Summary
Background
Survival from colorectal cancer has been shown to be lower in Denmark and England than in comparable high-income countries. We used data from national colorectal cancer registries to assess whether differences in the proportion of patients receiving resectional surgery could contribute to international differences in colorectal cancer survival.
Methods
In this population-based study, we collected data from all patients aged 18–99 years diagnosed with primary, invasive, colorectal adenocarcinoma from Jan 1, 2010, to Dec 31, 2012, in Denmark, England, Norway, and Sweden, from national colorectal cancer registries. We estimated age-standardised net survival using multivariable modelling, and we compared the proportion of patients receiving resectional surgery by stage and age. We used logistic regression to predict the resectional surgery status patients would have had if they had been treated as in the best performing country, given their individual characteristics.
Findings
We extracted registry data for 139 457 adult patients with invasive colorectal adenocarcinoma: 12 958 patients in Denmark, 97 466 in England, 11 450 in Norway, and 17 583 in Sweden. 3-year colon cancer survival was lower in England (63·9%, 95% CI 63·5–64·3) and Denmark (65·7%, 64·7–66·8) than in Norway (69·5%, 68·4–70·5) and Sweden (72·1%, 71·2–73·0). Rectal cancer survival was lower in England (69·7%, 69·1–70·3) than in the other three countries (Denmark 72·5%, 71·1–74·0; Sweden 74·1%, 72·7–75·4; and Norway 75·0%, 73·1–76·8). We found no significant differences in survival for patients with stage I disease in any of the four countries. 3-year survival after stage II or III rectal cancer and stage IV colon cancer was consistently lower in England (stage II rectal cancer 86·4%, 95% CI 85·0–87·6; stage III rectal cancer 75·5%, 74·2–76·7; and stage IV colon cancer 20·5%, 19·9–21·1) than in Norway (94·1%, 91·5–96·0; 83·4%, 80·1–86·1; and 33·0%, 31·0–35·1) and Sweden (92·9%, 90·8–94·6; 80·6%, 78·2–82·7; and 23·7%, 22·0–25·3). 3-year survival after stage II rectal cancer and stage IV colon cancer was also lower in England than in Denmark (stage II rectal cancer 91·2%, 88·8–93·1; and stage IV colon cancer 23·5%, 21·9–25·1). The total proportion of patients treated with resectional surgery ranged from 47 803 (68·4%) of 69 867 patients in England to 9582 (81·3%) of 11 786 in Sweden for colon cancer, and from 16 544 (59·9%) of 27 599 in England to 4106 (70·8%) of 5797 in Sweden for rectal cancer. This range was widest for patients older than 75 years (colon cancer 19 078 [59·7%] of 31 946 patients in England to 4429 [80·9%] of 5474 in Sweden; rectal cancer 4663 [45·7%] of 10 195 in England to 1342 [61·9%] of 2169 in Sweden), and the proportion of patients treated with resectional surgery was consistently lowest in England. The age gradient of the decline in the proportion of patients treated with resectional surgery was steeper in England than in the other three countries in all stage categories. In the hypothetical scenario where all patients were treated as in Sweden, given their age, sex, and disease stage, the largest increase in resectional surgery would be for patients with stage III rectal cancer in England (increasing from 70·3% to 88·2%).
Interpretation
Survival from colon cancer and rectal cancer in England and colon cancer in Denmark was lower than in Norway and Sweden. Survival paralleled the relative provision of resectional surgery in these countries. Differences in patient selection for surgery, especially in patients older than 75 years or individuals with advanced disease, might partly explain these differences in international colorectal cancer survival.
Funding
Early Diagnosis Policy Research Grant from Cancer Research UK (C7923/A18348).
Introduction
Colorectal cancer is among the three most common cancer diagnoses and causes of cancer death in women and men in Denmark, Norway, Sweden, and the UK.1 The deficit in survival from colorectal cancer seen in Denmark and England compared with that of Norway and Sweden2, 3, 4 might be explained partly by differences in disease stage distribution, arising from delays in diagnosis.5 The variations in stage-specific survival also suggest differences in treatment.5
Research in context.
Evidence before this study
To identify previous population-based international comparisons of colorectal cancer survival and treatment, we searched PubMed for articles published between Jan 1, 1980, and Jan 31, 2018, using the terms “population-based”, “cancer”, “survival”, “treatment”, “international”, “colorectal OR colon OR rectum”, without language restrictions. We manually searched the 95 references retrieved. In total, 22 articles assessed colorectal cancer outcomes in at least one of the countries included in our study. Additionally, we examined secondary references, national and international clinical guidelines, and other national reports for information on colorectal cancer management. Previous research showed that colorectal cancer survival was lower in England and Denmark than in other high-income countries with similar health-care coverage. The deficit in survival was partly explained by a more advanced stage distribution in Denmark and, potentially, by suboptimal care in England. Analysts who did this research pointed to the need for research into differences in stage-specific treatment between these countries.
Added value of this study
We used national population-based clinical data to compare stage-specific survival of patients diagnosed with primary colorectal adenocarcinoma in Denmark, England, Norway, and Sweden between 2010 and 2012, and to assess whether the international survival differences could be explained by differences in patient care. We considered stage-specific survival differences in relation to the proportion of patients who received resectional surgery. We showed that net survival up to 3 years after colon cancer was substantially lower in England and Denmark than in Norway and Sweden, and survival from rectal cancer was lower in England than in the other three countries. International differences were wider for patients with more advanced disease stage. The probability of receiving resectional surgery paralleled the survival outcomes, with patients in England substantially less likely to receive resectional surgery than in the other three countries. We also found a steep declining age gradient in the probability of receiving resectional surgery in England, which was less noticeable or not evident in the other countries.
Implications of all the available evidence
These findings have important policy implications, showing that the colorectal cancer survival deficit in England can be attributed partly to shortfalls in treatment. Patients older than 75 years, in particular, are less likely to receive surgery than patients with the same characteristics in Denmark, Norway, and Sweden. We highlight the need for more patient data on comorbidities, frailty, and additional therapies to understand these differences better.
The primary treatment for colorectal cancer is surgical removal of the main tumour or tumours and affected tissues. Total mesorectal excision became the standard surgery for rectal cancer in Denmark, Norway, and Sweden in the mid-1990s6, 7, 8 and some years later in England.9 This technique entails the removal of the rectum and surrounding tissues, including lymph nodes and fascia,10 and requires particular surgical training and skills to secure good results. The surgical principle of resection in the embryological plane, which is used in mesorectal excision, was later applied to colon cancer surgery, with favourable results in terms of recurrences and survival.11, 12
Preoperative (neo-adjuvant) or postoperative (adjuvant) radiotherapy or chemotherapy can be used to reduce the risk of recurrence and treat micrometastases.13, 14 The decision to treat patients with colorectal cancer with neo-adjuvant or adjuvant therapy depends on the extent of disease and risk of recurrence. In general, clinical guidelines do not recommend additional therapy for early-stage colon tumours or rectal tumours treated with surgery with adequate resection margins.13, 14, 15 The use of neo-adjuvant or adjuvant therapy for stage II or III tumours is variable between—and within16—countries, particularly for rectal tumours.17
We used population-based data from national colorectal cancer registries to estimate stage-specific and age-standardised net survival at 3 years of patients diagnosed with colorectal cancer in Denmark, England, Norway, and Sweden, and to compare the proportions of patients receiving resectional surgery in those countries by patient and tumour characteristics.
Methods
Study design and data sources
In this population-based study, we included all patients aged 18–99 years diagnosed with primary, invasive colorectal adenocarcinomas from Jan 1, 2010, to Dec 31, 2012. Patients diagnosed by their death certification alone and patients with records with invalid date sequences were excluded.18 In Denmark, England, and Norway, we extracted data from population-based national cancer registries. By linking individual patient records in Denmark to the Danish Colorectal Cancer Group database,19 additional clinical information was available for 11 746 (90·7%) patients registered with colorectal adenocarcinomas in the Danish National Cancer Registry. Similarly, 97 185 (99·7%) English national cancer registry records for patients with colorectal cancer were linked to at least one of the National Bowel Cancer Audit data, Hospital Episode Statistics inpatient and outpatient records, and the Cancer Waiting Times Monitoring Data Set. Norwegian national cancer registry data are routinely linked to the Norwegian Colorectal Cancer Registry, a specialised registry that contains detailed clinical information on all patients with colorectal cancer nationwide.8 The Swedish Colorectal Cancer Registry provided clinical data on patients with colorectal cancer in Sweden;7 its coverage for the study period was near complete.20 Definitions of clinical variables (site, stage, or treatment) were agreed with in-country clinicians and specialised cancer registry staff to reconcile differences in coding between the various data sources. The data specifications were agreed in advance with other countries through a prespecified data protocol. Some further discussions with clinicians and registry staff were held to understand and reconcile differences in coding and clinical practices in those countries.
All patients included in this study were followed up from time of diagnosis until death or until Dec 31, 2014, whichever occurred first. Last vital status was assessed by linking data to national death registry records.
Procedures
Colon cancer was defined by topographical codes C18.0–C19.9 and rectal cancer by codes C20.0–C20.9 of the International Classification of Diseases for Oncology, 3rd edition.21 Tumours located 15 cm or less from the anal verge were considered rectal cancers. Tumours with morphological codes for non-adenocarcinoma were excluded from analyses.
We applied consistent quality control measures to all records (appendix pp 1–2).18 In cases of multiple tumours diagnosed at the same site within 6 months of each other, we retained the date of diagnosis of the first tumour; where stage and type of surgery were inconsistent between records (n=327, 0·23%), we selected the most advanced stage and most extensive surgery. For all patients with record of more than one surgery, we selected the most extensive surgery.
Disease extent (stage), as defined by the Union for International Cancer Control TNM classification of malignant tumours, was characterised by applying a hierarchical algorithm previously described.22 Priority was given to pathological confirmation of tumour, lymph node extension, and distant metastases (if positive), over clinical TNM components. During 2010–12, Denmark and England used the 5th edition of TNM in their colorectal cancer registries, whereas the 7th edition was used in Norway and Sweden. There is broad comparability between the main stage categories among these TNM editions.22
We defined resectional surgery as the surgical removal of the primary tumour, irrespective of the intent and outcome of surgery, done within 9 months of diagnosis. Information regarding surgery for each patient was extracted from the specialised registry data for Denmark, Norway, and Sweden. For England, we derived this information from Hospital Episode Statistics and National Bowel Cancer Audit records, by identifying relevant codes from the Classification of Intervention and Procedures of the Office of Population Censuses and Surveys (OPCS), version 4.7 (OPCS 4.7),23 a standard classification of procedures done in National Health Service (NHS) hospitals in England. Local excisions were considered radical for stage I tumours alone. Non-resectional procedures that were purely diagnostic or symptom-alleviating (eg, stoma) were not considered as resectional surgery. In Denmark and England, surgical status was categorised as missing when patients were registered in the national cancer registry but were not recorded in the specialised colorectal cancer registry (or in Hospital Episode Statistics for England). We calculated the potential range of the proportion of patients that might have had resectional surgery in Denmark and England, first assuming that all patients with missing data were treated and then assuming that they were all untreated. We then estimated upper and lower limits of the probable distribution of resectional surgery in each of these countries.
Information regarding radiotherapy and planned chemotherapy within 6 months of diagnosis was extracted from colorectal cancer registry data in Denmark, Norway, and Sweden, and from National Bowel Cancer Audit and Cancer Waiting Times records in England.
We hold approvals from the UK Health Research Authority (reference ECC 3–04(i)/2011), the National Health Service Research Ethics Service (11/LO/0331), and the London School of Hygiene & Tropical Medicine (LSHTM, 12171). We have a data processing agreement with the Danish Cancer Society and approval from the Danish Data Protection Agency to use data from the Danish Colorectal Cancer Group; a data disclosure agreement with the Cancer Registry of Norway to use the Norwegian data; and ethical approval from the Regional Ethical Committee in Uppsala to use the Swedish data. Data preparation and analyses were done at the London School of Hygiene & Tropical Medicine. Data were extracted and transferred with a standard data structure protocol and file transmission procedure, in line with the CONCORD programme for the global surveillance of cancer survival.24
Outcomes
Our primary aim was to assess the estimated age-standardised net survival up to 3 years after diagnosis by country and disease stage and the estimated probability of patients receiving resectional surgery by stage and age in each country. We also estimated the hypothetical change in the probability of receiving resectional surgery that patients would have had if they had been treated as in the best performing country, given their individual characteristics.
Statistical analysis
We compared the demographic and clinical characteristics of patients with colorectal cancer diagnosed in Denmark, England, Norway, and Sweden, including patients' age, sex, and disease stage. We estimated net survival up to 3 years after diagnosis by country and disease stage, using the complete approach.25 Net survival controls for the hazard of death from other causes (background mortality) and is suitable for use in international comparisons of survival because background mortality differs between countries. In the absence of reliable information on the cause of death, the background mortality hazard was provided by life tables for the general population defined by country, sex, single year of age, and year.26 We used a multivariable modelling approach to estimate the excess mortality hazard (ie, due to colorectal cancer) and predict net survival. Survival models were stratified by country and disease stage. We used a model selection strategy to test for non-linearity and time dependence of the effects of sex and age on the excess mortality hazard and their interactions.27 Survival was predicted by age group, defined by the International Cancer Survival Standard. We used International Cancer Survival Standard weights to produce a weighted average of the survival estimates (age-standardisation), to allow for differences between countries in the age distribution of the population of patients with cancer.28 We used the Stata command strcs to fit flexible parametric survival models on the log-hazard scale.29
We used multivariate logistic regression models to compare the probability of receiving resectional surgery between countries. Models were developed for each disease stage and initially included country, age, and sex. We started with a full, saturated model that included main effects and all potential interactions. The main effects and important interactions between country and age were kept a priori, and we considered other interactions on the basis of the likelihood ratio test. Non-linearity was assessed by comparing the model with age as a categorical variable against a model with age as a continuous variable. If categorical age was chosen, the non-linear effect of age was modelled by use of a restricted cubic spline variable. Subsequently, we applied the model coefficients of the best performing country to individuals from the other countries, to assess the hypothetical change in the probability of receiving resectional surgery if patients had been treated as in the best performing country, given their observed characteristics. We used Stata 15 software for all statistical analyses.30
Role of the funding source
The funders of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the manuscript. The corresponding author had full access to all the data in the study, and the final responsibility for the decision to submit for publication.
Results
We included information from 139 457 patients diagnosed with colorectal adenocarcinoma in England, Denmark, Norway, and Sweden in our analyses. The distribution by topographical site varied slightly between countries. The age distribution of patients was similar between countries, with patients with rectal cancer being younger than patients with colon cancer overall (table 1). Median follow-up was similar between countries (table 2). Disease stage was known for 11 676 (90·1%) of 12 958 patients in Denmark, 81 108 (83·2%) of 97 466 in England, 10 174 (88·9%) of 11 450 in Norway, and 16 572 (94·3%) of 17 583 in Sweden. In patients with known disease stage, the proportion diagnosed with stage I–III colon cancer was higher in Sweden than in England, Norway, and Denmark; for rectal cancer, the proportion diagnosed with stage I–III rectal cancer was higher in England than in Sweden, Denmark, and Norway (table 1).
Table 1.
Characteristics of patients diagnosed with colorectal adenocarcinoma, 2010–12
|
Colon tumours |
Rectal tumours |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Denmark | England | Norway | Sweden | Denmark | England | Norway | Sweden | ||
| Mean age, years (SD) | 71·9 (11·3) | 72·4 (12·0) | 72·6 (11·9) | 72·7 (11·5) | 69·5 (11·5) | 70·0 (12·2) | 69·9 (12·2) | 70·3 (11·9) | |
| Age group, years | |||||||||
| 18–54 | 660 (7·7%) | 5700 (8·2%) | 656 (7·9%) | 863 (7·3%) | 481 (11·0%) | 3109 (11·3%) | 346 (11·1%) | 626 (10·8%) | |
| 55–64 | 1480 (17·3%) | 11 818 (16·9%) | 1345 (16·1%) | 1796 (15·2%) | 935 (21·3%) | 5936 (21·5%) | 679 (21·8%) | 1102 (19·0%) | |
| 65–74 | 2841 (33·2%) | 20 403 (29·2%) | 2441 (29·3%) | 3653 (31·0%) | 1502 (34·2%) | 8359 (30·3%) | 946 (30·4%) | 1900 (32·8%) | |
| 75–84 | 2567 (30·0%) | 21 942 (31·4%) | 2670 (32·0%) | 3907 (33·1%) | 1107 (25·2%) | 7289 (26·4%) | 809 (26·0%) | 1580 (27·3%) | |
| 85–99 | 1019 (11·9%) | 10 004 (14·3%) | 1227 (14·7%) | 1567 (13·3%) | 366 (8·3%) | 2906 (10·5%) | 331 (10·6%) | 589 (10·2%) | |
| Sex | |||||||||
| Men | 4160 (48·6%) | 37 279 (53·4%) | 4087 (49·0%) | 5875 (49·8%) | 2670 (60·8%) | 17 700 (64·1%) | 1836 (59·0%) | 3421 (59·0%) | |
| Women | 4407 (51·4%) | 32 588 (46·6%) | 4252 (51·0%) | 5911 (50·2%) | 1721 (39·2%) | 9899 (35·9%) | 1275 (41·0%) | 2376 (41·0%) | |
| Disease stage at diagnosis* | |||||||||
| Stage I | 839 (10·7%) | 7413 (12·8%) | 972 (13·0%) | 1462 (13·0%) | 793 (20·7%) | 5674 (24·4%) | 705 (26·0%) | 1247 (23·3%) | |
| Stage II | 2723 (34·7%) | 17 524 (30·3%) | 2482 (33·2%) | 3648 (32·5%) | 1032 (27·0%) | 5014 (21·6%) | 659 (24·3%) | 1264 (23·6%) | |
| Stage III | 2036 (25·9%) | 17 258 (29·8%) | 1931 (25·9%) | 3436 (30·6%) | 1062 (27·8%) | 7520 (32·4%) | 645 (23·8%) | 1551 (29·0%) | |
| Stage IV | 2256 (28·7%) | 15 679 (27·1%) | 2081 (27·9%) | 2670 (23·8%) | 935 (24·5%) | 5026 (21·6%) | 699 (25·8%) | 1294 (24·2%) | |
| Unknown stage | 713 (8·3%) | 11 993 (17·2%) | 873 (10·5%) | 570 (4·8%) | 569 (13·0%) | 4365 (15·8%) | 403 (13·0%) | 441 (7·6%) | |
| Received resectional surgery† | 6040 (70·5%) | 47 803 (68·4%) | 6023 (72·2%) | 9582 (81·3%) | 2982 (67·9%) | 16 544 (59·9%) | 2064 (66·3%) | 4106 (70·8%) | |
| Received radiotherapy‡ | 134 (1·6%) | 2097 (3·0%) | 109 (1·3%) | 54 (0·5%) | 1182 (26·9%) | 11 299 (40·9%) | 1321 (42·5%) | 2935 (50·6%) | |
| Received chemotherapy‡§ | 3272 (38·2%) | 18 640 (26·7%) | 1654 (19·8%) | 2525 (21·4%) | 2060 (46·9%) | 8484 (30·7%) | 931 (29·9%) | 1404 (24·2%) | |
| Unknown treatment status¶ | 949 (11·1%) | 214 (0·3%) | 0 (0·0%) | 0 (0·0%) | 263 (6·0%) | 67 (0·2%) | 0 (0·0%) | 0 (0·0%) | |
| Total | 8567 (100%) | 69 867 (100%) | 8339 (100%) | 11 786 (100%) | 4391 (100%) | 27 599 (100%) | 3111 (100%) | 5797 (100%) | |
Data are n (%), unless otherwise specified.
Proportions of total number of patients with known stage.
Defined as surgery to remove the primary tumour within 9 months of diagnosis, excluding diagnostic and palliative procedures.
Received within 6 months of diagnosis; sources and completeness of information on chemotherapy or radiotherapy varied greatly between countries, with a high proportion of missing information in England.
Planned chemotherapy in Norway and Sweden.
Proportion of patients not registered in specialised colorectal cancer registries (or Hospital Episode Statistics, Cancer Waiting Times Monitoring Data Set for England).
Table 2.
Age-standardised net survival of patients diagnosed with colorectal adenocarcinoma, 2010–12
|
Colon tumours |
Rectal tumours |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Denmark | England | Norway | Sweden | Denmark | England | Norway | Sweden | ||
| Median follow-up time, years (IQR) | 2·5 (0·9–3·5) | 2·4 (0·7–3·5) | 2·5 (1·1–3·7) | 2·7 (1·4–3·7) | 2·7 (1·5–3·7) | 2·6 (1·4–3·6) | 2·8 (1·9–3·8) | 2·8 (1·8–3·8) | |
| 1-year net survival (95% CI) | |||||||||
| All stages | 80·3 (79·5–81·0) | 78·2 (77·9–78·5) | 80·9 (80·1–81·6) | 83·9 (83·3–84·5) | 85·5 (84·5–86·5) | 84·6 (84·2–85·0) | 87·4 (86·0–88·6) | 87·6 (86·7–88·5) | |
| Stage I | 97·1 (95·6–98·1) | 98·7 (97·8–99·2) | 98·2 (96·4–99·1) | 100 (100·0–100·0) | 98·0 (95·8–99·1) | 98·8 (98·2–99·1) | 98·4 (95·9–99·4) | 99·8 (99·6–99·9) | |
| Stage II | 94·5 (93·4–95·4) | 94·9 (94·6–95·3) | 95·5 (94·4–96·3) | 96·8 (96·1–97·4) | 95·3 (93·9–96·3) | 94·6 (93·8–95·3) | 99·8 (99·8–99·8) | 97·5 (96·1–98·4) | |
| Stage III | 87·8 (86·4–89·2) | 87·5 (87·1–88·0) | 89·3 (87·8–90·6) | 90·7 (89·7–91·6) | 90·0 (87·9–91·7) | 91·1 (90·4–91·8) | 93·9 (92·3–95·2) | 93·6 (92·1–94·8) | |
| Stage IV | 52·9 (51·3–54·5) | 48·4 (47·8–49·1) | 57·3 (55·4–59·1) | 51·9 (50·2–53·6) | 61·6 (59·3–63·9) | 60·0 (58·9–61·0) | 66·6 (63·8–69·2) | 61·5 (59·4–63·6) | |
| Unknown stage | 62·8 (60·2–65·4) | 62·2 (61·3–63·0) | 58·0 (55·0–60·8) | 78·9 (76·1–81·4) | 78·9 (75·9–81·6) | 72·7 (71·6–73·8) | 75·2 (71·8–78·2) | 83·1 (80·4–85·5) | |
| 2-year net survival (95% CI) | |||||||||
| All stages | 71·9 (71·0–72·8) | 69·9 (69·5–70·2) | 73·9 (73·0–74·8) | 76·7 (75·9–77·5) | 78·2 (76·9–79·4) | 76·2 (75·7–76·8) | 80·3 (78·6–81·8) | 79·8 (78·6–81·0) | |
| Stage I | 96·2 (94·3–97·5) | 98·2 (97·1–98·9) | 98·2 (96·4–99·1) | 99·5 (97·2–100·0) | 97·0 (93·9–98·6) | 97·8 (96·9–98·4) | 98·4 (95·9–99·4) | 99·8 (97·5–100·0) | |
| Stage II | 92·3 (90·9–93·5) | 92·7 (92·2–93·2) | 94·7 (93·4–95·7) | 95·3 (94·3–96·2) | 93·0 (91·1–94·5) | 90·3 (89·2–91·3) | 98·9 (98·0–99·4) | 95·1 (93·2–96·4) | |
| Stage III | 81·4 (79·6–83·1) | 80·1 (79·5–80·7) | 82·1 (80·2–83·8) | 84·0 (82·7–85·3) | 83·0 (80·2–85·4) | 82·9 (81·9–83·9) | 88·2 (85·7–90·3) | 86·8 (84·9–88·6) | |
| Stage IV | 34·4 (32·8–36·1) | 30·5 (29·9–31·1) | 41·9 (39·9–44·0) | 33·6 (31·8–35·3) | 42·4 (39·9–44·9) | 39·7 (38·6–40·8) | 49·6 (46·6–52·4) | 39·8 (37·7–42·0) | |
| Unknown stage | 53·2 (50·4–56·0) | 52·8 (51·9–53·8) | 49·8 (46·6–52·8) | 73·0 (69·8–75·9) | 72·0 (68·4–75·2) | 63·5 (62·1–64·7) | 64·2 (60·3–67·9) | 77·1 (73·9–79·9) | |
| 3-year net survival (95% CI) | |||||||||
| All stages | 65·7 (64·7–66·8) | 63·9 (63·5–64·3) | 69·5 (68·4–70·5) | 72·1 (71·2–73·0) | 72·5 (71·1–74·0) | 69·7 (69·1–70·3) | 75·0 (73·1–76·8) | 74·1 (72·7–75·4) | |
| Stage I | 95·6 (93·4–97·0) | 97·8 (96·6–98·6) | 98·2 (96·4–99·1) | 99·3 (96·0–99·9) | 96·3 (92·4–98·2) | 96·9 (95·6–97·8) | 98·4 (95·9–99·4) | 99·3 (95·7–99·9) | |
| Stage II | 90·6 (88·9–92·0) | 91·0 (90·4–91·5) | 94·3 (93·0–95·4) | 94·1 (92·8–95·1) | 91·2 (88·8–93·1) | 86·4 (85·0–87·6) | 94·1 (91·5–96·0) | 92·9 (90·8–94·6) | |
| Stage III | 76·3 (74·1–78·4) | 74·1 (73·3–74·8) | 76·3 (74·0–78·4) | 78·4 (76·8–79·9) | 77·2 (73·9–80·1) | 75·5 (74·2–76·7) | 83·4 (80·1–86·1) | 80·6 (78·2–82·7) | |
| Stage IV | 23·5 (21·9–25·1) | 20·5 (19·9–21·1) | 33·0 (31·0–35·1) | 23·7 (22·0–25·3) | 30·4 (28·0–32·9) | 27·1 (26·0–28·2) | 38·5 (35·5–41·4) | 26·7 (24·7–28·8) | |
| Unknown stage | 47·4 (44·5–50·2) | 47·0 (45·9–48·0) | 45·2 (42·0–48·4) | 69·7 (66·3–72·9) | 67·3 (63·3–70·9) | 57·2 (55·8–58·6) | 56·7 (52·4–60·8) | 73·2 (69·8–76·3) | |
3-year age-standardised net survival from colon cancer was higher in Sweden and Norway than in Denmark and England. Rectal cancer survival was consistently higher than that for colon tumours in the study countries. 3-year survival from rectal cancer was generally similar between Denmark, Norway, and Sweden, and lower in England (table 2).
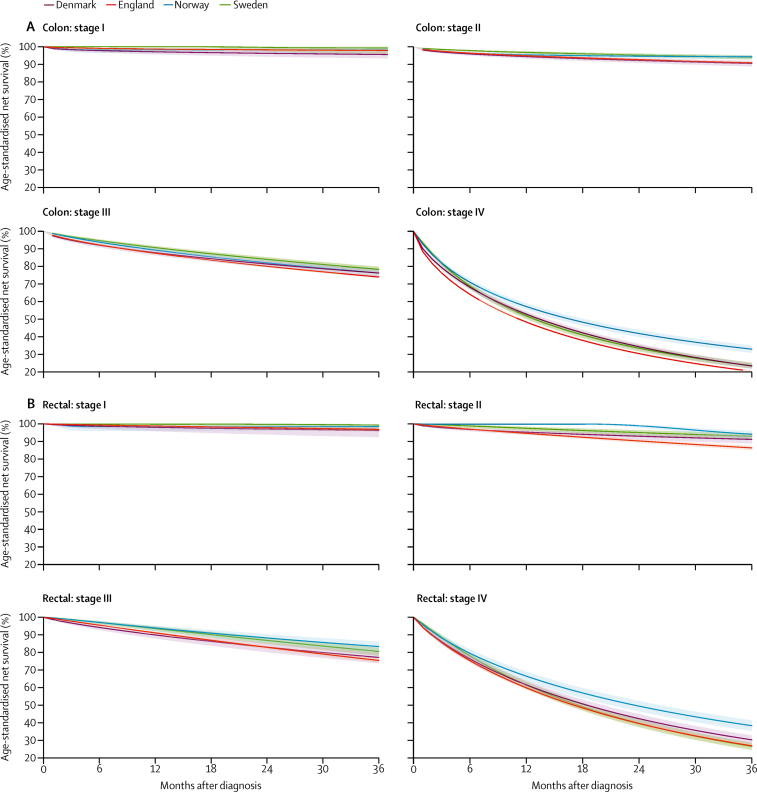
Net survival decreased with the increase in disease stage (table 2, figure 1). Age-standardised 3-year survival for stage I tumours was higher than 95% in all countries for both colon and rectal cancers. 3-year survival for patients with stage II tumours was about 90% or higher in all countries, although survival for patients with rectal or colon cancer in England and colon cancer in Denmark was notably lower than for patients in Sweden or Norway. Although generally higher than 75%, survival for patients with stage III colon and rectal cancer was lower in England than in Norway and Sweden up to 3 years after diagnosis, and in Denmark 1 year after diagnosis. Survival from stage IV colon cancer was consistently lowest in England and highest in Norway, reaching 20·5% for England and 33·0% for Norway at 3 years. For stage IV rectal cancer, survival was lowest in Sweden and England (26·7% in Sweden and 27·1% in England) and highest in Norway (38·5%) at 3 years (figure 1).
Figure 1.
Age-standardised stage-specific survival for colon (A) and rectal (B) adenocarcinoma diagnosed in 2010–12
Shaded areas represent 95% CI of survival estimates.
The overall proportion of patients who received resectional surgery was higher for colon than for rectal cancer in all countries (table 1). We could not establish the surgical status of some patients in Denmark because they were not registered in the Danish Colorectal Cancer Group database (949 [11·1%] of 8567 patients with colon cancer and 263 [6·0%] of 4391 patients with rectal cancer). Patients with colorectal cancer who were not registered in the Danish Colorectal Cancer Group database were more likely to have advanced stage disease or missing stage information and be slightly older than patients registered in the database. Data on surgery were unavailable for 0·3% of patients with colorectal cancer in England because their national cancer registry records could not be linked to the additional databases (Hospital Episode Statistics, National Bowel Cancer Audit, or Cancer Waiting Times). We report here analyses of the patients with known surgical status. The proportion of patients receiving resectional surgery was highest in Sweden and lowest in England, for both types of cancer (table 1).
The proportion of patients treated with resectional surgery was lower in individuals aged 75 years or older than in younger patients in each country, and international differences in resectional surgery use widened with increasing age of patients (table 3). The share of patients aged 75 years or older with colon cancer with evidence of resectional surgery varied from 19 078 (59·7%) of 31 946 patients in England to 4429 (80·9%) of 5474 in Sweden. In patients aged 75 years or older with rectal cancer, the share of those with resectional surgery varied from 4663 (45·7%) of 10 195 patients in England to 1342 (61·9%) of 2169 in Sweden. Sweden had the highest proportion of patients treated with resectional surgery for colon cancer, for each age group. For rectal cancer, Norway and Sweden had the highest proportions of resectional surgery for all but the youngest age group, where Denmark had the highest proportion of patients treated. England had the lowest proportion of patients treated with resectional surgery for rectal cancer in all age groups and for colon cancer in the two oldest age groups (table 3).
Table 3.
Proportion of patients diagnosed with colorectal adenocarcinoma in 2010–12 that received resectional surgery by age, sex, and disease stage
|
Colon tumours |
Rectal tumours |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Denmark | England | Norway | Sweden | Denmark | England | Norway | Sweden | ||
| Age group, years | |||||||||
| 18–54 | 463 (70·2%) | 4233 (74·3%) | 495 (75·5%) | 712 (82·5%) | 371 (77·1%) | 2065 (66·4%) | 248 (71·7%) | 465 (74·3%) | |
| 55–64 | 1091 (73·7%) | 8951 (75·7%) | 990 (73·6%) | 1442 (80·3%) | 705 (75·4%) | 4083 (68·8%) | 499 (73·5%) | 860 (78·0%) | |
| 65–74 | 2077 (73·1%) | 15 541 (76·2%) | 1844 (75·5%) | 2999 (82·1%) | 1072 (71·4%) | 5733 (68·6%) | 664 (70·2%) | 1439 (75·7%) | |
| 75–84 | 1818 (70·8%) | 14 834 (67·6%) | 1974 (73·9%) | 3255 (83·3%) | 698 (63·1%) | 3962 (54·4%) | 510 (63·0%) | 1090 (69·0%) | |
| 85–99 | 591 (58·0%) | 4244 (42·4%) | 720 (58·7%) | 1174 (74·9%) | 136 (37·2%) | 701 (24·1%) | 143 (43·2%) | 252 (42·8%) | |
| Sex | |||||||||
| Men | 2917 (70·1%) | 25 649 (68·8%) | 2925 (71·6%) | 4711 (80·2%) | 1843 (69·0%) | 10 820 (61·1%) | 1239 (67·5%) | 2427 (70·9%) | |
| Women | 3123 (70·9%) | 22 154 (68·0%) | 3098 (72·9%) | 4871 (82·4%) | 1139 (66·2%) | 5724 (57·8%) | 825 (64·7%) | 1679 (70·7%) | |
| Disease stage at diagnosis | |||||||||
| Stage I | 752 (89·6%) | 6916 (93·3%) | 836 (86·0%) | 1328 (90·8%) | 741 (93·4%) | 5100 (89·9%) | 605 (85·8%) | 1122 (90·0%) | |
| Stage II | 2528 (92·8%) | 16 438 (93·8%) | 2189 (88·2%) | 3541 (97·1%) | 928 (89·9%) | 3958 (78·9%) | 556 (84·4%) | 1145 (90·6%) | |
| Stage III | 1849 (90·8%) | 15 555 (90·1%) | 1688 (87·4%) | 3282 (95·5%) | 900 (84·7%) | 5289 (70·3%) | 539 (83·6%) | 1361 (87·7%) | |
| Stage IV | 882 (39·1%) | 6263 (39·9%) | 1158 (55·6%) | 1356 (50·8%) | 307 (32·8%) | 1293 (25·7%) | 331 (47·4%) | 376 (29·1%) | |
| Unknown stage | 29 (4·1%) | 2631 (21·9%) | 152 (17·4%) | 75 (13·2%) | 106 (18·6%) | 904 (20·7%) | 33 (8·2%) | 102 (23·1%) | |
| Total | 6040 (70·5%) | 47 803 (68·4%) | 6023 (72·2%) | 9582 (81·3%) | 2982 (67·9%) | 16 544 (59·9%) | 2064 (66·3%) | 4106 (70·8%) | |
Data are n (%). Resectional surgery defined as surgery to remove the primary tumour within 9 months of diagnosis, excluding diagnostic and palliative procedures.
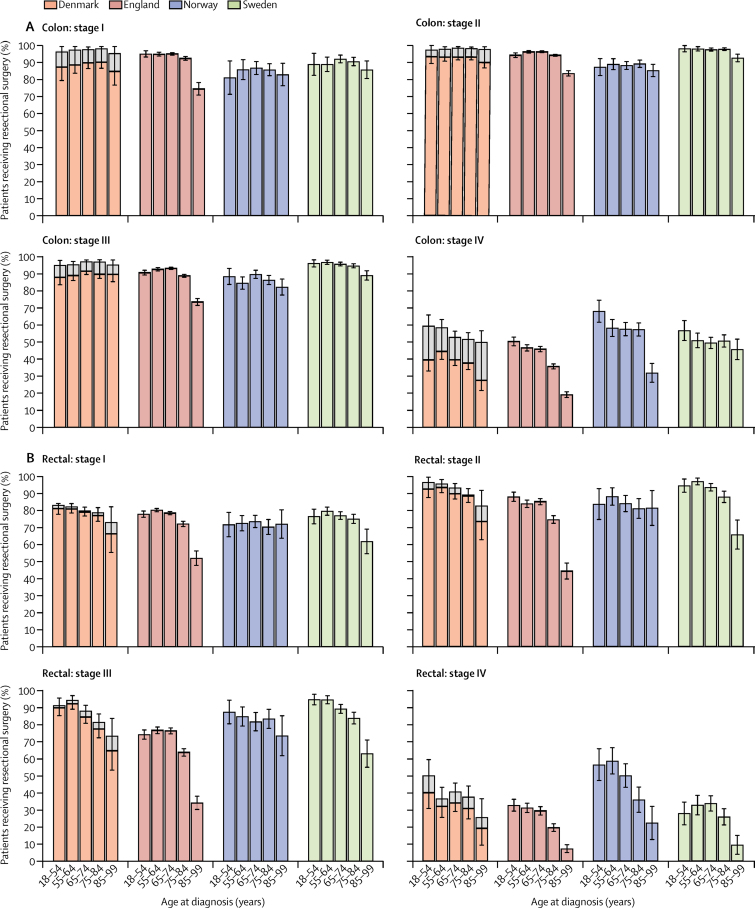
To account for differences in disease stage distribution, we examined the proportion of patients treated by stage and age group (figure 2). The proportion of patients with colon cancer with evidence of resectional surgery for each stage and age group was mostly similar between the four countries for stages I and II and in patients aged 75 years or younger. A higher proportion of patients younger than 85 years with stage I colon cancer in England had resectional surgery than in other countries. However, we observed a steep decline in the proportion of patients that had surgical treatment in the older age categories in England for each disease stage and for both types of cancer, which was not evident in the other countries (figure 2). For instance, a higher proportion of patients received resectional surgery for stage I colon tumours in the 75–84 age group than in the 85 and older age group in all countries, but the absolute difference between these age groups was 18·0% in England compared with 2·9–5·5% in the other countries. A similar pattern was noted in the other disease stage categories. The proportion of patients with colon cancer aged 85 years and older with evidence of resectional surgery was consistently highest in Sweden for each disease stage as compared with the other three countries.
Figure 2.
Proportion of patients who underwent resectional surgery for colon (A) and rectal (B) adenocarcinoma by disease stage at diagnosis and age group, for diagnoses 2010–12
Error bars are 95% CI. Resectional surgery is defined as surgery to remove the primary tumour within 9 months of diagnosis, excluding diagnostic and palliative procedures. Information on surgical status was available for all patients in Norway and Sweden. Information on surgery was missing for some patients in Denmark and for a small proportion of patients in England: light grey areas represent the proportion of patients with unknown surgical status by stage and age group; overall height of the bars shows the proportion of patients that would receive surgery if all patients with missing treatment data had surgical treatment.
Among patients younger than 85 years with rectal cancer who were diagnosed with stage I or II disease, we observed no significant differences in the likelihood of being treated with resectional surgery in any of the four countries. However, for patients with rectal cancer diagnosed with stage III–IV disease, the proportion treated was lower in England than in the other countries in our study, particularly in the oldest age groups (figure 2). We observed an age gradient in the proportion of patients receiving resectional surgery with all rectal cancer stages in Denmark, England, and Sweden, with lower proportions among patients aged 85 years or older than among younger patients. The age gradient was steeper in England than in other countries. For instance, between patients aged 75–84 years and those aged 85 years or older, the absolute difference in the proportion of patients who received resectional surgery for stage III rectal tumours was 29·6% in England, 20·9% in Sweden, and 12·8% in Denmark. We found no age gradient in the proportion of patients treated for rectal cancer in Norway, except for patients with stage IV cancer.
To assess the validity of our findings and check whether the age differences in the likelihood of patients receiving resectional surgery were driven by differences in the management of patients diagnosed at aged 90 years or older, we repeated the analyses with exclusion of this patient group. The patterns we observed persisted in this reanalysis (appendix pp 3, 6–7).
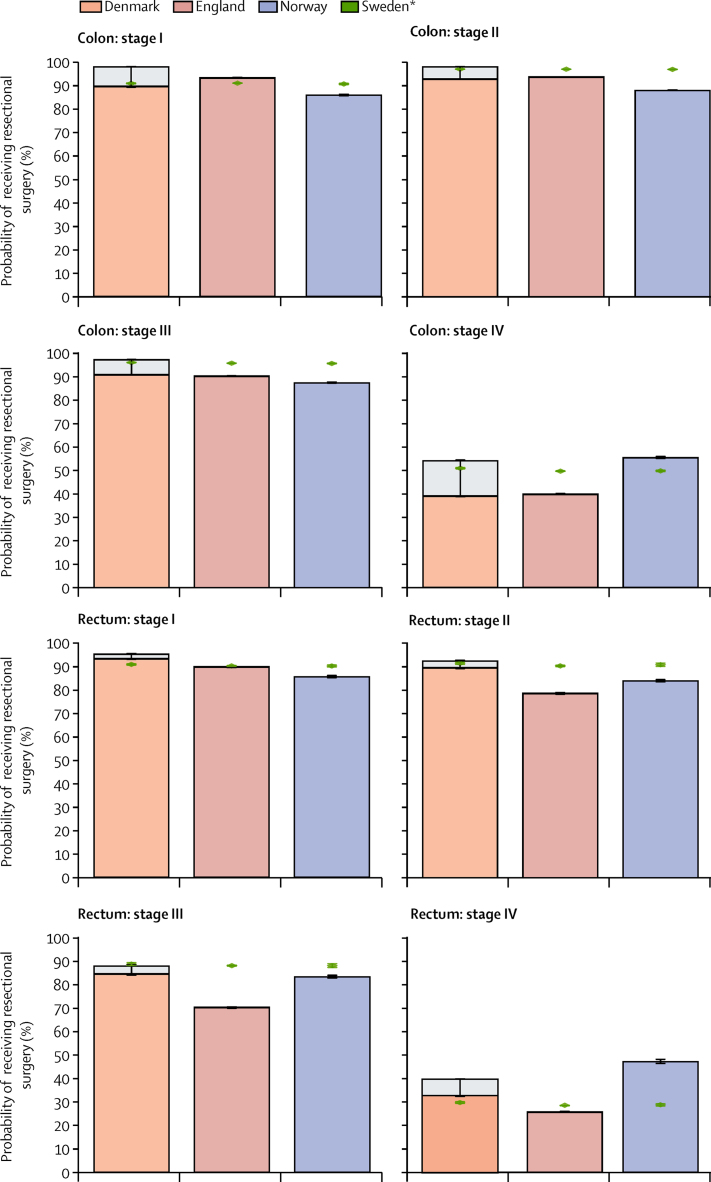
Overall, Sweden had the highest survival for colon and rectal cancer and the highest proportion of patients receiving resectional surgery, compared with those of the other countries (although outcomes in Norway were generally similar, or better in specific strata, to those in Sweden). To highlight any groups of patients who might be at a disadvantage in the likelihood of receiving resectional surgery compared with patients in other countries, we applied the coefficients for Sweden to data from the other three countries and interpreted it as the probability of a patient receiving resectional surgery if they had been treated as in Sweden, given their observed age, sex, and disease stage. The number of events per parameter was above the recommended threshold of ten in all categories,31 at 100 events per parameter for our most complex model in the category with fewest events (stage IV rectal cancer).
In this hypothetical scenario, changes in the proportion of patients with stage I colon or rectal cancer receiving resectional surgery would be minor (figure 3). In England, Denmark, and Norway there would be a higher proportion treated among patients with stage II and III colon and rectal cancer, if treated as in Sweden. Overall, the largest improvements in the proportion of patients receiving resectional surgery would be seen in patients with stage II (from 78·9% to 90·7%) or III (from 70·3% to 88·2%) rectal cancer in England. The proportion of patients receiving resectional surgery for stage IV colon cancer in Denmark and England would increase (from 39·1% to 51·1% in Denmark and from 40·0% to 49·8% in England)—whereas in Norway, the proportion would decrease (from 55·6% to 49·9%) if patients had been treated as in Sweden. The hypothetical decrease in the proportion of stage IV patients receiving surgery in Norway would be even larger for rectal cancer (from 47·4% to 28·8%).
Figure 3.
Predicted probability of receiving resectional surgery by patient characteristics (age and sex) and tumour characteristics (stage at diagnosis)
Error bars are 95% CI. Resectional surgery is defined as surgery to remove the primary tumour within 9 months of diagnosis, excluding diagnostic and palliative procedures. *Predicted probabilities of patients receiving resectional surgery by applying the coefficients of the Swedish logistic model to the cohorts of patients in each country, on the basis of the country-specific distributions of patient characteristics. Light grey areas at the top of the bars for Denmark and England represent the proportion of patients with unknown surgical status by stage and age group. The overall height of the bars shows the proportion we would observe if all patients with missing treatment data had received surgery.
Patients with missing disease stage were slightly older than the mean age for each country and cancer type (range 75·3–77·3 years for colon cancer and 74·5–75·4 years for rectal cancer). The proportion of patients with colon cancer without known disease stage who had evidence of having resectional surgery was lower than that of any known stage category in each country and higher in England than in the other three countries (table 3). The proportion of patients with rectal cancer without known disease stage who had evidence of having resectional surgery was higher in Sweden than in the other three countries. Survival of patients with colorectal cancer without known disease stage was higher in Sweden than in Denmark, England, and Norway. Additionally, survival of these patients was higher than that of patients with known stage III disease and lower than that of patients with known stage IV disease, in all four countries (table 2).
Discussion
In this study, to understand the mechanisms underlying international differences in cancer outcomes, we compared the characteristics of patients diagnosed with colorectal adenocarcinoma in Denmark, England, Norway, and Sweden. We provide updated figures of up to 3-year net survival in these countries, and our results support previous findings of lower survival for patients in England and, to a lesser degree, in Denmark, than in Sweden or Norway.2, 3, 4, 5 Our results also support findings that Denmark seems to be closing the survival gap with Sweden and Norway, particularly for rectal cancer.4, 6, 24 In the stage-specific analyses, we noted no significant differences between countries in survival for patients diagnosed with early stage (I or II) colorectal adenocarcinomas, but wider international survival differences in patients with more advanced disease stages (III or IV). Additional information on treatment, available from specialised colorectal cancer registries, helped us to understand these survival differences better.
Cancer survival is largely determined by receipt of potentially curative treatment, which, in the case of colorectal cancer, is primarily surgery. Treatment options mainly depend on disease stage and the underlying health status of patients, which is determined by their comorbidities, frailty, and age.
Clinical guidelines for cancer treatment aim to standardise and assure adequate cancer care for a population. The amount of detail in the national clinical guidelines regarding colorectal cancer management varied, with recommendations from England being generally less specific than guidelines from Denmark, Norway, and Sweden.14, 15, 32, 33 Nonetheless, indications for surgery were largely consistent between these countries, especially for rectal tumours. Treatment guidelines for colon cancer in Denmark, Norway, and Sweden explicitly recommend the removal of the part of the bowel that contains the tumour and its mesentery (and en-bloc resection, if the visceral fascia is compromised because of ingrowth of the tumour into neighbouring organs).13, 14, 32 By contrast, English guidelines recognise mesorectal excision as the standard surgery for most rectal tumours but do not explicitly recommend the corresponding procedure (dissection in the embryological plane) for colon tumours.15
None of the guidelines for the countries in our study mentions age as an exclusion criterion for receiving surgical treatment. However, we found stark differences between countries in the proportion of patients aged 75 years or older who received resectional surgery. Older patients (aged 75 years or older with stage II–IV rectal cancer or stage IV colon cancer and aged 85 years or older for the other stages of rectal and colon cancer) in England had a lower probability of receiving resectional surgery than patients of a similar age with similar disease extension in the other three countries, and a lower probability than younger patients in England. Countries with better survival—Norway and Sweden—had a higher proportion of older patients receiving resectional surgery than that in England for most stages of disease. England had the steepest negative age gradient in the proportion of patients receiving resectional surgery and had a survival deficit in comparison with the other three countries, particularly for rectal cancer. These patterns persisted even after we excluded patients aged 90 years or older from the analyses. Conversely, we noted a higher proportion of patients younger than 85 years who were diagnosed with stage I or II tumours who had evidence of resectional surgery in England than in the other three countries. Of the countries studied, only England had a colorectal cancer screening programme with national coverage during the study period.34 The diagnosis and treatment of asymptomatic disease through screening might explain the high proportion of patients surgically treated for early stage disease in the eligible age group in England. In the other countries in our study, screening was not implemented at national level during the study period34 and could not have affected the disease stage distribution or population-based survival.
Although less aggressive treatment for older patients might sometimes be justified because of comorbidity or frailty, concerns have been raised that some of the disparities in age-related cancer care in England arise because of clinical decision making on the basis of chronological age.35 A 2011 report36 showed lower resection rates in older patients with cancer in England during 2004–06 than those of younger patients, with less than 2% of patients aged 80 years or older having a major resection surgery for six of 13 cancers examined.
Although an increase in the proportion of patients receiving resectional surgery does not necessarily translate into better short-term survival because aggressive treatment might be associated with high short-term mortality, our findings suggest that international differences in survival are, at least in part, determined by differences in patient selection practices for surgical treatment. Choices in management of older patients with colorectal cancer might greatly affect population-based survival because the mean age at diagnosis is about 70 years.37
Patients in countries with a greater proportion of patients treated with surgery and better short-term survival might also have more access to laparoscopic surgery or better postoperative care than in the other countries. Although there is conflicting evidence about the long-term benefit of a laparoscopic versus an open surgical approach for rectal cancer,38, 39 laparoscopic surgery is associated with lower perioperative mortality and fewer complications than open surgery.40, 41 In Denmark, the increasing use of laparoscopic surgery for colorectal cancer has been associated with a reduction in perioperative mortality rates.42 Differences between countries in the use of this approach might explain some of the differences in the proportion of patients receiving surgical treatment and, potentially, in survival. However, we were not able to account for this information in our analysis because not all datasets had sufficiently complete data for this question.
Our study has some limitations. We were able to do this international comparison of detailed clinical characteristics and outcomes for patients with colorectal cancer because of the existence of specialised colorectal cancer registries. These include core variables that have previously been examined for comparability and validated—in these and other European colorectal cancer registries.43 However, some residual data quality issues might affect the comparability of results. Our analyses accounted for age, sex, and disease stage, but comparable information on comorbidities—an important determinant of treatment—was not available in all countries. Furthermore, comorbidity measures might not reveal a patient's overall health status. Performance status scales, which are commonly used to assess patients' general condition (such as their degree of independence) and eligibility for specific treatments,44 might not be ideal for older patients with cancer, especially those with multiple comorbidities.45 Although comprehensive geriatric assessment scales have been proposed and validated,45 they are rarely used, documented, or routinely collected.46 Nevertheless, at the population level, the burden of cardiovascular disease—the most common contraindication for surgery—is similar in the four countries included in our study.47 Population-based all-cause mortality and life expectancy in older ages are also similar in these four countries (appendix p 4), supporting the validity of the findings in our study.
The overall proportion of patients with unknown disease stage is notably higher in England than in Denmark, Norway, and Sweden. The reasons for stage information to be missing are likely to differ according to how high or low the proportion of unknown stage is and, therefore, probably differ between countries. Patients with unknown disease stage had a lower probability of receiving resectional surgery than patients with known stage, and had similar survival to that of patients with advanced disease stages. Therefore, our complete-case analysis might overestimate stage-specific survival and the proportion of patients receiving resectional surgery in England, and provides a conservative estimate of the disparities between England and the other three countries in our study.
In Denmark, 9·3% of patients were not registered in the Danish Colorectal Cancer Group database and thus, their treatment status remained undetermined. By contrast with the Danish National Cancer Registry, the Danish Colorectal Cancer Group database only includes adults with a first-time diagnosis of colorectal adenocarcinoma treated in Danish public hospitals who were in contact with a surgical department.19 Therefore, we calculated a potential range of the proportion of patients that might have had resectional surgery and estimated upper and lower limits of the probable distribution of resectional surgery in Denmark. Because of the Danish Colorectal Cancer Group database's exclusion criteria and the stage distribution of patients without a Danish Colorectal Cancer Group database record, it is probable that a substantial number of these patients did not undergo resectional surgery. Assuming that none of these patients received resectional surgery, the proportion of patients with rectal cancer who received resectional surgery was still higher in Denmark than in England and similar to that in Sweden for most combinations of age and disease stage. Nevertheless, these missing data might have masked some age and stage trends in the likelihood of patients undergoing resectional surgery, especially for colon cancer.
We were not able to ascertain treatment intent, residual disease status, venous invasion status, or postoperative complications in patients who received resectional surgery. We expect that the prognosis of patients who underwent resectional surgery but had residual disease or any postoperative complication was poorer than those without residual disease. Systematic differences in the distribution of such patients between these four countries, or differences in their perioperative management, might affect the between-country comparability of these results. Furthermore, patients with non-resectable tumours in better-performing countries might be more likely to be offered other treatment (such as treatment to prolong life or make tumours amenable to resection) than patients in worse-performing countries. For example, clinical guidelines in Norway and Sweden describe the importance of neo-adjuvant or conversion treatment of metastatic tumours to render them resectable,13, 14 whereas guidelines in England prioritise symptom control and state that initial systemic treatment followed by surgery should be considered only if both primary and metastatic tumours are judged to be resectable.15 Moreover, in countries that frequently use neo-adjuvant therapy with delayed surgery for rectal cancer, the resulting down-staging could cause an underestimation of the differences in stage-specific survival when these countries are compared with settings where neo-adjuvant treatment is more variable or followed by immediate surgery.
The completeness and granularity of the data collected by the colorectal cancer registries regarding chemotherapy and radiotherapy varied greatly during the study period—from complete registration of radiotherapy protocols in Norway and Sweden to a high proportion of missing information in England. These inconsistencies meant that including radiotherapy and chemotherapy in our analyses was not possible (appendix p 5). Adjuvant chemotherapy is associated with improved outcomes in stage III colon cancer and is used universally, but for stage II colon cancer and rectal cancer (in general) its value and therefore its use have been more variable.48, 49, 50 Neo-adjuvant radiotherapy decreases recurrence rates, but evidence for its effect on survival is conflicting and so use also varies within countries16, 51 and between countries.17 The use of targeted therapy in combination with chemotherapy might help to make tumours amenable to resection in some patients with metastatic or locally advanced disease,52 but no survival benefit has been shown for this combination.53 For patients with metastatic disease treated with non-curative intent, optimal use of systemic therapy contributes to longer survival.52, 54 Variability between countries in the use of these additional therapies, and other differences in oncological care beyond surgery, might also contribute to the observed differences in survival.
Despite the limitations of the data included in our study, we have identified important international differences in the distribution of resectional surgery by age and disease stage. The main data quality issue (the higher proportion of patients with unknown disease stage in England) is likely to have led to a conservative estimate of the differences found in stage-specific survival outcomes in England compared with those of other countries. We noted that differences in survival between patients with colorectal cancer treated in England, Denmark, Norway, and Sweden tended to increase with time after diagnosis, and it is possible that these differences between countries would widen with longer follow-up.
Changes in practice during and after the study period are likely to affect future trends. Over the past two or three decades, colorectal cancer outcomes have improved in all the countries compared in our study.3, 24, 55 The centralisation and specialisation of colorectal cancer surgery have been suggested as important drivers of this improvement.4 A 2012 Cochrane review56 found a clear association between operative mortality and 5-year survival with hospital and surgeon caseload and specialisation. Specialisation of surgeons has led to more widespread and aggressive treatment of metastatic disease, with increased use of chemotherapy and resection of metastases. However, it is likely that centralisation and specialisation vary between the four countries in our study, and these changes in practice are also likely to affect patient survival differently in these countries.4, 6
Expedited referral routes were initiated in England and Denmark in the 2000s, in response to low cancer survival related to system delays.57, 58 Similar rapid referral routes were introduced in Norway and Sweden in 2016, following the Danish experience. Although diagnostic delays are important factors in cancer care, the effect of these referral routes on cancer survival remains uncertain.
In Denmark, a nationwide screening programme with a monitoring database was introduced in 2014.59 The Norwegian Directorate of Health is planning to introduce a national colorectal cancer screening programme in Norway in 2019, offering a faecal immunochemical test or colonoscopy to individuals when they turn 55 years.60 Similarly, a national colorectal cancer screening programme is due to be implemented in Sweden in 2019, following regional screening programmes.61 In England, a one-off screening test with flexible sigmoidoscopy for people aged 55 years is being rolled out.62 With increases in diagnosis and treatment of asymptomatic disease, it is likely that survival and the proportion of patients treated for early stage disease will increase in the future.
Since 2013, surgeon-specific outcomes have been reported annually as quality measures in England.63 It is hoped that this increase in accountability will lead to improvements in patient care. However, these changes might also affect patient selection for surgery. The major reorganisation of the NHS in 2013,64 alongside substantial resource constraints in the NHS65 in the past decade, has had a potentially negative effect on cancer services. For instance, the 62-day treatment waiting time target—the aim that a patient should wait no more than 2 months from the date that the hospital receives an urgent referral for suspected cancer to the start of their treatment—has been missed for several quarters running, showing that services are unable to meet the demands placed on them.66 Given the ongoing financial pressures and austerity in the UK and the NHS, the future trends in survival for patients with cancer in England are uncertain.
Our findings have important policy implications, suggesting that the colorectal cancer survival deficit in England as compared with Denmark, Norway, and Sweden can be attributed partly to shortfalls in provision of surgical treatment. We showed that older patients in England, in particular, were less likely to receive resectional surgery than patients with similar characteristics in the other countries in our study. We posit that increases in the proportion of patients receiving resectional surgery might translate into better longer-term outcomes in England, provided that adequate postoperative care is also available.
Improving the capture of information on patients with colorectal cancer in specialised clinical registries, including data from individuals who are not eligible for surgery, would allow a more complete population-based comparison of colorectal cancer outcomes. Complete and comparable data on comorbidities, frailty, and additional therapies are required to improve understanding of international differences and inequalities in cancer outcomes.
Acknowledgments
Acknowledgments
This study was funded by an Early Diagnosis Policy Research Grant from Cancer Research UK to the Cancer Policy Programme at the London School of Hygiene & Tropical Medicine (LSHTM; award number C7923/A18348). We thank the national colorectal cancer registries in Denmark, England, Norway, and Sweden for their sustained efforts in collecting data for patients with colorectal cancer to the highest quality standards. We are indebted to Professor Lars Påhlman for encouraging us to design this study and for his support in its inception. We are grateful for advice received from members of the Cancer Policy Programme Scientific Advisory Group (Peter Sasieni, Deborah Ashby, Paul Aylin, Andrew Roddam, and Sally Vernon). We thank Claudia Allemani and the CONCORD Programme for the Global Surveillance of Cancer Survival for support with the data protocol and secure data transmission utility. We gratefully acknowledge the advice and support of members of the LSHTM Cancer Survival Group (CSG), especially Yuki Alencar (CSG Coordinator), Francisco Javier Rubio (Statistician), and Adrian Turculet (CSG Data Manager).
Contributors
SW, BR, MPC, and SBM designed the study. SBM did the literature review. CDG, MM, and MPC prepared the data protocol and data call. JC and LHI (Denmark), SBM and CDG (England), EAS and MGG (Norway), and KL and BG (Sweden) prepared national datasets according to the protocol. SBM developed and applied algorithms to derive and harmonise clinical variables. All authors contributed to the data harmonisation and variable definition process. CM and CDG prepared the survival model selection algorithm. SBM did the data analyses, and prepared the first draft of the manuscript, tables, and figures. SW and BR supervised the study. All authors had access to results at the data preparation and analysis stages, contributed to the interpretation of the results, revised and critically reviewed the manuscript, and approved the submitted version. SBM, CDG, SW, BR, and MPC had access to all the data in the study and take responsibility for its integrity and the accuracy of the analyses.
Declaration of interests
BR reports grants from the UK Department of Health, outside the submitted work. All other authors declare no competing interests.
Supplementary Material
References
- 1.International Agency for Research on Cancer GLOBOCAN 2012: estimated cancer incidence, mortality and prevalence worldwide in 2012. 2015. http://globocan.iarc.fr/Pages/fact_sheets_cancer.aspx?cancer=colorectal
- 2.Morris EJA, Sandin F, Lambert PC. A population-based comparison of the survival of patients with colorectal cancer in England, Norway and Sweden between 1996 and 2004. Gut. 2011;60:1087–1093. doi: 10.1136/gut.2010.229575. [DOI] [PubMed] [Google Scholar]
- 3.Coleman MP, Forman D, Bryant H. Cancer survival in Australia, Canada, Denmark, Norway, Sweden, and the UK, 1995–2007 (the International Cancer Benchmarking Partnership): an analysis of population-based cancer registry data. Lancet. 2011;377:127–138. doi: 10.1016/S0140-6736(10)62231-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Walters S, Benitez-Majano S, Muller P. Is England closing the international gap in cancer survival? Br J Cancer. 2015;113:848–860. doi: 10.1038/bjc.2015.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Maringe C, Walters S, Rachet B. Stage at diagnosis and colorectal cancer survival in six high-income countries: a population-based study of patients diagnosed during 2000–7. Acta Oncol. 2013;52:919–932. doi: 10.3109/0284186X.2013.764008. [DOI] [PubMed] [Google Scholar]
- 6.Iversen LH, Green A, Ingeholm P, Osterlind K, Gogenur I. Improved survival of colorectal cancer in Denmark during 2001–2012—the efforts of several national initiatives. Acta Oncol. 2016;55(suppl 2):10–23. doi: 10.3109/0284186X.2015.1131331. [DOI] [PubMed] [Google Scholar]
- 7.Kodeda K, Johansson R, Zar N. Time trends, improvements and national auditing of rectal cancer management over an 18-year period. Colorectal Dis. 2015;17:O168–O179. doi: 10.1111/codi.13060. [DOI] [PubMed] [Google Scholar]
- 8.Guren MG, Kørner H, Pfeffer F. Nationwide improvement of rectal cancer treatment outcomes in Norway, 1993–2010. Acta Oncol. 2015;54:1714–1722. doi: 10.3109/0284186X.2015.1034876. [DOI] [PubMed] [Google Scholar]
- 9.Richards MA. The size of the prize for earlier diagnosis of cancer in England. Br J Cancer. 2009;101(suppl 2):S125–S129. doi: 10.1038/sj.bjc.6605402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Heald RJ. A new approach to rectal cancer. Br J Hosp Med. 1979;22:277–281. [PubMed] [Google Scholar]
- 11.Hohenberger W, Weber K, Matzel K, Papadopoulos T, Merkel S. Standardized surgery for colonic cancer: complete mesocolic excision and central ligation—technical notes and outcome. Colorectal Dis. 2009;11:354–364. doi: 10.1111/j.1463-1318.2008.01735.x. [DOI] [PubMed] [Google Scholar]
- 12.Dimitriou N, Griniatsos J. Complete mesocolic excision: techniques and outcomes. World J Gastrointest Oncol. 2015;7:383–388. doi: 10.4251/wjgo.v7.i12.383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Påhlman L, Cedermark B, Bohe M. Onkologiskt Centrum, Norra Regionen; Umeå: 2008. Colorectal cancer, National care programme 2008. (in Swedish). [Google Scholar]
- 14.Norwegian Directorate of Health National operational guidelines for diagnosis, treatment and follow-up of cancer of the colon and rectum. 2017. https://helsedirektoratet.no/retningslinjer/nasjonalt-handlingsprogram-med-retningslinjer-for-diagnostikk-behandling-og-oppfolging-av-kreft-i-tykktarm-og-endetarm (in Norwegian).
- 15.National Institute for Health and Care Excellence . National Institute for Health and Care Excellence; London: 2011. Colorectal cancer: diagnosis and management. [Google Scholar]
- 16.Morris EJA, Finan PJ, Spencer K. Wide variation in the use of radiotherapy in the management of surgically treated rectal cancer across the English National Health Service. Clin Oncol (R Coll Radiol) 2016;28:522–531. doi: 10.1016/j.clon.2016.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Glimelius B, Myklebust TÅ, Lundqvist K, Wibe A, Guren MG. Two countries—two treatment strategies for rectal cancer. Radiother Oncol. 2016;121:357–363. doi: 10.1016/j.radonc.2016.11.010. [DOI] [PubMed] [Google Scholar]
- 18.Li R, Abela L, Moore J. Control of data quality for population-based cancer survival analysis. Cancer Epidemiol. 2014;38:314–320. doi: 10.1016/j.canep.2014.02.013. [DOI] [PubMed] [Google Scholar]
- 19.Ingeholm P, Gögenur I, Iversen LH. Danish Colorectal Cancer Group database. Clin Epidemiol. 2016;8:465–468. doi: 10.2147/CLEP.S99481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moberger P, Sköldberg F, Birgisson H. Evaluation of the Swedish Colorectal Cancer Registry: an overview of completeness, timeliness, comparability and validity. Acta Oncol. 2018 doi: 10.1080/0284186X.2018.1529425. published online Nov 26. [DOI] [PubMed] [Google Scholar]
- 21.Fritz AG, Percy C, Jack A, editors. International Classification of Diseases for Oncology (ICD-O) 3rd edn. World Health Organization; Geneva: 2000. [Google Scholar]
- 22.Benitez-Majano S, Fowler H, Maringe C, Di Girolamo C, Rachet B. Deriving stage at diagnosis from multiple population-based sources: colorectal and lung cancer in England. Br J Cancer. 2016;115:391–400. doi: 10.1038/bjc.2016.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Health and Social Care Information Centre NHS Classifications OPCS-4. 2016. https://isd.hscic.gov.uk/trud3/user/guest/group/0/pack/10
- 24.Allemani C, Matsuda T, Di Carlo V. Global surveillance of trends in cancer survival 2000–14 (CONCORD-3): analysis of individual records for 37 513 025 patients diagnosed with one of 18 cancers from 322 population-based registries in 71 countries. Lancet. 2018;391:1023–1075. doi: 10.1016/S0140-6736(17)33326-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brenner H, Gefeller O. Deriving more up-to-date estimates of long-term patient survival. J Clin Epidemiol. 1997;50:211–216. doi: 10.1016/s0895-4356(97)00280-1. [DOI] [PubMed] [Google Scholar]
- 26.London School of Hygiene & Tropical Medicine Cancer Survival Group Cancer Survival Group UK life tables. 2015. http://csg.lshtm.ac.uk/tools-analysis/uk-life-tables/
- 27.Royston P, Sauerbrei W. Multivariable modeling with cubic regression splines: a principled approach. Stata J. 2007;7:45. [Google Scholar]
- 28.Corazziari I, Quinn MJ, Capocaccia R. Standard cancer patient population for age standardising survival ratios. Eur J Cancer. 2004;40:2307–2316. doi: 10.1016/j.ejca.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 29.Bower H, Crowther MJ, Lambert PC. A command for fitting flexible parametric survival models on the log-hazard scale. Stata J. 2016;16:989–1012. [Google Scholar]
- 30.StataCorp . StataCorp LLC; College Station, TX: 2017. Stata Statistical Software: release 15. [Google Scholar]
- 31.Peduzzi P, Concato J, Kemper E, Holford TR, Feinstein AR. A simulation study of the number of events per variable in logistic regression analysis. J Clin Epidemiol. 1996;49:1373–1379. doi: 10.1016/s0895-4356(96)00236-3. [DOI] [PubMed] [Google Scholar]
- 32.Danish Colorectal Cancer Group Danish Colorectal Cancer Group's current guidelines. 2017. https://dccg.dk/retningslinjer/kolorektal-cancer/ (in Danish).
- 33.National Working Group. Regional Cancer Centres National Care Programme—Colorectal Cancer. 2016. https://www.cancercentrum.se/globalassets/cancerdiagnoser/tjock-och-andtarm-anal/vardprogram/nvpkolorektalcancer_2016-03-15.pdf (in Swedish).
- 34.Schreuders EH, Ruco A, Rabeneck L. Colorectal cancer screening: a global overview of existing programmes. Gut. 2015;64:1637–1649. doi: 10.1136/gutjnl-2014-309086. [DOI] [PubMed] [Google Scholar]
- 35.National Cancer Equality Initiative/Pharmaceutical Oncology Initiative . Department of Health; London: 2012. The impact of patient age on clinical decision-making in oncology. [Google Scholar]
- 36.National Cancer Intelligence Network . National Cancer Intelligence Network; London: 2011. Major surgical resections—England, 2004–2006. [Google Scholar]
- 37.Papamichael D, Audisio RA, Glimelius B. Treatment of colorectal cancer in older patients: International Society of Geriatric Oncology (SIOG) consensus recommendations 2013. Ann Oncol. 2015;26:463–476. doi: 10.1093/annonc/mdu253. [DOI] [PubMed] [Google Scholar]
- 38.Stevenson ARL. The future for laparoscopic rectal cancer surgery. Br J Surg. 2017;104:643–645. doi: 10.1002/bjs.10503. [DOI] [PubMed] [Google Scholar]
- 39.Chen K, Cao G, Chen B. Laparoscopic versus open surgery for rectal cancer: a meta-analysis of classic randomized controlled trials and high-quality nonrandomized studies in the last 5 years. Int J Surg. 2017;39:1–10. doi: 10.1016/j.ijsu.2016.12.123. [DOI] [PubMed] [Google Scholar]
- 40.Hamaker ME, Schiphorst AH, Verweij NM, Pronk A. Improved survival for older patients undergoing surgery for colorectal cancer between 2008 and 2011. Int J Colorectal Dis. 2014;29:1231–1236. doi: 10.1007/s00384-014-1959-y. [DOI] [PubMed] [Google Scholar]
- 41.Stormark K, Soreide K, Soreide JA. Nationwide implementation of laparoscopic surgery for colon cancer: short-term outcomes and long-term survival in a population-based cohort. Surg Endosc. 2016;30:4853–4864. doi: 10.1007/s00464-016-4819-8. [DOI] [PubMed] [Google Scholar]
- 42.Iversen LH, Ingeholm P, Gogenur I, Laurberg S. Major reduction in 30-day mortality after elective colorectal cancer surgery: a nationwide population-based study in Denmark 2001–2011. Ann Surg Oncol. 2014;21:2267–2273. doi: 10.1245/s10434-014-3596-7. [DOI] [PubMed] [Google Scholar]
- 43.van Gijn W, van den Broek CB, Mroczkowski P. The EURECCA project: data items scored by European colorectal cancer audit registries. Eur J Surg Oncol. 2012;38:467–471. doi: 10.1016/j.ejso.2012.01.005. [DOI] [PubMed] [Google Scholar]
- 44.Yates JW, Chalmer B, McKegney FP. Evaluation of patients with advanced cancer using the Karnofsky performance status. Cancer. 1980;45:2220–2224. doi: 10.1002/1097-0142(19800415)45:8<2220::aid-cncr2820450835>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 45.Repetto L, Fratino L, Audisio RA. Comprehensive geriatric assessment adds information to Eastern Cooperative Oncology Group performance status in elderly cancer patients: an Italian Group for Geriatric Oncology study. J Clin Oncol. 2002;20:494–502. doi: 10.1200/JCO.2002.20.2.494. [DOI] [PubMed] [Google Scholar]
- 46.Prince MJ, Wu F, Guo Y. The burden of disease in older people and implications for health policy and practice. Lancet. 2015;385:549–562. doi: 10.1016/S0140-6736(14)61347-7. [DOI] [PubMed] [Google Scholar]
- 47.Wilkins EWL, Wickramasinghe K, Bhatnagar P. European Heart Network; Brussels: 2017. European cardiovascular disease statistics 2017. [Google Scholar]
- 48.Tiselius C, Gunnarsson U, Smedh K, Glimelius B, Påhlman L. Patients with rectal cancer receiving adjuvant chemotherapy have an increased survival: a population-based longitudinal study. Ann Oncol. 2013;24:160–165. doi: 10.1093/annonc/mds278. [DOI] [PubMed] [Google Scholar]
- 49.van de Velde CJ, Boelens PG, Borras JM. EURECCA colorectal: multidisciplinary management: European consensus conference colon & rectum. Eur J Cancer. 2014;50:1–34. doi: 10.1016/j.ejca.2013.06.048. [DOI] [PubMed] [Google Scholar]
- 50.Breugom AJ, Swets M, Bosset JF. Adjuvant chemotherapy after preoperative (chemo)radiotherapy and surgery for patients with rectal cancer: a systematic review and meta-analysis of individual patient data. Lancet Oncol. 2015;16:200–207. doi: 10.1016/S1470-2045(14)71199-4. [DOI] [PubMed] [Google Scholar]
- 51.Asli LM, Johannesen TB, Myklebust TA, Moller B, Eriksen MT, Guren MG. Preoperative chemoradiotherapy for rectal cancer and impact on outcomes—a population-based study. Radiother Oncol. 2017;123:446–453. doi: 10.1016/j.radonc.2017.04.012. [DOI] [PubMed] [Google Scholar]
- 52.Van Cutsem E, Cervantes A, Adam R. ESMO consensus guidelines for the management of patients with metastatic colorectal cancer. Ann Oncol. 2016;27:1386–1422. doi: 10.1093/annonc/mdw235. [DOI] [PubMed] [Google Scholar]
- 53.Primrose J, Falk S, Finch-Jones M. Systemic chemotherapy with or without cetuximab in patients with resectable colorectal liver metastasis: the New EPOC randomised controlled trial. Lancet Oncol. 2014;15:601–611. doi: 10.1016/S1470-2045(14)70105-6. [DOI] [PubMed] [Google Scholar]
- 54.Sorbye H, Cvancarova M, Qvortrup C, Pfeiffer P, Glimelius B. Age-dependent improvement in median and long-term survival in unselected population-based Nordic registries of patients with synchronous metastatic colorectal cancer. Ann Oncol. 2013;24:2354–2360. doi: 10.1093/annonc/mdt197. [DOI] [PubMed] [Google Scholar]
- 55.Gatta G, Capocaccia R, Sant M. Understanding variations in survival for colorectal cancer in Europe: a EUROCARE high resolution study. Gut. 2000;47:533–538. doi: 10.1136/gut.47.4.533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Archampong D, Borowski D, Wille-Jorgensen P, Iversen LH. Workload and surgeon's specialty for outcome after colorectal cancer surgery. Cochrane Database Syst Rev. 2012;14 doi: 10.1002/14651858.CD005391.pub3. CD005391. [DOI] [PubMed] [Google Scholar]
- 57.Department of Health . Department of Health; London: 2000. The NHS Cancer Plan. [Google Scholar]
- 58.Probst HB, Hussain ZB, Andersen O. Cancer patient pathways in Denmark as a joint effort between bureaucrats, health professionals and politicians—a national Danish project. Health Policy. 2012;105:65–70. doi: 10.1016/j.healthpol.2011.11.001. [DOI] [PubMed] [Google Scholar]
- 59.Larsen MB, Njor S, Ingeholm P, Andersen B. Effectiveness of colorectal cancer screening in detecting earlier-stage disease— a nationwide cohort study in Denmark. Gastroenterology. 2018;155:99–106. doi: 10.1053/j.gastro.2018.03.062. [DOI] [PubMed] [Google Scholar]
- 60.Norwegian Directorate of Health . Norwegian Directorate of Health; Oslo: 2017. National screening programme against bowel cancer—status and recommendations. (in Norwegian). [Google Scholar]
- 61.Aronsson M, Carlsson P, Levin L-Å, Hager J, Hultcrantz R. Cost-effectiveness of high-sensitivity faecal immunochemical test and colonoscopy screening for colorectal cancer. Br J Surg. 2017;104:1078–1086. doi: 10.1002/bjs.10536. [DOI] [PubMed] [Google Scholar]
- 62.National Health Service Bowel scope screening. https://www.nhs.uk/conditions/bowel-cancer-screening/bowel-scope-screening/
- 63.Vallance AE, Fearnhead NS, Kuryba A. Effect of public reporting of surgeons' outcomes on patient selection, “gaming,” and mortality in colorectal cancer surgery in England: population based cohort study. BMJ. 2018;361:k1581. doi: 10.1136/bmj.k1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.UK Government Health and Social Care Act 2012. 2012. http://www.legislation.gov.uk/ukpga/2012/7/pdfs/ukpga_20120007_en.pdf
- 65.Porter M. British Medical Association; 2016. NHS funding and efficiency savings.https://www.bma.org.uk/collective-voice/influence/key-negotiations/nhs-funding/nhs-funding-and-efficiency-savings [Google Scholar]
- 66.MacMillan Cancer Support Patients in limbo as cancer waiting time targets missed for two years running. 2018. https://www.macmillan.org.uk/aboutus/news/latest_news/patients-in-limbo-as-cancer-waiting-time-targets-missed-for-two-years-running-.aspx
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.