Abstract
Purpose
The aim of this study was to determine the prevalence and risk factors for community-acquired urinary tract infections (CA-UTIs) caused by extended-spectrum β-lactamase (ESBL) producing Escherichia coli and Klebsiella species.
Materials and Methods
The patients diagnosed with CA-UTIs caused by E. coli or Klebsiella spp. were included in the study. All of the patients were compared to demographic characteristics, underlying diseases, urinary tract pathology, history of hospitalization, use of antibiotics according to ESBL positivity.
Results
A total of 322 urine isolates were studied. Sixty-six patients (37.1%) of a total of 178 patients were ESBL positive E. coli and Klebsiella spp. Being over the age of sixty (odds ratio [OR], 1.90; p=0.03), history of renal stone (OR, 3.00; p=0.03), urinary tract anatomical of physiological disorder (OR, 2.17; p=0.01), urologic intervention (OR, 3.43; p<0.001), history of urinary tract surgery (OR, 3.10; p=0.01), history of urinary catheterization (OR, 3.43; p<0.001), and hospitalization for last 1 year (OR, 3.70; p=0.01) and antibiotic usage in the last 3 months (OR, 1.90; p=0.04) were found as significant risk factors for the producing of ESBL. However, gender and underlying disease were not related for ESBL production.
Conclusions
In present study, high rate of ESBL positivity was detected in CA-UTIs. The increasing of infections caused by ESBL positive E. coli and Klebsiella spp. are bringing together a lot of the problem, such as antibiotic resistance and reducing treatment options for outpatients. Identification of underlying risk factors would be important for the development of preventive strategies.
Keywords: Community-acquired urinary tract infections, Escherichia coli, Extended-spectrum beta-lactamase, Klebsiella species
INTRODUCTION
Extended-spectrum β-lactamases (ESBLs) belong to a group of enzymes that are responsible for the development of resistance against several β-lactam-containing antibiotics, including penicillins, cephalosporins, and aztreonam. As the name suggests, these enzymes hydrolyze the four-atom ring (β-lactam) present in these antibiotics, thus making them ineffective. ESBLs are mainly produced by the strains belonging to the Gram-negative family Enterobacteriaceae, especially Escherichia coli and Klebsiella pneumoniae [1,2,3].
The ESBLs were initially isolated in the hospital settings. However, since 2001 [4], reports of community-acquired (CA) infections of ESBLs have started emerging, thus making the epidemiology of infections resulting from ESBL-producing bacteria yet more complex [2,5].
The increasing resistance to β-lactam antibiotics, used to treat urinary tract infections (UTIs), has made the treatment more difficult. E. coli and Klebsiella species are the most common causative agents of CA-UTIs and frequently resistant to many of the antimicrobial agents recommended for the treatment of such infections. Recent years have witnessed a surge in the emergence of ESBLproducing E. coli and Klebsiella spp. [6,7].
The increased resistance of the causative microorganisms to CA-UTIs is associated with increased mortality, morbidity, health care costs, and the need for introducing broad-spectrum antibiotics [8]. Identification of risk factors for antimicrobial resistance may contribute toward improved empirical treatment of CA-UTIs. The reported risk factors for developing a UTI with a CA ESBL-producing E. coli or Klebsiella spp. include old age, female sex, diabetes mellitus, recurrent UTIs, invasive urological procedures, and prior use of antibiotics, such as aminopenicillins, cephalosporins, and fluoroquinolones [4,5,9,10,11].
The present study identified the risk factors in patients with CA-UTIs caused by ESBL positive microorganisms as compared to infections caused by ESBL negative microbes.
MATERIALS AND METHODS
1. Design and data collection
This is a prospective case-control study, and carried out between 1 January 2012 and 31 December 2012. The study was conducted at the Department of Infectious Disease and Clinical Microbiology, Ankara Training and Research Hospital, situated in central region of Turkey. Our hospital is an 468 bed tertiary care teaching hospital. Patients diagnosed with CA-UTIs caused by E. coli or Klebsiella spp. and ≥18 years old were included in the study. UTI was defined as a growth in urine culture, pyuria, and at least one of the following symptoms: dysuria, frequency or urgency. Each patient was included once in the study and informed consent was obtained from participants. The following exclusion criteria were used:
i) contaminated urine culture (more than three microorganisms)
ii) were unable to answer our questionnaire
iii) had urinary tract symptoms, but not positive urine culture
iv) patients with health care associated UTI (i.e., hospitalized for 48 hours during the last 30 days)
v) the presence of other microorganisms in urine culture.
We recorded the presence of urinary tract abnormalities, immunosuppression, comorbidities, previous antibiotic treatment (in last three months), any interaction with the healthcare system in the previous 3 months or one year and invasive urological procedures. UTIs can be categorized as uncomplicated and complicated. Uncomplicated UTI is defined as an infection occurring in a structurally and neurologically normal urinary tract. Complicated UTI is defined as an infection in a urinary tract with functional or structural abnormalities, and UTI in men and pregnant women [12].
The study was approved by the Regional Committee for Medical and Health Research Ethics in Ankara Training and Research Hospital (approval number: 2012/3816).
2. Microbiological examination
Urine culture was performed by using blood and eosine methylene blue agar. E. coli and Klebsiella spp. were identified by using manual tests, such as colony morphology, Gram stain, oxidase test, triple sugar iron agar, urease, citrate, indol and methyl red tests. Antibacterial susceptibility testing and ESBL screening were performed by using disc diffusion method according to the Clinical and Laboratory Standards Institute [13] criteria in our clinical laboratory. Following antimicrobial agents (Bioanalyse, Ankara, Turkey) were used for antibiotic susceptibility testing: amoxicillin-clavulanic acid (20 µg/10 µg), aztreonam (30 µg), cefuroxim (30 µg), ceftriaxone (CRO, 30 µg), ceftazidime (CAZ, 30 µg), cefepime (30 µg), cefoxitine (30 µg), cefotaxime (30 µg), imipenem (10 µg), meropenem (10 µg), ertapenem (10 µg), ciprofloxacin (CIP, 5 µg), gentamicin (GEN, 120 µg), amikacin (AK, 30 µg), nitrofurantoin (F, 300 µg), fosfomycin (50 µg), piperacillin-tazobactam (10/1–110 µg). Quality was assured by testing E. coli ATCC 25922 and K. pneumoniae ATCC 700603 in every batch.
ESBL production was confirmed by using double disc synergy and E-tests (AB Biodisk, Stockholm, Sweden). Double disc synergy test determination was performed by using amoxicillin-clavulanic acid in the center and CAZ, CRO, cefoxitin, aztreonam around it with a 25 mm distance. Confirmation by E-test method was performed by using CAZ (0.5–32 µg/mL)/CAZ clavulanate (0.064–4 µg/mL) and cefotaxime (0.25–16 µg/mL)/cefotaxime clavulanate (0.016–1 µg/mL) E-test strips. If the proportion of CAZ/CAZ clavulanate and cefotaxime/cefotaxime clavulanate MIC values were ≥8 in E-test method or expansion of indicator cephalosporin inhibition zone towards amoxicillin-clavulanic acid disc by double disc synergy method were interpreted as production of ESBL enzyme (Figs. 1, 2).
Fig. 1. Double disk synergy method. Use amoxicillin clavulanate in proximity to cephalosporin discs. The distance between discs is critical. Any distorsion of zones indicates extended-spectrum β-lactamase.
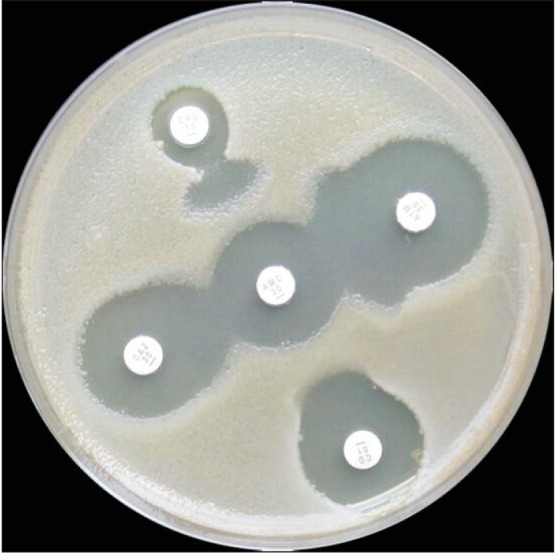
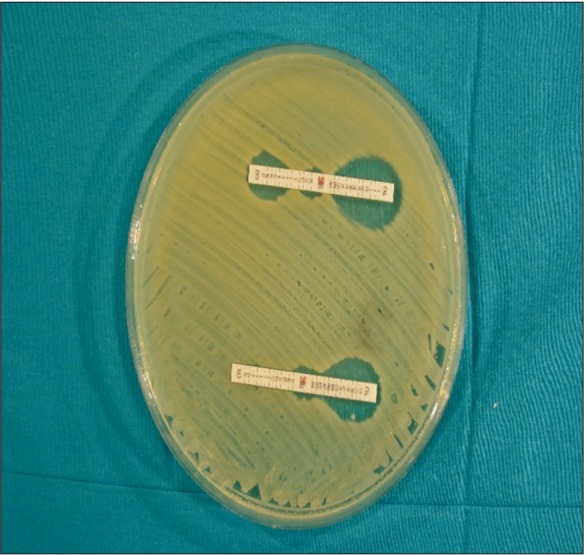
Fig. 2. Detection of extended-spectrum β-lactamase (ESBL) with an E-test. Ceftazidime (CAZ) MIC against Escherichia coli isolate in A is >32 µg/mL in the absence of clavulanate and 0.125 µg/mL in the presence of clavulanate. As the ratio of CAZ with and without clavulanate is ≥8, the isolates were phenotypically determined as ESBL producers.
3. Statistical analysis
The SPSS ver. 15.0 software (SPSS Inc., Chicago, IL, USA) was used for statistical analysis. Parametric tests were used to compare the variables between independent groups; the Shapiro Wilk and Kolmogorov-Smirnov tests were used to verify the assumptions. The Mann-Whitney U-test was used to compare two independent groups and the chi-square test was used to analyze the differences in categorical variables between these groups. Also, multiple logistic regression analysis was performed to determine independent risk factors that influenced the resistance to different antibiotics. The variables with a significant difference (p<0.05) were compared with multiple logistic regression analysis; the results of which were summarized with odds ratios [ORs], 95% confidence interval [CI], and p-values. Descriptive statistics was used to summarize the demographic data. The qualitative variables were expressed as rates and frequency, and quantitative variables were defined as medium (minimum-maximum) and/or mean±standard deviation. A p-value <0.05 was considered as statistically significant.
RESULTS
A total of 322 patients with CA-UTIs were enrolled and bacterial growth was detected in 264 patients (82.0%) of their urine samples. E. coli was the most commonly isolated species (60.5%), followed by Klebsiella spp. (6.5%), Proteus mirabilis (2.8%), Pseudomonas aeruginosa (2.2%) and other microorganisms (Acinetobacter baumanni, Enterobacter aerogenes, P. vulgaris, Stenotrophomonas maltophilia, E. cloacae) (3.4%). E. coli (161; 90.4%) and Klebsiella spp. (17; 9.6%) were identified in 178 patients meeting the study criteria. Fifty eight of E. coli isolates (36%) and eight of Klebsiella spp. isolates (47%) totally 66 of (37.1%) of them were phenotypically positive for ESBL production. Remaining, 112 of isolates (E. coli n=103; Klebsiella spp. n=9) were found as negative for ESBL production. We groupped the patients according to occurrence of ESBL positive or negative growth of E. coli and Klebsiella obtained from urine, and compared the groups for the risk factors. The study case (n=66) and control groups (n=112) were well matched in most of the demographics (such as age and sex) and clinical factors. The results were shown in Table 1.
Table 1. Patient characteristics, epidemiological and clinical variables associated with ESBL positive Escherichia coli and Klebsiella species CA-UTI (univariate analysis).
| Patient data | ESBL (+) (n=66) | ESBL (−) (n=112) | p-value |
|---|---|---|---|
| Sex | >0.05 | ||
| Male | 39 (59.1 ) | 35 (31.3) | |
| Female | 27 (40.9) | 77 (68.8) | |
| Age (y) | <0.05 | ||
| <60 | 27 (40.9) | 64 (57.1) | |
| >60 | 39 (59.1) | 48 (42.9) | |
| Type of infection | >0.05 | ||
| Uncomplicated | 36 (54.5) | 48 (42.9) | |
| Complicated | 30 (45.5) | 64 (57.1) | |
| Comorbidity | >0.05 | ||
| Diabetes mellitus | 19 (28.8) | 23 (20.5) | |
| Hypertension | 32 (48.5) | 41 (36.6) | |
| Chronic cardiovascular disease | 6 (9.1) | 12 (10.7) | |
| Chronic renal disease | 9 (13.6) | 8 (7.1) | |
| Another systemic diseasesa | 17 (25.8) | 17 (15.2) | |
| Urinary system malignancy | 6 (9.1) | 4 (3.6) | >0.05 |
| Urolithiasis | 22 (33.3) | 16 (14.3) | <0.05 |
| Urinary tract abnormalitiesb | 27 (40.9) | 24 (24.1) | |
| History of urinary catheterization | 36 (54.5) | 29 (25.9) | |
| Recurrent UTI | 18 (27.3) | 12 (10.7) | |
| History of urological surgery | 13 (19.7) | 8 (7.1) | |
| History of invasive urological procedures | 19 (28.8) | 7 (6.3) | |
| History of hospitalization in last 3 months | 15 (27.3) | 9 (8.0) | |
| History of hospitalization in last one year | 11 (16.7) | 11 (9.8) | |
| History of antibiotic use in last 3 months | 33 (50.0) | 38 (33.9) |
Values are presented as number (%).
ESBL, extended-spectrum β-lactamase; CA-UTI, community-acquired urinary tract infection.
a:Rheumatoid arthritis, Behcet disease, Gout disease.
b:Vesicoureteral reflux, benign prostatic hypertrophy, sistosel, neurogenic bladder.
The univariate analysis showed that >60 years old, urolithiasis, urinary tract abnormalities, urinary system malignancy, history of urinary catheterization, recurrent UTI, history of urinary system operation and invasive urological procedures, history of hospitalization in last 3 months or in the preceding year, history of antibiotic use in preceeding 3 months were significantly higher in the ESBL positive group (Table 1).
Antibiotic usage in the last three months and the effect of antibiotics on ESBL positivity was investigated. The results were shown in Table 2. Significant increase in the number of infections due to ESBL positive E. coli and Klebsiella spp. were found in patients with a history of previous use of antibiotics in the last 3 months, particularly with fluoroquinolones (OR, 2.11; 95% CI, 1.010–4.397; p=0.043) or nitrofurantoin (OR, 6.50; 95% CI, 1.314–32.400) use.
Table 2. Comparison of the antibiotic usage during the last 3 months in the study population with and without ESBL positive Escherichia coli and Klebsiella species.
| Antibiotics | ESBL (+) (n=66) | ESBL (−) (n=112) | p-value | OR (95% CI) |
|---|---|---|---|---|
| Penicillins | 0 (0.0) | 11 (9.8) | >0.05 | 1.65 (1.460–1.860) |
| Fluoroquinolones | 19 (28.8) | 18 (16.1) | <0.05 | 2.11 (1.010–4.397) |
| Cephalosporins | 5 (7.6) | 4 (3.6) | >0.05 | 2.21 (0.570–8.550) |
| Fosfomycin | 2 (3.0) | 3 (2.7) | >0.05 | 1.13 (0.180–6.970) |
| Nitrofurantoin | 7 (10.6) | 2 (1.8) | <0.05 | 6.50 (1.314–32.400) |
Values are presented as number (%).
ESBL, extended-spectrum β-lactamase; OR, odds ratio; CI, confidence interval.
The antibiotic susceptibility patterns of E. coli and Klebsiella spp. from cases and controls are shown in Table 3. The higher rates of resistance, particularly in ESBL positive strains, to cephalosporins, fluoroquinolones, amoxicillin clavulanate are noteworthy. In addition to carbapenems, AK, fosfomycin and F appear to be effective against ESBL positive strains.
Table 3. Antibiotic resistance rates of ESBL positive Escherichia coli and Klebsiella species.
| Antibiotic | ESBL (+) (n=66) | ESBL (−) (n=112) | p-value |
|---|---|---|---|
| AMP | 63 (95.5) | 45 (40.2) | <0.001 |
| AMC | 53 (80.3) | 26 (23.2) | <0.001 |
| CRO | 66 (100.0) | 1 (0.9) | <0.001 |
| CXM | 66 (100.0) | 13 (11.6) | <0.001 |
| FEP | 66 (100.0) | 2 (1.8) | <0.001 |
| CAZ | 66 (100.0) | 0 (0.0) | <0.001 |
| IMP | 0 (0.0) | 0 (0.0) | - |
| MEM | 0 (0.0) | 0 (0.0) | - |
| TM-SXT | 36 (54.5) | 21 (18.8) | <0.001 |
| FF | 4 (6.1) | 1 (0.9) | >0.05 |
| TZP | 24 (36.4) | 9 (8.0) | <0.001 |
| CIP | 50 (75.8) | 25 (22.3) | <0.001 |
| LEV | 49 (74.2) | 24 (21.4) | <0.001 |
| GEN | 20 (30.3) | 9 (8.0) | <0.001 |
| AK | 14 (21.2) | 6 (5.4) | <0.001 |
| F | 9 (13.6) | 6 (5.4) | >0.05 |
Values are presented as number (%).
ESBL, extended-spectrum β-lactamase; AMP, ampicillin; AMC, amoxicillin/clavulanate; CRO, ceftriaxone; CXM, cefuroxim; FEP, cefepime; CAZ, ceftazidime; IMP, imipenem; MEM, meropenem; TM-SXT, trimethoprim/sulfamethoxazole; FF, fosfomycin; TZP, piperacillin tazobactam; CIP, ciprofloxacin; LEV, levofloxacin; GEN, gentamicin; AK, amikacin; F, nitrofurantoin.
Multivariate analysis included all significant factors found in univariate analysis (age, urolithiasis, urinary tract abnormalities, urinary system malignancy, history of urinary catheterization, recurrent UTI, history of urinary system operation and invasive urological procedures, history of hospitalization in last 3 months or in the preceding year, history of antibiotic use in preceeding 3 months). In the final analysis urolithiasis (OR, 3.00; 95% CI, 0.14–0.17; p=0.01), history of invasive urological procedures (OR, 3.22; 95% CI, 0.10–0.19; p=0.03), history of urinary catheterization (OR, 2.32; 95% CI, 0.19–0.94; p=0.03) were identified as independent risk factors for ESBL positive E. coli and Klebsiella spp. CA-UTI (Table 4).
Table 4. Independent risk factors of ESBL positive Escherichia coli and Klebsiella species CA-UTI identified using multivariate logistic regression analysis.
| Comorbidities | p-value | OR | 95% CI |
|---|---|---|---|
| Urolithiasis | <0.05 | 3.00 | 0.14–0.70 |
| History of invasive urological procedures | <0.05 | 3.22 | 0.10–0.90 |
| History of urinary catheterization | <0.05 | 2.32 | 0.19–0.94 |
ESBL, extended-spectrum β-lactamase; CA-UTI, community-acquired urinary tract infection; OR, odds ratio; CI, confidence interval.
DISCUSSION
UTIs are in the second place after upper respiratory tract infections among the community acquired infections. CA-UTIs are one of the most common indications for prescribing antibiotics empirically. However, the increasing prevalence of infections caused by antibiotic-resistant Gram-negative bacteria makes the empirical treatment of CA-UTIs more difficult [14,15]. ESBL positive E. coli and Klebsiella spp. are emerging causative agents of CA-UTIs.
This prospective study, which was conducted in a tertiary care teaching hospital, found that approximately 36% of community acquired E. coli; 47% of Klebsiella spp. produced ESBL. Similar results were found in other studies in Turkey, also the rates of positivity has been increasing in recent years [10,15,16]. These indicate that ESBL-producing E. coli and Klebsiella spp. are not only a nosocomial problem, but are a growing comprehensive issue in CA-UTIs.
The resistance rates of antibiotics other than beta-lactams of ESBL positive E. coli and Klebsiella spp. strains are generally higher than ESBL negative E. coli and Klebsiella spp. strains (Table 3). F, Fosfomycin, and Pivmecillinam as f irst line treatment options for uncomplicated UTI whereas fluoroquinolones are recommended as second line treatment option because of a propensity for collateral damage for uncomplicated UTI in Infectious Diseases Society of America guidelines [17]. Quinolones should be preferred in CA-UTIs caused by E. coli in areas where trimethoprim/sulfamethoxazole (TMSXT) resistance reaches 20%. Although quinolones effective in the majority of uropathogens resistance in E. coli has been rising also. Therefore, it is important that prevalance of resistance rates must be continuously determined in each population. In our study, 54.5% of the ESBL positive strains; 18.8% of the ESBL negative strains were resistant to TMSXT; 75.8% of the ESBL positive strains and 22.3% of the ESBL negative strains were resistant to CIP and 30.3% of the ESBL positive strains; 8% of the ESBL negative strains were resistant to GEN. These high rates of resistance to non beta lactam antibiotics (particularly quinolones, TMSXT and aminoglycosides) are the most worrisome aspects of ESBL positive E. coli and Klebsiella spp. concerns. In a study; similar to our results the CIP resistance, was reported to be 31.5% in ESBL positive and 9.1% in ESBL negative E. coli isolates [4]. CIP resistance rates, published in 2015 from Taiwan, were reported to be even higher: 68.9% in ESBL positive and 18% in ESBL negative Gram-negative isolates [18]. Also resistance rates for CIP against uncomplicated UTI isolates was reported as 0%–14.7% in the ECO SENS Project, 2.5% in the USA and 1.2% in outpatients in Canada [19,20,21]. The most frequently seen beta-lactamase genotype is CTX-M-15 [22]. That is likely acquired from plasmid in fluoroquinolone resistant E. coli [23] which, in a part, explains the results of the present study where the resistance rates to fluoroquinolones were higher in ESBL positive strain than those in ESBL negative strain.
Inappropriate empirical antibiotic treatment has been associated increased morbidity and mortality in CA-UTIs [19,24]. High resistance rates for beta lactams, TM-SXT and CIP restrict empirical antibiotic use in the outpatient settings. For this reason, in uncomplicated UTIs caused by ESBL positive E. coli and Klebsiella spp. strains therapeutic options include fosfomycin or F [15,19,20,25]. In our study resistance rates for the ESBL positive group were 13.6% and 6.1% for F and fosfomycin, respectively and also F resistance was found to be higher than previously published data [15,19,20,25].
The study case (n=66) and control groups (n=112) were well matched in most of the demographics (such as age and sex) and clinical factors (Table 1). The univariate analysis showed that patients older than 60 years was significantly higher in the ESBL positive group than that of negative group (p=0.03). This result is indicating that advanced age may be a risk factor for the infections due to ESBL positivite microorganisms. Several studies in the literature support our findings [26,27].
When we evaluated the correlation between underlying systemic diseases and ESBL producing, we did not identify any significant underlying systemic diseases in ESBL positive CA-UTIs. Similar results were found in another studies [2,28,29]. However, further studies are required to support this correlation.
The multivariate analysis used in the current study found the presence of urolithiasis, history of invasive urological procedures, and history of urinary catheterization as independent risk factors for CA-UTIs caused by ESBL positive E. coli and Klebsiella spp. On the other hand, other studies have reported previous use of quinolones or secondgeneration cephalosporins, previous hospitalization, diabetes mellitus, the presence of a urinary catheter, and advanced age as the most frequently detected independent risk factors [1,2,3,4,7].
CONCLUSIONS
The production of ESBLs has emerged as a significant problem in the treatment of CA-UTIs worldwide, severely affecting the clinical outcomes. The present study found urolithiasis, history of invasive urological procedures, and history of urinary catheterization as potential risk factors associated with widespread resistance to CA-UTIs.
The identification of risk factors is necessary for effective treatment strategies to be developed, which, in turn, would reduce the spread of these infections and optimize the antibiotic use. Local antimicrobial susceptibility patterns of urinary isolates should be followed. Besides, clinicians should be aware of the resistance and should consider the risk factors, contributing to resistance, before prescribing appropriate antibiotics.
Footnotes
CONFLICTS OF INTEREST: The authors have nothing to disclose.
References
- 1.Tumbarello M, Spanu T, Sanguinetti M, Citton R, Montuori E, Leone F, et al. Bloodstream infections caused by extended-spectrum-beta-lactamase-producing Klebsiella pneumoniae: risk factors, molecular epidemiology, and clinical outcome. Antimicrob Agents Chemother. 2006;50:498–504. doi: 10.1128/AAC.50.2.498-504.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kuster SP, Hasse B, Huebner V, Bansal V, Zbinden R, Ruef C, et al. Risks factors for infections with extended-spectrum beta-lactamase-producing Escherichia coli and Klebsiella pneumoniae at a tertiary care university hospital in Switzerland. Infection. 2010;38:33–40. doi: 10.1007/s15010-009-9207-z. [DOI] [PubMed] [Google Scholar]
- 3.Hyle EP, Lipworth AD, Zaoutis TE, Nachamkin I, Fishman NO, Bilker WB, et al. Risk factors for increasing multidrug resistance among extended-spectrum beta-lactamase-producing Escherichia coli and Klebsiella species. Clin Infect Dis. 2005;40:1317–1324. doi: 10.1086/429239. [DOI] [PubMed] [Google Scholar]
- 4.Calbo E, Romaní V, Xercavins M, Gómez L, Vidal CG, Quintana S, et al. Risk factors for community-onset urinary tract infections due to Escherichia coli harbouring extended-spectrum beta-lactamases. J Antimicrob Chemother. 2006;57:780–783. doi: 10.1093/jac/dkl035. [DOI] [PubMed] [Google Scholar]
- 5.Rodríguez-Baño J, Alcalá JC, Cisneros JM, Grill F, Oliver A, Horcajada JP, et al. Community infections caused by extended-spectrum beta-lactamase-producing Escherichia coli. Arch Intern Med. 2008;168:1897–1902. doi: 10.1001/archinte.168.17.1897. [DOI] [PubMed] [Google Scholar]
- 6.Meier S, Weber R, Zbinden R, Ruef C, Hasse B. Extended-spectrum β-lactamase-producing Gram-negative pathogens in community-acquired urinary tract infections: an increasing challenge for antimicrobial therapy. Infection. 2011;39:333–340. doi: 10.1007/s15010-011-0132-6. [DOI] [PubMed] [Google Scholar]
- 7.Rodríguez-Baño J, Navarro MD, Romero L, Martínez-Martínez L, Muniain MA, Perea EJ, et al. Epidemiology and clinical features of infections caused by extended-spectrum beta-lactamase-producing Escherichia coli in nonhospitalized patients. J Clin Microbiol. 2004;42:1089–1094. doi: 10.1128/JCM.42.3.1089-1094.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Søraas A, Sundsfjord A, Sandven I, Brunborg C, Jenum PA. Risk factors for community-acquired urinary tract infections caused by ESBL-producing Enterobacteriaceae –a case-control study in a low prevalence country. PLoS One. 2013;8:e69581. doi: 10.1371/journal.pone.0069581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ben-Ami R, Rodríguez-Baño J, Arslan H, Pitout JD, Quentin C, Calbo ES, et al. A multinational survey of risk factors for infection with extended-spectrum beta-lactamase-producing Enterobacteriaceae in nonhospitalized patients. Clin Infect Dis. 2009;49:682–690. doi: 10.1086/604713. [DOI] [PubMed] [Google Scholar]
- 10.Yilmaz E, Akalin H, Ozbey S, Kordan Y, Sinirtaş M, Gürcüoglu E, et al. Risk factors in community-acquired/onset urinary tract infections due to extended-spectrum beta-lactamase-producing Escherichia coli and Klebsiella pneumoniae. J Chemother. 2008;20:581–585. doi: 10.1179/joc.2008.20.5.581. [DOI] [PubMed] [Google Scholar]
- 11.Azap OK, Arslan H, Serefhanoğlu K, Colakoğlu S, Erdoğan H, Timurkaynak F, et al. Risk factors for extended-spectrum beta-lactamase positivity in uropathogenic Escherichia coli isolated from community-acquired urinary tract infections. Clin Microbiol Infect. 2010;16:147–151. doi: 10.1111/j.1469-0691.2009.02941.x. [DOI] [PubMed] [Google Scholar]
- 12.Sobel JD, Kaye D. Urinary tract infections. In: Bennett JE, Dolin R, Douglas RG, Mandell GL, editors. Mandell, Douglas, and Bennett's principles and practice of infectious diseases. 6th ed. Philadelphia: Churchill Livingstone; 2005. pp. 875–905. [Google Scholar]
- 13.Matthew A Wikler Clinical and Laboratory Standards Institute. Performance standards for antimicrobial susceptibility testing: twentieth informational supplement. Wayne: Clinical and Laboratory Standards Institute; 2010. [Google Scholar]
- 14.Steinke DT, Seaton RA, Phillips G, MacDonald TM, Davey PG. Prior trimethoprim use and trimethoprim-resistant urinary tract infection: a nested case-control study with multivariate analysis for other risk factors. J Antimicrob Chemother. 2001;47:781–787. doi: 10.1093/jac/47.6.781. [DOI] [PubMed] [Google Scholar]
- 15.Arslan H, Azap OK, Ergönül O, Timurkaynak F Urinary Tract Infection Study Group. Risk factors for ciprofloxacin resistance among Escherichia coli strains isolated from community-acquired urinary tract infections in Turkey. J Antimicrob Chemother. 2005;56:914–918. doi: 10.1093/jac/dki344. [DOI] [PubMed] [Google Scholar]
- 16.Kurtaran B, Candevir A, Tasova Y, Kibar F, Inal AS, Komur S, et al. Antibiotic resistance in community-acquired urinary-tract infections: prevalence and risk factors. Med Sci Monit. 2010;16:CR246–CR251. [PubMed] [Google Scholar]
- 17.Gupta K, Hooton TM, Naber KG, Wullt B, Colgan R, Miller LG, et al. International clinical practice guidelines for the treatment of acute uncomplicated cystitis and pyelonephritis in women: a 2010 update by the Infectious Diseases Society of America and the European Society for Microbiology and Infectious Diseases. Clin Infect Dis. 2011;52:e103–e120. doi: 10.1093/cid/ciq257. [DOI] [PubMed] [Google Scholar]
- 18.Kung CH, Ku WW, Lee CH, Fung CP, Kuo SC, Chen TL, et al. Epidemiology and risk factors of community-onset urinary tract infection caused by extended-spectrum β-lactamase-producing Enterobacteriaceae in a medical center in Taiwan: a prospective cohort study. J Microbiol Immunol Infect. 2015;48:168–174. doi: 10.1016/j.jmii.2013.08.006. [DOI] [PubMed] [Google Scholar]
- 19.Karlowsky JA, Kelly LJ, Thornsberry C, Jones ME, Sahm DF. Trends in antimicrobial resistance among urinary tract infection isolates of Escherichia coli from female outpatients in the United States. Antimicrob Agents Chemother. 2002;46:2540–2545. doi: 10.1128/AAC.46.8.2540-2545.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kahlmeter G. An international survey of the antimicrobial susceptibility of pathogens from uncomplicated urinary tract infections: the ECO·SENS project. J Antimicrob Chemother. 2003;51:69–76. doi: 10.1093/jac/dkg028. [DOI] [PubMed] [Google Scholar]
- 21.Zhanel GG, Karlowsky JA, Harding GK, Carrie A, Mazzulli T, Low DE, et al. A Canadian national surveillance study of urinary tract isolates from outpatients: comparison of the activities of trimethoprim-sulfamethoxazole, ampicillin, mecillinam, nitrofurantoin and ciprofloxacin. Antimicrob Agents Chemother. 2000;44:1089–1092. doi: 10.1128/aac.44.4.1089-1092.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Castanheira M, Farrell SE, Krause KM, Jones RN, Sader HS. Contemporary diversity of β-lactamases among Enterobacteriaceae in the nine U.S. census regions and ceftazidime-avibactam activity tested against isolates producing the most prevalent β-lactamase groups. Antimicrob Agents Chemother. 2014;58:833–838. doi: 10.1128/AAC.01896-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nicolas-Chanoine MH, Bertrand X, Madec JY. Escherichia coli ST131, an intriguing clonal group. Clin Microbiol Rev. 2014;27:543–574. doi: 10.1128/CMR.00125-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hyle EP, Lipworth AD, Zaoutis TE, Nachamkin I, Bilker WB, Lautenbach E. Impact of inadequate initial antimicrobial therapy on mortality in infections due to extended-spectrum beta-lactamase-producing Enterobacteriaceae: variability by site of infection. Arch Intern Med. 2005;165:1375–1380. doi: 10.1001/archinte.165.12.1375. [DOI] [PubMed] [Google Scholar]
- 25.Alós JI, Serrano MG, Gómez-Garcés JL, Perianes J. Antibiotic resistance of Escherichia coli from community-acquired urinary tract infections in relation to demographic and clinical data. Clin Microbiol Infect. 2005;11:199–203. doi: 10.1111/j.1469-0691.2004.01057.x. [DOI] [PubMed] [Google Scholar]
- 26.Colodner R, Rock W, Chazan B, Keller N, Guy N, Sakran W, et al. Risk factors for the development of extended-spectrum beta-lactamase-producing bacteria in nonhospitalized patients. Eur J Clin Microbiol Infect Dis. 2004;23:163–167. doi: 10.1007/s10096-003-1084-2. [DOI] [PubMed] [Google Scholar]
- 27.Khanfar HS, Bindayna KM, Senok AC, Botta GA. Extended spectrum beta-lactamases (ESBL) in Escherichia coli and Klebsiella pneumoniae: trends in hospital and community settings. J Infect Dev Ctries. 2009;3:295–299. doi: 10.3855/jidc.127. [DOI] [PubMed] [Google Scholar]
- 28.Osthoff M, McGuinness SL, Wagen AZ, Eisen DP. Urinary tract infections due to extended-spectrum beta-lactamase-producing Gram-negative bacteria: identification of risk factors and outcome predictors in an Australian tertiary referral hospital. Int J Infect Dis. 2015;34:79–83. doi: 10.1016/j.ijid.2015.03.006. [DOI] [PubMed] [Google Scholar]
- 29.Lautenbach E, Patel JB, Bilker WB, Edelstein PH, Fishman NO. Extended-spectrum beta-lactamase-producing Escherichia coli and Klebsiella pneumoniae: risk factors for infection and impact of resistance on outcomes. Clin Infect Dis. 2001;32:1162–1171. doi: 10.1086/319757. [DOI] [PubMed] [Google Scholar]


