Abstract
Tryptophan catabolism is an attractive target for reducing tumor progression and improving antitumor immunity in multiple cancers. Tumor infiltration by CD8 T cells correlates with improved prognosis in triple-negative breast cancer (TNBC) and a significant effort is underway to improve CD8 T cell antitumor activity. In this study, primary human immune cells were isolated from the peripheral blood of patients and used to demonstrate that the tryptophan catabolite kynurenine induces CD8 T cell death. Furthermore, it is demonstrated that anchorage-independent TNBC utilizes the tryptophan catabolizing enzyme tryptophan 2,3-dioxygenase (TDO) to inhibit CD8 T cell viability. Publicly available data revealed that high TDO2, the gene encoding TDO, correlates with poor breast cancer clinical outcomes, including overall survival and distant metastasis-free survival, while expression of the gene encoding the more commonly studied tryptophan catabolizing enzyme IDO1 did not. Metabolomic analysis, using quantitative mass spectrometry, of tryptophan and its catabolites - including kynurenine - in the plasma from pre-surgical breast cancer patients (n=77) and 40 cancer-free donors (n=40) indicated a strong correlation between substrate and catabolite in both groups. Interestingly, both tryptophan and kynurenine were lower in the plasma from breast cancer patients compared to controls, particularly in women with estrogen receptor (ER)-negative and stage-3 and −4 breast cancer.
Keywords: TDO2, breast cancer, kynurenine, T cell, immunotherapy
Introduction
Triple-negative breast cancer (TNBC) is an aggressive disease that lacks FDA-approved targeted therapies for the majority of patients (1). Importantly, TNBCs with high levels of immune infiltrate have more favorable prognoses (2, 3). Specifically, a high ratio of tumor-infiltrating cytotoxic CD8 T cells to immunosuppressive FoxP3+ T regulatory cells correlates with improved breast cancer-specific survival in chemoresistant TNBC (4). Indeed, TNBC has high expression of programmed cell death 1-ligand 1 (PD-L1) compared to other breast cancer subtypes, suggestive of evasion from immune attack (5–7). Active clinical trials are investigating the efficacy of targeting the PD-1/PD-L1 axis and other immune-directed interventions as treatments for TNBC; however, results in TNBC patients treated with the anti-PD-1 agent pembrolizumab indicate promising but limited efficacy (8, 9).
We previously discovered that aberrant expression and activity of the hepatic tryptophan-catabolizing enzyme tryptophan-2,3-dioxygenase (TDO) in TNBC cell lines supports anchorage-independent cell growth and late-stage metastasis through an autocrine mechanism, whereby the tryptophan catabolite kynurenine activates the aryl-hydrocarbon receptor (AhR) in the breast cancer cells (10). However, it is clear that kynurenine also has suppressive paracrine effects on immune cells (11, 12). Most studies have focused on the tryptophan catabolizing enzyme indoleamine-2,3-dioxygenase (IDO) in cancer, and current inhibitors of this pathway in clinical trials only target IDO activity. IDO transcript correlates with poor prognosis and increased microvessel density in breast cancer (13). However, TDO2 (the gene encoding TDO), is significantly higher in estrogen receptor (ER) negative (–) compared to ER+ breast tumors and correlates with overall survival in breast cancer patients, suggesting a potential failure of current tryptophan catabolizing agents in effectively targeting tumor cell enzymatic activity (10). Importantly, recent negative clinical trial results in melanoma (the ECHO-301 trial) and pancreatic cancer (ECHO-203), suggest that IDO inhibitors are not sufficient for the reversal of tumor immune evasion (14) and indicate that it may be necessary to target TDO as well.
Alterations in blood tryptophan and kynurenine levels have been examined in multiple human cancers (15–17). Lower serum tryptophan correlates with poor prognosis in melanoma, consistent with an increase in tryptophan catabolism (17). A small-scale study showed that plasma from post-surgical (lumpectomy or mastectomy) breast cancer patients has reduced tryptophan and elevated kynurenine compared to cancer-free controls, as measured by ultra-high pressure liquid chromatography (UHPLC), but importantly this study may not have been able to measure any potential effects of the primary tumor on systemic levels of these molecules (15). Another breast cancer study found relatively higher intratumoral kynurenine in ER-versus ER+ tumors (16). The issue of whether decreased tryptophan or increased kynurenine levels in breast cancer patient plasma reflect the activity of tumor tryptophan catabolizing enzymes remains unresolved.
Here, we demonstrate that kynurenine increased primary human CD8 T cell death and that conditioned media from anchorage-independent TNBC cells suppressed primary human CD8 T cell function in a TDO-dependent manner, suggesting a mechanism of enhanced immune evasion during metastasis. Furthermore, we quantified tryptophan and kynurenine – as well as a series of tryptophan catabolites – in plasma from 77 pre-surgical breast cancer patients using UHPLC-MS and detected significant differences in plasma from women with breast cancer versus no breast cancer, ER+ versus negative tumors, and with increasing disease stage.
Materials and Methods
Cell Culture
BT549 cells, purchased from ATCC in 2008 and authenticated prior to use by Short Tandem Repeat DNA Profiling (Promega), were grown in RPMI Medium 1640 with 10% fetal bovine serum (FBS), penicillin/streptomycin (P/S) and insulin supplementation. Suspension culture was achieved using plates coated with 12 mg/mL poly(2-hydroxyethyl methacrylate) (Sigma-Aldrich) reconstituted in 95% ethanol. Ethanol evaporated overnight, and plates were washed with PBS prior to use.
Primary Human T Cell Isolation and Activation
Blood was collected by venipuncture into tubes with heparin (BD Biosciences Vacutainer Systems) from donors with written informed consent under a University of Colorado Anschutz Medical Campus Colorado Institutional Review Board (COMIRB)-approved protocol. Lymphocytes were isolated by adding 10 mL of Ficoll-Paque PLUS (GE Healthcare) per 20 mL of blood diluted in Hanks’ Balanced Salt Solution, then centrifuging at 2000 rpm for 25 minutes without deceleration. CD8 T cells were resuspended in PBS containing 0.5% BSA and 2 mM EDTA for selection using positive isolation kits (Dynabeads, Invitrogen). After isolation, cells were labeled with carboxyfluorescein succinimidyl ester (CFSE, Invitrogen) according to manufacturer’s instructions, then resuspended in RPMI medium containing 10% FBS, P/S, non-essential amino acids, sodium pyruvate, and HEPES. T cells were activated in plates coated with 0.5 μg/mL of CD3 antibody (clone OKT3, eBioscience) and 1.0 μg/mL of soluble CD28 antibody (clone CD28.2, eBioscience) for 5 days. For intracellular staining, T cells were stimulated for 4 hours after the activation using phorbol-12-myristate-13-acetate (PMA) at 20 ng/mL, ionomycin at 1 μg/mL, and GolgiStop (BD Biosciences).
Cell Staining and Flow Cytometry
T cells were stained with antibodies for CD3 (clone HIT3a, Biolegend) and CD8 (clone RPA-T8, Biolegend) at a 1:100 dilution and an intracellular stain for interferon gamma (clone 4S.B3, eBioscience) at a 1:50 dilution. Intracellular staining was achieved using an intracellular fixation and permeabilization buffer set (eBioscience). Dead cells were detected by positivity for a viability dye according to manufacturer’s instructions (Fixable Viability Dye BV421, eBioscience). Flow cytometry was conducted using an LSR II (BD Biosciences) and data were analyzed using FlowJo software (Tree Star, Inc).
TDO2 and AhR Inhibition
The TDO2 inhibitor 680C91 (Sigma-Aldrich) or the AhR antagonist CH-223191 (TOCRIS Bioscience) prepared in dimethyl sulfoxide (DMSO) were used.
Breast Cancer Patient Plasma
Blood and was acquired as described above from pre-surgical breast cancer patients with written informed consent under a COMIRB and HRPO approved protocol (COMIRB 15–2225 and HRPO A-18613). The characteristics of the cohort are described in Supplementary Table 1.
UHPLC-MS Targeted Metabolomic Analysis of Tryptophan Catabolism
Tryptophan (15N2) and kynurenine (ring-D4, 3,3-D2) were purchased from Cambridge Isotope Laboratories, Incorporated. To ensure linearity over 5 orders of magnitude, lysis buffer (LB), a 5:3:2 ratio of MeOH:acetonitrile:H2O, and 0.1% formic acid aliquots were each spiked with varying ranges of heavy-labeled tryptophan and kynurenine (50 μM, 5 μM, 500 nM, 50 nM, 5 nM, 500 pM) and analyzed by ultra-high pressure liquid chromatography coupled to mass spectrometry – UHPLC-MS. Plasma (20 μL) was extracted at 1:5 and 1:10 in LB containing 5 μM heavy labeled tryptophan and kynurenine as previously described, then diluted 1:5, 1:10 and 1:20 with H2O and run on UHPLC-MS (18, 19). Sample extracts were analyzed via UHPLC-MS (Vanquish, Q Exactive – Thermo Fisher, Bremen, Germany) using C18 reverse-phase chromatography and positive electrospray ionization (ESI) (18, 20). A Kinetex C18 column, 2.1× ×150 mm, 1.7 μm particle size (Phenomenex) was used and equipped with a C18 guard column (Phenomenex). The method is a variant of previously published methods (21). Samples were resolved at 45°C with a gradient elution over 4 minutes, flowing at 450 μL/min. Mobile phase A is 0.1% formic acid in water, mobile phase B is 0.1% formic acid in acetonitrile. 5% B and 95% A is held from 0.00–0.50 minutes. From 0.50–1.10 minutes, B increases to 95% B and 5% A. This condition is held from 1.10 – 2.75 minutes. From 2.75–3.00 minutes A decreases to the initial condition of 5% B and 95% A and is held from 3.00–4.00 minutes. The Q Exactive mass spectrometer was operated in positive ion mode using electrospray ionization, scanning in Full MS mode (1 μscan) from 100 to 1500 m/z at 70,000 resolution, with 4 kV spray voltage, 45 sheath gas,15 auxiliary gas. Calibration was performed prior to analysis using the Pierce Positive Ion Calibration Solution (Thermo Fisher Scientific). Metabolite assignments and heavy isotopologue detection for absolute quantitation against internal standards were determined against in house standard libraries and KEGG database searches through Maven (22). Technical reproducibility was assessed by monitoring internal heavy labeled standard mixes as reported (18). Calculation of absolute quantification for measured metabolites was performed using the following formula: [light] = (abundance light) / (abundance heavy) * [heavy] (dilution factor) where dilution factor is 10 for an extraction of 10 μL plasma in 90 μL lysis buffer.
Statistical Analyses
Prism Graphpad Version 7.02 was used for all statistical analyses. All tests are two-sided with statistical significance set at p<0.05. Statistical tests and sample sizes are described in the figure legends. *p<0.05, **p<0.01, ***p<0.001, ****p<0.0001.
Results
Primary human CD8 T cell response to kynurenine and cancer cell conditioned media
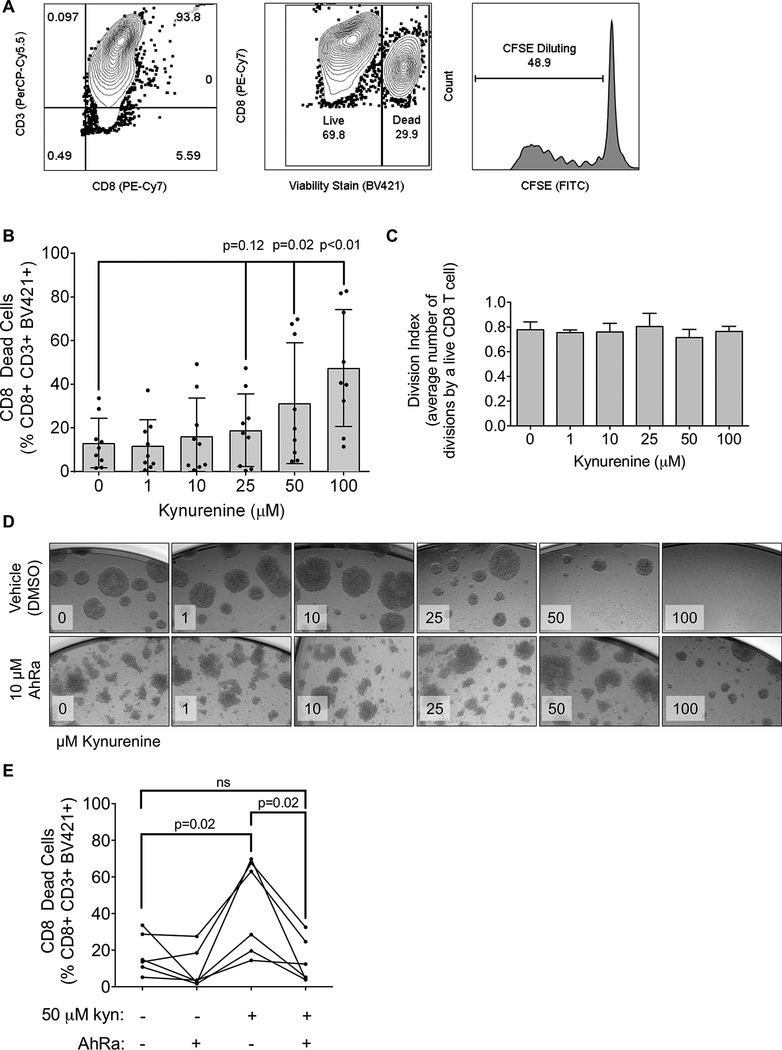
Infiltration into breast tumors by cytotoxic CD8 T cells correlates with improved prognosis in ER-breast cancer (4, 23–25). To test the effect of kynurenine on primary human peripheral blood cytotoxic T cells, CD8+ cells isolated from peripheral blood of healthy human donors (Figure 1A) were treated with increasing doses of kynurenine over a 5-day T cell receptor (TCR) activation protocol. At 50 and 100 μM kynurenine, CD8 T cell death increased as measured by a fixable viability dye (Figure 1B), while CFSE analysis of live CD8 T cells indicated no change in proliferation (Figure 1C), demonstrating a direct impact of kynurenine specifically on human CD8 T cell viability. To test whether kynurenine acts through AhR to affect CD8 T cell viability, CD8 T cells were treated with increasing doses of kynurenine with or without the AhR antagonist CH-223191 (26). Treatment with CH-223191 significantly reduced the cytotoxic effect of 50 μM kynurenine (Figure 1D-E), indicating that kynurenine acts in part on CD8 T cells through AhR.
Figure 1: Increased kynurenine leads to primary human CD8 T cell death, which is reversed by an AhR antagonist.
(A) Representative example of gating strategy for flow cytometric analysis: CD8 T cells were gated based on double positivity for CD8 (PE-Cy7) and CD3 (PerCP-Cy5.5). Cell death was determined by positive staining for the fixable viability stain BV421. Proliferation was measured by CFSE dilution. (B) Cells were activated with CD3 and CD28 antibodies for 5 days in the indicated concentration of kynurenine and CD8 T cell death was determined. Each dot represents cells from one human donor. N=9 donors, mean with standard deviation, one-way ANOVA. (C) Cells were treated with kynurenine as described in (B) and assayed for CD8 T cell proliferation. N=9 donors, one-way ANOVA. (D) Representative images (10× magnification) of CD8 T cells on activation Day 5 that were treated with indicated concentrations of kynurenine and either 10 μM of the AhR antagonist CH-223191 (AhRa) or DMSO. (E) CD8 T cells were activated, treated with 10 μM AhRa or vehicle control (DMSO), and assayed for cell death. N=6, mean with standard deviation, paired t-tests.
We previously reported that TNBC cells surviving under anchorage-independent culture conditions increased expression and activity of TDO, resulting in increased intracellular and secreted kynurenine (10). In the present study, we asked whether such anchorage-independent cells have enhanced immune evasion. BT549 TNBC cells were cultured in attached (ATT) or suspended (SUS) conditions for 24 or 48 hours. Primary human CD8 T cells were cultured in these conditioned media (CM) with CD3 and CD28 antibodies for 5 days. CD8 T cells cultured in SUS CM were less viable than those cultured in ATT CM (Figure 2A). Since kynurenine decreased interferon-gamma (IFNγ) mRNA in murine cytotoxic T cells (27), we tested whether CM from attached versus suspended TNBC cells would affect primary human CD8 T cell production of IFNγ. Indeed, the SUS CM significantly reduced IFNγ production compared to ATT CM (Figure 2B and 2C). To determine if the effects of SUS CM were due to increased TDO activity, TNBC cells were plated in ATT or SUS conditions with or without TDO-specific inhibitor 680C91 for 48 hours. While SUS CM again increased CD8 T cell death, this effect was significantly abrogated when SUS cells were treated with TDO inhibitor 680C91 (Figure 2D), suggesting that TDO activity is at least partially responsible for the CM effect. However, treating the CD8 T cells with the AhR antagonist CH-223191 did not rescue the effect of SUS CM, suggesting additional effects of TNBC TDO2 activity on the CD8 T cells (Figure 2E).
Figure 2: The effect of conditioned media from suspended TNBC cells on primary human CD8 T cells is similar to that of purified kynurenine, and is reversed by TDO2 inhibitor 680C91.
(A) Conditioned media was collected from BT549 TNBC cells grown in attached (ATT) or suspended (SUS) culture conditions for either 24 or 48 hours as indicated. CD8 T cells were isolated from the blood of normal donors and activated for 5 days with CD3 and CD28 antibodies in the conditioned media. Each line represents the response of CD8 T cells from one donor. Cell death was measured as described in Figures 1A and B. N=9, paired t-tests. (B) Representative flow cytometric analysis of CD8 T cells for IFNγ production is shown. (C) IFNγ production by CD8 T cells cultured in conditioned media for 48 hours. N=7, paired t-tests. (D) CD8 T cells were activated in conditioned media and either 0.1 μM 680C91 (to inhibit TDO activity) or vehicle control (DMSO). Each line represents the response of CD8 T cells from one human volunteer donor. N=5, paired t-tests. (E) CD8 T cells were activated in conditioned media and treated with either 10 μM CH-223191 (to inhibit AhR activation) or vehicle control (DMSO). N=5, paired t-tests.
TDO2 RNA expression correlates with breast cancer outcomes, and tryptophan catabolism is altered in breast cancer
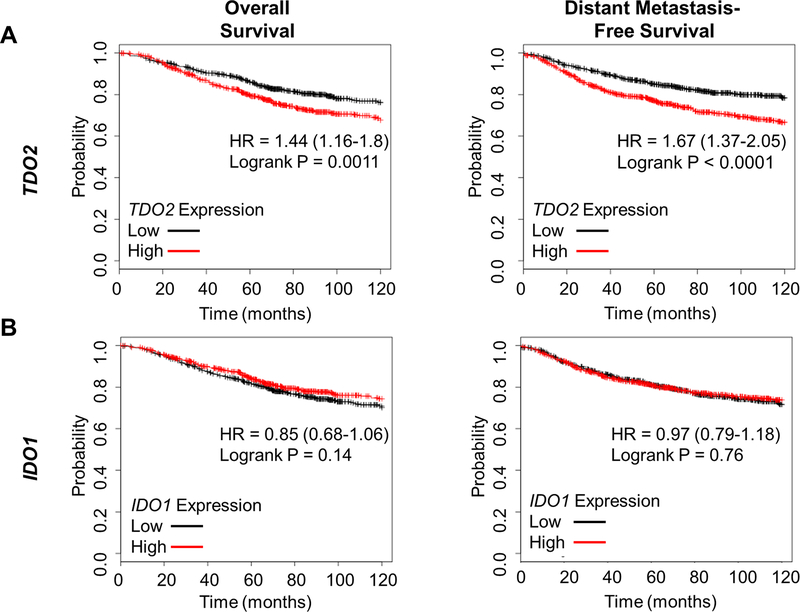
We previously reported that TDO2 is significantly higher in breast cancer versus normal breast tissue and in ER-negative versus ER-positive disease, and that above-median TDO2 correlated with poor overall survival in the Curtis et al dataset (a dataset containing gene expression analysis from 2000 breast tumors, extracted through the data-mining platform Oncomine) (10, 28). In the current study, we used the KM Plotter tool to investigate breast cancer outcomes associated with both TDO2 and IDO1 expression (29). High TDO2 in primary breast cancer correlated with worse overall survival and reduced distant metastasis-free survival (Figure 3A), while IDO1 did not (Figure 3B). Consistent with our findings that breast cancer cells have elevated tryptophan catabolism during anchorage independence, we also found, using the Curtis et al dataset (28), that the gene encoding the amino acid transporter LAT1 (SLCA5) (which uptakes tryptophan and other large neutral amino acids from the microenvironment into the cell) was significantly elevated in breast cancer versus normal breast (Supplemental Figure 1A), in ER-negative versus ER-positive disease (Supplemental Figure 1B), and in higher grade breast cancer (Supplemental Figure 1C). We also found using the Curtis et al dataset and KM Plotter tool that above-median LAT1 was associated with worse overall (Supplemental Figures 1D-E) as well as distant metastasis-free (Supplemental Figure 1F) survival as compared to below-median.
Figure 3: TDO2 expression correlates with breast cancer outcomes, while expression of the more commonly studied IDO1 does not.
(A) Patients were split into TDO2-high (red, n=699) or TDO2–low (black, n=703) groups based on median TDO2 gene expression. Overall survival (left) and distant metastasis-free survival (right) was plotted using KmPlot.com. (B) Patients were split into IDO1-high (red, n=701) or IDO1–low (black, n=701) groups based on median gene expression, and overall survival and distant-metastasis free survival was plotted. N=1402, hazard ratios are given with 95% confidence intervals, logrank test.
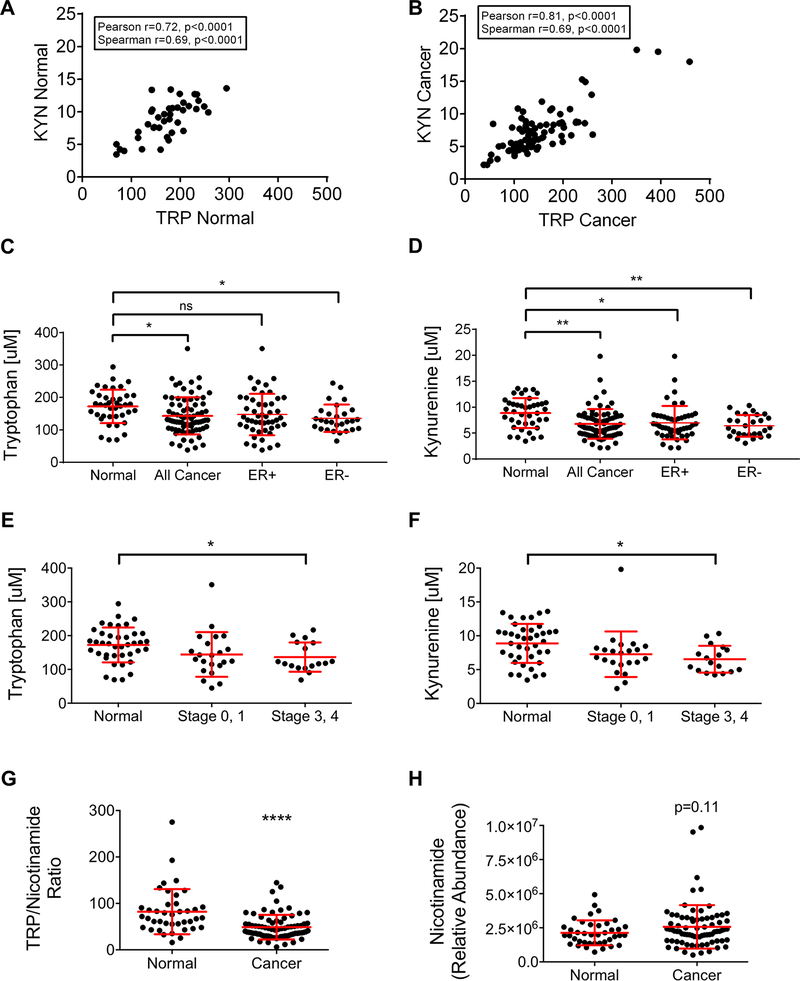
To further understand how tryptophan catabolism is altered in breast cancer subtypes, we measured plasma concentrations of tryptophan and its main catabolites, including kynurenine using UHPLC-MS (Supplementary Table 1). In the plasma from cancer-free “normal” women, the concentration of tryptophan ranged from 69.50–294.49 μM, while in women with breast cancer the range was 38.04–260.77 μM. Meanwhile, the plasma concentration of kynurenine in normal women ranged from 3.46–13.61 μM, and from 2.19–19.83 μM in women with breast cancer. Strikingly, there was a significant positive correlation between tryptophan and kynurenine both in plasma from normal women (Figure 4A) and women with breast cancer (Figure 4B). A comparison of the plasma concentrations of tryptophan and kynurenine in women with breast cancer versus normal controls demonstrated that women with breast cancer had significantly reduced tryptophan (mean = 141.39 μM) compared to normal (mean = 173.32 μM) (Figure 4C). Interestingly and surprisingly, the concentration of kynurenine was also significantly lower in the plasma of women with breast cancer (mean = 7.12 μM) compared to normal controls (mean = 9.02 μM) (Figure 4D).
Figure 4: Tryptophan and kynurenine are reduced in breast cancer plasma.
Ultra-high performance liquid chromatography/mass spectrometry was used to measure tryptophan and kynurenine concentrations and nicotinamide abundance in the plasma from normal women and women with breast cancer. The concentrations of tryptophan and kynurenine correlate significantly and positively in both normal (A) (n=40) and breast cancer patient (B) (n=77) plasma by Pearson and Spearman tests. Comparison of tryptophan (C) and kynurenine (D) concentrations in plasma from normal women, all breast cancer patients in the cohort, and ER+ versus ER-breast cancer patients. Mean with standard deviation, *p<0.05, **p<0.01, one-way ANOVA and Tukey’s multiple comparisons test. Comparison of tryptophan (E) and kynurenine (F) concentrations plotted by breast cancer stage is shown. Mean with standard deviation, one-way ANOVA and Tukey’s multiple comparisons test. (G) Comparison of the ratio of tryptophan (TRP) to nicotinamide between plasma from normal women and women with breast cancer. Mean with standard deviation, **** p<0.0001, unpaired t-test. (H) Comparison of the relative abundance of nicotinamide in the plasma from normal and breast cancer patient plasma. Mean with standard deviation, p=0.11, unpaired t-test.
Given previous reports that TDO2 is significantly higher in ER-versus ER+ disease, the plasma tryptophan and kynurenine concentrations in women with ER- and ER+ breast cancer were next compared (10). There was no significant difference in either plasma tryptophan or kynurenine in women with ER+ and ER-disease (Figure 4C-D), though lower levels of tryptophan and anthranilate were observed in plasma from women with ER-, HER2-disease (Supplemental Figure 2). However, ER-breast cancer patients had significantly reduced plasma tryptophan compared to normal controls, while ER+ breast cancer patients did not (Figure 4C). Plasma kynurenine from both ER- and ER+ breast cancer subsets was significantly lower than that from normal controls (Figure 4D). Of note, these decreases were more marked in women with non-pregnancy-associated breast cancer (PABC) in comparison to PABC patients (where PABC is defined as a diagnosis with 5 years of pregnancy), with the highest relative levels of tryptophan (and its catabolites hydroxytryptophan, kynurenine and kynurenic acid) detected in normal women who did not give birth within the past 5 years. Interestingly, women without a cancer diagnosis (normal), who had not given birth within the past five years, had intermediate levels of tryptophan and its catabolites in between the levels observed in normal women who had given birth within the past five years and PABC/non-PABC patients (Supplementary Figure 3).
To examine whether either plasma tryptophan or kynurenine correlated with breast cancer aggressiveness, breast cancer stage at diagnosis was used. Both tryptophan (Figure 4E) and kynurenine (Figure 4F) were significantly lower in stage 3/4 breast cancer patient plasma than in plasma from normal controls, while there was no difference in either tryptophan or kynurenine when comparing stage 0/1 patients versus normal (Figure 4E-F). Interestingly, there was also a decrease in the kynurenine catabolite kynurenic acid in stage 3/4 breast cancer patients compared to patients with stage 1/2 disease (Supplementary Figure 4).
Our finding that both tryptophan and kynurenine were reduced in the plasma of breast cancer patients versus normal controls led us to investigate whether there were differences in nicotinamide, the final metabolite in the kynurenine pathway of tryptophan catabolism, between breast cancer patient and normal plasma. To do this, we calculated the ratio of tryptophan/nicotinamide in our dataset. We found that the tryptophan/nicotinamide ratio was significantly lower in the plasma from breast cancer patients compared to normal controls, suggesting a potential overall increase in tryptophan catabolism not limited to the conversion of tryptophan to kynurenine (Figure 4G). While the average relative abundance of nicotinamide was slightly elevated in breast cancer patient plasma compared to controls, this difference was not statistically significant (Figure 4H). However, the ratio, which represents each individual’s substrate (tryptophan) versus metabolite, is likely more meaningful given the person to person variability.
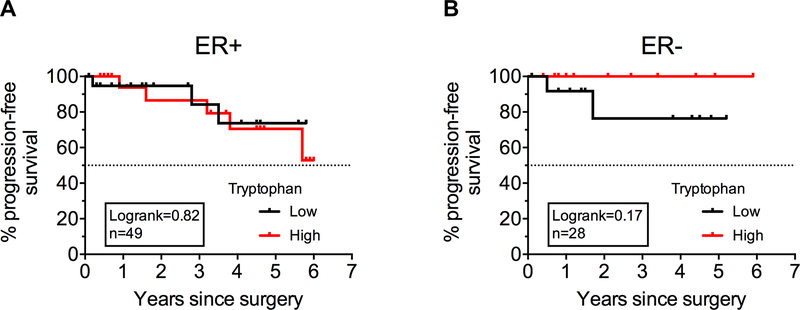
To examine whether tryptophan catabolism correlated with outcomes in this breast cancer data set, we divided the patient data into tryptophan-low and tryptophan-high groups based on the median concentration of plasma tryptophan, and compared the progression-free survival of these groups, where progression was defined as local, regional, or distant recurrence, or patient death. While there was no significant difference in progression-free survival between these groups in breast cancer patients with either ER+ (Figure 5A) or ER- (Figure 5B) disease, there was a trend which did not reach statistical significance towards patients with lower than median plasma tryptophan having poorer outcomes (Figure 5B), consistent with a negative impact of reduced tryptophan in ER-disease.
Figure 5: Breast cancer patient plasma tryptophan and progression-free survival.
Ultra-high performance liquid chromatography/mass spectrometry was used to measure tryptophan concentrations in the plasma from women with breast cancer. Kaplan-Meyer curves plotting progression-free survival of breast cancer patients with ER+ disease (n=49) (A) or ER-disease (n=28) (B) are shown, where progression was defined by local, regional, or distant recurrence, or patient death in the years after surgery. Logrank test.
Discussion
While tryptophan catabolism is known to mediate tumor immune evasion, the impact of IDO or TDO activity on CD8 T cells was thought to occur through indirect suppression via regulatory T cell activation. We demonstrate here that the direct impact of kynurenine on primary human CD8 T cells is to reduce viability in the setting of activation through the T cell receptor. We previously reported that TDO activity increased in TNBC cell lines under anchorage-independent conditions, resulting in increased intracellular and secreted kynurenine (10). Critically, we show here that conditioned media from TNBC cells cultured under anchorage-independent conditions decreases the viability and IFNγ production of primary human CD8 T cells, and that this effect is abrogated by the specific TDO inhibitor 680C91, indicating that anchorage-independent TNBC cells are highly immunosuppressive in part through TDO-mediated activity. A high ratio of cytotoxic CD8 T cells to Treg cells correlates with improved clinical outcomes in TNBC (30, 31). Thus, the power of TDO-positive metastatic TNBC cells to suppress CD8 T cells both directly and indirectly via the induction of Tregs (12) could critically contribute to disease progression. Interestingly, the concentration of kynurenine in the conditioned media was lower than the concentration of purified kynurenine that reduced CD8 T cell viability, a phenomenon which could be explained by the milieu of other factors that are secreted by suspended TNBC cells which might have immune-modulatory effects (10).
Although inhibition of TDO activity in suspended TNBC cells abrogated the effects of conditioned media on the viability and function of CD8 cells, inhibition of the receptor for kynurenine, AhR, in the CD8 cells was not sufficient to mitigate this effect. One possible explanation for the differential effects of TDO as compared to AhR inhibition is that the effect of TDO activity on CD8 T cells is due to the combined effects of both tryptophan depletion and kynurenine secretion. Kynurenines combined with tryptophan depletion downregulates the TCR zeta-chain and decreases cytokine production in murine CD8 T cells (27), and IDO-mediated tryptophan depletion inhibits mTOR and PKC-θ activation to induce murine T cell anergy and autophagy (32). AhR antagonism would interfere with kynurenine-mediated AhR activation, but would not abrogate tryptophan depletion.
To our knowledge, the present study includes the largest metabolomic analysis of tryptophan and kynurenine in plasma from pre-surgery breast cancer patients, allowing assessment of the impact of an existing breast tumor on systemic tryptophan and kynurenine levels. Given that others have reported higher kynurenine and lower tryptophan levels in breast cancer patients compared to healthy donors (15), our finding of a strong maintenance of balanced levels of substrate to catabolite in plasma from both breast cancer patients and cancer-free donors was unexpected. This may be attributable to the strong ability of the kidneys to handle increased kynurenine and it is documented that increased TDO/IDO activity does not necessarily result in increased plasma kynurenine (33, 34). Interestingly, we found that compared to healthy donors, tryptophan was only significantly lower in the plasma of patients with ER-negative tumors. This finding suggests a particularly important role of elevated tumor tryptophan catabolism in ER-negative disease. An expanded follow-up metabolomic study that includes more patients with ER- and stage 3/4 disease is imperative to determine if plasma tryptophan and kynurenine reflect tumoral enzymatic activity and correlate with outcome. Although currently limited by lack of quality antibodies for IHC, it is likely that measurement of TDO/IDO protein levels or activity in primary tumor and/or circulating tumor cells would be more informative than plasma levels of tryptophan and kynurenine. Additionally, levels of the enzymes in tumors should be correlated with tumor-infiltrating lymphocyte composition in future studies. It is possible that further flux through the kynurenine pathway in tumor cells could account for the comparatively low kynurenine concentration in plasma from women with breast cancer. In a previous study, we found that expression of KYNU, the gene encoding kynureninase (an enzyme downstream of TDO) is elevated in suspended TNBC cell lines and in the context of increased inflammatory signaling (10), which would be consistent with tryptophan catabolism further down the pathway. Indeed, our finding that the tryptophan/nicotinamide ratio was significantly reduced in breast cancer patient plasma compared to controls is consistent with the possibility of an overall increase in tryptophan catabolic activity not limited to the initial step in the pathway. Metabolomic tracing of tryptophan could help elucidate the ultimate fate of this amino acid in tumors. Development of better antibodies to measure TDO and IDO or gauge activity will be a significant hurdle to overcome in the quest for biomarkers indicative of patients who may benefit from tryptophan catabolism-targeted therapies.
Supplementary Material
Implications.
Implications: This study underscores the importance of tryptophan catabolism, particularly in aggressive disease, and suggests that future pharmacological efforts should focus on developing drugs that target both TDO and IDO1.
Acknowledgements
The authors wish to thank the University of Colorado School of Medicine Biological Mass Spectrometry Core Facility for their contributions to this manuscript. We also acknowledge the shared resources of the University of Colorado Cancer Center NCI Support Grant (P30CA046934), particularly the Tissue Culture Core. We thank Emily Rozzo and Michelle Borakove for assistance in patient sample collection and analysis guidance. Finally, we thank the blood donors that made these studies possible.
Financial Support
DOD BCRP Award Number W81XWH-15–1-0039 to JKR and NRSA F31 Award Number CA203486–01A1 to LIG.
Footnotes
Conflict of Interest Disclosure Statement
The authors declare no conflicts of interest.
References
- 1.Liedtke C, Mazouni C, Hess KR, Andre F, Tordai A, Mejia JA, et al. Response to neoadjuvant therapy and long-term survival in patients with triple-negative breast cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2008;26(8):1275–81. [DOI] [PubMed] [Google Scholar]
- 2.Aaltomaa S, Lipponen P, Eskelinen M, Kosma VM, Marin S, Alhava E, et al. Lymphocyte infiltrates as a prognostic variable in female breast cancer. Eur J Cancer. 1992;28A(4–5):859–64. [DOI] [PubMed] [Google Scholar]
- 3.Denkert C, von Minckwitz G, Brase JC, Sinn BV, Gade S, Kronenwett R, et al. Tumor-infiltrating lymphocytes and response to neoadjuvant chemotherapy with or without carboplatin in human epidermal growth factor receptor 2-positive and triple-negative primary breast cancers. J Clin Oncol. 2015;33(9):983–91. [DOI] [PubMed] [Google Scholar]
- 4.Miyashita M, Sasano H, Tamaki K, Hirakawa H, Takahashi Y, Nakagawa S, et al. Prognostic significance of tumor-infiltrating CD8+ and FOXP3+ lymphocytes in residual tumors and alterations in these parameters after neoadjuvant chemotherapy in triple-negative breast cancer: a retrospective multicenter study. Breast cancer research : BCR. 2015;17:124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ali HR, Glont SE, Blows FM, Provenzano E, Dawson SJ, Liu B, et al. PD-L1 protein expression in breast cancer is rare, enriched in basal-like tumours and associated with infiltrating lymphocytes. Ann Oncol. 2015;26(7):1488–93. [DOI] [PubMed] [Google Scholar]
- 6.Mittendorf EA, Philips AV, Meric-Bernstam F, Qiao N, Wu Y, Harrington S, et al. PD-L1 expression in triple-negative breast cancer. Cancer Immunol Res. 2014;2(4):361–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sabatier R, Finetti P, Mamessier E, Adelaide J, Chaffanet M, Ali HR, et al. Prognostic and predictive value of PDL1 expression in breast cancer. Oncotarget. 2015;6(7):5449–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nanda R, Chow LQ, Dees EC, Berger R, Gupta S, Geva R, et al. Pembrolizumab in Patients With Advanced Triple-Negative Breast Cancer: Phase Ib KEYNOTE-012 Study. J Clin Oncol. 2016;34(21):2460–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kwa MJ, Adams S. Checkpoint inhibitors in triple-negative breast cancer (TNBC): Where to go from here. Cancer. 2018. [DOI] [PubMed] [Google Scholar]
- 10.D’Amato NC, Rogers TJ, Gordon MA, Greene LI, Cochrane DR, Spoelstra NS, et al. A TDO2-AhR signaling axis facilitates anoikis resistance and metastasis in triple-negative breast cancer. Cancer Res. 2015;75(21):4651–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Opitz CA, Litzenburger UM, Sahm F, Ott M, Tritschler I, Trump S, et al. An endogenous tumour-promoting ligand of the human aryl hydrocarbon receptor. Nature. 2011;478(7368):197–203. [DOI] [PubMed] [Google Scholar]
- 12.Mezrich JD, Fechner JH, Zhang X, Johnson BP, Burlingham WJ, Bradfield CA. An interaction between kynurenine and the aryl hydrocarbon receptor can generate regulatory T cells. J Immunol. 2010;185(6):3190–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wei L, Zhu S, Li M, Li F, Wei F, Liu J, et al. High Indoleamine 2,3-Dioxygenase Is Correlated With Microvessel Density and Worse Prognosis in Breast Cancer. Frontiers in immunology. 2018;9:724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Companies Scaling Back IDO1 Inhibitor Trials. Cancer discovery. 2018. [DOI] [PubMed] [Google Scholar]
- 15.Lyon DE, Walter JM, Starkweather AR, Schubert CM, McCain NL. Tryptophan degradation in women with breast cancer: a pilot study. BMC Res Notes. 2011;4:156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tang X, Lin CC, Spasojevic I, Iversen ES, Chi JT, Marks JR. A joint analysis of metabolomics and genetics of breast cancer. Breast cancer research : BCR. 2014;16(4):415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Weinlich G, Murr C, Richardsen L, Winkler C, Fuchs D. Decreased serum tryptophan concentration predicts poor prognosis in malignant melanoma patients. Dermatology. 2007;214(1):8–14. [DOI] [PubMed] [Google Scholar]
- 18.D’Alessandro A, Nemkov T, Yoshida T, Bordbar A, Palsson BO, Hansen KC. Citrate metabolism in red blood cells stored in additive solution-3. Transfusion. 2017;57(2):325–36. [DOI] [PubMed] [Google Scholar]
- 19.Nemkov T, Hansen KC, D’Alessandro A. A three-minute method for high-throughput quantitative metabolomics and quantitative tracing experiments of central carbon and nitrogen pathways. Rapid Commun Mass Spectrom. 2017;31(8):663–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Clasquin MF, Melamud E, Rabinowitz JD. LC-MS data processing with MAVEN: a metabolomic analysis and visualization engine. Curr Protoc Bioinformatics. 2012;Chapter 14:Unit14 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.D’Alessandro A, Nemkov T, Hansen KC, Szczepiorkowski ZM, Dumont LJ. Red blood cell storage in additive solution-7 preserves energy and redox metabolism: a metabolomics approach. Transfusion. 2015;55(12):2955–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gray N, Lewis MR, Plumb RS, Wilson ID, Nicholson JK. High-Throughput Microbore UPLC-MS Metabolic Phenotyping of Urine for Large-Scale Epidemiology Studies. J Proteome Res. 2015;14(6):2714–21. [DOI] [PubMed] [Google Scholar]
- 23.Mahmoud SM, Paish EC, Powe DG, Macmillan RD, Grainge MJ, Lee AH, et al. Tumor-infiltrating CD8+ lymphocytes predict clinical outcome in breast cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2011;29(15):1949–55. [DOI] [PubMed] [Google Scholar]
- 24.Ali HR, Provenzano E, Dawson SJ, Blows FM, Liu B, Shah M, et al. Association between CD8+ T-cell infiltration and breast cancer survival in 12,439 patients. Ann Oncol. 2014;25(8):1536–43. [DOI] [PubMed] [Google Scholar]
- 25.Liu F, Lang R, Zhao J, Zhang X, Pringle GA, Fan Y, et al. CD8(+) cytotoxic T cell and FOXP3(+) regulatory T cell infiltration in relation to breast cancer survival and molecular subtypes. Breast Cancer Res Treat. 2011;130(2):645–55. [DOI] [PubMed] [Google Scholar]
- 26.Kim SH, Henry EC, Kim DK, Kim YH, Shin KJ, Han MS, et al. Novel compound 2-methyl-2H-pyrazole-3-carboxylic acid (2-methyl-4-o-tolylazo-phenyl)-amide (CH-223191) prevents 2,3,7,8-TCDD-induced toxicity by antagonizing the aryl hydrocarbon receptor. Mol Pharmacol. 2006;69(6):1871–8. [DOI] [PubMed] [Google Scholar]
- 27.Fallarino F, Grohmann U, You S, McGrath BC, Cavener DR, Vacca C, et al. The combined effects of tryptophan starvation and tryptophan catabolites down-regulate T cell receptor zeta-chain and induce a regulatory phenotype in naive T cells. J Immunol. 2006;176(11):6752–61. [DOI] [PubMed] [Google Scholar]
- 28.Curtis C, Shah SP, Chin SF, Turashvili G, Rueda OM, Dunning MJ, et al. The genomic and transcriptomic architecture of 2,000 breast tumours reveals novel subgroups. Nature. 2012;486(7403):346–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Szasz AM, Lanczky A, Nagy A, Forster S, Hark K, Green JE, et al. Cross-validation of survival associated biomarkers in gastric cancer using transcriptomic data of 1,065 patients. Oncotarget. 2016;7(31):49322–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Asano Y, Kashiwagi S, Goto W, Kurata K, Noda S, Takashima T, et al. Tumour-infiltrating CD8 to FOXP3 lymphocyte ratio in predicting treatment responses to neoadjuvant chemotherapy of aggressive breast cancer. Br J Surg. 2016;103(7):845–54. [DOI] [PubMed] [Google Scholar]
- 31.Miyashita M, Sasano H, Tamaki K, Chan M, Hirakawa H, Suzuki A, et al. Tumor-infiltrating CD8+ and FOXP3+ lymphocytes in triple-negative breast cancer: its correlation with pathological complete response to neoadjuvant chemotherapy. Breast Cancer Res Treat. 2014;148(3):525–34. [DOI] [PubMed] [Google Scholar]
- 32.Metz R, Rust S, Duhadaway JB, Mautino MR, Munn DH, Vahanian NN, et al. IDO inhibits a tryptophan sufficiency signal that stimulates mTOR: A novel IDO effector pathway targeted by D-1-methyl-tryptophan. Oncoimmunology. 2012;1(9):1460–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kolodziej LR, Paleolog EM, Williams RO. Kynurenine metabolism in health and disease. Amino Acids. 2011;41(5):1173–83. [DOI] [PubMed] [Google Scholar]
- 34.Takikawa O, Yoshida R, Kido R, Hayaishi O. Tryptophan degradation in mice initiated by indoleamine 2,3-dioxygenase. J Biol Chem. 1986;261(8):3648–53. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.