms. The cell membrane potential was held at 0 mV throughout the experiment. (B) Summary data of currents at a voltage step of −100 mV and holding potential of 0 mV. All values are significantly different from each other (p <0.0001). (Forsk = forskolin, Iva = ivacaftor, Resv = Resveratrol)
DISCUSSION
For Class 3 gating mutations such as G551D, combined potentiator therapy has important clinical implications since they exhibit extremely low Po.29 In this study, we investigated the effects of ivacaftor, resveratrol, and their joint applications on G551D-CFTR and identified that combining the agents significantly increased CFTR-mediated Cl− transport in FRT cells and primary HSNE cells expressing G551D CFTR compared to either agent alone. Furthermore, when R-domains were maximally phosphorylated via cAMP-dependent pathways with the addition of forskolin, the total capacity for Cl− secretion was also markedly improved with both drugs. At the optimal dose, ivacaftor (10 uM) was determined to be more effective than resveratrol (100 uM) at improving CFTR-mediated Cl− transport (by whole cell patch clamp) and overall capacity of Cl− secretion following application of forskolin. Importantly, resveratrol and ivacaftor stimulated significantly more Cl− secretion through G551D-CFTR channels, that is, G551D-CFTR channel activity that was maximally potentiated by combining both drugs was stronger than the additive effect of both agents alone. Similar effects were identified in both FRT and primary HSNE. Thus, we conclude that resveratrol and ivacaftor affect G551D-CFTR through different, but perhaps complementary gating mechanisms. Effects may be mediated by improved efficiency of one or both drugs at their active binding site.
Resveratrol and ivacaftor both increase wild type CFTR currents in excised patches, and thus their target is within the CFTR molecule itself.3,30,31 Both agents affect ATP-dependent gating of CFTR channels with increased channel Po, consistent with a target site at the NBD dimer. Mounting evidence suggests that most CFTR potentiators act through binding to the NBDs to promote the activity of the protein and then, with lower affinity, to an inhibitory site.32 Thus, at higher doses, potentiators start to inhibit capacity for CFTR-mediated Cl− secretion. A biphasic dose–response relationship for resveratrol has been observed previously with optimally sustained CFTR-mediated anion secretion at 100 μM without altering the maximal capacity for Cl− secretion (forskolin-activated).30,31 Ivacaftor has been observed to confer optimal effects at 10μM and has an EC50 of 100 nM in G551D CFTR expressing cells. Interactions at the NBDs are complex and beyond the scope of this paper. However, one would expect application of both drugs at these concentrations to impart either similar or decreased CFTR-mediated Cl− transport (more saturation of the inhibitory site or competitive antagonism) if the active sites were identical.
Further studies are required to understand the combined effects of resveratrol and ivacaftor on G551D-CFTR, but our findings suggest combining these 2 CFTR potentiators might confer improved clinical benefit over what is currently achieved with FDA approved ivacaftor in this CF population. Indeed, CFTR-mediated Cl− secretion in the presence of forskolin reached levels approximately 60% of wild type CFTR in FRT cells and, in primary G551D/F508del HSNE, was actually similar in magnitude to what is detected in forskolin-stimulated primary non-CF HSNE cells. Unfortunately, resveratrol exhibits poor bioavailability with attainable maximum plasma levels of approximately 2 μM – well below the optimum concentration.16 However, multiple therapies for CF involve topical application of drugs to the sinuses and lungs, including hypertonic saline, dornase alpha, and antibiotic therapies.33–35 The sinuses in particular are an excellent target area for topical CFTR potentiators delivered in saline rinse or aerosol. Thus, strategies to deliver topical resveratrol to the sinuses or lower airway in G551D CF individuals who are already taking ivacaftor are certainly justified and should be further evaluated.
CONCLUSION
Additive improvement in G551D CFTR-mediated Cl− secretion with resveratrol and ivacaftor suggests distinct mechanisms and/or binding sites for these potentiators. Future clinical studies employing topical delivery of resveratrol to the sinuses to enhance ivacaftor therapy in G551D CF-associated CRS are planned.
Acknowledgments
Funding Support: This work was supported by National Institutes of Health (NIH)/National Heart, Lung, and Blood Institute (1 R01 HL133006-02), National Institute of Diabetes and Digestive and Kidney Diseases (5P30DK072482-05, CF Research Center Pilot Award), and CF Smackdown seed grant to B.A.W.
Footnotes
Level of Evidence: NA.
Disclosures: All authors have read and approved the manuscript. Dr. Bradford A. Woodworth is a consultant for Cook Medical and Olympus. An oral presentation of this work was presented at the American Rhinologic Society’s Spring Meeting on April 19th, 2018 in Baltimore, Maryland.
REFERENCES
- 1.Moller W, Haussinger K, Ziegler-Heitbrock L, Heyder J. Mucociliary and long-term particle clearance in airways of patients with immotile cilia. Respir Res. 2006;7:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Accurso FJ, Rowe SM, Clancy JP, et al. Effect of VX-770 in persons with cystic fibrosis and the G551D-CFTR mutation. N Engl J Med.363(21):1991–2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Van Goor F, Hadida S, Grootenhuis PD, et al. Rescue of CF airway epithelial cell function in vitro by a CFTR potentiator, VX-770. Proceedings of the National Academy of Sciences of the United States of America. 2009;106(44):18825–18830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bradbury NA, Jilling T, Berta G, Sorscher EJ, Bridges RJ, Kirk KL. Regulation of plasma membrane recycling by CFTR. Science. 1992;256(5056):530–532. [DOI] [PubMed] [Google Scholar]
- 5.Noone PG, Pue CA, Zhou Z, et al. Lung disease associated with the IVS8 5T allele of the CFTR gene. American journal of respiratory and critical care medicine. 2000;162(5):1919–1924. [DOI] [PubMed] [Google Scholar]
- 6.Noone PG, Zhou Z, Silverman LM, Jowell PS, Knowles MR, Cohn JA. Cystic fibrosis gene mutations and pancreatitis risk: relation to epithelial ion transport and trypsin inhibitor gene mutations. Gastroenterology. 2001;121(6):1310–1319. [DOI] [PubMed] [Google Scholar]
- 7.McKone EF, Emerson SS, Edwards KL, Aitken ML. Effect of genotype on phenotype and mortality in cystic fibrosis: a retrospective cohort study. Lancet. 2003;361(9370):1671–1676. [DOI] [PubMed] [Google Scholar]
- 8.Virgin FW, Rowe SM, Wade MB, et al. Extensive surgical and comprehensive postoperative medical management for cystic fibrosis chronic rhinosinusitis. American journal of rhinology & allergy. 2012;26(1):70–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Springsteel MF, Galietta LJ, Ma T, et al. Benzoflavone activators of the cystic fibrosis transmembrane conductance regulator: towards a pharmacophore model for the nucleotide-binding domain. Bioorg Med Chem. 2003;11(18):4113–4120. [DOI] [PubMed] [Google Scholar]
- 10.Galietta LJ, Springsteel MF, Eda M, et al. Novel CFTR chloride channel activators identified by screening of combinatorial libraries based on flavone and benzoquinolizinium lead compounds. J Biol Chem. 2001;276(23):19723–19728. [DOI] [PubMed] [Google Scholar]
- 11.Caci E, Folli C, Zegarra-Moran O, et al. CFTR activation in human bronchial epithelial cells by novel benzoflavone and benzimidazolone compounds. Am J Physiol Lung Cell Mol Physiol. 2003;285(1):L180–188. [DOI] [PubMed] [Google Scholar]
- 12.Moran O, Galietta LJ, Zegarra-Moran O. Binding site of activators of the cystic fibrosis transmembrane conductance regulator in the nucleotide binding domains. Cell Mol Life Sci. 2005;62(4):446–460. [DOI] [PubMed] [Google Scholar]
- 13.Melin P, Thoreau V, Norez C, Bilan F, Kitzis A, Becq F. The cystic fibrosis mutation G1349D within the signature motif LSHGH of NBD2 abolishes the activation of CFTR chloride channels by genistein. Biochem Pharmacol. 2004;67(12):2187–2196. [DOI] [PubMed] [Google Scholar]
- 14.Gray MA, Greenwell JR, Argent BE. Secretin-regulated chloride channel on the apical plasma membrane of pancreatic duct cells. J Membr Biol. 1988;105(2):131–142. [DOI] [PubMed] [Google Scholar]
- 15.Verkman AS, Galietta LJ. Chloride channels as drug targets. Nat Rev Drug Discov. 2009;8(2):153–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sohma Y, Yu YC, Hwang TC. Curcumin and genistein: the combined effects on disease-associated CFTR mutants and their clinical implications. Curr Pharm Des. 2013;19(19):3521–3528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bhargave G, Woodworth BA, Xiong G, Wolfe SG, Antunes MB, Cohen NA. Transient receptor potential vanilloid type 4 channel expression in chronic rhinosinusitis. American journal of rhinology. 2008;22(1):7–12. [DOI] [PubMed] [Google Scholar]
- 18.Cohen NA, Zhang S, Sharp DB, et al. Cigarette smoke condensate inhibits transepithelial chloride transport and ciliary beat frequency. The Laryngoscope. 2009;119(11):2269–2274. [DOI] [PubMed] [Google Scholar]
- 19.Virgin FW, Azbell C, Schuster D, et al. Exposure to cigarette smoke condensate reduces calcium activated chloride channel transport in primary sinonasal epithelial cultures. The Laryngoscope. 2010;120(7):1465–1469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Woodworth BA, Antunes MB, Bhargave G, et al. Murine nasal septa for respiratory epithelial air-liquid interface cultures. Biotechniques. 2007;43(2):195–204. [DOI] [PubMed] [Google Scholar]
- 21.Woodworth BA, Antunes MB, Bhargave G, Palmer JN, Cohen NA. Murine tracheal and nasal septal epithelium for air-liquid interface cultures: A comparative study Am J Rhinol. 2007;21(5):533–537. [DOI] [PubMed] [Google Scholar]
- 22.Woodworth BA, Tamashiro E, Bhargave G, Cohen NA, Palmer JN. An in vitro model of Pseudomonas aeruginosa biofilms on viable airway epithelial cell monolayers Am J Rhinol. 2008;22(3):234–238. [DOI] [PubMed] [Google Scholar]
- 23.Zhang S, Fortenberry JA, Cohen NA, Sorscher EJ, Woodworth BA. Comparison of vectorial ion transport in primary murine airway and human sinonasal air-liquid interface cultures, models for studies of cystic fibrosis, and other airway diseases. American journal of rhinology & allergy. 2009;23(2):149–152. [DOI] [PubMed] [Google Scholar]
- 24.Dean N, Ranganath NK, Jones B, et al. Porcine nasal epithelial cultures for studies of cystic fibrosis sinusitis. International forum of allergy & rhinology. 2014;4(7):565–570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Conger BT, Zhang S, Skinner D, et al. Comparison of cystic fibrosis transmembrane conductance regulator (CFTR) and ciliary beat frequency activation by the CFTR Modulators Genistein, VRT-532, and UCCF-152 in primary sinonasal epithelial cultures. JAMA otolaryngology-- head & neck surgery. 2013;139(8):822–827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tipirneni KE, Grayson JW, Zhang S, et al. Assessment of acquired mucociliary clearance defects using micro-optical coherence tomography. International forum of allergy & rhinology. 2017;7(9):920–925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Illing EA, Cho DY, Zhang S, et al. Chlorogenic Acid Activates CFTR-Mediated Cl-Secretion in Mice and Humans: Therapeutic Implications for Chronic Rhinosinusitis. Otolaryngology--head and neck surgery : official journal of American Academy of Otolaryngology-Head and Neck Surgery. 2015;153(2):291–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang S, Skinner D, Hicks SB, et al. Sinupret activates CFTR and TMEM16A-dependent transepithelial chloride transport and improves indicators of mucociliary clearance. PloS one. 2014;9(8):e104090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bompadre SG, Sohma Y, Li M, Hwang TC. G551D and G1349D, two CF-associated mutations in the signature sequences of CFTR, exhibit distinct gating defects. J Gen Physiol. 2007;129(4):285–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Woodworth BA. Resveratrol ameliorates abnormalities of fluid and electrolyte secretion in a hypoxia-Induced model of acquired CFTR deficiency. The Laryngoscope. 2015;125 Suppl 7:S1–S13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang S, Blount AC, McNicholas CM, et al. Resveratrol enhances airway surface liquid depth in sinonasal epithelium by increasing cystic fibrosis transmembrane conductance regulator open probability. PloS one. 2013;8(11):e81589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yu YC, Miki H, Nakamura Y, et al. Curcumin and genistein additively potentiate G551D-CFTR. Journal of cystic fibrosis : official journal of the European Cystic Fibrosis Society. 2011;10(4):243–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chiu AG, Antunes MB, Palmer JN, Cohen NA. Evaluation of the in vivo efficacy of topical tobramycin against Pseudomonas sinonasal biofilms. J Antimicrob Chemother. 2007;59(6):1130–1134. [DOI] [PubMed] [Google Scholar]
- 34.Mainz JG, Schumacher U, Schadlich K, et al. Sino nasal inhalation of isotonic versus hypertonic saline (6.0%) in CF patients with chronic rhinosinusitis - Results of a multicenter, prospective, randomized, double-blind, controlled trial. Journal of cystic fibrosis : official journal of the European Cystic Fibrosis Society. 2016;15(6):e57–e66. [DOI] [PubMed] [Google Scholar]
- 35.Mainz JG, Schiller I, Ritschel C, et al. Sinonasal inhalation of dornase alfa in CF: A double-blind placebo-controlled cross-over pilot trial. Auris Nasus Larynx. 2011;38(2):220–227. [DOI] [PubMed] [Google Scholar]


