Abstract
Background and Aims:
De novo lipogenesis is increased in livers of patients with nonalcoholic steatohepatitis (NASH). Acetyl-CoA carboxylase catalyzes the rate-limiting step in this process. We evaluated the safety and efficacy of GS-0976, an inhibitor of acetyl-CoA carboxylase in liver, in a phase 2, randomized, placebo-controlled trial of patients with NASH.
Methods:
We analyzed data from 126 patients with hepatic steatosis ≥8%, based on the magnetic resonance imaging-estimated proton density fat fraction (MRI-PDFF), and liver stiffness ≥2.5 kPa, based on magnetic resonance elastography measurement, or historical biopsy consistent with NASH and F1-F3 fibrosis. Patients were randomly assigned (2:2:1) to groups given GS-0976 (20 mg), GS-0976 (5mg), or placebo daily for 12 weeks, from August 8, 2016 through July 18, 2017. Measures of hepatic steatosis, stiffness, serum markers of fibrosis, and plasma metabolomics were evaluated. The primary aims were to confirm previous findings and evaluate the relationship between dose and efficacy.
Results:
A ≥30% relative decrease from baseline in MRI-PDFF (PDFF response) occurred in 48% of patients given 20 mg GS-0976 (P=.004 vs placebo), 23% given 5 mg GS-0976 (P=.43 vs placebo), and 15% given placebo. Median relative decreases in MRI-PDFF were greater in patients given 20 mg GS-0976 (decrease of 29%) than those given placebo (decrease of 8%) (P=.002). Changes in magnetic resonance elastography-measured stiffness did not differ among groups, but a dose-dependent reduction in the fibrosis marker TIMP1 was observed in patients given 20 mg GS-0976. Plasma levels of acylcarnitine species also decreased in patients with a PDFF response given 20 mg GS-0976. GS-0976 was safe, but median relative increases of 11% and 13% in serum levels of triglycerides were observed in patients given GS-0976.
Conclusions:
In a randomized, placebo-controlled trial of patients with NASH, we found 12 weeks administration of 20 mg GS-0976 to reduce hepatic steatosis, selected markers of fibrosis, and liver biochemistry. ClinicalTrials.gov no: NCT02856555
Keywords: DNL, MRE, TIMP metallopeptidase inhibitor 1, effects
INTRODUCTION
Nonalcoholic steatohepatitis (NASH), which is estimated to affect up to 5% of the United States population, is characterized by the presence of hepatic steatosis, inflammation, hepatocyte ballooning, and variable degrees of fibrosis in patients who do not consume excess alcohol.1 Patients with advanced fibrosis due to NASH have the greatest risk of liver-related morbidity and mortality attributable to hepatic decompensation and hepatocellular carcinoma. NASH is the second leading indication for liver transplantation in the United States and its burden is projected to increase two-fold by 2030.2-4
A central feature of NASH is the aberrant regulation of lipids within hepatocytes. Increased endogenous fatty acid biosynthesis or de novo lipogenesis (DNL), impaired fatty acid oxidation, and the generation of biologically active fatty acid signaling molecules are important factors in NASH pathogenesis.5, 6 For instance, DNL is significantly increased in patients with NASH, even under fasting conditions.7, 8 It has been proposed that an imbalance in fatty acid biosynthesis, the inability of mitochondria to adequately metabolize fatty acids, along with the inability to store or export excess free fatty acids as neutral triglycerides results in lipotoxicity.5 This manifests in ballooning degeneration of hepatocytes, the stimulation of pro-inflammatory pathways, and the subsequent activation of hepatic stellate cells into collagen-producing myofibroblasts that mount a pro-fibrotic response.5, 9
The regulation of DNL plays a central role in fatty acid synthesis and catabolism. The rate-limiting step in DNL is conversion of acetyl-coenzyme A (acetyl-CoA) to malonyl-CoA by the enzyme acetyl-CoA carboxylase (ACC). ACC has two isoforms, which have distinct cellular distributions and hence unique functional roles in fatty acid metabolism. The ACC1 isoform catalyzes the formation of malonyl-CoA, the main substrate for fatty acid biosynthesis in the cytosol. ACC2 is localized in mitochondria where malonyl-CoA serves as a potent allosteric inhibitor of carnitine palmitoyl-transferase (CPT) 1, the carrier protein of fatty acids into mitochondria for β-oxidation.10, 11 Since inhibition of ACC1 and ACC2 isoforms would be expected to reduce DNL and enhance mitochondrial β-oxidation, respectively, these complementary roles support ACC inhibition as a potentially attractive therapeutic target in NASH.12-14
GS-0976 is a liver-targeted, small-molecule allosteric inhibitor of both ACC1 and ACC2 in development for the treatment of NASH. In pre-clinical models of diet and genetically-induced obesity, ACC blockade decreased hepatic steatosis, inflammation, and insulin resistance.12 A pilot study in overweight and obese individuals demonstrated that administration of a single dose of GS-0976 led to a marked dose-dependent inhibition of hepatic DNL.6 In an open label proof-of-concept trial, 10 NASH patients treated with GS-0976 20 mg daily for 12 weeks had a median decrease of 29% in hepatic DNL and significant reductions in hepatic fat by magnetic resonance imaging-proton density fat fraction (MRI-PDFF), liver stiffness by magnetic resonance elastography (MRE), and serum tissue inhibitor of metalloproteinase 1 (TIMP1), a marker of fibrogenesis.15
The objectives of the current phase 2 study were to: 1) confirm these findings in a larger patient population with a placebo control group; 2) evaluate whether a dose-response relationship exists for these efficacy parameters by comparing GS-0976 20 mg and 5 mg daily; and 3) confirm the safety of GS-0976 in patients with NASH.
METHODS
Patients
Eligible patients 18 to 75 years of age with a clinical diagnosis of nonalcoholic fatty liver disease (NAFLD) on the basis of imaging or liver biopsy within two years of screening were considered. Inclusion criteria included hepatic steatosis by MRI-PDFF ≥8% and liver stiffness by MRE ≥2.5 kPa during screening. Alternatively, patients with a liver biopsy within 12 months of screening consistent with a diagnosis of NASH with F1-F3 fibrosis according to the NASH Clinical Research Network (CRN) classification were eligible.16 We excluded patients with histologic or imaging evidence of cirrhosis; prior history of decompensated liver disease; or a FibroSure/FibroTest (LabCorp, Burlington, NC) result of ≥0.75 (unless a biopsy within 12 months excluded cirrhosis). Patients with alanine aminotransferase (ALT) >5 × the upper limit of normal (ULN), total bilirubin >1 × ULN, international normalized ratio (INR) >1.2, body mass index (BMI) <18 kg/m2, or platelet count less than 100,000/mL3 were also excluded.
Study Design
Patients in this phase 2, randomized, double-blind, placebo-controlled study were randomly assigned in a 2:2:1 ratio to receive GS-0976 20 mg, GS-0976 5 mg, or placebo orally once daily (without regard to food) for 12 weeks. Randomization was stratified by the presence or absence of diabetes mellitus as determined by medical history, use of medication for diabetes mellitus, or screening lab values if previously undiagnosed (ie, hemoglobin A1c [HbA1c] ≥6.5% or fasting plasma glucose ≥126 mg/dL).
Efficacy Assessments
Imaging
Longitudinal changes in liver fat and liver stiffness from baseline to week 12 were assessed using MRI-PDFF and two-dimensional MRE (60 Hz), respectively. An experienced central radiology group (University of California San Diego Radiology Reading Center) blinded to clinical and histologic data performed radiographic assessments as previously described.17, 18 Specifically, changes in these parameters in co-localized regions of interest were performed.18, 19 The proportion of patients who had at least a 30% relative decrease from baseline at week 12 in MRI-PDFF (herein referred to as PDFF response) was determined. This magnitude of MRI-PDFF reduction has been associated with histologic improvement in patients with NASH.20 Where available, liver stiffness and hepatic fat content by Controlled Attenuation Parameter (CAP) were also assessed using transient elastography (FibroScan®; Echosens, Paris, France) at baseline and week 12.
Serum Markers of Fibrosis and Liver Injury
Noninvasive markers of fibrosis, including the ELF test (Siemens, Tarrytown, NY) and its components (TIMP1, procollagen III N-terminal peptide [PIIINP], and hyaluronic acid [HA]) and FibroSure/FibroTest were measured. Serum markers of liver injury and function including ALT, asparate aminotransferase (AST), bilirubin, gamma-glutamyl transferase (GGT), and alkaline phosphatase were also collected. Change from baseline in serum levels of cytokeratin (CK)-18 M30 and M65 fractions were measured as indicators of hepatocellular apoptosis and necrosis, respectively (M30 Apoptosense and M65 ELISA; Diapharma, West Chester, OH).
Plasma Acylcarnitine Measurements
Blood was collected into EDTA tubes and processed to plasma from patients fasted at least 8 hours. Samples were stored at −80°C prior to analysis by Metabolon (Morrisville, NC, USA). Acylcarnitine species measurements were performed with Ultrahigh Performance Liquid Chromatography-Tandem Mass Spectroscopy (UHPLC-MS; Waters Acquity UHPLC, Waters Corporation, Milford, MA USA) and Hybrid Quadrupole-Orbitrap mass spectometer (Q-Exactive Thermo Fischer Scientific, Waltham, MA, USA) platforms in electrospray ionisation-positive and -negative modes, as previously described.21 Acylcarnitine species were identified by comparison to an in-house library of purified standards. Relative quantification of peaks was performed as area-under-the-curve detector ion counts by peak area integration. The mean relative standard deviation of internal standards for this study was 6%. The relative change in acylcarnitine species from baseline was assessed according to subgroups defined by treatment arm and the presence or absence of a PDFF response.
Safety Assessments
Safety was evaluated by assessment of clinical laboratory tests, physical examinations, vital signs measurements, and by documentation of adverse events. All safety data was collected from the time of the first dose of study drug to 30 days after the last dose of study drug.
Statistical Analyses
A formal sample size calculation was not performed; rather, the number of included patients was based on clinical experience with similar phase 2 studies. However, we calculated that if 4% of the planned 25 patients receiving placebo and 32% of the planned 50 patients receiving GS-0976 20 mg had a ≥30% relative reduction in hepatic steatosis by MRI-PDFF at week 12, this sample size would provide 80% power to detect a difference between the groups based on a two-sided Fisher’s exact test at a significance level of p=0.05. All statistical analyses were performed using SAS, version 9.4 (SAS Institute Inc., Cary, NC, USA).
The primary endpoint of the study was safety. Safety and efficacy endpoints were analyzed in all patients who were randomized and received at least one dose of study drug. Exploratory efficacy endpoints included change from baseline in steatosis as measured by MRI-PDFF and CAP, and the proportion of patients with ≥30% reduction in MRI-PDFF from baseline to week 12. We also assessed changes in liver stiffness (as measured by MRE and transient elastography), and noninvasive markers of fibrosis and liver injury. Since obesity may influence the reliability of measurements of liver stiffness and CAP by transient elastography, subgroup analyses were conducted according to the type of probe used (M vs. XL) for these measurements.22, 23
Study Oversight
The study was approved by the institutional review board or independent ethics committee at participating sites and conducted in compliance with the Declaration of Helsinki, Good Clinical Practice guidelines, and local regulatory requirements. The study was designed and conducted by the sponsor (Gilead Sciences) in collaboration with the principal investigator, according to the protocol. The sponsor collected the data, monitored study conduct, and performed the statistical analyses. An independent data safety monitoring committee reviewed the progress of the study. All authors had access to the data and assumed responsibility for the integrity and completeness of the reported data. All authors reviewed and approved the final manuscript.
RESULTS
Baseline Characteristics
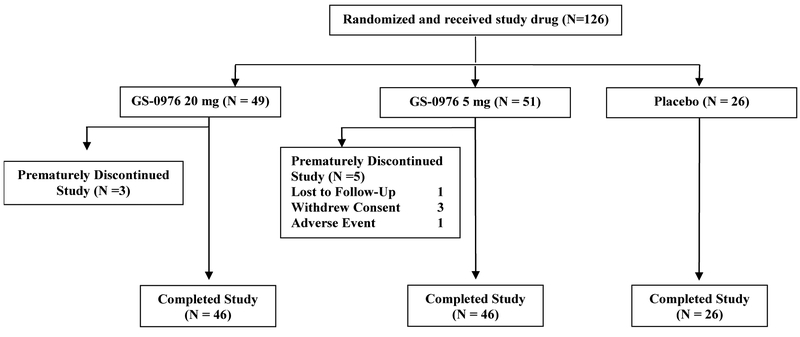
Between August 8, 2016 and July 18, 2017, a total of 433 patients were screened and 127 were randomly assigned to treatment at 41 sites in the United States (Figure 1). Reasons for screen failure are provided in the Supplementary Appendix. One patient randomized to receive 20 mg of GS-0976 did not initiate treatment and was excluded from the analysis. The baseline characteristics of the study population are provided in Table 1. Patient characteristics were similar across treatment groups, with several exceptions. Overall, the median age was 56 years (range, 21 to 74) and the majority were female (65%), white (87%), and had diabetes mellitus (60%). Compared with the placebo group, a larger proportion of patients in the GS-0976 5 mg group were male (27% vs. 41%) and more patients in the GS-0976 20 mg group were Hispanic/Latino (31% vs. 47%) and had diabetes (58% vs. 65%). At baseline, the median (IQR) hepatic fat content by MRI-PDFF was 14.4% (11.1, 19.0) and median liver stiffness by MRE and transient elastography were 3.4 kPa (3.0, 4.2) and 8.7 kPa (6.8, 14.3), respectively. In total, 41% of patients had an MRE-stiffness ≥3.64 kPa, consistent with advanced (F3-F4) fibrosis.24
Figure 1. Flow Diagram of Study Participants.
Table 1.
Demographics and Baseline Characteristics
| Characteristic | GS-0976 20 mg (n=49) |
GS-0976 5 mg (n=51) |
Placebo (n=26) |
|---|---|---|---|
| Age, year (range) | 56 (46, 62) | 55 (47, 61) | 58 (49, 64) |
| Male, n (%) | 16 (33) | 21 (41) | 7 (27) |
| White race, n (%) | 44 (90) | 42 (82) | 23 (89) |
| Weight, kg | 93 (82, 108) | 93 (81, 112) | 93 (79, 109) |
| BMI, kg/m2 | 33 (28, 37) | 33 (30, 36) | 33 (28, 35) |
| Diabetes, n (%) | 32 (65) | 29 (57) | 15 (58) |
| ALT, U/L | 54 (40, 76) | 61 (39, 89) | 47 (34, 55) |
| AST, U/L | 37 (29, 59) | 46 (30, 67) | 37 (27, 50) |
| ALP, U/L | 93 (72, 112) | 77 (62, 100) | 88 (74, 108) |
| GGT, U/L | 52 (33, 102) | 52 (37, 118) | 38 (29, 91) |
| Total bilirubin, mg/dL | 0.4 (0.3, 0.6) | 0.5 (0.4, 0.6) | 0.5 (0.4, 0.7) |
| Fasting glucose, mg/dL | 117 (97, 149) | 102 (93, 120) | 121 (94, 157) |
| Fasting insulin, μIU/mL | 26.1 (17.0, 48.9) | 16.4 (10.7, 30.4) | 19.9 (14.2, 34.4) |
| HbA1c, % | 6.5 (5.8, 7.8) | 5.9 (5.4, 6.8) | 6.0 (5.7, 8.2) |
| HOMA-IR | 8.7 (5.3, 13.0) | 4.1 (2.5, 8.9) | 6.6 (3.8, 8.8) |
| Cholesterol, mg/dL | 179 (152, 203) | 188 (151, 225) | 194 (171, 226) |
| LDL-C, mg/dL | 99.0 (81.7, 125.0) | 103.6 (76.4, 132.1) | 112.4 (96.2, 126.6) |
| HDL-C, mg/dL | 42 (35, 53) | 48 (39, 55) | 48 (41, 57) |
| Triglycerides, mg/dL | 160 (125, 201) | 154 (105, 209) | 152 (116, 185) |
| MRI-PDFF, % | 15.9 (10.9, 20.1) | 15.7 (11.3, 19.6) | 13.6 (9.4, 15.2) |
| CAP, dB/m | 331 (304, 359) | 311 (286, 364) | 342 (272, 350) |
| MRE-stiffness, kPa | 3.40 (2.96, 3.99) | 3.42 (2.89, 4.47) | 3.46 (3.15, 4.20) |
| Liver stiffness by transient elastography, kPa |
8.5 (6.6, 14.5) | 9.0 (7.1, 16.5) | 8.7 (7.2, 12.5) |
| ELF score | 9.24 (8.75, 10.34) | 9.79 (8.94, 10.28) | 9.47 (8.80, 10.18) |
| FibroSure/FibroTest | 0.29 (0.13, 0.43) | 0.24 (0.14, 0.39) | 0.27 (0.15, 0.42) |
| CK18 M30 | 306 (219, 410) | 374 (200, 567) | 247 (188, 417) |
| CK18 M65 | 436 (247, 679) | 540 (206, 1172) | 369 (196, 886) |
Unless otherwise specified, values are median (IQR) or n (%).
Hepatic Steatosis by MRI-PDFF and CAP
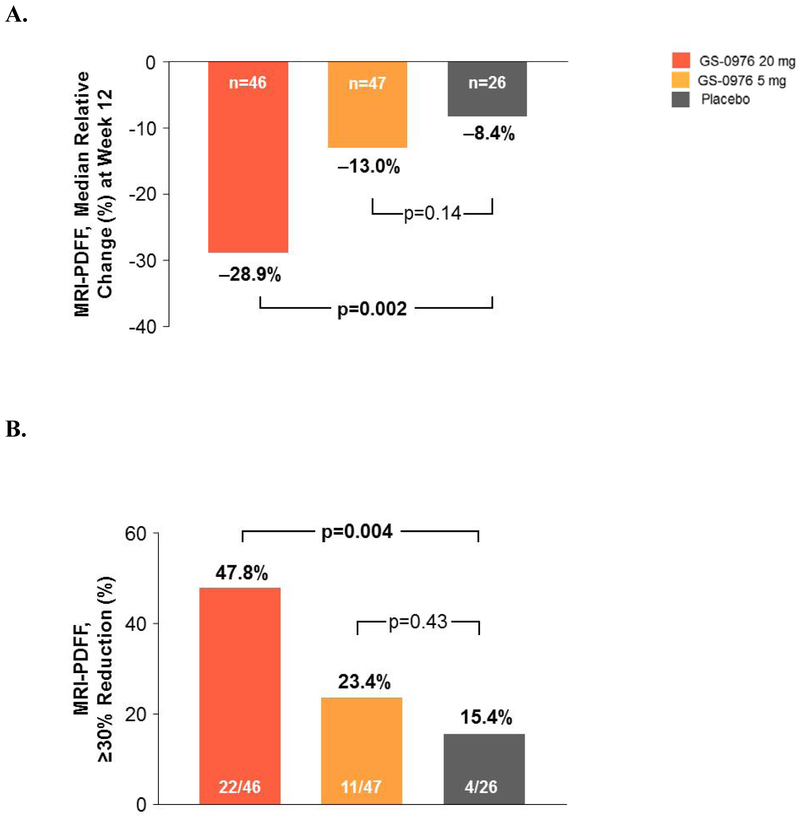
MRI-PDFF and MRE assessments at baseline and week 12 were available for 46 of the 49 patients randomized to receive 20 mg of GS-0976, 47 of the 51 patients randomized to receive 5 mg of GS-0976, and for all 26 of the patients on placebo. The median (IQR) relative percent change in MRI-PDFF from baseline to week 12 was −29% (−48, −12) in patients receiving GS-0976 20 mg, −13% (−29, 0.6) for patients receiving GS-0976 5 mg, and −8% (−18, 10) for those receiving placebo (Figure 2A). Compared with placebo, the difference between MRI-PDFF reductions in patients receiving GS-0976 20 mg was statistically significant (p=0.002), but not for those receiving GS-0976 5 mg (p=0.14). Relative reductions in MRI-PDFF of at least 30% were seen in 48% of patients (22/46) receiving GS-0976 20 mg (p=0.004 vs placebo), 23% of patients (11/47) receiving GS-0976 5 mg (p=0.43 vs placebo), and 15% of patients (4/26) receiving placebo (Figure 2B). When available, hepatic steatosis was also measured by CAP. Dose-dependent reductions in CAP were observed in GS-0976-treated patients that paralleled changes in MRI-PDFF, but these differences were not statistically significant (Supplementary Figure 1A). Differences in changes in CAP between GS-0976-treated patients and those on placebo were most evident when measured using the XL probe versus the M probe (Supplementary Figure 1B).
Figure 2. Reduction in MRI-PDFF with GS-0976.
(A) Relative changes in hepatic steatosis by MRI-PDFF between baseline and week 12 according to treatment group. Data are median (IQR).
(B) Proportion of patients that achieved a ≥30% relative reduction in hepatic steatosis by MRI-PDFF between baseline and week 12.
Liver Stiffness by MRE and Transient Elastography
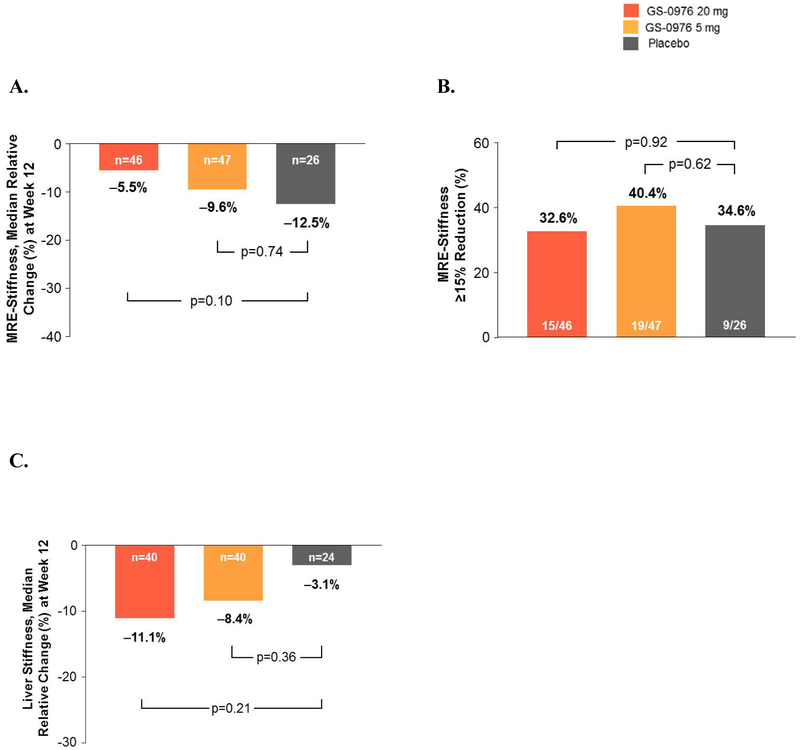
Changes in liver stiffness measured by MRE did not differ among the treatment groups. Median (IQR) relative percent change from baseline to week 12 was −6% (−17, 8) for patients receiving GS-0976 20 mg, −10% (−23, 3) for patients receiving GS-0976 5 mg, and −13% (−23, −2) for those receiving placebo (Figure 3A). Reductions in MRE-stiffness of at least 15% were also similar between treatment groups (Figure 3B). Liver stiffness measurements by transient elastography at baseline and week 12 were available for 40 of the 49 patients randomized to receive GS-0976 20 mg, 40 of the 51 patients randomized to receive GS-0976 5 mg, and for 24 of the 26 patients on placebo. Liver stiffness reductions from baseline to week 12 were greater in GS-0976-treated patients versus placebo, but these differences were not statistically significant (Figure 3C). Changes in liver stiffness by transient elastography appeared dependent upon the type of probe used, as well as consistent use of the same type of probe over time. Using the M probe, no differences in change of liver stiffness from baseline to week 12 were observed between treatment groups (Supplementary Figure 2A). However, in patients measured with the XL probe, a statistically significant decrease in liver stiffness was observed in GS-0976-treated patients compared with placebo. Specifically, median (IQR) relative changes from baseline to week 12 were −7.2% (−32.6, 7.8) in patients treated with GS-0976 20 mg (p=0.032 vs placebo), −3.6% (−23.8, 8.2) in patients treated with GS-0976 5 mg (p=0.039 vs placebo), and +30.6% (4.5, 63.8) in those treated with placebo (Supplementary Figure 2A). Consistent use of the same probe between measurements was also associated with declines in liver stiffness in the GS-0976-treated groups compared with placebo (Supplementary Figure 2B).
Figure 3. Liver stiffness measured by MRE and Transient Elastography.
(A) Relative changes in MRE-stiffness between baseline and week 12 according to treatment group. Data are median (IQR).
(B)Proportion of patients that achieved a ≥15% relative reduction in MRE-stiffness between baseline and week 12.
(C)Relative changes in liver stiffness by transient elastography between baseline and week 12 according to treatment group. Data are median (IQR).
Liver Biochemistry and Markers of Fibrosis and Liver Injury
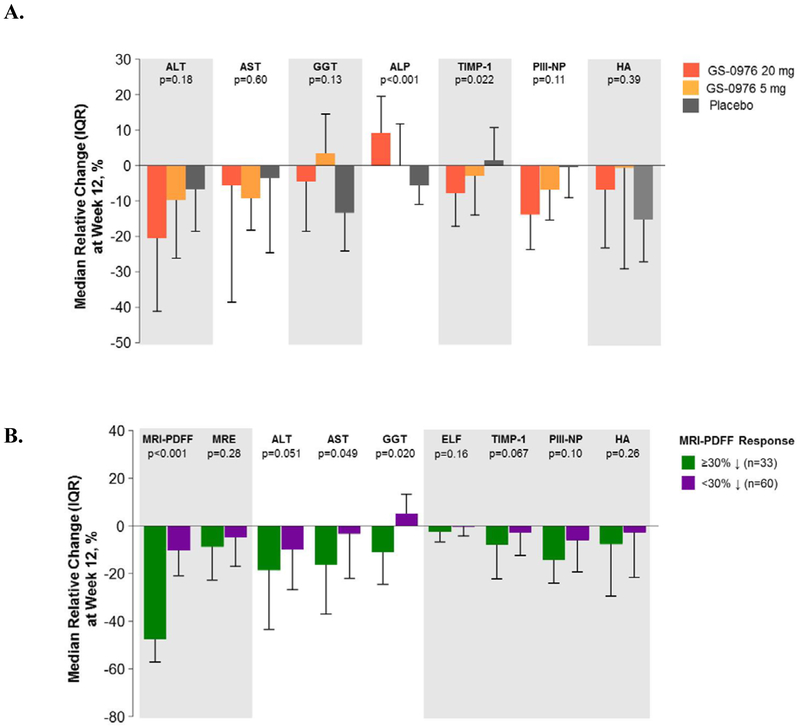
Patients receiving GS-0976 had greater median relative reductions in ALT, TIMP1, and PIII-NP than patients receiving placebo, with only TIMP1 being statistically significant between patients receiving GS-0976 20 mg versus placebo (p=0.022) (Figure 4A). Median alkaline phosphatase increased by 9% in patients receiving GS-0976 20 mg (p<0.001 vs placebo), but was unchanged or decreased in those on GS-0976 5 mg and placebo, respectively. Changes in other liver biochemistry tests and in the ELF score were not significantly different among the treatment groups. Compared to PDFF-nonresponders (n=60), the 33 patients who achieved at least a 30% relative reduction from baseline in MRI-PDFF showed consistent decreases in liver biochemistry (ALT, AST, GGT) and the ELF score and its components (Figure 4B).
Figure 4. Liver biochemistry and serum markers of fibrosis are decreased with GS-0976 treatment.
(A) Changes in liver biochemistry and serum markers of fibrosis between baseline and week 12 according to treatment group. Data are median (IQR).
(B) Changes in liver biochemistry and serum markers of fibrosis between baseline and week 12 according to PDFF response. Data are median (IQR).
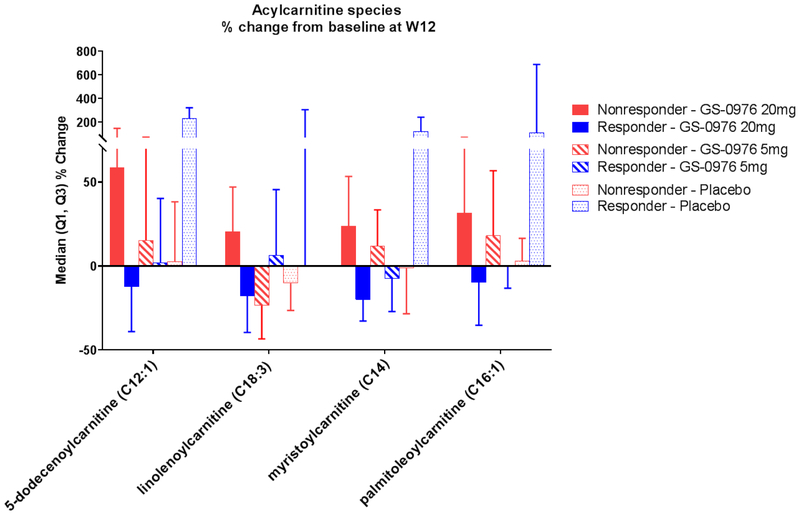
Acylcarnitine Species Decrease in Patients on GS-0976 20 mg with a PDFF Response
Since the relative abundance of peripheral acylcarnitine species reflects the efficiency of mitochondrial β-oxidation,25, 26 an unbiased plasma-based metabolomics screen was performed to identify changes relevant to ACC inhibition with GS-0976 treatment. Patients were categorized across treatment groups and according to MRI-PDFF response. Although minimal differences were observed between treatment groups, multiple long and medium-chain acylcarnitine species were significantly suppressed in the GS-0976 20 mg treated group who achieved a PDFF response versus those without a PDFF response (Figure 5, Supplementary Table 1). On the contrary, patients in the GS-0976 5 mg or placebo groups showed minimal changes in acylcarnitine species when compared according to PDFF response. These data support measurement of plasma acylcarnitines as potential peripheral markers of improved fatty acid catabolism with ACC inhibition by GS-0976.
Figure 5.
Relative changes of plasma acylcarnitine species between baseline and week 12 in patients treated with GS-0976 according to PDFF response.
Safety
The primary assessment of this study was the safety and tolerability of GS-0976. Adverse events (AEs) were experienced by 71% of patients receiving both 20 mg and 5 mg of GS-0976, and by 62% of patients receiving placebo (Table 2). The most common AEs among patients receiving GS-0976 were nausea, abdominal pain, diarrhea, and headache. Five patients experienced serious AEs (three on GS-0976 20 mg and two on GS-0976 5 mg); none of these were considered study drug-related (Table 2). Two patients, both in the GS-0976 5 mg group, prematurely discontinued study treatment due to AEs. A 65-year-old woman discontinued study drug on day 26 due to night sweats (deemed by the investigator to be related to study drug) and a 46-year old man discontinued study drug on day 83 due to diverticulitis (deemed unrelated).
Table 2.
Adverse Events
| Event | GS-0976 20 mg (n=49) |
GS-0976 5 mg (n=51) |
Placebo (n=26) |
|---|---|---|---|
| Any TEAE | 35 (71) | 36 (71) | 16 (62) |
| TEAEs leading to premature study discontinuation | 0 | 2 (4) | 0 |
| Grade 3/4 TEAEs | 4 (8) | 1 (2) | 1 (4) |
| Serious AEs | 3 (4)† | 2 (4)‡ | 0 |
| Common AEs * | |||
| Nausea | 7 (14) | 4 (8) | 2 (8) |
| Abdominal pain | 6 (12) | 2 (4) | 1 (4) |
| Diarrhea | 6 (12) | 5 (10) | 0 |
| Influenza | 5 (10) | 0 | 1 (4) |
| Vomiting | 5 (10) | 1 (2) | 0 |
| Upper abdominal pain | 4 (8) | 2 (4) | 1 (4) |
| Fatigue | 4 (8) | 2 (4) | 3 (12) |
| Headache | 4 (8) | 5 (10) | 1 (4) |
| Sinusitis | 3 (6) | 2 (4) | 3 (12) |
| Patients with grade 3/4 laboratory abnormalities | 11 (22) | 14 (27) | 1 (4) |
| ALT | 0 | 2 (4) | 0 |
| AST | 2 (4) | 2 (4) | 0 |
| GGT | 2 (4) | 0 | 1 (4) |
| Glucose | 7 (14) | 3 (6) | 0 |
| Phosphate | 0 | 1 (2) | 0 |
| Sodium | 0 | 2 (4) | 1 (4) |
| Triglycerides | 7 (14) | 9 (18) | 0 |
Unless otherwise specified, values are n (%).
AE, adverse event; TEAE, treatment-emergent.adverse event.
AEs observed in >5% of patients in any group.
One 58-year-old woman with a history of hypertension and diabetes experienced transient cerebral ischemia 10 days after the end of treatment, and sepsis 12 days after the end of treatment; and one 47-year-old woman experienced abdominal pain and worsening hepatic encephalopathy 20 days after the end of treatment. One 61 year-old woman with a history of diabetes was diagnosed with B-cell lymphoma 274 days after the end of treatment.
One 56-year-old woman had acute febrile illness on day 74 of treatment, and one 46-year-old man had diverticulitis on day 83 of treatment.
The most frequent grade 3 or 4 laboratory abnormality was clinically asymptomatic hypertriglyceridemia (>500 mg/dL), observed in 14% of patients receiving GS-0976 20 mg, 18% of patients receiving GS-0976 5 mg, and none of the patients receiving placebo. After 12 weeks of treatment, the median increase in triglycerides from baseline was +14 mg/dL (+11%) among patients receiving GS-0976 20 mg, +16 mg/dL (+13%) for patients receiving GS-0976 5 mg, and –6 mg/dL (−4%) among patients receiving placebo (Supplementary Figure 3A). In total, 16 patients treated with GS-0976 developed grade 3 or 4 hypertriglyceridemia. In a multivariate logistic regression model including demographics, body weight, liver biochemistry, serum fibrosis markers, imaging parameters, glycemic indices, and lipids at baseline, grade 3 or 4 hypertriglyceridemia was associated with baseline triglycerides ≥250 mg/dL (67% [10/15] vs. 7% [6/84] of patients with baseline triglycerides <250 mg/dL; odds ratio [OR] 105.46; 95% CI 12.81-868.20 [p<0.001]) and liver stiffness by MRE (OR per 1-kPa: 1.93; 95% CI 1.20-3.09 [p=0.007]). Among patients with baseline triglycerides <250 mg/dL, only 1 patient (2%), who was treated with GS-0976 5 mg, experienced grade 4 hypertriglyceridemia (>1000 mg/dL). Four of the 16 patients with treatment-emergent triglycerides >500 mg/dL received fibrates or fish oil, and all of these patients had decreases in triglycerides to <500 mg/dL by week 12. By the end of study drug dosing, seven of the 12 remaining patients experienced decreases of their triglycerides to <500 mg/dL despite ongoing treatment with GS-0976 (Supplementary Figure 3B). Given the elevation in serum TG, advanced lipoprofile testing by NMR was performed (Supplementary Table 2). The lipoprofile showed that treatment with GS-0976 20 mg led to an increase in very low density lipoprotein (VLDL) particles and VLDL triglyceride that peaked at week 1 and returned to baseline by week 12. However, total cholesterol, high density lipoprotein (HDL) cholesterol, and low density lipoprotein (LDL) cholesterol and particle number, and the number of small LDL particles, did not differ at week 12 versus baseline. There were no significant changes in glucose, insulin, HbA1c, or body weight with GS-0976 20 or 5 mg compared to placebo (Supplementary Figure 4).
DISCUSSION
In this randomized, double-blind, placebo-controlled phase 2 trial in non-cirrhotic patients with NASH, treatment with GS-0976 20 mg daily for 12 weeks was safe and led to significant reductions in hepatic fat content by MRI-PDFF and decreased serum levels of TIMP1, an endogenous regulator of matrix metalloproteinases that promotes liver fibrosis.27 Moreover, consistent reductions in liver biochemistry in those patients who achieved ≥30% reduction in MRI-PDFF, indicative of reduced liver injury, were observed. Patients who achieved this level of MRI-PDFF reduction on GS-0976 20 mg daily also demonstrated decreased peripheral levels of medium and long-chain acylcarnitine species, a surrogate marker of increased beta oxidation of fatty acids.28
An important efficacy endpoint of the study was the proportion of patients who achieved a ≥30% relative reduction in liver fat by MRI-PDFF at 12 weeks versus baseline. Nearly 50% of the patients in the 20mg arm achieved this endpoint, which has been associated with histologic improvement in patients with NASH.20 Although there were no significant differences in MRE-stiffness between groups, we observed a dose-dependent trend toward reduced liver stiffness when measurements were made using transient elastography. Measurements using the XL probe or those taken with the same probe throughout the study identified significant reductions in liver stiffness in GS-0976-treated patients versus those on placebo. The reductions in serum TIMP1 and PIII-NP in PDFF responders further suggest that GS-0976 may have beneficial effects on liver fibrosis. Anti-fibrotic effects have been reported with ACC inhibition in pre-clinical models of NASH and fibrosis.29 A statistically significant reduction in TIMP1 and decrease in PIIINP were also observed in an independent 12 week proof-of-concept study with GS-0976.15 Whether these anti-fibrotic effects are due to a direct effect (e.g. on activated myofibroblasts) or secondary to reduced lipotoxicity warrants further investigation.
A notable finding of this study is the decrease in multiple, medium and long-chain acylcarnitine species observed in patients treated with GS-0976 20 mg that experienced a PDFF response. Previous studies of patients with NASH have demonstrated increased levels of both plasma and hepatic acylcarnitine metabolites.28, 30 Excess acylcarnitines may reflect the inability of mitochondria to metabolize fatty acids, although whether mitochondria have reduced or improved function in human NAFLD remains controversial.31-34 In mitochondria, ACC2 has a major functional role in inhibiting β-oxidation through its production of malonyl-CoA which inhibits CPT1, the major transport shuttle for fatty acids to enter the mitochondria. This is evident with genetic deletion of ACC2 in mice, where hepatic triglycerides are reduced and fatty acid oxidation continues unabated even in the presence of insulin challenge or high fat diets.35, 36 Thus, the reduction of acylcarnitine species in PDFF responders in this study suggests that mitochondrial function may be improved with GS-0976 and validates the strategy of targeting both ACC isoforms. Future studies will evaluate the utility of targeted profiling of these acylcarnitine metabolites for monitoring the pharmacodynamic activity of GS-0976.
Although GS-0976 treatment was safe and well tolerated, asymptomatic and dose-independent hypertriglyceridemia (>500 mg/dL) was observed in 16% of GS-0976-treated patients. In a recent study of another liver-targeted, small molecule ACC inhibitor (MK-4074), Kim and colleagues reported near doubling of serum triglyceride concentrations after four weeks of treatment in healthy volunteers.37 Based on data from a genetic model of ACC ablation (ACC double knockout mice), hypertriglyceridemia with MK-4074 was attributed to a marked increase in hepatic VLDL export due to SREBP-1c activation.38 A recent pre-clinical paper identified distinct mechanisms of triglyceride elevation in rodents with pharmacologic ACC 1/2 blockade versus those with genetic deletion of ACC.39 With ACC inhibition, PPARα expression was diminished at levels of DNL inhibition achieved in our study with GS-0976. This led to reduced trans-repression of apolipoprotein C3 (apoC3), higher serum apoC3 levels, diminished lipoprotein lipase activity, and reduced clearance of triglycerides from the serum.39 These effects of reduced triglyceride clearance were more pronounced than the contribution of increased VLDL export due to SREBP-1c activation. This increase in serum triglycerides was mitigated by fibrate administration in rodents. Similar results were also observed in patients who were treated with fenofibrate in the current study (see below). Overall, these findings in pre-clinical models of NASH and in patients in our study suggest that the mechanism of increased triglycerides seen with pharmacologic blockade of ACC differs from that seen with ACC 1 and 2 double knockout mice.
Functional differences exist between the current study with GS-0976 and the data reported by Kim and colleagues. In contrast to the near complete suppression of DNL in nearly all healthy volunteers given MK-4704,37 NASH patients treated with GS-0976 20 mg once daily experienced a median relative decline of 29% in hepatic DNL after 12 weeks of treatment in a proof-of-concept study. This relatively modest degree of DNL inhibition likely explains the comparatively small increase in serum triglycerides observed in the current study. Of note, grade 3 or 4 hypertriglyceridemia (>500 mg/dL) in this study was observed predominantly in patients with pre-existing triglycerides >250 mg/dL, likely due to a pre-existing defect in lipoprotein metabolism. These data suggest that restriction of GS-0976 treatment to patients with serum triglycerides <250 mg/dL at baseline may be a useful strategy to mitigate on-treatment hypertriglyceridemia with this agent. Importantly, despite ongoing GS-0976 therapy in all patients, triglyceride elevations appeared to peak after 1 week of therapy, resolved spontaneously in many affected individuals, and were not associated with changes in atherogenic lipoproteins (e.g. LDL cholesterol or particle number). These data suggest that these lipid changes are transient and unlikely to be associated with increased cardiovascular risk. However, longer term studies are necessary to confirm this hypothesis considering the increased background risk of cardiovascular disease in patients with NASH. Finally, the resolution of grade 3 or 4 hypertriglyceridemia in all patients started on fibrates or fish oil support available pre-clinical data (see above) and suggest that co-administration of these agents is an effective management strategy.
Another laboratory finding observed during the study was the small, but statistically significant, increase in serum alkaline phosphatase concentration (~9%) in the GS-0976 20 mg group. The etiology and clinical significance of this finding are unclear; however, no patients reported symptoms suggestive of hepatobiliary or bone disease.
Our study has several limitations that warrant discussion. First, due to the short 12-week duration, liver biopsies were not considered feasible to confirm the histologic benefits of ACC inhibition with GS-0976. Second, patients with cirrhosis were excluded due to a lack of pharmacokinetic data for GS-0976 in this patient population when this study was initiated. Nevertheless, in patients with evidence of advanced fibrosis at baseline (MRE-stiffness >3.64 kPa), which comprised approximately 40% of the cohort, similar reductions in MRI-PDFF and TIMP1 as observed in the entire cohort treated with GS-0976 20 mg were observed (data not shown). Since hepatic DNL is elevated across the spectrum of NASH severity, including patients with cirrhosis, future studies will evaluate the safety and efficacy of GS-0976 in patients with compensated cirrhosis due to NASH.
In summary, in this phase 2, randomized placebo-controlled trial of patients with NASH, 12 week therapy with GS-0976 20 mg, a liver-targeted, small molecule inhibitor of ACC, demonstrated significant improvements in hepatic steatosis and markers of fibrosis. Reductions of multiple plasma acylcarnitine species in GS-0976-treated patients with a PDFF response suggest that these metabolites may be useful surrogate markers of enhanced mitochondrial β-oxidation during GS-0976 treatment. These results support future studies to evaluate the safety and efficacy of targeting ACC with GS-0976 in patients with NASH.
Supplementary Material
Acknowledgments
Grant support: Supported by Gilead Sciences.
Abbreviations:
- ACC
acetyl-coA carboxylase
- MRI-PDFF
magnetic resonance imaging-proton density fat fraction
- MRE
magnetic resonance elastography
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Role of the study sponsor: The study was designed and conducted by the sponsor (Gilead Sciences) in collaboration with the principal investigator, according to the protocol. The sponsor collected the data, monitored study conduct, and performed the statistical analyses.
Conflicts of Interest:
Rohit Loomba has served on advisory committees or review panels for Galmed Inc, Tobira Inc, Arrowhead Research Inc, has served as a consultant for Gilead Inc, Corgenix Inc, Janssen and Janssen Inc, Zafgen Inc, Celgene Inc, Alnylam Inc, Inanta Inc, Deutrx Inc, and has received grant/research support from Daiichi Sankyo Inc, AGA, Merck Inc, Promedior Inc, Kinemed Inc, Immuron Inc, and Adheron Inc.
Zeid Kayali discloses no conflicts of interest.
Mazen Noureddin has served on advisory committees or review panels for EchoSense, OWL, Abbott, Intercept, has received grant/research support from Genfit, Galectin, Shire, Conatus, Galmed, Zydus, Gilead, has served as a speaker for Echosense, and holds stock in Anaeots.
Peter Ruane discloses no conflicts of interest.
Eric J. Lawitz has served on advisory committees or review panels for AbbVie, Achillion Pharmaceuticals, Regulus, Theravance, Enanta, Idenix Pharmaceuticals, Janssen, Merck & Co, Novartis, Gilead, has received grant/research support from AbbVie, Achillion Pharmaceuticals, Boehringer Ingelheim, Bristol-Myers Squibb, Gilead Sciences, GlaxoSmithKline, Idenix Pharmaceuticals, Intercept Pharmaceuticals, Janssen, Merck & Co, Novartis, Nitto Denko, Theravance, Salix, Enanta, has served as a speaker for Gilead, Janssen, AbbVie, Bristol Meyers Squibb, Merck, Intercept.
Michael Bennett discloses no conflicts of interest.
Lulu Wang, Eliza Harting, Jacqueline Tarrant, Bryan J. McColgan, Chuhan Chung, Adrian S. Ray, G. Mani Subramanian, and Robert P. Myers, are employees of and hold stock in Gilead Sciences, Inc.
Michael S. Middleton has served as a consultant for Bracco, Merge, has received grant/research support from Merck, Janssen, Alexion, AstraZeneca, Bristol-Myers Squibb, Galmed, General Electric, Guerbet, NuSirt, Sanofi, Shire, Celgene, Genentech, Intercept, Zydus, Gilead, Isis, Genzyme, Pfizer, Siemens, Bayer, Synageva, has served as an independent contractor for Takeda, and holds stock in Pfizer, General Electric.
Michelle Lai discloses no conflicts of interest.
Michael Charlton has served as a consultant for Novartis, Gilead, Intercept, NGM Bio, and has received grant/research support from Novartis, Gilead, Intercept, Genfit, Bristol Myers Squibb
Stephen A. Harrison has served on advisory committees or review panels for Fibrogen, Allergan, Intercept, has served as a consultant for NGM Bio, Madrigal, Octeta, Pfizer, Perspectum, Chronic Liver Disease Foundation, Genfit, has received grant/research Support from Galectin, Conatus, and has served as a speaker for Echosens.
REFERENCES
- 1.Chalasani N, Wilson L, Kleiner DE, et al. Relationship of steatosis grade and zonal location to histological features of steatohepatitis in adult patients with non-alcoholic fatty liver disease. J Hepatol 2008;48:829–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Younossi ZM, Blissett D, Blissett R, et al. The economic and clinical burden of nonalcoholic fatty liver disease in the United States and Europe. Hepatology 2016;64:1577–1586. [DOI] [PubMed] [Google Scholar]
- 3.Estes C, Razavi H, Loomba R, et al. Modeling the epidemic of nonalcoholic fatty liver disease demonstrates an exponential increase in burden of disease. Hepatology 2018;67:123–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wong RJ, Aguilar M, Cheung R, et al. Nonalcoholic steatohepatitis is the second leading etiology of liver disease among adults awaiting liver transplantation in the United States. Gastroenterology 2015;148:547–55. [DOI] [PubMed] [Google Scholar]
- 5.Neuschwander-Tetri BA. Hepatic lipotoxicity and the pathogenesis of nonalcoholic steatohepatitis: the central role of nontriglyceride fatty acid metabolites. Hepatology 2010;52:774–88. [DOI] [PubMed] [Google Scholar]
- 6.Stiede K, Miao W, Blanchette HS, et al. Acetyl-coenzyme A carboxylase inhibition reduces de novo lipogenesis in overweight male subjects: A randomized, double-blind, crossover study. Hepatology 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Donnelly KL, Smith CI, Schwarzenberg SJ, et al. Sources of fatty acids stored in liver and secreted via lipoproteins in patients with nonalcoholic fatty liver disease. J Clin Invest 2005;115:1343–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lambert JE, Ramos-Roman MA, Browning JD, et al. Increased de novo lipogenesis is a distinct characteristic of individuals with nonalcoholic fatty liver disease. Gastroenterology 2014;146:726–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Koyama Y, Brenner DA. Liver inflammation and fibrosis. J Clin Invest 2017;127:55–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Abu-Elheiga L, Brinkley WR, Zhong L, et al. The subcellular localization of acetyl-CoA carboxylase 2. Proc Natl Acad Sci U S A 2000;97:1444–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Abu-Elheiga L, Jayakumar A, Baldini A, et al. Human acetyl-CoA carboxylase: characterization, molecular cloning, and evidence for two isoforms. Proc Natl Acad Sci U S A 1995;92:4011–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Harriman G, Greenwood J, Bhat S, et al. Acetyl-CoA carboxylase inhibition by ND-630 reduces hepatic steatosis, improves insulin sensitivity, and modulates dyslipidemia in rats. Proc Natl Acad Sci U S A 2016;113:E1796–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Harwood HJ Jr. Acetyl-CoA carboxylase inhibition for the treatment of metabolic syndrome. Curr Opin Investig Drugs 2004;5:283–9. [PubMed] [Google Scholar]
- 14.Harwood HJ Jr. Treating the metabolic syndrome: acetyl-CoA carboxylase inhibition. Expert Opin Ther Targets 2005;9:267–81. [DOI] [PubMed] [Google Scholar]
- 15.Lawitz EJ, Coste A, Poordad F, et al. Acetyl-CoA Carboxylase Inhibitor GS-0976 for 12 Weeks Reduces Hepatic De Novo Lipogenesis and Steatosis in Patients with Nonalcoholic Steatohepatitis. Clin Gastroenterol Hepatol 2018. [DOI] [PubMed] [Google Scholar]
- 16.Kleiner DE, Brunt EM, Van Natta M, et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology 2005;41:1313–21. [DOI] [PubMed] [Google Scholar]
- 17.Asrani SK, Talwalkar JA, Kamath PS, et al. Role of magnetic resonance elastography in compensated and decompensated liver disease. J Hepatol 2014;60:934–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Loomba R, Lawitz E, Mantry PS, et al. The ASK1 inhibitor selonsertib in patients with nonalcoholic steatohepatitis: A randomized, phase 2 trial. Hepatology 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Venkatesh SK, Yin M, Ehman RL. Magnetic resonance elastography of liver: technique, analysis, and clinical applications. J Magn Reson Imaging 2013;37:544–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Patel J, Bettencourt R, Cui J, et al. Association of noninvasive quantitative decline in liver fat content on MRI with histologic response in nonalcoholic steatohepatitis. Therap Adv Gastroenterol 2016;9:692–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Evans AM, DeHaven CD, Barrett T, et al. Integrated, nontargeted ultrahigh performance liquid chromatography/electrospray ionization tandem mass spectrometry platform for the identification and relative quantification of the small-molecule complement of biological systems. Anal Chem 2009;81:6656–67. [DOI] [PubMed] [Google Scholar]
- 22.Myers RP, Pomier-Layrargues G, Kirsch R, et al. Feasibility and diagnostic performance of the FibroScan XL probe for liver stiffness measurement in overweight and obese patients. Hepatology 2012;55:199–208. [DOI] [PubMed] [Google Scholar]
- 23.Myers RP, Pollett A, Kirsch R, et al. Controlled Attenuation Parameter (CAP): a noninvasive method for the detection of hepatic steatosis based on transient elastography. Liver Int 2012;32:902–10. [DOI] [PubMed] [Google Scholar]
- 24.Loomba R, Cui J, Wolfson T, et al. Novel 3D Magnetic Resonance Elastography for the Noninvasive Diagnosis of Advanced Fibrosis in NAFLD: A Prospective Study. Am J Gastroenterol 2016;111:986–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ribel-Madsen A, Ribel-Madsen R, Brons C, et al. Plasma acylcarnitine profiling indicates increased fatty acid oxidation relative to tricarboxylic acid cycle capacity in young, healthy low birth weight men. Physiol Rep 2016;4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sampey BP, Freemerman AJ, Zhang J, et al. Metabolomic profiling reveals mitochondrial-derived lipid biomarkers that drive obesity-associated inflammation. PLoS One 2012;7:e38812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yoshiji H, Kuriyama S, Miyamoto Y, et al. Tissue inhibitor of metalloproteinases-1 promotes liver fibrosis development in a transgenic mouse model. Hepatology 2000;32:1248–54. [DOI] [PubMed] [Google Scholar]
- 28.Kalhan SC, Guo L, Edmison J, et al. Plasma metabolomic profile in nonalcoholic fatty liver disease. Metabolism 2011;60:404–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bates J, Hollenback D, Zagorska A, et al. Combination of ASK1 and ACC Inhibitors Increases Efficacy in Rodent Models of NASH. Hepatology 2017;66:A425. [Google Scholar]
- 30.Lake AD, Novak P, Shipkova P, et al. Branched chain amino acid metabolism profiles in progressive human nonalcoholic fatty liver disease. Amino Acids 2015;47:603–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sanyal AJ, Campbell-Sargent C, Mirshahi F, et al. Nonalcoholic steatohepatitis: association of insulin resistance and mitochondrial abnormalities. Gastroenterology 2001;120:1183–92. [DOI] [PubMed] [Google Scholar]
- 32.Sunny NE, Parks EJ, Browning JD, et al. Excessive hepatic mitochondrial TCA cycle and gluconeogenesis in humans with nonalcoholic fatty liver disease. Cell Metab 2011;14:804–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Koliaki C, Szendroedi J, Kaul K, et al. Adaptation of hepatic mitochondrial function in humans with nonalcoholic fatty liver is lost in steatohepatitis. Cell Metab 2015;21:739–46. [DOI] [PubMed] [Google Scholar]
- 34.Perry RJ, Peng L, Cline GW, et al. Non-invasive assessment of hepatic mitochondrial metabolism by positional isotopomer NMR tracer analysis (PINTA). Nat Commun 2017;8:798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Abu-Elheiga L, Matzuk MM, Abo-Hashema KA, et al. Continuous fatty acid oxidation and reduced fat storage in mice lacking acetyl-CoA carboxylase 2. Science 2001;291:2613–6. [DOI] [PubMed] [Google Scholar]
- 36.Abu-Elheiga L, Oh W, Kordari P, et al. Acetyl-CoA carboxylase 2 mutant mice are protected against obesity and diabetes induced by high-fat/high-carbohydrate diets. Proc Natl Acad Sci U S A 2003;100:10207–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kim CW, Addy C, Kusunoki J, et al. Acetyl CoA Carboxylase Inhibition Reduces Hepatic Steatosis but Elevates Plasma Triglycerides in Mice and Humans: A Bedside to Bench Investigation. Cell Metab 2017;26:394–406 e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Moon YA, Hammer RE, Horton JD. Deletion of ELOVL5 leads to fatty liver through activation of SREBP-1c in mice. J Lipid Res 2009;50:412–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Goedeke L, Bates J, Vatner DF, et al. Acetyl-CoA Carboxylase Inhibition Reverses NAFLD and Hepatic Insulin Resistance but Promotes Hypertriglyceridemia in Rodents. Hepatology 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.