Abstract
Introduction
The objective of the study was to investigate the efficacy and safety of repeated incobotulinumtoxinA injections for the treatment of upper-limb post-stroke spasticity in adults.
Methods
Adults 18–80 years of age with post-stroke upper-limb spasticity who completed the 12-week randomized, double-blind, placebo-controlled main period (MP) of a phase 3 trial (NCT01392300) were eligible to enrol in the 36-week open-label extension period (OLEX). The OLEX included three treatment cycles at fixed 12-week injection intervals; subjects were injected with 400 U incobotulinumtoxinA into the affected upper limb. Efficacy assessments included evaluation of muscle tone using the Ashworth Scale (AS) and the Global Impression of Change Scale (GICS) assessed by the investigator, subject, and caregiver. The incidence of adverse events (AEs) was monitored throughout the OLEX.
Results
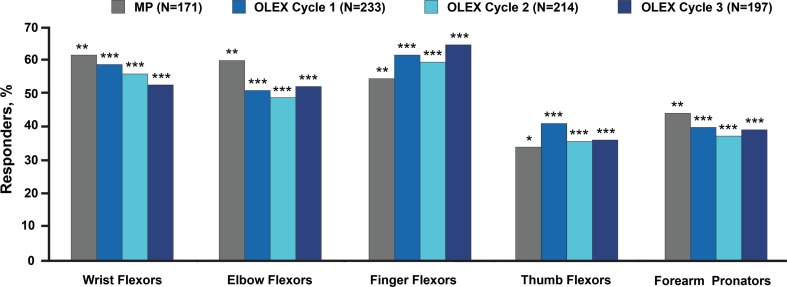
A total of 296 of 299 subjects (99.0%) who completed the MP received incobotulinumtoxinA in the OLEX, and 248 subjects completed the 36-week OLEX. The proportion of subjects with at least a 1-point improvement in AS score from each incobotulinumtoxinA treatment to the respective 4-week post-injection visit ranged by cycle from 52.3% to 59.2% for wrist flexors, 49.1% to 52.3% for elbow flexors, 59.8% to 64.5% for finger flexors, 35.5% to 41.2% for thumb flexors, and 37.4% to 39.9% for forearm pronators (P < 0.0001 for all). Over 90% of subjects were assessed by the investigator to be at least minimally improved (4 weeks post-injection) on the GICS during each injection cycle; 61.0% in the 1st cycle, 58.2% in the 2nd cycle, and 57.4% in the 3rd cycle were considered much improved or very much improved on the GICS. Three percent of subjects (9/296) reported treatment-related AEs; the most frequently reported were pain in the extremity (n = 2, 0.7%) and constipation (n = 2, 0.7%). Serious AEs were reported by 22 subjects (7.4%); however, none were considered treatment-related.
Conclusions
Repeated injections of incobotulinumtoxinA for the treatment of post-stroke upper-limb spasticity led to significant improvements in muscle tone and investigator’s global impression of change. Treatment was well tolerated, with no serious treatment-related AEs.
Funding
Merz Pharmaceuticals GmbH.
Keywords: IncobotulinumtoxinA, Neurology, Spasticity, Stroke
Introduction
Botulinum neurotoxin injections are a guideline-recommended, first-line treatment for post-stroke spasticity affecting the upper limb in adults [1–4]. Currently, three serotype A botulinum neurotoxin preparations (incobotulinumtoxinA, onabotulinumtoxinA, and abobotulinumtoxinA) are approved in the US and European commercial markets for the treatment of upper-limb spasticity in adults.
IncobotulinumtoxinA (Xeomin®, Merz Pharmaceuticals GmbH) is a purified botulinum neurotoxin type A formulation that is free of accessory, complexing proteins. When compared with other pharmaceutical preparations of botulinum neurotoxin type A, incobotulinumtoxinA has the highest specific neurotoxin activity and a lower immunogenicity [5, 6]. Clinical studies have demonstrated the therapeutic equivalence of incobotulinumtoxinA to onabotulinumtoxinA when the same number of units was used to treat various neurologic conditions, including cervical dystonia and blepharospasm [7–10], as well as non-neurological conditions [11–13].
Randomized, placebo-controlled clinical trials have previously demonstrated the safety and efficacy of a single incobotulinumtoxinA treatment, as noted by significant improvements in muscle tone, global and functional outcomes among subjects with post-stroke upper-limb spasticity, and treatment was well tolerated [14, 15]. However, patients with spasticity often require repeated, long-term botulinum toxin therapy [16]. In an open-label extension (OLEX) study previously conducted in Europe up to 69 weeks, repeated treatments with incobotulinumtoxinA resulted in sustained improvements in muscle tone among adults with post-stroke upper-limb spasticity and were also well tolerated [16].
In this randomized, placebo-controlled phase 3 clinical trial with an OLEX (NCT01392300; EudraCT 2010-023043-15), the safety and efficacy of repeated incobotulinumtoxinA treatments in adults with post-stroke spasticity of the upper limb were investigated. Data from the main period (MP) have previously been published [15]; here, we present data from the OLEX.
Methods
The study design and MP results have previously been reported [15], but the methodology is briefly summarized below.
This prospective study consisted of a parallel-group MP (12-week duration) with a single treatment of 400 U incobotulinumtoxinA or placebo (randomization ratio: 2:1). Subjects could continue into a 36-week OLEX to receive three further treatment cycles. Each cycle began with a single treatment session (400 U incobotulinumtoxinA), followed by 12 weeks of observation and assessment. The full study was conducted at 46 sites in the Czech Republic, Germany, Hungary, India, Poland, Russia, and the USA between September 2011 and February 2014 and in accordance with the Declaration of Helsinki. The study protocol, informed consent, and other appropriate study-related documents were approved by the independent ethics committees and institutional review boards responsible for each participating site.
Subjects and Treatment
The inclusion and exclusion criteria for the MP have been reported previously [15]; briefly, adult subjects (18–80 years) with spasticity of the upper limb due to stroke (at least 3 months after the last stroke) were eligible for participation in the MP. Participants had to have a flexed elbow, flexed wrist, and clenched fist clinical pattern of spasticity with muscle tone score of at least 2 on the Ashworth Scale (AS) at each site, and a clinical need for a total dose of 400 U incobotulinumtoxinA into the affected upper limb. The primary criteria for inclusion in the OLEX were completion of the preceding 12-week MP interval and a clinical need (in the opinion of the local site investigator) for continued treatment with 400 U incobotulinumtoxinA in the affected upper limb; safety criteria (e.g., minimum body weight, negative pregnancy test) were also applied.
The OLEX was designed to closely mirror the treatment of upper-limb spasticity in everyday clinical practice. Whereas the study MP required a primary target clinical pattern to be chosen by the investigator and associated muscles treated using a predefined total fixed dose and range within the pattern [15], in the OLEX the treatment of muscle groups associated with each clinical pattern of spasticity (e.g., flexed wrist, clenched fist, flexed elbow, pronated forearm, thumb-in-palm) was determined at the discretion of the investigator. Dosing was determined according to the subject’s clinical need, within a range of doses predefined for each muscle (Table 1). The total fixed dose for each OLEX injection cycle was 400 U. If the investigator determined at the 12-week follow-up for any given cycle that the subject did not require treatment with 400 U incobotulinumtoxinA, the subject exited the study. All injections were guided by electromyography, electrical muscle stimulation, or ultrasound.
Table 1.
Dose range per muscle for treatment of upper-limb spasticity during the OLEX
| Clinical pattern Muscle |
Units (range)a | Injection sites per muscle |
|---|---|---|
| Flexed wrist | ||
| Flexor carpi radialis | 25–100 | 1–2 |
| Flexor carpi ulnaris | 20–100 | 1–2 |
| Clenched fist | ||
| Flexor digitorum superficialis | 25–100 | 2 |
| Flexor digitorum profundus | 25–100 | 2 |
| Flexed elbow | ||
| Brachioradialis | 25–100 | 1–3 |
| Biceps | 75–200 | 1–4 |
| Brachialis | 25–100 | 1–2 |
| Pronated forearm | ||
| Pronator quadratus | 10–50 | 1 |
| Pronator teres | 25–75 | 1–2 |
| Thumb-in-palm | ||
| Flexor pollicis longus | 10–50 | 1 |
| Adductor pollicis | 5–30 | 1 |
| Flexor pollicis brevis/Opponens pollicis | 5–30 | 1 |
aA 100 U/2 mL dilution was used. For all muscles, the largest volume to be injected at a single injection site was 1.0 mL
OLEX open-label extension period
Efficacy Assessments
Changes in muscle tone were assessed using the AS [17–19], a 5-point scale for the quantitative determination of muscle tone ranging from 0 (no increase in muscle tone) to 4 (limb rigid in flexion or extension). AS assessments were performed prior to each injection and at follow-up visits 4 weeks post-injection. Responders were defined as subjects with at least a 1-point improvement in AS score for a particular muscle group at the 4-week follow-up visit. Mean changes in AS scores at the OLEX cycle control visits were determined relative to the study baseline injection visit (day 0 of MP), and to the respective cycle’s baseline injection visit for each treated muscle group.
Efficacy of the previous OLEX injection cycle was further evaluated using Global Impression of Change Scale (GICS) [15]. This subjective outcome measure was based on the investigator’s clinical assessment of each subject’s global change in upper-limb spasticity at the 4-week, follow-up visit when compared to the respective injection cycle baseline visit. GICS assessments were also completed by the subject and the subject’s caregiver (if applicable). A 7-point, balanced Likert scale was used to quantify responses as follows: – 3 = very much worse; – 2 = much worse; – 1 = minimally worse; 0 = no change; + 1 = minimally improved; + 2 = much improved; and + 3 = very much improved.
Safety Assessments
Subjects were actively prompted to report any adverse events (AEs) during each visit or via telephone contact. A specific questionnaire designed to elicit information related to an AE of special interest (AESI), defined as an AE occurring after treatment that may indicate toxin spread within the injected limb or more diffusely, was administered to subjects at each visit. Standard physical and neurological examinations were performed at the OLEX interval visits. International normalized ratio and pregnancy testing, where applicable, were conducted prior to each study injection. Clinical laboratory evaluations included hematologic and chemistry blood tests at week 12 of each cycle, and assessments of glucose and alkaline phosphatase levels were additionally performed at week 4. Safety assessments also included testing for anti-botulinum neurotoxin antibodies at weeks 4 and 12 of each cycle by fluorescent immunoassay, and for those showing a positive result in the screening test for neutralizing antibodies, by mouse hemidiaphragm assay [20, 21].
Statistical Analyses
The safety evaluation set (SES) was the subset of all subjects who were exposed to incobotulinumtoxinA at least once in the OLEX period. The full analysis set was the subset of SES subjects in the OLEX for whom at least one AS score value for the primary target clinical pattern was available in the OLEX and who were randomized after the amended MP protocol became effective [15]. Efficacy and safety data were analyzed descriptively, without imputation for missing data (observed case analysis). Changes in AS scores were analyzed using a Wilcoxon signed-rank test for specific pairwise comparisons.
Results
Subjects and Treatments Administered
A total of 299 subjects who completed the placebo-controlled MP entered the OLEX at 46 investigational sites. Of these subjects, 296/299 (99.0%) received incobotulinumtoxinA in the OLEX, as three subjects did not require treatment with 400 U incobotulinumtoxinA 12 weeks after the MP and discontinued participation in the OLEX. Of the 296 subjects who received treatment during the OLEX, 99 subjects (33.4%) had been randomized to placebo during the MP. As a result of the study design, subjects who had received placebo in the MP received one fewer treatment with incobotulinumtoxinA over the entire study than those who had received incobotulinumtoxinA in the MP. A total of 248 subjects (82.9%) completed all three injection cycles during the OLEX period. Reasons for subject discontinuation included withdrawal of consent (n = 19), predefined discontinuation criteria (n = 13), AEs (n = 10, including four subject deaths that were unrelated to treatment), loss to follow-up (n = 6), lack of efficacy (n = 3), and noncompliance (n = 1). Four subjects died during the OLEX, none as a result of treatment-related events. Subject demographics were similar to those reported for the MP and are summarized in Table 2.
Table 2.
Subject characteristics at OLEX baseline
| Characteristic | Subjects receiving ≥ 1 incobotulinumtoxinA injection (N = 296) |
|---|---|
| Mean (SD) age, years | 56.3 (11.5) |
| Male gender, n (%) | 169 (57.1) |
| Mean (SD) time since first diagnosis of upper-limb spasticity, months | 38.2 (55.2) |
| Clinical pattern of upper-limb spasticity, n (%) | |
| Flexed wrist | 296 (100.0) |
| Flexed elbow | 296 (100.0) |
| Clenched fist | 296 (100.0) |
| Thumb-in-palm | 243 (82.1) |
| Pronated forearm | 272 (91.9) |
OLEX open-label extension period, SD standard deviation
During the OLEX, 296 subjects received at least one incobotulinumtoxinA treatment, 276 received at least two treatments, and 257 subjects received three treatments. In all but one case, where a subject received only 375 U, subjects who were treated during a given injection cycle received a total dose of 400 U incobotulinumtoxinA in a total volume of 8 mL.
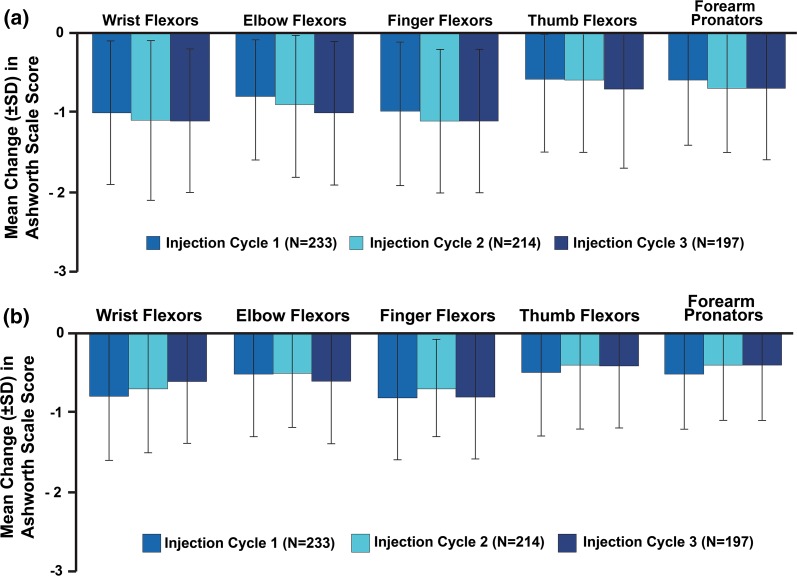
Efficacy Outcomes
For all subjects in the OLEX, including those who received placebo in the MP, improvements in mean AS scores were observed during each injection cycle (Table 3). Improvements were also observed at the 4-week post-injection visit compared with the MP baseline (Fig. 1a) and with each respective cycle’s baseline visit (Fig. 1b). During the first OLEX cycle, those who received incobotulinumtoxinA in the MP demonstrated similar changes in mean (standard deviation) AS score to those who received placebo in the MP (subjects treated in the first OLEX cycle, who had received placebo in the MP, at the 4-week post-injection visit compared with the MP baseline: wrist flexors, − 0.8 (0.9) [study baseline 2.6 (0.6)]; elbow flexors, − 0.7 (0.8) [study baseline 2.8 (0.6)]; finger flexors, − 1.0 (0.9) [study baseline 2.8 (0.6)]; thumb flexors, − 0.6 (1.0) [study baseline 2.0 (0.9)]; forearm pronators, − 0.5 (0.7) [study baseline 2.2 (0.9)]). Throughout the OLEX, there were no marked differences in AS responses between those in the two MP treatment groups. For all subjects in the OLEX, including those who received placebo in the MP, AS responder analysis revealed that repeated incobotulinumtoxinA treatments improved muscle tone from each treatment visit to the respective 4-week post-injection visit in all treated upper-limb muscle groups (P < 0.0001 for all; Wilcoxon signed-rank test); however, the responses for the thumb and pronator groups were somewhat lower than for the other muscle groups (Fig. 2). Across all clinical patterns, the proportions of responders were similar between the MP (incobotulinumtoxinA treatment group; N = 171) and subsequent OLEX cycles (Fig. 2).
Table 3.
Mean (± SD) Ashworth Scale scores for each OLEX injection cycle
| Cycle | N | Wrist flexors | Elbow flexors | Finger flexors | Thumb flexors | Forearm pronators |
|---|---|---|---|---|---|---|
| OLEX cycle 1 | ||||||
| Inj. visit | 234 | 2.4 ± 0.7 | 2.4 ± 0.7 | 2.6 ± 0.7 | 1.9 ± 0.8 | 2.0 ± 0.9 |
| Week 4 | 233 | 1.7 ± 0.9 | 1.9 ± 0.8 | 1.8 ± 0.9 | 1.4 ± 0.8 | 1.6 ± 0.9 |
| EOC | 230 | 2.1 ± 0.8 | 2.2 ± 0.7 | 2.4 ± 0.8 | 1.8 ± 0.8 | 1.9 ± 0.9 |
| OLEX cycle 2 | ||||||
| Inj. visit | 227 | 2.1 ± 0.8 | 2.3 ± 0.7 | 2.4 ± 0.8 | 1.8 ± 0.8 | 1.9 ± 0.9 |
| 4-week visit | 214 | 1.5 ± 0.8 | 1.8 ± 0.8 | 1.7 ± 0.8 | 1.4 ± 0.8 | 1.5 ± 0.9 |
| EOC | 208 | 2.1 ± 0.8 | 2.2 ± 0.8 | 2.3 ± 0.8 | 1.7 ± 0.9 | 1.9 ± 0.8 |
| OLEX cycle 3 | ||||||
| Inj. visit | 205 | 2.1 ± 0.7 | 2.2 ± 0.8 | 2.4 ± 0.8 | 1.7 ± 0.8 | 1.9 ± 0.8 |
| Week 4 | 197 | 1.5 ± 0.8 | 1.6 ± 0.8 | 1.6 ± 0.8 | 1.3 ± 0.8 | 1.5 ± 0.8 |
| EOC | 200 | 1.8 ± 0.9 | 1.9 ± 0.9 | 2.0 ± 0.8 | 1.6 ± 0.8 | 1.8 ± 0.9 |
EOC end of cycle, Inj injection, OLEX open-label extension period, SD standard deviation
Fig. 1.
Mean change in Ashworth Scale scores for each OLEX injection cycle compared with a main period baseline or b each respective cycle’s injection visit. OLEX open-label extension period, SD standard deviation
Fig. 2.
Responder analysis for each clinical pattern muscle group by OLEX injection cycle, observed cases. Subjects with an improvement (reduction) of ≥1 point on the Ashworth Scale at the 4-week post-injection visit were classified as responders. Main period values reported from [15] for comparison. *P = 0.028 vs placebo, **P < 0.001 vs placebo, logistic regression; ***P < 0.0001 vs cycle baseline value, Wilcoxon signed-rank test. MP main period, OLEX open-label extension period
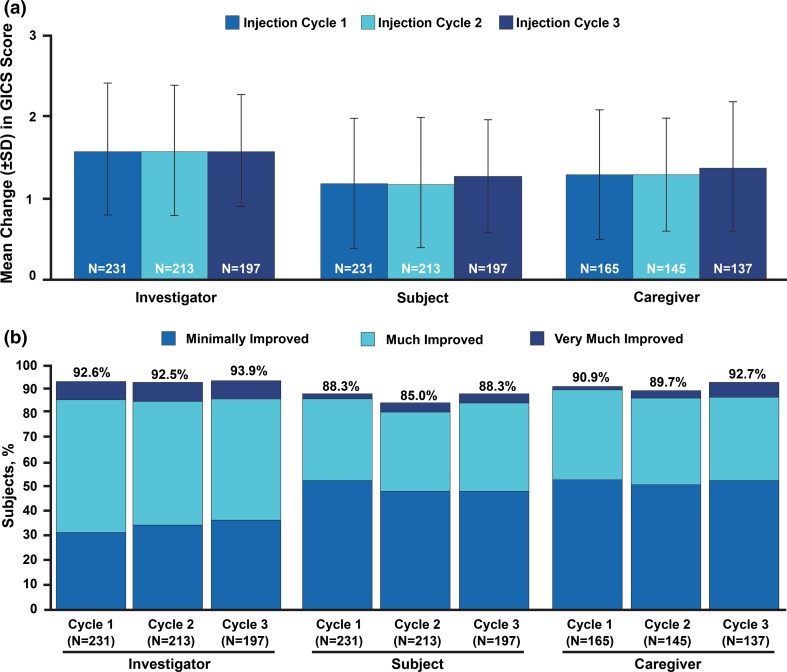
For all subjects in the OLEX, including those who received placebo in the MP, the mean investigator-assessed improvement on the GICS observed for each injection cycle was 1.6 points; similar improvements were noted for subject- and caregiver-assessed GICS scores across each injection cycle (Fig. 3a). A high proportion of subjects (> 85%) were considered at least minimally improved (i.e., a score of 1, 2, or 3) on the GICS for assessments by the investigator, subject, or caregiver during each injection cycle (Fig. 3b). Frequency distributions by score for the investigator-assessed GICS were similar across cycles and consistent with those observed for the MP [15]: 31.6–36.5% were considered minimally improved; 49.7–53.7% were considered much improved; and 7.4–7.6% were considered very much improved.
Fig. 3.
Improvement during OLEX on the GICS 4 weeks after each injection. Mean changes in GICS scores as assessed by the investigators, subjects, and caregivers are shown in a. The proportions of subjects with a score ≥ 1 on the GICS (i.e., minimally improved, much improved, or very much improved) are shown in b. GICS Global Impression of Change Scale, OLEX open-label extension period
Safety
The mean total doses administered to individual muscles during the OLEX were comparable between the three treatment cycles (Table 4). AEs were recorded in 93 of 296 (31.4%) subjects throughout the three incobotulinumtoxinA treatment cycles (Table 5). Treatment-related AEs were reported by 9/296 subjects (3.0%) across all treatment cycles, which was similar to the proportion of subjects reporting related AEs during the MP [15]. None of the treatment-related AEs were considered serious, and none of the four deaths were considered related to treatment (causes of death included acute myocardial infarction, pseudobulbar palsy [pseudobulbar syndrome as the result of vascular encephalopathy], cellulitis with septic shock and acute renal failure, and cardiac death [cardiac arrest]). All but one of the treatment-related AEs (increased level of γ-glutamyl transferase) had resolved by the end of the study; however, this subject had a high γ-glutamyl transferase level at study baseline. None of the AEs leading to premature discontinuation was considered treatment-related; these AEs included endocarditis, neck fracture, epilepsy and ischemic stroke, upper respiratory tract infection, dysuria, gastroesophageal reflux disease, and pneumonia/cerebral hemorrhage/respiratory failure. During the OLEX, observed AESIs included constipation (n = 5), diplopia (n = 1), muscular weakness (n = 1), pelvic floor muscle weakness (n = 1), and dyspnea (n = 1); however, not all AESIs were considered related to treatment (Table 5). Throughout the study, there were no cases of clinical non-responsiveness with a corresponding positive test for neutralizing antibodies.
Table 4.
Mean (± SD) doses (U) administered to individual muscles during the OLEX
| Muscle group | Muscle | Cycle 1 (N = 296) | Cycle 2 (N = 276) | Cycle 3 (N = 257) |
|---|---|---|---|---|
| All | All | 400.0 ± 0.0 | 399.9 ± 1.5 | 400.0 ± 0.0 |
| Elbow flexors | All | 147.4 ± 51.0 | 150.6 ± 55.8 | 152.3 ± 49.3 |
| Biceps | 93.6 ± 20.9 | 98.6 ± 28.4 | 100.4 ± 26.7 | |
| Brachialis | 47.4 ± 23.4 | 48.9 ± 24.8 | 50.9 ± 25.7 | |
| Brachioradialis | 44.8 ± 18.6 | 44.3 ± 20.4 | 42.8 ± 16.5 | |
| Wrist flexors | All | 108.9 ± 44.3 | 105.8 ± 40.7 | 106.2 ± 41.2 |
| Flexor carpi radialis | 57.0 ± 23.4 | 55.4 ± 21.7 | 55.2 ± 21.2 | |
| Flexor carpi ulnaris | 53.9 ± 22.8 | 53.0 ± 20.7 | 53.8 ± 20.7 | |
| Finger flexors | All | 119.9 ± 42.8 | 119.4 ± 40.7 | 119.3 ± 41.7 |
| Flexor digitorum profundus | 62.9 ± 23.0 | 61.1 ± 21.4 | 61.4 ± 21.5 | |
| Flexor digitorum superficialis | 60.7 ± 22.2 | 62.4 ± 21.8 | 62.3 ± 22.6 | |
| Forearm pronators | All | 52.9 ± 26.4 | 54.4 ± 26.8 | 56.0 ± 27.3 |
| Pronator quadratus | 27.4 ± 14.3 | 27.9 ± 14.1 | 29.3 ± 14.9 | |
| Pronator teres | 42.6 ± 16.4 | 42.2 ± 16.5 | 44.0 ± 15.6 | |
| Thumb flexors/adductors | All | 36.8 ± 24.5 | 37.8 ± 22.6 | 40.4 ± 25.3 |
| Adductor pollicis | 14.3 ± 8.7 | 15.1 ± 8.5 | 15.4 ± 9.9 | |
| Flexor pollicis brevis/o.p. | 14.0 ± 8.7 | 15.5 ± 8.8 | 16.5 ± 9.3 | |
| Flexor pollicis longus | 27.0 ± 15.4 | 29.7 ± 15.1 | 30.8 ± 16.0 |
OLEX open-label extension period, SD standard deviation
Table 5.
Summary of adverse events during OLEX
| Adverse events | Subjects, n (%), N = 296 |
|---|---|
| Any AE | 93 (31.4) |
| Any serious AE related to treatment | 0 |
| Deaths related to treatment | 0 |
| Any AE leading to discontinuation related to treatment | 0 |
| Any AE related to treatmenta | 9 (3.0) |
| Constipation | 2 (0.7) |
| Pain in extremity | 2 (0.7) |
| Dermatitis, allergic | 1 (0.3) |
| γ-Glutamyl transferase increased | 1 (0.3) |
| Injection-site bruising | 1 (0.3) |
| Muscular weakness | 1 (0.3) |
| Pelvic-floor muscle weakness | 1 (0.3) |
| Presyncope | 1 (0.3) |
| Uveitis | 1 (0.3) |
AE adverse event, OLEX open-label extension period
aAll treatment-related AEs were resolved by the end of the study, except one case of increased γ-glutamyl transferase in a subject who also had high levels of γ-glutamyl transferase at study baseline
During the OLEX, mean changes in vital signs (e.g., systolic and diastolic blood pressure, pulse, respiratory rate) were minimal, and median changes were zero. Body weight and BMI both increased nominally, by 0.2–0.4 kg and 0.1 kg/m2, respectively. For all clinical laboratory parameters, mean and median values were generally within normal ranges, with a few exceptions. Mean creatinine, γ-glutamyl transferase, and glucose values were near or slightly above the upper limit of normal ranges in each OLEX injection cycles, whereas the median values for each of these parameters fell within the normal ranges. Hematologic assessments (e.g., differential blood counts) also fell within normal ranges. None of the observed clinical laboratory values suggested a tendency toward a systemic, clinically relevant change during the OLEX.
Discussion
Results observed during this OLEX demonstrate that repeated injections enabled subjects to achieve improvements in muscle tone and the investigator’s GICS scores at the 4-week post-injection visit of each cycle. At the conclusion of the MP and OLEX of this study, subjects had received 3–4 total injections of 400 U incobotulinumtoxinA over 48 weeks. Overall, the mean changes in AS scores and the proportions of AS responders across all clinical patterns of upper-limb spasticity investigated during the OLEX were similar to those observed in previous studies [14, 16] and the MP of this study [15]. The general trend of these observations was that the extended treatment stabilized the tone reductions following active treatment in the MP. Notably, for all five clinical patterns, baseline-to-baseline comparisons suggest that there may have been some cycle-to-cycle carry-over effects. The changes in responder rates (number of subjects with at least a 1-point AS score change) in each OLEX cycle assessed at 4 weeks post-injection were statistically significant at the exploratory level (P < 0.0001 vs cycle baseline throughout) for each of the five treated clinical patterns, with a lower response rate observed for the thumb and pronator muscle groups than for the other muscle groups. Assessments by the investigators, subjects, and caregivers using the GICS confirmed that the vast majority of subjects showed clinically meaningful improvements in upper-limb spasticity after each treatment.
The volumes of incobotulinumtoxinA injected were similar between muscle groups and in all three injection cycles. The safety profile observed during the OLEX was similar to the study MP, with no new or unexpected AEs. Repeated incobotulinumtoxinA injections were well tolerated overall, consistent with previous studies of botulinum neurotoxin type A formulations in the treatment of post-stroke spasticity [14, 16, 22–25], including a study investigating the long-term safety of repeated high doses of incobotulinumtoxinA over 2 years [26].
Study limitations previously discussed for the MP [15] also apply to the OLEX. The total dose and injection intervals were all fixed by the study protocol, and although the protocol allowed more flexibility with regard to the doses per muscle for the OLEX, the treatments administered may not be entirely reflective of clinical practice. In real-world settings, the severity and pattern of upper-limb spasticity can differ markedly from patient to patient, and physicians may vary doses and injection intervals to meet individual patient needs. Indeed, a recent survey of physicians treating spasticity with botulinum neurotoxin injections reveals a common belief that better outcomes are linked to individualized dosing regimens, which may include shortening injection intervals to minimize the risk of wear-off phenomenon [27]. A recent study showed that a higher total dose results in improvements in muscle tone, goal attainment, and other functional measures [28]. Given the low immunogenicity of incobotulinumtoxinA [29], emerging literature supports the use of flexible injection intervals to maintain patients’ therapeutic benefits [29, 30]; however, there has been limited study of more frequent incobotulinumtoxinA injections in patients with spasticity [31]. It is also important to note that instrumented guidance was used to administer injections during this study, which likely contributed to the favorable and consistent improvements in AS and GICS scores observed; previous studies also support the use of guidance techniques vs manual needle placement to maximize clinical outcome measures [32–34].
Overall, these data support previous findings that incobotulinumtoxinA improves muscle tone in post-stroke upper-limb spasticity and confirm that repeated incobotulinumtoxinA injections at 12-week intervals are well tolerated, safe, and effective for the treatment of post-stroke upper-limb spasticity in adults.
Conclusions
Repeated injections of 400 U incobotulinumtoxinA in the upper extremities at regular 12-week intervals over 36 weeks led to significant and sustained improvements in muscle tone and meaningful investigator-assessed clinical improvements overall. Treatment was well tolerated, with no serious treatment-related AEs. These results support and extend previous findings from randomized controlled trials demonstrating that incobotulinumtoxinA is a safe and effective treatment for post-stroke upper-limb spasticity in adults.
Acknowledgements
The authors wish to thank all of the participants and investigators who contributed to this clinical study: Czech Republic: Michal Bar, Martin Bares, Edvard Ehler, Robert Jech, Petr Kaňovský, Radim Mazanec, Stanislav Vohanka, Oldrich Vysata; Germany: Reiner Benecke; France: Dominique Mazevet; Hungary: Csilla Rozsa, Zoltan Denes, Peter Dioszeghy, Laszlo Vecsei; India: Asad Abbas, Madhuri Behari, Mohit Bhatt, Shamsher Dwivedee, R. Srinivasa, Cannigaiper U. Velmurugendran, Krishnan Vijayan; Poland: Andrzej Bogucki, Anna Czlonkowska, Pawel Jan Polrola, Monika Rudzinska, Jaroslow W. Slawek, Zbigniew Stelmasiak, Adam Stepien, Andrzej Tutaj, Jaroslaw Wronka, Jacek Zaborski; Russia: Igor Boyev, Elena Kostenko, Dmitry Pokhabov, Denis Zakharov; USA: Ziyad Ayyoub, David Bowers, Allison Brashear, Andrew Dubin, Elie P. Elovic (coordinating investigator), Stanley Fisher, Richard Harvey, Richard T. Jermyn, Michael Y. Lee, Ian B. Maitin, Christina Marciniak, Shyamal H. Mehta, Michael C. Munin, Atul T. Patel, Bruce S. Rubin, Daniel D. Truong.
Funding
The article processing charges and open access fee were supported by Merz Pharmaceuticals GmbH.
Medical Writing and/or Editorial Assistance
Editorial assistance, under the direction of the authors, was provided by Kimberley Haines, MSc, of CMC CONNECT, a division of Complete Medical Communications, Glasgow, UK, funded by Merz Pharmaceuticals GmbH, in accordance with Good Publication Practice (GPP3) guidelines.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published. All authors had full access to all of the data in this study and take complete responsibility for the integrity of the data and accuracy of the data analysis.
Disclosures
Christina C Marciniak’s institution received financial support from Merz Pharmaceuticals for the conduct of this study and the institution also receives research support from Ipsen Innovation. Michael Munin received research funding from Allergan and Ipsen, and has participated in advisory board meetings with Allergan and Merz. Allison Brashear has consulted for Ipsen and Revance and has salary support from NINDS. Research with Merz, Ipsen, Allergan, Revance, NINDS. All research funds are paid directly to Wake Forest School of Medicine. Wake Forest School of Medicine manages Dr. Brashear’s conflict of interest. Angelika Hanschmann is an employee of Merz Pharmaceuticals GmbH (Frankfurt am Main, Germany). Reinhard Hiersemenzel is an employee of Merz Pharmaceuticals GmbH (Frankfurt am Main, Germany). Elie P Elovic participates in speaker bureau programs by Allergan and Ipsen.
Compliance with Ethics Guidelines
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1964, as revised in 2013. Informed consent was obtained from all subjects included in the study.
Data Availability
The datasets obtained during and/or analyzed during the current study are available from the corresponding author on reasonable request. The data will also become available on clinicaltrials.gov (https://clinicaltrials.gov/ct2/show/NCT01392300).
Footnotes
Enhanced Digital Features
To view enhanced digital features for this article go to 10.6084/m9.figshare.7284806.
References
- 1.Simpson DM, Hallett M, Ashman EJ, et al. Practice guideline update summary: botulinum neurotoxin for the treatment of blepharospasm, cervical dystonia, adult spasticity, and headache: report of the Guideline Development Subcommittee of the American Academy of Neurology. Neurology. 2016;86(19):1818–1826. doi: 10.1212/WNL.0000000000002560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Esquenazi A, Albanese A, Chancellor MB, et al. Evidence-based review and assessment of botulinum neurotoxin for the treatment of adult spasticity in the upper motor neuron syndrome. Toxicon. 2013;67:115–128. doi: 10.1016/j.toxicon.2012.11.025. [DOI] [PubMed] [Google Scholar]
- 3.Esquenazi A, Novak I, Sheean G, Singer BJ, Ward AB. International consensus statement for the use of botulinum toxin treatment in adults and children with neurological impairments–introduction. Eur J Neurol. 2010;17(Suppl 2):1–8. doi: 10.1111/j.1468-1331.2010.03125.x. [DOI] [PubMed] [Google Scholar]
- 4.Wissel J, Ward AB, Erztgaard P, et al. European consensus table on the use of botulinum toxin type A in adult spasticity. J Rehabil Med. 2009;41(1):13–25. doi: 10.2340/16501977-0303. [DOI] [PubMed] [Google Scholar]
- 5.Frevert J. Content of botulinum neurotoxin in Botox®/Vistabel®, Dysport®/Azzalure®, and Xeomin®/Bocouture®. Drugs R D. 2010;10(2):67–73. doi: 10.2165/11584780-000000000-00000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kukreja R, Chang T-W, Cai S, et al. Immunological characterization of the subunits of type A botulinum neurotoxin and different components of its associated proteins. Toxicon. 2009;53(6):616–624. doi: 10.1016/j.toxicon.2009.01.017. [DOI] [PubMed] [Google Scholar]
- 7.Benecke R, Jost WH, Kaňovský P, Ruzicka E, Comes G, Grafe S. A new botulinum toxin type A free of complexing proteins for treatment of cervical dystonia. Neurology. 2005;64(11):1949–1951. doi: 10.1212/01.WNL.0000163767.99354.C3. [DOI] [PubMed] [Google Scholar]
- 8.Jost WH, Kohl A, Brinkmann S, Comes G. Efficacy and tolerability of a botulinum toxin type A free of complexing proteins (NT 201) compared with commercially available botulinum toxin type A (BOTOX) in healthy volunteers. J Neural Transm. 2005;112(7):905–913. doi: 10.1007/s00702-004-0234-8. [DOI] [PubMed] [Google Scholar]
- 9.Roggenkämper P, Jost WH, Bihari K, Comes G, Grafe S, Team NBS. Efficacy and safety of a new botulinum toxin type A free of complexing proteins in the treatment of blepharospasm. J Neural Transm. 2006;113(3):303–312. doi: 10.1007/s00702-005-0323-3. [DOI] [PubMed] [Google Scholar]
- 10.Wohlfarth K, Müller C, Sassin I, Comes G, Grafe S. Neurophysiological double-blind trial of a botulinum neurotoxin type A free of complexing proteins. Clin Neuropharmacol. 2007;30(2):86–94. doi: 10.1097/01.WNF.0000240951.18821.50. [DOI] [PubMed] [Google Scholar]
- 11.Kane MA, Gold MH, Coleman WP, 3rd, et al. A randomized, double-blind trial to investigate the equivalence of incobotulinumtoxinA and onabotulinumtoxinA for glabellar frown lines. Dermatol Surg. 2015;41(11):1310–1319. doi: 10.1097/DSS.0000000000000531. [DOI] [PubMed] [Google Scholar]
- 12.Sattler G, Callander MJ, Grablowitz D, et al. Noninferiority of incobotulinumtoxinA, free from complexing proteins, compared with another botulinum toxin type A in the treatment of glabellar frown lines. Dermatol Surg. 2010;36(Suppl 4):2146–2154. doi: 10.1111/j.1524-4725.2010.01706.x. [DOI] [PubMed] [Google Scholar]
- 13.Prager W, Huber-Vorlander J, Taufig AZ, et al. Botulinum toxin type A treatment to the upper face: retrospective analysis of daily practice. Clin Cosmet Investig Dermatol. 2012;5:53–58. doi: 10.2147/CCID.S26550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kaňovský P, Slawek J, Denes Z, et al. Efficacy and safety of botulinum neurotoxin NT 201 in poststroke upper limb spasticity. Clin Neuropharmacol. 2009;32(5):259–265. doi: 10.1097/WNF.0b013e3181b13308. [DOI] [PubMed] [Google Scholar]
- 15.Elovic EP, Munin MC, Kaňovský P, Hanschmann A, Hiersemenzel R, Marciniak C. Randomized, placebo-controlled trial of incobotulinumtoxinA for upper-limb post-stroke spasticity. Muscle Nerve. 2016;53(3):415–421. doi: 10.1002/mus.24776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kaňovský P, Slawek J, Denes Z, et al. Efficacy and safety of treatment with incobotulinum toxin A (botulinum neurotoxin type A free from complexing proteins; NT 201) in post-stroke upper limb spasticity. J Rehabil Med. 2011;43(6):486–492. doi: 10.2340/16501977-0796. [DOI] [PubMed] [Google Scholar]
- 17.Ashworth B. Preliminary trial of carisoprodol in multiple sclerosis. Practitioner. 1964;192:540–542. [PubMed] [Google Scholar]
- 18.Lee K-C, Carson L, Kinnin E, Patterson V. The Ashworth Scale: a reliable and reproducible method of measuring spasticity. Neurorehabil Neural Repair. 1989;3(4):205–259. doi: 10.1177/136140968900300406. [DOI] [Google Scholar]
- 19.Bohannon RW, Smith MB. Interrater reliability of a modified Ashworth scale of muscle spasticity. Phys Ther. 1987;67(2):206–207. doi: 10.1093/ptj/67.2.206. [DOI] [PubMed] [Google Scholar]
- 20.Göschel H, Wohlfarth K, Frevert J, Dengler R, Bigalke H. Botulinum A toxin therapy: neutralizing and nonneutralizing antibodies—therapeutic consequences. Exp Neurol. 1997;147(1):96–102. doi: 10.1006/exnr.1997.6580. [DOI] [PubMed] [Google Scholar]
- 21.Sesardic D, Jones RG, Leung T, Alsop T, Tierney R. Detection of antibodies against botulinum toxins. Mov Disord. 2004;19(Suppl 8):S85–91. doi: 10.1002/mds.20021. [DOI] [PubMed] [Google Scholar]
- 22.Dressler D, Rychlik R, Kreimendahl F, Schnur N, Lambert-Baumann J. Long-term efficacy and safety of incobotulinumtoxinA and conventional treatment of poststroke arm spasticity: a prospective, non-interventional, open-label, parallel-group study. BMJ Open. 2015;5(12):e009358. doi: 10.1136/bmjopen-2015-009358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bakheit AM, Thilmann AF, Ward AB, et al. A randomized, double-blind, placebo-controlled, dose-ranging study to compare the efficacy and safety of three doses of botulinum toxin type A (Dysport) with placebo in upper limb spasticity after stroke. Stroke. 2000;31(10):2402–2406. doi: 10.1161/01.STR.31.10.2402. [DOI] [PubMed] [Google Scholar]
- 24.Simpson DM, Gracies JM, Yablon SA, Barbano R, Brashear A, BoNT/TZD Study Team Botulinum neurotoxin versus tizanidine in upper limb spasticity: a placebo-controlled study. J Neurol Neurosurg Psychiatry. 2009;80(4):380–385. doi: 10.1136/jnnp.2008.159657. [DOI] [PubMed] [Google Scholar]
- 25.Brashear A, Gordon MF, Elovic E, et al. Intramuscular injection of botulinum toxin for the treatment of wrist and finger spasticity after a stroke. N Engl J Med. 2002;347(6):395–400. doi: 10.1056/NEJMoa011892. [DOI] [PubMed] [Google Scholar]
- 26.Santamato A, Panza F, Intiso D, et al. Long-term safety of repeated high doses of incobotulinumtoxinA injections for the treatment of upper and lower limb spasticity after stroke. J Neurol Sci. 2017;378:182–186. doi: 10.1016/j.jns.2017.04.052. [DOI] [PubMed] [Google Scholar]
- 27.Bensmail D, Hanschmann A, Wissel J. Satisfaction with botulinum toxin treatment in post-stroke spasticity: results from two cross-sectional surveys (patients and physicians) J Med Econ. 2014;17(9):618–625. doi: 10.3111/13696998.2014.925462. [DOI] [PubMed] [Google Scholar]
- 28.Wissel J, Bensmail D, Ferreira JJ, et al. Safety and efficacy of incobotulinumtoxinA doses up to 800 U in limb spasticity: the TOWER study. Neurology. 2017;88(14):1321–1328. doi: 10.1212/WNL.0000000000003789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Evidente VGH, Fernandez HH, LeDoux MS, et al. A randomized, double-blind study of repeated incobotulinumtoxinA (Xeomin®) in cervical dystonia. J Neural Transm. 2013;120(12):1699–1707. doi: 10.1007/s00702-013-1048-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Truong DD, Gollomp SM, Jankovic J, et al. Sustained efficacy and safety of repeated incobotulinumtoxinA (Xeomin®) injections in blepharospasm. J Neural Transm (Vienna) 2013;120(9):1345–1353. doi: 10.1007/s00702-013-0998-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Trompetto C, Marinelli L, Mori L, et al. Do flexible inter-injection intervals improve the effects of botulinum toxin A treatment in reducing impairment and disability in patients with spasticity? Med Hypotheses. 2017;102:28–32. doi: 10.1016/j.mehy.2017.03.011. [DOI] [PubMed] [Google Scholar]
- 32.Picelli A, Lobba D, Midiri A, et al. Botulinum toxin injection into the forearm muscles for wrist and fingers spastic overactivity in adults with chronic stroke: a randomized controlled trial comparing three injection techniques. Clin Rehabil. 2014;28(3):232–242. doi: 10.1177/0269215513497735. [DOI] [PubMed] [Google Scholar]
- 33.Santamato A, Micello MF, Panza F, et al. Can botulinum toxin type A injection technique influence the clinical outcome of patients with post-stroke upper limb spasticity? A randomized controlled trial comparing manual needle placement and ultrasound-guided injection techniques. J Neurol Sci. 2014;347:39–43. doi: 10.1016/j.jns.2014.09.016. [DOI] [PubMed] [Google Scholar]
- 34.Grigoriu AI, Dinomais M, Remy-Neris O, Brochard S. Impact of injection-guiding techniques on the effectiveness of botulinum toxin for the treatment of focal spasticity and dystonia: a systematic review. Arch Phys Med Rehabil. 2015;96(11):pp. 2067–78 e1. doi: 10.1016/j.apmr.2015.05.002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets obtained during and/or analyzed during the current study are available from the corresponding author on reasonable request. The data will also become available on clinicaltrials.gov (https://clinicaltrials.gov/ct2/show/NCT01392300).