Abstract
Healthy body is one of the principle requirements of human beings, but the highly rated growth of harmful pathogens has challenged researchers for investigation of antimicrobial reagents. Infection of textile materials is a result of microbial adherence on the surface, and it is one of the vital clinical complications, which causes a high rate of mortality. New challenges as well as new opportunities in manufacturing of antimicrobial cellulosic textiles are the future concerns for textile and apparel industry. The major applications of antimicrobial textile could be ascribed according consumer demands, represented in more comfort, easy care, health, and durable to laundering. Such numerous properties could be achieved by the development of innovative methodologies with various finishing agents. Thus, the current review introduced an overview for the application of recent organic antimicrobial reagents in cellulosic textile finishing. The organic reagents are classified into two main categories; natural (chitosan, cyclodextrins and natural dyes) and synthetic (quaternary ammonium salts, triclosan, halogenated phenols and metal organic frameworks). The interaction between cellulose and such reagents, biological action mechanisms and factors affecting biocidal actions are all presented. For improvement of the durability and mechanical properties, pre-activation of cellulosic textile or using of cross-linkers is properly performed.
Keywords: Cellulosic textiles, Organic antimicrobial, Natural agents, Synthetic agents
Introduction
Since 1941; Textile industry had considered developing antimicrobial fibers, as people had realized that textile could act in protection of wearers against the spread of bacteria and diseases (Hirschmann and Robinson 1941). Therefore, textiles were designed to be widely used in various antimicrobial treatments and rapidly became a prerequisite to be used in hospitals, hotels, sports, and personal care industries. Textile antimicrobial finishing must not only kill undesirable microbes and inhibit the spreading of diseases, but also must realize three other basic requirements (Mao 2001); which could be pointed as follows; (1) safety: the product must be nontoxic to human and its application on textile materials must not result in skin allergy and irritation, (2) compatibility: the as-used antimicrobial finishing agent should not result in negative effects for the textile properties or appearances and must be compatible with common textile industrialization, and (3) durability: the reagent must be capable of enduring laundry, drying, and leaching (Mao 2001; Hirschmann and Robinson 1941). Medical textiles are manufactured to be applied in hygiene, healthiness, and private care as well as surgical uses. The medical textiles could be produced in knitted, woven, and non-woven structures based on their applications. Antimicrobial textile as one of medical textiles act by protecting users from hygienic problems resulting from exposure to pathogenic or odor-generating microbes, where, the growth of microorganisms results in reduction of functionality by undesirable aesthetic changes or rotting damage. Consequently, marketing of different antimicrobial textile finishing agents has dramatically increased. These antimicrobial textile finishing agents are characterized by different chemical structures, cost effectiveness, method of processing, and their environmental impact (Gao and Cranston 2008; Schindler and Hauser 2004; Dring 2003; Mahltig et al. 2005; Vigo 1983).
Cellulose is known world wide as the most abundant, renewable, and an almost inexhaustible polymeric raw material with fascinating chemical structure and properties. Additionally, cellulose is ascribed as poly-disperse linear stiff-chain homopolymer, which is composed of regio- and enantio-selectively β-1, 4-glycoside-bonded d-glucopyranose monomeric units. Cellulose polymer with its hydroxyl groups and the oxygen atoms of both the pyranose ring and the glycosidic bond, ordered hydrogen bond systems and acts in formation of various types of supra-molecular semi-crystalline structures. Therefore, cellulosic polymer is characterized by its chirality, highly functionality, biodegradability, wide chemical modifying capacity, and its capability to form versatile semi-crystalline fibers (Klemm et al. 1998, 2005).
Marketing of cellulose fibers has extensively grown in the past few years, as the expense of raising demand from the textile industry. Hence, functionalization of cellulose-based textiles is of increasing interest and a broad range of cellulose fabric products with special function can be obtained. When it comes to functional cellulosic textiles in sports and casual clothing, consumers are not only seeking fashion, but ergonomics, especially comfort and health. It is now a common practice in industrialized countries to announce new functional cellulosic textiles on a regular basis. Functionalization of cellulosic textiles has been an active research field of particular interest including antibacterial against both type Gram-positive and Gram-negative and antifungal properties (Ilić et al. 2009; Lee et al. 2003; Ahmed et al. 2015, 2018; El-Rafie et al. 2014; Emam et al. 2013, 2014, 2015a, b, 2017a, b; Ahmed and Emam 2016; Zahran et al. 2014).
Generally, antimicrobial textile finishing reagents belong could be divided to two kinds; organic and inorganic compounds, where, the as-mentioned two kinds are mainly differing in the mechanism of inhibiting or killing bacteria (Kalyon and Olgun 2001; An et al. 1996; Taylor et al. 1998; Kim et al. 2002; Kawahara et al. 2000; Yi et al. 2003; Yu et al. 2003; Top and Ülkü 2004). According to literatures (Kalyon and Olgun 2001; An et al. 1996; Kim et al. 2002; Top and Ülkü 2004), inorganic antimicrobial reagents are found to require longer time rather than organic ones. Therefore, the represented review summarizes some of the recent organic antimicrobial finishing agents for revolutionizing the field of medical cellulosic textile (fibers/fabrics) in the upcoming years. The organic antimicrobial agents can be classified into natural in origins (e.g., chitosan, cyclodextrins, and natural dyes) and synthetic in origin (quaternary ammonium salts, triclosan, halogenated phenols, and metal organic frameworks).
Functionalization strategies
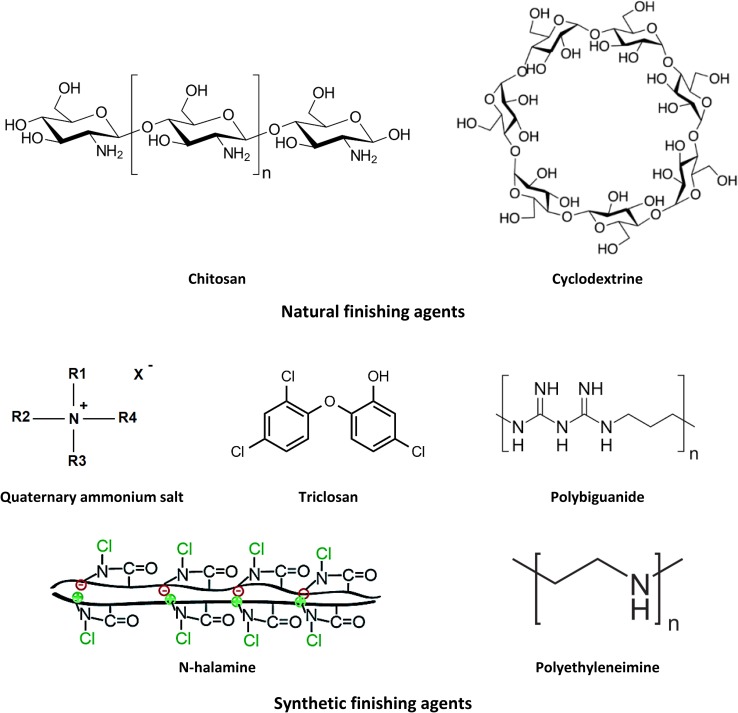
Most of the as-mentioned antimicrobial agents will be summarized in the following sections, which were basically classified into two main categories; naturally and synthetically resourced functionalizing agents. Chemical formulas of some antimicrobial agents (naturally and synthetic) were presented in Fig. 1. Due to environmental awareness, the newly acquired properties not only ascribed as the intrinsic function and long service life of the antimicrobial textile but also a manufacturing process that must be considered as environment-friendly method. Therefore, research on environment-friendly antimicrobial agents based on natural resources for cellulosic textile finishing is considered world wide and is acquiring high popularity because of the inherent properties and applications (Joshi et al. 2009; Yusuf et al. 2012, 2016). On the other hand, use of natural resources as antimicrobial agents has some limitations represented in the cost effectiveness, lower efficiency, and giving limited functions. Therefore, the researchers started to overcome the aforementioned problems by synthesizing new organic antimicrobial agents or using already synthesized materials to impart microbial resistance for textiles, named synthetically resourced antimicrobial agents.
Fig. 1.
Structures of naturally and synthetically resourced functionalizing agents for cellulosic textiles
Naturally resourced antimicrobial agents
Chitosan
Chitosan is defined as the de-acetylated derivative of chitin, which is the second most abundant polysaccharide found worldwide after cellulose. Chitosan has found to exhibit various applications in different fields including pharmaceutical and medical purposes, paper production, waste water treatment, biotechnology, cosmetics, food processing, and agriculture, in addition to cellulosic textile finishing (Shabbir and Mohammad 2016; Lim and Hudson 2003). Chitosan is employed in textile finishing for functionalization, in enhancement of dyeability and physical characters, wrinkle resistance and bioactivity (Masri et al. 1978; Rippon 1984; Bhuiyan et al. 2017; Munna et al. 2017) as summarized in Table 1. The bactericidal action of chitosan biopolymer mainly relied on positive charge density, molecular weight, concentration, hydrophilic/hydrophobic property, chelating capacity, and the physical state of the biopolymer. In addition to, ionic strength in medium, pH, temperature, reactive time and type microbial strain are also ascribed to play a role in the biocidal activities of chitosan. Kong et al. (2010). Antimicrobial activity of chitosan as biopolymer with amphoteric nature is pH dependent, and it was reached the maximum activity in the acidic medium. This is due to the better solubility of chitosan in acidic forming poly-cation. On the other hand, there are no reports approving that, chitosan has any of antimicrobial effects under basic conditions (Lim and Hudson 2004). The treatment with chitosan can significantly enhance the antimicrobial activity and physical characteristics such as abrasion resistance and crease recovery property of treated fabric samples (Bhuiyan et al. 2017). However, a slight deterioration in strength, and elongation is observed, which is probably caused by acidic treatment during surface-coating with chitosan (Bhuiyan et al. 2017). Therefore, Munna et al., used citric acid as crosslinking agent to enhance the mechanical properties of cotton fabrics during the treatment with chitosan under gamma radiation (Munna et al. 2017).
Table 1.
Antimicrobial cellulosic textiles using naturally resourced functionalizing agents
| Functionalizing agents | Functions | References |
|---|---|---|
| Chitosan | ||
| Chitosan | Antibacterial | Kong et al. (2010), Ignatova et al. (2007), Jeon et al. (2001), Hu et al. (2007a), Takahashi et al. (2008), Munna et al. (2017) |
| Chitosan | Antibacterial, enhancement of the dyeability and wrinkle resistance | Kong et al. (2010), Lim and Hudson (2004), Bhuiyan et al. (2017) |
| Nano-chitosan | Enhancement the dyeability | Chattopadhyay and Inamdar (2013) |
| Chitosan/poly(sodium-4-styrene sulfonate) | Antibacterial without affecting on breathability, flexibility or feel | Joshi et al. (2011) |
| Chitosan/poly(butyl acrylate) Chitosan/poly(Nisopropylamide) | Durable antibacterial | Ye et al. (2006) |
| Chitosan/N-halamine | Durable antibacterial | Cheng et al. (2014) |
| Methoxypolyethylene glycol-N-chitosan | Durable antibacterial | Abdel-Mohsen et al. (2012) |
| Chitosan-O-polyethylene glycol | Antimicrobial, anti-crease with comfortable sensation | Aly et al. (2010) |
| Cyclodextrins | ||
| Monochlorotriazine β-cyclodextrin | Enhance the release of essential oil | Bilia et al. (2014), Khanna and Chakraborty (2017) |
| β-Cyclodextrin | Capturing of organic pollutants | Alzate-Sánchez et al. (2016) |
| Cyclodextrin/triclosan | Antibacterial with control release | Cabrales et al. (2012) |
| β-Cyclodextrin/acrylic acid/AgNPs | Antibacterial | Hebeish et al. (2011) |
| Monochlorotriazinyl-β-cyclodextrin/acrylic acid | Antibacterial | Hebeish et al. (2014) |
| Natural dyes (plant extract) | ||
| Food wastes | Coloration | Yusuf et al. (2016) |
| Timber wastes | Coloration | Bechtold et al. (2006) |
| Agricultural industries wastes | Coloration | Shahid and Mohammad (2013) |
| Walnut shell | Coloration | Tutak and Korkmaz (2012) |
| Horse chestnut | Coloration | Tutak and Korkmaz (2012) |
| Pomegranate peel | Coloration, antibacterial, UV-protection | Tutak and Korkmaz (2012), Gawish et al. (2017) |
| Berberis vulgaris root | Coloration | Tutak and Korkmaz (2012) |
| Thyme | Coloration | Tutak and Korkmaz (2012) |
| Sage tea | Coloration | Tutak and Korkmaz (2012) |
| Henna leaves | Coloration, UV-protection | Tutak and Korkmaz (2012), Hasan et al. (2015), Omer et al. (2015) |
| Madder root | Coloration, UV-protection | Tutak and Korkmaz (2012), Gawish et al. (2016) |
| Aloe vera | Coloration, antibacterial | Ammayappan and Moses (2009) |
| Curcumin | Coloration, antibacterial | Ammayappan and Moses (2009), Gawish et al. (2017) |
| Cutch | Coloration, antibacterial | Gawish et al. (2017) |
| Red onion peel | Coloration, UV-protection | Gawish et al. (2016, 2017) |
| Mediterranean flora | Coloration, UV-protection | Grifoni et al. (2014) |
| Chamomile | Coloration, UV-protection | Gawish et al. (2016) |
| Chitosan–curcumin | Antioxidant, antibacterial, coloration | Zemljič et al. (2014) |
It has been reported that, water-soluble chitosan derivatives prepared by saccharization, alkylation, acylation, quaternization and metallization were found to exhibit higher antimicrobial effect than the native one (Xie et al. 2007; Ignatova et al. 2007). Chemical modification in the structural form of chitosan by incorporating certain groups with higher charge density, such as asparagine N-conjugated chitosan oligosaccharide and guanidinylated chitosan (Jeon et al. 2001; Hu et al. 2007a), was reported to acquire chitosan for higher antimicrobial action. In contrast to, N-carboxyethyl chitosan failed in showing any antimicrobial properties, which could be attributed to lacking free amino groups (Yancheva et al. 2007).
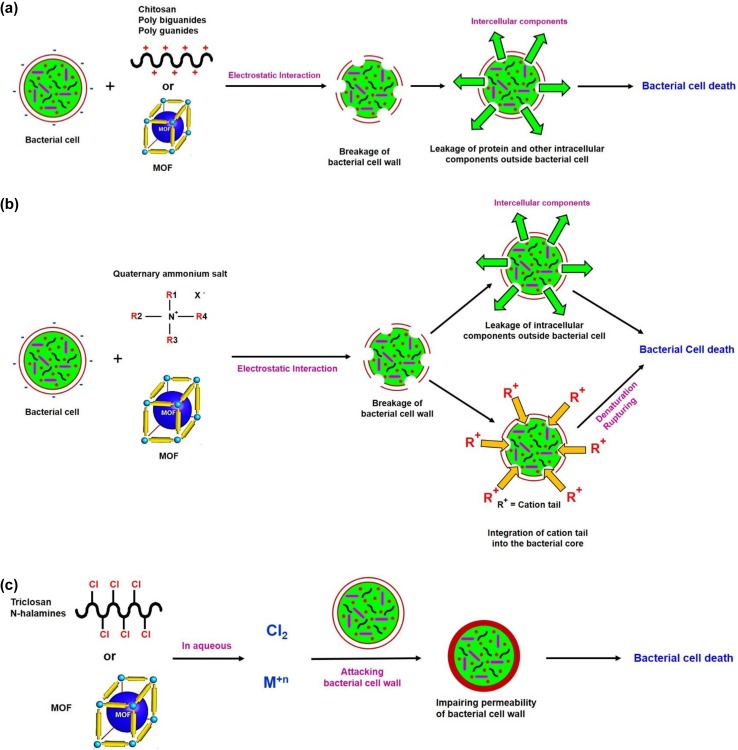
It is well known that, positive charge density shows enhanced electrostatic interaction of biopolymers with bacterial cells (Fig. 2a). Increment the degree of deacetylation for chitosan was found to increase the charge density for higher electrostatic interaction and thus enhanced biocidal activities (Takahashi et al. 2008). The interaction between positively charged chitosan macromolecules and the negatively charged bacterial cell membranes results in the leakage of protein and other intracellular components. Such ascribed interaction is mainly dependent on pH. At a pH less than pKa, the protonation of amino groups of chitosan takes a place which in turn results in electrostatic interaction between polymer and microbial cell wall. But at a pH higher than pKa, the hydrophobic interaction and chelation were reported to be responsible for chitosan antimicrobial activity (Hu et al. 2007b; Kong et al. 2008).
Fig. 2.
Interaction of quaternary ammonium salts with cellulose
Cellulosic fabrics are characterized by being less susceptible for effective dyeing and this disadvantage was overcome using chitosan as finishing agent before dyeing processing. Where, both cotton and chitosan are basically characterized by similar structures, and thus, their molecular backbones are bonded through Van der Waals forces and cross-linking by the formation of Schiff base between cellulosic reducing end groups and the amino groups of chitosan (Rippon 1984). Due to the cationic nature of chitosan in acidic conditions, it is found to exhibit ease of absorption with anionic dyes such as direct, acid, and reactive dyes through electrostatic attraction. Additionally, wrinkle resistance as another function can be acquired by cellulosic textiles by chitosan treatment, where, wrinkle resistance can be effectively enhanced as durable press finishing in cellulosic fabrics (Younsook and Dong 1998). The mechanical properties of cotton fabrics are negatively affected by the treatment and are oppositely proportional with the molecular weight of chitosan (Younsook and Dong 1998). The shrinkage resistance is linearly proportional with the molecular weight of chitosan used in the treatment of cellulosic textiles (Julia et al. 1998). The most interesting property imparted to cellulosic textiles by treatment with chitosan macromolecules is the antimicrobial activities, as the chitosan-finished cellulosic textiles were found to be effectively applicable as medical textiles (Shin et al. 1999; Lee et al. 1999; Zhang et al. 2003; Joshi et al. 2011; Naebe et al. 2016). Chitosan was mixed with anionic polyelectrolyte of poly(sodium-4-styrene sulfonate) for layer-by-layer coating of cotton textile to impart antimicrobial properties without affecting textile breathability, flexibility or feel (Joshi et al. 2011). Naebe et al., pre-activated the cotton fabrics by atmospheric helium/oxygen plasma to increase the adsorption amount of chitosan (Naebe et al. 2016). Dye uptake, wash fastness and antibacterial potency for the cotton fabrics were all improved by treatment with nano-chitosan (Chattopadhyay and Inamdar 2013). The treatment with chitosan did not affect the mechanical properties of cotton fabric, while the finishing with nano-chitosan resulted in slight improvement in tenacity and observable decrement in elongation at break (Chattopadhyay and Inamdar 2013). For durable antibacterial finishing, chitosan-poly(butyl acrylate) and chitosan-poly(N-isopropylamide) in the form of core–shell particle was applied onto cotton fabrics using pad-dry-cure technique (Ye et al. 2006), while, modified chitosan by N-halamine was used (Cheng et al. 2014). Break tensile strength and elongation for fabrics are both improved after functionalization and use of crosslinkers resulted in further improvement in the mechanical properties (Ye et al. 2006), while using of chitosan/N-halamine led to decrease in the breaking strength (Cheng et al. 2014). Abdel-Mohsen et al., reported a method for biomedical functionalization of cotton fabrics based on use of methoxypolyethylene glycol-N-chitosan graft copolymer in the presence of citric acid (Abdel-Mohsen et al. 2012). Chitosan-O-polyethylene glycol/citric acid was used as multifunctional finishing agent (antimicrobial, anti-crease and comfortable sensation) for cotton fabrics (Aly et al. 2010). Due to use of citric acid as crosslinkers, the mechanical properties of cotton fabrics are improved after functionalization (Aly et al. 2010; Abdel-Mohsen et al. 2012).
Cyclodextrins
Cyclodextrins have internal lipophilic cavities and external hydrophilic surfaces and consequently, the main activity of cyclodextrins is basically related to their capacity for including various guest molecules (Bilia et al. 2014; Alzate-Sánchez et al. 2016). Cotton was grafted with monochlorotriazine β-cyclodextrin to improve the release properties of essential oils (Bilia et al. 2014; Khanna and Chakraborty 2017). The tensile strength of cotton fabrics was insignificantly changed by the grafting with monocholortriazine β-cyclodextrin; however, the grafting yield exceeded 13% (Khanna and Chakraborty 2017). Porous β-cyclodextrin was covalently bonded with cotton fabric for capturing organic pollutants from the contaminated environment (water and air) (Alzate-Sánchez et al. 2016). The complexation or release of substances by fixed cyclodextrins can also be employed for medical purposes such as medical diagnostics by testing substrates from sweat of patients encapsulated via cyclodextrins (Buschmann et al. 2001). Interactive cyclodextrin derivatives, were found to intensively bond to cellulosic backbone of cotton textiles (Cabrales et al. 2012; Hebeish et al. 2011, 2014).
Inclusion substrates like cyclodextrins were reported to be successively grafted to cotton textiles, followed by inclusion of triclosan as antimicrobial agent to control its release capabilities, and the treated cotton was found to exhibit excellent action (Cabrales et al. 2012). Durable antibacterial cotton fabrics were performed by Hebeish et al., via applying a β-cyclodextrin-containing composite (Hebeish et al. 2011, 2014). β-cyclodextrin-grafted poly acrylic acid copolymer was used in preparation of nanosilver and the composite was applied onto fabrics (Hebeish et al. 2011), while cotton fabrics were pretreated with reactive copolymer of monochlorotriazinyl-β-cyclodextrin-grafted acrylic acid or β-cyclodextrin grafted acrylic acid prior to in situ reduction of nanosilver (Hebeish et al. 2014).
Natural dyes
Dyes have been applied before the progression of textile functionalization industry to prepare finished textiles with simultaneous coloration. Color shading of textile materials by synthetic dyes is necessary for the textile industry. However, the discharge of these dyes from textile industry in wastewater was found to be greatly dangerous to the aquatic flora and fauna due to their toxicity, non-biodegradability and their carcinogenic effects. Therefore, dyes extracted from natural origin like plants gained a high popularity over synthetic ones (Shabbir and Mohammad 2016; Yusuf et al. 2012; Ghaheh et al. 2014). These materials were mainly advantageous with lower cost effectiveness, giving a new generation of textile dyes and add new functions such as antimicrobial activities (Table 1). Additionally, the application of by-products and wastes from food, timber and agricultural industries can be expressed to be good alternatives for synthetic dyes (Bechtold et al. 2006; Shahid and Mohammad 2013; Yusuf et al. 2016).
Cellulosic textiles could be dyed by natural dyes through the coordination bonding of cellulose molecules with the aid of various metal ions. Most of the natural dyes require the use of a mordant (e.g., copper sulphate, ferric sulphate, potassium aluminum sulphate), to have a coloring power on cellulosic textiles (Tutak and Korkmaz 2012; Hasan et al. 2015). Pre- and post-mordanting could be performed to improve the fastness properties (Tutak and Korkmaz 2012; Hasan et al. 2015). By changing the applied extract or mordant, different resulted colors could be obtained. Furthermore, the finished cellulosic textiles were reported to exhibit antimicrobial potency (Ammayappan and Moses 2009; Gawish et al. 2017). Cotton fabrics treated with Aloe vera as a natural colorant, exhibited antibacterial activity higher than that treated with chitosan (Ammayappan and Moses 2009). Extracts of chamomile, red onion peel, madder, Mediterranean flora and henna leaves were used for coloring the cotton fabrics by imparting UV-protective and/or antibacterial properties (Gawish et al. 2016, 2017; Grifoni et al. 2014; Omer et al. 2015). Beside coloration, the effect of mordant on the added functions (antimicrobial activity and UV-protection) was studied for different dyed fabrics including cotton (Gawish et al. 2017). By incorporation of chitosan–curcumin, antioxidant, and antibacterial functionalized viscose fabrics were produced (Zemljič et al. 2014).
Synthetically resourced functionalizing agents
Quaternary ammonium salts
Quaternary ammonium salts as a type of functionalizing agent with molecular formula of nitrogen in cationic form, are ascribed as active reagents against different species of microorganisms. The biological activities of quaternary ammonium salts are principally affected by the length of the alkyl chain, the presence of the per-halogenated group and the number of cationic ammonium groups in the molecule (Shabbir and Mohammad 2016; Simoncic and Tomsic 2010). According to the literature (Gilbert and Moore 2005) quaternary ammonium salts with alkyl chain composed of 12–14 of alkyl groups were found to exhibit excellent bactericidal action against Gram-positive bacterial strains. While, alkyl chain with 14–16 alkyl groups showed better biocidal action against Gram-negative bacteria. Initial interaction between quaternary ammonium salts and bacterial wall is reported to be a result of electrostatic interaction between positively charged quaternary ammonium salt and the negatively charged microbial membranes (Fig. 2b). This is followed by the integration of quaternary ammonium salt hydrophobic tail into the bacterial hydrophobic membrane core, where it acts in denaturation of structural proteins and enzymes. Additionally, quaternary ammonium salts were found to induce dose- and time-dependent ultra-structural changes in antibiotic-resistant Escherichia coli (E. coli) (Ioannou et al. 2007). On the other hand, counter ion which is strongly bonded to quaternary ammonium salts was found to cause lower biocidal action, which could be due to slow and lower release of free ions into the surrounding medium (Chen et al. 2000).
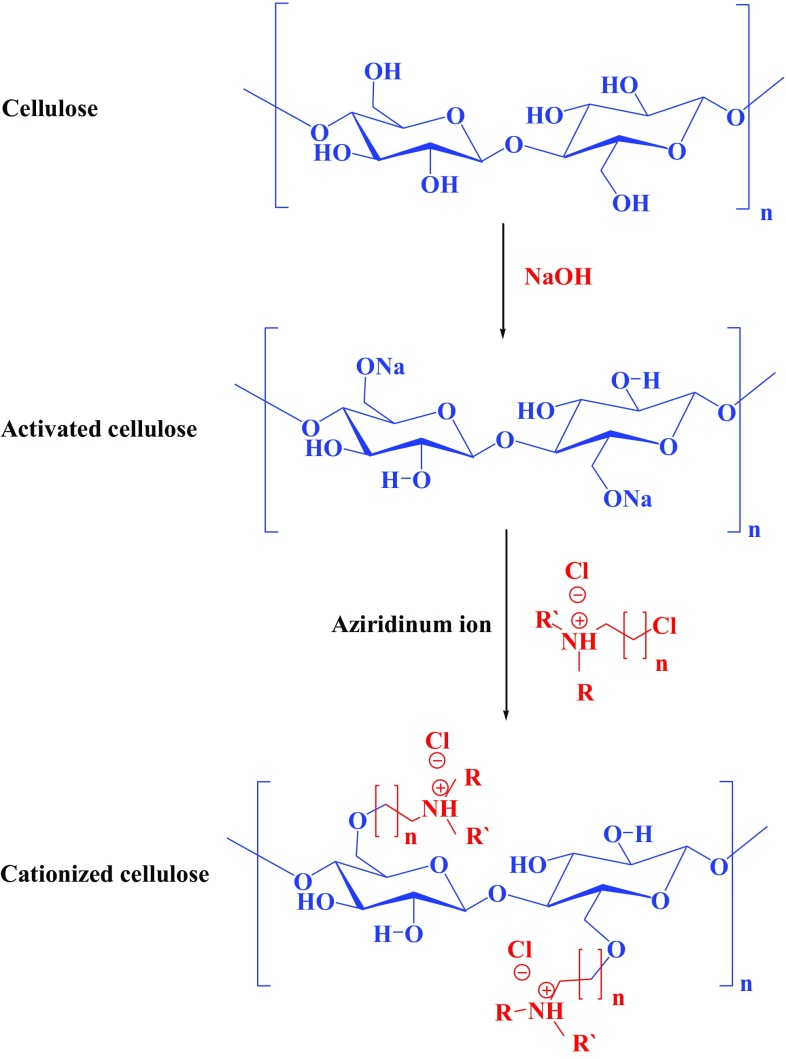
Many research works interested in the functionalization of cellulosic textiles were focused on modification of cellulose by different quaternary ammonium salts (Table 2). Figure 3 shows the interaction between cellulose and quaternary ammonium salts. Due to the loss of the physical bonding and leaching of these salts, the treated textiles with quaternary ammonium salts have poor washing durability (Simoncic and Tomsic 2010). Polymerizable quaternary ammonium salts with acrylate or methacrylate groups were carried out and sol–gel method for uploading/fixing on textile surfaces was reported (Owusu-Adom and Guymon 2008; Caillier et al. 2009). A more recent report on a novel environmentally friendly antimicrobial cotton fabrics finished with reactive siloxane sulfopropyl-betaine was studied to extend the research of quaternary ammonium salts (Chen et al. 2011). The results showed that the finished cotton fabrics exhibit good biological activity against bacteria and fungi, being nontoxic to animals and also does not induce skin stimulation. In addition to antibacterial activity and enhancement the hydrophobicity, the finishing with siloxane sulfopropyl-betaine is accompanied by large improvement in tensile strength of cotton textiles (Chen et al. 2011). Moreover, quaternary ammonium salts were used to cationize the cellulosic textile to easily dye with anionic dyes. A 3-chloro-2-hydroxypropyl trimethylammonium chloride, polyamino chlorohydrin quaternary ammonium, 2,3-epoxypropyl trimethyl ammonium chloride and glycidyltrimethyl ammonium chloride were used to modify cotton fabrics to improve its dyeing with salt-free reactive dye to overcome the problem of slat usage (Hauser and Tabba 2001; Hashem et al. 2003; Acharya et al. 2014; Arivithamani and Dev 2017a, b; Ramasamy and Kandasaamy 2005; Ma et al. 2017; Kamel et al. 2009, 2011).
Table 2.
Antimicrobial cellulosic textiles based on synthetically resourced functionalizing agents
| Functionalizing agents | Functions | References |
|---|---|---|
| Quaternary ammonium salts | ||
| Quaternary ammonium surfactant/poly acrylate/clay | Antibacterial | Owusu-Adom and Guymon (2008) |
| Quaternary ammonium group/methacrylic acid | Antibacterial | Caillier et al. (2009) |
| Siloxane sulfopropyl-betaine | Antimicrobial, enhancement of hydrophobicity and tensile strength | Chen et al. (2011) |
| 2,3-Epoxypropyl trimethyl ammonium chloride | Enhancement the dyeability | Hauser and Tabba (2001) |
| 3-Chloro-2-hydroxypropyl trimethylammonium chloride | Enhancement the dyeability | Hashem et al. (2003), Acharya et al. (2014), Arivithamani and Dev (2017a, b), Ramasamy and Kandasaamy (2005), kamel et al. (2011) |
| Polyamino chlorohydrin quaternary ammonium | Enhancement the dyeability | Ramasamy and Kandasaamy (2005), Kame et al. (2009) |
| Glycidyl trimethyl ammonium chloride | Enhancement the dyeability | Ma et al. (2017) |
| Triclosan | ||
| Triclosan/Ag | Antimicrobial | Dhiman and Chakraborty (2015) |
| Triclosan/methyl ester-containing silicon | Antimicrobial and hydrophobic | O’Lenick (2002) |
| Triclosan/dimethyl dihydroxy ethylene urea | Antibacterial, anti-crease, enhanced surface softness | Ibrahim et al. (2010) |
| Triclosan/butan tetracarboxylic acid or citric acid | Durable antibacterial | Orhan et al. (2009) |
| Triclosan/β-cyclodextrin | Durable antimicrobial | Peila et al. 2013 |
| Triclosan/polylactide microspheres | Antibacterial | Goetzendorf-Grabowska et al. (2004) |
| Triclosan/melamine–formaldehyde | Antimicrobial | Ocepek et al. (2012) |
| Triclosan/AgNPs-PVP/microwave curing | Antimicrobial and coloration | Ibrahim et al. (2013a) |
| Triclosan/chitosan/Aloe vera/TiO2-NPs/silicone softener | Antimicrobial, UV-protection, soft-handle, water repellent | Ibrahim et al. (2013b) |
| N-halamines | ||
| Methylol-5,5-dimethylhydantoin | Antimicrobial | Schindler and Hauser (2004), Vigo (2001) |
| Polybiguanides | ||
| Poly(hexamethylene biguanide) | Antimicrobial | Zhao and Chen (2016), Gao and Cranston (2008), Blackburn et al. (2006) |
| Polyethyleneimine | ||
| N-Alkyl-polyethyleneimine | Antimicrobial, antibiotic | Milović et al. (2005), Lin et al. (2003) |
| Metal organic frameworks | ||
| Copper-benzene tri-carboxylic acid (Cu-BTC) | Antibacterial | Rodríguez et al. (2014), Wang et al. (2015) |
Fig. 3.
Schematic diagram illustrated the antibacterial action mechanism of the organic antimicrobial agents used in finishing of cellulosic textiles
Triclosan
Production of biologically active reagents including triclosan or halogenated phenols is motivated by increment in consumer requirement. Triclosan (2, 4, 4′-trichloro2′-hydroxydiphenyl ether) is a highly effective biological reagent against most of the bacterial species (Fig. 2c); however, it has poor antifungal properties. Triclosan and its derivatives are highly preferable for textile finishing due to its low skin toxicity, in addition to its antimicrobial action (Gao and Cranston 2008). Therefore, triclosan was applied in household materials for antimicrobial action and in cellulosic textile finishing also simultaneously for the same purpose as presented in Table 2. Dhiman and Chakraborty, studied the usage of triclosan, triclosan with Ag-dispersed metal and triclosan–chitosan composite in functionalization of cotton fabrics via pad-dry-cure method (Dhiman and Chakraborty 2015). The best antimicrobial results were detected for the fabrics treated with triclosan/Ag-dispersed metal and the mechanical properties of the finished cotton fabrics are inconsiderably affected, whatever the antimicrobial agent applied (Dhiman and Chakraborty 2015). Triclosan as a finishing agent is found to exhibit low durability to washing because it is highly volatile at high temperatures and highly soluble at high pH (O’Lenick 2002). Triclosan was employed for manufacturing silicone-functionalized antimicrobial compounds, through the reaction with certain methyl ester-containing silicone compounds, where the final product was found to be greater in washing durability and have long-lasting antimicrobial finish (O’Lenick 2002). The as-mentioned compounds could be successively used in personal care and textile functionalization, with greater long-lasting durable antimicrobial, germicidal, and fungicidal actions (Li 2001). Treatment of cotton fabrics with triclosan in combination with dimethyl dihydroxy ethylene urea produced finished fabrics with superior antibacterial and anti-crease activities, and the fabric softness was enhanced (Ibrahim et al. 2010). A study for the application of triclosan was reported for durable antibacterial finishing of cotton/polyester blended fabrics through pad-dry-cure method (Ibrahim et al. 2010).
The addition of butane tetracarboxylic acid and citric acid enhanced the durability of the antimicrobial coating against laundering (Orhan et al. 2009). The treatment with polycarboxylic acid alone exhibited decrement in mechanical properties of cotton fabrics with increment in acid concentration. However, the treatment with triclosan/polycarboxylic acid does not significantly affect the mechanical properties of fabrics (Orhan et al. 2009). Peila’s et al., reported using two different ways of making antimicrobial cotton fabrics through adding triclosan into β-cyclodextrin cavity (Peila et al. 2013). The β-cyclodextrin-treated cotton fabrics followed by finishing with triclosan to produce fabrics with durable antimicrobial properties. Non-woven viscose fabrics were finished with triclosan encapsulated in biodegradable polylactide as a carrier (Goetzendorf-Grabowska et al. 2004). Good antimicrobial cotton fabrics without substantially changing in its properties, were obtained via treatment with melamine–formaldehyde polymer micro-capsulated with triclosan using screen printing (Ocepek et al. 2012). Due to use of melamine–formaldehyde, the mechanical properties of cotton fabrics are not substantially changed by treatment (Ocepek et al. 2012). For cellulosic fabrics’ pigmentation with acquiring antimicrobial activity, triclosan was first incorporated to the fabrics and then followed by microwave curing (Ibrahim et al. 2013a). Moreover, print paste including chitosan, Aloe vera, triclosan, TiO2-NPs and silicone softener was applied onto cotton/wool and viscose/wool-blended fabrics followed by microwave activation (Ibrahim et al. 2013b). The produced fabrics showed multi-functional properties represented in antimicrobial activity, UV-protection, soft-handle and water-repellent property.
N-halamines
N-halamines are well defined as heterocyclic compounds which contain, in the main skeleton, one or two covalent bonds between nitrogen and a halide, where the halide is usually chloride. N-halamines were reported to be characterized by biocidal activities against a broad spectra of bacteria, fungi, and viruses (Worley et al. 1988; Sun et al. 1995; Shabbir and Mohammad 2017). The antimicrobial action of such referred class of organic finishing agents is correlated to the halogen atom as presented in Fig. 2c. These oxidizing halogens act in direct transferring of and dissociation to give free halogen in aqueous media, where, these reactive free halogens play the role of inhibiting or inactivating the bacterial cell (Denyer and Stewart 1998). Due to the as-explained biocidal action of N-halamines, such compounds were successively applied as powerful antimicrobial functionalizing agent for cellulosic textile finishing (Table 2). Certain compounds were incorporated to improve the washing durability. Schindler and Hauser reported that, antimicrobial cellulosic textiles were produced by treatment with methylol-5,5-dimethylhydantoin in the presence of hypochlorite, results in the formation of chloramines on the fiber surface (Schindler and Hauser 2004). However, high concentration of chloramines was deposited on cellulosic surface, gave problems of fabric yellowness and strength-loss (Vigo 2001).
Polybiguanides
Polybiguanides as polymeric polycationic amines, is mainly composed of cationic biguanide repeating units with hydrocarbon chain linkers of identical or dissimilar length. Compared to N-halamines, polybiguanides exhibited much greater antimicrobial properties (Zanoaga and Tanasa 2014). Polyguanidines and polybiguanides are well known as a vital class of biocidal polymers, due to its high hydrophilicity, excellent biocidal efficiency against wide microbial spectrum and non-toxicity. It was reported that, when acrylate monomers were associated with biguanide groups, it exhibited good antibacterial action, owing to the electrostatic interaction with microbial cell membranes as schematic in Fig. 2a. Additionally, it displayed higher antimicrobial activity against Gram-positive bacteria rather than Gram-negative bacteria; this could be attributed to less complicated structure of Gram-positive bacteria, which facilitate the penetration of high molecular-weighted biocidal polymer (Ikeda et al. 1984). Therefore, recent approach has studied their application as new generation of antimicrobial finishing agents for textile functionalization. Cationic biguanide-interactive groups can bind with the negatively charged groups (COOH) of cellulose. One of the most applicable biguanide-based polymers is poly(hexamethylenebiguanide) (PHMB), because of its low toxicity and water solubility. Hence, padding and exhaustion as conventional application methods were used for applying PHMB onto cellulosic textile to imply antimicrobial potency (Zhao and Chen 2016; Gao and Cranston 2008; Blackburn et al. 2006) (Table 2).
Polyethyleneimine
Polyethyleneimine (PEI) is well known as a non-biodegradable, cationic and synthetic polymer containing primary, secondary, and tertiary amino functional groups. It is manufactured in both branched and linear-shaped polymer via acid-catalyzed polymerization of aziridine and ring opening polymerization of 2-ethyl-2-oxazoline followed by hydrolysis (Brissault et al. 2003; Samal et al. 2012). Due to the presence of amino groups in the backbone of polyethyleneimine, it has been widely exposed to chemical modifications for acquiring some of desirable physicochemical properties. It was reported that, the un-substituted polyethyleneimine did not exhibit any of antimicrobial activities. However, it was realized that hydrophobicity and positive charge density are vital requirements for antimicrobial activity. Therefore, polyethyleneimine was modified to be incorporated with alkyl groups to potentiate both of these requirements (Lin et al. 2002). Different researches studied certain methods for incorporation of N-alkyl-polyethyleneimine in all commercial plastics, textiles, and glass, for acquiring these immobilized surfaces complete inactivation of bacteria (both waterborne and airborne bacteria), and fungi (including pathogenic and antibiotic-resistant strains), where, the biocidal action was interoperated via cell-membrane rupturing (Milović et al. 2005). N-alkylated polyethyleneimine was also reported to be immobilized over woven cotton textiles (Table 2), and the modified textiles were found to exhibit strong antimicrobial properties against numerous airborne Gram-positive and Gram-negative bacterial strains. The application of high molecular weighted polyethyleneimine resulted in acquirement of excellent antimicrobial activity; while the application of low molecular-weighted polymer displayed negligible activities (Lin et al. 2003).
Metal organic frameworks
The metal organic frameworks (MOFs) are the coordination polymers, composed of the inorganic center presented in metal ion and organic coordinating linker presented in poly-functional organic acid (Abdelhameed et al. 2017c, 2018b). Porosity, crystalline feature, different dimensional structures (1D, 2D, 3D) and high surface area are characteristic properties which permit MOFs to be widely applicable in diverse fields (Horcajada et al. 2010; Sumida et al. 2011; Hu et al. 2014; Liu et al. 2014; Abdelhameed et al. 2017a, b). The insertion of MOFs within cellulosic textiles was recently studied to impart various functions (Abdelhameed et al. 2016, 2017d, e, 2018a; Emam and Abdelhameed 2017a, b; Emam et al. 2018a). Concerning antimicrobial applications, cobalt imidazolate metal–organic frameworks were used as biocidal materials (Quirós et al. 2015; Aguado et al. 2014). The biocidal activity of MOFs, mainly corresponds to the release of metal from the bulk MOFs and subsequently it is linearly proportional with the ease of metal release (Wyszogrodzka et al. 2016). Furthermore, the biological active ligands and incorporation of active substances both exhibited biocide effect (Wyszogrodzka et al. 2016). The action of MOFs ingredients to kill bacterial cell could occur by destruction of bacterial cell wall, penetration inside bacteria and interaction with protein, or disruption of bacterial cell wall permeability (Wyszogrodzka et al. 2016; Quirós et al. 2015; Lu et al. 2014; Zhuang et al. 2012). In case of biocidal textiles by MOFs material, few studies were published. MOF based on copper-benzene tri-carboxylic acid (Cu-BTC) was introduced inside synthetic and proteinic textiles to acquire antimicrobial activity (Emam et al. 2018b; Abbasi et al. 2012). As shown in Table 2, antibacterial cellulosic materials were produced by the direct generation of Cu-BTC MOF within the fibers’ structure (Rodríguez et al. 2014; Wang et al. 2015). The work in biological active cellulosic textiles based on MOFs materials is still limited and just few studies were found in literature, and hence it could be prophesied that MOFs will be largely used in near future to produce antimicrobial cellulosic textiles.
Conclusion
The current review provides an overview about the recent advancements in antimicrobial finishing of cellulosic textile. The represented antimicrobial reagents offer prolonged biocidal actions without environmental toxicity. The emergence of microbial resistant species is one of the vital defects with small molecular antibiotics as a result of their specific targets of action; however, in case of the currently reviewed antimicrobial reagents; physically destroying microbial cell membranes is reported to be the main route for preventing the microbial growth. In this review, the application of organic antimicrobial finishing agents (natural and synthetic in origin), as one of the main classes for antimicrobial textile finishing agents, has been widely illustrated. The natural origin-based textile antimicrobial agents were revealed to be environment-safe, such as chitosan, cyclodextrins, and natural dyes. The application of synthetic resourced antimicrobial agents, such as, polybiguanides, N-halamines, quaternary ammonium salts, polyethyleneimine, and metal organic frameworks were also well discussed. From all the previously mentioned antimicrobial agents, quaternary ammonium salts and metal organic frameworks, could be predicted to be the most commonly applicable organic compounds in future perspectives, and still not widely studied for biologically active cellulosic textiles.
Compliance with ethical standards
Conflict of interest
Author wants to declare that no scientific or financial conflicts of interest exist.
References
- Abbasi AR, Akhbari K, Morsali A. Dense coating of surface mounted CuBTC metal–organic framework nanostructures on silk fibers, prepared by layer-by-layer method under ultrasound irradiation with antibacterial activity. Ultrasonics Sonochem. 2012;19(4):846–852. doi: 10.1016/j.ultsonch.2011.11.016. [DOI] [PubMed] [Google Scholar]
- Abdelhameed RM, Abdel-Gawad H, Elshahat M, Emam HE. Cu–BTC@ cotton composite: design and removal of ethion insecticide from water. RSC Adv. 2016;6(48):42324–42333. [Google Scholar]
- Abdelhameed R, Abdel-Gawad H, Silva C, Rocha J, Hegazi B, Silva A. Kinetic and equilibrium studies on the removal of 14 C-ethion residues from wastewater by copper-based metal–organic framework. Int J Environ Sci Technol. 2017;15:2283–2294. [Google Scholar]
- Abdelhameed RM, Abdel-Gawad H, Taha M, Hegazi B. Separation of bioactive chamazulene from chamomile extract using metalorganic framework. J Pharm Biomed Anal. 2017;146:126–134. doi: 10.1016/j.jpba.2017.08.020. [DOI] [PubMed] [Google Scholar]
- Abdelhameed RM, Ananias D, Silva AM, Rocha J. Building light-emitting metalorganic frameworks by post-synthetic modification. Chem Sel. 2017;2(1):136–139. [Google Scholar]
- Abdelhameed RM, Emam HE, Rocha J, Silva AM. Cu-BTC metalorganic framework natural fabric composites for fuel purification. Fuel Process Technol. 2017;159:306–312. [Google Scholar]
- Abdelhameed RM, Kamel OM, Amr A, Rocha J, Silva AM. Antimosquito activity of a titanium–organic framework supported on fabrics. ACS Appl Mater Interfaces. 2017;9(27):22112–22120. doi: 10.1021/acsami.7b03164. [DOI] [PubMed] [Google Scholar]
- Abdelhameed RM, Rehan M, Emam HE. Figuration of Zr-based MOF@ cotton fabric composite for potential kidney application. Carbohyd Polym. 2018;195:460–467. doi: 10.1016/j.carbpol.2018.04.122. [DOI] [PubMed] [Google Scholar]
- Abdelhameed RM, Tobaldi DM, Karmaoui M. Engineering highly effective and stable nanocomposite photocatalyst based on NH2-MIL-125 encirclement with Ag3PO4 nanoparticles. J Photochem Photobiol A. 2018;351:50–58. [Google Scholar]
- Abdel-Mohsen A, Aly A, Hrdina R, Montaser A, Hebeish A. Biomedical textiles through multifunctioalization of cotton fabrics using innovative methoxypolyethylene glycol-N-chitosan graft copolymer. J Polym Environ. 2012;20(1):104–116. [Google Scholar]
- Acharya S, Abidi N, Rajbhandari R, Meulewaeter F. Chemical cationization of cotton fabric for improved dye uptake. Cellulose. 2014;21(6):4693–4706. [Google Scholar]
- Aguado S, Quirós J, Canivet J, Farrusseng D, Boltes K, Rosal R. Antimicrobial activity of cobalt imidazolate metal–organic frameworks. Chemosphere. 2014;113:188–192. doi: 10.1016/j.chemosphere.2014.05.029. [DOI] [PubMed] [Google Scholar]
- Ahmed HB, Emam HE. Layer by layer assembly of nanosilver for high performance cotton fabrics. Fibers Polymers. 2016;17(3):418–426. [Google Scholar]
- Ahmed HB, El-Rafie MH, Zahran MK. Bactericidal evaluation of nano-coated cotton fabrics. Am J Nano Res Appl. 2015;3(6):105–112. [Google Scholar]
- Ahmed HB, Emam HE, Mashaly HM, Rehan M. Nanosilver leverage on reactive dyeing of cellulose fibers: Color shading, color fastness and biocidal potentials. Carbohydr Polym. 2018;186:310–320. doi: 10.1016/j.carbpol.2018.01.074. [DOI] [PubMed] [Google Scholar]
- Aly A, Abdel-Mohsen A, Hebeish A. Innovative multifinishing using chitosan-O-PEG graft copolymer/citric acid aqueous system for preparation of medical textiles. J Text Inst. 2010;101(1):76–90. [Google Scholar]
- Alzate-Sánchez DM, Smith BJ, Alsbaiee A, Hinestroza JP, Dichtel WR. Cotton fabric functionalized with a β-cyclodextrin polymer captures organic pollutants from contaminated air and water. Chem Mater. 2016;28(22):8340–8346. [Google Scholar]
- Ammayappan L, Moses JJ. Study of antimicrobial activity of aloevera, chitosan, and curcumin on cotton, wool, and rabbit hair. Fibers Polym. 2009;10(2):161–166. [Google Scholar]
- An Y, Stuart G, McDowell S, McDaniel S, Kang Q, Friedman R. Prevention of bacterial adherence to implant surfaces with a crosslinked albumin coating in vitro. J Orthop Res. 1996;14(5):846–849. doi: 10.1002/jor.1100140526. [DOI] [PubMed] [Google Scholar]
- Arivithamani N, Dev VRG. Cationization of cotton for industrial scale salt-free reactive dyeing of garments. Clean Technol Environ Policy. 2017;19(9):2317–2326. [Google Scholar]
- Arivithamani N, Dev VRG. Sustainable bulk scale cationization of cotton hosiery fabrics for salt-free reactive dyeing process. J Clean Prod. 2017;149:1188–1199. [Google Scholar]
- Bechtold T, Mussak R, Mahmud-Ali A, Ganglberger E, Geissler S. Extraction of natural dyes for textile dyeing from coloured plant wastes released from the food and beverage industry. J Sci Food Agric. 2006;86(2):233–242. [Google Scholar]
- Bhuiyan MR, Hossain M, Zakaria M, Islam M, Uddin MZ. Chitosan coated cotton fiber: physical and antimicrobial properties for apparel use. J Polym Environ. 2017;25(2):334–342. [Google Scholar]
- Bilia AR, Guccione C, Isacchi B, Righeschi C, Firenzuoli F, Bergonzi MC. Essential oils loaded in nanosystems: a developing strategy for a successful therapeutic approach. Evid Based Complement Altern Med. 2014;2014:651593. doi: 10.1155/2014/651593. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Blackburn RS, Harvey A, Kettle LL, Payne JD, Russell SJ. Sorption of poly (hexamethylenebiguanide) on cellulose: mechanism of binding and molecular recognition. Langmuir. 2006;22(13):5636–5644. doi: 10.1021/la053002b. [DOI] [PubMed] [Google Scholar]
- Brissault B, Kichler A, Guis C, Leborgne C, Danos O, Cheradame H. Synthesis of linear polyethylenimine derivatives for DNA transfection. Bioconj Chem. 2003;14(3):581–587. doi: 10.1021/bc0200529. [DOI] [PubMed] [Google Scholar]
- Buschmann H-J, Knittel D, Schollmeyer E. New textile applications of cyclodextrins. J Incl Phenom Macrocycl Chem. 2001;40(3):169–172. [Google Scholar]
- Cabrales L, Abidi N, Hammond A, Hamood A. Cotton fabric functionalization with cyclodextrins. Surfaces. 2012;6:8. [Google Scholar]
- Caillier L, de Givenchy ET, Levy R, Vandenberghe Y, Géribaldi S, Guittard F. Synthesis and antimicrobial properties of polymerizable quaternary ammoniums. Eur J Med Chem. 2009;44(8):3201–3208. doi: 10.1016/j.ejmech.2009.03.031. [DOI] [PubMed] [Google Scholar]
- Chattopadhyay D, Inamdar M. Improvement in properties of cotton fabric through synthesized nano-chitosan application. Indian J Fibre Textile Res. 2013;38(1):14–21. [Google Scholar]
- Chen CZ, Beck-Tan NC, Dhurjati P, van Dyk TK, LaRossa RA, Cooper SL. Quaternary ammonium functionalized poly (propylene imine) dendrimers as effective antimicrobials: structure—activity studies. Biomacromolecules. 2000;1(3):473–480. doi: 10.1021/bm0055495. [DOI] [PubMed] [Google Scholar]
- Chen S, Chen S, Jiang S, Xiong M, Luo J, Tang J, Ge Z. Environmentally friendly antibacterial cotton textiles finished with siloxane sulfopropylbetaine. ACS Appl Mater interfaces. 2011;3(4):1154–1162. doi: 10.1021/am101275d. [DOI] [PubMed] [Google Scholar]
- Cheng X, Ma K, Li R, Ren X, Huang T. Antimicrobial coating of modified chitosan onto cotton fabrics. Appl Surf Sci. 2014;309:138–143. [Google Scholar]
- Denyer SP, Stewart G. Mechanisms of action of disinfectants. Int Biodeterior biodegrad. 1998;41(3–4):261–268. [Google Scholar]
- Dhiman G, Chakraborty J. Antimicrobial performance of cotton finished with triclosan, silver and chitosan. Fash Text. 2015;2(1):13. [Google Scholar]
- Dring I. Antimicrobial, rotproofing and hygiene finishes. In: Heywood W, editor. Textile finishing. England: Society of Dyers and Colourists Bradford; 2003. pp. 351–371. [Google Scholar]
- El-Rafie M, Ahmed HB, Zahran M. Characterization of nanosilver coated cotton fabrics and evaluation of its antibacterial efficacy. Carbohydr Polym. 2014;107:174–181. doi: 10.1016/j.carbpol.2014.02.024. [DOI] [PubMed] [Google Scholar]
- Emam HE, Abdelhameed RM. Anti-UV radiation textiles designed by embracing with nano-MIL (Ti, In)-metal organic framework. ACS Appl Mater Interfaces. 2017;9(33):28034–28045. doi: 10.1021/acsami.7b07357. [DOI] [PubMed] [Google Scholar]
- Emam HE, Abdelhameed RM. In-situ modification of natural fabrics by Cu-BTC MOF for effective release of insect repellent (N, N-diethyl-3-methylbenzamide) J Porous Mater. 2017;24(5):1175–1185. [Google Scholar]
- Emam HE, Manian AP, Široká B, Duelli H, Redl B, Pipal A, Bechtold T. Treatments to impart antimicrobial activity to clothing and household cellulosic-textiles—why “Nano”-silver? J Clean Prod. 2013;39:17–23. [Google Scholar]
- Emam HE, Manian AP, Široká B, Duelli H, Merschak P, Redl B, Bechtold T. Copper (I) oxide surface modified cellulose fibers—synthesis, characterization and antimicrobial properties. Surf Coat Technol. 2014;254:344–351. [Google Scholar]
- Emam HE, El-Rafie M, Ahmed HB, Zahran M. Room temperature synthesis of metallic nanosilver using acacia to impart durable biocidal effect on cotton fabrics. Fibers Polym. 2015;16(8):1676–1687. [Google Scholar]
- Emam HE, Saleh N, Nagy KS, Zahran M. Functionalization of medical cotton by direct incorporation of silver nanoparticles. Int J Biol Macromol. 2015;78:249–256. doi: 10.1016/j.ijbiomac.2015.04.018. [DOI] [PubMed] [Google Scholar]
- Emam HE, Ahmed HB, Bechtold T. In-situ deposition of Cu2O micro-needles for biologically active textiles and their release properties. Carbohydr Polym. 2017;165:255–265. doi: 10.1016/j.carbpol.2017.02.044. [DOI] [PubMed] [Google Scholar]
- Emam HE, El-Hawary NS, Ahmed HB. Green technology for durable finishing of viscose fibers via self-formation of AuNPs. Int J Biol Macromol. 2017;96:697–705. doi: 10.1016/j.ijbiomac.2016.12.080. [DOI] [PubMed] [Google Scholar]
- Emam HE, Abdelhamid HN, Abdelhameed RM. Self-cleaned photoluminescent viscose fabric incorporated lanthanide-organic framework (Ln-MOF) Dyes Pigm. 2018;159:491–498. [Google Scholar]
- Emam HE, Darwesh OM, Abdelhameed RM. In-growth metal organic framework/synthetic hybrids as antimicrobial fabrics and its toxicity. Colloids Surf B. 2018;165:219–228. doi: 10.1016/j.colsurfb.2018.02.028. [DOI] [PubMed] [Google Scholar]
- Gao Y, Cranston R. Recent advances in antimicrobial treatments of textiles. Text Res J. 2008;78(1):60–72. [Google Scholar]
- Gawish S, Helmy H, Ramadan A, Farouk R, Mashaly H. UV protection properties of cotton, wool, silk and nylon fabrics dyed with red onion peel, madder and chamomile extracts. J Text Sci Eng. 2016;6(4):1. [Google Scholar]
- Gawish S, Mashaly H, Helmy H, Ramadan A, Farouk R. Effect of mordant on UV protection and antimicrobial activity of cotton, wool, silk and nylon fabrics dyed with some natural dyes. J Nanomed Nanotechnol. 2017;8(421):2. [Google Scholar]
- Ghaheh FS, Mortazavi SM, Alihosseini F, Fassihi A, Nateri AS, Abedi D. Assessment of antibacterial activity of wool fabrics dyed with natural dyes. J Clean Prod. 2014;72:139–145. [Google Scholar]
- Gilbert P, Moore L. Cationic antiseptics: diversity of action under a common epithet. J Appl Microbiol. 2005;99(4):703–715. doi: 10.1111/j.1365-2672.2005.02664.x. [DOI] [PubMed] [Google Scholar]
- Goetzendorf-Grabowska B, Królikowska H, Gadzinowski M. Polymer microspheres as carriers of antibacterial properties of textiles: a preliminary study. Fibres Text East Eur. 2004;12(4):62–64. [Google Scholar]
- Grifoni D, Bacci L, Di Lonardo S, Pinelli P, Scardigli A, Camilli F, Sabatini F, Zipoli G, Romani A. UV protective properties of cotton and flax fabrics dyed with multifunctional plant extracts. Dyes Pigm. 2014;105:89–96. [Google Scholar]
- Hasan MM, Abu Nayem K, Anwarul Azim AYM, Ghosh NC. Application of purified lawsone as natural dye on cotton and silk fabric. J Text. 2015;2015:932627. doi: 10.1155/2015/932627. [DOI] [Google Scholar]
- Hashem M, Hauser P, Smith B. Reaction efficiency for cellulose cationization using 3-chloro-2-hydroxypropyl trimethyl ammonium chloride. Text Res J. 2003;73(11):1017–1023. [Google Scholar]
- Hauser PJ, Tabba AH. Improving the environmental and economic aspects of cotton dyeing using a cationised cotton. Color Technol. 2001;117(5):282–288. [Google Scholar]
- Hebeish A, El-Shafei A, Sharaf S, Zaghloul S. Novel precursors for green synthesis and application of silver nanoparticles in the realm of cotton finishing. Carbohydr Polym. 2011;84(1):605–613. [Google Scholar]
- Hebeish A, El-Shafei A, Sharaf S, Zaghloul S. In situ formation of silver nanoparticles for multifunctional cotton containing cyclodextrin. Carbohydr Polym. 2014;103:442–447. doi: 10.1016/j.carbpol.2013.12.050. [DOI] [PubMed] [Google Scholar]
- Hirschmann D, Robinson H. Testing impregnated fabrics for antibacterial properties. Soap Paint Chem. 1941;17:94–119. [Google Scholar]
- Horcajada P, Chalati T, Serre C, Gillet B, Sebrie C, Baati T, Eubank JF, Heurtaux D, Clayette P, Kreuz C. Porous metal–organic-framework nanoscale carriers as a potential platform for drug delivery and imaging. Nat Mater. 2010;9(2):172. doi: 10.1038/nmat2608. [DOI] [PubMed] [Google Scholar]
- Hu Y, Du Y, Yang J, Kennedy JF, Wang X, Wang L. Synthesis, characterization and antibacterial activity of guanidinylated chitosan. Carbohydr Polym. 2007;67(1):66–72. [Google Scholar]
- Hu Y, Du Y, Yang J, Tang Y, Li J, Wang X. Self-aggregation and antibacterial activity of N-acylated chitosan. Polymer. 2007;48(11):3098–3106. [Google Scholar]
- Hu Z, Deibert BJ, Li J. Luminescent metal–organic frameworks for chemical sensing and explosive detection. Chem Soc Rev. 2014;43(16):5815–5840. doi: 10.1039/c4cs00010b. [DOI] [PubMed] [Google Scholar]
- Ibrahim NA, Hashem M, El-Sayed WA, El-Husseiny S, El-Enany E. Enhancing antimicrobial properties of dyed and finished cotton/polyester fabrics. AATCC Rev. 2010;10(1):55–63. [Google Scholar]
- Ibrahim N, Eid B, Elmaaty TA, El-Aziz EA. A smart approach to add antibacterial functionality to cellulosic pigment prints. Carbohydr Polym. 2013;94(1):612–618. doi: 10.1016/j.carbpol.2013.01.040. [DOI] [PubMed] [Google Scholar]
- Ibrahim N, Khalil H, El-Zairy E, Abdalla W. Smart options for simultaneous functionalization and pigment coloration of cellulosic/wool blends. Carbohydr Polym. 2013;96(1):200–210. doi: 10.1016/j.carbpol.2013.03.084. [DOI] [PubMed] [Google Scholar]
- Ignatova M, Manolova N, Rashkov I. Novel antibacterial fibers of quaternized chitosan and poly (vinyl pyrrolidone) prepared by electrospinning. Eur Polym J. 2007;43(4):1112–1122. [Google Scholar]
- Ikeda T, Yamaguchi H, Tazuke S. New polymeric biocides: synthesis and antibacterial activities of polycations with pendant biguanide groups. Antimicrob Agents Chemother. 1984;26(2):139–144. doi: 10.1128/aac.26.2.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ilić V, Šaponjić Z, Vodnik V, Potkonjak B, Jovančić P, Nedeljković J, Radetić M. The influence of silver content on antimicrobial activity and color of cotton fabrics functionalized with Ag nanoparticles. Carbohydr Polym. 2009;78(3):564–569. [Google Scholar]
- Ioannou CJ, Hanlon GW, Denyer SP. Action of disinfectant quaternary ammonium compounds against Staphylococcus aureus. Antimicrob Agents Chemother. 2007;51(1):296–306. doi: 10.1128/AAC.00375-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeon Y-J, Park P-J, Kim S-K. Antimicrobial effect of chitooligosaccharides produced by bioreactor. Carbohydr Polym. 2001;44(1):71–76. [Google Scholar]
- Joshi M, Ali SW, Purwar R, Rajendran S. Ecofriendly antimicrobial finishing of textiles using bioactive agents based on natural products. Indian J Fibre Textile Res. 2009;34(3):295–304. [Google Scholar]
- Joshi M, Khanna R, Shekhar R, Jha K. Chitosan nanocoating on cotton textile substrate using layer-by-layer self-assembly technique. J Appl Polym Sci. 2011;119(5):2793–2799. [Google Scholar]
- Julia M, Cot M, Erra P, Jocic D, Canal J. The use of chitosan on hydrogen peroxide pretreated wool. Text Chem Colorist. 1998;30(8):78–83. [Google Scholar]
- Kalyon BD, Olgun U. Antibacterial efficacy of triclosan-incorporated polymers. Am J Infect Control. 2001;29(2):124–125. doi: 10.1067/mic.2001.113229. [DOI] [PubMed] [Google Scholar]
- Kamel M, El Zawahry M, Ahmed N, Abdelghaffar F. Ultrasonic dyeing of cationized cotton fabric with natural dye. Part 1: cationization of cotton using Solfix E. Ultrason Sonochem. 2009;16(2):243–249. doi: 10.1016/j.ultsonch.2008.08.001. [DOI] [PubMed] [Google Scholar]
- Kamel M, El Zawahry M, Ahmed N, Abdelghaffar F. Ultrasonic dyeing of cationized cotton fabric with natural dye. Part 2: cationization of cotton using Quat 188. Indus Crops Prod. 2011;34(3):1410–1417. doi: 10.1016/j.ultsonch.2008.08.001. [DOI] [PubMed] [Google Scholar]
- Kawahara K, Tsuruda K, Morishita M, Uchida M. Antibacterial effect of silver-zeolite on oral bacteria under anaerobic conditions. Dent Mater. 2000;16(6):452–455. doi: 10.1016/s0109-5641(00)00050-6. [DOI] [PubMed] [Google Scholar]
- Khanna S, Chakraborty J. Optimization of monochlorotriazine β-cyclodextrin grafting on cotton and assessment of release behavior of essential oils from functionalized fabric. Fash Text. 2017;4(1):6. [Google Scholar]
- Kim J-C, Song M-E, Kim M-J, Lee E-J, Park S-K, Rang M-J, Ahn H-J. Preparation and characterization of Triclosan-containing vesicles. Colloids Surf B. 2002;26(3):235–241. [Google Scholar]
- Klemm JD, Schreiber SL, Crabtree GR. Dimerization as a regulatory mechanism in signal transduction. Ann Rev Immunol. 1998;16(1):569–592. doi: 10.1146/annurev.immunol.16.1.569. [DOI] [PubMed] [Google Scholar]
- Klemm D, Heublein B, Fink HP, Bohn A. Cellulose: fascinating biopolymer and sustainable raw material. Angew Chem Int Ed. 2005;44(22):3358–3393. doi: 10.1002/anie.200460587. [DOI] [PubMed] [Google Scholar]
- Kong M, Chen XG, Liu CS, Liu CG, Meng XH, Yu LJ. Antibacterial mechanism of chitosan microspheres in a solid dispersing system against E. coli. Colloids Surf B. 2008;65(2):197–202. doi: 10.1016/j.colsurfb.2008.04.003. [DOI] [PubMed] [Google Scholar]
- Kong M, Chen XG, Xing K, Park HJ. Antimicrobial properties of chitosan and mode of action: a state of the art review. Int J Food Microbiol. 2010;144(1):51–63. doi: 10.1016/j.ijfoodmicro.2010.09.012. [DOI] [PubMed] [Google Scholar]
- Lee S, Cho J-S, Cho G. Antimicrobial and blood repellent finishes for cotton and nonwoven fabrics based on chitosan and fluoropolymers. Text Res J. 1999;69(2):104–112. [Google Scholar]
- Lee H, Yeo SY, Jeong SH. Antibacterial effect of nanosized silver colloidal solution on textile fabrics. J Mater Sci. 2003;38(10):2199–2204. [Google Scholar]
- Li S (2001) Esterified triclosan derivatives as improved textile antimicrobial agents. USA patent, US5968207A
- Lim S-H, Hudson SM. Review of chitosan and its derivatives as antimicrobial agents and their uses as textile chemicals. J Macromol Sci Part C Polym Rev. 2003;43(2):223–269. [Google Scholar]
- Lim S-H, Hudson SM. Synthesis and antimicrobial activity of a water-soluble chitosan derivative with a fiber-reactive group. Carbohydr Res. 2004;339(2):313–319. doi: 10.1016/j.carres.2003.10.024. [DOI] [PubMed] [Google Scholar]
- Lin J, Qiu S, Lewis K, Klibanov AM. Bactericidal properties of flat surfaces and nanoparticles derivatized with alkylated polyethylenimines. Biotechnol Prog. 2002;18(5):1082–1086. doi: 10.1021/bp025597w. [DOI] [PubMed] [Google Scholar]
- Lin J, Qiu S, Lewis K, Klibanov AM. Mechanism of bactericidal and fungicidal activities of textiles covalently modified with alkylated polyethylenimine. Biotechnol Bioeng. 2003;83(2):168–172. doi: 10.1002/bit.10651. [DOI] [PubMed] [Google Scholar]
- Liu J, Chen L, Cui H, Zhang J, Zhang L, Su C-Y. Applications of metal–organic frameworks in heterogeneous supramolecular catalysis. Chem Soc Rev. 2014;43(16):6011–6061. doi: 10.1039/c4cs00094c. [DOI] [PubMed] [Google Scholar]
- Lu X, Ye J, Zhang D, Xie R, Bogale RF, Sun Y, Zhao L, Zhao Q, Ning G. Silver carboxylate metal–organic frameworks with highly antibacterial activity and biocompatibility. J Inorg Biochem. 2014;138:114–121. doi: 10.1016/j.jinorgbio.2014.05.005. [DOI] [PubMed] [Google Scholar]
- Ma W, Shen K, Xiang N, Zhang S. Combinative scouring, bleaching, and cationization pretreatment of greige knitted cotton fabrics for facilely achieving salt-free reactive dyeing. Molecules. 2017;22(12):2235. doi: 10.3390/molecules22122235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahltig B, Haufe H, Böttcher H. Functionalisation of textiles by inorganic sol–gel coatings. J Mater Chem. 2005;15(41):4385–4398. [Google Scholar]
- Mao J. Durable freshness for textiles. AATCC Rev. 2001;1:28–31. [Google Scholar]
- Milović NM, Wang J, Lewis K, Klibanov AM. Immobilized N-alkylated polyethylenimine avidly kills bacteria by rupturing cell membranes with no resistance developed. Biotechnol Bioeng. 2005;90(6):715–722. doi: 10.1002/bit.20454. [DOI] [PubMed] [Google Scholar]
- Munna MKH, Chinyerenwa AC, Kamruzzaman M, Hossain MA, Ahamed MK, Wahab MA. Effect of gamma radiation on cotton fabric with chitosan to improve the mechanical properties. Int J Text Sci. 2017;6(1):1–6. [Google Scholar]
- Naebe M, Li Q, Onur A, Denning R. Investigation of chitosan adsorption onto cotton fabric with atmospheric helium/oxygen plasma pre-treatment. Cellulose. 2016;23(3):2129–2142. [Google Scholar]
- O’Lenick AJ Jr (2002) Silicone functionalized triclosan. USA patent, US6384173B1
- Ocepek B, Boh B, Šumiga B, Tavčer PF. Printing of antimicrobial microcapsules on textiles. Color Technol. 2012;128(2):95–102. [Google Scholar]
- Omer K, Tao Z, Seedahmed A. New approach for dyeing and UV protection properties of cotton fabric using natural dye extracted from henna leaves. Fibres Text East Eur. 2015;5(113):60–65. [Google Scholar]
- Orhan M, Kut D, Gunesoglu C. Improving the antibacterial activity of cotton fabrics finished with triclosan by the use of 1, 2, 3, 4-butanetetracarboxylic acid and citric acid. J Appl Polym Sci. 2009;111(3):1344–1352. [Google Scholar]
- Owusu-Adom K, Guymon CA. Photopolymerization kinetics of poly (acrylate)-clay composites using polymerizable surfactants. Polymer. 2008;49(11):2636–2643. [Google Scholar]
- Peila R, Vineis C, Varesano A, Ferri A. Different methods for β-cyclodextrin/triclosan complexation as antibacterial treatment of cellulose substrates. Cellulose. 2013;20(4):2115–2123. [Google Scholar]
- Quirós J, Boltes K, Aguado S, de Villoria RG, Vilatela JJ, Rosal R. Antimicrobial metal–organic frameworks incorporated into electrospun fibers. Chem Eng J. 2015;262:189–197. [Google Scholar]
- Ramasamy M, Kandasaamy P. Effect of cationization of cotton on it’s dyeability. Indian J Fibre Textile Res. 2005;30(3):315–323. [Google Scholar]
- Rippon JA. Improving the dye coverage of immature cotton fibres by treatment with chitosan. Color Technol. 1984;100(10):298–303. [Google Scholar]
- Rodríguez HS, Hinestroza JP, Ochoa-Puentes C, Sierra CA, Soto CY. Antibacterial activity against Escherichia coli of Cu-BTC (MOF-199) metalorganic framework immobilized onto cellulosic fibers. J Appl Polym Sci. 2014;131:19. [Google Scholar]
- Samal SK, Dash M, Van Vlierberghe S, Kaplan DL, Chiellini E, Van Blitterswijk C, Moroni L, Dubruel P. Cationic polymers and their therapeutic potential. Chem Soc Rev. 2012;41(21):7147–7194. doi: 10.1039/c2cs35094g. [DOI] [PubMed] [Google Scholar]
- Schindler WD, Hauser PJ. Chemical finishing of textiles. Amsterdam: Elsevier; 2004. [Google Scholar]
- Shabbir M, Mohammad F. Natural polymers: scope in textile functionalization. In: Ikram S, Ahmed S, editors. Natural polymers: derivatives, blends and composites. 1. New York: Nova Science Publisher; 2016. [Google Scholar]
- Shabbir M, Mohammad F. Insights into the functional finishing of textile materials using nanotechnology. In: Muthu S, editor. Textiles and clothing sustainability. Singapore: Springer; 2017. pp. 97–115. [Google Scholar]
- Shahid M, Mohammad F. Perspectives for natural product based agents derived from industrial plants in textile applications—a review. J Clean Prod. 2013;57:2–18. [Google Scholar]
- Shin Y, Yoo DI, Min K. Antimicrobial finishing of polypropylene nonwoven fabric by treatment with chitosan oligomer. J Appl Polym Sci. 1999;74(12):2911–2916. [Google Scholar]
- Simoncic B, Tomsic B. Structures of novel antimicrobial agents for textiles—a review. Text Res J. 2010;80(16):1721–1737. [Google Scholar]
- Sumida K, Rogow DL, Mason JA, McDonald TM, Bloch ED, Herm ZR, Bae T-H, Long JR. Carbon dioxide capture in metal–organic frameworks. Chem Rev. 2011;112(2):724–781. doi: 10.1021/cr2003272. [DOI] [PubMed] [Google Scholar]
- Sun G, Chen T, Sun W, Wheatley W, Worley S. Preparation of novel biocidal N-halamine polymers. J Bioact Compat Polym. 1995;10(2):135–144. [Google Scholar]
- Takahashi T, Imai M, Suzuki I, Sawai J. Growth inhibitory effect on bacteria of chitosan membranes regulated with deacetylation degree. Biochem Eng J. 2008;40(3):485–491. [Google Scholar]
- Taylor R, Toasaksiri S, Reid R. Determination of antibacterial quaternary ammonium compounds in lozenges by capillary electrophoresis. J Chromatogr A. 1998;798(1–2):335–343. doi: 10.1016/s0021-9673(97)00986-2. [DOI] [PubMed] [Google Scholar]
- Top A, Ülkü S. Silver, zinc, and copper exchange in a Na-clinoptilolite and resulting effect on antibacterial activity. Appl Clay Sci. 2004;27(1–2):13–19. [Google Scholar]
- Tutak M, Korkmaz NE. Environmentally friendly natural dyeing of organic cotton. J Nat Fibers. 2012;9(1):51–59. [Google Scholar]
- Vigo TL. Protection of textiles from biological attack. Handbook of fiber science and technology: vol II. In: Lewin M, Sello SB, editors. Chemical processing of fibers and fabrics. New York/USA: Marcel Dekker Inc; 1983. pp. 367–426. [Google Scholar]
- Vigo TL. Antimicrobial polymers and fibers: retrospective and prospective. Washington, DC: ACS Publications; 2001. [Google Scholar]
- Wang C, Qian X, An X. In situ green preparation and antibacterial activity of copper-based metal–organic frameworks/cellulose fibers (HKUST-1/CF) composite. Cellulose. 2015;22(6):3789–3797. [Google Scholar]
- Worley SD, Williams D, Crawford RA. Halamine water disinfectants. Crit Rev Environ Sci Technol. 1988;18(2):133–175. [Google Scholar]
- Wyszogrodzka G, Marszałek B, Gil B, Dorożyński P. Metalorganic frameworks: mechanisms of antibacterial action and potential applications. Drug Discov Today. 2016;21(6):1009–1018. doi: 10.1016/j.drudis.2016.04.009. [DOI] [PubMed] [Google Scholar]
- Xie Y, Liu X, Chen Q. Synthesis and characterization of water-soluble chitosan derivate and its antibacterial activity. Carbohydr Polym. 2007;69(1):142–147. [Google Scholar]
- Yancheva E, Paneva D, Maximova V, Mespouille L, Dubois P, Manolova N, Rashkov I. Polyelectrolyte complexes between (cross-linked) N-carboxyethylchitosan and (quaternized) poly [2-(dimethylamino) ethyl methacrylate]: preparation, characterization, and antibacterial properties. Biomacromol. 2007;8(3):976–984. doi: 10.1021/bm061029j. [DOI] [PubMed] [Google Scholar]
- Ye W, Xin JH, Li P, Lee KLD, Kwong TL. Durable antibacterial finish on cotton fabric by using chitosan-based polymeric core-shell particles. J Appl Polym Sci. 2006;102(2):1787–1793. [Google Scholar]
- Yi Y, Wang Y, Liu H. Preparation of new crosslinked chitosan with crown ether and their adsorption for silver ion for antibacterial activities. Carbohydr Polym. 2003;53(4):425–430. [Google Scholar]
- Younsook S, Dong Y. Use of chitosan to improve dyeability of Dp-finished cotton (II) J Appl Polym Sci. 1998;67(9):1515–1521. [Google Scholar]
- Yu D-G, Teng M-Y, Chou W-L, Yang M-C. Characterization and inhibitory effect of antibacterial PAN-based hollow fiber loaded with silver nitrate. J Membr Sci. 2003;225(1–2):115–123. [Google Scholar]
- Yusuf M, Ahmad A, Shahid M, Khan MI, Khan SA, Manzoor N, Mohammad F. Assessment of colorimetric, antibacterial and antifungal properties of woollen yarn dyed with the extract of the leaves of henna (Lawsonia inermis) J Clean Prod. 2012;27:42–50. [Google Scholar]
- Yusuf M, Khan MA, Mohammad F. Investigations of the colourimetric and fastness properties of wool dyed with colorants extracted from Indian madder using reflectance spectroscopy. Optik Int J Light Electron Opt. 2016;127(15):6087–6093. [Google Scholar]
- Zahran M, Ahmed HB, El-Rafie M. Surface modification of cotton fabrics for antibacterial application by coating with AgNPs—alginate composite. Carbohydr Polym. 2014;108:145–152. doi: 10.1016/j.carbpol.2014.03.005. [DOI] [PubMed] [Google Scholar]
- Zanoaga M, Tanasa F. Antimicrobial reagents as functional finishing for textiles intended for biomedical applications. I. Synthetic organic compounds. Chem J Mold. 2014;9(1):14–32. [Google Scholar]
- Zemljič LF, Volmajer J, Ristić T, Bracic M, Sauperl O, Kreže T. Antimicrobial and antioxidant functionalization of viscose fabric using chitosan–curcumin formulations. Text Res J. 2014;84(8):819–830. [Google Scholar]
- Zhang Z, Chen L, Ji J, Huang Y, Chen D. Antibacterial properties of cotton fabrics treated with chitosan. Text Res J. 2003;73(12):1103–1106. [Google Scholar]
- Zhao T, Chen Q. Halogenated phenols and polybiguanides as antimicrobial textile finishes. In: Sun G, editor. Antimicrobial textiles. 1. Cambridge: Woodhead Publishing, Sawston; 2016. [Google Scholar]
- Zhuang W, Yuan D, Li JR, Luo Z, Zhou HC, Bashir S, Liu J. Highly potent bactericidal activity of porous metalorganic frameworks. Adv Healthc Mater. 2012;1(2):225–238. doi: 10.1002/adhm.201100043. [DOI] [PubMed] [Google Scholar]