Abstract
Metastasis is the most common cause of death in colorectal cancer (CRC) patients. Fatty acid synthase (FASN) and sphingosine kinase-1 and −2 (SPHK1 and 2) are overexpressed in many cancers, including CRC. However, the contribution of FASN-mediated upregulation of sphingolipid metabolism to CRC metastasis and the potential of these pathways as targets for therapeutic intervention remain unknown. This study, determined that SPHKs are overexpressed in CRC as compared to normal mucosa. FASN expression significantly correlated with SPHK2 expression in data sets from The Cancer Genome Atlas (TCGA) and a CRC tumor microarray. FASN, SPHK1 and SPHK2 co-localized within invadopodia of primary CRC cells. Moreover, FASN inhibition decreased SPHK2 expression and the levels of dihydrosphingosine 1-phosphate (DH-S1P) and sphingosine 1-phosphate (S1P) in CRC cells and tumor tissues. Inhibition of FASN using TVB-3664 and sphingolipid metabolism using FTY-720 significantly inhibited the ability of primary CRC cells to proliferate, migrate, form focal adhesions and degrade gelatin. Inhibition of the FASN/SPHK/S1P axis was accompanied by decreased activation of p-MET, p-FAK and p-PAX. S1P treatment rescued FASN-mediated inhibition of these proteins, suggesting that FASN promotes metastatic properties of CRC cells, in part, through an increased sphingolipid metabolism. These data demonstrate that upregulation of the FASN/SPHK/S1P axis promotes CRC progression by enhancing proliferation, adhesion and migration.
Keywords: colorectal cancer, FASN, SPHK, S1P, metastasis
INTRODUCTION
Metabolic alterations are the hallmark of tumor progression and metastasis (1). Most cancers are characterized by increased de novo lipid biosynthesis (2). Abnormally elevated lipid synthesis provides cancer cells with membrane building blocks, signaling lipid molecules, post-translational modifications of proteins, and energy supply to support rapid cell proliferation (3). Fatty acid synthase (FASN), a key enzyme of de novo lipid synthesis, is overexpressed in many solid cancers, including CRC (4). Elevated expression of FASN is associated with advanced stages of CRC and poor prognosis suggesting that it may play a role in progression of CRC to metastatic disease (4–6). We previously reported that shRNA-mediated inhibition of FASN in CRC cells attenuated activation of MET, Akt, FAK, and paxillin, which are known to regulate adhesion, migration, and invasion. Furthermore, we found that inhibition of FASN dramatically reduces lung and hepatic metastases and inhibits tumor angiogenesis in vivo (4,7).
Potent and selective FASN small molecule inhibitors (TVB-2640, TVB-3166, TVB-3693 and TVB-3664) have recently been described (8–10). TVBs demonstrate significantly improved potency and selectivity and are typically well tolerated in animals as compared to older compounds such as celulenin, orlistat and C-75 which are widely used in preclinical studies (8). The first Phase I study (3V2640-CLIN-002) showed that TVB-2640 demonstrates a favorable tolerability profile with no significant adverse events and a biomarker profile demonstrating inhibition of FASN in solid tumor patients (8,11). TVB-2640 is currently being tested in phase II clinical trials in combination with other therapeutic agents. Its analogs, TVB-3166 and TVB-3664, have shown anti-cancer activity in lung, prostate and ovarian cancer models in vitro and in vivo (9,10). Our laboratory recently reported that oral once-daily TVB-3664 was well tolerated in mice and showed selective anti-tumor growth properties in CRC patient derived-xenografts (PDXs) (12).
Accumulating evidence suggests that many cancers, including CRC, have alterations in sphingolipids and their metabolizing enzymes (13). Sphingosine kinase (SPHK) is an evolutionary conserved lipid kinase with two mammalian isoforms, SPHK1 and SPHK2, which catalyze the phosphorylation of sphingosine and dihydrosphingosine to form sphingosine 1-phosphate (S1P) and dihydrosphingosine 1-phosphate (DH-S1P), respectively (14). The dynamic balance between intracellular S1P versus sphingosine and ceramide, and the consequent regulation of opposing signaling pathways are important factors that determine whether cancer cells survive or die (15,16).
Condensation of palmitate and serine to form 3-ketosphinganine, a reaction catalyzed by serine palmitoyltransferase, is believed to be the key regulatory and rate-limiting step of de novo ceramide synthesis (17), suggesting that overexpression of FASN and an increase in the flux of de novo palmitate to sphingolipid metabolism may increase the activity of SPHKs and production of DH-S1P and S1P. DH-S1P and S1P have been implicated in the regulation of proliferation, migration and angiogenesis, and strongly correlate with cancer metastasis (18,19). S1P plays an important role in development of colitis-associated cancer (20); however, its role in CRC metastasis remains unclear. FTY-720 is an agonist of S1P receptors as well as a substrate for sphingosine kinases that have demonstrated anticancer properties in multiple pre-clinical studies (21).
While it is known that upregulation of de novo lipid synthesis is critically important for malignant transformation, tumor growth and metastasis, the contribution of de novo lipogenesis to CRC progression via sphingolipid metabolism is not known. Our study is the first to show the crucial role of de novo lipid synthesis in activating metastatic capabilities of CRC cells via upregulation of sphingolipid metabolism in CRC. The results of our study demonstrate that upregulation of FASN enhances sphingolipid metabolism and leads to activation of signaling pathways associated with an increase in adhesion, migration and invasion in CRC. The results of this study suggest that targeting sphingolipid metabolism should be further explored as a potential target for advanced CRC.
METHODS
Human Primary CRC Cells
Primary CRC cells in Patient (Pt) 93, Pt 130, Pt 2377 Primary Tumor (PT) and Pt 2377 Liver Metastasis (LM) were isolated and established from patient-derived xenograft (PDX) tumors using a previously described protocol of cancer cell isolation from tissues (22). Pt 2377 PT and matched Pt 2377 LM were established from the same patient. Pt 93 was diagnosed with metastatic medullary cancer (T3N1bM1); Pt 130 was diagnosed with metastatic colonic adenocarcinoma (T3N0M1a); and Pt 2377 was diagnosed with metastatic colonic adenocarcinoma (T3N0M1a), and tissues were collected from both primary tumor and liver metastases.
For the present study, cells were maintained as monolayer culture in DMEM supplemented with 10% fetal bovine serum (FBS, Sigma-Aldrich, St. Louis, MO) and 1% penicillin–streptomycin. Primary Pt 93 and Pt 130 cells were authenticated using short tandem repeat DNA profiling in March 2016 (Genetica, Cincinnati, OH). Furthermore, the mutation profiles of 198 cancer associated genes were determined using targeted next-generation sequencing and were matched to original patient tissues and PDX models for our ongoing studies (Markey Cancer Center Oncogenomics Shared Resource Facility).
Tissue Microarray (TMA)
TMA sections were prepared from 56 tumor tissues and 57 matched normal colon mucosa of patients who were diagnosed with Stage I-IV CRC and had surgery at University of Kentucky Chandler Medical Center (TMA ID BH15991A). Scoring was carried out blindly by a pathologist; immunoreactivity score was determined by multiplication of the values for staining intensity (0, no staining; 1, weak staining; 2, moderate staining; 3, strong staining) and the values for percentage of positive tumor cells (0, no positive cells; 1, 0–10% positive; 2, 11–50% positive; 3, 51–100% positive).
mRNA expression
Microarrays were downloaded from the Oncomine database (PMID:22810696). RNA sequencing data were downloaded from Genomic Data Commons (GDC) for TCGA COAD data (accessed March 2018) and normalized to logarithm of transcripts per kilobase million. Gene expressions between tumor and normal samples were compared using linear mixed models. Spearman’s correlation coefficients were used to quantify correlations between expressions of two genes.
FASN, SPHK1 and/or SPHK2 expression from microarray data (primary solid tumor, n =215; solid tissue normal, n = 22) as well as RNASeq data (primary solid tumor, n = 456; solid tissue normal, n = 41) in The Cancer Genome Atlas (TCGA) COAD study were analyzed.
Lipid Extraction and Liquid Chromatography-Tandem Mass Spectrometry
Sphingolipids were extracted and quantified as described previously (23). Extracted lipids were quantified using the Applied Biosystems 4000 Q-Trap mass spectrometer. The measurements used hexadeuterated S1P as an internal standard and were normalized to total lipid phosphorous determined after wet digestion of total lipid extracts in perchloric acid.
Plasmids / siRNAs / Antibodies
FASN cDNA (ID6172538; Open Biosystem, Chicago, IL) was cloned into the pEGFP vector. Stable overexpression was established by transfecting SW480 and Pt 130 cells with pEGFP-FASN vector and Gentamicin (Invitrogen, Austin, TX) selection. SPHK1 (ID 1181 and s16957) and SPHK2 (ID 1677 and 1587) siRNAs were purchased from ThermoFisher (Waltham, MA). Antibodies for western blot and immunofluorescent staining were purchased from Cell Signaling: FASN (#3180), pMET (#3129), MET (#3148), p-paxillin (#2541), paxillin (#2542), pFAK (#3283), FAK (#3285), pAkt (#4058L), Akt (#4691L), pERK1/2 (#4695), ERK1/2 (#9107) and RhoA (#2117). Alexa Fluor 568 phalloidin was purchased from ThermoFisher (Cat # A12380).
Western blot data were analyzed using ImageJ software. The quantification of band intensities was performed using the Gel Analysis method outlined in the ImageJ documentation: http://rsb.info.nih.gov/ij/docs/menus/analyze.html#gels.
Proliferation Assay
To determine the IC50, primary CRC cells were plated in 96-well plates at a density of 3,000 cells per well, cultured overnight, and then treated with TVB-3664 (3-V Biosciences, Inc.) (7 days) and FTY-720 (48h) in serial dilutions and analyzed by MTT assay (Promega, Madison, WI). For cell count experiments (Beckman-Coulter Vi Cell, Fullerton, CA), cells were seeded in 12-well plates at a confluence of 50,000 cells per well and then treated with TVB-3664 (0.2 μM, 7 days) and 80,000 cells/well and then treated with FTY-720 (2.5 μM, 48h).
Cell Migration Assay
Cells were plated at low densities on glass-bottom dishes (Cellvis) coated with 5 μg/ml fibronectin in DMEM containing 1% FBS and 20 ng/ml HGF, and cultured for 3h in a CO2 incubator. Cells were tracked for 8h with images acquired every 20 min using a Nikon Biostation IMQ (20X objective). All cells that remained entirely within the field of view, did not touch other cells, and did not divide, were tracked and analyzed. Cell paths were generated and analyzed using NIS-Elements AR (Nikon).
Focal Adhesion Staining
Cells were seeded on glass-bottom dishes pre-coated with 5 μg/ml fibronectin, cultured for 6h, then fixed with 4% paraformaldehyde for 15 min, permeablized with 0.5% Triton X-100 for 15 min, and finally blocked with 5% BSA in PBS for 1h. Cells were incubated with p-Paxillin (Y118) or p-FAK (Y397) antibodies, washed three times with PBS and incubated with a Dylight 480-labeled goat anti-rabbit secondary antibody and Alexa Fluor 568 phalloidin for 30 min. After final washes with PBS, the images were captured with a Nikon Eclipse Ti total internal reflection fluorescence (TIRF) microscope equipped with a 60, 1.45 numerical aperture objective, CoolSNAP HQ2 charge-coupled camera (Roper Scientific). Focal adhesion area distribution was analyzed with NIS-Elements software (Nikon).
Invadopodia Formation (Gelatin Degradation) Assay
Glass-bottom dishes were coated with warm Alexa 488-conjugated gelatin (100 μg/ml) in PBS. The coated dishes were dried, fixed with pre-chilled glutaraldehyde solution (0.3%) for 10 min, washed with PBS, and then reduced with 5 mg/ml of sodium borohydride in PBS for 3 min. The dishes were washed extensively with PBS and incubated with DMEM containing 10% FBS and antibiotics for 2h. Cells were seeded at low confluence in the dishes and cultured for 10h, then fixed with 4% paraformaldehyde, permeabilized with 0.5% Triton X-100 and stained with Alexa 647-Src and 561-phalloidin. Images were captured using a TIRF microscope and analyzed with NIS-Elements software (Nikon).
In Vivo Studies
Six- to eight-week-old NSG mice (NOD.Cg-Prkdc Il2rg /SzJ) purchased from The Jackson Laboratory (Bar Harbor, ME) were used for implantation of tumor tissues and establishment of PDX models. For evaluation of the effect of TVB-3664 on signaling pathways associated with migration, we utilized tissue samples from Pt 2402 PDX model established from a patient diagnosed with metastatic adenocarcinoma (lung) consistent with colon primary tumor (12). Briefly, mice were given a vehicle (30% PEG400) or 6 mg/kg of TVB-3664 by oral gavage daily for 4 weeks. Tissues were lysed and western blot was performed. To evaluate the effect of FTY-720 on expression of migration / invasion markers, mice were injected with 5 mg/kg of FTY-720 i.p. daily for 5 weeks and tumor tissues were lysed and analyzed by western blot.
RESULTS
FASN, SPHK1 and SPHK2 are highly expressed in CRC and co-localized within invadopodia in primary CRC cells
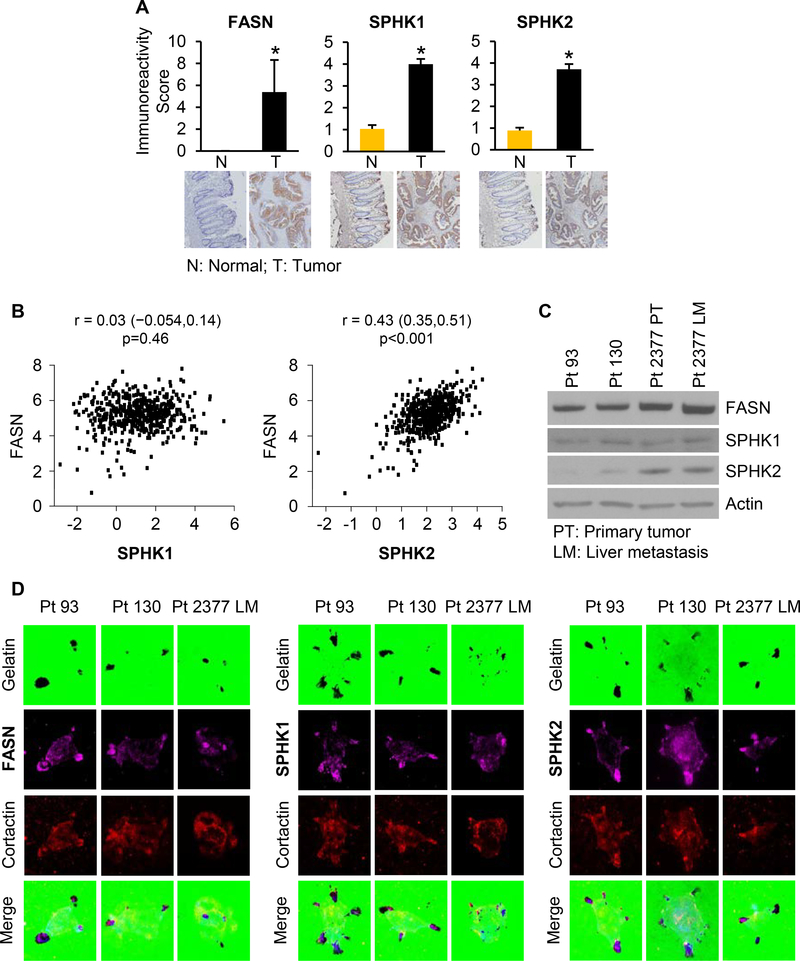
Multiple studies, including ours, have reported that FASN is overexpressed in CRC (4,7,24). Consistently, statistical evaluation of immunoreactivity scores showed increased FASN expression in a TMA from 56 patients with Stage I-IV CRC who had surgery at UK Chandler Medical Center. TMA analysis also revealed that sphingosine kinases, SPHK1 and SPHK2, are overexpressed in primary CRC as compared to normal colon mucosa. SPHK1 expression was observed in cytosol, while SPHK2 expression was detected in both cytosol and nucleus (Figure 1A). Statistical analysis of correlations among FASN, SPHK1 and SPHK2 expression levels revealed a significant correlation between FASN and SPHK2 (Spearman’s r = 0.27894, N = 57, p = 0.0374). Spearman analysis showed that expression of SPHK1 is positively correlated with expression of SPHK2 in CRC tissues (Spearman’s r = 0.52237, N = 57, p < 0.0001). Interestingly, expression of SPHK1 in normal colon mucosa also showed a strong positive correlation with SPHK2 expression (Spearman’s r = 0.80422, N = 57, p < 0.0001). The expression levels of FASN, SPHK1 and SPHK2 were also high in liver metastases as compared to matched normal colon tissues (Supplemental Figure 1A).
Figure 1. FASN, SPHK1 and SPHK2 are highly expressed in CRC and co-localized within invadopodia in primary CRC cells.
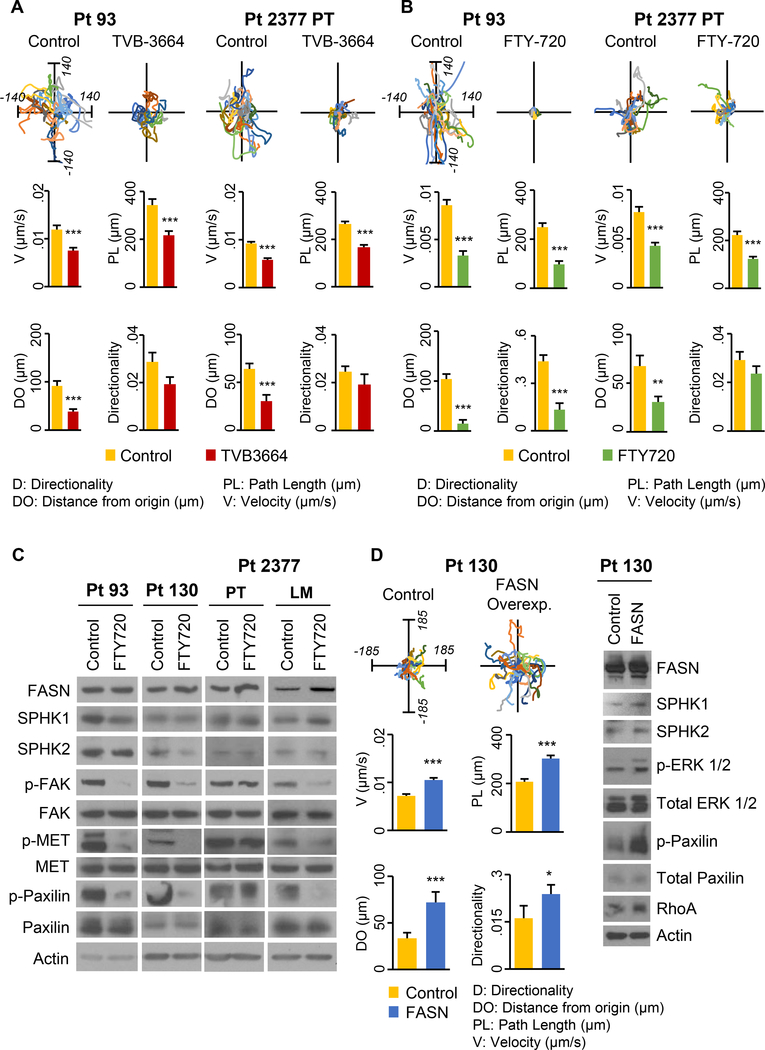
(A) Immunoreactivity score of FASN, SPHK1 and SPHK2 expression was analyzed in matched normal colon mucosa and tumor tissues (n = 57 normal and 56 tumor tissues; *p<0.001 as compared to normal tissue; TMA ID BH15991A). (B) Correlation of FASN with either SPHK1 or SPHK2 was determined based on RNASeq data (primary solid tumor, n = 456; solid tissue normal, n = 41) from The Cancer Genome Atlas. (C) Expression of FASN, SPHK1 and SPHK2 in primary Pt 93, Pt 130 and Pt 2377 PT and Pt 2377 LM CRC cells. (D) Co-localization of FASN, SPHK1 and SPHK2 (anti-rabbit 647 labeled purple color secondary antibodies) with cortactin (561 labeled red color) at invadopodia degraded areas in Pt 93, Pt 130 and Pt 2377 LM cells. Background gelatin is stained with Alexa Fluor 488 (green).
To confirm these findings we analyzed FASN, SPHK1 and SPHK2 mRNA expression using Genomic Data Commons (tumor, n = 456; normal, n = 41) and Oncomine (tumor, n = 215; normal, n = 22) databases. Consistent with the results from the TMA analysis, expression of FASN and SPHK1 mRNA was higher in tumor tissues as compared to normal mucosa. Interestingly, based on both datasets, expression of SPHK2 mRNA was lower in tumor tissues than in normal tissues (Supplemental Figure 1B-C). However, similar to TMA data analysis, we determined significant correlation between FASN and SPHK2 mRNA expression in tumor tissues in both microarray and RNA sequencing datasets. No significant correlation was observed between expression of FASN and SPHK1 mRNA (Figure 1B and Supplemental Figure 1D).
Pt 93, Pt 130, Pt 2377 PT and Pt 2377 LM primary cell lines were established from PDX tumors as previously described (12). Pt 2377 PT and Pt 2377 LM cells were established from primary tumor (PT) and liver metastasis (LM) from the same patient, Pt 2377. Western blot analysis revealed that primary CRC cells have high expression of FASN and SPHK1. Pt 2377 LM cells had the highest level of FASN expression as compared to matched Pt 2377 PT cells and to Pt 93 and Pt 130 cells. The levels of SPHK2 expression varied among primary cell lines. The high level of FASN expression was associated with the high level of SPHK2 in Pt 2377 PT and Pt 2377 LM cells (Figure 1C).
Invadopodia are actin-rich membrane protrusions through which cancer cells grip the substratum and degrade the extracellular matrix to enable cancer cells to invade and metastasize (25,26). Using TIRF microscopy, we demonstrated that FASN, SPHK1 and SPHK2 were co-localized within CRC cell invadopodia with cortactin, a protein associated with increased cancer cell migration and metastatic potential (27). Invadopodia structures of cells were detected as degraded dark spots of background Alexa Fluor 488-labeled gelatin (Figure 1D). All together, these results demonstrate interconnection between de novo lipid synthesis and sphingolipid metabolism in human CRC and suggest FASN, SPHK1 and SPHK2 might play a role in activating invadopodia, and thus promoting CRC cell invasion and metastasis.
Inhibition of FASN is associated with a decrease in DH-S1P and S1P in CRC
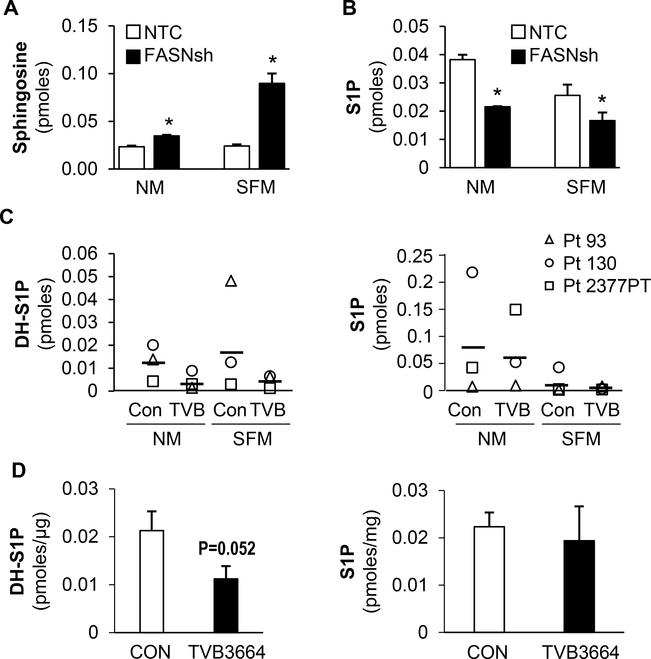
Sphingolipids have emerged as bioeffector molecules, controlling various aspects of cell growth, survival and metastasis in cancer (19,20,28). Using a [13C]-sodium acetate tracer, we previously demonstrated that stable inhibition of FASN significantly decreased the level of de novo production of palmitate (4). Since palmitate is a preferred substrate for the first step of sphingolipid synthesis (29), we examined the effect of FASN inhibition on sphingolipid metabolism. Figure 2A-B demonstrates that stable knockdown of FASN in HCT116 cells increases the level of sphingosine and decreases the level of S1P in both normal medium and serum-free conditions, suggesting that overexpression of FASN enhances synthesis of S1P in CRC.
Figure 2. Inhibition of FASN is associated with a decrease in DH-S1P and S1P in CRC.
Total level of (A) sphingosine and (B) S1P in NTC and FASNsh HCT116 CRC cells as detected by mass spectrometry (NM-normal medium; SFM-serum free medium; *p < 0.05 vs. control). (C) The effect of TVB-3664 treatment (0.2 μM, 7 days) on the levels of DH-S1P and S1P in cell medium from Pt 93, Pt130 and Pt 2377 PT cells when cultured in NM and SFM conditions. (D) The effect of TVB-3664 treatment (4 weeks, 3 mg/kg) on the levels of DH-S1P and S1P in tumor tissues in Pt 2449 PT PDXs (control n=3; TVB n=3). (C-D) DH-S1P and S1P measurements were performed using HPLC-MS/MS technologies (MUSC Lipidomics Core, SC). Phosphate concentrations were used to normalize samples.
S1P biosynthesis is not directly linked to de novo sphingoid base transformations and is dependent on catabolic generation of sphingosine from complex sphingolipids. On the other hand, DH-S1P is considered the de novo synthesized sphingolipid and SPHKs direct the metabolic flow of newly formed sphingoid bases from ceramide formation toward the synthesis of DH-S1P (30). Therefore, we decided to test whether FASN inhibition affects the level of DH-S1P and S1P in primary CRC cells and PDX tumors. Figure 2C shows that TVB-3664 treatment of Pt 93, Pt 130 and Pt 2377 PT primary CRC cell lines significantly decreased the level of DH-S1P in cell medium in both normal and serum free conditions. Changes in the level of S1P were not consistent among cell lines; however, the average level of S1P was lower in TVB-3664 treated cells as compared to control in both normal and serum free conditions. Furthermore, analysis of DH-S1P levels in tumor samples from control and TVB-3664 treated mice PDX Pt 2449 PT from our concurrent studies shows that FASN inhibition is also associated with a decrease in DH-S1P level. However, this change was not statistically significant. We did not observe changes in the S1P level due to TVB-3664 treatment in this model (Figure 2D).
Together, these data suggest that overexpression of FASN alters sphingolipid metabolism in both normal and serum free conditions.
Down regulation of de novo lipid synthesis and sphingolipid metabolism inhibits proliferation in primary CRC cells
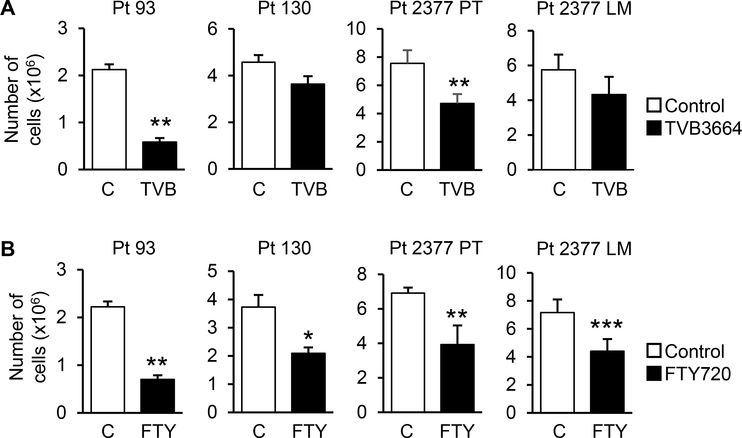
In addition to the synthesis of DNA and proteins, production of lipids is a prerequisite for cell growth and proliferation. To determine whether de novo lipogenesis and sphingolipid metabolism are required for cellular proliferation in CRC, primary CRC cells were treated with 0.2 μM TVB-3664, a FASN inhibitor (9), or 2.5 μM FTY-720, a structural analog of sphingosine that alters de novo sphingolipid synthesis and decreases the level of intracellular S1P (31,32). As shown in Figure 3A, FASN inhibition significantly decreased proliferation of Pt 93 and Pt 2377 PT cells. TVB-3664 treatment did not have a significant effect on proliferation of Pt 130 and Pt 2377 LM. In contrast, FTY-720 treatment significantly decreased proliferation in all four CRC cell lines including Pt 130 and Pt 2377 LM cells (Figure 3B). Together, these data suggest that sphingolipid metabolism downstream of FASN reinforces proliferative potential of primary CRC cells.
Figure 3. Inhibition of de novo lipid synthesis and sphingolipid metabolism decreases proliferation in primary CRC cells.
Cellular proliferation of primary Pt 93, Pt 130, and Pt 2377 PT and Pt 2377 LM CRC cells treated with (A) 0.2 μM TVB-3664 for 7 days and (B) 2.5 μM FTY-720 for 48h (Data shown as mean ± SEM. All experiments were performed three times using multiple replicates; *p<0.05, **p<0.01 and ***p<0.001 vs. control).
Inhibition of FASN and sphingolipid metabolism inhibits formation of focal adhesion complexes
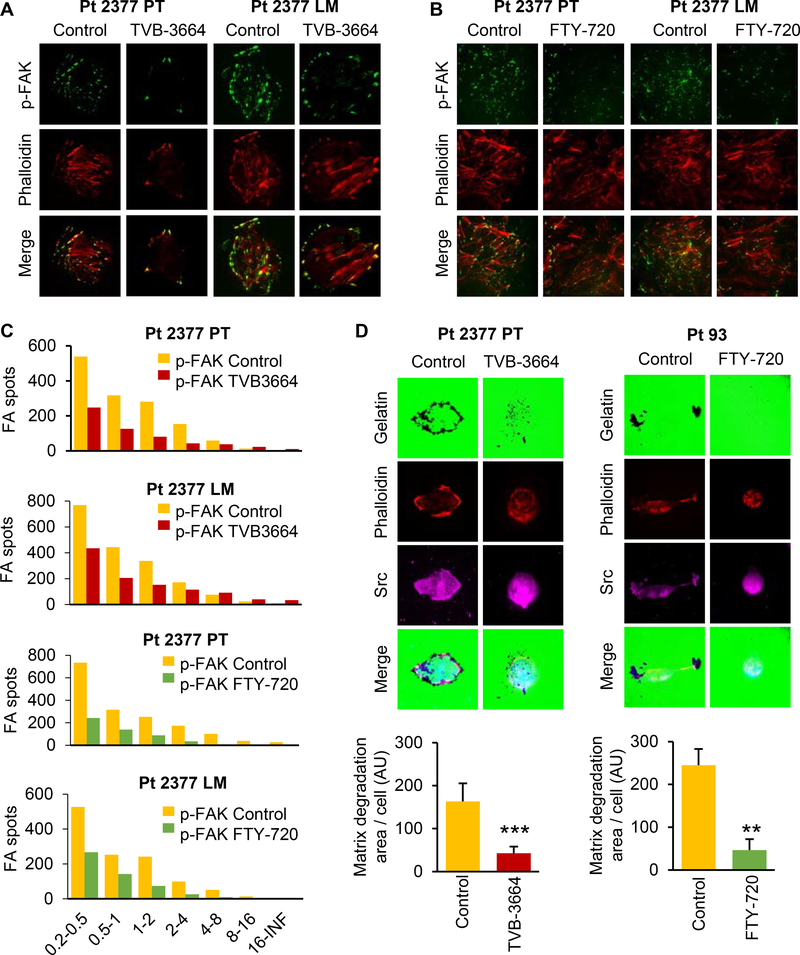
Focal adhesion structures are required for cellular interactions with the extracellular matrix and, thus, are crucially important in the migration process (33). We have previously reported that stable knockdown of FASN attenuates the activation of MET, Akt, FAK, and paxillin, which are known to regulate adhesion, migration, and invasion (4). To elucidate the effect of pharmacological inhibition of de novo lipogenesis and sphingolipid metabolism on focal adhesion formation, we utilized immunofluorescent staining and TIRF microscopy. Our results demonstrate that inhibition of FASN activity decreases the number of focal adhesions in Pt 2377 PT and Pt 2377 LM cells as analyzed by quantifying pFAK (Y397) staining (Figure 4A, C). Similarly, FTY-720 treatment of Pt 2377 PT and Pt 2377 LM cells decreased the number of focal adhesions (Figure 4B, C), suggesting the contribution of both metabolic pathways to focal adhesion dynamics in CRC cells. These results were confirmed by quantification of p-Paxilin (Y118) staining in Pt 2377 PT, Pt 2377 LM and Pt 130 cell lines when cells were treated with either TVB-3664 or FTY-720 (Supplemental Figure 2A-C). To demonstrate that the effect of FTY720 on formation of focal adhesions is indeed mediated by SPHKs/S1P, Pt 130 and Pt 93 cells were transfected with SPHK1 and SPHK2 siRNAs and the number of focal adhesions was determined by quantification of p-Paxilin (Y118) staining (Supplemental Figure 3). Both inhibition of SPHK1 and SPHK2 decreased formation of focal adhesions (Supplemental Figure 3A-B). Consistently, we observed attenuated activation of pMET and pFAK in these cell lines (Supplemental Figure 3C).
Figure 4. Inhibition of FASN and sphingolipid metabolism diminishes formation of focal adhesion complexes.
Primary Pt 2377 PT and Pt 2377 LM CRC cells were treated with (A) 0.2 μM TVB-3664 for 7 days and (B) 2.5 μM FTY-720 for 48h, stained with focal adhesion marker p-FAK (Y397) antibody (green) and phalloidin (red) and images were captured using TIRF microscopy. Representative images are shown. (C) Number of focal adhesions in different size classes was quantified using NIS-Elements. (D) Primary Pt 2377 PT cells were treated with 0.2 μM TVB-3664 for 7 days. Primary Pt 93 CRC cells were treated with 2.5 μM FTY-720 for 48h. Gelatin degradation ability of cells was assessed. Background gelatin is stained with Alexa Fluor 488 (green). Graphs display quantification of gelatin degradation per cell in both control and treated cells.
Since FASN, SPHK1 and SPHK2 are co-localized in invadopodia structures (Figure 1D), we tested the effect of FASN and sphingolipid metabolism inhibition on the gelatin degradation capability of CRC cells. It has been shown that Src contributes to invadopodia formation and extracellular matrix (ECM) degradation in human cancer cells (34). To assess the gelatin degradation capability of control and TVB-3664 treated primary CRC cells, staining for Src and Phalloidin was performed. As shown in Figure 4D, both TVB-3664 and FTY-720 treatments decreased gelatin degradation capability of Pt 2377 PT and Pt 93 cells, suggesting the vital role of the FASN/SPHK/S1P axis in invasion of CRC cells.
Together, these findings suggest that de novo lipogenesis and sphingolipid metabolism contribute to CRC cell migration and invasion by activation of focal adhesions and invadopodia.
Inhibition of FASN and sphingolipid metabolism decreases migration of primary CRC cells
Cancer cell motility is an essential part of the metastatic cascade (35). To determine the functional importance of de novo lipid synthesis and sphingolipid metabolism in cell migration, primary CRC cells were pre-treated with 0.2 μM TVB-3664 (7 days) or 2.5 μM FTY-720 (48h) and cell migration was monitored using Live Cell Imaging for 8h. Data analysis demonstrated that inhibition of FASN significantly decreased cell velocity and path length parameters in Pt 93, Pt 2377 PT and Pt 2377 LM cells, and significantly decreased distance from origin in all cell lines. A significant reduction in cell directionality was observed in Pt 130 and Pt 2377 LM cell lines treated with TVB-3664 as compared to control cells (Figure 5A and Supplemental Figure 4A). Consistently, stable knockdown of FASN decreased directed migration and Golgi polarization in HCT116 cells (Supplemental Figure 4C-D). FTY-720 treatment had a more prominent effect on inhibition of cell migration as compared to TVB-3664 treatment. FTY-720 significantly decreased all migration parameters in Pt 93, Pt 130, Pt 2377 PT and Pt 2377 LM cells with the exception of directionality in Pt 2377 PT where changes did not reach statistical significance (Figure 5B and Supplemental Figure 4B). Inhibition of cell migration by FTY-720 (2.5 μM) was accompanied by a significant decrease in activation of pFAK, pMET and pPAX in all cell lines (Figure 5C).
Figure 5. Inhibition of FASN and sphingolipid metabolism decreases migration of primary CRC cells.
Primary Pt 93 and Pt 2377 PT CRC cells were treated with (A) 0.2 μM TVB-3664 for 7 days and (B) 2.5 μM FTY-720 for 48h and cell migration was assessed using Live Cell Imaging. At least 25 cells from two independent experiments were analyzed. Each line with unique color represents an individual cell’s migration path. Migration potential is analyzed in four parameters: velocity (V), path length (PL), distance from origin (DO) and directionality (D). (C) The effect of FTY-720 treatment (48h) on p-Met, p-Fak and p-Paxilin activation in primary Pt 93, Pt 130, Pt 2377 PT and Pt 2377 LM CRC cells. (D) The effect of overexpression of FASN in Pt 130 cells on cell migration and activation of signaling pathways associated with cell migration. Equal numbers of cells were cultured for 24h prior cell analysis.
To test whether overexpression of FASN promotes cell migration, Pt 130 and SW480 cells were stably transfected with pEGFP-control or pEGFP-FASN plasmids (7). Overexpression of FASN resulted in a significant increase in all cell migration parameters in Pt 130 and velocity, distance from origin, and path length in SW480 cell lines. Interestingly, FASN overexpression led to an increase in SPHK1 expression in both cell lines and SPHK2 expression in SW480 cells. We also observed that FASN overexpression increased activation of pPAX and pErk1/2, which was previously reported to activate both SPHK1 and SPHK2 (36,37) (Figure 5D and Supplemental Figure 4E).
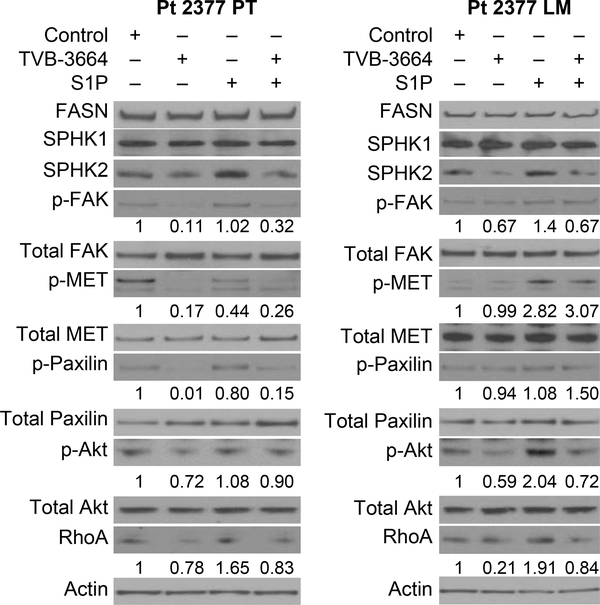
To further confirm that FASN-mediated changes in the signaling pathways associated with cell migration occur through upregulation of sphingolipid metabolism, we treated primary CRC cells with 0.2 μM of TVB-3664 for 7 days and then treated them with 1 μM S1P for 20 min. Consistent with the functional data (Figure 4–5), TVB-3664 treatment significantly decreased activation of p-MET, p-FAK, p-PAX, p-Akt and RhoA in CRC cells. Furthermore, inhibition of FASN expression led to a decrease in SPHK2 expression in Pt 2377 PT and Pt 2377 LM. S1P treatment rescued the inhibitory effects of TVB-3664 on p-Met, p-FAK, p-PAX and pAkt, suggesting that the effect of FASN on activation of these pathways is S1P dependent (Figure 6). Similar results were observed in Pt 93 and Pt 130 cells (Supplemental Figure 5). Together, these data suggest that upregulation of the FASN/SPHK/S1P axis promotes migratory capabilities of primary CRC cells.
Figure 6. S1P treatment rescues the effect of TVB-3664 on inhibition of the signaling pathway associated with cell migration and invasion.
Activation of p-Met, p-Fak, p-Akt, RhoA and p-Paxilin was assessed in Pt 2377 PT and Pt 2377 LM primary CRC cells treated with 0.2 μM TVB-3664 (7 d) and 1 μM S1P (20 min) alone or in combination.
Inhibition of de novo lipid synthesis and sphingolipid metabolism prevents activation of pro-metastatic signaling pathways in patient-derived xenografts tumors
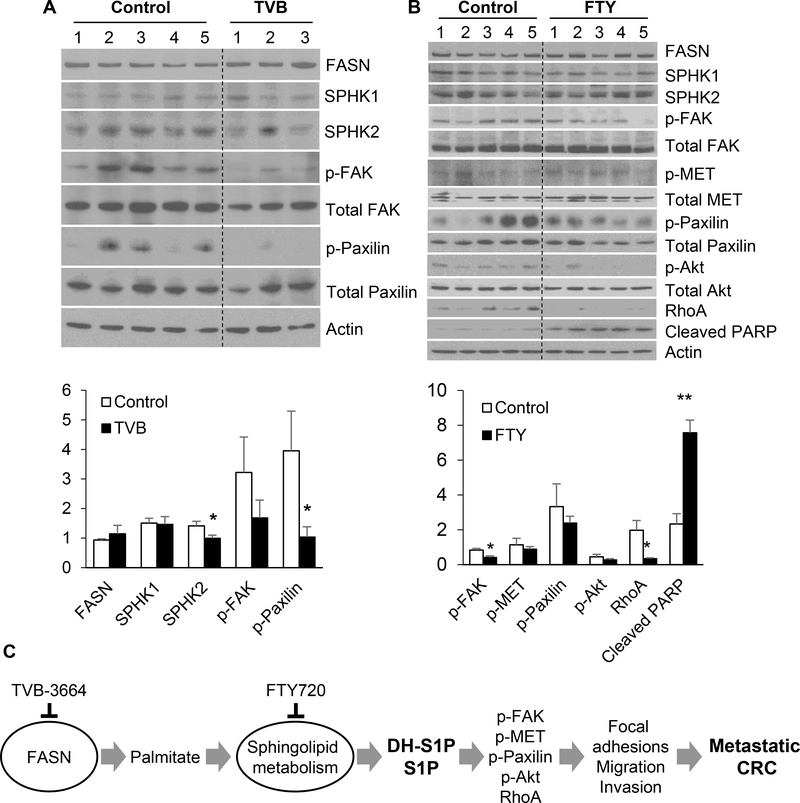
To test whether inhibition of FASN/SPHKs/S1P axis has a similar effect on activation of markers associated with adhesion and migration such as pMET, pFAK pPAX, RhoA and pAkt in vivo, we performed western blot analysis on tumor tissues collected from PDX model Pt 2402, which was established from a patient diagnosed with metastatic CRC to lung (12). PDXs were treated with either TVB-3664 (6 mg/kg daily by oral gavage for 4 weeks) or FTY-720 (5 mg/kg daily i.p. for 5 weeks). Consistent with our findings in primary CRC cells, TVB-3664 treatment led to a significant decrease in the expression of SPHK2 and activation of p-FAK and p-Paxilin; however no effect was noted in activation of PARP or Akt and MET pathways (Figure 7A). FTY-720 treatment did not affect the levels of SPHK1 and SPHK2, but decreased the levels of pFAK, pPAX, pAkt and RhoA (Figure 7B). Interestingly, FTY-720 treatment also led to a significant induction of PARP cleavage, which further supports the reported effect of FTY-720 on apoptosis (32). FTY-720 treatment led to approximately 30% reduction in tumor weight (Supplemental Figure 6).
Figure 7. Inhibition of de novo lipid synthesis and sphingolipid metabolism hinders activation of pro-metastatic signaling pathways in PDX tumors.
(A) Pt 2402 PDX tumors were treated with TVB-3664 (6 mg/kg for 4w) and tumor tissues were analyzed for expression of SPHK1, SPHK2 and other signaling proteins associated with cell migration and invasion by western blot. Band intensities were quantified using ImageJ software. (B) Pt 2402 PDX tumors were treated with FTY-720 (5 mg/kg daily i.p. for 5 weeks) and tumor tissues were analyzed for expression of SPHK1, SPHK2 and other signaling proteins associated with cell migration and invasion by western blot. (C) Schematic illustration of regulation of cell adhesion, migration and invasion by the FASN/SPHK/S1P axis.
In summary, our data suggest that de novo lipogenesis and sphingolipid metabolism activate signal transductions involved in cell adhesion, migration and invasion in vitro and in vivo (Figure 7C) and warrant further investigation of these metabolic pathways in regulation of adhesion, migration and invasion in metastatic animal models.
DISCUSSION
In this study, we investigated the role of the FASN/SPHK/S1P axis in regulation of the metastatic potential of primary CRC cells. Our group and others have reported that upregulation of lipogenic enzymes, including FASN, contributes to cancer cell progression and metastasis (4,7,38,39). However, the mechanisms behind the effect of FASN on CRC growth and metastasis are not fully understood. Here, we report that alteration of sphingolipid metabolism, including overexpression and activation of SPHKs and an increase in production of DH-S1P and S1P downstream of FASN, plays a vital role in regulation of CRC cells growth, migration and invasion.
Altered sphingolipid metabolism has been shown to contribute to cancer progression (40). According to the ceramide-sphingosine-S1P rheostat model, both ceramide and sphingosine promote apoptosis, whereas DH-S1P/S1P enhances cell survival (19,30). In agreement with this model, we demonstrated an increase in the total sphingosine level in HCT116 cells with knockdown of FASN, while the level of S1P was significantly decreased. Our study also showed that pharmacological inhibition of FASN is also associated with a decrease in DH-S1P in conditioned medium from primary CRC cells and in PDX tumors. We did not observe changes in the levels of S1P in PDX tumors, which can be explained by the complexity of S1P signaling in the presence of stroma in PDX tumors (15,19). Since S1P acts extracellularly in a paracrine and autocrine manner through five specific G protein-coupled receptors S1P1–S1P5 (15,16), another potential explanation is a change in expression/availability of S1P receptors due to alterations in a cell membrane composition associated with FASN inhibition (10).
In agreement with our presented results, TVB-3166 treatment significantly alters intermediates of de novo sphingolipid metabolism of breast cancer cells (41). Consistently, our recent studies showed that prolonged treatment of CRC PDXs with TVB-3664 also leads to significant alterations in different sphingolipids (12). Moreover, the important role of de novo lipid synthesis upstream of sphingolipid metabolism was also suggested in a study showing that overexpression of FASN is associated with inhibition of drug-induced ceramide production, thus, causing drug resistance in breast cancer cells (42).
An interconnection between FASN and SPHK2 is strongly suggested by the significant correlation between expression of these enzymes in CRC tissues at mRNA and protein levels. Furthermore, our data show that inhibition of FASN significantly decreases expression of SPHK2 in vitro and in vivo, while overexpression of FASN in Pt 130 and SW480 CRC cells increases expression of both SPHK1 and SPHK2. The mechanisms of regulation of sphingolipid metabolism downstream of de novo lipid synthesis are currently under investigation in our laboratory.
SPHK1 is located mainly in the cytoplasm and the cell membrane, whereas SPHK2 is mainly located in the mitochondria, nucleus and the endoplasmic reticulum (43). Surprisingly, we observed localization of FASN and SPHKs in invadopodia, suggesting their role in invadopodia biogenesis and cell migration and invasion. These findings further support our hypothesis that an increase in de novo lipid synthesis alters sphingolipid metabolism to promote metastatic potential of CRC cells. The role of sphingolipid metabolism in CRC metastasis is also supported by recent studies showing that upregulation of SPHK1 significantly correlates with parameters such as lymph node metastasis, liver metastasis, and advanced TNM stage (44) and SPHK2 is implicated in CRC cell proliferation and invasion by enhancing MYC expression (45). Interestingly, in agreement with the latter study, our data show that inhibition of sphingolipid metabolism using FTY-720 significantly decreases cellular proliferation in all cell lines tested, and the effect of FTY-720 is more prominent than the effect of FASN inhibition, which seems to be more cell-line specific.
Our published studies showed that stable knockdown of FASN inhibits CRC metastasis by alteration of cell migration, adhesion and secretion of angiogenic factors (4,7). Cerulenin, an antifungal antibiotic that inhibits fatty acid and steroid biosynthesis, also suppresses liver metastasis of colon cancer in mice (46). In agreement with these studies, our data show that pharmacological inhibition of FASN using TVB-3664 leads to suppression of CRC cell growth, migration, focal adhesion and invadopodia formation.
Our results revealed that FTY-720 treatment is more effective not only in suppressing cellular proliferation, but also in inhibiting migration of CRC cells. These findings are consistent with another study that reported FTY-720 significantly decreases migration and invasion of glioblastoma cells (47). Our data suggest that, at least in CRC, activity of SPHKs and production of DH-S1P and S1P are more vital in cell motility than de novo-produced palmitate, which can contribute to diverse cell functions through multiple mechanisms (24). Since sphingolipids are major components of the plasma membrane and lipid raft and also act as signaling molecules, we do not exclude the possibility that intermediates of sphingolipid metabolism, other than DH-S1P and S1P, contribute to the effects of TVB-3664 and FTY-720 on cell migration (40).
Even though the effects of FTY-720 on proliferation and cell motility in cancer cells have been reported (32), its effect on metastatic capabilities of cells and the mechanisms behind it have not yet been elucidated in CRC. Our data revealed that FTY-720 blocks cell migration and invasion through suppression of focal adhesion formation and abolishment of cells’ ability to form invadopodia and digest gelatin. Inhibition of formation of focal adhesions and activation of pFAK and pMET was also observed when expression of SPHK1 and SPHK2 is down regulated by siRNAs suggesting that these effects indeed are mediated by SPHKs/S1P axis. These findings are consistent with published data from our group showing that FASN upregulates MMP9 in CRC cell lines (7), and with a study demonstrating that FASN inhibition significantly decreases expression of MMP2 and MMP9 in osteosarcoma cells (48). In agreement with our in vitro studies, we observed that inhibition of FASN and sphingolipid metabolism in PDX models established from a patient who was diagnosed with CRC metastasized to lung leads to inhibition of p-FAK, p-PAX, RhoA and pAkt activation. We are currently exploring anti-cancer properties of FTY-720 in metastatic CRC models in vivo.
Our results showing that S1P treatment can rescue the effect of FASN inhibition on activation of p-FAK, p-MET, p-Akt and p-PAX provide further evidence that the effect of TVB-3664 on the signaling pathways associated with migration and invasion, at least in part, occurs through alteration of S1P synthesis by SPHKs. Even though both S1P and DH-S1P are generated in response to activation of SPHKs, their contribution to the activation of signaling pathways may be different (14). Therefore, further studies are required to better understand the role of FASN overexpression in de novo biosynthesis of sphingolipids and its association with a selective increase in intracellular content of DH-S1P or S1P. Moreover, it is very important to understand the contribution of SPHK1 and SPHK2 to CRC progression and metastasis using genetic manipulations of these enzymes and to understand how the phosphorylation status of these enzymes contributes to their functions in CRC. We are currently pursuing these projects in our laboratory.
In recent years, lipogenic enzymes have become an attractive target for therapeutic intervention (8,32). Our study demonstrates that FASN overexpression alters sphingolipid metabolism, increases DH-S1P and S1P production and, thus, activates signaling pathways associated with cancer cell motility and invasion to promote CRC metastasis. The compelling data demonstrating the effect of FTY-720 on cellular proliferation and metastatic potential of CRC cells provide a strong rational to further explore the role of the FASN/SPHK/S1P axis in metastatic CRC, which could lead to identification of new therapeutic targets and strategies in CRC.
Supplementary Material
ACKNOWLEDGMENTS
The University of Kentucky Markey Cancer Center’s Research Communications Office and Donna Gilbreath assisted in preparation of this manuscript. The authors thank the Biospecimen and Tissue Procurement Shared Resource Facility (SRF), Redox Metabolism SRF, and Biostatistics and Bioinformatics SRF of the University of Kentucky Markey Cancer Center (supported by National Cancer Institute grant P30 CA177558).
Financial support: This work is supported by grants from 3-V Biosciences, Inc. (YYZ); ACS IRG 85–001-25 (YYZ); NCI K22 CA197193 (YYZ); P20 GM121327 (YYZ), R01 CA133429 (TG) and R01 CA208343 (BME/TG).
Footnotes
Conflict of interest statement: The authors declare no potential conflicts of interest.
REFERENCES
- 1.Seyfried TN, Flores R, Poff AM, D’Agostino DP. Cancer as a metabolic disease: implications for novel therapeutics. Carcinogenesis 2014;35:515–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Swinnen JV, Brusselmans K, Verhoeven G. Increased lipogenesis in cancer cells: new players, novel targets. Current Opinion in Clinical Nutrition & Metabolic Care 2006;9:358–65 [DOI] [PubMed] [Google Scholar]
- 3.Zhang F, Du G. Dysregulated lipid metabolism in cancer. World J Biol Chem 2012;3:167–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zaytseva YY, Rychahou PG, Gulhati P, Elliott VA, Mustain WC, O’Connor K, et al. Inhibition of fatty acid synthase attenuates CD44-associated signaling and reduces metastasis in colorectal cancer. Cancer research 2012;72:1504–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cai Y, Wang J, Zhang L, Wu D, Yu D, Tian X, et al. Expressions of fatty acid synthase and HER2 are correlated with poor prognosis of ovarian cancer. Medical oncology 2015;32:391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sounni NE, Cimino J, Blacher S, Primac I, Truong A, Mazzucchelli G, et al. Blocking lipid synthesis overcomes tumor regrowth and metastasis after antiangiogenic therapy withdrawal. Cell metabolism 2014;20:280–94 [DOI] [PubMed] [Google Scholar]
- 7.Zaytseva YY, Elliott VA, Rychahou P, Mustain WC, Kim JT, Valentino J, et al. Cancer cell-associated fatty acid synthase activates endothelial cells and promotes angiogenesis in colorectal cancer. Carcinogenesis 2014;35:1341–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Buckley D, Duke G, Heuer TS, O’Farrell M, Wagman AS, McCulloch W, et al. Fatty acid synthase - Modern tumor cell biology insights into a classical oncology target. Pharmacol Ther 2017;177:23–31 [DOI] [PubMed] [Google Scholar]
- 9.Heuer TS, Ventura R, Mordec K, Lai J, Fridlib M, Buckley D, et al. FASN Inhibition and Taxane Treatment Combine to Enhance Anti-tumor Efficacy in Diverse Xenograft Tumor Models through Disruption of Tubulin Palmitoylation and Microtubule Organization and FASN Inhibition-Mediated Effects on Oncogenic Signaling and Gene Expression. EBioMedicine 2017;16:51–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ventura R, Mordec K, Waszczuk J, Wang Z, Lai J, Fridlib M, et al. Inhibition of de novo Palmitate Synthesis by Fatty Acid Synthase Induces Apoptosis in Tumor Cells by Remodeling Cell Membranes, Inhibiting Signaling Pathways, and Reprogramming Gene Expression. EBioMedicine 2015;2:806–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.National Cancer Institute. 2017 FASN Inhibitor TVB-2640 in Treating Patients with Colon or Other Cancers That Can Be Removed by Surgery. National Institutes of Health; <https://www.cancer.gov/about-cancer/treatment/clinical-trials/search/v?id=NCI-2016-01710&r=1>. [Google Scholar]
- 12.Zaytseva YY, Rychahou PG, Le A-T, Scott TL, Flight RM, Kim JT, et al. Preclinical evaluation of novel fatty acid synthase inhibitors in primary colorectal cancer cells and a patient-derived xenograft model of colorectal cancer. Oncotarget 2018;9:24787–800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Garcia-Barros M, Coant N, Truman JP, Snider AJ, Hannun YA. Sphingolipids in colon cancer. Biochimica et biophysica acta 2014;1841:773–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bu S, Kapanadze B, Hsu T, Trojanowska M. Opposite effects of dihydrosphingosine 1-phosphate and sphingosine 1-phosphate on transforming growth factor-beta/Smad signaling are mediated through the PTEN/PPM1A-dependent pathway. J Biol Chem 2008;283:19593–602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Spiegel S, Milstien S. Sphingosine 1-phosphate, a key cell signaling molecule. J Biol Chem 2002;277:25851–4 [DOI] [PubMed] [Google Scholar]
- 16.Huwiler A, Zangemeister-Wittke U. Targeting the conversion of ceramide to sphingosine 1-phosphate as a novel strategy for cancer therapy. Critical reviews in oncology/hematology 2007;63:150–9 [DOI] [PubMed] [Google Scholar]
- 17.Don AS, Lim XY, Couttas TA. Re-configuration of sphingolipid metabolism by oncogenic transformation. Biomolecules 2014;4:315–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Morad SA, Cabot MC. Ceramide-orchestrated signalling in cancer cells. Nature reviews Cancer 2013;13:51–65 [DOI] [PubMed] [Google Scholar]
- 19.Pyne NJ, Pyne S. Sphingosine 1-phosphate and cancer. Nature reviews Cancer 2010;10:489–503 [DOI] [PubMed] [Google Scholar]
- 20.Liang J, Nagahashi M, Kim EY, Harikumar KB, Yamada A, Huang WC, et al. Sphingosine-1-phosphate links persistent STAT3 activation, chronic intestinal inflammation, and development of colitis-associated cancer. Cancer cell 2013;23:107–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.White C, Alshaker H, Cooper C, Winkler M, Pchejetski D. The emerging role of FTY720 (Fingolimod) in cancer treatment. Oncotarget 2016;7:23106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kondo J, Endo H, Okuyama H, Ishikawa O, Iishi H, Tsujii M, et al. Retaining cell-cell contact enables preparation and culture of spheroids composed of pure primary cancer cells from colorectal cancer. Proc Natl Acad Sci U S A 2011;108:6235–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mathews TP, Kennedy AJ, Kharel Y, Kennedy PC, Nicoara O, Sunkara M, et al. Discovery, biological evaluation, and structure-activity relationship of amidine based sphingosine kinase inhibitors. J Med Chem 2010;53:2766–78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Menendez JA, Lupu R. Fatty acid synthase and the lipogenic phenotype in cancer pathogenesis. Nature reviews Cancer 2007;7:763–77 [DOI] [PubMed] [Google Scholar]
- 25.Yamaguchi H, Takeo Y, Yoshida S, Kouchi Z, Nakamura Y, Fukami K. Lipid rafts and caveolin-1 are required for invadopodia formation and extracellular matrix degradation by human breast cancer cells. Cancer research 2009;69:8594–602 [DOI] [PubMed] [Google Scholar]
- 26.Artym VV, Zhang Y, Seillier-Moiseiwitsch F, Yamada KM, Mueller SC. Dynamic interactions of cortactin and membrane type 1 matrix metalloproteinase at invadopodia: defining the stages of invadopodia formation and function. Cancer research 2006;66:3034–43 [DOI] [PubMed] [Google Scholar]
- 27.Weed SA, Parsons JT. Cortactin: coupling membrane dynamics to cortical actin assembly. Oncogene 2001;20:6418–34 [DOI] [PubMed] [Google Scholar]
- 28.Spiegel S, Milstien S. Sphingosine-1-phosphate: an enigmatic signalling lipid. Nature reviews Molecular cell biology 2003;4:397–407 [DOI] [PubMed] [Google Scholar]
- 29.Merrill AH Jr., Wang E, Mullins RE Kinetics of long-chain (sphingoid) base biosynthesis in intact LM cells: effects of varying the extracellular concentrations of serine and fatty acid precursors of this pathway. Biochemistry 1988;27:340–5 [DOI] [PubMed] [Google Scholar]
- 30.Berdyshev EV, Gorshkova IA, Usatyuk P, Zhao Y, Saatian B, Hubbard W, et al. De novo biosynthesis of dihydrosphingosine-1-phosphate by sphingosine kinase 1 in mammalian cells. Cell Signal 2006;18:1779–92 [DOI] [PubMed] [Google Scholar]
- 31.Berdyshev EV, Gorshkova I, Skobeleva A, Bittman R, Lu X, Dudek SM, et al. FTY720 inhibits ceramide synthases and up-regulates dihydrosphingosine 1-phosphate formation in human lung endothelial cells. J Biol Chem 2009;284:5467–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.White C, Alshaker H, Cooper C, Winkler M, Pchejetski D. The emerging role of FTY720 (Fingolimod) in cancer treatment. Oncotarget 2016;7:23106–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.De Pascalis C, Etienne-Manneville S. Single and collective cell migration: the mechanics of adhesions. Molecular Biology of the Cell 2017;28:1833–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Murphy DA, Courtneidge SA. The ‘ins’ and ‘outs’ of podosomes and invadopodia: characteristics, formation and function. Nat Rev Mol Cell Biol 2011;12:413–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Paul CD, Mistriotis P, Konstantopoulos K. Cancer cell motility: lessons from migration in confined spaces. Nature reviews Cancer 2017;17:131–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pitson SM, Moretti PA, Zebol JR, Lynn HE, Xia P, Vadas MA, et al. Activation of sphingosine kinase 1 by ERK1/2-mediated phosphorylation. EMBO J 2003;22:5491–500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hait NC, Bellamy A, Milstien S, Kordula T, Spiegel S. Sphingosine kinase type 2 activation by ERK-mediated phosphorylation. J Biol Chem 2007;282:12058–65 [DOI] [PubMed] [Google Scholar]
- 38.Jiang L, Wang H, Li J, Fang X, Pan H, Yuan X, et al. Up-regulated FASN expression promotes transcoelomic metastasis of ovarian cancer cell through epithelial-mesenchymal transition. International journal of molecular sciences 2014;15:11539–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zaytseva YY, Harris JW, Mitov MI, Kim JT, Butterfield DA, Lee EY, et al. Increased expression of fatty acid synthase provides a survival advantage to colorectal cancer cells via upregulation of cellular respiration. Oncotarget 2015;6:18891–904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ryland LK, Fox TE, Liu X, Loughran TP, Kester M. Dysregulation of sphingolipid metabolism in cancer. Cancer biology & therapy 2011;11:138–49 [DOI] [PubMed] [Google Scholar]
- 41.Benjamin DI, Li DS, Lowe W, Heuer T, Kemble G, Nomura DK. Diacylglycerol Metabolism and Signaling Is a Driving Force Underlying FASN Inhibitor Sensitivity in Cancer Cells. ACS chemical biology 2015;10:1616–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Liu H, Wu X, Dong Z, Luo Z, Zhao Z, Xu Y, et al. Fatty acid synthase causes drug resistance by inhibiting TNF-alpha and ceramide production. Journal of lipid research 2013;54:776–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hatoum D, Haddadi N, Lin Y, Nassif NT, McGowan EM. Mammalian sphingosine kinase (SphK) isoenzymes and isoform expression: challenges for SphK as an oncotarget. Oncotarget 2017;8:36898–929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Long J, Xie Y, Yin J, Lu W, Fang S. SphK1 promotes tumor cell migration and invasion in colorectal cancer. Tumor Biology 2016;37:6831–6 [DOI] [PubMed] [Google Scholar]
- 45.Zhang L, Liu X, Zuo Z, Hao C, Ma Y. Sphingosine kinase 2 promotes colorectal cancer cell proliferation and invasion by enhancing MYC expression. Tumor Biology 2016;37:8455–60 [DOI] [PubMed] [Google Scholar]
- 46.Murata S, Yanagisawa K, Fukunaga K, Oda T, Kobayashi A, Sasaki R, et al. Fatty acid synthase inhibitor cerulenin suppresses liver metastasis of colon cancer in mice. Cancer science 2010;101:1861–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhang L, Wang H, Zhu J, Ding K, Xu J. FTY720 reduces migration and invasion of human glioblastoma cell lines via inhibiting the PI3K/AKT/mTOR/p70S6K signaling pathway. Tumor Biology 2014;35:10707–14 [DOI] [PubMed] [Google Scholar]
- 48.Liu ZL, Mao JH, Peng AF, Yin QS, Zhou Y, Long XH, et al. Inhibition of fatty acid synthase suppresses osteosarcoma cell invasion and migration via downregulation of the PI3K/Akt signaling pathway in vitro. Molecular medicine reports 2013;7:608–12 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.