Abstract
Background
The tamarind seeds have a lot of nutrients that may be used to control cholesterol or glucose levels.
Objective(s)
The effects of tamarind seeds (T) on lipid and carbohydrate metabolism in rats were studied. Rats were offered basal diet (BD) with T (2%, 4% or 8%) or without T.
Materials and methods
Feeding and growth performance in rats were measured and samples of liver and blood were analyzed for glycogen content and levels of cholesterol and glucose respectively.
Results
The inclusion of T in the diet influences the feeding and growth performance in rats. The serum cholesterol level was reduced (p < 0.05) in Sprague Dawley (SD) rats fed on basal diet (BD) containing 4% and 8% T (0.24 ± 0.14 g/l and 0.31 ± 0.06 g/l respectively) compared to control (0.79 ± 0.04 g/l). The serum glucose levels in the spontaneous hypertensive rats (SHR) was lower (50.74 ± 2.50 mg/dl; p < 0.05) than control (93.52 ± 10.83 mg/dl) at 4% T. Incorporation of increasing doses of T resulted in linear increase of glycogen storage in livers of SD rats fed on BD and high sucrose diet.
Conclusion
Tamarind seeds can lower blood glucose and serum cholesterol and enhance storage of glycogen in rats.
Keywords: Tamarind seeds, Glucose, Glycogen, Cholesterol, Spontaneous hypertensive rats, Feeding and growth performance
1. Introduction
Hypercholesterolemia is an abnormal condition characterized by high cholesterol concentration of low density lipoprotein (LDL) and a lower concentration of functional high density lipoprotein (HDL) which could lead to cardiovascular diseases due to development and increase of atheroma (atherosclerosis) [6]. It could also lead to myocardial infarction (heart attack), stroke and peripheral vascular disease [10]. Generally, the balance in LDL and HDL is genetically determined but can be affected by body build, medications, food choices and other factors [19]. It is suggested that total blood cholesterol (HDL + VLDL) of less than 200 mg/dl is desirable for the body [43]. Serum cholesterol levels of 200–239 mg/dl can be regarded as borderline while values greater than 240 mg/dl are considered high [32].
Hyperglycemia is a metabolic disorder in which the circulation of blood glucose level is excessive in the blood plasma resulting from defects in insulin secretion, insulin action or both [3]. In addition, postprandial glycemia (related insulinaemia and lipidaemia) has been associated in chronic metabolic diseases such as type 2 diabetes mellitus and cardiovascular disease [9].
Dietary manipulation through the consumption of specific plant materials containing phytochemicals has proven to regulate the level of glucose and lipids in the blood [30]. Studies have shown that Tamarindus indica can be used to reduce visceral fat accumulation and improve hyperlipidemia and hyperglycemia in rats [28], [40]. T. indica contains phenolic compounds like catenin, procyanidin B2, epicatechin, tartaric acid, mucilage, pectin, arabinose, xylose, tannins, galactose, glucose, uronic acid and triterpene [7] as well as all essential amino acids except tryptophan [36], [1], [25]. The tamarind seeds also have similar properties found to be important and used in traditional therapies [25]. Till date, there are intensive bioactivity studies on tamarind pulp. However, the tamarind seed which is basically considered a waste product is an under-utilized resource. Therefore, the aim of the present study is to determine in vivo effects of tamarind seeds inclusion (2%, 4% and 8%) in diets on parameters related to diabetics and hypertension such as blood glucose, glycogen in liver and cholesterol levels in three rat models: normal, hypertensive and exposed to hyperglycemic condition by feeding on high sucrose diet (HSD) during 4 weeks of feeding period. Additionally, evaluation of feeding and growth performance of rats was also carried out.
2. Materials and methods
2.1. Materials
2.1.1. Animal feed
Rat chow made from soybean meal was purchased from local feed manufacturer (Gold Coin Malaysia). Tamarind and soybean milk powder (Nutrisoy Inc.) were purchased from local markets.
2.1.2. Experimental animals
Male Sprague–Dawley rats (SD; n = 56) and spontaneously hypertensive rats (SHR; n = 28) out bred of Wistar-Kyoto rats, 6–8 weeks old were randomly selected from University of Malaya animal house. These animals were individually caged (600 × 380 × 200 mm3) at all time and had access to rat chow and fresh water ad libitum.
2.2. Methods
2.2.1. Preparation of ground tamarind seeds
The pulp of fresh tamarind fruits was removed and the seeds were washed out of residual flesh. A total weight of about 8 kg of seeds from the fruits was obtained and the seeds were dried in the oven (50 °C) and ground separately into fine particles of about 50 μm particle size.
2.2.2. Preparation of diets containing ground tamarind seeds
The basal diet (BD) was mixed with ground tamarind seeds at the following concentration: 2%, 4% and 8% w/w. High sucrose diet (HSD) was prepared by adding 30% w/w sucrose to the BD. Equivalent weight of soymilk was added into the diets to balance the difference in nitrogen and energy content as a result of the seeds inclusion. Proximate analysis of rat chow and fresh ground tamarind seeds is shown in Table 1. Furthermore, proximate analysis of the basal diet and high sucrose diet containing different concentration of tamarind seeds are shown in Table 2, Table 3 respectively.
Table 1.
Proximate analysis of rat chow and fresh ground seeds (%).
| Materials | Rat chow | Tamarind seeds |
|---|---|---|
| Dry matter | 88.00 ± 0.10 | 95.10 ± 0.10 |
| Lipids | 2.20 ± 0.10 | 2.90 ± 0.10 |
| Protein | 19.10 ± 0.10 | 11.80 ± 0.11 |
| Ash | 4.60 ± 0.10 | 4.50 ± 0.00 |
| Total carbohydrates | 62.00 ± 2.03 | 75.80 ± 3.21 |
Data are presented as the mean of four observations ± standard error mean.
Table 2.
Proximate analysis of the basal diet containing different concentration of tamarind seeds.
| Materials | 0% | 2% | 4% | 8% |
|---|---|---|---|---|
| Dry matter | 88.00 ± 0.20 | 88.00 ± 0.10 | 88.8 ± 0.20 | 88.3 ± 0.60 |
| Lipid | 2.30 ± 0.30 | 2.20 ± 0.30 | 2.20 ± 0.30 | 2.20 ± 0.30 |
| Protein | 19.10 ± 0.10 | 19.10 ± 1.10 | 19.10 ± 1.20 | 19.10 ± 1.30 |
| Ash | 4.60 ± 0.10 | 4.60 ± 0.10 | 4.60 ± 0.10 | 4.60 ± 0.01 |
| Total carbohydrates | 62.00 ± 2.00 | 62.10 ± 1.50 | 62.90 ± 2.10 | 62.40 ± 1.80 |
Data are presented as the mean of four observations ± standard error mean.
Table 3.
Proximate analysis of high sucrose diet containing different concentration of tamarind seeds.
| Materials | 0% | 2% | 4% | 8% |
|---|---|---|---|---|
| Dry matter | 87.80 ± 0.20 | 87.70 ± 0.10 | 87.90 ± 0.20 | 87.80 ± 0.60 |
| Lipid | 2.20 ± 0.30 | 2.10 ± 0.30 | 2.20 ± 0.30 | 2.2 ± 0.30 |
| Protein | 19.10 ± 0.10 | 19.10 ± 1.10 | 19.10 ± 1.00 | 19.10 ± 1.40 |
| Ash | 4.60 ± 0.30 | 4.60 ± 0.10 | 4.60 ± 0.20 | 4.60 ± 0.00 |
| Total carbohydrates | 61.90 ± 2.00 | 61.90 ± 1.50 | 62.80 ± 2.10 | 62.10 ± 1.80 |
Data are presented as the mean of four observations ± standard error mean.
2.2.3. Experimental procedures
Three different and independent experiments were conducted. In the first experiment, SD rats (n = 28) were randomly assigned into seven groups of four each and served with test diets (BD) containing tamarind seeds at various concentrations of 2, 4 and 8%, respectively where 0% served as control in the group. In the second experiment, the same procedures were carried out except that each group was fed on HSD containing 2, 4 and 8% tamarind seeds respectively and 0% tamarind seeds served for control group. In the third experiment, SHR rats were assigned into seven groups accordingly and fed with BD. Initial body weight (IBW), body weight gain (BWG), feed intake (FI) and faecal output from each rat were measured at the beginning and every seven days thereafter for 4 weeks.
The animals were sacrificed by cervical dislocation at the end of the feeding period. Blood samples were collected into heparinized tubes and after centrifugation (2500 g; 10 min 4 °C) the plasma was harvested and kept cold at 4 °C for further analysis. Liver samples of approximately 5 g were collected and stored at −20 °C for liver glycogen estimation.
2.2.4. Feeding performance analysis
-
1)
The amount of weekly diet ingested was calculated as the difference in the total weight of feed offered at the beginning and balance at the end of the week. The weekly data collected were then used to calculate daily feed intake according to Ennouri et al. [15] with the following formula:
-
2)
Fecal dry matter (DM) was determined after drying faeces collected in 24 h at 105 °C to constant weight [15].
-
3)
Macro nutrients digestibility were assessed as the difference between daily DM intake and 24 h DM excretion in faeces according to Ennouri et al. [15]:
-
4)
The feed conversion efficiency (FCE) was determined by the following formula [15]:
-
5)
The protein efficiency ratio (PER) is the weight gain of the growing rat divided by total protein intake during the feeding period [15] according to the following formula:
2.2.5. Enzymatic determination of total cholesterol
The total cholesterol was determined using commercial kits from Chemo Lab (Malaysia). The cholesterol level was evaluated by mixing thoroughly 10 μl of serum or standard solution with 1.0 ml of kit reagent. The mixture was allowed to stand for 5 min at 37 °C prior to absorbance reading at 500 nm.
The cholesterol concentration was calculated using the following formula:
2.2.6. Estimation of blood glucose concentration
Serum glucose level was determined according to modified Trinder method [15]. Serum glucose content was estimated by mixing 0.1 ml of serum with 1 ml of water, 1 ml of 5.0% zinc sulfate and 1 ml of 0.25 N sodium hydroxide. The mixture was then centrifuged (2500 g, 10 min) and the supernatant (1 ml) was transferred into a test tube containing 1 ml of alkaline copper reagent followed by boiling in water bath for 10 min. The mixture was cooled by placing the tubes under running water for 3 min. Arseno-molybdate reagent (1 ml) was added to the resultant solution and the volume was made up to 10 ml with water. The optical density was read at 500 nm against a blank set at zero. The glucose concentration in the samples was then calculated from a glucose calibration curve which was also run at the same time with the glucose analysis.
2.2.7. Estimation of liver glycogen content
Liver glycogen content was determined according to Vats et al. [42]. Liver samples (200 mg) were rinsed with ice-cold saline and then solubilized by incubating with 2 ml of 30% potassium hydroxide at 55 °C for 30 min. The solubilized liver tissue (0.2 ml) was placed on ice bath and then neutralized with 0.2 ml of 1 M HCL, 0.8 ml of water and 2 ml anthrone reagent (0.2 g anthrone/100 ml of 95% H2SO4). The mixture was then incubated at 100 °C for 10 min. Absorbance was measured at 620 nm and the liver glycogen content (mg glycogen/g tissue) was calculated using glucose standard curve.
2.2.8. Statistical analysis
All results presented are means of three independent measurements. Data were presented as mean ± standard error mean using one-way analysis of variance (ANOVA) by SPSS software version 16. The statistical significance was tested at p < 0.05 using post hoc Tukey's analysis at 95% least significant difference (LSD).
3. Results
3.1. Feeding and growth performance in rats
3.1.1. Feed intake and body weight gain of SD rats fed on BD containing tamarind seeds
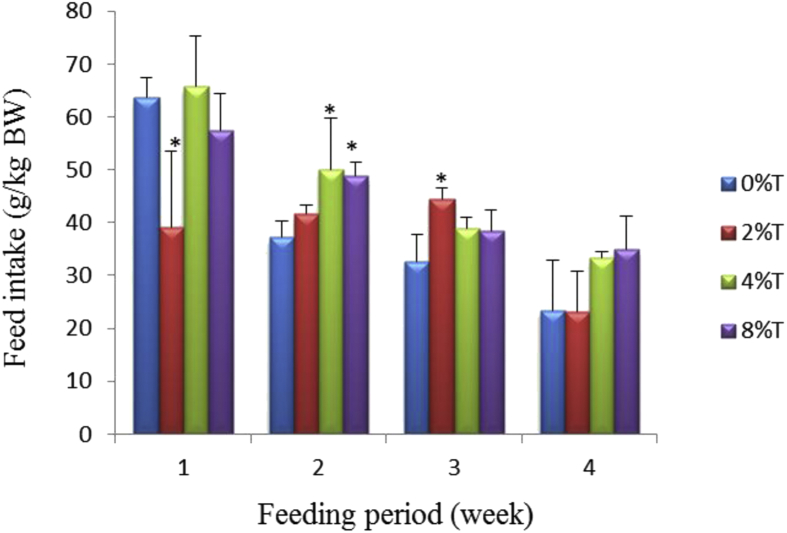
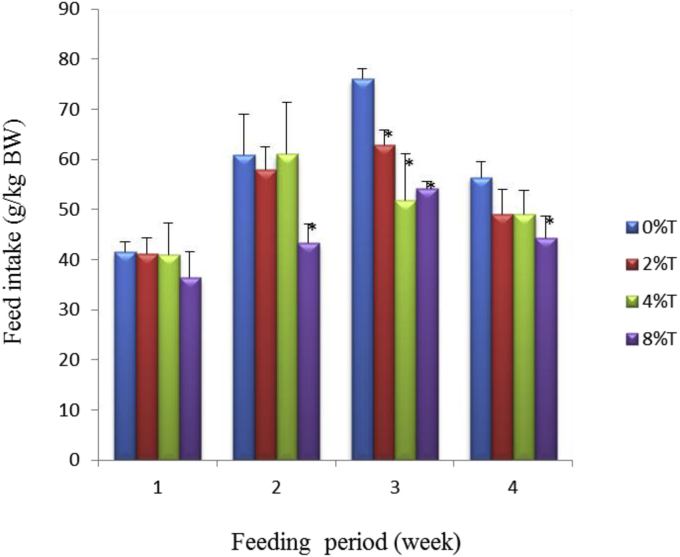
The control group consumed feed of 63 ± 4 g/kg BW/day during the first week of feeding and this value reduced gradually to 23 ± 10 g/kg BW/day by the end of week four (Fig. 1). The inclusion of 2% tamarind seeds into BD resulted in reduced feed intake (FI) to 38 ± 15 g/kg BW/day (p < 0.05) during week 1 of feeding whereas BD containing 4% or 8% tamarind seeds was not different from control. Intake of diets containing tamarind seeds during the next 3 weeks was not different from control except at 4% and 8% of tamarind seeds in week 2, and 2% in week 3 (p < 0.05; Fig. 1).
Fig. 1.
Feed intake (g/kg BW) of SD rats fed on BD containing different concentration of tamarind seeds (T) during 4 weeks of feeding period. Values are presented as mean ± SEM (n = 4). *p < 0.05 compared to control (0% tamarind seeds).
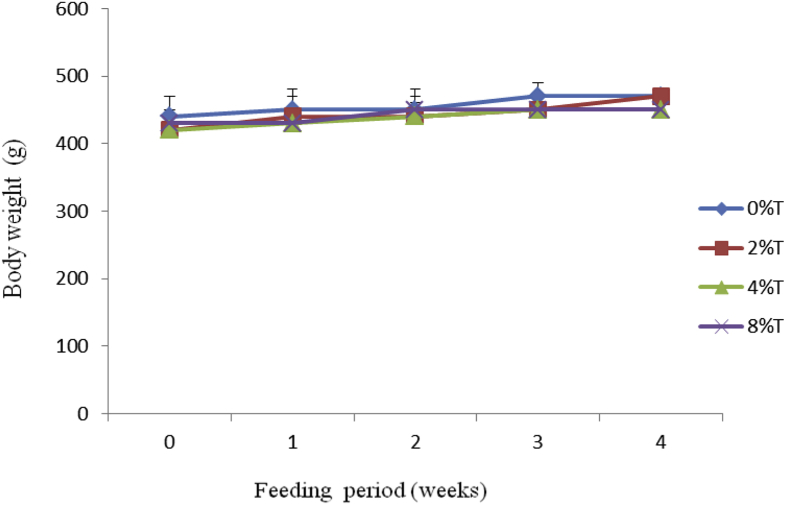
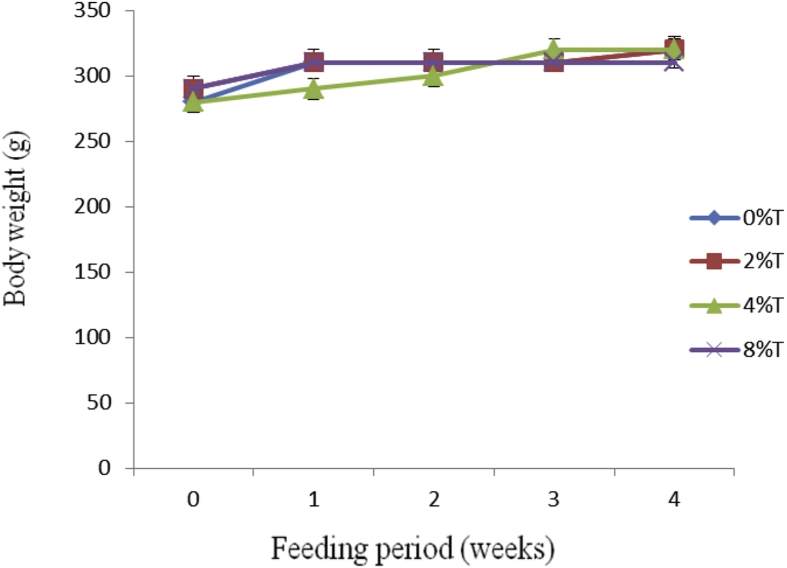
All treated groups had a marginal increase in BWG of between 15 and 47 g during the 4 weeks of feeding (Fig. 2). The increase in BWG was linear and similar (25–47 g) for groups consumed 0%, 2% and 4% tamarind seeds whereas group fed on 8% showed the least increase in BWG (15 g). The average BWG (g) to IBW (kg) during the entire feeding period showed that control group had the lowest BWG (57 ± 15 g/kg BW) compared to those fed on tamarind seeds (105 ± 27 (p < 0.05), 69 ± 22 (p > 0.05) and 71 ± 38 (p > 0.05) g/kg BW) for 2%, 4% and 8% respectively (data not shown).
Fig. 2.
Body weight (g) of SD rats fed on BD containing different concentration of tamarind seeds (T) during 4 weeks of feeding period. Values are presented as mean ± SEM (n = 4).
3.1.2. Feed intake and body weight gain of SD rats fed on HSD containing tamarind seeds
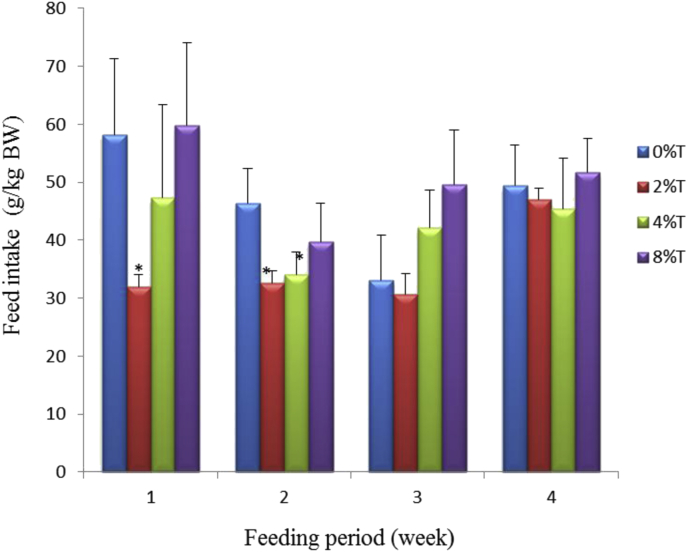
Average daily FI of treated groups fed on HSD containing 0%, 4% and 8% of tamarind seeds ranged 50–60 g/kg BW at the first week (Fig. 3). These values reduced (p > 0.05) to about 33–48 g/kg BW during the subsequent 3 weeks of feeding. Treated group fed on HSD containing 2% of tamarind seeds had lower (p < 0.05) initial FI (30 g/kg BW) that remained for the first 3 weeks of feeding before increasing to 44 g/kg BW at the 4th week (Fig. 3).
Fig. 3.
Feed intake (g/kg BW) of SD rats fed on HSD containing different concentrations of tamarind seeds (T) during 4 weeks of feeding period. Values are presented as mean ± SEM (n = 4). *p < 0.05 compared to control (0% tamarind seeds).
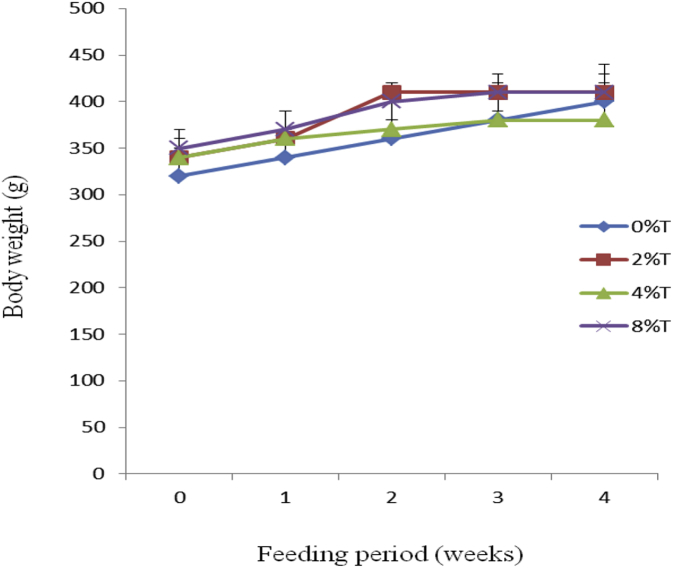
All treated groups were increased in BWG compared to IBW during the feeding (Fig. 4). The difference in IBW and final body weight of the rats was in the range of 20%–40%. The inclusion of tamarind seeds (2% and 4%) into the diet reduced (p < 0.05) average BWG. The average BWG of the control group (282 ± 36 g/kg BW; p < 0.05) was higher than treated groups (155 ± 22, 118 ± 36 and 156 ± 26 g/kg BW) for 2%, 4%, and 8% tamarind seeds respectively (data not shown).
Fig. 4.
Body weight (g) of SD rats fed on HSD containing different concentration of tamarind seeds (T) during 4 weeks of feeding period. Values are presented as mean ± SEM (n = 4).
3.1.3. Feed intake and body weight gain of SHR fed on BD containing tamarind seeds
FI of the control group increased significantly from 42 g/kg BW to 73 g/kg BW (p < 0.05) during the first three weeks of feeding (Fig. 5). However, this value reduced (p < 0.05) to about 55 g/kg BW by week 4. The inclusion of tamarind seeds (2%, 4% and 8%) to the BD did not affect the FI compared to control (Fig. 5).
Fig. 5.
Feed intake (g/kg BW) of SHR fed on BD containing different concentration of tamarind seeds (T) during 4 weeks of feeding period. Values are presented as mean ± SEM (n = 4). *p < 0.05 compared to control (0% tamarind seeds).
Treated group fed on BD containing 4% of tamarind seeds showed the highest BWG (Fig. 6). All rats showed an increase in BWG within 15–25% by the end of the feeding. However, increasing the inclusion of tamarind seeds to 8% into the diet reduced (p < 0.05) average BWG (95 ± 18 g/kg BW) compared to control (134 ± 21 g/kg BW) (data not shown).
Fig. 6.
Body weight (g) of SHR fed on BD containing different concentrations of tamarind seeds (T) during 4 weeks of feeding period. Values are presented as mean ± SEM (n = 4).
3.1.4. Effect of tamarind seeds on the digestibility of diets in rats
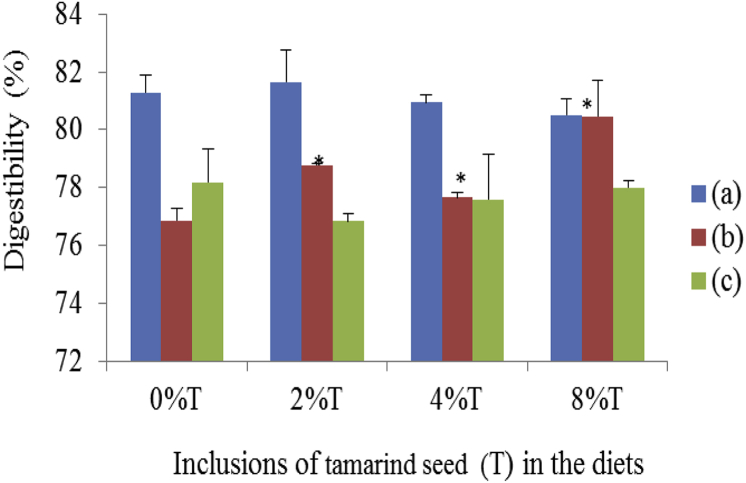
BD was more digestible than when sucrose was included (Fig. 7). Furthermore, BD was more digestible when consumed by SD rats than by SHR. The inclusion of tamarind seeds did not change the digestibility of BD in SD rats and SHR. However, the digestibility of HSD tended to improve (p < 0.05) with increasing inclusion of tamarind seeds, the optimum effect was achieved at 8% (80.46 ± 1.23%) compared to control (76.86 ± 0.42%).
Fig. 7.
The digestibility of BD and HSD containing different concentrations of tamarind seeds fed to SD rats and SHR. (a) SD rats fed on BD, (b) SD rats fed on HSD and (c) SHR fed on BD. Values are presented as mean ± SEM (n = 4). *p < 0.05 compared to control (0% tamarind seeds).
3.1.5. Effect of tamarind seeds on feed conversion efficiency (FCE) of diets in rats
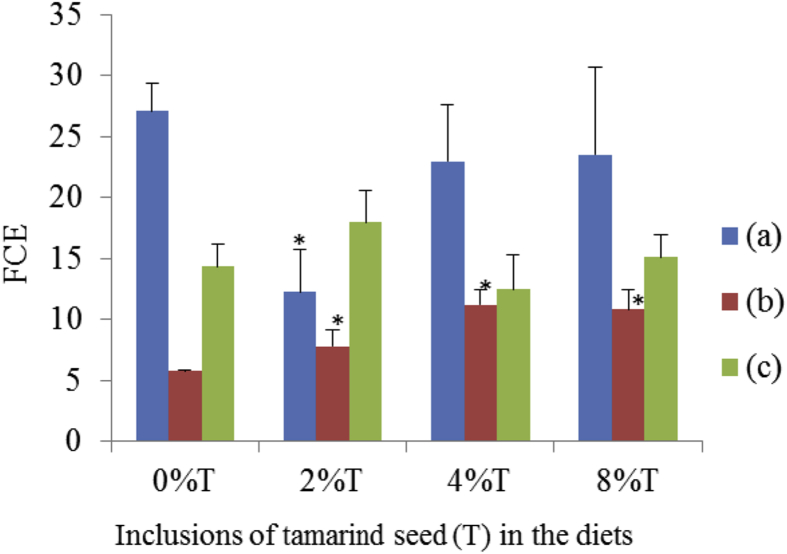
SD rats group fed on BD showed the least FCE (27 ± 5) followed by SHR fed on BD (14 ± 4) and SD fed on HSD (5.3 ± 0.2; Fig. 8). The inclusion of 2% tamarind seeds increased (p < 0.05) FCE up to 50% in SD rats fed on BD. The addition of the tamarind seeds did not improve (p > 0.05) FCE in SD rats and SHR fed on HSD and BD respectively.
Fig. 8.
The feed conversion efficiency (FCE) of BD and HSD containing different concentrations of tamarind seeds fed to SD rats and SHR. (a) SD rats fed on BD, (b) SD rats fed on HSD and (c) SHR fed on BD. Values are presented as mean ± SEM (n = 4). *p < 0.05 compared to control (0% tamarind seeds).
3.1.6. Effect of tamarind seeds on protein efficiency ratio (PER) of diets in rats
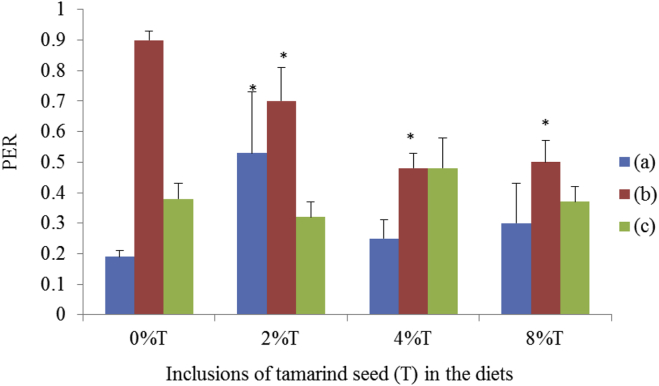
Inclusion of 2% tamarind seeds resulted in the highest PER (0.54 ± 0.20; p < 0.05) in SD rats fed on BD compared to control (0.19 ± 0.02; Fig. 9). SD rats fed on HSD had the highest PER (0.90 ± 0.03) but the inclusion of tamarind seeds (4% and 8%) reduced (p < 0.05) PER to the lowest values (0.5). The inclusion of tamarind seeds in BD had no significant effect on the PER of SHR as compared to control (Fig. 9).
Fig. 9.
The protein efficiency ratio (PER) of BD and HSD containing different concentrations of tamarind seeds fed to SD rats and SHR. (a) SD rats fed on BD, (b) SD rats fed on HSD and (c) SHR fed on BD. Values are presented as mean ± SEM (n = 4). *p < 0.05 compared to control (0% tamarind seeds).
3.2. Serum cholesterol concentration of rats fed on BD and HSD containing tamarind seeds
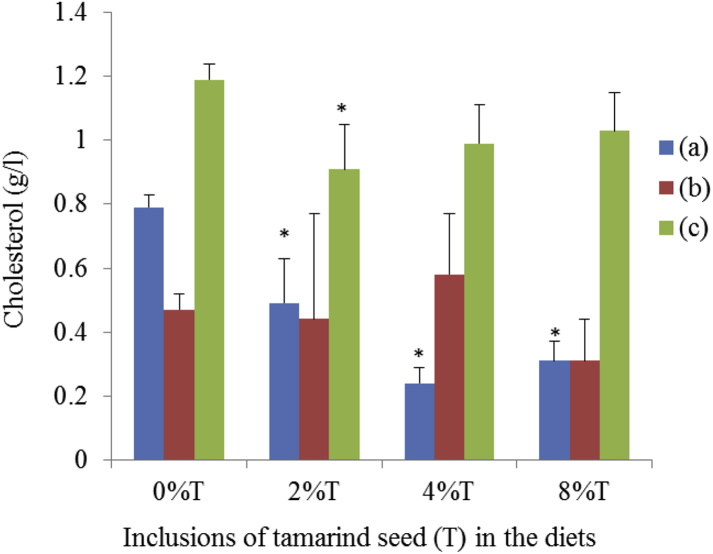
SD rats fed on BD had blood cholesterol of 0.79 ± 0.04 g/l (Fig. 10). The blood cholesterol level was lowered (p < 0.05) with the inclusion of tamarind seeds at all doses in BD. The inclusion of different doses of tamarind seeds did not affect significantly the cholesterol level of the SD rats fed on HSD. SHR (control group) had the highest blood cholesterol (1.19 ± 0.05 g/l). The addition of tamarind seeds resulted in lowering of blood cholesterol but a significant effect was only seen at 2% of tamarind seeds inclusion (0.91 ± 0.14 g/l; Fig. 10).
Fig. 10.
The serum cholesterol of SHR and SD rats fed on BD and HSD containing different concentrations of tamarind seeds. (a) SD rats fed on BD, (b) SD rats fed on HSD and (c) SHR fed on BD. Values are presented as mean ± SEM (n = 4). *p < 0.05 compared to control (0% tamarind seeds).
3.3. Serum glucose concentration of rats fed on BD and HSD containing tamarind seeds
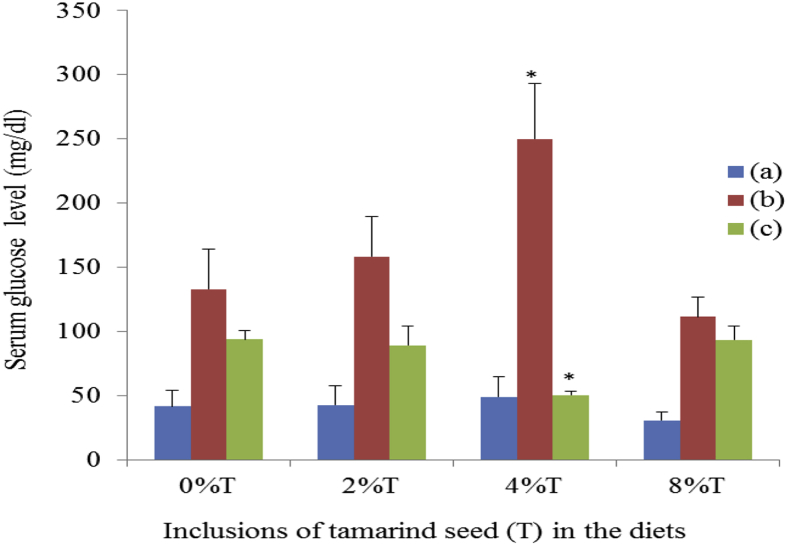
The lowest blood glucose was shown in SD rats fed on BD (41.72 ± 12.46 mg/dl) followed by SHR fed on BD (93.52 ± 10.83 mg/dl) and SD rats fed on HSD (133.08 ± 31.24 mg/dl) (Fig. 11). The addition of tamarind seeds did not affect serum glucose levels except for SHR, which showed a significant reduction (p < 0.05) of serum glucose to 50 ± 3 mg/dl at 4% tamarind seeds inclusion. However, the inclusion of 4% tamarind seeds in HSD resulted in increased (p < 0.05) serum glucose level in SD rats (250 ± 44 mg/dl) compared to control.
Fig. 11.
The serum glucose concentration of SHR and SD rats fed on BD and HSD containing different concentrations of tamarind seeds. (a) SD rats fed on BD, (b) SD rats fed on HSD and (c) SHR fed on BD. Values are presented as mean ± SEM (n = 4). *p < 0.05 compared to control (0% tamarind seeds).
3.4. Liver glycogen content of rats fed on BD and HSD containing tamarind seeds
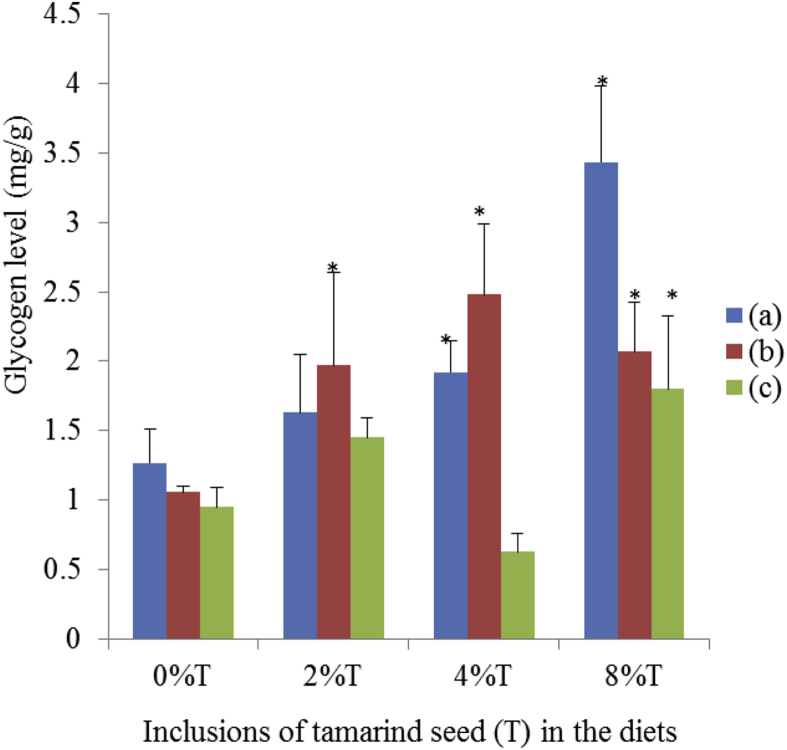
All control groups fed on diet with no tamarind seeds showed similar liver glycogen content (0.95–1.27 mg/g; Fig. 12). Incorporation of increasing doses of tamarind seeds resulted in a linear increase of glycogen storage in livers of SD rats fed on BD with maximum level recorded at 8% tamarind seeds (3.43 ± 0.55 mg/g, p < 0.05). There was also evidence of increased liver glycogen storage with increasing tamarind seeds inclusion for SD rats fed on HSD ranged from 2–2.5 mg/g compared to control (Fig. 12). The inclusion of tamarind seeds brought about an increased (p < 0.05) liver glycogen content at 2% and 8% (1.45 ± 0.14 and 1.80 ± 0.53 mg/g respectively) than control in SHR (Fig. 12).
Fig. 12.
The liver glycogen content of SHR and SD rats fed on BD and HSD containing different concentrations of tamarind seeds. (a) SD rats fed on BD, (b) SD rats fed on HSD and (c) SHR fed on BD. Values are presented as mean ± SEM (n = 4). *p < 0.05 compared to control (0% tamarind seeds).
4. Discussion
4.1. Effects of tamarind seeds on feed intake and body weight gain
Feed intake can be affected by diet palatability, flavor and odor [22] whereas body weight gain is determined by the energy and nitrogen content of food consumed [41]. Previous attempts on tamarind seeds inclusion in the diet showed lower feed intake in cow [8] and pig [27]. This has been suggested to be due to the presence of tannin [16] in seed which in general could cause feed avoidance as the percentage inclusion of seed in the compound feed increased. In the present study, the inclusion of 2–8% tamarind seeds in the diets was shown not to affect the feed intake of the SD rats during the period which is in agreement with Kumar and Bhattacharya [24]. Nevertheless, there were some variations (10–40%) in the quantity of feed consumed (g/kg BW) per day between control and treatments during the trial and this occurred possibly due to differences in adaptations of the rats to test diets [38]. In general these variations did not result in significant change in feed intake over the period in normal SD rats; however in SHR, the negative effect of the tamarind seeds secondary compounds (tannins) resulted in lowering voluntary feed intake after two weeks of feeding possibly by decreasing palatability of the ration because of astringent effects of tannin on the oral cavity [45].
The inclusion of tamarind seeds had no effect on body weight gain of SD rats fed on BD although there were some variations in body weight gain between the treated and control which may be attributed to the differences in animal's efficiency in converting absorbed nutrients into body mass [29]. Previous study showed that rats fed on high carbohydrate meal (e.g. sucrose) gained more weight than rats fed on normal rat chow [17] which was also seen in rats fed on HSD in the present study. However, the apparent beneficial effect of sucrose on body weight gain was lowered when tamarind seeds were included in the diet. Tannins in these feed may have acted as anti-nutritive factors since it was demonstrated that this plant's secondary metabolites lower absorption and post digestive assimilation of nutrients into body mass in animals [39], [5]. In SHR fed on BD, the inclusion of tamarind seeds lowered the body weight gain of the rats when compared with control and this may be explained by reduced feed intake by SHR (Fig. 3).
4.2. Effects of tamarind seeds on digestibility, FCE and PER
Digestibility is described as the difference in feed intake and fecal excretion in relation to feed intake [29]. Thus, when consumption of feed is high and fecal excretion is also high, the value of digestibility is low i.e. feed is not properly digested and/or absorption is impaired. The values of digestibility from the present study ranged from 77 to 80% which are comparable to previous report using normal rats [15] and hypercholesterolemic rats [29]. The addition of tamarind seeds in HSD may however provide additional beneficial effect of the phytochemical compounds of tamarind seeds.
FCE is defined as a measure of an animal's efficiency in converting feed mass into increased body mass, which is expressed as the mass of the food eaten divided by the body mass gain over a specified period of time [29]. Low values of FCE implies high efficiencies and vice versa. The lowest FCE of control groups were recorded in BD-fed SD rats followed by BD-fed SHR and HSD-fed SD rats. This shows that the same diet may have different effect on rats of different physiological status and thus the same rat may perform differently on different diet [38], [21]. The increased (p < 0.05) FCE up to 50% in SD rats fed on BD containing 2% of tamarind seeds may associated with high growth performance (Fig. 2).
PER relates the body weight gain over the protein consumed with the implication that a high PER value indicates an efficient feed as a protein source [15]. There was an evidence of improved PER in BD-fed SD but it occurred only at 2% of tamarind seeds (Fig. 9). In general, tamarind seeds inclusion in the diet did not have a profound effect on PER improvement. In fact, tamarind seeds inclusion may even reduce PER when the diet also contains sucrose.
4.3. Effects of tamarind seeds on serum cholesterol, glucose and liver glycogen content in rats
According to American Heart Association [4] high total cholesterol content (>2.5 g/l) indicates hypercholesterolemia while values less than 2.0 g/l is considered normal. The lowering of blood cholesterol as a result of tamarind seeds inclusion in the BD fed SD rats and SHR may be occur due to the presence of the phytochemicals. Tamarind seeds contain phenolic compounds such as phytosterols [14], [1] in a concentration of 590 mg/kg dry weight [26]. These phytosterols, especially the beta-sitosterols are recognized to decrease plasma lipoprotein and cholesterol levels [20] via reducing the cholesterol solubility and absorption across the intestinal barrier [18], [44]. In fact, phytosterols' hydrophobicity is more readily to mix with bile salt and acid micelles [34] than can animal cholesterol [13]. This causes the excretion of a greater part of unabsorbed cholesterol particularly the low density lipoprotein with the faeces [35].
Blood sugar concentration in the body has a range of 64.8–104.4 mg/dl in a normal person [2]. Blood glucose is the primary source of energy in the body obtained from carbohydrates breakdown [23]. The elevation or reduction of blood glucose occurs as a result of certain conditions like illness, stress, surgery or intake of a particular substance [11]. Phenolic compounds in plants can have blood glucose lowering capacity [12], [31], [46], [15]. However, tamarind seeds appeared not to be a strong suppressor of blood glucose elevation in SD rats fed on HSD and BD as compared to control. This could be due to the physiological conditions of the animals [37]. The difference in response of these rats reflects the importance of several facets of blood glucose homeostasis.
Glycogen is stored in the liver or muscles and it functions as secondary energy storage in animals which is reconverted back to glucose by glycogenolysis in low energy state [33]. The increase of glucose intake or use additives or phenolic compounds could enhance glycogen storage [15]. In the present study, the inclusion of tamarind seeds had positive effects on glycogen storage which occurred in a dose dependent manner. The improved liver glycogen storage could be associated with the presence of phenolic compounds in tamarind seeds.
5. Conclusion
The inclusion of tamarind seeds into the diet influenced the feeding and growth performance to some extent. The inclusion of tamarind seeds (4% and 8%) in basal diet lowered the cholesterol levels of normal rats. In addition, 4% of tamarind seeds suppressed high blood glucose of SHR. Increased doses of tamarind seeds enhanced glycogen storage in the liver in all the three models of rats. Thus, tamarind seeds could be useful in treating people with hyperglycemia and/or hypercholesteremia. The present investigation may discover the promising values of tamarind seeds as a source of energy, protein as well as bioactive phytochemical for health improvement. Further studies are needed to identify the principal phenolic compounds in tamarind seeds which are responsible for lowering cholesterol and blood glucose levels in rats.
Sources of funding
IPPP University of Malaya.
Conflict of interest
None.
Acknowledgement
We are grateful to the Vice Chancellor University of Malaya, Prof Datuk Dr. Ghauth Jasmon and to the IPPP University of Malaya for their dynamic support and also to the Dean faculty of science, University of Malaya Professor Datin Dr. Saadah, Abdul Rahman for his kind gestures.
Footnotes
Peer review under responsibility of Transdisciplinary University, Bangalore.
References
- 1.Aengwanich W., Maitree S., Chaleerin P., Thangklang P., Kapan S., Srikhun T. Antimicrobial effect of polyphenolic compound extracted from tamarind seed coat on productive performance of broilers. Int J Appl Res Vet Med. 2009;7:112–115. [Google Scholar]
- 2.American Diabetes Association Clinical practice recommendations – standards of medical care in diabetes. Diab Care. 2008;31:S12–S54. doi: 10.2337/dc08-S012. [DOI] [PubMed] [Google Scholar]
- 3.American Diabetes Association Diagnosis and classification of diabetes mellitus. Diab Care. 2009;32:S62–S67. doi: 10.2337/dc09-S062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.American Heart Association Phytochemicals and cardiovascular disease. Circulation. 2006;114:82–96. [Google Scholar]
- 5.Andres C., Lars B., Gina B., Abraham L.B. Effect of black bean tannins on in vitro carbohydrate digestion and absorption. J Nutr Biochem. 1996;7:445–450. [Google Scholar]
- 6.Aronow W.S. Treatment of hypercholesterolemia. J Clin Exp Cardiol. 2013;4:1–8. [Google Scholar]
- 7.Bhadoriya S.S., Mishra V., Raut S., Ganeshpurkar A., Jain S.K. Anti-inflammatory and antinociceptive activities of a hydroethanolic extract of Tamarindus indica leaves. Sci Pharm. 2012;80(3):685–700. doi: 10.3797/scipharm.1110-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bhatta R., Krishnamoorthy U., Mohammed F. Effects of tamarind (Tamarindus indica) seed husk tannis on in vitro rumen fermentation. Anim Feed Sci Technol. 2001;90:143–152. [Google Scholar]
- 9.Blaak E.E., Antoine J.M., Benton D., Björck I., Bozzetto L., Brouns F. Impact of postprandial glycaemia on health and prevention of disease. Obes Rev. 2012;13(10):923–984. doi: 10.1111/j.1467-789X.2012.01011.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brunzell J.D., Facp M.D., Davidson Michael, Facc C.D., Furberg M.D., Ronald B. Lipoprotein management in patients with cardiometabolic risk. J Am Coll Cardiol. 2008;51:1512–1524. doi: 10.1016/j.jacc.2008.02.034. [DOI] [PubMed] [Google Scholar]
- 11.Daly M.E., Vale C., Walker M., Littlefield A., Alberti K.G., Mathers J.C. Acute effects on insulin sensitivity and diurnal metabolic profiles of a high-sucrose compared with a high-starch diet. Am J Clin Nutr. 1998;67:1186–1196. doi: 10.1093/ajcn/67.6.1186. [DOI] [PubMed] [Google Scholar]
- 12.Delaney B., Luke S., Wade S., James H., Steve M., John H. Oral absorption of phytosterols and emulsified phytosterols by Sprague–Dawley rats. J Nutr Biochem. 2004;15:289–295. doi: 10.1016/j.jnutbio.2003.08.013. [DOI] [PubMed] [Google Scholar]
- 13.Elkin R.G., Lorenz E.S. Feeding laying hens a bio available soy sterol mixture fails to enrich their eggs with phytosterols or elicit egg yolk compositional changes. Meta Nutr Poult Sci. 2009;88:152–158. doi: 10.3382/ps.2008-00271. [DOI] [PubMed] [Google Scholar]
- 14.Engel R., Schubert H. Formulation of phytosterols in emulsions for increased dose response in functional foods. Innov Food Sci Emerg Technol. 2005;6:233–237. [Google Scholar]
- 15.Ennouri M., Fetoui H., Bourret E., Zeghal N., Attia H. Evaluation of some biological parameters of Opuntia ficus indica. 1. Influence of seed oil supplemented diet on rats. Bioresour Techonol. 2006;97:1382–1386. doi: 10.1016/j.biortech.2005.07.010. [DOI] [PubMed] [Google Scholar]
- 16.Gu L., Kelm M.A., Hammerstone J.F., Zhang Z., Beecher G., Holden J. Liquid chromatographic/electrospray ionization mass spectrometric studies of procyanidins in foods. J Mass Spectrom. 2003;38:1272–1280. doi: 10.1002/jms.541. [DOI] [PubMed] [Google Scholar]
- 17.Hansen M., Baunsgaard D., Autrup H., Vogel U.B., Moller P., Lindecrona R. Sucrose, glucose, and fructose have similar genotoxicity in the rat colon and affect the metabolome. Food Chem Toxicol. 2008;46:752–760. doi: 10.1016/j.fct.2007.09.110. [DOI] [PubMed] [Google Scholar]
- 18.Heinemann T., Axtmann G., Von Bergmann K. Comparison of intestinal absorption of cholesterol with different plant sterol in man. Eur J Clin Investig. 1993;23:827–831. doi: 10.1111/j.1365-2362.1993.tb00737.x. [DOI] [PubMed] [Google Scholar]
- 19.Huff M.W., Assini J.M., Hegele R.A. Gene therapy for hypercholesterolemia. Circ Res. 2014;115:542–545. doi: 10.1161/CIRCRESAHA.114.304800. [DOI] [PubMed] [Google Scholar]
- 20.Ikeda I., Tanaka K., Sugano M., Vahouny G.V., Gallo L.L. Inhibition of cholesterol absorption in rats by plants sterols. J Lipid Res. 1998;29:1573–1582. [PubMed] [Google Scholar]
- 21.Imafidon K.E. Liver function status of hypertensive and normotensive rats administered Persea americana mill. (Avocado) seeds. Acad J Plant Sci. 2010;3:130–133. [Google Scholar]
- 22.Jacela J.Y., Derouchey J.M., Tokach M.D. Feed additives for swine: fact sheets – flavors and mold inhibitors, mycotoxin binders, and antioxidants. J Swine Heal Product. 2010;18:27–32. [Google Scholar]
- 23.Kaye F.P., Sussanna H.A., Janette C.B.M. International table of glycemic index and glycemic load values. Am J Clin Nutr. 2002;76:5–56. doi: 10.1093/ajcn/76.1.5. [DOI] [PubMed] [Google Scholar]
- 24.Kumar C.S., Bhattacharya S. Tamarind seed: properties, processing and utilization. Crit Rev Food Sci Nutr. 2008;48:1–20. doi: 10.1080/10408390600948600. [DOI] [PubMed] [Google Scholar]
- 25.Kuru P. Tamarindus indica and its health related effects. Asian Pac. J Trop Biomed. 2014;4:676–681. [Google Scholar]
- 26.Leroux M., Kaye H.S., Brand-Miller J. International table of glycemic index and glycemic load values: 2002. Am J Clin Nutr. 2002;76:5–56. doi: 10.1093/ajcn/76.1.5. [DOI] [PubMed] [Google Scholar]
- 27.Mahto B., Singh A.K., Mahto D.K., Kumar B. Effect of tamarind seed (Tamarind indica) feeding on feed intake and nutrient utilization in Desi pig. Agri Res Commun Cent. 2010;44:205–207. [Google Scholar]
- 28.Maiti R., Das U.K., Ghosh D. Attenuation of hyperglycemia and hyperlipidemia in streptozotocin-induced diabetic rats by aqueous extract of seed of Tamarindus indica. Biol Pharm Bull. 2005;28(7):1172–1176. doi: 10.1248/bpb.28.1172. [DOI] [PubMed] [Google Scholar]
- 29.Makni M., Fetoui H., Gargouri N.K., Garoui E.M., Jaber H., Makni J. Hypolipidemic and hepatoprotective effects of flax and pumpkin seed mixture rich in ω-3 and ω-6 fatty acids in hypercholesterolemic rats. Food Chem Toxicol. 2008;46:3714–3720. doi: 10.1016/j.fct.2008.09.057. [DOI] [PubMed] [Google Scholar]
- 30.Meng S., Cao J., Feng Q., Peng J., Hu Y. Roles of chlorogenic acid on regulating glucose and lipids metabolism: a review. Evid Based Compl Altern Med. 2013;11 doi: 10.1155/2013/801457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Naczk M., Shahidi F.E. Extraction and analysis of phenolics in food. J Chromatogr A. 2004;1054:95–111. [PubMed] [Google Scholar]
- 32.National Cholesterol Education Program Report of the national cholesterol education program expert panel on detection, evaluation, and treatment of high blood cholesterol in adults. The expert panel. Arch Intern Med. 1988;148:36–69. [PubMed] [Google Scholar]
- 33.Pedersen D.J., Lessard S.J., Coffey V.G. High rates of muscle glycogen re-synthesis after exhaustive exercise when carbohydrate is co-ingested with caffeine. J Appl Physiol. 2008;105:7–13. doi: 10.1152/japplphysiol.01121.2007. [DOI] [PubMed] [Google Scholar]
- 34.Piironen V., Lindsay D.G., Miettinen T.A., Toivo J., Lampi A.M. Plant sterols: biosynthesis, biological function and their importance to human nutrition. J Sci Food Agri. 2000;80:939–966. [Google Scholar]
- 35.Pouteau E., Monnard I., Piguet-Welsch C., Groux M.J.A., Sagalowicz L., Berger A. Non-esterified plant sterols solubilized in low fat milks inhibit cholesterol absorption: a stable isotope double-blind crossover study. Eur J Nutr. 2003;42:154–164. doi: 10.1007/s00394-003-0406-6. [DOI] [PubMed] [Google Scholar]
- 36.Pumthong G. Chiang Mai University; Thailand: 1999. Antioxidative activity of polyphenolic compounds extracted from seed coat of Tamarindus indicus Linn. [Google Scholar]
- 37.Roske I., Nieber K., Oehme P. Stress-induced dependence on endogenous opioid peptides–a fundamental process in the pathophysiology of a disturbed adaptation–its influence by substance. Pharmazie. 1990;45:517–521. [PubMed] [Google Scholar]
- 38.Shi G., Leray V., Scarpignato C., Bentouimou N., Bruley des Varannes S., Cherbut C. Specific adaptation of gastric emptying to diets with differing protein content in the rat: is endogenous cholecystokinin implicated. J Gastroenterol Hepatol. 1997;41:612–618. doi: 10.1136/gut.41.5.612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Simon M., Rogler J.C., Carlos J.M., Larry G.B. Herbivore growth reduction by tannins: use of waldbauer ratio techniques and manipulation of salivary protein production to elucidate mechanisms of action. Biochem Syst Ecol. 1990;18:183–197. [Google Scholar]
- 40.Sole S.S., Srinivasan B.P. Aqueous extract of tamarind seeds selectively increases glucose transporter-2, glucose transporter-4, and islets' intracellular calcium levels and stimulates β-cell proliferation resulting in improved glucose homeostasis in rats with streptozotocin-induced diabetes mellitus. Nutr Res. 2012;32(8):626–636. doi: 10.1016/j.nutres.2012.06.015. [DOI] [PubMed] [Google Scholar]
- 41.Tran-Duy A., Smit B., Dam A., Schrama W.J. Effects of dietary starch and energy levels on maximum feed intake, growth and metabolism of Nile tilapia, Oreochromis niloticus. Aquaculture. 2008;277:213–219. [Google Scholar]
- 42.Vats V., Yadav S.P., Grover J.K. Ethanolic extract of Ocimum sanctum leaves partially attenuates streptozotocin-induced alterations in glycogen content and carbohydrate metabolism in rats. J Enthnophannacol. 2004;90:155–160. doi: 10.1016/j.jep.2003.09.034. [DOI] [PubMed] [Google Scholar]
- 43.Warren B.Z., Nicole S., Edmund C., Jenkins T.K., Urv B.T., Wayne S. Cholesterol level, statin use and Alzheimer's disease in adults with Down syndrome. Neurosci Lett. 2007;416:279–284. doi: 10.1016/j.neulet.2007.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wasan K.M., Najafi S., Wong J., Kwong M., Pritchard P.H. Assessing plasma lipid levels, body weight, and hepatic and renal toxicity following chronic oral administration of a water-soluble phystanol compound, FM-VP4, to gerbils. J Pharm Sci. 2001;4:228–234. [PubMed] [Google Scholar]
- 45.Yang M.H., Wang C.H., Chen H.L. Green, oolong and black tea extracts modulate lipid metabolism in hyperlipidemia rats fed high-sucrose diet. J Nutr Biochem. 2001;12:14–20. doi: 10.1016/s0955-2863(00)00140-6. [DOI] [PubMed] [Google Scholar]
- 46.Yao L.H., Jian Y.M., Shi J., Tomas-Barberan F.A., Datta N., Singanusong R. Flavonoids in food and their health benefits. Plant Food Hum Nutr. 2004;59:113. doi: 10.1007/s11130-004-0049-7. [DOI] [PubMed] [Google Scholar]