Abstract
Metastatic lung cancer is common in lung adenocarcinoma patients, but the molecular mechanisms of metastasis remain incompletely resolved. MicroRNA (miR) regulate gene expression and contribute to cancer development and progression. This report identifies miR-576–3p and its mechanism of action in lung cancer progression. miR-576–3p was determined to be significantly decreased in clinical specimens of late-stage lung adenocarcinoma. Overexpression of miR-576–3p in lung adenocarcinoma cells decreased mesenchymal marker expression and inhibited migration and invasion. Inhibition of miR-576–3p in non-malignant lung epithelial cells increased migration and invasion as well as mesenchymal markers. Serum/glucocorticoid regulated kinase 1 (SGK1) was a direct target of miR-576–3p, and modulation of miR-576–3p levels led to alterations in SGK1 protein and mRNA as well as changes in activation of its downstream target linked to metastasis, N-myc downstream regulated 1 (NDRG1). Loss of the ability of miR-576–3p to bind the 3’-UTR of SGK1, rescued the inhibition in migration and invasion observed with miR-576–3p overexpression. Additionally, increased SGK1 levels were detected in lung adenocarcinoma patient samples expressing mesenchymal markers, and pharmacological inhibition of SGK1 resulted in a similar inhibition of migration and invasion of lung adenocarcinoma cells as observed with miR-576–3p overexpression. Together, these results reveal miR-576–3p downregulation is selected for in late-stage lung adenocarcinoma due to its ability to inhibit migration and invasion by targeting SGK1. Furthermore, these results also support targeting SGK1 as a potential therapeutic for lung adenocarcinoma.
Keywords: lung cancer, SGK1, miRNA, migration, invasion
INTRODUCTION
Lung cancer is the leading cause of cancer deaths in the United States, with lung adenocarcinoma being the most prevalent form of lung cancer1. The 5-year survival rate of lung cancer patients in late-stage disease with distant metastasis is <5%1. This late-stage disease, characterized by metastasis to nearby and distant organs, represents a significant problem in the treatment of lung cancer and leads to poor patient outcome. However, the molecular mechanisms that propel lung cancer cells to metastasize have not been fully elucidated. A better understanding of these mechanisms should lead to improved treatment of patients.
miRNA have important functions in cancer development and progression, including tumor initiation, proliferation, invasion, and/or metastasis by regulating gene expression2. Multiple miRNA have been reported to contribute to lung cancer3, 4. For example, miR-31 is overexpressed in lung adenocarcinoma and is a lung tumor initiator through its targeting of multiple negative regulators of the RAS/MAPK pathway5. In contrast, the miR-34 family has a tumor suppressive function in lung cancer, causing its expression to be downregulated in lung adenocarcinoma6–8. For lung cancer progression, miR-218 downregulation was recently reported to lead to increased migration and invasion by altering the expression of genes that regulate epithelial to mesenchymal transition (EMT)9. Therefore, dysregulated miRNA can impact lung tumorigenesis from initiation through progression.
Although the function of numerous miRNA have been determined, there are many miRNA whose function is unknown or not fully characterized. For example, primate-specific miR-576–3p was reported to suppress proliferation of bladder cancer cells by targeting cyclin D110, but its role in other cancer types and whether it has other targets is unknown. We determined miR-576–3p was significantly downregulated in lung adenocarcinoma and this facilitated lung adenocarcinoma cell migration and invasion capabilities. Mechanistically, miR-576–3p directly targeted Serum and Glucocorticoid Regulated Kinase 1 (SGK1), and altered the phosphorylation of its downstream target N-Myc Downstream Regulated 1 (NDRG1). NDRG1 regulates EMT to exert its anti-metastatic effects11, 12. Our data indicate miR-576–3p has an important function in lung cancer progression by inhibiting cell movement through SGK1 signaling.
MATERIALS AND METHODS
Cell culture, transfections, and retroviral production
All cell lines were previously obtained, authenticated, and cultured as we previously reported5. Mycoplasma testing was performed prior to and at intervals during experimentation. Transfections were performed with Lipofectamine 2000 (ThermoFisher) in serum free RPMI1640 using 50 nM miR-576–3p mimic, 200nM miR-576–3p inhibitor, or a corresponding negative control (Dharmacon), and the transfection media was replaced with serum containing culture media 6 hours after transfection. miR-576 (GeneCopoeia, Rockville, MD) was overexpressed in cells using pBABE retroviral vectors with puromycin selection markers. Puromycin selection was performed for 5 days and stably infected cells were used for subsequent experiments.
Patient samples
All de-identified human samples (normal lung and lung adenocarcinoma) were previously obtained from the Vanderbilt University Medical Center Lung Biorepository5, which banked samples following informed patient consent. Clinicopathologic features of the samples evaluated are in Supplemental Tables S1 and S2.
Cell growth and transformation assays
For measurement of proliferation following overexpression or inhibition of miRNA, cells were transfected or infected as described above. MTT assays were performed every 24 hours for 4 days, as we previously reported5, 13, and absorbance was measured on a Cytation 5 reader (BioTek). Clonogenic and soft-agar assays were conducted as previously reported5 with lung adenocarcinoma cells following infection with a pBABE-puro retrovirus encoding miR-576 or a scrambled control and puromycin selection (described above). Colonies were counted using an Olympus CKX53 inverted microscope (10×). Colonies counted between days 11–14.
qPCR array and quantitative real-time PCR (qRT-PCR)
RNA was isolated using TRIzol (ThermoFisher). The qPCR array to detect 754 miRNA was previously conducted14. For qRT-PCR, cDNA synthesis and qRT-PCR using TaqMan probes (ThermoFisher) for miRNA and SYBR green (Qiagen) for mRNA was performed as described previously15, 16. RNU6B was the endogenous control for miRNA, and β-ACTIN levels were used for normalization of mRNA. All assays were performed in triplicate. Data are displayed as 2−deltaCt. Primer sequences for SGK1 are forward, 5’-CAATCCTCATGCTAAACC and reverse, 5’-TTCCAGGAAGCAGCGTTC.
Migration and invasion assays
Cells were resuspended in serum free RPMI1640 and placed in migration chambers (8.0 μm pores, BioCoat insert) or invasion chambers (8.0 μm pores, Corning BioCoat Matrigel invasion chamber). Transwell migration and invasion assays were performed and inserts stained after 18 hours, blinded, and counted using an Olympus CKX53 inverted microscope (10×) as previously reported17. Images were captured by Cytation 5 imaging reader (4×). Numbers of cells used for migration assays (2.5×104 - 5×104) and invasion assays (5×104 - 1×105) was dependent on the cell line. For the experiments with the SGK inhibitor, EMD638683(ApexBio), cells were treated for 24 hours before collecting cells for western blot analysis (see below) or performing migration and invasion assays.
Western Blotting
Whole cell lysates were Western blotted as previously described18. Antibodies specific for: VIMENTIN (R28), SNAIL (C15D3), SGK1 (D27C11), NDRG1 (D8G9), P-NDRG1 (D98G11) were from Cell Signaling, and α-TUBULIN (B-5–1-2) was from Sigma.
Luciferase assays
The entire 3’-UTR of SGK1 was amplified from human genomic DNA using PCR with the primer sequences: forward, 5’-ATAGCTAGCACCCTGTTAGGGCTTG and reverse, 5’-ATATCTCGAGCAACGGCTCTGACTG. The 3’-UTR was inserted into pmiRGLO Luciferase miRNA Target Expression Vector (Promega) and the sequence was confirmed by Sanger sequencing. Luciferase assays were conducted as previously reported following transfection with miRNA mimics or controls15. β–Galactadase plasmid was co-transfected for normalization.
Analysis of TCGA RNA-seq data
We obtained normalized RNA-seq V2 data for mRNA expression from 515 lung adenocarcinoma patient samples, available in The Cancer Genome Atlas (TCGA) project. For each sample, we calculated the average mRNA expression values of literature curated mesenchymal signature genes (AXL, CDH2, FN1, SNAI1, SNAI2, TWIST1, VIM, and ZEB1). Tumor samples were then ranked based on the average gene expression values. Samples were denoted as mesenchymal or epithelial subtype specific if they were in the highest or lowest quartile, respectively. SGK1 expression was determined in the samples, and differential expression of SGK1 was measured using unpaired, two-tailed t test.
Statistics
Graphs represent the mean ±SEM. Two-tailed Student’s t tests were used for comparisons, except for patient sample analysis, which used a one-tailed t test, and RNA seq analysis, which used unpaired, two-tailed t test. P-values are indicated in figure legends.
RESULTS
miR-576–3p expression is reduced in human lung adenocarcinoma.
To determine the miRNA that are differentially expressed in lung adenocarcinoma and that may contribute to its development or progression, we compared miRNA levels in the lung adenocarcinoma cell line A549 to those in the non-transformed bronchial epithelial cell line 16HBE, using a qPCR miRNA array. All miRNA probes used were specific for the mature form of miRNA to avoid detecting the primary and precursor forms. Expression levels of miRNA were obtained and analyzed. miR-296, miR-203, miR-576–3p, were the top three most decreased miRNA in A549 cells compared to non-transformed 16HBE control cells (Table 1). Previous studies have shown that miR-296 and miR-203 levels are both reduced in lung cancer19, 20, indicating the qPCR array identified known downregulated miRNA. In addition, both miR-296 and miR-203 are reported to inhibit migration and invasion in lung adenocarcinoma19, 21. However, there is a paucity of data on the role of miR-576–3p, a primate-specific miRNA, in cancer with only reports that it is reduced in bladder cancer22, 23.
Table 1.
Top 3 downregulated miRNA in lung adenocarcinoma
| miRNA | Fold Change* |
|---|---|
| miR-296 | 5.7×10−3 |
| miR-203 | 1.0×10−2 |
| miR-576–3p | 1.6×10−2 |
qPCR array comparing A549 to 16HBE cells
To investigate miR-576–3p in lung cancer, we first evaluated miR-576–3p expression in a panel of lung adenocarcinoma cell lines. miR-576–3p was reduced in at least 80% (8 of 10) of the lung cancer cell lines evaluated compared to immortalized human bronchial epithelial cells (Figure 1A). To assess whether these results were reflected in patients, we examined miR-576–3p levels in human lung adenocarcinoma and normal lung samples. miR-576–3p levels were significantly reduced in lung adenocarcinoma samples compared to levels in normal lung (Figure 1B). When the patients were stratified according to early-stage (I and II) and late-stage (III and IV) disease, miR-576–3p expression trended towards decreased expression in early-stage, but was only significantly reduced in late-stage adenocarcinoma (Figure 1C). Further, separation of patient samples by pathological stage showed that miR-576–3p levels were significantly decreased in both stage III and stage IV (Supplemental Figure 1A). A qPCR miRNA array performed on stage I lung adenocarcinoma samples with matched normal lung from the same patient also showed a trend towards decreased miR-576–3p expression in malignant samples (9 of 11), but did not reach statistical significance (Supplemental Figure 1B). Therefore, miR-576–3p is downregulated in late-stage lung adenocarcinoma.
Figure 1. miR-576–3p expression is downregulated in human lung adenocarcinoma.
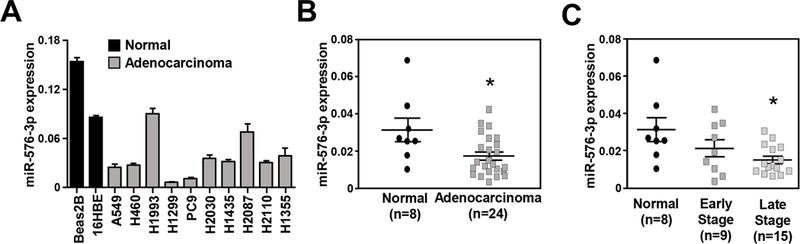
(A) qRT-PCR analysis of miR-576–3p in two bronchial epithelial cell lines and a panel of lung adenocarcinoma cell lines; (triplicates) SEM. (B) qRT-PCR analysis of normal human lung and lung adenocarcinoma patient samples; (triplicates) SEM; *P=5.8×10−3, two-tailed t-test. (C, D) qRT-PCR data from B grouped as early-stage (I & II) and late-stage (III & IV) (C) or separated into tumor stage (D); (triplicates) SEM; late-stage, C, *P=3×10−3; Stage III, D, *P=8.3×10−3; Stage IV, D, *P=0.038, one-tailed t-test. All data are relative to the endogenous control RNU6B small RNA.
miR-576–3p does not affect lung adenocarcinoma proliferation but inhibits colony formation.
To test the functional relevance of miR-576–3p expression in lung epithelial cells, we either inhibited or overexpressed miR-576–3p and assessed proliferation. Previously, it was reported that miR-576–3p regulated proliferation of bladder cancer cells10. However, inhibition of miR-576–3p in the immortalized, non-malignant bronchial epithelial line Beas2B, which expressed increased levels of miR-576–3p compared to lung adenocarcinoma cells, resulted in no change in growth rate compared to control inhibitor (Figure 2A). Similarly, overexpression of miR-576–3p in the lung adenocarcinoma cell lines A549 and H460 resulted in no change in proliferation (Figure 2A). As a control, we used miR-31, which we previously reported induces proliferation of lung cells5. miR-31 overexpression induced proliferation and inhibition of miR-31 reduced proliferation (Figure 2A). Thus, miR-576–3p does not appear to function as a regulator of proliferation in lung cells.
Figure 2. miR-576–3p does not affect proliferation, but inhibits colony formation.
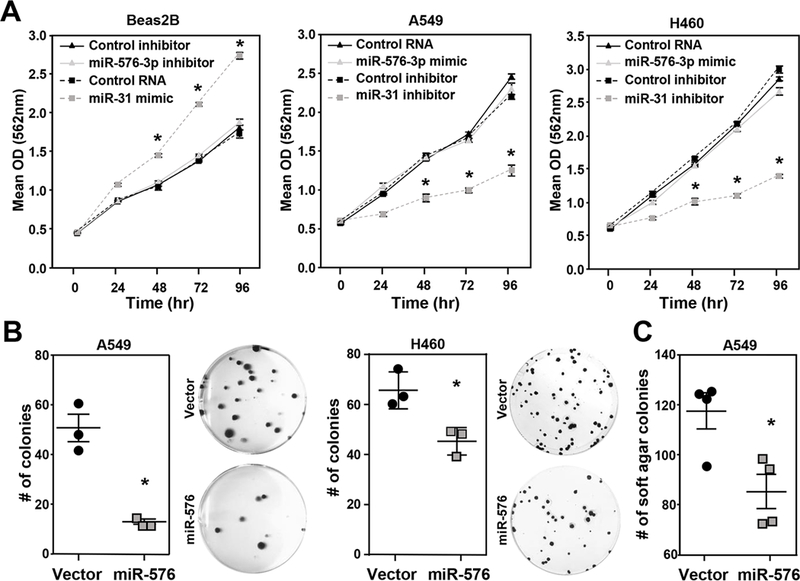
(A) The indicated cell lines were transfected with miR-576–3p mimic, miR-576–3p inhibitor, or controls, including a miR-31 mimic and inhibitor as a positive control. MTT assays (quadruplicate) were performed at the indicated intervals; SEM, *P<0.001, two-tailed t-test. (B, C) Colony formation (B) and soft agar (C) assays (both triplicates) were performed with the indicated lung adenocarcinoma cells retrovirally overexpressing miR-576 or scrambled control. Representative images (10×) shown. Data are representative of three independent experiments; SEM; B, A549 *P=7.0×10−3; H460 *P=1.9×10−2; C, *P<0.02, two-tailed t-test.
To further investigate the role of miR-576–3p in processes critical for lung cancer growth and progression, we measured the ability of miR-576–3p to influence colony formation under conditions of low cell density and loss of surface contact. Lung adenocarcinoma cells were infected with a miR-576 encoded retrovirus to obtain stable long-term miR-576 overexpression that would be needed for these assays (Supplemental Figure 2A). Similar to transfection with miR-576–3p mimic, retroviral overexpression of miR-576 had no effect on proliferation (Supplemental Figure 2B). However, miR-576 overexpressing lung adenocarcinoma cells had a significantly reduced number of colonies from low density cultures compared to cells with scrambled control (Figure 2B). miR-576 overexpression also resulted in a significantly reduced number of colonies in soft agar assays compared to scrambled controls (Figure 2C). The data indicate miR-576–3p is not altering proliferation, but appears to be influencing the ability of lung cells to grow under stressful conditions.
Elevated miR-576–3p reduces migratory and invasive capabilities of lung adenocarcinoma cells.
Given that we observed significantly decreased levels of miR-576–3p in late-stage disease (Figures 1C and 1D) and that miR-576–3p impacts lung adenocarcinoma growth under stress, we evaluated the role of miR-576–3p in stressful cellular processes important for metastasis, cell migration and invasion. To determine whether miR-576–3p influences migration and invasion, we modulated miR-576–3p levels in lung adenocarcinoma cells and non-cancerous bronchial epithelial cells and performed transwell migration and invasion assays. Inhibition of miR-576–3p in non-malignant Beas2B cells resulted in a significantly higher number of migrated cells compared to cells with control inhibitor (Figure 3A). Elevated levels of miR-576–3p in A549 and H460 lung adenocarcinoma cells resulted in a significantly decreased number of migrated cells compared to control RNA (Figure 3B). Testing of additional lung adenocarcinoma cells also showed reduced cell migration with increased miR-576–3p levels (Supplemental Figure 3A).
Figure 3. miR-576–3p alters mesenchymal marker expression and inhibits migration and invasion.
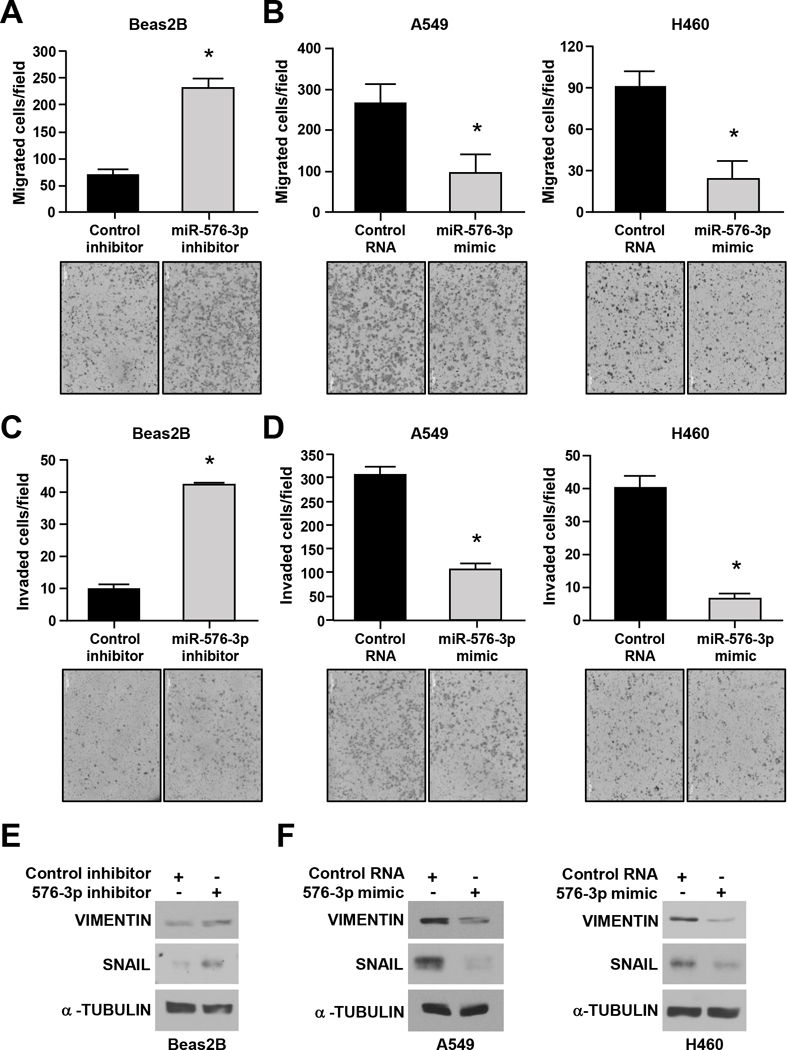
The indicated cell lines were transfected with the miR-576–3p inhibitor (A, C, E), miR-576–3p mimic (B, D, F), or control. (A-D) Transwell migration and invasion assays were performed; SEM; A, *P=9.7×10−3; B, A549, *P=0.028; H460, *P=2.0×10−3; C, *P=1.7×10−3; D, A549, *P=2.5×10−3; H460, *P=1.6×10−4; two-tailed t-test. Representative images shown (10×). (E, F) Western blots for the indicated proteins were performed. Data are representative of at least three independent experiments.
Since miR-576–3p inhibits lung adenocarcinoma cell migration, we then evaluated whether it would influence cell invasion. Invasion assays performed using matrigel transwell chambers with inhibition of miR-576–3p in non-malignant Beas2B cells resulted in an increased number of invading cells compared to inhibitor control cells (Figure 3C). Upon miR-576–3p overexpression, there was a significant reduction in the number of invading lung adenocarcinoma cells (Figure 3D and Supplemental Figure 3B). These results show that miR-576–3p inhibits cell migration and invasion and that lower levels induce cell migration and invasion.
A cellular process linked to migration and invasion is EMT, which occurs when epithelial cells upregulate mesenchymal markers (e.g., VIMENTIN, SNAIL) and gain migratory and invasive properties24. We tested the effects of altered miR-576–3p levels on EMT markers in non-malignant and malignant lung cell lines. Inhibition of miR-576–3p in Beas2B cells resulted in increased expression of the mesenchymal markers SNAIL and VIMENTIN (Figure 3E). Overexpression of miR-576–3p in A549 and H460 cells led to decreased expression of these mesenchymal markers compared to controls (Figure 3F). These data indicate that markers associated with cell movement are altered by changing miR-576–3p levels, and correlate with changes in migration and invasion.
SGK1 is a direct target of miR-576–3p.
To study the molecular mechanism by which miR-576–3p affects migration and invasion, we utilized publically available databases (TargetScan, mirPath, TargetMiner) to identify potential targets that impact these biological processes. We identified a potential miR-576–3p binding site in the 3’-UTR of the human SGK1 gene in all three databases (Figure 4A). SGK1 has previously been linked to regulation of cell motility and lung cancer cell migration25, 26. To test whether SGK1 was a novel target of miR-576–3p, we performed a series of experiments. We first assessed direct binding of miR-576–3p to the 3’-UTR of SGK1 using a luciferase reporter assay. Luciferase activity was significantly decreased in cells transfected with miR-576–3p or miR-133b, a miRNA previously reported to bind the 3’-UTR of SGK127 (Figure 4B). Luciferase activity was unaltered in cells transfected with the control RNA or miR-31, which is not predicted to bind any sequence within the SGK1 3’-UTR. These results indicate SGK1 is a direct target of miR-576–3p.
Figure 4. SGK1 is a direct target of miR-576–3p.
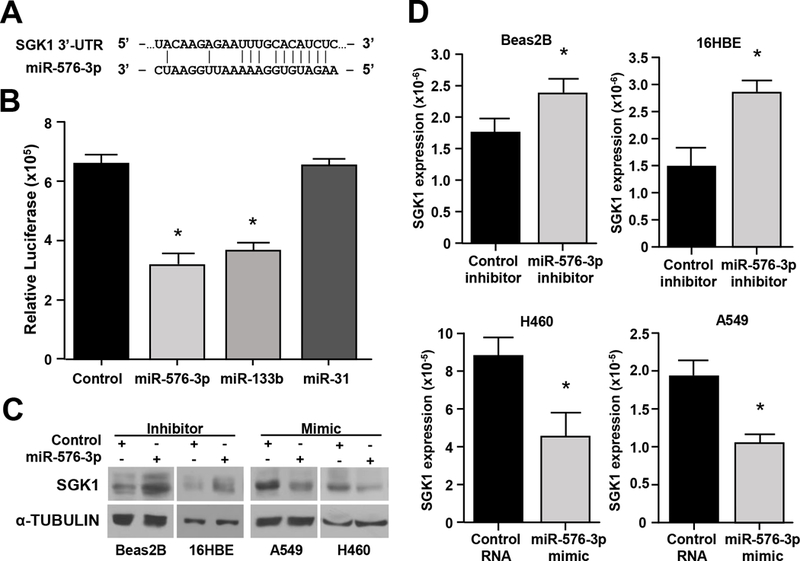
(A) Schematic of the SGK1 3’-UTR binding site for miR-576–3p. (B) Luciferase activity (triplicates) was measured after transfection of the indicated miRNA mimic or control RNA into 293T cells; SEM; miR-576–3p, *P=8.8×10−4; miR-133b, *P=5.9×10−4, two-tailed t-test. miR-133b and miR-31 mimics served as positive and negative controls, respectively. (C, D) Western blots (C) and qRT-PCR (triplicate, D) were performed with cells transfected with miR-576–3p mimic, miR-576–3p inhibitor, or controls; SEM; Beas2B, *P=0.021; 16HBE, *P=8.7×10−3; H460, *P=8.3×10−3; A549, *P=2.4×10−3, two-tailed t-test. Data are representative of three independent experiments.
We next assessed whether miR-576–3p affected expression of SGK1. Inhibition of miR-576–3p in Beas2B and 16HBE cells increased SGK1 protein levels (Figure 4C). Overexpression of miR-576–3p in A549 and H460 lines resulted in a decrease of SGK1 protein levels (Figure 4C). Because miRNA frequently also lead to reductions in the mRNA they target, we assessed the effect of miR-576–3p on SGK1 mRNA levels. Inhibition of miR-576–3p in Beas2B and 16HBE cells significantly increased SGK1 mRNA levels (Figure 4D). Overexpression of miR-576–3p in A549 and H460 cells resulted in a significant decrease in SGK1 mRNA in both cell lines (Figure 4D). Therefore, SGK1 is a direct target of miR-576–3p and altering miR-576–3p expression results in changes of SGK1 protein and mRNA levels.
miR-576–3p regulation of SGK1 is necessary for inhibition of migration and invasion.
To determine whether miR-576–3p targeting SGK1 caused the observed changes in invasion and migration, we generated a vector encoding SGK1 lacking its 3’-UTR. H460 cells containing SGK1 with a deleted 3’-UTR did not show a reduction in SGK1 protein following transfection of miR-576–3p mimic (Figure 5A), whereas cells with vector control did. Additionally, miR-576–3p overexpression inhibited H460 migration and invasion in the vector control cells (Figure 5B and 5C). However, overexpression of miR-576–3p in the cells expressing SGK1 with the deleted 3’-UTR were still able to migrate and invade (Figure 5B and 5C). Therefore, miR-576–3p inhibits migration and invasion, at least in part, through direct targeting of SGK1.
Figure 5. Deletion of the miR-576–3p binding site in SGK1 rescues the inhibition of migration and invasion from miR-576–3p.
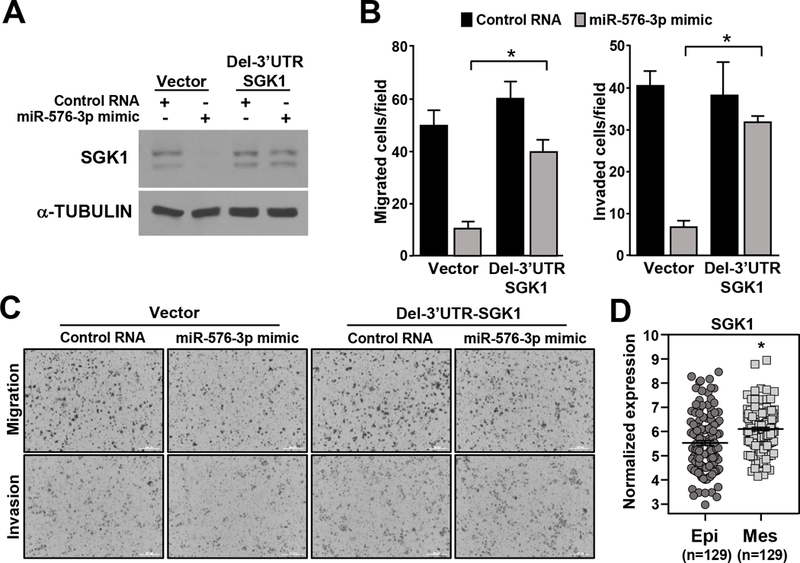
H460 cells infected with an empty retrovirus or a retrovirus expressing SGK1 lacking its 3’-UTR (Del-3’UTR SGK1) were transfected with miR-576–3p mimic or control RNA. (A) Western blots were performed for the proteins indicated. (B) Transwell migration and invasion assays were performed; SEM; migration, *P=8.9×10−6; invasion, *P=1.6×10−7 two-tailed t-test. (C) Representative images of B (10×). Data are representative of three independent experiments. (D) SGK1 expression in TCGA RNA-seq data divided into mesenchymal and epithelial subtypes, *P<1.43 ×10−5.
Increased SGK1 levels in lung adenocarcinoma patient samples expressing mesenchymal markers.
Because our data indicate miR-576–3p regulates migration and invasion of lung epithelial cells through SGK1 and these cellular processes are linked to EMT, we assessed SGK1 levels in lung adenocarcinoma patient samples that have undergone EMT. Lung adenocarcinoma samples from TCGA were separated into mesenchymal and epithelial subtypes based on gene expression. SGK1 expression was then assessed in both subtypes. SGK1 levels were significantly higher in patient lung adenocarcinoma samples expressing a mesenchymal gene signature (Figure 5D). These results indicate SGK1 levels are higher in lung adenocarcinoma cells that have an increased ability to migrate and invade.
Downstream signaling of SGK1 is affected by changes in miR-576–3p.
Our data show that miR-576–3p targets SGK1, and SGK1 is a kinase that contributes to metastasis by phosphorylating downstream targets, such as NDRG128. NDRG1 has been implicated as a suppressor of metastasis, and its ability to inhibit metastasis is regulated by phosphorylation29. We evaluated SGK1 activity by assessing phosphorylation of NDRG1 at threonine-346 by Western blot. As would be predicted for miR-576–3p targeting SGK1, inhibition of miR-576–3p in Beas2B cells resulted in an increase in phospho-NDRG1, and overexpression of miR-576–3p in A549 cells led to a decrease in phospho-NDRG1 (Figure 6A). These data indicate downstream signaling of SGK1 is altered with changes in miR-576–3p levels, which provides further evidence for miR-576–3p targeting SGK1.
Figure 6. Pharmacological SGK inhibition prevents downstream target phosphorylation and cell migration and invasion.
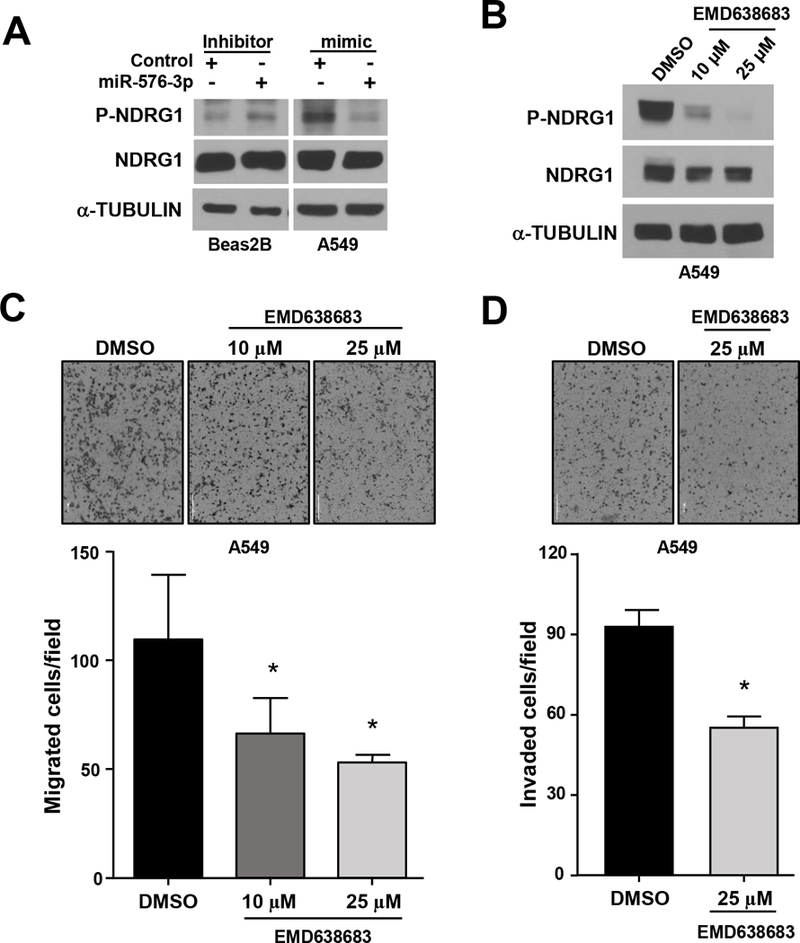
(A) The indicated cells were transfected with miR-576–3p mimic, inhibitor, or controls. Phospho-NDRG1 (Thr346) and total NDRG1 levels were determined by Western blot. (B-D) A549 cells were treated with vehicle control (DMSO) or with EMD638683 at the concentrations indicated. Western blots (B) and transwell migration (C) and invasion (D) assays performed (representative images shown, 4×); SEM; C, 10μM *P=0.045, 25μM *P=0.025; D, *P=5.4×10−5, two-tailed t-test. Data are representative of three independent experiments.
Pharmacologically inhibiting SGK1 reduces migratory and invasive capabilities of lung adenocarcinoma cells.
Given that SGK1 has a critical role in regulating metastasis25, 26, we tested the effects of SGK1 inhibition in lung adenocarcinoma cells separate from its regulation by miR-576–3p. We pharmacologically inhibited SGK1 activity with the SGK inhibitor EMD63868330. Treatment of lung adenocarcinoma cells with EMD638683 resulted in an inhibition of SGK1 activity, as detected by a dose-dependent decrease of phospho-NDRG1 (Figure 6B and Supplemental Figure 4A). Moreover, lung adenocarcinoma cells treated with EMD638683 had significantly reduced migration (Figure 6C and Supplemental Figure 4B). The decrease in migration was dose dependent with an increased concentration inhibiting migration to a greater extent. Invasion of lung adenocarcinoma cells was also significantly decreased with EMD638683 treatment (Figure 6D and Supplemental Figure 4C). Thus, similar to what is observed with miR-576–3p overexpression, pharmacologically inhibiting SGK1 decreased downstream signaling of SGK1, as well as migration and invasion. Together, the data indicate targeting of SGK1 by miR-576–3p or pharmacologically is inhibitory to lung adenocarcinoma migratory and invasive capabilities.
DISCUSSION
The contributions of miRNA in the molecular mechanisms of lung tumor progression have not been fully elucidated. In this study, we identified a mechanism that contributes to lung adenocarcinoma cell migration and invasion, cellular processes that are crucial for tumor metastasis. Utilizing patient samples, we determined miR-576–3p was significantly downregulated in late-stage lung adenocarcinoma, suggesting miR-576–3p may have an inhibitory role in tumor progression. In support of this concept, our data show miR-576–3p inhibited migration and invasion of lung adenocarcinoma cells through direct targeting of SGK1, a gene previously linked to regulation of cell motility and lung cancer cell migration25, 26. Our data further showed that SGK1 expression was significantly increased in lung adenocarcinoma patient samples that contained a mesenchymal gene signature. Our results on miR576–3p and SGK1 provide new insight into a mechanism exploited by lung cancer cells to metastasize.
miRNA have been linked to all stages of lung cancer, including initiation and progression3. Our evaluation of miRNA in lung adenocarcinoma patient samples and cell lines revealed miR-576–3p was significantly downregulated compared to normal lung tissue and immortalized bronchial epithelial cells. Little was known about miR-576–3p prior to our study. Previously, expression of miR-576–3p was reported reduced in bladder cancer22, 23. Moreover, lower levels of miR-576–3p were associated with advanced disease and lymph node metastasis in bladder cancer and correlated with poor overall survival in bladder cancer patients22. Our data showed that miR-576–3p was significantly downregulated in late-stage (stages III and IV) lung adenocarcinoma, but not early stages of disease, suggesting it has a preferential function in inhibiting cellular processes involved in late-stage lung cancer. Overexpression of miR-576–3p decreased the ability of lung adenocarcinoma cells to migrate and invade, whereas inhibition of miR-576–3p increased cell migration and invasion. Additionally, overexpression of miR-576–3p in lung adenocarcinoma cells reduced the expression of mesenchymal markers associated with EMT and cell movement, whereas inhibition of miR-576–3p increased their expression. Together, the data indicate that miR-576–3p downregulation is a prerequisite to both lung adenocarcinoma and bladder cancer progression.
In bladder cancer, miR-576–3p was reported to target cyclin D1 and alter proliferation10, but how this would impact cancer cell movement is unclear. In contrast, we detected no changes in proliferation in lung adenocarcinoma or bronchial epithelial cells with modulation of miR-576–3p. Instead, our data show that miR-576–3p exerted its effects on cancer cell migration and invasion through direct targeting of SGK1. SGK1 is a component of the PI3K pathway, whose signaling is linked to cellular proliferation, metabolism, and migration31. PI3K inhibitors have been developed and tested as potential cancer therapy for many different malignancies32, and one, BYL719, is currently in clinical trials for treatment of solid tumors33, 34. Resistance to the PI3K inhibitor, BYL719, is reported to be mediated through SGK1 signaling34. SGK1 shares homology with the AKT family and has been understudied in cancer. There are three members of the SGK family, including SGK1, SGK2 and SGK331. All three SGK family members have been implicated in cell movement26, 35–37, but SGK1 is the only member that contains a miR-576–3p binding site in its 3’-UTR. Our data show miR-576–3p directly targets SGK1, leading to a decrease in SGK1 protein and RNA and inhibition of lung adenocarcinoma migration and invasion. Specificity of miR-576–3p for SGK1 and its effects on cell movement were determined, in part, by deleting the miR-576–3p binding site in the 3’-UTR of SGK1. Loss of the miR-576–3p binding site in SGK1 prevented miR-576–3p from downregulating SGK1 and inhibiting lung adenocarcinoma cell migration and invasion. We obtained additional evidence that the mechanism of miR-576–3p inhibition of cell movement was through SGK1 by evaluating a downstream target of SGK1, NDRG1. NDRG1 was previously reported to be a target of the SGK family28 and a known suppressor of metastasis in multiple cancers29, 38. We determined that modulation of miR-576–3p levels altered phosphorylation of NDRG1 at Thr346, an SGK1 phosphorylation site28. Therefore, our data reveal that miR-576–3p targets SGK1 to prevent cell movement, and lung adenocarcinoma cells downregulate miR-576–3p to facilitate migration and invasion. Moreover, our data link increased SGK1 expression with a mesenchymal gene signature in patient samples, providing additional evidence that SGK1 contributes to lung cancer migratory and invasive capabilities.
SGK1 inhibition has been proposed as a potential therapy for colorectal cancer39. Given our results that miR-576–3p targets SGK1 in lung adenocarcinoma, we used the SGK1 inhibitor EMD638683 to inhibit its activity in lung cancer cells. We determined this SGK1 inhibitor leads to a decrease in NDRG1 phosphorylation, as well as a decrease in lung adenocarcinoma migration and invasion. Although additional tests and significant optimization of an SGK inhibitor would be needed, our data indicate SGK1 inhibition may be an effective approach to inhibit lung adenocarcinoma metastasis and have therapeutic potential for lung adenocarcinoma patients.
Supplementary Material
IMPLICATIONS.
This study reveals SGK1 inhibition with miR-576–3p or pharmacologically inhibits migration and invasion of lung adenocarcinoma, providing mechanistic insights into late stage lung adenocarcinoma and a new treatment avenue.
ACKNOWLEDGEMENTS
The authors thank Drs. David Carbone and Rosana Eisenberg for lung patient samples and the members of the Eischen lab for helpful suggestions. These studies were supported by NCI grant R01CA177786 (CME), NCI Cancer Center support grant P30CA056036 and the Sidney Kimmel Cancer Center.
Financial support: These studies were supported by NCI grant R01CA177786 (CME), NCI Cancer Center support grant P30CA056036, and the Sidney Kimmel Cancer Center.
Footnotes
Conflict of interest: The authors declare no conflicts of interest.
REFERENCES
- 1.Siegel RL, Miller KD, and Jemal A, Cancer statistics, 2018. CA Cancer J Clin 2018;68:7–30. [DOI] [PubMed] [Google Scholar]
- 2.Peng Y and Croce CM, The role of MicroRNAs in human cancer. Signal Transduct Target Ther 2016;1:15004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Inamura K and Ishikawa Y, MicroRNA In Lung Cancer: Novel Biomarkers and Potential Tools for Treatment. J Clin Med 2016;5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Legras A, Pecuchet N, Imbeaud S, Pallier K, Didelot A, Roussel H, et al. , Epithelial-to-Mesenchymal Transition and MicroRNAs in Lung Cancer. Cancers (Basel) 2017;9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Edmonds MD, Boyd KL, Moyo T, Mitra R, Duszynski R, Arrate MP, et al. , MicroRNA-31 initiates lung tumorigenesis and promotes mutant KRAS-driven lung cancer. J Clin Invest 2016;126:349–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.He L, He X, Lim LP, de Stanchina E, Xuan Z, Liang Y, et al. , A microRNA component of the p53 tumour suppressor network. Nature 2007;447:1130–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bommer GT, Gerin I, Feng Y, Kaczorowski AJ, Kuick R, Love RE, et al. , p53-mediated activation of miRNA34 candidate tumor-suppressor genes. Curr Biol 2007;17:1298–307. [DOI] [PubMed] [Google Scholar]
- 8.Kasinski AL and Slack FJ, miRNA-34 prevents cancer initiation and progression in a therapeutically resistant K-ras and p53-induced mouse model of lung adenocarcinoma. Cancer Res 2012;72:5576–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shi ZM, Wang L, Shen H, Jiang CF, Ge X, Li DM, et al. , Downregulation of miR-218 contributes to epithelial-mesenchymal transition and tumor metastasis in lung cancer by targeting Slug/ZEB2 signaling. Oncogene 2017;36:2577–2588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liang Z, Li S, Xu X, Xu X, Wang X, Wu J, et al. , MicroRNA-576–3p inhibits proliferation in bladder cancer cells by targeting cyclin D1. Mol Cells 2015;38:130–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ureshino H, Murakami Y, Watari K, Izumi H, Kawahara A, Kage M, et al. , N-myc downstream regulated gene 1 (NDRG1) promotes metastasis of human scirrhous gastric cancer cells through epithelial mesenchymal transition. PLoS One 2012;7:e41312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hu ZY, Xie WB, Yang F, Xiao LW, Wang XY, Chen SY, et al. , NDRG1 attenuates epithelial-mesenchymal transition of nasopharyngeal cancer cells via blocking Smad2 signaling. Biochim Biophys Acta 2015;1852:1876–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Grieb BC, Gramling MW, Arrate MP, Chen X, Beauparlant SL, Haines DS, et al. , Oncogenic protein MTBP interacts with MYC to promote tumorigenesis. Cancer Res 2014;74:3591–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Edmonds MD and Eischen CM, Differences in miRNA expression in early stage lung adenocarcinomas that did and did not relapse. PLoS One 2014;9:e101802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McGirt LY, Adams CM, Baerenwald DA, Zwerner JP, Zic JA, and Eischen CM, miR-223 regulates cell growth and targets proto-oncogenes in mycosis fungoides/cutaneous T-cell lymphoma. J Invest Dermatol 2014;134:1101–1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang P, Lushnikova T, Odvody J, Greiner TC, Jones SN, and Eischen CM, Elevated Mdm2 expression induces chromosomal instability and confers a survival and growth advantage to B cells. Oncogene 2008;27:1590–8. [DOI] [PubMed] [Google Scholar]
- 17.Mitra R, Chen X, Greenawalt EJ, Maulik U, Jiang W, Zhao Z, et al. , Decoding critical long non-coding RNA in ovarian cancer epithelial-to-mesenchymal transition. Nat Commun 2017;8:1604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gramling MW and Eischen CM, Suppression of Ras/Mapk pathway signaling inhibits Myc-induced lymphomagenesis. Cell Death Differ 2012;19:1220–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vaira V, Faversani A, Martin NM, Garlick DS, Ferrero S, Nosotti M, et al. , Regulation of lung cancer metastasis by Klf4-Numb-like signaling. Cancer Res 2013;73:2695–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang S, Zhu L, Zuo W, Zeng Z, Huang L, Lin F, et al. , MicroRNA-mediated epigenetic targeting of Survivin significantly enhances the antitumor activity of paclitaxel against non-small cell lung cancer. Oncotarget 2016;7:37693–37713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chi Y, Jin Q, Liu X, Xu L, He X, Shen Y, et al. , miR-203 inhibits cell proliferation, invasion, and migration of non-small-cell lung cancer by downregulating RGS17. Cancer Sci 2017;108:2366–2372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Meng FM, Meng FM, and Song XL, MiR-576–3p is a novel marker correlated with poor clinical outcome in bladder cancer. Eur Rev Med Pharmacol Sci 2017;21:973–977. [PubMed] [Google Scholar]
- 23.Tatarano S, Chiyomaru T, Kawakami K, Enokida H, Yoshino H, Hidaka H, et al. , miR-218 on the genomic loss region of chromosome 4p15.31 functions as a tumor suppressor in bladder cancer. Int J Oncol 2011;39:13–21. [DOI] [PubMed] [Google Scholar]
- 24.Brabletz T, Kalluri R, Nieto MA, and Weinberg RA, EMT in cancer. Nat Rev Cancer 2018;18:128–134. [DOI] [PubMed] [Google Scholar]
- 25.Schmidt EM, Gu S, Anagnostopoulou V, Alevizopoulos K, Foller M, Lang F, et al. , Serum- and glucocorticoid-dependent kinase-1-induced cell migration is dependent on vinculin and regulated by the membrane androgen receptor. FEBS J 2012;279:1231–42. [DOI] [PubMed] [Google Scholar]
- 26.Xiaobo Y, Qiang L, Xiong Q, Zheng R, Jianhua Z, Zhifeng L, et al. , Serum and glucocorticoid kinase 1 promoted the growth and migration of non-small cell lung cancer cells. Gene 2016;576:339–46. [DOI] [PubMed] [Google Scholar]
- 27.Kong C, Sun L, Zhang M, Ding L, Zhang Q, Cheng X, et al. , miR-133b Reverses the Hydrosalpinx-induced Impairment of Embryo Attachment Through Down-regulation of SGK1. J Clin Endocrinol Metab 2016;101:1478–89. [DOI] [PubMed] [Google Scholar]
- 28.Murray JT, Campbell DG, Morrice N, Auld GC, Shpiro N, Marquez R, et al. , Exploitation of KESTREL to identify NDRG family members as physiological substrates for SGK1 and GSK3. Biochem J 2004;384:477–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fang BA, Kovacevic Z, Park KC, Kalinowski DS, Jansson PJ, Lane DJ, et al. , Molecular functions of the iron-regulated metastasis suppressor, NDRG1, and its potential as a molecular target for cancer therapy. Biochim Biophys Acta 2014;1845:1–19. [DOI] [PubMed] [Google Scholar]
- 30.Ackermann TF, Boini KM, Beier N, Scholz W, Fuchss T, and Lang F, EMD638683, a novel SGK inhibitor with antihypertensive potency. Cell Physiol Biochem 2011;28:137–46. [DOI] [PubMed] [Google Scholar]
- 31.Di Cristofano A, SGK1: The Dark Side of PI3K Signaling. Curr Top Dev Biol 2017;123:49–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fruman DA, Chiu H, Hopkins BD, Bagrodia S, Cantley LC, and Abraham RT, The PI3K Pathway in Human Disease. Cell 2017;170:605–635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Juric D, Rodon J, Tabernero J, Janku F, Burris HA, Schellens JHM, et al. , Phosphatidylinositol 3-Kinase alpha-Selective Inhibition With Alpelisib (BYL719) in PIK3CA-Altered Solid Tumors: Results From the First-in-Human Study. J Clin Oncol 2018:JCO2017727107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Castel P, Ellis H, Bago R, Toska E, Razavi P, Carmona FJ, et al. , PDK1-SGK1 Signaling Sustains AKT-Independent mTORC1 Activation and Confers Resistance to PI3Kalpha Inhibition. Cancer Cell 2016;30:229–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tian S, Wang X, and Proud CG, Oncogenic MNK signalling regulates the metastasis suppressor NDRG1. Oncotarget 2017;8:46121–46135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liu J, Zhang G, Lv Y, Zhang X, Ying C, Yang S, et al. , SGK2 promotes hepatocellular carcinoma progression and mediates GSK-3beta/beta-catenin signaling in HCC cells. Tumour Biol 2017;39:1010428317700408. [DOI] [PubMed] [Google Scholar]
- 37.Schmid E, Bhandaru M, Nurbaeva MK, Yang W, Szteyn K, Russo A, et al. , SGK3 regulates Ca(2+) entry and migration of dendritic cells. Cell Physiol Biochem 2012;30:1423–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bandyopadhyay S, Pai SK, Gross SC, Hirota S, Hosobe S, Miura K, et al. , The Drg-1 gene suppresses tumor metastasis in prostate cancer. Cancer Res 2003;63:1731–6. [PubMed] [Google Scholar]
- 39.Liang X, Lan C, Jiao G, Fu W, Long X, An Y, et al. , Therapeutic inhibition of SGK1 suppresses colorectal cancer. Exp Mol Med 2017;49:e399. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


