Abstract
Background:
Low vitamin B-6 status has been linked to depressive symptomatology. However, most studies have been cross-sectional, may not have controlled for relevant confounders, and have examined this association in non-Hispanic white populations. We examined the longitudinal association of vitamin B-6 status with depressive symptomatology across 3 time points over ~ 5–7 y in a cohort of older Hispanic adults of Puerto Rican descent, a population previously identified to be at high risk for depressive symptomatology and clinical depression.
Methods:
We used two-level hierarchical linear regression models (HLM) for continuous outcomes. Level-1 data included three measures of participant’s depressive symptomatology collected at baseline, 2y follow-up and 5y follow-up. Participants constituted level-2 data. Vitamin B-6 status was associated with depressive symptomatology across these time points.
Results:
Plasma pyridoxyl-5’-phosphate (PLP) concentration, a time-varying predictor, was significantly associated with depressive symptomatology. Study participants with PLP deficiency, vs. optimal PLP, had higher baseline depressive symptoms (CES-D score of 22±14, vs. 20±13); this differential remained constant over time and persisted after controlling for age, sex, education, BMI, smoking and alcohol use, other relevant nutritional factors, perceived stress, stressful life events, allostatic load, and use of antidepressant medication. However, PLP concentration was not associated with the rate of change in depressive symptomatology over time.
Conclusions:
Suboptimal plasma PLP is associated with higher depressive symptomatology in older Puerto Rican adults and this appears to persist over time. Our data suggest that identification and treatment of vitamin B-6 deficiency may be a useful preventive approach in this population.
Keywords: vitamin B-6 deficiency, depressive symptoms, plasma PLP, older Latino adults, longitudinal association
Introduction
Major depressive disorder (MDD) is a chronic health condition with significant consequences for health care costs, disability, quality of life, medical morbidity and mortality. 1 The World Health Organization (WHO) has ranked depression as the 4th leading cause of disability, projected to become the 2nd by 2020, and estimates than 350 million people are affected worldwide. 2 While depression is treatable, only 39% of individuals with severe depression contact a mental health professional, 3 up to 45% achieve remission after trying one antidepressant, 4 approximately 20% are treatment resistant (i.e., nonresponse to two different pharmacologic types taken as directed for sufficient periods of time), and up to 50% of patients will experience recurrence of symptoms. 5 Current outcomes of antidepressant treatments focused on the monoamine hypothesis point to the need for research that explores alternative mechanisms to treat and prevent the onset a recurrence of major depression. 6
The significant role of nutrition in major depressive disorders is recognized.7–9 Both malnutrition resulting from inadequate diets,10,11 and specific micro-nutrient deficiencies12,13 have been associated with depression, particularly in older adults.14 Ageing related conditions, metabolic dysregulation, poor nutrient absorption, polypharmacy, and social stress may decrease quality of food intake or bioavailability of nutrients, in turn, leading to nutrient deficiencies.15 Optimal micronutrient status is necessary for well-functioning neurological health.16 An association between depressive symptoms and low vitamin B-6 status, evidenced by low concentration of plasma pyridoxal-5’-phosphate (PLP), has been suggested, 19 although the evidence is mixed. 20
Vitamin B-6 is a water soluble compound that comprises three different pyridine derivatives, pyridoxine (PN), pyridoxal (PL), and pyridoxamine (PM), of which pyridoxal-5’– phosphate (PLP) is the biologically most active form. 21 The coenzyme pyridoxal-5’– phosphate (PLP) is an essential cofactor for amino acid decarboxylases involved in the synthesis of neurotransmitters implicated in depression including dopamine, norepinephrine, serotonin or 5-hydroxytryptamine (5-HT), and γ-amino butyric acid (GABA). 22,23 Immune dysregulation and activation of the inflammatory response system (IRS) are also characteristic of major depression. 24 The role of vitamin B-6 in the metabolism of tryptophan and one-carbon metabolism makes vitamin B-6 a relevant cofactor in the body’s immune response. 22 Vitamin B-6 prevents the accumulation of neurotoxic intermediates produced during tryptophan metabolism, acting as a cofactor in the metabolism of tryptophan through the kynurenine aminotransferase and kynureninase enzymes. 25 Several epidemiological and treatment studies have established an association between low vitamin B-6 status and depressive symptomatology. However, research gaps in the current vitamin B-6 and depression association include the cross-sectional design of the majority of current investigations, the failure to control for relevant confounders, and the dearth of studies examining this association in ethnically diverse populations at high risk for depressive symptomatology. 26
Our objective, then, was to examine individual differences in the longitudinal association of vitamin B-6 status, measured at three time points as plasma PLP, with participants’ initial status and rate of change in depressive symptomatology before and after adjusting for relevant confounders, in a cohort of older Puerto Rican adults residing in the U.S. mainland, a population previously identified to be at high risk for experiencing depressive symptomatology 27 and major depression 28, compared to non-Hispanic whites as well as other Latino groups.
Chronic stress and/or stressful life events are significantly associated with an increased incidence and relapse of major depression disorders.29–31 Social stress models of depression posit that individual variance in stress exposure, perception and coping mechanisms of stress, and capacity for resilience are fundamental for the onset and prevalence of depression and depressive symptomatology.32–35 Similarly, stress has been linked to increased food intake 36,37 and low nutrient food choices, 38–42 in turn, influencing nutritional status. We, therefore, examine the effect of PLP on depressive symptomatology trajectories after controlling for the effects of stress and, to capture the complex role of stress on depressive symptomatology, we control for three different types of stress. Perceived stress, a subjective measure of stress; lifetime stressful life events, an objective measure of stress; and allostatic load, a multi-system physiological measure hypothesized to capture a lifetime exposure to multiple stressors.43–45
A number of micro-nutrients are important for optimal mental health,7 empirical studies evidence associations between depression and micro-nutrient deficiencies. In older adults, in particular, deficiencies in the mineral magnesium, 5,46–49 vitamin D,50,51 and vitamin B12 and folate, 52 are associated with higher depressive symptoms. Therefore, we control for magnesium, vitamin D, vitamin B12 and folate in our models.
We hypothesized that suboptimal plasma PLP, a time-varying predictor, would be significantly associated with higher score on the Center for Epidemiologic Studies-Depression Scale (CES-D) at baseline and would interact significantly with time, before and after adjusting for age, sex, education and BMI; smoking and alcohol use; perceived stress, experiences of stressful life events, and allostatic load; time-varying nutritional factors related to depression including, plasma vitamin concentrations of vitamins B-12, D, and folate; and baseline dietary intake of magnesium; and use of antidepressant medication.
Data and Methods
We used data from the Boston Puerto Rican Health Study, a population-based cohort study of Puerto Rican adults between the ages of 45 and 75 y at baseline, residing in an urban metropolitan area of the northeastern United States. The study was approved by the Institutional Review Boards at Tufts Medical Center, Northeastern University and the University of Massachusetts Lowell. All participants provided signed informed consent, in their language of choice (Spanish or English). A total of 1,500 participants were recruited at baseline between 2004 and 2009 (specifics of the study and recruitment methodology are described in detail elsewhere). 53 Two follow-up interviews were completed at approximately 2-y and 5-y time points after the baseline. Completion for the 2-y interview was 86% and for the 5-y interview, 64% of baseline participants. In addition to the survey questionnaire, biomarker collection at each wave included a fasting blood sample for measurement of nutritional and other biological markers. Fasting blood samples were obtained by venipuncture, processed, placed on ice, and delivered within three hours to the Nutrition Evaluation Laboratory, Jean Mayer USDA Human Nutrition Research Center on Aging, Tufts University. The current analysis included 1446 participants with complete depressive symptomatology and plasma PLP data at baseline. The longitudinal analysis incorporated additional data from 1223 baseline participants interviewed at 2y follow-up, and 790 baseline participants interviewed at 5y follow-up (Figure 1).
Figure 1.
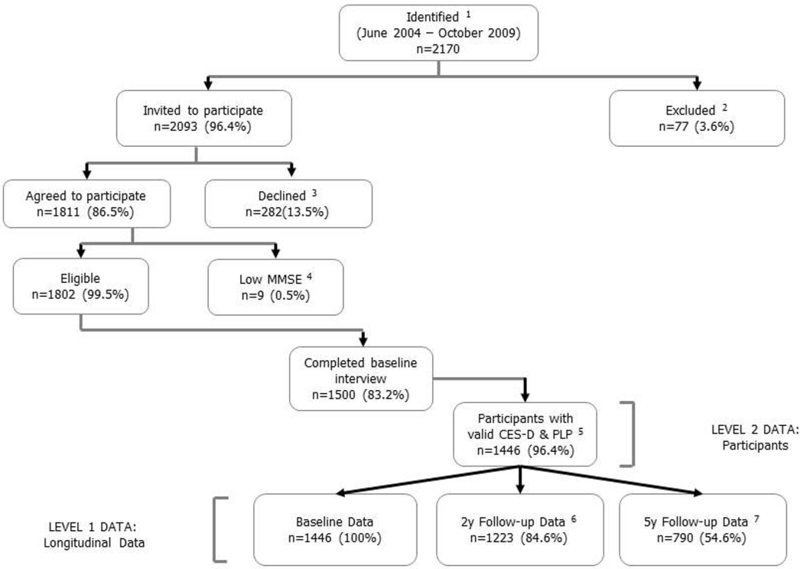
Flow diagram of participants included in the current analysis. 1 Identified as of Puerto Rican descent. 2 Met the exclusion criteria (eg, serious illness, moved from the study area, homeless, or hostile). 3 Reasons for declining participation (eg, not interested, too busy, did not want blood drawn, length of study, or doctor/spouse advised against participation). 4 Low Mini-Mental State Examination Score (MMSE<10). 5 Participants with valid depressive symptomatology and plasma PLP data. 6 Baseline participants with 2y follow-up data. 7 Baseline participants with 5y follow-up data.
Measures
Depressive symptomatology was measured with the Center for Epidemiologic Studies-Depression Scale (CES-D-20), a 20-item response scale that evaluates the frequency of symptoms associated with depression, such as poor appetite, feeling lonely, restless sleep, crying spells, and feeling sad. 54 Responses are measured using a 4-point Likert scale, from 0=rarely/never, 1=some or few times, 2=occasionally/or a moderate amount, to 3=most/all of the time. Scores range from 0 to 60, with higher scores indicating greater depressive symptomatology. A cutoff score of 16 or greater is generally used to identify individuals at risk of clinical depression. This instrument has shown good reliability and discriminating features in Puerto Ricans. 27,55 The Cronbach’s alpha for the CES-D scale components in this sample was high (α=.90). We used the CES-D as a continuous variable measured at three points in time (Baseline, and 2y and 5y follow-up).
Pyridoxal 5’-phosphate (PLP), the active form of vitamin B-6, was measured enzymatically using tyrosine decarboxylase (CV=16%). 56 Vitamin B-6 deficiency is generally defined by plasma PLP concentration <20 nmol/L, with marginal impairment defined at < 30 nmol/L. 25 We created a dichotomous variable to identify study participants with suboptimal vitamin B-6 (0=no if PLP concentration ≥30 nmol/L; 1=yes if PLP < 30 nmol/L). We defined plasma PLP as a time-varying predictor, as PLP plasma concentrations may vary over time. We used PLP data collected at baseline, 2y and 5y follow-up.
We adjusted for baseline health indicators and behaviors, including body mass index (BMI = weight (kg) divided by height (m2)), smoking (never=ref; vs. past, or current) and alcohol use -not currently drinking=ref; vs. moderate (≤ 1 drink/day for women, or ≤ 2 drinks/day for men), or heavy (greater than the latter).
Associations between stress and depression have been documented; 57,58 therefore, we adjusted for baseline reports of objective and subjective stress measured with the Life Events Questionnaire (LEQ) 59 and the Perceived Stress Scale (PSS) 60 respectively. The LEQ, an 82-item inventory list, targets life events that are primarily acute, unpleasant or threatening, occurred during past year.61 Participants indicated first whether or not they had experienced the event (0=no; 1=yes), and if so, they were asked to categorize the event as “good” or “bad”, and to rate its effect using a 4-point Likert scale, from 1=no effect, 2=some effect, 3=moderate effect, and 4=great effect. For the current analysis, we used the sum score of the effects of all bad stressful life events reported at baseline. The Perceived Stress 14-item Scale (PSS) 60 was used to assess participant’s assessment of stressful circumstances in their lives without asking for specific events. The PSS evaluated the level of perceived stress during the last month using a 5-point Likert scale, from 0=never, 1=almost never, 2=every now and then, 3=often, 4=very often. The Cronbach’s alpha for the PSS-14 scale components in this sample was high (α=.85). We used the PSS-14 as a continuous variable.
We included an allostatic load score to measure the body’s physiological response to cumulative psychosocial and environmental challenges. The allostatic load score used in this study consists of 11 biomarkers that represent the function of neuroendocrine (serum dehydroepiandrosterone sulfate (DHEA-S), urinary cortisol, urinary norepinephrine, urinary epinephrine), immune (C-reactive protein (CRP)), cardiovascular (systolic blood pressure (SBP), diastolic blood pressure (DBP)), and metabolic (plasma total and HDL cholesterol, plasma HbA1c and central adiposity (waist circumference)) systems. Overnight fasting blood samples for biochemical analyses of dehydroepiandrosterone sulfate (DHEA-S), glycosylated hemoglobin (HbA1c), C-reactive protein (CRP), total cholesterol (TC), high density lipoprotein cholesterol (HDL-C), plasma triglycerides and glucose, were drawn by a certified phlebotomist. Measures for cortisol, epinephrine and norepinephrine were obtained from 12-hour urine collection samples. Allostatic load, as a composite score for this study, has been described previously. 62 Briefly, a summary score was constructed for the number of biomarkers in which participants fell into the upper or lower clinically defined cutoff point, except for serum DHEA-S and urinary epinephrine and norepinephrine, for which the lower (DHEA-S) and upper (epinephrine and norepinephrine) quartile was used as the cutoff point. To account for medication use, a point was assigned if a participant was taking medication for hypertension, diabetes, hyperlipidemia, or testosterone, but the respective parameter did not fall within the defined cutoff (see Table 1). Final allostatic load scores ranges from 0 to 11, scores from 0 to 2 are considered low, 3 to 5 high, and greater than 6 very high.
Table 1.
Definition of Allostatic Load in the Boston Puerto Rican Study (Mattei et al 2010)
| Biomarker | Cut-off Points |
|---|---|
| Neuroendocrine System | |
| DHEA-S (ng/mL) a | Men <= 589.5 or taking medications |
| Women <= 368.5 or taking medications | |
| Cortisol: (μg/g creatinine) b | Men >= 41.5 |
| Women >= 49.5 | |
| Norepinephrine: (μg/g creatinine) b | Men >= 30.5 |
| Women >= 46.9 | |
| Epinephrine: (μg/g creatinine) b | Men >= 2.8 |
| Women >= 3.6 | |
| Immune System | |
| C-reactive protein: CRP (mg/L) c | > 3 |
| Cardiovascular system | |
| Systolic Blood Pressure: SBP (mmHg) d | > 140 or taking medications |
| Diastolic Blood Pressure: DBP (mmHg) d | > 90 or taking medications |
| Metabolic System | |
| Lipid metabolism | |
| Total Cholesterol: TC (mg/dl) e | >= 240 or taking medications |
| High Density Lipoprotein: HDL-C (mg/dl) e | < 40 or taking medications |
| Glucose metabolism | |
| Glycosylated hemoglobin: HbA1c (%) f | > 7 or taking medications |
| Adipose tissue deposition | |
| Waist Circumference: WC (cm) g | Men > 102 |
| Women > 88 |
Trivedi and Khaw (2001);
Goldman et al (2004);
Pearson etal (2003);
NHLBI (2004);
NHLBI (2002);
ADA (2008);
NHLBI (2002)
Micronutrients
Overnight fasting blood samples (12 h) were collected and analyzed for plasma vitamins, (25-hydroxyvitamin vitamin D (ng/mL), vitamins B-6, B-12 and folate). Plasma 25-hydroxyvitamin D (ng/mL) was measured with a 125I radioimmunoassay kit procedure (DiaSorin Inc) following manufacturer’s specifications (68100E). The intra- and inter-assay CVs were 10.8% and 9.4%, respectively.
Plasma pyridoxal-5’-phosphate (PLP) was determined enzymatically using tyrosine decarboxylase, based on the principles described by Shin-Buehring et al. 63 In this method, PLP activity in the plasma sample is determined on the basis of release of tritiated tyramine following the incubation of tyrosine decarboxylase apoenzyme with the supernatant fraction of TCA-precipitated serum samples and tritium-labeled tyrosine. The coefficient of variation for this assay in our laboratory is 16%.
Baseline plasma folate and vitamin B12 concentrations were determined by a radioassay method using a commercially available kit from Biorad. Coefficients of variation for these assays in our laboratory are 7% for vitamin B12 and 10% for folate. In March 2007, the Bio-Rad was discontinued. We replaced it with the Immunoassay kits from Siemens Medical Solutions Diagnostics for use on the Immulite 1000, and the matrix for the analysis of these measures changed from plasma to serum. Folate was analyzed using a competitive, liquid-phase ligand labeled protein chemiluminescent assay (LKFO1) and vitamin B12 using a solid-phase, competitive chemiluminescent assay (LKVB1).
Dietary intake of magnesium was assessed with a semi-quantitative food-frequency questionnaire (FFQ), which has been adapted and validated for this population. 64 Dietary data were linked to the Minnesota Nutrient Data System 1999, version 25 (University of Minnesota Nutrition Coordinating Center, Minneapolis, MN, USA) for nutrient analysis. Dietary intake of magnesium was adjusted for energy intake using the residual method. 65
Analytical Strategy
Two level hierarchical linear regression models (HLM) for continuous outcomes were estimated using STATA statistical software version 13.0. The level-1 data included three measures of participant’s depressive symptomatology collected at baseline, 2y follow-up and 5y follow-up. Participant’s constituted the Level-2 data. Depressive symptomatology measures were, therefore, nested within participants. HLM models account for the clustering nature of the data, avoid underestimation of standard errors, and reduce type I error. 66 Two level hierarchical linear regression models were run to examine differences between-participants in the effect of suboptimal plasma PLP, a time-varying covariate, at baseline, and the rate of change in depressive symptoms, i.e., intercepts and slopes, over the course of five-seven years after adjusting for age, sex, education, and BMI in model 1. In model 2, we additionally adjusted for smoking and alcohol use.
We subsequently added baseline experiences of perceived stress, stressful life events, and allostatic load, to control for the effect of subjective and social stressors, and the physiological response to stress on depressive symptoms. In model 4, we adjusted for the time-varying effect of plasma vitamins B-12 and folate, and baseline effect of plasma 25(OH) vitamin D and nutrient intake of magnesium. Vitamins D, B-12 and folate, and dietary intake of magnesium were log-transformed to normalize data distributions.
In final model 5, we controlled for the time-varying effect of antidepressant medication use. We used the log likelihood ratio (LL), the Akaike Information Criterion (AIC) and the Bayesian Information Criterion (BIC) statistics to assess model fit. 66 The smaller the LL, AIC, and BIC, the better fit the model provides. The AIC and BIC consider error and parsimony concurrently. HLM allows the modeling of time invariant and time-varying predictors of change in participant’s depressed symptomatology over repeated measurement points. 67 HLM models use maximum-likelihood estimation to take into account unbalanced designs, i.e., participants missing data at different time points. 66
Results
Our final sample included 1,157 participants. Data included 843 (73%) women, and 314 (27%) men from baseline; 710 (74%) women, and 245 (26%) men from 2 y follow-up; and 227 (79%) women, and 60 (21%) men from 5–7 y follow-up. The average age of participants was 57y (SD±7.5) at baseline, 59.2 (SD±7.5) at 2 y follow-up, and 64.5y (SD±7.0) at 5–7 y follow-up. At initial assessment, 21% of participants had <5th grade education, 25% had completed middle school, 38% high school, and 15% had at least some college education. At baseline, participants with suboptimal plasma PLP concentration had significantly higher BMI and allostatic load (Table 2); significantly lower plasma vitamins D, B-12, and folate, and significantly lower dietary intake of magnesium than participants with adequate plasma PLP concentration (Table 3). A greater proportion of participants with suboptimal plasma PLP were smokers or used antidepressant medication (Table 2).
Table 2.
Characteristics of the Puerto Rican Health Study participants at baseline, n=1,446 1
| Participants by Baseline plasma PLP status | ||||||
|---|---|---|---|---|---|---|
| ≥30 nmol/L | <30 nmol/L | |||||
| n=1,011 | n=435 | |||||
| Mean | (±SD) | Mean | (±SD) | t-test | p-value | |
| Age in years | 57 | (±7.6) | 57 | (±7.4) | 0.43 | 0.67 |
| Body Mass Index (BMI) | 31 | (±6.3) | 33 | (±7.4) | −3.3 | <0.001 |
| Plasma vitamin B-12 | 561 | (±282) | 502 | (±278) | 3.7 | <0.001 |
| Plasma folate | 20 | (±8.9) | 16 | (±6.8) | 8.5 | <0.0001 |
| Plasma 25-hydroxyvitamin D (ng/mL) | 18 | (±8.1) | 16 | (±6.6) | 6.2 | <0.0001 |
| Energy adjusted magnesium diet intake, (mg) | 328 | (±72.8) | 314 | (±61.5) | 3.4 | <0.001 |
| Stressful life events | 8 | (±8.0) | 8 | (±7.9) | 0.8 | 0.41 |
| Perceived stress | 24 | (±9.2) | 24 | (±9.9) | −0.8 | 0.41 |
| Allostatic Load | 4 | (±1.9) | 5 | (±1.9) | −4.7 | <0.0001 |
| n | % | n | % | χ 2 | p-value | |
| Sex | 0.0003 | 0.99 | ||||
| Male-ref | 297 | 29% | 128 | 29% | ||
| Female | 714 | 71% | 307 | 71% | ||
| Education | 9.0 | 0.03 | ||||
| No schooling or <5th grade(ref) | 211 | 21% | 97 | 22% | ||
| 5th-8th grade | 250 | 25% | 113 | 26% | ||
| 9-th-12th grade | 376 | 37% | 177 | 41% | ||
| College/graduate | 174 | 17% | 48 | 11% | ||
| Smoking | 11 | 0.001 | ||||
| Never -Ref | 483 | 48% | 168 | 39% | ||
| Yes | 526 | 52% | 267 | 61% | ||
| Alcohol use | 6.6 | 0.04 | ||||
| Not currently drinking (ref) | 535 | 53% | 258 | 60% | ||
| Moderate (<=1 drink/d-Women; <=2 drink/d-Men) | 379 | 38% | 146 | 34% | ||
| Heavy (>1 drink/d-Women; >2 drink/d-Men) | 89 | 9% | 26 | 6% | ||
| Use of antidepressant medication | 5.0 | 0.03 | ||||
| No –Ref | 670 | 67% | 262 | 60% | ||
| Yes | 337 | 33% | 172 | 40% | ||
Not all categories add to n=1446 due to missing data in some variables (<1%)
Table 3.
Micronutrients and CES-D by PLP status
| Baseline* | 2y Follow-Up* | 5y Follow-Up* | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n=1,446 | n=1,223 | n=790 | ||||||||||||||||
| ≥30 nmol/L | <30 nmol/L | ≥30 nmol/L | <30 nmol/L | ≥30 nmol/L | <30 nmol/L | |||||||||||||
| η=1,011 | η=435 | η=844 | η=379 | η=617 | η=173 | |||||||||||||
| Mean | (±SD) | Mean | (±SD) | t-test | p-value | Mean | (±SD) | Mean | (±SD) | t-test | p-value | Mean | (±SD) | Mean | (±SD) | t-test | p-value | |
| CES-D (outcome) | 20 | (±13) | 22 | (±14) | −2.7 | 0.008 | 17 | (±12) | 20 | (±13) | −2.9 | 0.004 | 16 | (±11) | 19 | (±11) | −2.9 | 0.004 |
| Vitamin B-12 | 561 | (±282) | 502 | (±278) | 3.7 | 0.0003 | 589 | (±305) | 517 | (±288) | 3.8 | 0.0001 | 568 | (±272) | 463 | (±236) | 3.2 | 0.002 |
| Folate | 20 | (±9) | 16 | (±7) | 8.5 | <0.0001 | 20 | (±9) | 15 | (±6) | 10.3 | <0.0001 | 22 | (±10) | 15 | (±7) | 5.9 | <0.0001 |
| Vitamin D | 18 | (±8) | 16 | (±7) | 6.2 | <0.0001 | − | - | ||||||||||
| Magnesium intake (mg) | 328 | (±73) | 314 | (±61) | 3.4 | 0.001 | - | - | ||||||||||
Unadjusted values
Overall mean depressive symptomatology scores in the sample were 20.3 (SD=13.3) at baseline, 18.1 (SD=12.5) at 2 y follow-up, and 16.5 (SD=11.1) at 5–7 y follow-up. A cutoff score of 16 or greater is generally used to identify individuals at risk of clinical depression. 57%, 51% and 45% of participants had CES-D scores above this cutoff at baseline, 2 y and 5–7 y, respectively, suggesting high risk for clinical depression in this population. Almost a third of participants at baseline (30.1%) and 2 y follow-up (30.1%), and more than one fifth at 5–7 y follow-up (21.9%) had suboptimal plasma PLP concentration (PLP<30 nmol/L). Depressive symptomatology, plasma vitamin B-12 and plasma folate were significantly and consistently lower in participants with suboptimal, vs. adequate plasma PLP concentration, at baseline, 2 y and 5–7 y follow-up (Table 3).
In HLM models (Table 4), initial CES-D score was not associated with suboptimal PLP plasma concentration before or after adjusting for sex, age, education and BMI (Model 1); or for smoking and alcohol use (Model 2). After adjustment for stressful life events, perceived stress, and allostatic load in Model 3, participants with suboptimal PLP plasma concentration, vs. adequate PLP, had significantly higher depressive symptomatology (β=1.30; SE=0.50; P=0.01) at baseline, and this persisted over time (lines remain parallel, Figure 2). The effect of time-varying suboptimal PLP on depressive symptomatology remained significant (β=1.29; SE=0.56; P=0.02) after controlling for time-varying plasma vitamins B-12 and folate, and baseline dietary intake of magnesium and plasma vitamin D (Model 4); as well as additionally controlling for time-varying antidepressant medication use (β=1.14; SE=0.55; P=0.04) in Model 5 (Table 4).
Table 4.
Longitudinal Associations between Depressive symptomatology (CES-D) and time-varying suboptimal PLP status in the Puerto Rican Health study participants
| Model 1 a | Model 2 b | Model 3 c | Model 4 d | Model 5 e | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| β | (SE) | p-value | β | (SE) | p-value | β | (SE) | p-value | β | (SE) | p-value | β | (SE) | p-value | |
| Suboptimal Vitamin B-6 (plasma PLP<30 nmol/L) | |||||||||||||||
| No -ref | - | - | - | - | |||||||||||
| Yes | 0.72 | 0.54 | 0.18 | 0.70 | 0.54 | 0.20 | 1.30 | 0.50 | 0.01 | 1.29 | 0.56 | 0.02 | 1.14 | 0.55 | 0.04 |
| Time | −0.68 | 0.07 | <0.0001 | −0.67 | 0.07 | <0.0001 | −0.66 | 0.08 | <0.0001 | −0.79 | 0.10 | <0.0001 | −0.81 | 0.10 | <0.0001 |
| Suboptimal Vitamin B-6 * Time | |||||||||||||||
| No -ref | - | - | - | - | |||||||||||
| Yes | 0.08 | 0.16 | 0.61 | 0.03 | 0.16 | 0.83 | −0.12 | 0.16 | 0.48 | 0.07 | 0.22 | 0.73 | 0.11 | 0.22 | 0.63 |
| Goodness of fit | |||||||||||||||
| Df | 12 | 14 | 17 | 21 | 22 | ||||||||||
| N (CES-D score: Level-1 data) | 3427 | 3393 | 3099 | 2407 | 2399 | ||||||||||
| N (participants: Level-2 data) | 1446 | 1435 | 1304 | 1169 | 1169 | ||||||||||
| Log Likelihood (LL) | −13036 | −12896 | −11381 | −8821 | −8766 | ||||||||||
| Akaike Information Criterion (AIC) | 26097 | 25819 | 22796 | 17677 | 17575 | ||||||||||
| Bayesian Information Criterion (BIC) | 26170 | 25905 | 22899 | 17775 | 17703 | ||||||||||
Model 1: adjusted for age + sex + education + BMI
Model 2: Model 1 + smoking, and alcohol use
Model 3: Model 2 + stressful life events, perceived stress, and allostatic load
Model 4: Model 3 + time-varying plasma vitamins B-12, Folate, baseline plasma vitamin D + baseline energy adjusted magnesium diet intake
Model 5: Model 4 + time-varying antidepressant drug use
Figure 2.
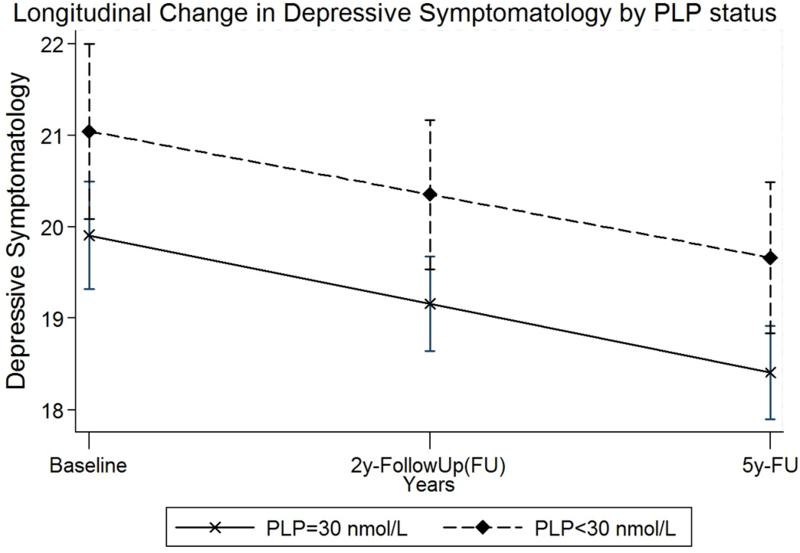
Longitudinal change in depressive symptomatology by PLP status after adjusting for age, sex, education, BMI, smoking and alcohol use; perceived stress, stressful life events, and allostatic load; micronutrients including plasma vitamin B-12, folate, D, energy adjusted dietary intake of magnesium and antidepressant medication use.
On average, CES-D score declined significantly by 0.81 points per year after controlling for all covariates (SE=0.10; P<0.0001) (Model 5, Table 4). The slope of the decline in depressive symptomatology over time was slightly steeper in participants with PLP ≥30 nmol/L, vs. PLP <30, plasma concentration (Figure 2); however, this difference was not statistically significant in any of the HLM models (Table 4). LL, AIC, and BIC statistics decreased considerably after addition of covariates in models 1 through 5 (Table 4) indicating better model fit.
Discussion
In this cohort of older adult Puerto Ricans residing in the northeastern U.S. mainland, there was a significant association between suboptimal plasma PLP status, measured as <30mg/dL PLP, and depressive symptomatology at baseline. This association remained significant over time, and after adjusting for a number of individual, health behavior, medical conditions, subjective and objective stress variables, and related nutritional factors.
Previous longitudinal research on vitamin B-6 and depression is limited, but our results are in line with two identified longitudinal studies. In one, higher intake (food and supplementation) of vitamin B-6 in adults aged 65 y and older was associated with lower incidence of depression for up to 12 y of follow-up, after adjusting for socio-demographic variables, antidepressant medication use, and a number of health behaviors and health conditions. 68 In the second, incidence of major depression was significantly associated with lower vitamin B-6 intake from food in women from the NuAge cohort study (68–82 y) after a three year period. 69 The overall decline in depressive symptomatology in our study is consistent with previous studies investigating depressive symptoms across the adult life span that show U-shaped patterns, with symptoms being high during young adulthood, decreasing by the end of middle adulthood, and increasing again over the age of 70. 70 The causes of the U-shape in depressive symptomatology are not clear, but it has been suggested that the increase in later decades may be related to social capital loses and declines in health. 71
Significant associations between depression and insufficient dietary intake of B-6 are well documented. 26,68,72 Good functioning of the nervous and immune systems appear to be strongly influenced by adequate vitamin B6 levels. 73
Vitamin B-6 and the nervous system.
The coenzyme pyridoxal phosphate, the active form of B-6, is an essential cofactor for the amino acid decarboxylases involved in the synthesis of the neurotransmitters implicated in depression i.e. dopamine, norepinephrine, serotonin and ϒ-aminobutyric acid (GABA). 22,23 In animal models, moderate PLP deficiency is characterized by decreases in GABA and serotonin without a change in dopamine, norepinephrine, and epinephrine in various brain areas. 74 Decreases in serotonin are, in turn, associated with the regulation of the hypothalamus-pituitary-end organ systems, as well as regulation of sleep, blood presure, secretion of melatonin, and reward behaviors. 74 Studies in humans have reported reduced expression of brain-derived neurotrophic factor (BDNF) in patients with major depression, with restored BDNF reported after antidepressant treatment. 75 There is evidence in humans of the neuroprotective properties of vitamin B-6 23,76 and its significance in the treatment of metabolic syndrome and hypertension, two frequent comorbid conditions of depression.23
Vitamin B-6 and the immune system.
Inflammation has been implicated in the etiology of MDD. 77 Individuals with vs. without depression have been shown to have higher C-reactive protein (CRP) and other inflammatory markers. 78 Inflammation promotes the production of pro-inflammatory cytokines i.e., IL-6, TNF-α, and CRP. 79 Increases in cytokines affect the proper function of the hypothalamic-pituitary-adrenal (HPA) axis by increasing system peripheral glucocorticoid resistance 80 which, in turn, promotes the conversion of tryptophan to kynurenine rather than to serotonin, resulting in lower utilization of serotonin, a mechanism implicated in depression. 81 Inflammation and associated increases in proinflammatory cytokines are associated with reduced expression of nerve growth factors in the hippocampal and prefrontal cortex seen in patients with major depression. 82
Although epidemiological data have reported bidirectional results on the inflammation-depression association in humans, a recent meta-analysis concluded that inflammatory cytokines were likely to contribute to depressive symptoms, rather than the other way around. 83 The role of vitamin B-6 in the metabolism of tryptophan and in one-carbon metabolism makes vitamin B-6 a relevant cofactor in the body’s immune response. 22,25 The direct effect of vitamin B-6 on immune response is evidenced by the associations between low pyridoxal-5′-phosphate (PLP) and higher functional indices and biomarkers of inflammation; 84 and by the cellular decrease of PLP with more severe inflammation. 85 Although, the exact mechanism implicating PLP involvement during inflammation remains to be specified, there is indication that PLP precedes the formation of inflammation markers in its role as an essential cofactor for serine hydroxymethyltransferase-stimulated inflammation. 84 The indirect role of PLP in the degradation of tryptophan, through the kynurenine pathway, which is dependent on many PLP enzymes and responsible for more than 95% of tryptophan degradation, is another potential mechanism linking PLP and inflammation. 84 Indoleamine 2,3-dioxygenase (IDO), one of the enzymes implicated in the conversion of tryptophan to kynurenine, is upregulated by inflammatory stimuli. It is suggested that the stimulation of IDO in response to inflammatory stimuli will mobilize plasma PLP for use in the degradation of kynurenine. 84 It is therefore also possible that the low PLP concentrations seen during inflammation may reflect the uptake of vitamin B-6 coenzymes from the circulation to meet higher demands in other tissues. 86
Major strengths of this study include the use of biomarkers to assess B-6 status, and the longitudinal assessment of the link between B-6 and high depressive symptomatology. Prevention efforts intended to identify and treat nutritional deficiencies in individuals experiencing high depressive symptomatology may be cost effective in reducing the overall incidence and persistent prevalence of major depression by targeting the mental health condition before it becomes chronic. The significant increase in MDD observed in adults aged 50 y and older between 2005 and 2010 in the United States 1 demonstrates the need for earlier preventive efforts. Further research with diverse racial and ethnic groups in this area is warranted, as evidence suggests that depression is more common in adult ethnic minorities than in non-Hispanic whites. 1 Minority older adults are more likely to live in poverty, face food insecurity, and experience more chronic health conditions. 87 Nutritional prevention efforts may aid in reducing complex interactions between mental health symptoms, chronic diseases, medication use, and nutrient intake. Translational implications of the role of PLP in inhibiting proinflammatory cytokines or their signaling pathways offer the unique opportunity to test and monitor the therapeutic efficacy of PLP to reduced depressive symptomatology and/or to increase treatment response to conventional antidepressant medication in selected populations.88
In addition to the longitudinal design, this study adds to the current literature by examining the association between plasma vitamin B-6 and depression after adjusting for a number of important confounders that have been overlooked in previous research, such as objective and subjective stress, and the physiological response to environmental challenges, i.e., allostatic load. A link between stress and nutrition has been established. 89 Under conditions of persistent stress or conditions perceived as such, the adrenal glands increase the release of cortisol. Elevated stress induced cortisol may disrupt the antagonistic effects of insulin and glucocorticoids creating an unbalance that may lead to desensitization of satiety signals and increased appetite. 90 Research shows that stress may affect food preferences toward non-nutritious foods high in fats or sugars or both. 89 Previous work with this Puerto Rican cohort found that greater perceived stress was associated with lower fruit, vegetable, and protein intake, and greater consumption of salty snacks. 40 The authors found a positive and significant association between cortisol and stress in those without diabetes, and with higher insulin and BMI, independent of diabetes; providing support for a link between stress, cortisol, and dietary patterns. 40 In turn, stress itself may contribute to depressive symptomatology. 34,91,92
The findings of the present study must be interpreted in light of some limitations. The sample included adult Puerto Ricans residing in a Northeast city of the U.S., and their experiences may not reflect those of Puerto Ricans residing in other regions, or to other Latino groups in the U.S. Unlike other Latino subgroups, the migration of Puerto Ricans is officially classified as internal migration as they enjoy the social and political benefits of U.S. citizenship. Future studies including nationally representative samples of individuals and geographic regions are needed to replicate our findings. Second, despite the longitudinal design, the observational nature of this study limits causal inference due to a threat to internal validity by unmeasured potential confounders.
Conclusion
We found significantly higher depressive symptoms in participants with suboptimal plasma PLP concentration compared to participants with adequate PLP concentration. These results were consistent over time, and were robust to adjustment for a large number of potential confounders.
Additional prospective cohort studies designed to study the association between nutrient deficiency and risk of depression are needed. Studies that examine whether the systematic use of vitamin B-6 supplementation decreases depressive symptomatology in older adults are warranted. The complex etiology of depression in adults –interplay between biological and fix factors, i.e., sex and other genetic traits, and modifiable factors, i.e., cerebrovascular disease and other medical conditions, stress, and diet behavior –poses a significant challenge in prevention and treatment efforts. Dietary intake, a modifiable behavior, offers a valuable opportunity to improving present efforts. Our results suggest that interventions to reduce depressive symptoms among older adults may focus on improving nutritional status, as well as addressing the effects of life stressors, both objective and perceived, given the relevance of stress on nutritional behavior and on behavioral change.
Acknowledgements:
We would like to express our gratitude to the study participants who have been part of the study over the years.
Sources of Support: This study was funded by the National Institutes of Health (Grants P01 AG023394 and P50 HL105185, Katherine L. Tucker, PhD, Principal Investigator)
Footnotes
Ethics approval The data presented here was collected after obtaining informed consent as approved by the approved by the Institutional Review Boards at Tufts Medical Center, Northeastern University and the University of Massachusetts Lowell.
Conflict of interest: All authors declare no conflicts of interest to disclose.
References
- 1.Greenberg PE, Fournier AA, Sisitsky T, Pike CT, Kessler RC. The economic burden of adults with major depressive disorder in the United States (2005 and 2010). The Journal of clinical psychiatry. 2015;76(2):155–162. [DOI] [PubMed] [Google Scholar]
- 2.The World Health Organization W. Fact sheet: Depression. 2016.
- 3.Dobalian A, Rivers PA. Racial and ethnic disparities in the use of mental health services. The journal of behavioral health services & research. 2008;35(2):128–141. [DOI] [PubMed] [Google Scholar]
- 4.Thase ME, Entsuah AR, Rudolph RL. Remission rates during treatment with venlafaxine or selective serotonin reuptake inhibitors. The British journal of psychiatry : the journal of mental science. 2001;178:234–241. [DOI] [PubMed] [Google Scholar]
- 5.Eby GA 3rd, Eby KL Magnesium for treatment-resistant depression: a review and hypothesis. Medical hypotheses. 2010;74(4):649–660. [DOI] [PubMed] [Google Scholar]
- 6.Réus GZ, Jansen K, Titus S, Carvalho AF, Gabbay V, Quevedo J. Kynurenine pathway dysfunction in the pathophysiology and treatment of depression: Evidences from animal and human studies. Journal of Psychiatric Research. 2015;68:316–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Popa TA, Ladea M. Nutrition and depression at the forefront of progress. Journal of medicine and life. 2012;5(4):414–419. [PMC free article] [PubMed] [Google Scholar]
- 8.Rao TS, Asha MR, Ramesh BN, Rao KS. Understanding nutrition, depression and mental illnesses. Indian journal of psychiatry. 2008;50(2):77–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lang UE, Beglinger C, Schweinfurth N, Walter M, Borgwardt S. Nutritional Aspects of Depression. Cellular Physiology and Biochemistry. 2015;37(3):1029–1043. [DOI] [PubMed] [Google Scholar]
- 10.Opie RS, Itsiopoulos C, Parletta N, et al. Dietary recommendations for the prevention of depression. Nutr Neurosci. 2017;20(3):161–171. [DOI] [PubMed] [Google Scholar]
- 11.Opie RS, O’Neil A, Itsiopoulos C, Jacka FN. The impact of whole-of-diet interventions on depression and anxiety: a systematic review of randomised controlled trials. Public health nutrition. 2015;18(11):2074–2093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Davison KM, Kaplan BJ. Nutrient Intakes Are Correlated With Overall Psychiatric Functioning in Adults With Mood Disorders. Can J Psychiatry. 2012;57(2):85–92. [DOI] [PubMed] [Google Scholar]
- 13.Gougeon L Nutritional predictors of depression in a cohort of community-dwelling elderly Canadians: NuAge cohort. Applied physiology, nutrition, and metabolism = Physiologie appliquee, nutrition et metabolisme. 2014;39(12):1412. [Google Scholar]
- 14.Polivka D, von Arnim CAF. [Vitamins and nutritional supplements in older persons]. Vitamine und Nahrungsergänzung bei älteren Menschen. Der Internist. 2015;56(11):1318–1324. [DOI] [PubMed] [Google Scholar]
- 15.Brito Noronha M, Almeida Cunha N, Agra Araujo D, Flaminio Abrunhosa S, Nunes Rocha A, Freitas Amaral T. Undernutrition, Serum Vitamin B12, Folic Acid And Depressive Symptoms In Older Adults. Nutricion hospitalaria. 2015;32(1):354–361. [DOI] [PubMed] [Google Scholar]
- 16.Bourre JM. Effects of nutrients (in food) on the structure and function of the nervous system: update on dietary requirements for brain. Part 1: micronutrients. The journal of nutrition, health & aging. 2006;10(5):377–385. [PubMed] [Google Scholar]
- 17.Rucklidge JJ, Kaplan BJ. Broad-spectrum micronutrient formulas for the treatment of psychiatric symptoms: a systematic review. Expert review of neurotherapeutics. 2013;13(1):49–73. [DOI] [PubMed] [Google Scholar]
- 18.Lai JS, Hiles S, Bisquera A, Hure AJ, McEvoy M, Attia J. A systematic review and meta-analysis of dietary patterns and depression in community-dwelling adults. The American journal of clinical nutrition. 2014;99(1):181–197. [DOI] [PubMed] [Google Scholar]
- 19.Spinneker A, Sola R, Lemmen V, Castillo MJ, Pietrzik K, Gonzalez-Gross M. Vitamin B6 status, deficiency and its consequences--an overview. Nutricion hospitalaria. 2007;22(1):7–24. [PubMed] [Google Scholar]
- 20.Williams AL, Cotter A, Sabina A, Girard C, Goodman J, Katz DL. The role for vitamin B-6 as treatment for depression: a systematic review. Family practice. 2005;22(5):532–537. [DOI] [PubMed] [Google Scholar]
- 21.Wu XY, Lu L. Vitamin B6 deficiency, genome instability and cancer. Asian Pacific journal of cancer prevention : APJCP. 2012;13(11):5333–5338. [DOI] [PubMed] [Google Scholar]
- 22.Kennedy DO. B Vitamins and the Brain: Mechanisms, Dose and Efficacy--A Review. Nutrients. 2016;8(2):68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dakshinamurti S, Dakshinamurti K. Antihypertensive and neuroprotective actions of pyridoxine and its derivatives. Canadian journal of physiology and pharmacology. 2015;93(12):1083–1090. [DOI] [PubMed] [Google Scholar]
- 24.Berk M, Williams LJ, Jacka FN, et al. So depression is an inflammatory disease, but where does the inflammation come from? BMC medicine. 2013;11:200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lotto V, Choi SW, Friso S. Vitamin B6: a challenging link between nutrition and inflammation in CVD. The British journal of nutrition. 2011;106(2):183–195. [DOI] [PubMed] [Google Scholar]
- 26.Murakami K, Sasaki S. Dietary intake and depressive symptoms: a systematic review of observational studies. Molecular nutrition & food research. 2010;54(4):471–488. [DOI] [PubMed] [Google Scholar]
- 27.Falcon LM, Tucker KL. Prevalence and correlates of depressive symptoms among Hispanic elders in Massachusetts. The journals of gerontology Series B, Psychological sciences and social sciences. 2000;55(2):S108–116. [DOI] [PubMed] [Google Scholar]
- 28.Alegria M, Canino G, Shrout PE, et al. Prevalence of mental illness in immigrant and non-immigrant U.S. Latino groups. The American journal of psychiatry. 2008;165(3):359–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kessler RC. The Effects of Stressful Life Events on Depression. Annu Rev Psyc 1997;48:191–214. [DOI] [PubMed] [Google Scholar]
- 30.Kendler KS, Karkowski LM, Prescott CA. Causal Relationship Between Stressful Life Events and the Onset of Major Depression. American Journal of Psychiatry 1999;156:837–841. [DOI] [PubMed] [Google Scholar]
- 31.Mazure CM. Life stressors as risk factors in depression. Clinical Psychology: Science and Practice. 1998;5(3):291–313. [Google Scholar]
- 32.Avison WR, Turner RJ. Stressful Life Events and Depressive Symptoms: Disaggregating the Effects of Acute Stressors and Chronic Strains. Journal of Health and Social Behavior. 1988;29(3):253–264. [PubMed] [Google Scholar]
- 33.Turner JJ. Social Support and Coping In: Horwitz AV, Scheid TL, eds. A Handbook for the Study of Mental Health: Social Context, Theories, and Systems. New York, NY: Cambridge University Press; 1999:198–210. [Google Scholar]
- 34.Pearlin LI, Schieman S, Fazio EM, Meersman SC. Stress, health, and the life course: Some conceptual perspectives. Journal of Health and Social Behavior. 2005;46(2):205–219. [DOI] [PubMed] [Google Scholar]
- 35.Aneshensel CS, Phelan JC. The Sociology of Mental Health: Surveying the Field In: Phelan CSAaJc, ed. Handbook of the Sociology of Mental Health. New York: Springer; 1999:3–18. [Google Scholar]
- 36.Rutters F, Nieuwenhuizen AG, Lemmens SG, Born JM, Westerterp-Plantenga MS. Acute stress-related changes in eating in the absence of hunger. Obesity (Silver Spring, Md). 2009;17(1):72–77. [DOI] [PubMed] [Google Scholar]
- 37.Papier K, Ahmed F, Lee P, Wiseman J. Stress and dietary behaviour among first-year university students in Australia: sex differences. Nutrition (Burbank, Los Angeles County, Calif). 2015;31(2):324–330. [DOI] [PubMed] [Google Scholar]
- 38.Oliver G, Wardle J, Gibson EL. Stress and food choice: A laboratory study. Psychosomatic Medicine. 2000;62(6):853–865. [DOI] [PubMed] [Google Scholar]
- 39.Tryon MS, DeCant R, Laugero KD. Having your cake and eating it too: a habit of comfort food may link chronic social stress exposure and acute stress-induced cortisol hyporesponsiveness. Physiology & behavior. 2013;114–115:32–37. [DOI] [PubMed] [Google Scholar]
- 40.Laugero KD, Falcon LM, Tucker KL. Relationship between perceived stress and dietary and activity patterns in older adults participating in the Boston Puerto Rican Health Study. Appetite. 2011;56(1):194–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Oliver G, Wardle J, Gibson EL. Stress and food choice: a laboratory study. Psychosom Med. 2000;62(6):853–865. [DOI] [PubMed] [Google Scholar]
- 42.Zellner DA, Loaiza S, Gonzalez Z, et al. Food selection changes under stress. Physiology & behavior. 2006;87(4):789–793. [DOI] [PubMed] [Google Scholar]
- 43.McEwen BS. Brain on stress: How the social environment gets under the skin. Proceedings of the National Academy of Sciences of the United States of America. 2012;109(Suppl 2):17180–17185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Danese A, McEwen BS. Adverse childhood experiences, allostasis, allostatic load, and age-related disease. Physiology & behavior. 2012;106(1):29–39. [DOI] [PubMed] [Google Scholar]
- 45.Seeman T, Epel E, Gruenewald T, Karlamangla A, McEwen BS. Socio-economic differentials in peripheral biology: cumulative allostatic load. Annals of the New York Academy of Sciences. 2010;1186:223–239. [DOI] [PubMed] [Google Scholar]
- 46.E P, B S, E K, P W, A P, G. N Antidepressant- and anxiolytic-like activity of magnesium in mice. Pharmacol Biochem Behav. 2004;78:7–12. [DOI] [PubMed] [Google Scholar]
- 47.Eby GA, Eby KL. Rapid recovery from major depression using magnesium treatment. Medical hypotheses. 2006;67(2):362–370. [DOI] [PubMed] [Google Scholar]
- 48.Eby GA, Eby KL, Murck H. Magnesium and Major Depression In: Nechifor RVaM, ed. Magnesium in the Central Nervous System. Adelaide, South Australia: University of Adelaide Press; 2011. [PubMed] [Google Scholar]
- 49.HH R, PB M, IW J Depression and magnesium deficiency. Int J Psychiatry Med. 1989;19:57–63. [DOI] [PubMed] [Google Scholar]
- 50.Parker GB, Brotchie H, Graham RK. Vitamin D and depression. Journal of affective disorders. 2017;208:56–61. [DOI] [PubMed] [Google Scholar]
- 51.Okereke OI, Singh A. The role of vitamin D in the prevention of late-life depression. Journal of affective disorders. 2016;198:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Almeida OP, Ford AH, Flicker L. Systematic review and meta-analysis of randomized placebo-controlled trials of folate and vitamin B12 for depression. International Psychogeriatrics. 2015;27(5):727–737. [DOI] [PubMed] [Google Scholar]
- 53.Tucker KL, Mattei J, Noel SE, et al. The Boston Puerto Rican Health Study, a longitudinal cohort study on health disparities in Puerto Rican adults: challenges and opportunities. BMC public health. 2010;10:107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Radloff LS. The CES-D Scale: A Self-Report Depression Scale for Research in the General Population. Applied Psychological Measurement. 1977;1(3):385–401. [Google Scholar]
- 55.Mahard RE. The CES-D as a measure of depressive mood in the elderly Puerto Rican population. Journal of Gerontology. 1988;43(1):24–25. [DOI] [PubMed] [Google Scholar]
- 56.Shin YS, Rasshofer R, Friedrich B, Endres W. Pyridoxal-5’-phosphate determination by a sensitive micromethod in human blood, urine and tissues; its relation to cystathioninuria in neuroblastoma and biliary atresia. Clin Chim Acta. Clin Chim Acta 1983;127:77–85. [DOI] [PubMed] [Google Scholar]
- 57.Charney DS, Manji HK. Life stress, genes, and depression: multiple pathways lead to increased risk and new opportunities for intervention. Science’s STKE : signal transduction knowledge environment. 2004;2004(225):re5. [DOI] [PubMed] [Google Scholar]
- 58.Marin MF, Lord C, Andrews J, et al. Chronic stress, cognitive functioning and mental health. Neurobiol Learn Mem. 2011;96(4):583–595. [DOI] [PubMed] [Google Scholar]
- 59.Norbeck JS. The Norbeck Social Support Questionnaire. Birth defects original article series. 1984;20(5):45–57. [PubMed] [Google Scholar]
- 60.Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. Journal of Health and Social Behavior. 1983;24:385–396. [PubMed] [Google Scholar]
- 61.Monroe SM, Reid MW. Gene-Environment Interactions in Depression Research : Genetic Polymorphisms and Life-Stress Polyprocedures. Psychological Science. 2008;19(10):947–956. [DOI] [PubMed] [Google Scholar]
- 62.Mattei J, Demissie S, Falcon LM, Ordovas JM, Tucker KL. Allostatic load is associated with chronic conditions in the Boston Puerto Rican Health Study. Socia Science and Medicine. 2010;70(12):1988–1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Shin-Buehring Y, Rasshofer R, Endres W. A new enzymatic method for pyridoxal-5’-phosphate determination. Journal of Inherited Metabolic Disorders. 1981;4:123–124. [Google Scholar]
- 64.Tucker KL, Bianchi LA, Maras J, Bermudez OI. Adaptation of a food frequency questionnaire to assess diets of Puerto Rican and non-Hispanic adults. American journal of epidemiology. 1998;148(5):507–518. [DOI] [PubMed] [Google Scholar]
- 65.Willett WC, Howe GR, Kushi LH. Adjustment for total energy intake in epidemiologic studies. The American journal of clinical nutrition. 1997;65(4 Suppl):1220S–1228S; discussion 1229S-1231S. [DOI] [PubMed] [Google Scholar]
- 66.Raudenbush S, Bryk AS. Hierarchical linear models: Applications and data analysis methods. Vol 2nd ed Thousand Oaks, CA: Sage Publications; 2002. [Google Scholar]
- 67.Singer JD, Willett JB. Applied Longitudinal Data Analysis: Modeling Change and Event Occurrence. New York: Oxford University Press; 2003. [Google Scholar]
- 68.Skarupski KA, Tangney C, Li H, Ouyang B, Evans DA, Morris MC. Longitudinal association of vitamin B-6, folate, and vitamin B-12 with depressive symptoms among older adults over time. The American journal of clinical nutrition. 2010;92(2):330–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Gougeon L, Payette H, Morais JA, Gaudreau P, Shatenstein B, Gray-Donald K. Intakes of folate, vitamin B6 and B12 and risk of depression in community-dwelling older adults: the Quebec Longitudinal Study on Nutrition and Aging. European journal of clinical nutrition. 2016;70(3):380–385. [DOI] [PubMed] [Google Scholar]
- 70.Tomitaka S, Kawasaki Y, Furukawa T. Right Tail of the Distribution of Depressive Symptoms Is Stable and Follows an Exponential Curve during Middle Adulthood. PloS one. 2015;10(1):e0114624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sutin AR, Terracciano A, Milaneschi Y, An Y, Ferrucci L, Zonderman AB. The trajectory of depressive symptoms across the adult life span. JAMA Psychiatry. 2013;70(8):803–811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Murakami K, Miyake Y, Sasaki S, Tanaka K, Arakawa M. Dietary folate, riboflavin, vitamin B-6, and vitamin B-12 and depressive symptoms in early adolescence: the Ryukyus Child Health Study. Psychosom Med. 2010;72(8):763–768. [DOI] [PubMed] [Google Scholar]
- 73.Logan AC, Jacka FN. Nutritional psychiatry research: an emerging discipline and its intersection with global urbanization, environmental challenges and the evolutionary mismatch. Journal of physiological anthropology. 2014;33:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Dakshinamurti K, Lal KJ, Sharma SK. Pyridoxine (vitamin B6), neurotransmitters and hypertension In: Marino G, Sannia G, Bossa F, eds. Biochemistry of Vitamin B6 and PQQ. Basel: Birkhäuser Basel; 1994:331–335. [Google Scholar]
- 75.Castren E, Rantamaki T. The role of BDNF and its receptors in depression and antidepressant drug action: Reactivation of developmental plasticity. Developmental neurobiology. 2010;70(5):289–297. [DOI] [PubMed] [Google Scholar]
- 76.Sharma S Beyond Diet & Depression: Disease-Specific Depression and Biomarkers Vol 1 New York: Nova Science Publishers; 2015. [Google Scholar]
- 77.Young JJ, Bruno D, Pomara N. A review of the relationship between proinflammatory cytokines and major depressive disorder. Journal of affective disorders. 2014;169:15–20. [DOI] [PubMed] [Google Scholar]
- 78.Miller AH, Maletic V, Raison CL. Inflammation and its discontents: the role of cytokines in the pathophysiology of major depression. Biol Psychiatry. 2009;65(9):732–741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Dowlati Y, Herrmann N, Swardfager W, et al. A meta-analysis of cytokines in major depression. Biol Psychiatry. 2010;67(5):446–457. [DOI] [PubMed] [Google Scholar]
- 80.Miller GE, Cohen S, Ritchey AK. Chronic psychological stress and the regulation of pro-inflammatory cytokines: a glucocorticoid-resistance model. 2002(0278–6133 (Print)). [DOI] [PubMed]
- 81.Myint AM, Kim YK, Verkerk R, Scharpe S, Steinbusch H, Leonard B. Kynurenine pathway in major depression: evidence of impaired neuroprotection. 2007(0165–0327 (Print)). [DOI] [PubMed]
- 82.Bremner JD, Narayan M, Anderson ER, Staib LH, Miller HL, Charney DS. Hippocampal volume reduction in major depression. The American journal of psychiatry. 2000;157(1):115–118. [DOI] [PubMed] [Google Scholar]
- 83.Hannestad J, DellaGioia N, Bloch M. The Effect of Antidepressant Medication Treatment on Serum Levels of Inflammatory Cytokines: A Meta-Analysis. Neuropsychopharmacology. 2011;36(12):2452–2459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ueland PM, McCann A, Midttun O, Ulvik A. Inflammation, vitamin B6 and related pathways. Molecular aspects of medicine. 2016. [DOI] [PubMed] [Google Scholar]
- 85.Sakakeeny L, Roubenoff R, Obin M, et al. Plasma pyridoxal-5-phosphate is inversely associated with systemic markers of inflammation in a population of U.S. adults. The Journal of nutrition. 2012;142(7):1280–1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Chiang EP, Smith DE, Selhub J, Dallal G, Wang YC, Roubenoff R. Inflammation causes tissue-specific depletion of vitamin B6. Arthritis research & therapy. 2005;7(6):R1254–1262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Berkowitz SA, Gao X, Tucker KL. Food-Insecure Dietary Patterns Are Associated With Poor Longitudinal Glycemic Control in Diabetes: Results From the Boston Puerto Rican Health Study. Diabetes Care. 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Almeida OP, Ford AH, Flicker L. Systematic review and meta-analysis of randomized placebo-controlled trials of folate and vitamin B12 for depression. International psychogeriatrics / IPA. 2015;27(5):727–737. [DOI] [PubMed] [Google Scholar]
- 89.Barrington WE, Beresford SA, McGregor BA, White E. Perceived stress and eating behaviors by sex, obesity status, and stress vulnerability: findings from the vitamins and lifestyle (VITAL) study. Journal of the Academy of Nutrition and Dietetics. 2014;114(11):1791–1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Adam TC, Epel ES. Stress, eating and the reward system. Physiology & behavior. 2007;91(4):449–458. [DOI] [PubMed] [Google Scholar]
- 91.Raikkonen K, Matthews KA, Kuller LH. Depressive symptoms and stressful life events predict metabolic syndrome among middle-aged women: a comparison of World Health Organization, Adult Treatment Panel III, and International Diabetes Foundation definitions. Diabetes Care. 2007;30(4):872–877. [DOI] [PubMed] [Google Scholar]
- 92.Turner RJ, Lloyd DA. The Stress Process and the Social Distribution of Depression. Journal of Health and Social Behavior 1999;40:374–404. [PubMed] [Google Scholar]


