Management of primary testicular lymphoma (PTL), a rare disease accounting for less than 2% of non-Hodgkin lymphomas, poses unique challenges.[1] PTL arises from an immune-privileged anatomic site and has distinct molecular features similar to primary central nervous system (CNS) lymphoma. Proper initial management is paramount given frequent relapses in extranodal sites, particularly in the contralateral testicle, CNS, skin, or pleura, and poor survival after relapse.[1–3] Because of poor penetration of chemotherapy through the blood-testis barrier, and bilateral involvement of PTL in 10% of patients, the National Comprehensive Cancer Network (NCCN) guidelines have recommended 25–30 gray (Gy) radiation therapy (RT) to the contralateral testis as part of treatment for PTL of any stage, yet adherence to this guideline in clinical practice is uncertain.[4] In prior retrospective series, RT was delivered to 24–80% of patients after completion of immunochemotherapy.[3,5–8] Although no randomized trial has been conducted, RT has been included in key prospective studies of PTL.[9] Observational data suggest that RT may reduce contralateral testicular relapses from 42% to less than 10%.[10] Moreover, in a retrospective cohort from the International Extranodal Lymphoma Study Group, RT was associated with a significant improvement in 5-year progression-free survival (PFS, 70% versus 36%), and overall survival (OS, 66% versus 38%).[2] Using nationwide data from the US, we examined factors associated with receipt of RT and subsequent OS of patients with PTL treated in the era of modern immunochemotherapy.
We conducted a retrospective cohort study (approved by the Institutional Review Board at Rhode Island Hospital) using the National Cancer Data Base (NCDB), a joint project of the Commission on Cancer of the American College of Surgeons and the American College of Surgeons.[11] The NCDB files contain over 34 million records from over 1,500 US hospital-based cancer registries, including about 84% of all newly diagnosed lymphomas in the US, with high-quality data on treatment modalities, and minimum 90% follow-up rate for survival.[11] We extracted records of 2,446 male patients diagnosed with primary testicular diffuse large B-cell lymphoma (DLBCL) in 2004–2015, and excluded 576 cases who did not receive multi-agent chemotherapy, 49 who received RT prior to chemotherapy or had uncertain RT administration status, and 16 with missing data on the zipcode of residence. For adjustment in multivariable models, we included patients’ socio-demographic characteristics, Charlson-Deyo comorbidity index (as a measure of baseline mortality), history of prior malignancy, stage of the lymphoma, presence of B symptoms, type of treating hospital (categorized as academic/research or community), and its distance from the patient’s residence, as previously described.[12] Specific chemotherapy drugs, regimens, doses, duration, or depth of response to chemotherapy were not available. OS (available for patients diagnosed in 2004–2014) was the main survival outcome. Sites of relapse and PFS were not recorded.
For analysis of factors associated with the use of RT, we used multivariable robust Poisson regression to compute adjusted relative risk (RR) with 95% confidence intervals (95%CI). For survival analysis, we applied two strategies to overcome immortal-time bias related to the fact that some patients died prior to receiving RT. First, for patients treated with RT, we reclassified time-at-risk prior to starting RT into the “untreated” group.[13] We then plotted OS using Simon-Makuch curves,[14] and analyzed OS in an extended multivariable Cox model, reporting adjusted hazard ratio (HR).[13] Additionally, we used landmark analysis including only patients who survived >12 months from diagnosis as an alternative approach, and examined OS using standard Kaplan-Meier curves and a straightforward Cox model in this subcohort. All multivariable models used the same set of explanatory variables (listed in Table 1), regardless of statistical significance.
Table 1.
Characteristics of patients with primary testicular DLBCL (2004–2015, N=1,805), stratified by receipt of radiation therapy (RT—note that percentages are given “per row”), and multivariable model for the relative risk (RR) of receiving RT.
| Variable | RT administered |
P a | RR for receiving RT |
||||
|---|---|---|---|---|---|---|---|
| No, N (%) | Yes, N (%) | RR | 95% CI | ||||
| N | 907 | (50.2) | 898 | (49.8) | |||
| Age group | <.001 | ||||||
| 18 to 50 years | 103 | (51.8) | 96 | (48.2) | 0.85 | (0.72–1.00) | |
| 51 to 60 years | 141 | (46.1) | 165 | (53.9) | 0.94 | (0.83–1.06) | |
| 61 to 70 years | 233 | (42.9) | 310 | (57.1) | Reference | ||
| 71 to 80 years | 285 | (51.8) | 265 | (48.2) | 0.86 | (0.76–0.96) | |
| >80 years | 145 | (70.0) | 62 | (30.0) | 0.55 | (0.44–0.68) | |
| Race / ethnicity | .017 | ||||||
| White non-Hispanic | 776 | (49.1) | 806 | (50.9) | Reference | ||
| White Hispanic | 44 | (59.5) | 30 | (40.5) | 0.84 | (0.64–1.11) | |
| Black | 34 | (68.0) | 16 | (32.0) | 0.65 | (0.44–0.97) | |
| Asian or other | 53 | (53.5) | 46 | (46.5) | 0.89 | (0.72–1.10) | |
| Comorbidity index | <.001 | ||||||
| 0 | 688 | (48.0) | 744 | (52.0) | Reference | ||
| ≥1 | 219 | (58.7) | 154 | (41.3) | 0.82 | (0.72–0.93) | |
| Prior malignancy | <.001 | ||||||
| No | 725 | (48.3) | 776 | (51.7) | Reference | ||
| Yes | 182 | (59.9) | 122 | (40.1) | 0.80 | (0.69–0.92) | |
| Health insurance | .08 | ||||||
| Private or Medicare | 850 | (49.7) | 860 | (50.3) | Reference | ||
| Medicaid | 26 | (54.2) | 22 | (45.8) | 1.02 | (0.75–1.39) | |
| Uninsured | 31 | (66.0) | 16 | (34.0) | 0.68 | (0.46–1.02) | |
| Income b | .040 | ||||||
| < $38,000 | 139 | (57.4) | 103 | (42.6) | 0.84 | (0.71–0.99) | |
| $38,000 to $47,999 | 208 | (52.5) | 188 | (47.5) | 0.92 | (0.81–1.04) | |
| $48,000 to $62,999 | 246 | (48.5) | 261 | (51.5) | 0.99 | (0.88–1.10) | |
| ≥ $63,000 | 314 | (47.6) | 346 | (52.4) | Reference | ||
| Ann Arbor stage | <.001 | ||||||
| I | 377 | (42.6) | 508 | (57.4) | Reference | ||
| II | 175 | (54.7) | 145 | (45.3) | 0.79 | (0.69–0.90) | |
| III/IV | 279 | (59.6) | 189 | (40.4) | 0.74 | (0.65–0.83) | |
| Unrecorded | 76 | (57.6) | 56 | (42.4) | 0.76 | (0.62–0.93) | |
| B symptoms | <.001 | ||||||
| Absent or unrecorded | 808 | (49.2) | 835 | (50.8) | Reference | ||
| Present | 99 | (61.1) | 63 | (38.9) | 0.84 | (0.70–1.02) | |
| Distance to facility | .38 | ||||||
| < 20 miles | 674 | (50.9) | 651 | (49.1) | Reference | ||
| ≥ 20 miles | 233 | (48.5) | 247 | (51.5) | 1.06 | (0.96–1.18) | |
| Cancer program | .99 | ||||||
| Community | 614 | (50.2) | 608 | (49.8) | Reference | ||
| Academic/research | 293 | (50.3) | 290 | (49.7) | 0.97 | (0.88–1.07) | |
P for univariate comparison using a chi-squared test
Median yearly household income in the zipcode of residence according to the 2012 American Community Survey data, grouped by national quartiles.
Among 1,805 men with PTL included in the analysis, median age was 68 years (interquartile range [IQR], 59–76). Two thirds of patients had stage IE or IIE DLBCL, 9% had B symptoms at diagnosis, and 18 (1%) were HIV positive. Overall, 898 (49.8%) received RT (Table 1), a proportion which remained unchanged over the years (P for trend = 0.13). Median dose of RT was 30 Gy, with 77% of patients receiving 30–39.9 Gy, delivered over median 16 treatments (IQR, 15–18).
In univariate comparisons (Table 1), RT recipients were on average younger (median age 67 versus 70 years), more frequently white non-Hispanic, and more likely to have no comorbidities, or stage IE lymphoma. In a multivariable model, compared with the reference group of patients aged 61 to 70, those who were younger than 50 were 15% less likely, those aged 71 to 80 years were 14% less likely, and those aged >80 years were 45% less likely to receive RT. Other factors significantly associated with lower use of RT included black race, living in areas with the lowest median income, Ann Arbor stage II-IV, presence of comorbidities, and history of prior malignancy. We observed no significant differences by distance to treating facility, or whether the facility was designated as community or academic.
With a median follow-up of 6.8 years, median OS for the entire cohort was 9.0 years (95%CI, 8.1–9.4), with an estimated OS of 76.4% (95%CI, 74.3–78.5) at 3 years, and 68.0% (95%CI, 65.4–70.5) at 5 years. Among patients who received RT, OS was 86.4% (95%CI, 83.7–88.7) at 3 years and 79.0% (95%CI, 75.6–81.9) at 5 years (Fig. 1A), whereas for those who did not it was 66.6% (95%CI, 63.2–69.8) at 3 years and 57.2% (95%CI, 53.3–60.8) at 5 years. Fewer than 10% of patients died within 12 months of diagnosis. In a multivariable extended Cox model accounting for immortal-time bias, and adjusting for all factors from Table 1, receipt of RT was associated with significantly lower mortality (adjusted HR, 0.66; 95%CI, 0.54–0.79). The result was consistent in the landmark analysis (Fig. 1B, adjusted HR, 0.68; 95%CI, 0.56–0.84), and stable in a sensitivity analysis varying the landmark between 6 and 18 months of initial survival (HR, 0.63 to 0.68). To overcome a possible misclassification of disseminated lymphomas with secondary testicular involvement in the registries, we conducted an analysis limited to patients with stage I/II DLBCL and testicle recorded as the primary site. Among these 1205 patients, 5-year OS was 82.5% (95%CI, 78.8–85.7) in the group that received RT, and 62.7% (95%CI, 57.6–67.4) among those who did not (Fig. 1C–D). In multivariable models, receipt of RT was similarly associated with longer survival (HR 0.60; 05%CI 0.47–0.77 in the extended Cox model, and HR 0.65; 95%CI 0.50–0.84 in 12-month landmark analysis).
Figure 1.
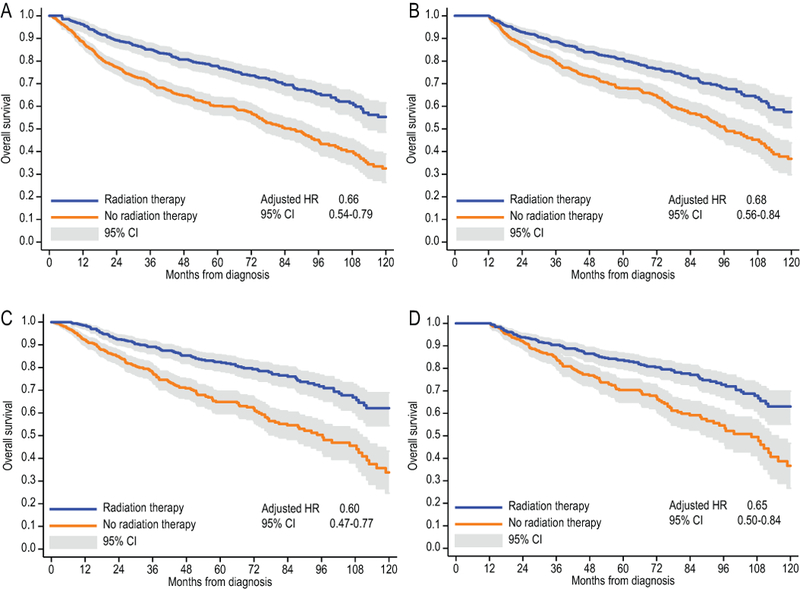
Overall survival of patients with PTL of any stage (A, B), or limited to stage I/II only (C, D); Simon-Makuch survival curves adjusting for immortal-time bias (A, C), and Kaplan-Meier curves using landmark analysis at 12 months of survival (B, D). Adjusted hazard ratios for radiation therapy use are calculated from multivariable survival models including all variables from Table 1.
Despite a benefit supported by retrospective and prospective trials,[2,5,9] nearly half of PTL patients in this “real-world” analysis did not receive RT. Although this proportion is better than in a prior study using data from 1980–2005,[7] our cohort was limited to recipients of multi-agent chemotherapy, and thus excluded patients too ill to receive standard curative treatment. Our community-derived survival estimates replicate exactly outcomes in a recent multi-institutional case series of 280 patients with 5-year OS of 68%. In that study, 95% of patients achieved complete remission with immunochemotherapy, so occurrence of progressive disease could not explain the low rate of RT delivery[3]. While prior authors hoped for an improvement over time, we observed no increase in the percentage of patients receiving RT, despite guidelines consistently recommending RT throughout the study period. Although the NCDB is limited by lack of details on chemotherapy regimens, PFS data, and insight into reasons for omission of RT, our analysis, using advanced methods to account for immortal-time bias, reinforces the association between receipt of RT and survival in PTL. We acknowledge that many disease-specific prognostic factors, like the International Prognostic Index, CNS involvement and CNS-directed therapy, or bulky disease, may confound this association.[2] Nevertheless, OS advantage among RT recipients was present even in the subgroup surviving >1 year from diagnosis, who would be reasonably expected to achieve remission with the initial course of therapy. Our analysis for the first time, to our knowledge, examined factors associated with receipt of RT, and found a concerning association with non-medical factors like black race or living in a socio-economically deprived area—factors previously described in the context of survival disparities in lymphoma.[15] RT requires multiple daily visits delaying return to work, and may incur high out-of-pocket costs, creating a selective barrier for socio-economically disadvantaged individuals. Omission of RT in older patients and those with comorbidities may also be clinically undesirable, as these groups may be the least likely to tolerate aggressive salvage therapies needed at relapse. Surprisingly, distance to treating facility was not predictive of RT administration, and we saw no difference between academic and community centers. We contend that even with acknowledged limitations, when combined with reduction in relapse rate defined in prior studies,[2,5] the large difference in OS borne out in this contemporary dataset underscores the importance of guideline-adherent therapy in PTL, which should include scrotal RT and CNS prophylaxis even in the era of efficacious chemoimmunotherapy. Further research should examine if the delivery of these treatments in the context of socio-economic disparities may be an indicator of equitable, expert quality care in PTL, or whether persistent clinical or systemic barriers to effective delivery of RT exist.
Acknowledgements
Dr. Olszewski’s work was supported by the grant 128608-RSGI-15-211-01-CPHPS from the American Cancer Society, and U54GM115677 from the National Institute of General Medical Sciences. The data used in the study are derived from a de-identified NCDB file. The American College of Surgeons and the Commission on Cancer have not verified and are not responsible for the analytic or statistical methodology employed, or the conclusions drawn from these data by the investigator. Preliminary results of this study were presented at the 2018 American Society of Clinical Oncology Annual Meeting, June 1–5, Chicago, IL.
Footnotes
Conflict of interest:
The authors declare no conflict of interest.
References
- 1.Cheah CY, Wirth A, Seymour JF. Primary testicular lymphoma. Blood 2014;123:486–493. [DOI] [PubMed] [Google Scholar]
- 2.Zucca E, Conconi A, Mughal TI, et al. Patterns of outcome and prognostic factors in primary large-cell lymphoma of the testis in a survey by the International Extranodal Lymphoma Study Group. J Clin Oncol 2003;21:20–27. [DOI] [PubMed] [Google Scholar]
- 3.Deng L, Xu-Monette ZY, Loghavi S, et al. Primary testicular diffuse large B-cell lymphoma displays distinct clinical and biological features for treatment failure in rituximab era: a report from the International PTL Consortium. Leukemia 2016;30:361–372. [DOI] [PubMed] [Google Scholar]
- 4.Zelenetz AD, Gordon LI, Wierda WG, et al. Diffuse Large B-Cell Lymphoma Version 1.2016. J Natl Compr Canc Netw 2016;14:196–231. [DOI] [PubMed] [Google Scholar]
- 5.Ho JC, Dabaja BS, Milgrom SA, et al. Radiation therapy improves survival in patients with testicular diffuse large B-cell lymphoma(). Leuk Lymphoma 2017;58:2833–2844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Olszewski AJ, Falah J, Castillo JJ. Survival Claims From Observational Data on Cancer Therapy. J Clin Oncol 2016;34:1425–1427. [DOI] [PubMed] [Google Scholar]
- 7.Gundrum JD, Mathiason MA, Moore DB, Go RS. Primary testicular diffuse large B-cell lymphoma: a population-based study on the incidence, natural history, and survival comparison with primary nodal counterpart before and after the introduction of rituximab. J Clin Oncol 2009;27:5227–5232. [DOI] [PubMed] [Google Scholar]
- 8.Kridel R, Telio D, Villa D, et al. Diffuse large B-cell lymphoma with testicular involvement: outcome and risk of CNS relapse in the rituximab era. Br J Haematol 2017;176:210–221. [DOI] [PubMed] [Google Scholar]
- 9.Vitolo U, Chiappella A, Ferreri AJ, et al. First-line treatment for primary testicular diffuse large B-cell lymphoma with rituximab-CHOP, CNS prophylaxis, and contralateral testis irradiation: final results of an international phase II trial. J Clin Oncol 2011;29:2766–2772. [DOI] [PubMed] [Google Scholar]
- 10.Conrad AL, Go RS. Contralateral testicular relapse after prophylactic radiation in a patient with primary testicular diffuse large B-cell lymphoma. Eur J Haematol 2009;83:603–605. [DOI] [PubMed] [Google Scholar]
- 11.Boffa DJ, Rosen JE, Mallin K, et al. Using the National Cancer Database for Outcomes Research: A Review. JAMA Oncol 2017;3:1722–1728. [DOI] [PubMed] [Google Scholar]
- 12.Olszewski AJ, Ollila T, Reagan JL. Time to treatment is an independent prognostic factor in aggressive non-Hodgkin lymphomas. Br J Haematol 2018. [DOI] [PMC free article] [PubMed]
- 13.Giobbie-Hurder A, Gelber RD, Regan MM. Challenges of guarantee-time bias. J Clin Oncol 2013;31:2963–2969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Simon R, Makuch RW. A non-parametric graphical representation of the relationship between survival and the occurrence of an event: application to responder versus non-responder bias. Stat Med 1984;3:35–44. [DOI] [PubMed] [Google Scholar]
- 15.Tao L, Foran JM, Clarke CA, Gomez SL, Keegan TH. Socioeconomic disparities in mortality after diffuse large B-cell lymphoma in the modern treatment era. Blood 2014;123:3553–3562. [DOI] [PMC free article] [PubMed] [Google Scholar]


