Abstract
Anxiety disorders are associated with enhanced error-related negativity (ERN) across development but it remains unclear whether alterations in brain electrophysiology are linked to the timing of puberty. Pubertal timing and alterations of prefrontal and limbic development are implicated in risk for depression, but the interplay of these factors on the ERN-anxiety association has not been assessed. We examined the unique and interactive effects of pubertal timing and depression on the ERN in a sample of youth 10–19 years old with anxiety disorders (n=30) or no history of psychopathology (n=30). Earlier pubertal maturation was associated with an enhanced ERN. Amongst early, but not late maturing youth, higher depressive symptoms were associated with a reduced ERN. The magnitude of neural reactivity to errors is sensitive to anxiety, depression, and development. Early physical maturation and anxiety may heighten neural sensitivity to errors yet predict opposing effects in the context of depression.
Keywords: pediatric anxiety, error-related negativity, puberty, depression, EEG
The ability to self-detect errors and dynamically adjust goal-directed behavior is one critical aspect of adaptive cognitive development (Tamnes, Walhovd, Torstveit, Sells, & Fjell, 2013). The neural basis of error monitoring is observable in scalp-recorded electroencephalogram (EEG) as a fronto-central negativity for error versus correct responses, termed the error-related negativity (ERN, (Falkenstein, Hohnsbein, Hoormann, & Blanke, 1990; Gehring, Goss, Coles, Meyer, & Donchin, 1993). The ERN was initially localized primarily to the anterior cingulate cortex (van Veen & Carter, 2002), a region of the brain where information about pain, threat, and punishment is integrated to change behavior (Shackman et al., 2011). Additionally, more recent work suggests that activity within the posterior cingulate cortex (Buzzell et al., 2017), superior/medial prefrontal cortex (Hochman, Eviatar, Breznitz, Nevat, & Shaul, 2009; Holmes & Pizzagalli, 2008), and insular cortex (Czobor et al., 2017), which operate within a fronto-limbic network involved in emotional salience and attention, also provide a substantial contribution to the scalp-recorded ERN. An enhanced ERN is postulated to be involved in conflict monitoring (Yeung, Botvinick, & Cohen, 2004), increased sensitivity to endogenous threat (Weinberg, Meyer, et al., 2016), and reinforcement learning (Holroyd & Coles, 2002). These cognitive processes have shown relevance to the etiology of anxiety disorders (Moser, Moran, Schroder, Donnellan, & Yeung, 2013; Weinberg, Meyer, et al., 2016), and indeed, a more negative ERN has consistently differentiated adults with heterogeneous anxiety disorders from healthy counterparts across over 40 studies, with an overall effect size of r = 0.35 (Cavanagh & Shackman, 2015; Moser et al., 2013).
Increasingly, an enhanced ERN is also observable in clinically anxious youth, including those with obsessive compulsive disorder (OCD) (Carrasco, Harbin, et al., 2013; Carrasco, Hong, et al., 2013; Hajcak, Franklin, Foa, & Simons, 2008; Hanna et al., 2012), social anxiety disorder (Kujawa, Weinberg, et al., 2016), and other mixed anxiety disorders (Ladouceur, Dahl, Birmaher, Axelson, & Ryan, 2006; Meyer, Hajcak, Glenn, Kujawa, & Klein, 2017; Meyer et al., 2013). Several studies report an association between anxiety severity and increases in the ERN (Bress, Meyer, & Hajcak, 2015; Meyer, Weinberg, Klein, & Hajcak, 2012; Santesso, Segalowitz, & Schmidt, 2006; Weinberg, Meyer, et al., 2016), though this linear relationship has not been consistently observed in clinical samples (Carrasco, Hong, et al., 2013; Hajcak et al., 2008; Hanna et al., 2012; Ladouceur et al., 2006). Variability in the anxiety-ERN association may in part, be related to co-morbid depression, which has been purported to have an opposing effect on the ERN (Weinberg, Klein, & Hajcak, 2012; Weinberg, Kotov, & Proudfit, 2015). In support of this, a blunted ERN has been reported in relation to depressive symptoms among youth (Weinberg, Meyer, et al., 2016) and in offspring of mothers with recurrent depression (Meyer, Bress, Hajcak, & Gibb, 2016). Of note, in adult studies, however, the effects of depression are quite mixed, with reduced (Olvet, Klein, & Hajcak, 2010; Weinberg et al., 2012), equivocal (Schoenberg, 2014; Weinberg et al., 2015), and even larger ERN’s reported (Chiu & Deldin, 2007; Holmes & Pizzagalli, 2010).
Although both anxiety and depression are related to alterations in the ERN amongst youth, it is less clear whether anxiety and depression relate to or interact with developmental changes in the error-monitoring system. Not all studies include a wide enough age range to chart the ERN across childhood, adolescence, and adulthood (Lo et al., 2016; Meyer et al., 2013; Santesso et al., 2006). Yet, others have reported interactions between age and symptoms of anxiety and depression (Meyer et al., 2012; Weinberg, Meyer, et al., 2016). For instance, Hanna et al (2012) found that the ERN increased prematurely in youth with OCD relative to controls (Hanna et al., 2012). By contrast, Ladouceur et al (2012) found that youth with major depression did not exhibit the expected increase in ERN amplitudes as a function of age (Ladouceur et al., 2012). Thus, while youth likely undergo changes in the ERN across development, further work is needed to specify how development interacts with dimensions of psychopathology.
In particular, one important aspect of development likely to have direct implications for brain development and possible downstream influence on risk for psychopathology is the hormonal changes associated with puberty, as both puberty ratings and sex hormones have shown a relationship with the ERN (Gorday & Meyer, 2018). Puberty is a process that is marked by reproductive maturation, via elevated secretion of gonadal steroid hormones (Sisk & Zehr, 2005). Although the brain is a target organ for gonadal steroid hormones throughout life, the adolescent brain is particularly sensitive to these exposures (Sisk, 2017). Therefore, puberty may partly account for changes in brain structure and function that source localization studies have also implicated in the ERN. For instance, changes in pubertal sex hormones across late childhood to early adolescence have been linked to grey matter density in the anterior cingulate and prefrontal cortex (Brouwer et al., 2015), as well as increased functional activation in these areas (Op de Macks et al., 2016). Additionally, pubertal stage has been associated with volume changes in subcortical brain areas involved in salience and decision-making (Goddings et al., 2014). Moreover, the effects of pubertal status on the brain have been shown to interact with age (Goddings et al., 2014), suggesting that the timing with which the brain is exposed to gonadal hormones has the potential to produce time-sensitive neural development and behavioral maturation (Blakemore, Burnett, & Dahl, 2010). Accordingly, there may be value and precision that is gained through assessing the impact of pubertal timing on neural development - that is, the effect of puberty that is not explained by age (Blakemore et al., 2010).
Pubertal timing may be a particularly relevant measure of development in clinical samples because rates of depression (Alloy, Hamilton, Hamlat, & Abramson, 2016) and anxiety (Graber, 2013) increase during puberty, for which early developing youth are at highest risk. The underlying causes for this may relate to physiological and hormonal changes during puberty that alter neural circuits directly (Buchanan, Eccles, & Becker, 1992; Schulz, Molenda-Figueira, & Sisk, 2009). Alternatively, the physiological changes associated with early puberty may alter an adolescent’s social experiences during puberty, and in turn, increase risk for psychopathology (Graber, 2013). It is also possible that the neural and social changes associated with early pubertal development may moderate endogenous threat sensitivity in a way that predisposes anxious youth to depression (Silk, Davis, McMakin, Dahl, & Forbes, 2012). However, the impact of pubertal timing on ERN-anxiety and ERN-depression associations has not yet been examined.
Accordingly, this study sought to address the relationships between the ERN, anxiety, and depression in a developmental context. We examined the impact of pubertal timing and depressive symptoms on the ERN in youth with heterogeneous anxiety disorders (AD) and healthy controls (HC). We hypothesized that AD youth would exhibit an enhanced ERN and that the ERN would be larger amongst early developing youth. We also hypothesized that by contrast youth with greater depressive symptoms would exhibit a blunted ERN. We expected that depression would moderate the effects of anxiety and pubertal timing, such that high levels of depression would be associated with a reduced ERN only amongst early developing, anxious youth.
Methods
Participants
Participants were youth recruited through the University of Michigan and University of Illinois at Chicago. Patients were recruited through outpatient clinics at each university and healthy controls were recruited through the surrounding communities (Bunford et al., 2017; Burkhouse et al., 2017; Kujawa, MacNamara, Fitzgerald, Monk, & Phan, 2015; Kujawa, Swain, et al., 2016; Kujawa, Weinberg, et al., 2016). We have previously published ERN data from this sample of youth and a sample of young adults before and after treatment for anxiety (Kujawa, Weinberg, et al., 2016). A total of 60 participants between the ages of 10–19 (n = 30 AD youth with a current diagnosis of social, separation, or generalized anxiety disorder and n = 30 HC with no psychiatric history) provided usable electroencephalogram (EEG) and questionnaire data. Diagnoses were obtained through the semi-structured Schedule of Affective Disorders and Schizophrenia for School-Age Children (Kaufman et al., 1997) by master’s- or doctoral-level clinicians. History of mania, psychotic symptoms, intellectual disability, pervasive developmental disorders, current substance use disorders, or current suicidal ideation was exclusionary. Patients with secondary comorbid depressive disorders were included (Table 1). Diagnoses of attention deficit hyperactivity disorder (ADHD) and oppositional defiant disorder (ODD) were not exclusionary in AD participants; however in the current sample, no participants met criteria for ODD and only 2 participants (AD group) had ADHD.
Table 1.
Demographic and clinical characteristics of anxious and healthy youth
| AD (n = 30) | HC (n = 30) | Overall (n = 60) | ||||
|---|---|---|---|---|---|---|
| M | SD | M | SD | M | SD | |
| Age | 15.00 | 2.86 | 16.13 | 2.70 | 15.57 | 2.82 |
| CDI Depression T Score* | 55.54 | 11.96 | 38.38 | 3.57 | 46.81 | 12.26 |
| Puberty Raw Score | 3.32 | .91 | 3.35 | .72 | 3.27 | .82 |
| Pubertal Timinga | .01 | 1.18 | .06 | .87 | 0.04 | 1.03 |
| MASC Anxiety T Scoreb* | 60.74 | 13.52 | 38.93 | 9.22 | 49.26 | 15.80 |
| Accuracy (% Correct) | 93.39 | .04 | 92.69 | .05 | 93.04 | .05 |
| Congruent Trial (# Errors) | 5.27 | 5.12 | 5.27 | 7.00 | 5.27 | 6.10 |
| Incongruent Trial (# Errors) | 16.53 | 9.43 | 18.87 | 11.80 | 17.70 | 10.66 |
| Response Times | ||||||
| Congruent Correct | 437.25 | 78.03 | 419.86 | 90.76 | 428.56 | 84.37 |
| Congruent Incorrect | 360.08 | 165.12 | 400.77 | 176.72 | 380.42 | 170.69 |
| Incongruent Correct | 523.01 | 95.91 | 486.39 | 106.44 | 504.70 | 102.13 |
| Incongruent Incorrect | 422.25 | 191.68 | 380.49 | 83.50 | 401.37 | 148.09 |
| N | % | N | % | N | % | |
| Sex (% female) | 16 | 53.3 | 16 | 53.3 | 32 | 53.3 |
| Race | ||||||
| White | 14 | 46.7 | 18 | 60.0 | 32 | 53.3 |
| Black | 4 | 13.3 | 6 | 20.0 | 10 | 16.7 |
| Asian | 3 | 10.0 | 5 | 16.7 | 8 | 13.3 |
| Native Hawaiian | 1 | 3.3 | 0 | 0.0 | 1 | 1.7 |
| Pacific Islander | ||||||
| Multiple | 3 | 10.0 | 0 | 0.0 | 3 | 5.0 |
| Other | 5 | 16.7 | 1 | 3.3 | 6 | 10.0 |
| Ethnicity (% Hispanic) | 7 | 23.3 | 3 | 10.0 | 10 | 16.7 |
| Site (% UIC) | 18 | 60.0 | 22 | 73.3 | 40 | 66.7 |
| Lifetime Diagnosesc | ||||||
| Social Anxiety | 20 | 66.7 | -- | -- | -- | -- |
| Separation Anxiety | 5 | 16.7 | -- | -- | -- | -- |
| Generalized Anxiety | 22 | 73.3 | -- | -- | ||
| Specific Phobia | 6 | 20.0 | -- | -- | ||
| Depressive Disorderd | 10 | 33.3 | -- | -- | -- | -- |
Residual of sex-corrected z-score regressed on age;
Included for descriptive, but not analytic, purposes
Diagnoses not mutually exclusive;
Includes lifetime history of major depressive disorder, dysthymia, or depression not otherwise specified;
Denotes significantly different at p <.05
Pubertal Timing
The Pubertal Development Scale (PDS) is a self-report questionnaire that assesses pubertal development (Petersen, Crockett, Richards, & Boxer, 1988). The PDS evaluates height, weight, body hair, body hair growth, breast change (girls only), facial hair growth (boys only), and menstruation (girls only). All items except menstruation (binary coded [yes=4, no = 0]) are rated on an ordinal scale. Total scores are calculated by summing across items, with higher raw scores indicating more mature pubertal status. The PDS has previously demonstrated good psychometric properties and convergent validity based on self- and physician-rated Tanner stages (Petersen et al., 1988), including in ethnically diverse samples (Siegel, Yancey, Aneshensel, & Schuler, 1999). Given that boys tend to show an age lag in pubertal onset (Graber, 2013), prior to conducting analyses, we calculated sex-corrected z-scores using the mean and standard deviation of total PDS score for boys and girls separately. Consistent with previous research, the PDS sex-corrected z-score was regressed onto age and the residual obtained was used as a continuous measure of pubertal timing (Belsky et al., 2007; Dorn, Dahl, Woodward, & Biro, 2006; Dorn, Susman, & Ponirakis, 2003). Accordingly, positive pubertal timing scores indicate earlier onset of puberty than expected for age relative to same sex peers and negative pubertal timing scores indicate later onset of puberty than expected for age relative to same sex peers. Pubertal timing scores and puberty total scores (sex-corrected) were moderately correlated (r = .61, p <.001). Additionally, age was moderately correlated with puberty total scores (r = .57, p <.001).
Depressive & Anxiety Symptoms
Self-report of depressive symptoms was collected using the Children’s Depression Inventory (CDI; (Kovacs, 1981)), a reliable and valid measure of depressive symptoms. Higher scores indicate greater depression severity, with raw scores of 15, 21, and 25 reflective of mild, moderate, and severe depression, respectively (Bang, Park, & Kim, 2015). For the purposes of this study, raw scores were transformed to age and sex corrected T-scores with a mean of 50 and a standard deviation of 10. Self-report anxiety symptoms were recorded using the Multidimensional Anxiety Scale for Children (March, Parker, Sullivan, Stallings, & Conners, 1997).
Error Task
Participants completed a flanker task, which has been shown to reliably elicit an ERN in youth (Meyer, Bress, & Proudfit, 2014). On each trial, horizontally aligned arrowheads were presented for 200 ms, followed by an intertrial interval between 2300 and 2800 ms. The task included 11 blocks of 30 trials (330 trials total), with half of the trials compatible (>>>>> or <<<<<) and half incompatible (<<><< or >><>>). Participants were instructed to press the left or right mouse button to indicate the direction of the center arrow. Participants first completed a practice block of 30 trials. During the task, participants received feedback on their performance at the end of each block in order to ensure a sufficient number of error trials. The message “Please try to be more accurate” was displayed if accuracy was < 75%, and “Please try to respond faster” was displayed if accuracy was above 90%. Otherwise, the message “You’re doing a great job” was displayed. The administrative procedures for the flanker task were identical across study sites; specifically, the task was administered in a quiet room at both sites with only one observer present. Sites used the same version of the flanker task, which was run using the same software, so as to minimize any possible impact of the size of the visual stimuli on the data.
EEG Data Acquisition and Preprocessing
Data were collected using a BioSemi (Amsterdam, Netherlands) 34- channel cap (32 channels plus FCz and Iz). EEG data acquisition and preprocessing steps were identical across study sites. Specifically, electrodes were placed on the left and right mastoids, and electrooculogram recorded from four facial electrodes. Data were digitized at 24-bit resolution with a Least Significant Bit (LSB) value of 31.25 nV and a sampling rate of 1024 Hz, and processed offline using Brain Vision Analyzer software (Brain Products, Gilching, Germany). Data were converted to a linked mastoid reference, filtered with high-pass and low-pass filters of 0.1 and 30 Hz, and segmented 500 ms before the response and continuing for 1000 ms after the response. Eyeblinks were corrected (Gratton, Coles, & Donchin, 1983), and semi-automated artifact rejection procedures removed artifacts with a voltage step of >50 μV between sample points, voltage difference of 175 μV within 400 ms intervals, maximum voltage difference of <0.5 μV within 100 ms intervals, and additional artifacts removed using visual inspection. ERPs to errors and correct responses were averaged separately and baseline corrected to the window 500–300 ms prior to responses. The ERN was scored at the frontocentral site (FCz) where amplitude was maximal, 50 to 100 ms after the response (Figure 1; AD and HC shown separately, prior to adjusting for other variables of interest, in Supplemental Figure 1). Analyses focused on the error minus correct response difference score (ΔERN) to isolate variation in the ERP wave related to performance monitoring (Luck, 2005). More negative ΔERN indicates greater differentiation between errors and correct responses.
Figure 1.
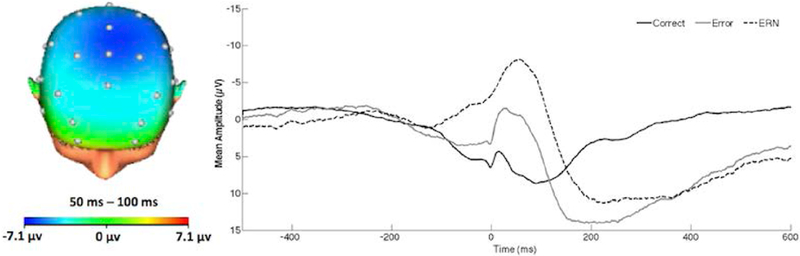
On the left, topographic map of neural activity (error minus correct) across the entire sample. On the right, response-locked ERP waveform for correct and error trials, as well as the difference waves (error-related negativity; ΔERN) at FCz across the entire sample. Increases in the ΔERN are represented by values that are more negative in polarity.
Data Analytic Plan
To test our hypotheses, we conducted a generalized multiple linear regression analysis with the ΔERN as the dependent variable. All continuous variables were mean centered prior to analysis. The model included the main effects of diagnosis (coded [HC = 0], [AD =1], pubertal timing, and CDI depression T scores, as well as all two-way interactions, and a diagnosis × pubertal timing × CDI depression three-way interaction. We initially ran a model including biological sex, but did not result in any main or moderating effects of sex, and so sex was removed to achieve the most parsimonious model.
A significant interaction was followed-up using a standard simple slopes approach (Aiken, West, & Reno, 1991). Specifically, the moderator was either re-centered at 1 SD above the mean for “high scores” and 1 SD below the mean for “low scores” for continuous variables (i.e. pubertal timing) or the simple slopes were tested at each level of the categorical moderator variable (i.e. diagnosis). Post-hoc additional follow-up linear regression models were run at high and low symptoms and plotted at these levels for display.
Results
Descriptive Statistics
Demographic and descriptive statistics of the sample are presented in Table 1. Participants were matched on age and sex and were comparable in terms of mean pubertal timing across diagnostic groups. Amongst participants with an anxiety disorder, 30.0% met criteria for a lifetime depressive disorder. Moreover, there was a range of depressive symptomatology within the AD group; 43.3% of CDI total raw scores fell in the mild to moderate severity range. There was no significant effect of age in relation to depressive symptoms (main effect, b = .05, se = .58, p = .932) or any age × diagnostic group interactions in relation to depressive symptoms (interaction, b = −.76, se = 13.09, p = .347). Depressive symptoms were significantly correlated with pubertal development amongst the AD group using a directional one-test (positive association, r = .32, p = .042), an effect that was reduced to a trend level when using a non-directional, two-tailed test (p = .084).
Behavioral Results
With regard to behavioral performance during the flanker task, participants committed an average of 22.97 ± 15.66 errors, corresponding to an overall 93.04% accuracy rate. Accuracy rates did not differ between AD (M = 93.39%, SD = .04) and HC (M = 92.69%, SD = .05) youth. Accuracy was also unrelated to pubertal timing (r = .19, p = .14), anxiety symptoms (MASC Total T-Score, r = .09, p = .49), or depressive symptoms (CDI Total T-Score, r = .17, p = .21). Additionally, diagnostic group did not moderate the associations between accuracy and depressive symptoms (B = −.002, SE = .003, p = .41), anxiety symptoms (B = −.001, SE = .002, p = .39), or pubertal timing (B = −.005, SE = .01, p = .97).
Response times for congruent and incongruent trials, stratified by correct and incorrect responses are reported overall and for each group in Table 1. Overall (across all participants), responses were faster for incorrect versus correct responses to congruent, F(1, 59) = 22.37, p = <.001, and incongruent trials, F(1, 59) = 28.22, p = <.001. The interaction between diagnosis and congruence for response time to correct trials was not significant, F(1, 58) = .86, p =.359. The interaction between diagnosis and congruence was also not significant for error rate, F(1, 58) = 1.46, p =.232
Impact of Pubertal Timing and Depressive Symptoms
Results of the generalized multiple linear regression model are presented in Table 2. There was a main effect of diagnosis; as expected, the ΔERN was more pronounced (more negative) in AD participants, after controlling for the impact of pubertal timing and depression. There was also a main effect of pubertal timing, such that earlier onset of puberty was associated with a more enhanced ΔERN (Figure 2). There was no main effect of depressive symptoms. There was, however, a two-way interaction between diagnostic group and depressive symptoms. Follow-up analyses indicated that among AD participants, higher depression was associated with an attenuated ΔERN, b = .14, se = .07, p <.05 (Figure 3), but not among HC participants, b = −.34, se = .30, p =.26. There was also a two-way interaction between depressive symptoms and pubertal timing. Follow-up analyses indicated that with earlier onset of puberty (‘high’ pubertal timing scores), increases in depressive symptoms were associated with a reduced ΔERN, b = .18, se = .08, p = .04 (Figure 4). Depressive symptoms were unrelated to the ΔERN amongst youth with late pubertal timing (‘low’ pubertal timing scores), b = −.10, se = .11, p = .21. The three-way interaction between pubertal timing, mood, and diagnostic group did not reach significance.
Table 2.
Generalized linear regression model examining the impact of anxiety disorder diagnosis, pubertal timing, and depression on error-related negativity. Increases in the ΔERN are represented by values that are more negative in polarity.
| ΔERN | |||
|---|---|---|---|
| Parameter | b | SE | p |
| Diagnosis | 3.28 | 1.30 | .012 |
| Pubertal Timing | –1.19 | .42 | .005 |
| Depression | 0.12 | 0.08 | .122 |
| Diagnosis × Pubertal Timing | –1.86 | 1.48 | .284 |
| Diagnosis × Depression | –0.80 | 0.33 | .016 |
| Pubertal Timing × Depression | 0.22 | 0.07 | .003 |
| Diagnosis × Pubertal Timing × Depression | 0.50 | .38 | .182 |
A model was also evaluated excluding youth with ADHD (n = 2) from the AD group and no substantive changes in the results were observed.
A model was also evaluated using the Meyer et al (2017) guidelines, calculating an ERN standardized residual score, by saving the variance leftover in a regression where the CRN was entered, predicting the ERN. Using the ERN residual as the dependent variable, we did not observe substantive changes to the parameter estimates and the overall pattern of findings was the same as those yielded by the ΔERN.
Last, we evaluated the sensitivity of our findings by testing a model predicting the ΔERN, derived using a baseline of both 400 to 200 milliseconds (ms) and 150 to 50 ms prior to the response; the results were unchanged using a baseline 400 to 200 ms prior to response. Using a baseline of 150 to 50 ms prior to response, the diagnosis × depression effect was unchanged; the pubertal timing main effect and interaction with depression were reduced to a trend (with direction of findings remaining unchanged). These parameter estimates are reported in Supplemental Table 1
Figure 2.
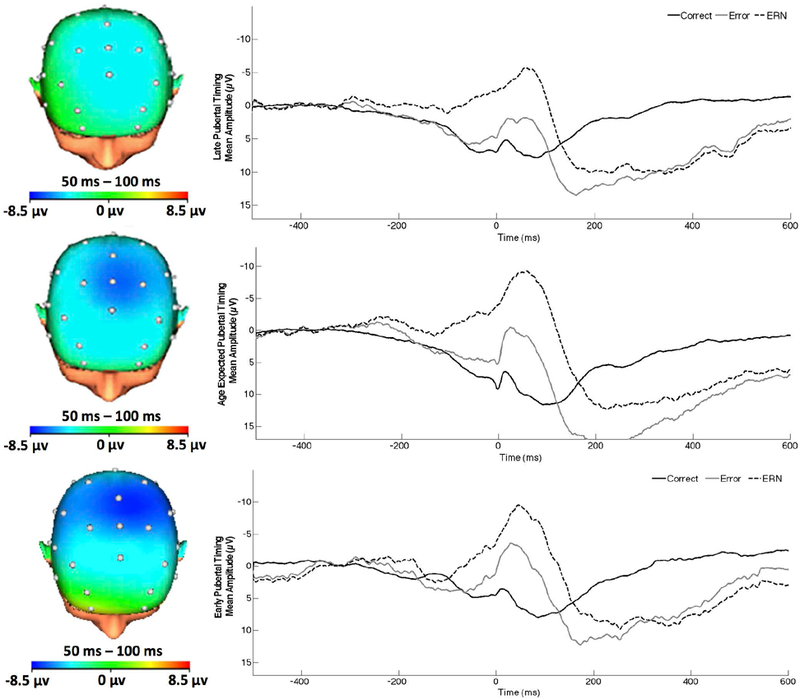
On the left, topographic map of neural activity (error minus correct) for youth with late, average, and early pubertal timing (from top to bottom, respectively). On the right, response-locked ERP waveform for correct and error trials, as well as the difference waves (error-related negativity; ΔERN) at FCz for late, average, and early pubertal timing (from top to bottom, respectively). Increases in the ΔERN are represented by values that are more negative in polarity.
Figure 3.
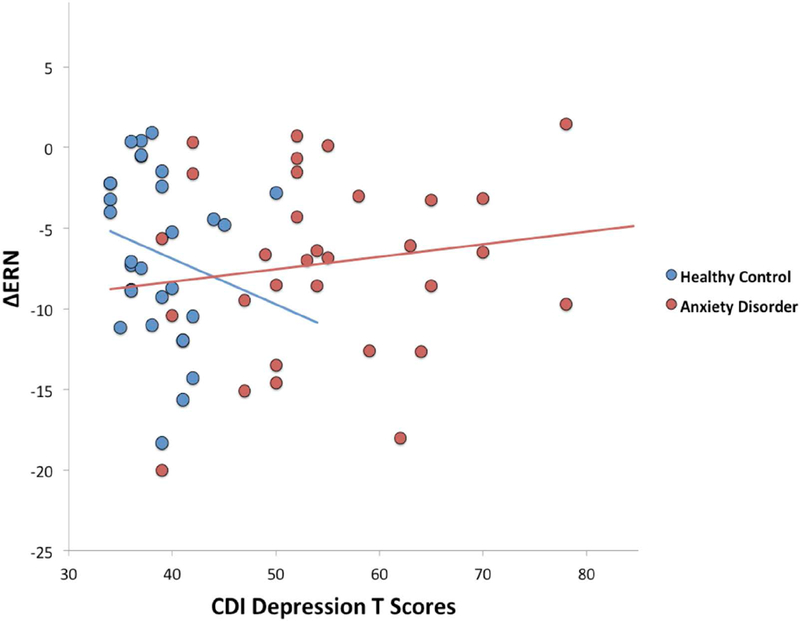
Scatterplot illustrating the effect of depressive symptoms on the ΔERN by diagnostic group (anxiety disorder vs. healthy controls). Increases in the ΔERN are represented by values that are more negative in polarity. ΔERN = difference between error-related negativity and correct-related negativity; CDI= Children’s Depression Inventory
Figure 4.
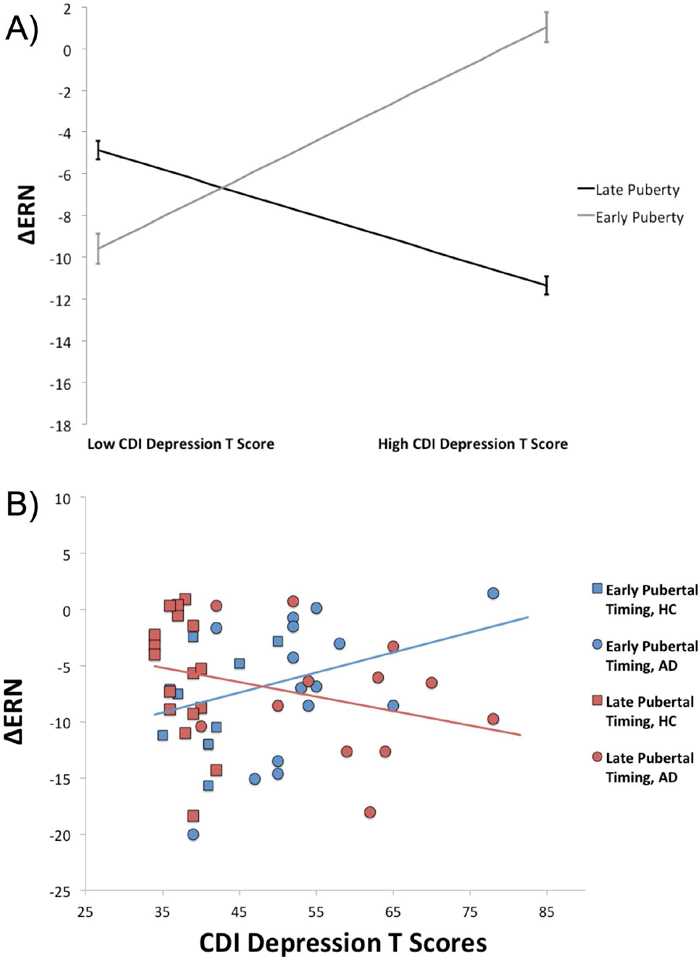
A) Plot of simple slopes depicting the effect of depressive symptoms on the ΔERN with early and late pubertal timing (± 1 standard deviation from the mean). B) Scatterplot depicting the effect of depression on the ERN at early and late pubertal timing (median split). Increases in the ΔERN are represented by values that are more negative in polarity. ΔERN = difference between error-related negativity and correct-related negativity; CDI= Children’s Depression Inventory
Exploratory Analyses
To evaluate the potential effect of different baselines on these results, these analyses were repeated with a baseline of both 400 to 200 milliseconds (ms) and 150 to 50 ms prior to the response, the results of which are reported in Supplemental Table 1. We also conducted an additional regression model to assess whether dimensional anxiety symptoms were related to the ERN or interacted with pubertal timing in relation the ERN. There was no main effect of anxiety symptoms (b = −.05, se = .04, p = .261, nor did anxiety symptoms interact with pubertal timing (b = .04, se = .05, p = .457).
Discussion
We examined error monitoring in a sample of youth with heterogeneous anxiety disorders in relation to pubertal timing and depressive symptoms. Our primary finding provides preliminary evidence that in both anxious and healthy youth, early pubertal timing is associated with enhanced ERN amplitude. This finding builds on prior work linking puberty stage with an enhanced ERN (Gorday & Meyer, 2018), demonstrating that pubertal timing may provide another layer of specificity into how developmental changes impact the ERN. Our results also replicate prior work (for a review see;(Meyer, 2017)), that controlling for depressive symptoms, child and adolescent anxiety disorders are marked by an increased ERN. Depressive symptoms attenuated these effects, both amongst early developing and anxious youth, such that increasing depressive symptoms were associated with a reduced ERN (Weinberg, Meyer, et al., 2016). Taken together, these findings indicate that the magnitude of neural reactivity to errors is sensitive to variations in pubertal development, anxiety, and depression.
An enhanced ERN is robustly associated with anxiety disorders in adults (Cavanagh & Shackman, 2015; Moser et al., 2013), and increasingly among youth (Meyer, 2017). Consequently, the question of when and how this neural sensitivity develops becomes important to understand in what way this biomarker of anxiety differs across typical development. Indeed, the observed main effect of pubertal timing enhances our precision in understanding when sensitive periods for the development of the ERN are most likely to occur. By correcting for chronological age in our analyses, we show that the timing of pubertal onset is related to the development of the ERN such that it is enhanced in early maturing youth and attenuated amongst late maturing youth. This extends previous evidence of age-related changes in the ERN, (Davies, Segalowitz, & Gavin, 2004; Hanna et al., 2012; Meyer et al., 2012; Tamnes et al., 2013; Weinberg, Meyer, et al., 2016), showing that the timing of physical maturation compared to same-aged peers can predict the neural signature of youth’s sensitivity to their own errors. Although longitudinal designs, including the assessment of sex steroid hormones (Brown & Spencer, 2013), are needed to draw firm conclusions, this also means that the timing of puberty in relation to fluctuations in cortical maturation could reflect a mechanism by which the ERN becomes enhanced over time (and generally, with age).
Although early physical maturation was related to heightened neural sensitivity to errors overall, symptoms of depression exerted an opposing influence of the magnitude of the ERN; high depressive symptoms amongst early maturing youth were associated with a reduced ERN. This finding poses a good example of why the transition from childhood to adolescence may be a particularly important time period to consider multiple levels of the RDoC negative valence system and their interactions (Garvey, Avenevoli, & Anderson, 2016), in relation to the development of psychopathology. It is possible that a blunted ERN is a pre-existing risk factor or state marker of depression and masks the enhancement in the ERN that is typically observed across pubertal development (Holder & Blaustein, 2014). Conversely, given that childhood and adolescence is characterized by continued, but uneven, cortical maturation, there may be a time-sensitive impact of sex steroids that disproportionately modulates (especially) limbic circuitry during an early pubertal phase (Peper, Hulshoff Pol, Crone, & van Honk, 2011), and in turn, increases propensity for depression and differential internalization of the value of mistakes (Guyer, Silk, & Nelson, 2016). It is also possible that early puberty may indirectly affect the ERN via social-affective processes. Indeed, social scrutiny and victimization is common amongst early developing youth (Sontag & Graber, 2010; Sontag, Graber, & Clemans, 2011). Many youth respond to social-evaluative threat with withdrawal and develop an increased tolerance to negative social feedback (Allen et al., 2006; Lee, Hankin, & Mermelstein, 2010), which could be reflected at the neural level in terms of reduced sensitivity to endogenous threat (Silk et al., 2012).
In anxious youth, a reduced ERN in relation to depressive, but not anxiety symptoms is consistent with prior work in a community sample of adolescents (Meyer et al., 2016; Weinberg, Meyer, et al., 2016). Although evaluations of the ERN in child and adolescent major depression are generally sparse (but see (Ladouceur et al., 2012; Meyer et al., 2016)), this finding also converges with adult studies where depression severity is inversely associated with sensitivity to errors (Olvet et al., 2010; Weinberg, Liu, & Shankman, 2016) and a moderator of ERN magnitude in anxiety disorders (Weinberg et al., 2012; Weinberg et al., 2015). This study also offers leverage for RDoC-inspired methodological approaches, highlighting that variation in symptom dimensions of co-occurring psychopathologies are at least partially distinguishable in terms of patterns of neural response. Accordingly, the ERN may be a useful marker to track variations in trans-diagnostic symptoms and appears sensitive to phenotypes. In contrast, there was no association between depression and the ERN amongst HC where the range of symptoms was more restricted. Moreover, it was somewhat surprising that in exploratory analyses, anxiety symptoms did not relate to an enhanced ERN. On the other hand, these findings are consistent with evidence suggesting that the ERN is a trait-like marker of anxiety, based on evidence that the ERN does not change after successful treatment for instance, in OCD (Hajcak et al., 2008; Riesel, Endrass, Auerbach, & Kathmann, 2015), nor does it correlated with the degree of anxiety symptom change (e.g. (Kujawa, Weinberg, et al., 2016), but see (Gorka et al., 2018)), and can be present in unaffected first-degree relatives of patients with anxiety (Carrasco, Harbin, et al., 2013; Riesel, Endrass, Kaufmann, & Kathmann, 2011). Therefore, put together, future studies should utilize a depression comparison group to determine if this effect is specific to the co-occurrence of depression and anxiety or also seen in the absence of anxiety.
Although speculative, taken together with the dynamic relationship between early pubertal timing and depressive symptoms, a blunted ERN may be one mechanism that places early maturing anxious youth at high risk for co-occurring depression. In line with this possibility, prior work suggests there is a degree of normative developmental increase in the ERN (Buzzell et al., 2017). Theoretically, increased ERN in adolescence is thought to reflect the transition from sensitivity to external fear to greater internal saliency of threats related to ones’ behavior (Meyer, 2017). However, early pubertal timing may increase the likelihood that ERN development departs from a typical age-related trajectory (Sisk, 2017). For example, if the error-monitoring system fails to develop appropriately, lack of attention to behavioral competence, performance, and social threat could be observed, which may potentiate future risk for depression (Ladouceur et al., 2012). Conversely, it is possible that an enhanced ERN may protect early maturing anxious youth from developing depression. For instance, enhanced neural activity supporting the monitoring and salience of one’s performance could be an adaptive and compensatory response to the tendency of depressed individuals to exhibit reduced sensitivity to reward and reinforcement (Kumar et al., 2018). Future longitudinal designs would help to further unpack the emergence of internalizing symptom dimensions in relation to the neural changes occurring across the transition from pre- to post-pubertal status and in relation to the timing of when this transition occurs.
The primary limitation of this study is the absence of a comparison group of youth with depression and without anxiety disorders. Although the notion of ERN blunting in depression is empirically driven (Ladouceur et al., 2012; Meyer et al., 2016; Olvet et al., 2010; Weinberg et al., 2012; Weinberg et al., 2015; Weinberg, Liu, et al., 2016; Weinberg, Meyer, et al., 2016), in the present sample we can only comment on the relationship of emergent depression with the ERN in the context of primary anxiety and cannot confidently extrapolate as to whether depression shows a similar association with error-monitoring in the absence of anxiety symptoms. Second, although we speculate about the neural changes associated with pubertal timing in relation to the activation of the hypothalamic-pituitary-gonadal axis that is characteristic of this developmental period, an ideal design would include concurrent assessment of sex steroid hormones (Brown & Spencer, 2013) and their interactions with other neuroendocrine systems (Handa & Weiser, 2014) to unravel the mechanisms by which pubertal changes exert their effects. As previously mentioned, an optimal design would be longitudinal and evaluate individual differences as youth transition through puberty. One recent cross-sectional study indicated that dehydroepiandrosterone hormone level was associated with an enhanced ERN in female youth, even when adjusting for age and puberty scores (Gorday & Meyer, 2018). Third, the sample size was small and heterogeneous with regard to primary diagnosis and prior treatment, precluding the examination of effects of diagnostic comorbidity (as opposed to symptoms assessed dimensionally) and their interactions with development. Finally, though we did not observe any effects of biological sex on the results (and corrected for sex differences in pubertal timing), given the small sample size we cannot rule out the possibility that we were underpowered to detect the ways in which sex might interact with age, puberty, diagnosis, depression, or their combination. For instance, it was somewhat surprising that age was not related to depression in this sample. It is possible that age effects are of a small magnitude and that a larger sample would be needed to detect significance within the AD group. Alternatively, it may also be the case that because this sample is seeking treatment for anxiety with substantial comorbid depression, that it is over enriched for internalizing symptoms, obscuring an association that might be observed more naturalistically. For these reasons, the findings reported herein should be interpreted as preliminary and hypothesis generating.
The current study also benefited from several strengths, such as the inclusion of a relatively wide age-span of healthy and anxious children and adolescents to understand typical and atypical maturational changes in performance monitoring. Our study addresses a gap in the literature by being the first to examine how timing of physical development influences neural substrates of error monitoring in relation to dimensions of internalizing psychopathology. It seems clear from these data that the ERN as a marker of anxiety in youth does not operate independently from dimensions of psychopathology, variations in development, and their interactions, illustrating the utility of the ERN as a sensitive measure of trans-diagnostic phenotypes during complex developmental epochs. Methodologically, the blunted phenotype associated with depression helps guide future studies in that it is important to isolate depression from anxiety and early puberty when assessing the ERN. Particularly amongst early developing youth, assessment of the ERN may help to predict vulnerability to anxiety, depression, or their combination. An exciting avenue for future work will be investigating the potential for error-related brain activity to prospectively predict developmental trajectories of internalizing psychopathology.
Supplementary Material
On the left, topographic map of neural activity (error minus correct) for AD and HC youth (from top to bottom, respectively) prior to adjusting for pubertal timing and depression. On the right, response-locked ERP waveform for correct and error trials, as well as the difference waves (error-related negativity; ΔERN) at FCz for AD and HC youth (from top to bottom, respectively). Increases in the ΔERN are represented by values that are more negative in polarity. AD = Anxiety Disorders; HC = Healthy Control.
Acknowledgments
This work was supported by National Institute of Mental Health Grant R01-MH086517 to CSM and KLP. ATP is supported by National Institute of Mental Health Grant F31- MH108258–03. KLB is supported by National Institute of Mental Health Grant K23-MH113793–01.
References
- Aiken LS, West SG, & Reno RR (1991). Multiple regression: Testing and interpreting interactions: Sage. [Google Scholar]
- Allen JP, Insabella G, Porter MR, Smith FD, Land D, & Phillips N (2006). A social-interactional model of the development of depressive symptoms in adolescence. J Consult Clin Psychol, 74(1), 55–65. doi: 10.1037/0022-006X.74.1.55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alloy LB, Hamilton JL, Hamlat EJ, & Abramson LY (2016). Pubertal Development, Emotion Regulatory Styles, and the Emergence of Sex Differences in Internalizing Disorders and Symptoms in Adolescence. Clin Psychol Sci, 4(5), 867–881. doi: 10.1177/2167702616643008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bang YR, Park JH, & Kim SH (2015). Cut-Off Scores of the Children’s Depression Inventory for Screening and Rating Severity in Korean Adolescents. Psychiatry Investig, 12(1), 23–28. doi: 10.4306/pi.2015.12.1.23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belsky J, Steinberg LD, Houts RM, Friedman SL, DeHart G, Cauffman E, Susman E (2007). Family rearing antecedents of pubertal timing. Child development, 78(4), 1302–1321. [DOI] [PubMed] [Google Scholar]
- Blakemore SJ, Burnett S, & Dahl RE (2010). The role of puberty in the developing adolescent brain. Hum Brain Mapp, 31(6), 926–933. doi: 10.1002/hbm.21052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bress JN, Meyer A, & Hajcak G (2015). Differentiating anxiety and depression in children and adolescents: evidence from event-related brain potentials. J Clin Child Adolesc Psychol, 44(2), 238–249. doi: 10.1080/15374416.2013.814544 [DOI] [PubMed] [Google Scholar]
- Brouwer RM, Koenis MM, Schnack HG, van Baal GC, van Soelen IL, Boomsma DI, & Hulshoff Pol HE (2015). Longitudinal development of hormone levels and grey matter density in 9 and 12-year-old twins. Behav Genet, 45(3), 313–323. doi: 10.1007/s10519-015-9708-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown GR, & Spencer KA (2013). Steroid hormones, stress and the adolescent brain: a comparative perspective. Neuroscience, 249, 115–128. doi: 10.1016/j.neuroscience.2012.12.016 [DOI] [PubMed] [Google Scholar]
- Buchanan CM, Eccles JS, & Becker JB (1992). Are adolescents the victims of raging hormones: evidence for activational effects of hormones on moods and behavior at adolescence. Psychol Bull, 111(1), 62–107. [DOI] [PubMed] [Google Scholar]
- Bunford N, Kujawa A, Swain JE, Fitzgerald KD, Monk CS, & Phan KL (2017). Attenuated neural reactivity to happy faces is associated with rule breaking and social problems in anxious youth. Eur Child Adolesc Psychiatry, 26(2), 215–230. doi: 10.1007/s00787-016-0883-9 [DOI] [PubMed] [Google Scholar]
- Burkhouse KL, Kujawa A, Klumpp H, Fitzgerald KD, Monk CS, & Phan KL (2017). Neural correlates of explicit and implicit emotion processing in relation to treatment response in pediatric anxiety. J Child Psychol Psychiatry, 58(5), 546–554. doi: 10.1111/jcpp.12658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buzzell GA, Richards JE, White LK, Barker TV, Pine DS, & Fox NA (2017). Development of the error-monitoring system from ages 9–35: Unique insight provided by MRI-constrained source localization of EEG. Neuroimage, 157, 13–26. doi: 10.1016/j.neuroimage.2017.05.045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrasco M, Harbin SM, Nienhuis JK, Fitzgerald KD, Gehring WJ, & Hanna GL (2013). Increased error-related brain activity in youth with obsessive-compulsive disorder and unaffected siblings. Depress Anxiety, 30(1), 39–46. doi: 10.1002/da.22035 [DOI] [PubMed] [Google Scholar]
- Carrasco M, Hong C, Nienhuis JK, Harbin SM, Fitzgerald KD, Gehring WJ, & Hanna GL (2013). Increased error-related brain activity in youth with obsessive-compulsive disorder and other anxiety disorders. Neurosci Lett, 541, 214–218. doi: 10.1016/j.neulet.2013.02.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavanagh JF, & Shackman AJ (2015). Frontal midline theta reflects anxiety and cognitive control: meta-analytic evidence. J Physiol Paris, 109(1–3), 3–15. doi: 10.1016/j.jphysparis.2014.04.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu PH, & Deldin PJ (2007). Neural evidence for enhanced error detection in major depressive disorder. Am J Psychiatry, 164(4), 608–616. doi: 10.1176/ajp.2007.164.4.608 [DOI] [PubMed] [Google Scholar]
- Czobor P, Kakuszi B, Nemeth K, Balogh L, Papp S, Tombor L, & Bitter I (2017). Electrophysiological indices of aberrant error-processing in adults with ADHD: a new region of interest. Brain Imaging Behav, 11(6), 1616–1628. doi: 10.1007/s11682-016-9610-x [DOI] [PubMed] [Google Scholar]
- Davies PL, Segalowitz SJ, & Gavin WJ (2004). Development of response-monitoring ERPs in 7- to 25-year-olds. Dev Neuropsychol, 25(3), 355–376. doi: 10.1207/s15326942dn2503_6 [DOI] [PubMed] [Google Scholar]
- Dorn LD, Dahl RE, Woodward HR, & Biro F (2006). Defining the boundaries of early adolescence: A user’s guide to assessing pubertal status and pubertal timing in research with adolescents. Applied Developmental Science, 10(1), 30–56. [Google Scholar]
- Dorn LD, Susman EJ, & Ponirakis A (2003). Pubertal timing and adolescent adjustment and behavior: Conclusions vary by rater. Journal of Youth and Adolescence, 32(3), 157–167. [Google Scholar]
- Falkenstein M, Hohnsbein J, Hoormann J, & Blanke L (1990). Effects of errors in choice reaction tasks on the ERP under focused and divided attention. Psychophysiological brain research, 1, 192–195. [Google Scholar]
- Garvey M, Avenevoli S, & Anderson K (2016). The National Institute of Mental Health Research Domain Criteria and Clinical Research in Child and Adolescent Psychiatry. J Am Acad Child Adolesc Psychiatry, 55(2), 93–98. doi: 10.1016/j.jaac.2015.11.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gehring WJ, Goss B, Coles MG, Meyer DE, & Donchin E (1993). A neural system for error detection and compensation. Psychological science, 4(6), 385–390. [Google Scholar]
- Goddings AL, Mills KL, Clasen LS, Giedd JN, Viner RM, & Blakemore SJ (2014). The influence of puberty on subcortical brain development. Neuroimage, 88, 242–251. doi: 10.1016/j.neuroimage.2013.09.073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorday JY, & Meyer A (2018). Linking puberty and error-monitoring: Relationships between self-reported pubertal stages, pubertal hormones, and the error-related negativity in a large sample of children and adolescents. Dev Psychobiol. doi: 10.1002/dev.21625 [DOI] [PubMed] [Google Scholar]
- Gorka SM, Burkhouse KL, Klumpp H, Kennedy AE, Afshar K, Francis J, Phan KL (2018). Error-related Brain Activity as a Treatment Moderator and Index of Symptom Change during Cognitive-Behavioral Therapy or Selective Serotonin Reuptake Inhibitors. Neuropsychopharmacology, 43(6), 1355–1363. doi: 10.1038/npp.2017.289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graber JA (2013). Pubertal timing and the development of psychopathology in adolescence and beyond. Horm Behav, 64(2), 262–269. doi: 10.1016/j.yhbeh.2013.04.003 [DOI] [PubMed] [Google Scholar]
- Gratton G, Coles MG, & Donchin E (1983). A new method for off-line removal of ocular artifact. Electroencephalogr Clin Neurophysiol, 55(4), 468–484. [DOI] [PubMed] [Google Scholar]
- Guyer AE, Silk JS, & Nelson EE (2016). The neurobiology of the emotional adolescent: From the inside out. Neurosci Biobehav Rev, 70, 74–85. doi: 10.1016/j.neubiorev.2016.07.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajcak G, Franklin ME, Foa EB, & Simons RF (2008). Increased error-related brain activity in pediatric obsessive-compulsive disorder before and after treatment. Am J Psychiatry, 165(1), 116–123. doi: 10.1176/appi.ajp.2007.07010143 [DOI] [PubMed] [Google Scholar]
- Handa RJ, & Weiser MJ (2014). Gonadal steroid hormones and the hypothalamo-pituitary-adrenal axis. Front Neuroendocrinol, 35(2), 197–220. doi: 10.1016/j.yfrne.2013.11.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanna GL, Carrasco M, Harbin SM, Nienhuis JK, LaRosa CE, Chen P, Gehring WJ (2012). Error-related negativity and tic history in pediatric obsessive-compulsive disorder. J Am Acad Child Adolesc Psychiatry, 51(9), 902–910. doi: 10.1016/j.jaac.2012.06.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hochman EY, Eviatar Z, Breznitz Z, Nevat M, & Shaul S (2009). Source localization of error negativity: additional source for corrected errors. Neuroreport, 20(13), 1144–1148. doi: 10.1097/WNR.0b013e32832f84ed [DOI] [PubMed] [Google Scholar]
- Holder MK, & Blaustein JD (2014). Puberty and adolescence as a time of vulnerability to stressors that alter neurobehavioral processes. Front Neuroendocrinol, 35(1), 89–110. doi: 10.1016/j.yfrne.2013.10.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes AJ, & Pizzagalli DA (2008). Spatiotemporal dynamics of error processing dysfunctions in major depressive disorder. Arch Gen Psychiatry, 65(2), 179–188. doi: 10.1001/archgenpsychiatry.2007.19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes AJ, & Pizzagalli DA (2010). Effects of task-relevant incentives on the electrophysiological correlates of error processing in major depressive disorder. Cogn Affect Behav Neurosci, 10(1), 119–128. doi: 10.3758/CABN.10.1.119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holroyd CB, & Coles MGH (2002). The neural basis of human error processing: reinforcement learning, dopamine, and the error-related negativity. Psychol Rev, 109(4), 679–709. doi: 10.1037/0033-295X.109.4.679 [DOI] [PubMed] [Google Scholar]
- Kaufman J, Birmaher B, Brent D, Rao U, Flynn C, Moreci P Ryan N (1997). Schedule for affective disorders and schizophrenia for school-age children-present and lifetime version (K-SADS-PL): initial reliability and validity data. Journal of the American Academy of Child & Adolescent Psychiatry, 36(7), 980–988. [DOI] [PubMed] [Google Scholar]
- Kovacs M (1981). Rating scales to assess depression in school-aged children. Acta Paedopsychiatrica: International Journal of Child & Adolescent Psychiatry. [PubMed] [Google Scholar]
- Kujawa A, MacNamara A, Fitzgerald KD, Monk CS, & Phan KL (2015). Enhanced Neural Reactivity to Threatening Faces in Anxious Youth: Evidence from Event-Related Potentials. J Abnorm Child Psychol, 43(8), 1493–1501. doi: 10.1007/s10802-015-0029-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kujawa A, Swain JE, Hanna GL, Koschmann E, Simpson D, Connolly S, Phan KL (2016). Prefrontal Reactivity to Social Signals of Threat as a Predictor of Treatment Response in Anxious Youth. Neuropsychopharmacology, 41(8), 1983–1990. doi: 10.1038/npp.2015.368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kujawa A, Weinberg A, Bunford N, Fitzgerald KD, Hanna GL, Monk CS, Phan KL (2016). Error-related brain activity in youth and young adults before and after treatment for generalized or social anxiety disorder. Prog Neuropsychopharmacol Biol Psychiatry, 71, 162–168. doi: 10.1016/j.pnpbp.2016.07.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar P, Goer F, Murray L, Dillon DG, Beltzer ML, Cohen AL, Pizzagalli DA (2018). Impaired reward prediction error encoding and striatal-midbrain connectivity in depression. Neuropsychopharmacology. doi: 10.1038/s41386-018-0032-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ladouceur CD, Dahl RE, Birmaher B, Axelson DA, & Ryan ND (2006). Increased error-related negativity (ERN) in childhood anxiety disorders: ERP and source localization. J Child Psychol Psychiatry, 47(10), 1073–1082. doi: 10.1111/j.1469-7610.2006.01654.x [DOI] [PubMed] [Google Scholar]
- Ladouceur CD, Slifka JS, Dahl RE, Birmaher B, Axelson DA, & Ryan ND (2012). Altered error-related brain activity in youth with major depression. Dev Cogn Neurosci, 2(3), 351–362. doi: 10.1016/j.dcn.2012.01.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee A, Hankin BL, & Mermelstein RJ (2010). Perceived social competence, negative social interactions, and negative cognitive style predict depressive symptoms during adolescence. J Clin Child Adolesc Psychol, 39(5), 603–615. doi: 10.1080/15374416.2010.501284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo SL, Schroder HS, Fisher ME, Durbin CE, Fitzgerald KD, Danovitch JH, & Moser JS (2016). Associations between Disorder-Specific Symptoms of Anxiety and Error-Monitoring Brain Activity in Young Children. J Abnorm Child Psychol. doi: 10.1007/s10802-016-0247-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luck SJ (2005). An introduction to the event-related potential technique MIT press; Cambridge, Ma, 45–64. [Google Scholar]
- March JS, Parker JD, Sullivan K, Stallings P, & Conners CK (1997). The Multidimensional Anxiety Scale for Children (MASC): factor structure, reliability, and validity. Journal of the American Academy of Child & Adolescent Psychiatry, 36(4), 554–565. [DOI] [PubMed] [Google Scholar]
- Meyer. (2017). A biomarker of anxiety in children and adolescents: A review focusing on the error-related negativity (ERN) and anxiety across development. Developmental Cognitive Neuroscience, 27(Supplement C), 58–68. doi: 10.1016/j.dcn.2017.08.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer, Bress JN, Hajcak G, & Gibb BE (2016). Maternal Depression Is Related to Reduced Error-Related Brain Activity in Child and Adolescent Offspring. J Clin Child Adolesc Psychol, 1–12. doi: 10.1080/15374416.2016.1138405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer, Hajcak G, Glenn CR, Kujawa AJ, & Klein DN (2017). Error-related brain activity is related to aversive potentiation of the startle response in children, but only the ERN is associated with anxiety disorders. Emotion, 17(3), 487–496. doi: 10.1037/emo0000243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer, Weinberg A, Klein DN, & Hajcak G (2012). The development of the error-related negativity (ERN) and its relationship with anxiety: evidence from 8 to 13 year-olds. Dev Cogn Neurosci, 2(1), 152–161. doi: 10.1016/j.dcn.2011.09.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer A, Bress JN, & Proudfit GH (2014). Psychometric properties of the error-related negativity in children and adolescents. Psychophysiology, 51(7), 602–610. doi: 10.1111/psyp.12208 [DOI] [PubMed] [Google Scholar]
- Meyer A, Hajcak G, Torpey DC, Kujawa A, Kim J, Bufferd S, Klein DN (2013). Increased error-related brain activity in six-year-old children with clinical anxiety. J Abnorm Child Psychol, 41(8), 1257–1266. doi: 10.1007/s10802-013-9762-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moser JS, Moran TP, Schroder HS, Donnellan MB, & Yeung N (2013). On the relationship between anxiety and error monitoring: a meta-analysis and conceptual framework. Front Hum Neurosci, 7, 466. doi: 10.3389/fnhum.2013.00466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olvet DM, Klein DN, & Hajcak G (2010). Depression symptom severity and error-related brain activity. Psychiatry Res, 179(1), 30–37. doi: 10.1016/j.psychres.2010.06.008 [DOI] [PubMed] [Google Scholar]
- Op de Macks ZA, Bunge SA, Bell ON, Wilbrecht L, Kriegsfeld LJ, Kayser AS, & Dahl RE (2016). Risky decision-making in adolescent girls: The role of pubertal hormones and reward circuitry. Psychoneuroendocrinology, 74, 77–91. doi: 10.1016/j.psyneuen.2016.08.013 [DOI] [PubMed] [Google Scholar]
- Peper JS, Hulshoff Pol HE, Crone EA, & van Honk J (2011). Sex steroids and brain structure in pubertal boys and girls: a mini-review of neuroimaging studies. Neuroscience, 191, 28–37. doi: 10.1016/j.neuroscience.2011.02.014 [DOI] [PubMed] [Google Scholar]
- Petersen AC, Crockett L, Richards M, & Boxer A (1988). A self-report measure of pubertal status: Reliability, validity, and initial norms. Journal of Youth and Adolescence, 17(2), 117–133. [DOI] [PubMed] [Google Scholar]
- Riesel A, Endrass T, Auerbach LA, & Kathmann N (2015). Overactive Performance Monitoring as an Endophenotype for Obsessive-Compulsive Disorder: Evidence From a Treatment Study. Am J Psychiatry, 172(7), 665–673. doi: 10.1176/appi.ajp.2014.14070886 [DOI] [PubMed] [Google Scholar]
- Riesel A, Endrass T, Kaufmann C, & Kathmann N (2011). Overactive error-related brain activity as a candidate endophenotype for obsessive-compulsive disorder: evidence from unaffected first-degree relatives. Am J Psychiatry, 168(3), 317–324. doi: 10.1176/appi.ajp.2010.10030416 [DOI] [PubMed] [Google Scholar]
- Santesso DL, Segalowitz SJ, & Schmidt LA (2006). Error-related electrocortical responses are enhanced in children with obsessive-compulsive behaviors. Dev Neuropsychol, 29(3), 431–445. doi: 10.1207/s15326942dn2903_3 [DOI] [PubMed] [Google Scholar]
- Schoenberg PL (2014). The error processing system in major depressive disorder: cortical phenotypal marker hypothesis. Biol Psychol, 99, 100–114. doi: 10.1016/j.biopsycho.2014.03.005 [DOI] [PubMed] [Google Scholar]
- Schulz KM, Molenda-Figueira HA, & Sisk CL (2009). Back to the future: The organizational-activational hypothesis adapted to puberty and adolescence. Horm Behav, 55(5), 597–604. doi: 10.1016/j.yhbeh.2009.03.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shackman AJ, Salomons TV, Slagter HA, Fox AS, Winter JJ, & Davidson RJ (2011). The integration of negative affect, pain and cognitive control in the cingulate cortex. Nat Rev Neurosci, 12(3), 154–167. doi: 10.1038/nrn2994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel JM, Yancey AK, Aneshensel CS, & Schuler R (1999). Body image, perceived pubertal timing, and adolescent mental health. Journal of adolescent health, 25(2), 155–165. [DOI] [PubMed] [Google Scholar]
- Silk JS, Davis S, McMakin DL, Dahl RE, & Forbes EE (2012). Why do anxious children become depressed teenagers? The role of social evaluative threat and reward processing. Psychol Med, 42(10), 2095–2107. doi: 10.1017/S0033291712000207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sisk CL (2017). Development: Pubertal Hormones Meet the Adolescent Brain. Curr Biol, 27(14), R706–R708. doi: 10.1016/j.cub.2017.05.092 [DOI] [PubMed] [Google Scholar]
- Sisk CL, & Zehr JL (2005). Pubertal hormones organize the adolescent brain and behavior. Front Neuroendocrinol, 26(3–4), 163–174. doi: 10.1016/j.yfrne.2005.10.003 [DOI] [PubMed] [Google Scholar]
- Sontag LM, & Graber JA (2010). Coping with perceived peer stress: gender-specific and common pathways to symptoms of psychopathology. Dev Psychol, 46(6), 1605–1620. doi: 10.1037/a0020617 [DOI] [PubMed] [Google Scholar]
- Sontag LM, Graber JA, & Clemans KH (2011). The role of peer stress and pubertal timing on symptoms of psychopathology during early adolescence. J Youth Adolesc, 40(10), 1371–1382. doi: 10.1007/s10964-010-9620-8 [DOI] [PubMed] [Google Scholar]
- Tamnes CK, Walhovd KB, Torstveit M, Sells VT, & Fjell AM (2013). Performance monitoring in children and adolescents: a review of developmental changes in the error-related negativity and brain maturation. Dev Cogn Neurosci, 6, 1–13. doi: 10.1016/j.dcn.2013.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Veen V, & Carter CS (2002). The anterior cingulate as a conflict monitor: fMRI and ERP studies. Physiol Behav, 77(4–5), 477–482. [DOI] [PubMed] [Google Scholar]
- Weinberg A, Klein DN, & Hajcak G (2012). Increased error-related brain activity distinguishes generalized anxiety disorder with and without comorbid major depressive disorder. J Abnorm Psychol, 121(4), 885–896. doi: 10.1037/a0028270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberg A, Kotov R, & Proudfit GH (2015). Neural indicators of error processing in generalized anxiety disorder, obsessive-compulsive disorder, and major depressive disorder. J Abnorm Psychol, 124(1), 172–185. doi: 10.1037/abn0000019 [DOI] [PubMed] [Google Scholar]
- Weinberg A, Liu H, & Shankman SA (2016). Blunted neural response to errors as a trait marker of melancholic depression. Biol Psychol, 113, 100–107. doi: 10.1016/j.biopsycho.2015.11.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberg A, Meyer A, Hale-Rude E, Perlman G, Kotov R, Klein DN, & Hajcak G (2016). Error-related negativity (ERN) and sustained threat: Conceptual framework and empirical evaluation in an adolescent sample. Psychophysiology, 53(3), 372–385. doi: 10.1111/psyp.12538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeung N, Botvinick MM, & Cohen JD (2004). The neural basis of error detection: conflict monitoring and the error-related negativity. Psychol Rev, 111(4), 931–959. doi: 10.1037/0033-295X.111.4.939 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
On the left, topographic map of neural activity (error minus correct) for AD and HC youth (from top to bottom, respectively) prior to adjusting for pubertal timing and depression. On the right, response-locked ERP waveform for correct and error trials, as well as the difference waves (error-related negativity; ΔERN) at FCz for AD and HC youth (from top to bottom, respectively). Increases in the ΔERN are represented by values that are more negative in polarity. AD = Anxiety Disorders; HC = Healthy Control.


