Abstract
Background:
Recently, we identified the circadian rhythm protein Period 2 (PER2) in robust cardioprotection from myocardial ischemia (MI). Based on findings that perioperative MI is the most common major cardiovascular complication and that anesthetics can alter the expression of PER2, we hypothesized that an anesthesia mediated downregulation of PER2 could be detrimental if myocardial ischemia and reperfusion (IR) would occur.
Methods and Results:
We exposed mice to pentobarbital, fentanyl, ketamine, propofol, midazolam or isoflurane and determined cardiac Per2 mRNA levels. Unexpectedly, only midazolam treatment resulted in an immediate and significant downregulation of Per2 transcript levels. Subsequent studies in mice pretreated with midazolam using an in-situ mouse model for myocardial (IR)-injury revealed a significant and dramatic increase in infarct sizes or Troponin-I serum levels in the midazolam treated group when compared to controls. Using the recently identified flavonoid, nobiletin, as a PER2 enhancer completely abolished the deleterious effects of midazolam during myocardial IR-injury. Moreover, nobiletin treatment alone significantly reduced infarct sizes or Troponin I levels in wildtype but not in Per2−/− mice. Pharmacological studies on nobiletin like flavonoids revealed that only nobiletin and tangeritin, both found to enhance PER2, were cardioprotective in our murine model for myocardial IR-injury.
Conclusion:
We identified midazolam mediated downregulation of cardiac PER2 as an underlying mechanism for a deleterious effect of midazolam pretreatment in myocardial IR-injury. These findings highlight PER2 as a cardioprotective mechanism and suggest the PER2 enhancers nobiletin or tangeritin as preventative therapy for myocardial IR-injury in the perioperative setting where midazolam pretreatment occurs frequently.
Keywords: Per2, nobiletin, perioperative myocardial ischemia, midazolam, flavonoid, circadian
Introduction
More than 230 million major surgeries are performed annually worldwide, and this number grows continuously [1]. Cardiac complications, including nonfatal myocardial infarction (MI), pose a significant risk to patients undergoing major non-cardiac surgery. In fact, perioperative Ml is the most common major cardiovascular complication [2]. Moreover, based on the prospective cohort VISION study (vascular events in noncardiac surgery patients cohort evaluation) [3], each year non-cardiac surgery accounts for approximately 8 million myocardial injuries with a 30-day mortality rate of 10% worldwide, indicating the need for novel cardioprotective therapies. Recently, the circadian rhythm protein Period2 (PER2) was identified as an important regulator of hypoxia and ischemia dependent pathways in the heart [4–6]. PER2 is believed to be a key mediator of cardioprotection through optimization of hypoxia inducible factor 1 alpha (HIF1A) dependent carbohydrate metabolism during myocardial ischemia [4, 5, 7, 8]. Interestingly, it has been found that anesthetics used in the perioperative setting can change the expression of circadian proteins including PER2 [9–11]. However, if this would also lead to an increased susceptibility to myocardial ischemia and reperfusion injury is unknown. Based on the recently identified cardioprotective role of PER2, we hypothesized that anesthesia mediated alteration of PER2 expression would be detrimental if myocardial ischemia and reperfusion would occur. Using a well-established in situ-mouse model of myocardial IR-injury [12, 13] we found that the benzodiazepine midazolam, the most commonly used anesthetic prior to surgical procedures worldwide [14], is deleterious on the heart by downregulating PER2. Pharmacological studies using the PER2 enhancer nobiletin completely reversed the midazolam mediated effects on the heart. In addition, pretreatment of mice with nobiletin alone revealed robust cardioprotection which was abolished in Per2−/− mice, indicating that nobiletin is PER2 specific. Finally, in depth pharmacological studies comparing compounds similar to nobiletin identified a prominent role for PER2 enhancers in cardioprotection from myocardial IR-injury.
Methods
Mouse Experiments.
Experimental protocols were approved by the Institutional Review Board (Institutional Animal Care and Use Committee [IACUC]) at the University of Colorado Denver, USA. They were in accordance with the NIH guidelines for use of live animals. Mice were housed in a 14/10-h light-dark cycle and all mouse experiments were conducted at the same time point (7AM-12PM). To eliminate gender- and age-related variations, we routinely used 12- to 16-week-old male mice [4, 6].
Pharmacological compounds.
Nobiletin, tangeretin, sinensetin, 5,6,7-trimethoxyflavone, flavone, solutol (Kolliphor® HS 15) and pentobarbital sodium salt were purchased from Sigma-Aldrich (St. Louis, MO, USA). 3’,4’,7,8-tetramethoxyflavone was purchased from Alfa Aesar (Tewksbury, MA, USA). Midazolam, ketamine and fentanyl were obtained from Pfizer Inc. (NY, NY, USA). Propofol was obtained from Fresenius Kabi (Lake Zurich, IL, USA). Isoflurane was purchased from Baxter (Deerfield, IL, USA). The total volume of all administered drugs was 0.5 ml.
Transcriptional analysis.
C57BL/6J wildtype mice were treated with a single dose of either intraperitoneally (i.p.) pentobarbital (70 mg/kg), fentanyl (1 mg/kg), ketamine (200 mg/kg), midazolam (200 mg/kg) or propofol (200 mg/kg), while isoflurane (1% inhaled) was maintained throughout. Two hours later mice were euthanized, and the heart tissue was harvested. Protocol details are given in Fig. 1A (I). Myocardial cells were treated with midazolam or vehicle (0.9% NaCl) for 6 h. Total RNA was isolated from whole heart tissue or murine primary cardiac myocytes, primary cardiac endothelia and primary cardiac fibroblasts by Qiazol Reagent (Qiagen) and chloroform extraction in conjunction with the RNeasy Mini Kit (Qiagen), following the manufacturer’s instructions (SA-Biosciences, Qiagen). cDNA from mRNA was generated using iScript (Bio-Rad) and transcript levels were determined by real-time RT-PCR (iCycler; Bio-Rad Laboratories Inc.) [15]. The PCR reactions contained 1 μM sense and 1 μM antisense oligonucleotides with SYBR Green (Bio-Rad, 170–8880). Each target sequence was amplified as follows: 1× (95°C for 3 min), 40× (95°C for 15 sec, 55°C for 30 sec, 72°C for 10 sec), 1× (72°C for 1 min), 4°C hold. Primer sets for mouse Per2 were from Qiagen (Mm_Per2_SG QuantiTect Primer Assay). Primer sets for mouse beta-Actin were from Qiagen (Mm_Actb_2_SG QuantiTect Primer Assay).
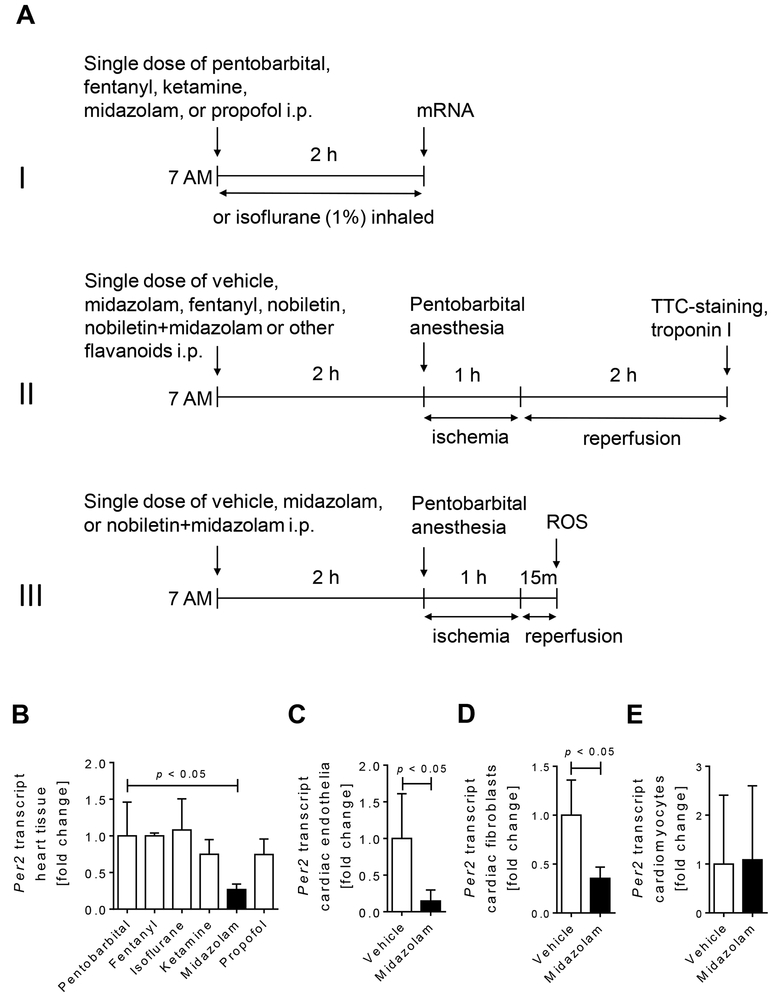
Figure 1. Studies of cardiac Per2 regulation following exposure of wildtype mice to anesthetics.
Wildtype mice were exposed to a single dose of pentobarbital (70 mg/kg i.p.), fentanyl (1 mg/kg i.p.), isoflurane (1% inhaled), ketamine (200mg/kg i.p.), propofol (200 mg/kg i.p.), or midazolam (200 mg/kg i.p.). Two hours later cardiac Per2 mRNA expression levels were analyzed. In a subset of experiments murine endothelia, fibroblasts or cardiomyocytes were exposed to vehicle (NaCl 0.9%) or midazolam for 6 hours. Total RNA was isolated by Qiazol Reagent (Qiagen) and chloroform extraction in conjunction with the RNeasy Mini Kit (Qiagen), following the manufacturer’s instructions (SA-Biosciences, Qiagen). cDNA from mRNA was generated using iScript (Bio-Rad) and transcript levels were determined by real-time RT-PCR (iCycler; Bio-Rad Laboratories Inc.). (A) Overview and timeline of all in vivo studies. (I) Screening of different anesthetics for their effect on mouse heart Per2 mRNA levels. (II) Myocardial ischemia and reperfusion studies. (III) Reactive oxygen species (ROS) measurements following myocardial ischemia. (B) Mouse cardiac Per2 mRNA levels two hours after exposure to different anesthetics. (C) Per2 mRNA levels from murine cardiac endothelia after 6 h of midazolam (50μM) exposure. (D) Per2 mRNA levels from murine cardiac fibroblasts after 6 h of midazolam (50μM) exposure. (E) Per2 mRNA levels from isolated murine cardiomyocytes after 6 h of midazolam (50μM) exposure; (mean±SD, n=4–6; p<0.05).
Cell culture experiments.
All experiments were conducted after serum starvation in order to synchronize cells for PER2 expression [4].
Isolation of adult cardiomyocytes [4, 16].
The protocol for isolation of adult cardiomyocytes was adapted from O’Connell et al [17]. C57BL6/J mice were anesthetized, and the heart was quickly removed from the chest cavity and immediately placed in KHB buffer. The aorta was cannulated, and the heart perfused with Ca2+-free KHB for 3 min followed by 8–12 min perfusion with KHB containing 40 μM Ca2+ in and collagenase II (Worthington Biochemical Corp). After perfusion, ventricles were removed, minced and incubated in 15 mL collagenase solution for an additional 3–7 min. An equal volume of stopping buffer (KHB containing 10% FBS, 12.5 μM Ca2+, and 2 mM ATP) was added to the digestion solution. Myocytes were allowed to sediment by gravity for 3 min at room temperature and centrifuged at 20 × g for 3 min. The pellet was resuspended in 100 μM Ca2+ and sedimentation followed by centrifugation was repeated for subsequent 400 μM and 900 μM Ca2+ in a slow calcium re-introduction process. Myocytes were resuspended in MEM (Gibco 11575–032) supplemented with 10% FBS, 10 mM BDM, 100 U/mL Pen/Strep, and 2 mM ATP and plated on laminin-coated plates (10 μg/mL laminin in PBS). Myocytes were incubated in a 37°C, 2% CO2 incubator. After healthy myocyte adhesion, media was exchanged for MEM supplemented with 10 mM BDM, 1 X ITS (final concentrations of 5 μg/mL insulin, 5 μg/mL transferrin, and 5 ng/mL selenium), 100 U/mL Pen/Strep, and 1 mg/mL BSA. Myocytes were treated with midazolam or vehicle (0.9% NaCl) in this culture medium for 6 h and thereafter immediately resuspended in Qiazol for mRNA isolation and gene expression analysis [4].
Murine cardiac endothelial cells.
C57BL/6 mouse primary cardiac endothelial cells were obtained from Cell Biologics (C57–6024) and handled following the manufacturer’s instructions in complete mouse endothelial cell medium supplemented with VEGF, ECGS, heparin, EGF, hydrocortisone, L-glutamine, antibiotic-antimycotic solution, and FBS (M1168). After cells reached confluency, endothelia were exposed to midazolam or vehicle (0.9% NaCl) for 6 h and thereafter immediately resuspended in Qiazol for mRNA analysis.
Isolation of fibroblasts.
Heart tissue from C57BL6/J mice was minced and digested using Collagenase Type II solution (Worthington Biochemical Corporation) at 37°C, 100 rpm, collecting the supernatant every 10 minutes for 90 minutes and replacing with fresh collagenase solution until the heart tissue was fully digested. Fibroblasts were isolated after plating and incubation of the cell suspension in a cell culture incubator with 5% CO2 for 2 h. 2 h upon plating alive and healthy fibroblasts were adhered to the dish. After cells reached confluency, fibroblasts were exposed to midazolam or vehicle (0.9% NaCl) for 6 h and thereafter immediately resuspended in Qiazol for mRNA analysis.
Murine Model for myocardial ischemia and reperfusion injury [4, 6, 12, 13, 16, 18–21].
C57BL/6J or Per2−/− mice (Per2tm1Brd Tyrc-Brd/J) were obtained from the Jackson Laboratories [22, 23]. Two hours prior to ischemia mice were pre-treated with a single dose of either vehicle (0.9% NaCl or solutol:0.9%NaCl [1:100] i.p.), fentanyl (1 mg/kg i.p.), midazolam (200 mg/kg i.p.), nobiletin (1 mg/kg i.p.) in solutol:0.9% NaCl 0.9% [1:100 ratio], nobiletin+midazolam or flavone (0.55 mg/kg), tangeritin (0.93 mg/kg), sinensetin (0.93 mg/kg), 5,6,7-trimethoxyflavone (0.78 mg/kg), and 3’,4’,7,8-tetramethoxyflavone (0.85 mg/kg) in solutol:0.9%NaCl (ratio 1:100). Protocol details are given in Fig.1A (II). Anesthesia was induced with 10–70mg/kg i.p. and maintained with 10 mg/kg/h i.p. of pentobarbital as necessary. Myocardial ischemia and reperfusion injury in mice was performed as described previously [4, 6, 12, 13, 16, 18–21]. Infarct sizes were determined by calculating the percentage of infarcted myocardium to the AAR using a double staining technique with Evan’s blue and triphenyltetrazolium chloride (TTC). Using planimetry via the NIH software Image 1.0 (National Institutes of Health, Bethesda, MA), the AAR and the infarct size were determined. All animals were under deep anesthesia while performing surgical procedures. After study completion, animals were euthanized with an overdose of pentobarbital and exsanguination.
Heart Enzyme Measurement.
Blood was collected by central venous puncture for troponin I (cTnI) measurements using a quantitative rapid cTnI assay (Life Diagnostics, Inc., West Chester, PA, USA). cTnI is highly specific for myocardial ischemia and has a well-documented correlation with the infarct size in mice [12, 18, 19, 21] and humans [24].
Hydrogen peroxide assay.
Hydrogen peroxide levels were measured using the hydrogen peroxide assay kit (Abcam, Cambridge, UK) according to the manufacturer’s protocol. In brief, the left ventricle (area at risk) was harvested after 60 min of ischemia and 15 min of reperfusion (Fig.1A (III)). Heart tissues were homogenized in assay buffer and spun down at 13,000 g, 4°C for 5 min. Supernatants were deproteinized by adding ice-cold perchloric acid. The samples were again centrifuged at 13,000×g, 4 °C for 2 min, and the supernatant was precipitated by adding ice-cold potassium hydroxide. Next, samples were centrifuged at 13,000×g, 4 °C for 15 min, and hydrogen peroxide was measured in the supernatant. Using a 96-well microplate, samples were added, and reactions were initiated immediately by adding OxiRed. Fluorescence was measured on Synergy 2 Multi-Mode Microplate reader (Biotech, Winooski, VT, USA) in excitation range of 540/25 nm and emission detection of 620/40 nm. Fluorescence levels were normalized to the protein concentration of samples before deproteinization.
Data analysis.
Data were compared by an unpaired one-way ANOVA with Tukey’s post-hoc test, or by an unpaired Student’s t-test where appropriate. Correlation analysis was performed using liner regression. Values are expressed as mean (±SD). P<0.05 was considered statistically significant. For all statistical analysis, GraphPad Prism 6.0 software was used. The authors had full access to and take full responsibility for the integrity of the data. All authors have read and agree to the manuscript as written.
Results
Midazolam downregulates cardiac expressed PER2 in wildtype mice.
Based on observations that anesthesia can alter the expression of circadian rhythm proteins [9, 10, 25] we exposed mice to anesthetics used frequently in the clinical setting. Experimental design and timeline of in vivo studies are given in Fig. 1A. Pentobarbital (70 mg/kg), fentanyl (1 mg/kg), ketamine (200 mg/kg), midazolam (200 mg/kg) or propofol (200 mg/kg), were given intraperitoneally (i.p.) as a single dose, while isoflurane (1%) was maintained throughout, which caused a loss of righting reflex and loss of the pedal withdrawal reflex [26]. Two hours later animals were euthanized with an overdose of pentobarbital and we analyzed cardiac Per2 mRNA expression levels. While fentanyl, isoflurane, ketamine, or propofol had no significant effects on Per2 expression, the benzodiazepine midazolam revealed a robust and significant downregulation of cardiac Per2 transcript levels (0.3-fold, p<0.05; Fig. 1B). To understand if this was a direct effect on cardiac tissue, we obtained cardiac endothelia, or isolated cardiac fibroblasts and cardiomyocytes from C57BL/6 wildtype mice to establish a primary murine cell line. After confirmation of cell viability, primary murine endothelia, fibroblasts or cardiomyocytes were exposed to midazolam (50 μM) or 0.9% NaCl vehicle for 6 hours. As shown in Fig. 1C-E, midazolam significantly reduced Per2 mRNA expression in murine cardiac endothelial cells or fibroblasts but had no effect on primary murine cardiomyocytes. Taken together, when comparing fentanyl, propofol, ketamine, isoflurane, and midazolam, only the benzodiazepine midazolam significantly reduces murine cardiac Per2 mRNA levels.
Midazolam administration increases infarct sizes and Troponin-I levels in an in-situ mouse model for myocardial ischemia and reperfusion injury.
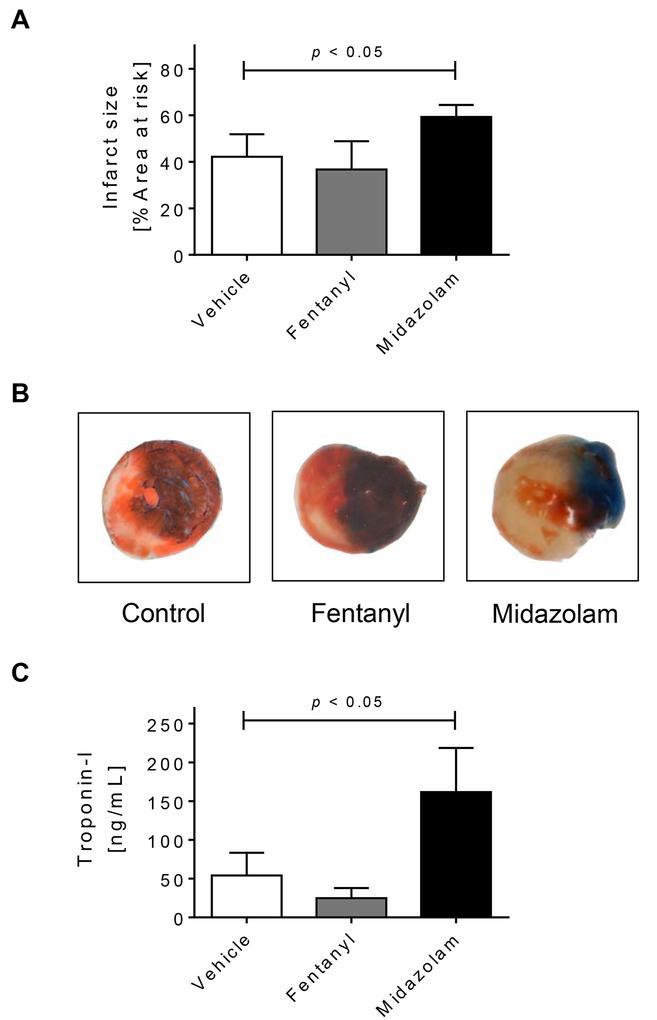
After we found that midazolam significantly downregulated cardiac Per2 mRNA levels, we next pursued myocardial IR-studies following midazolam administration. Here, we used fentanyl as a secondary control as it had no effect on cardiac Per2 mRNA levels (Fig.1B). C57BL/6 wildtype mice were pretreated with a single dose i.p. of 200 mg/kg of midazolam, 1mg/kg fentanyl, or vehicle (0.9% NaCl). Two hours later wildtype mice underwent 60 min of myocardial ischemia followed by 120 min of reperfusion. Anesthesia was induced and maintained with pentobarbital. As shown in Fig.2A-B, fentanyl pretreatment had no significant effect on infarct sizes when compared to our standard control using pentobarbital. However, midazolam pretreatment significantly increased infarct sizes when compared to control or fentanyl treated animals (mean ± SD, control: 42.2 ± 9.7 % or fentanyl: 36.7 ± 12.1% vs midazolam 59.3 ± 5.2%, p<0.05; Fig.2A-B). To confirm our results from the infarct size analysis which were obtained via TTC and Evans Blue double staining, we next determined Troponin-I serum levels using ELISA. Indeed, mice administered midazolam prior to IR-injury had significantly higher Troponin-I serum levels when compared to vehicle or fentanyl controls (mean ± SD: control: 54.1 ± 29.1 ng/ml or fentanyl: 24.9 ± 13.1 ng/ml vs midazolam 161.7 ± 57.0 ng/ml, p<0.05; Fig.2C). We next investigated if midazolam mediated changes in Per2 transcript levels would also correlate with Troponin-I serum levels. A shown in Fig. 2D, regression analysis revealed a significant negative correlation between Per2 transcript and Troponin-I levels (r=−0.79, p<0.01). Taken together, midazolam mediated downregulation of cardiac expressed and cardioprotective murine PER2 is associated with deleterious consequences following myocardial ischemia and reperfusion injury.
Figure 2. Midazolam in myocardial ischemia and reperfusion injury.
Mice were pretreated with vehicle (NaCl 0.9%), fentanyl (1mg/kg) or midazolam (200mg/kg) i.p. 2 hours prior to myocardial ischemia. Myocardial ischemia consisted of 60 min of ischemia followed by 120 minutes of reperfusion. Infarct sizes were measured by double staining with Evan’s blue and triphenyl-tetrazolium chloride. Infarct sizes are expressed as the percent of the area at risk (AAR) that underwent infarction. Serum troponin I concentrations were measured by enzyme-linked immunosorbent assay (ELISA). (A) Infarct sizes as the percent of AAR; (B) Representative infarct staining; (C) Serum troponin I concentrations; (D) Linear Regression analysis between Per2 transcript and troponin I levels; (n=5–7; mean±SD; p<0.05).
The PER2 enhancer nobiletin reverses the deleterious effects of midazolam and is cardioprotective in a PER2 dependent manner.
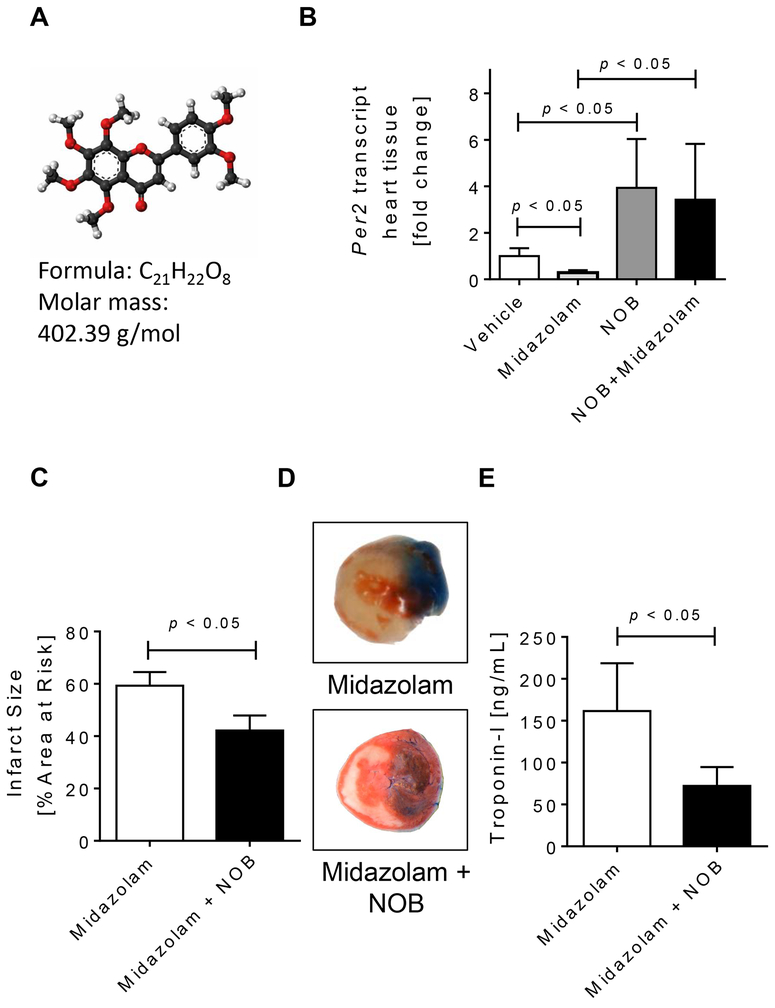
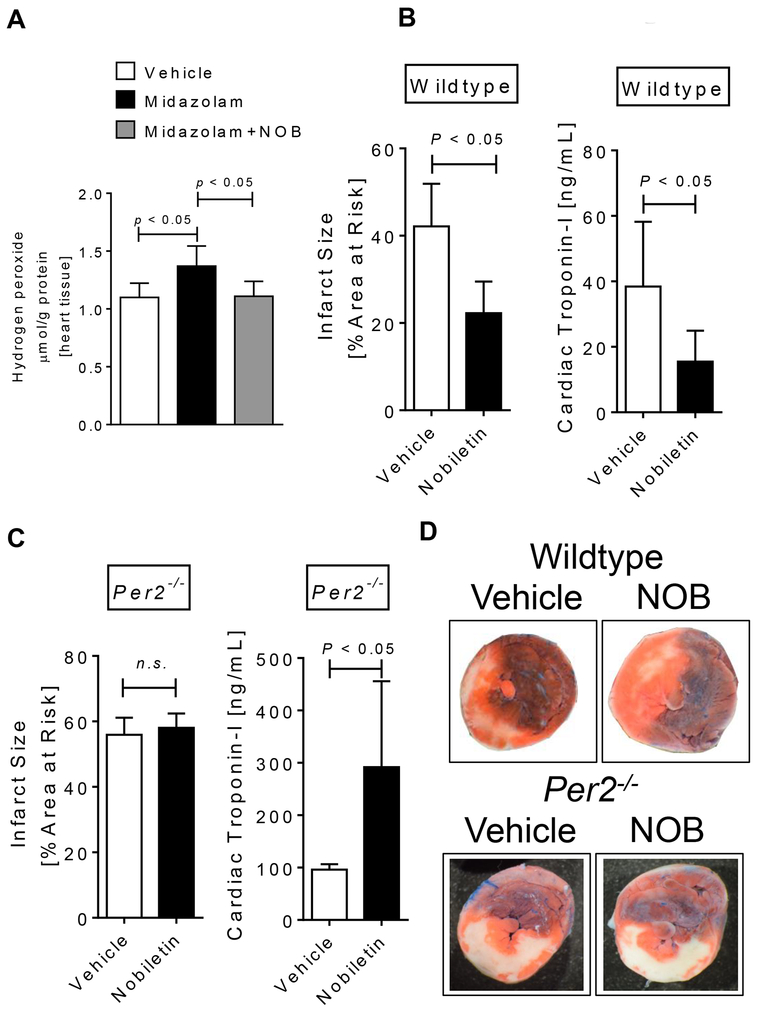
After we found that a midazolam mediated downregulation of cardiac Per2 was associated with increased infarct sizes or Troponin-I levels following myocardial IR-injury, we next investigated if we could reverse these effects by using a PER2 enhancer. A recent large-scale screen identified nobiletin, a flavonoid from citrus peels, as a potent PER2 enhancer [27] (Fig. 3A). Next, we treated mice with vehicle (solutol in 0.9% NaCl, solutol:NaCl 0.9% [1:100 ratio]), nobiletin (1mg/kg i.p. in solutol:NaCl 0.9% [1:100 ratio]) alone or together with midazolam and determined cardiac Per2 mRNA levels 2 hours later. As seen in Fig. 3B, nobiletin significantly increased cardiac Per2 transcript levels (3.9-fold, p<0.05) and reversed midazolam mediated downregulation of cardiac Per2. Following co-administration of midazolam and nobiletin 2 hours prior to myocardial ischemia, the deleterious effects of midazolam during myocardial IR-injury were fully reversed. In fact, co-administration of midazolam and nobiletin significantly reduced myocardial infarct sizes and Troponin-I serum levels, when compared to midazolam treated mice (mean ± SD: infarct sizes: midazolam: 59.3 ± 5.2% vs nobiletin + midazolam: 42.15 ± 5.7% or Troponin-I: midazolam: 161.7 ± 57.0 ng/ml vs nobiletin + midazolam: 72.1 ± 22.6 ng/ml, Fig. 3C-E), resulting in infarct sizes or serum Troponin-I levels similar to vehicle treated mice (Fig. 2). Based on the increased infarct sizes following midazolam administration, we next investigated if midazolam would also increase the production of reactive oxygen species (ROS) during reperfusion injury. We therefore determined H2O2 tissue levels in the left ventricle after 60 minutes of ischemia and 15 min or reperfusion. As shown in Fig. 4A, while midazolam significantly increased ROS production when compared to saline treated controls, nobiletin + midazolam treatment resulted in ROS levels similar to vehicle treated mice. Next, we analyzed the cardioprotective effects of nobiletin alone. Pretreatment of wildtype mice with nobiletin 2 hours prior to myocardial ischemia significantly reduced myocardial infarcts sizes (mean ± SD, vehicle: 42.2 ± 9.7 % vs nobiletin: 22.2 ± 7.2%, p<0.05; Fig. 4B, C) or Troponin-I levels (mean ± SD: vehicle: 54.1 ± 29.1 ng/ml vs nobiletin: 13.0 ± 6.8 ng/ml, p<0.05; Fig. 4D) when compared to vehicle (solutol in 0.9% NaCl, solutol:NaCl 0.9% [1:100 ratio]) treated controls. To understand if nobiletin would be PER2 specific we administered nobiletin to Per2−/− mice prior to IR-injury. As shown in Fig. 4C, D nobiletin revealed no cardioprotection in Per2−/− mice (mean infarct sizes ± SD, vehicle: 55.9 ± 5.2 % vs nobiletin: 58.0 ± 4.4%, n.s. and mean Troponin-I ± SD, vehicle: 96.2 ± 10.4 % vs nobiletin: 291.2 ± 164.0%, p<0.05). Taken together, the PER2 enhancer nobiletin abolishes the deleterious effects of midazolam and is cardioprotective in a PER2 dependent manner during myocardial ischemia and reperfusion injury.
Figure 3. Nobiletin reverses the deleterious effects of midazolam.
Mice were treated i.p. with vehicle (solutol in 0.9% NaCl [ratio 1:100]), nobiletin in solutol:0.9%NaCl (ratio 1:100; 1mg/kg), midazolam (200mg/kg) or midazolam + nobiletin. Two hours later cardiac Per2 mRNA expression levels were analyzed, or mice underwent myocardial ischemia. Myocardial ischemia consisted of 60 min of ischemia followed by 120 minutes of reperfusion. Infarct sizes were measured by double staining with Evan’s blue and triphenyl-tetrazolium chloride. Infarct sizes are expressed as the percent of the area at risk (AAR) that underwent infarction. Serum troponin I concentrations were measured by enzyme-linked immunosorbent assay (ELISA). (A) Molecular formula of nobiletin, a potent PER2 enhancer. (B) Mouse cardiac Per2 mRNA levels two hours after exposure to vehicle, midazolam, nobiletin or midazolam+nobiletin. (C) Infarct sizes as the percent of AAR; (D) Representative infarct staining; (E) Serum troponin I concentrations; (n=5–7; mean±SD; p<0.05; NOB=nobiletin).
Figure 4. Nobiletin is cardioprotective via PER2 and reverses midazolam induced ROS production.
Mice were treated i.p. with vehicle (solutol in 0.9% NaCl [ratio 1:100]), nobiletin in solutol:0.9%NaCl (ratio 1:100; 1mg/kg), midazolam (200mg/kg) or midazolam + nobiletin 2 hours prior to myocardial ischemia. Myocardial ischemia consisted of 60 min of ischemia followed by 120 minutes of reperfusion. For ROS (H2O2) measurements reperfusion consisted of 15 minutes after 60 min of ischemia. Infarct sizes were measured by double staining with Evan’s blue and triphenyl-tetrazolium chloride. Infarct sizes are expressed as the percent of the area at risk (AAR) that underwent infarction. Serum troponin I concentrations were measured by enzyme-linked immunosorbent assay (ELISA). (A) ROS production determined by H2O2 measurement in the area at risk of wildtype mice. (B) Infarct sizes as the percent of AAR and serum troponin I levels in wildtype mice treated with vehicle or nobiletin (1mg/kg) 2 hours prior to myocardial ischemia. (C) Infarct sizes as the percent of AAR and serum troponin I levels in Per2−/− mice treated with vehicle or nobiletin (1mg/kg) 2 hours prior to myocardial ischemia. (D) Representative infarct staining from wildtype and Per2−/− mice; (n=7–8; mean±SD; p<0.05; NOB=nobiletin).
The flavonoids and PER2 enhancer nobiletin and tangeritin are cardioprotective.
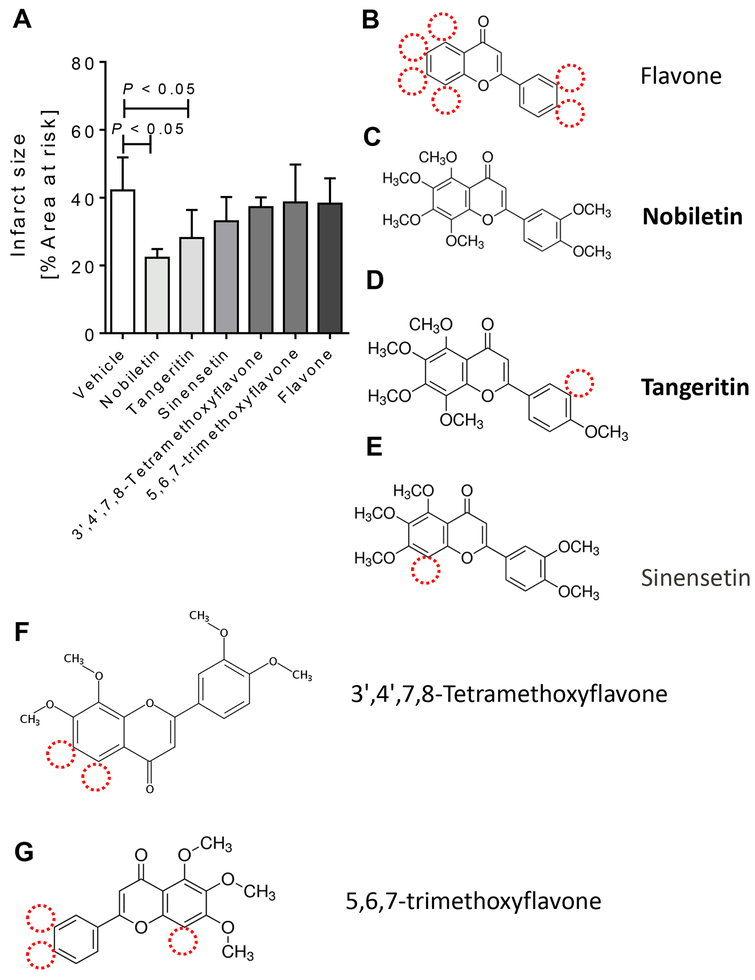
Based on our findings that the flavonoid nobiletin had a robust cardioprotective effect during myocardial ischemia and reperfusion injury, we evaluated if this was a general flavonoid mediated effect [28]. We therefore performed a comparison of compounds similar to nobiletin or basic flavonoid structured compounds during our murine model of IR-injury. C57BL/6 wildtype mice received nobiletin (1 mg/kg) equimolar doses in solutol:saline [ratio 1:100] of flavone (0.55 mg/kg), tangeritin (0.93 mg/kg), sinensetin (0.93 mg/kg), 5,6,7-trimethoxyflavone (0.78 mg/kg), or 3’,4’,7,8-tetramethoxyflavone (0.85 mg/kg) 2 hours prior to myocardial ischemia via intraperitoneal injection. While most compounds are very similar to nobiletin, only tangeritin was also found to be a PER2 enhancer [27]. As shown in Fig. 5, only mice who received tangeritin or nobiletin had significantly smaller infarct sizes when compared to vehicle (solutol in 0.9% NaCl, solutol:NaCl 0.9% [1:100 ratio]) treated mice (mean ± SD, vehicle: 42.2 ± 9.7 % vs nobiletin: 22.2 ± 7.2% or tangeritin 28.1 ± 8.3%, p<0.05). Taken together, using nobiletin similar flavonoids during myocardial ischemia and reperfusion injury, only the PER2 enhancer tangeritin revealed similar cardioprotective effects like nobiletin.
Figure 5. Flavonoids in cardioprotection from IR injury.
Mice underwent 60 min of ischemia and 120 min of reperfusion. Infarct sizes were measured by double staining with Evan’s blue and triphenyl-tetrazolium chloride. Infarct sizes are expressed as the percent of the area at risk (AAR) that underwent infarction. C57BL/6 wildtype mice received vehicle (solutol in 0.9% NaCl [ratio 1:100]) or nobiletin (1mg/kg) equimolar doses of flavone (0.55 mg/kg), tangeritin (0.93 mg/kg), sinensetin (0.93 mg/kg), 5,6,7-trimethoxyflavone (0.78 mg/kg), or 3’,4’,7,8-tetramethoxyflavone (0.85 mg/kg) in solutol:0.9%NaCl (ratio 1:100) 2 hours prior to myocardial ischemia via intraperitoneal injection. (A) Infarct sizes as the percent of AAR; (B) flavone; (C) nobiletin; (C) tangeritin; (E) sinensetin; (F) 3’,4’,7,8-tetramethoxyflavone; (G) 5,6,7-trimethoxyflavone; (n=5–8; mean±SD; p<0.05).
Discussion
Our novel findings in this study are: 1) comparing different anesthetics, only midazolam treatment of mice results in a robust and direct downregulation of cardiac expressed PER2; 2) midazolam, if given prior to murine myocardial ischemia, has significant deleterious effects on the myocardium; 3) the flavonoid and PER2 enhancer nobiletin completely reverses the deleterious effects of midazolam during murine myocardial IR-injury; 4) treatment of Per2−/− mice with nobiletin reveals no cardioprotection, suggesting that nobiletin is PER2 specific; and 5) the PER2 enhancers tangeritin and nobiletin represent a novel therapy in murine myocardial IR-injury.
Midazolam first came into use in 1976 and is on the World Health Organization’s List of Essential Medicines, the most effective and safe medicines needed in a health system. The benzodiazepine binds to receptor sites in the gamma-aminobutyric acid (GABA) system. While midazolam is the benzodiazepine most frequently used for procedural sedation, recent clinical data suggest that midazolam is associated with severe clinical complications. As such midazolam has been associated with the occurrence of delirium and is therefore not recommended anymore as first line sedative on critical care units [29–32]. Moreover, a recent study found that continuous infusion of benzodiazepines is linked to an increased likelihood of death among patients who receive mechanical ventilation, when compared to the sedative propofol [11]. In fact, there is growing body of evidence that shows that benzodiazepines are associated with poorer patient outcomes, including increased brain dysfunction, time on mechanical ventilation, and ICU length of stay [33]. However, if midazolam use prior to perioperative myocardial ischemia could be deleterious is unknown. As each year non-cardiac surgery accounts for approximately 8 million myocardial injuries worldwide with a 30-day mortality rate of 10% [3], a deleterious effect of midazolam on heart ischemia could have major implications for current clinical practice. In fact, midazolam is the most commonly used anesthetic prior to surgical procedures worldwide [14].
Interestingly, animal studies have shown that midazolam abolishes the cardioprotective effects of ischemic preconditioning (IPC), while flumazenil, a midazolam antagonist, is cardioprotective in rabbits [34]. IPC, where the heart is pretreated with short non-lethal ischemic periods prior to a longer ischemia time is believed to be the strongest cardioprotective effect at the bench [35]. Mechanistic studies on midazolam in abolishing cardiac IPC suggest that midazolam via GABA receptors inhibits the mitochondrial adenosine triphosphate-sensitive potassium (K-ATP) channels, which in turn can activate the cardioprotective protein kinase C-ε (PKC-ε) – an important component within the preconditioning cascade [34]. Another study had similar findings using a cultured chick embryonic cardiomyocyte model of hypoxia and re-oxygenation [36]. In fact, the peripheral benzodiazepine receptor, a mitochondrial inner membrane protein, was found to play a role in mitochondrial function during cardiac IR-injury using perfused rat hearts [37]. These studies are in support of our findings and suggest that the observed deleterious effect of midazolam during IR-injury could have been a result of mitochondrial K-ATP channel blockade.
Other mechanistic studies on cardiac IPC identified a pivotal role of extracellular adenosine generation via CD73 and signaling through the adenosine receptor ADORA2B in myocardial tissue protection [4, 19, 21]. Following microarray studies in mice with deletion of ADORA2B signaling pathways during in situ myocardial IPC pointed towards the circadian rhythm protein PER2 [4]. Metabolic in vivo studies with labeled tracers indicated a limited ability of Per2−/− mice to use carbohydrates for oxygen-efficient glycolysis during myocardial ischemia or IPC. These studies further found that PER2 facilitated the optimization of carbohydrate metabolism through interaction with HIF1A. These studies support the findings of the current study and suggest that midazolam mediated downregulation of cardiac PER2 inhibits PER2 mediated cardioprotective effects. In fact, Per2−/− mice are not protected by IPC and have larger infarct sizes than controls [4], indicating a critical role for PER2 in IPC and IR-injury.
Midazolam increases GABAA signaling and our findings indicating midazolam downregulates PER2 are supported by recent studies on GABAA signaling as an important component of circadian rhythm protein regulation [38]. In fact it has been shown that GABAA activation can inhibit the expression of Per2 mRNA [39]. Moreover, GABAA receptor protein has been found to be present in mouse whole heart, with greater expression in the left ventricle and aorta and with a less expression in the atria and right ventricle [40]. While other anesthetics such as propofol or isoflurane also act partly on GABAA receptors, only midazolam primarily activates GABAA signaling, further supporting our current findings.
Recently, a wide search for circadian rhythm modifying molecules identified several small molecules including nobiletin, that could increase PER2 signaling [41]. In follow up studies, nobiletin, a flavonoid from citrus peels, was found not only to enhance the expression of PER2 but was also able to protect from a metabolic syndrome in mice [27]. While flavonoids have been implicated in protection from IR-injury in earlier studies [28], it seems striking to us that from the nobiletin similar flavonoids investigated in this study, only tangeritin, which also increases PER2 expression [27] was found to be cardioprotective.
In summary, this is the first report on how midazolam mediated alterations of PER2 expression could have functional consequences during IR-injury of the heart. Future studies are warranted to elucidate the clinical relevance of midazolam use on myocardial injury and PER2 signaling in the perioperative setting.
Acknowledgments
Source of financial support for the work:
National Heart, Lung, and Blood Institute (NIH-NHLBI) 5R01HL122472 Grant to T.E.; American Heart Association (AHA) Predoctoral Fellowship 16PRE30510006 and Colorado Clinical & Translational Sciences Institute (CCTSI) TL1 TR001081 to C.M.B.
Footnotes
Conflict of Interest
The authors declare there are no conflicts of interest.
References
- [1].Landesberg G, Beattie WS, Mosseri M, Jaffe AS, Alpert JS. Perioperative myocardial infarction. Circulation, 2009; 119: 2936–44. [DOI] [PubMed] [Google Scholar]
- [2].Goldman L, Caldera DL, Nussbaum SR, Southwick FS, Krogstad D, Murray B, Burke DS, O’Malley TA, Goroll AH, Caplan CH, Nolan J, Carabello B, Slater EE. Multifactorial index of cardiac risk in noncardiac surgical procedures. N Engl J Med, 1977; 297: 845–50. [DOI] [PubMed] [Google Scholar]
- [3].Botto F, Alonso-Coello P, Chan MT, Villar JC, Xavier D, Srinathan S, Guyatt G, Cruz P, Graham M, Wang CY, Berwanger O, Pearse RM, Biccard BM, Abraham V, Malaga G, Hillis GS, Rodseth RN, Cook D, Polanczyk CA, Szczeklik W, Sessler DI, Sheth T, Ackland GL, Leuwer M, Garg AX, Lemanach Y, Pettit S, Heels-Ansdell D, Luratibuse G, Walsh M, Sapsford R, Schunemann HJ, Kurz A, Thomas S, Mrkobrada M, Thabane L, Gerstein H, Paniagua P, Nagele P, Raina P, Yusuf S, Devereaux PJ, Devereaux PJ, Sessler DI, Walsh M, Guyatt G, McQueen MJ, Bhandari M, Cook D, Bosch J, Buckley N, Yusuf S, Chow CK, Hillis GS, Halliwell R, Li S, Lee VW, Mooney J, Polanczyk CA, Furtado MV, Berwanger O, Suzumura E, Santucci E, Leite K, Santo JA, Jardim CA, Cavalcanti AB, Guimaraes HP, Jacka MJ, Graham M, McAlister F, McMurtry S, Townsend D, Pannu N, Bagshaw S, Bessissow A, Bhandari M, Duceppe E, Eikelboom J, Ganame J, Hankinson J, Hill S, Jolly S, Lamy A, Ling E, Magloire P, Pare G, Reddy D, Szalay D, Tittley J, Weitz J, Whitlock R, Darvish-Kazim S, Debeer J, Kavsak P, Kearon C, Mizera R, O’Donnell M, McQueen M, Pinthus J, Ribas S, Simunovic M, Tandon V, Vanhelder T, Winemaker M, Gerstein H, McDonald S, O’Bryne P, Patel A, Paul J, Punthakee Z, Raymer K, Salehian O, Spencer F, Walter S, Worster A, Adili A, Clase C, Cook D, Crowther M, Douketis J, Gangji A, Jackson P, Lim W, Lovrics P, Mazzadi S, Orovan W, Rudkowski J, Soth M, Tiboni M, Acedillo R, Garg A, Hildebrand A, Lam N, Macneil D, Mrkobrada M, Roshanov PS, Srinathan SK, Ramsey C, John PS, Thorlacius L, Siddiqui FS, Grocott HP, McKay A, Lee TW, Amadeo R, Funk D, McDonald H, Zacharias J, Villar JC, Cortes OL, Chaparro MS, Vasquez S, Castaneda A, Ferreira S, Coriat P, Monneret D, Goarin JP, Esteve CI, Royer C, Daas G, Chan MT, Choi GY, Gin T, Lit LC, Xavier D, Sigamani A, Faruqui A, Dhanpal R, Almeida S, Cherian J, Furruqh S, Abraham V, Afzal L, George P, Mala S, Schunemann H, Muti P, Vizza E, Wang CY, Ong GS, Mansor M, Tan AS, Shariffuddin II, Vasanthan V, Hashim NH, Undok AW, Ki U, Lai HY, Ahmad WA, Razack AH, Malaga G, Valderrama-Victoria V, Loza-Herrera JD, De Los Angeles Lazo M, Rotta-Rotta A, Szczeklik W, Sokolowska B, Musial J, Gorka J, Iwaszczuk P, Kozka M, Chwala M, Raczek M, Mrowiecki T, Kaczmarek B, Biccard B, Cassimjee H, Gopalan D, Kisten T, Mugabi A, Naidoo P, Naidoo R, Rodseth R, Skinner D, Torborg A, Paniagua P, Urrutia G, Maestre ML, Santalo M, Gonzalez R, Font A, Martinez C, Pelaez X, De Antonio M, Villamor JM, Garcia JA, Ferre MJ, Popova E, Alonso-Coello P, Garutti I, Cruz P, Fernandez C, Palencia M, Diaz S, Del Castillo T, Varela A, de Miguel A, Munoz M, Pineiro P, Cusati G, Del Barrio M, Membrillo MJ, Orozco D, Reyes F, Sapsford RJ, Barth J, Scott J, Hall A, Howell S, Lobley M, Woods J, Howard S, Fletcher J, Dewhirst N, Williams C, Rushton A, Welters I, Leuwer M, Pearse R, Ackland G, Khan A, Niebrzegowska E, Benton S, Wragg A, Archbold A, Smith A, McAlees E, Ramballi C, Macdonald N, Januszewska M, Stephens R, Reyes A, Paredes LG, Sultan P, Cain D, Whittle J, Del Arroyo AG, Sessler DI, Kurz A, Sun Z, Finnegan PS, Egan C, Honar H, Shahinyan A, Panjasawatwong K, Fu AY, Wang S, Reineks E, Nagele P, Blood J, Kalin M, Gibson D, Wildes T, Vascular events In noncardiac Surgery patIents cOhort evaluatioN Writing Group oboTVeInSpceI, Appendix 1. The Vascular events In noncardiac Surgery patIents cOhort evaluatio NSIWG, Appendix 2. The Vascular events In noncardiac Surgery patIents cOhort evaluatio NOC, Vascular events In noncardiac Surgery patIents cOhort evaluatio NVSI. Myocardial injury after noncardiac surgery: a large, international, prospective cohort study establishing diagnostic criteria, characteristics, predictors, and 30-day outcomes. Anesthesiology, 2014; 120: 564–78. [DOI] [PubMed] [Google Scholar]
- [4].Eckle T, Hartmann K, Bonney S, Reithel S, Mittelbronn M, Walker LA, Lowes BD, Han J, Borchers CH, Buttrick PM, Kominsky DJ, Colgan SP, Eltzschig HK. Adora2b-elicited Per2 stabilization promotes a HIF-dependent metabolic switch crucial for myocardial adaptation to ischemia. Nat Med, 2012; 18: 774–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Bartman CM, Oyama Y, Brodsky K, Khailova L, Walker L, Koeppen M, Eckle T. Intense light-elicited upregulation of miR-21 facilitates glycolysis and cardioprotection through Per2-dependent mechanisms. PLoS One, 2017; 12: e0176243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Bonney S, Kominsky D, Brodsky K, Eltzschig H, Walker L, Eckle T. Cardiac Per2 functions as novel link between fatty acid metabolism and myocardial inflammation during ischemia and reperfusion injury of the heart. PLoS One, 2013; 8: e71493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Brainard J, Gobel M, Scott B, Koeppen M, Eckle T. Health implications of disrupted circadian rhythms and the potential for daylight as therapy. Anesthesiology, 2015; 122: 1170–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Oyama Y, Bartman CM, Gile J, Eckle T. Circadian MicroRNAs in Cardioprotection. Curr Pharm Des, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Dispersyn G, Pain L, Touitou Y. Propofol anesthesia significantly alters plasma blood levels of melatonin in rats. Anesthesiology, 2010; 112: 333–7. [DOI] [PubMed] [Google Scholar]
- [10].Dispersyn G, Touitou Y, Coste O, Jouffroy L, Lleu JC, Challet E, Pain L. Desynchronization of daily rest-activity rhythm in the days following light propofol anesthesia for colonoscopy. Clin Pharmacol Ther, 2009; 85: 51–5. [DOI] [PubMed] [Google Scholar]
- [11].Lonardo NW, Mone MC, Nirula R, Kimball EJ, Ludwig K, Zhou X, Sauer BC, Nechodom K, Teng C, Barton RG. Propofol is associated with favorable outcomes compared with benzodiazepines in ventilated intensive care unit patients. Am J Respir Crit Care Med, 2014; 189: 1383–94. [DOI] [PubMed] [Google Scholar]
- [12].Eckle T, Grenz A, Kohler D, Redel A, Falk M, Rolauffs B, Osswald H, Kehl F, Eltzschig HK. Systematic evaluation of a novel model for cardiac ischemic preconditioning in mice. Am J Physiol Heart Circ Physiol, 2006; 291: H2533–40. [DOI] [PubMed] [Google Scholar]
- [13].Eckle T, Koeppen M, Eltzschig H. Use of a hanging weight system for coronary artery occlusion in mice. J Vis Exp, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Matthes K, Urman R, Ehrenfeld J. Anesthesiology: A Comprehensive Board Review for Primary and Maintenance of Certification. Oxford University Press; 2013. [Google Scholar]
- [15].Eltzschig HK, Ibla JC, Furuta GT, Leonard MO, Jacobson KA, Enjyoji K, Robson SC, Colgan SP. Coordinated adenine nucleotide phosphohydrolysis and nucleoside signaling in posthypoxic endothelium: role of ectonucleotidases and adenosine A2B receptors. J Exp Med, 2003; 198: 783–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Seo SW, Koeppen M, Bonney S, Gobel M, Thayer M, Harter PN, Ravid K, Eltzschig HK, Mittelbronn M, Walker L, Eckle T. Differential Tissue-Specific Function of Adora2b in Cardioprotection. J Immunol, 2015; 195: 1732–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].O’Connell TD, Rodrigo MC, Simpson PC. Isolation and culture of adult mouse cardiac myocytes. Methods Mol Biol, 2007; 357: 271–96. [DOI] [PubMed] [Google Scholar]
- [18].Eckle T, Kohler D, Lehmann R, El Kasmi K, Eltzschig HK. Hypoxia-inducible factor-1 is central to cardioprotection: a new paradigm for ischemic preconditioning. Circulation, 2008; 118: 166–75. [DOI] [PubMed] [Google Scholar]
- [19].Warth A, Eckle T, Kohler D, Faigle M, Zug S, Klingel K, Eltzschig HK, Wolburg H. Upregulation of the water channel aquaporin-4 as a potential cause of postischemic cell swelling in a murine model of myocardial infarction. Cardiology, 2007; 107: 402–10. [DOI] [PubMed] [Google Scholar]
- [20].Koeppen M, Harter PN, Bonney S, Bonney M, Reithel S, Zachskorn C, Mittelbronn M, Eckle T. Adora2b signaling on bone marrow derived cells dampens myocardial ischemia-reperfusion injury. Anesthesiology, 2012; 116: 1245–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Kohler D, Eckle T, Faigle M, Grenz A, Mittelbronn M, Laucher S, Hart ML, Robson SC, Muller CE, Eltzschig HK. CD39/ectonucleoside triphosphate diphosphohydrolase 1 provides myocardial protection during cardiac ischemia/reperfusion injury. Circulation, 2007; 116: 1784–94. [DOI] [PubMed] [Google Scholar]
- [22].Zheng B, Larkin DW, Albrecht U, Sun ZS, Sage M, Eichele G, Lee CC, Bradley A. The mPer2 gene encodes a functional component of the mammalian circadian clock. Nature, 1999; 400: 169–73. [DOI] [PubMed] [Google Scholar]
- [23].Ma X, Kumar M, Choudhury SN, Becker Buscaglia LE, Barker JR, Kanakamedala K, Liu MF, Li Y. Loss of the miR-21 allele elevates the expression of its target genes and reduces tumorigenesis. Proc Natl Acad Sci U S A, 2011; 108: 10144–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Vasile VC, Babuin L, Giannitsis E, Katus HA, Jaffe AS. Relationship of MRI-determined infarct size and cTnI measurements in patients with ST-elevation myocardial infarction. Clin Chem, 2008; 54: 617–9. [DOI] [PubMed] [Google Scholar]
- [25].Matsuo I, Iijima N, Takumi K, Higo S, Aikawa S, Anzai M, Ishii H, Sakamoto A, Ozawa H. Characterization of sevoflurane effects on Per2 expression using ex vivo bioluminescence imaging of the suprachiasmatic nucleus in transgenic rats. Neurosci Res, 2016; 107: 30–7. [DOI] [PubMed] [Google Scholar]
- [26].Dhir A, Zolkowska D, Rogawski MA. Seizure protection by intrapulmonary delivery of midazolam in mice. Neuropharmacology, 2013; 73: 425–31. [DOI] [PubMed] [Google Scholar]
- [27].He B, Nohara K, Park N, Park YS, Guillory B, Zhao Z, Garcia JM, Koike N, Lee CC, Takahashi JS, Yoo SH, Chen Z. The Small Molecule Nobiletin Targets the Molecular Oscillator to Enhance Circadian Rhythms and Protect against Metabolic Syndrome. Cell Metab, 2016; 23: 610–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Akhlaghi M, Bandy B. Mechanisms of flavonoid protection against myocardial ischemia-reperfusion injury. J Mol Cell Cardiol, 2009; 46: 309–17. [DOI] [PubMed] [Google Scholar]
- [29].Gile J, Scott B, Eckle T. The Period 2 Enhancer Nobiletin as Novel Therapy in Murine Models of Circadian Disruption Resembling Delirium. Crit Care Med, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Kudchadkar SR. Benzodiazepines and Delirium in the Young and Old: Truth Be Told or Still Not Sold? Crit Care Med, 2017; 45: 1562–1564. [DOI] [PubMed] [Google Scholar]
- [31].Scott BK. Disruption of Circadian Rhythms and Sleep in Critical Illness and its Impact on the Development of Delirium. Curr Pharm Des, 2015; 21: 3443–52. [DOI] [PubMed] [Google Scholar]
- [32].Smith HAB, Gangopadhyay M, Goben CM, Jacobowski NL, Chestnut MH, Thompson JL, Chandrasekhar R, Williams SR, Griffith K, Ely EW, Fuchs DC, Pandharipande PP. Delirium and Benzodiazepines Associated With Prolonged ICU Stay in Critically Ill Infants and Young Children. Crit Care Med, 2017; 45: 1427–1435. [DOI] [PubMed] [Google Scholar]
- [33].Hughes CG, McGrane S, Pandharipande PP. Sedation in the intensive care setting. Clin Pharmacol, 2012; 4: 53–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Rivo J, Raphael J, Drenger B, Berenshtein E, Chevion M, Gozal Y. Flumazenil mimics whereas midazolam abolishes ischemic preconditioning in a rabbit heart model of ischemia-reperfusion. Anesthesiology, 2006; 105: 65–71. [DOI] [PubMed] [Google Scholar]
- [35].Colgan SP, Eltzschig HK, Eckle T, Thompson LF. Physiological roles for ecto-5’-nucleotidase (CD73). Purinergic Signal, 2006; 2: 351–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Zhang HY, McPherson BC, Liu H, Baman TS, Rock P, Yao Z. H(2)O(2) opens mitochondrial K(ATP) channels and inhibits GABA receptors via protein kinase C-epsilon in cardiomyocytes. Am J Physiol Heart Circ Physiol, 2002; 282: H1395–403. [DOI] [PubMed] [Google Scholar]
- [37].Leducq N, Bono F, Sulpice T, Vin V, Janiak P, Fur GL, O’Connor SE, Herbert JM. Role of peripheral benzodiazepine receptors in mitochondrial, cellular, and cardiac damage induced by oxidative stress and ischemia-reperfusion. J Pharmacol Exp Ther, 2003; 306: 828–37. [DOI] [PubMed] [Google Scholar]
- [38].Myung J, Hong S, DeWoskin D, De Schutter E, Forger DB, Takumi T. GABA-mediated repulsive coupling between circadian clock neurons in the SCN encodes seasonal time. Proc Natl Acad Sci U S A, 2015; 112: E3920–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Ehlen JC, Novak CM, Karom MC, Gamble KL, Albers HE. Interactions of GABA A receptor activation and light on period mRNA expression in the suprachiasmatic nucleus. J Biol Rhythms, 2008; 23: 16–25. [DOI] [PubMed] [Google Scholar]
- [40].Tyagi N, Lominadze D, Gillespie W, Moshal KS, Sen U, Rosenberger DS, Steed M, Tyagi SC. Differential expression of gamma-aminobutyric acid receptor A (GABA(A)) and effects of homocysteine. Clin Chem Lab Med, 2007; 45: 1777–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Chen Z, Yoo SH, Park YS, Kim KH, Wei S, Buhr E, Ye ZY, Pan HL, Takahashi JS. Identification of diverse modulators of central and peripheral circadian clocks by high-throughput chemical screening. Proc Natl Acad Sci U S A, 2012; 109: 101–6. [DOI] [PMC free article] [PubMed] [Google Scholar]