Abstract
Untreated organic contaminants in municipal wastewater, such as endocrine-disrupting chemicals (EDCs), have become a significant issue in aquatic ecosystems, particularly in freshwater bodies that receive wastewater discharge. This has raised concerns about the accumulation of EDCs in aquatic species via continuous exposure. This study evaluated the uptake of EDCs by quagga mussels (Dreissena bugensis), an invasive species in a water supply reservoir. The field sampling results showed that steroid hormones were not detected in the water samples, and only pharmaceuticals and personal care products were present (0.49 to 36 ng/L). Additionally, testosterone was the most abundant steroid in the mussel tissue (6.3 to 20 ng/g dry weight), and other synthetic chemicals (i.e., bisphenol A, triclosan, and salicylic acid) were also detected in the mussel tissue (24 to 47 ng/g dry weight). After being exposed to exogenous EDCs for 7, 21, and 42 days under controlled laboratory conditions, testosterone was not detected in the mussel anymore, but bisphenol A, triclosan, and salicylic acid were found at relatively high levels in the mussel tissue, although the concentrations did not increase over time. Overall, the study demonstrated the uptake of EDCs in quagga mussels, which suggests that this species can be used to reflect water quality deterioration in aquatic ecosystems.
Keywords: Endocrine-disrupting chemicals, Steroidal hormones, Pharmaceuticals, Invasive species, Bioaccumulation
Introduction
Municipal wastewater treatment plants (WWTPs) often discharge organic contaminants at trace levels in the effluents, such as naturally occurring and synthetic hormones as well as pharmaceuticals and personal care products (PPCPs). These unregulated contaminants have become a significant concern in the environment because of their potential effects on the health of wildlife and humans at trace levels. These trace organic chemicals are frequently found at levels of parts per trillion to parts per billion in wastewater effluents (Jones et al. 2005; Lubliner et al. 2010; Miao et al. 2004; Miao et al. 2002; Soulet et al. 2002). Previous studies have also reported the presence of these contaminants at significant levels in surface waters worldwide (Ellis 2006; Kolpin et al. 2002; Lin and Reinhard 2005; Stan and Heberer 1997; Ternes et al. 2002). Therefore, the long-term ecological and human health effects, as well as the exposure routes, of untreated contaminants in aquatic ecosystems need to be evaluated.
Many emerging contaminants are documented to cause adverse health effects, such as endocrine disruption, in wildlife and humans (Brian et al. 2005; Brian et al. 2007). Fish and other organisms downstream from WWTPs are chronically exposed to endocrine-disrupting chemicals (EDCs) that may cause inappropriate sexual differentiation or development (Guillette et al. 1995; McLachlan 2001; Vajda et al. 2008). In addition to fish species, studies also reported the presence and uptake of EDCs in other aquatic species such as mollusks (Fernandes et al. 2011; Giusti and Joaquim-Justo 2013; Janer and Porte 2007; Scott 2012), mussels (Fernandes et al. 2010; Hallmann et al. 2016; Peck et al. 2007; Schwarz et al. 2017), algae (Bai and Acharya 2016; Bai and Acharya 2017; Maes et al. 2014; Zhang et al. 2014), and duckweed (Shi et al. 2010). Therefore, research activities attempt to identify the contaminants that can biomagnify in food chains and accumulate at harmful concentrations in higher trophic level organisms, including human beings. However, little information is available about the uptake of EDCs in bivalves, especially for synthetic chemicals that behave as xenoestrogens. The role that bivalves play in the accumulation and/or food web transfer of EDCs in aquatic ecosystems is still unclear.
Mussels have been used to indicate bioavailable concentrations of heavy metals and organic pollutants in aquatic environments. Mussels are efficient filter feeders that consume plankton and organic detritus, and they accumulate contaminants directly from the water column and from particulate matter (Richman and Somers 2005). The quagga mussel (Dreissena bugensis) is an invasive species that has spread through freshwaters across the United States, especially in the southwestern States. In southern Nevada, Lake Mead exhibits year-round warm temperatures, high calcium levels, and a lack of natural predators, all of which are strongly favorable conditions for the growth of quagga mussels. Additionally, Lake Mead represents a water supply reservoir that is affected by anthropogenic activities and used for drinking water, as well as recreation activities and a habitat for diverse wildlife species. Lake Mead is highly influenced by wastewater discharge from multiple WWTPs in the Las Vegas metropolitan area, where numerous untreated organic contaminants have been detected previously (Bai and Acharya 2017; Boyd and Furlong 2002; Rosen et al. 2010; Snyder and Benotti 2010). Studies conducted over the past two decades have shown continuous endocrine disruption in common carp (Cyprinus carpio) and largemouth bass (Micropterus salmoides) in Lake Mead (Bevans et al. 1996; Goodbred et al. 2015; Patino et al. 2003, 2015). Additionally, the Las Vegas Wash, constructed and naturally created wetlands that receive wastewater discharges and urban runoff, was found to have a significantly high estrogenicity response (Jones-Lepp et al. 2012). Therefore, quagga mussel may be exposed to EDCs in this aquatic ecosystem as well.
The goal of this study was to understand the uptake of EDCs by quagga mussels in an aquatic ecosystem using both field monitoring and laboratory experiments. The objectives of this research were to (1) measure ambient concentrations of a suite of EDCs in water samples and quagga mussels collected from different locations in Lake Mead, NV and (2) determine the bioaccumulation of EDCs by quagga mussels under controlled laboratory conditions via direct exposure to EDCs in the water or via food chain (i.e., algae feeding). This research provides much-needed insights into the occurrence, exposure routes, and environmental risks of EDC accumulation in invertebrates.
Materials and methods
Mussel and water sampling
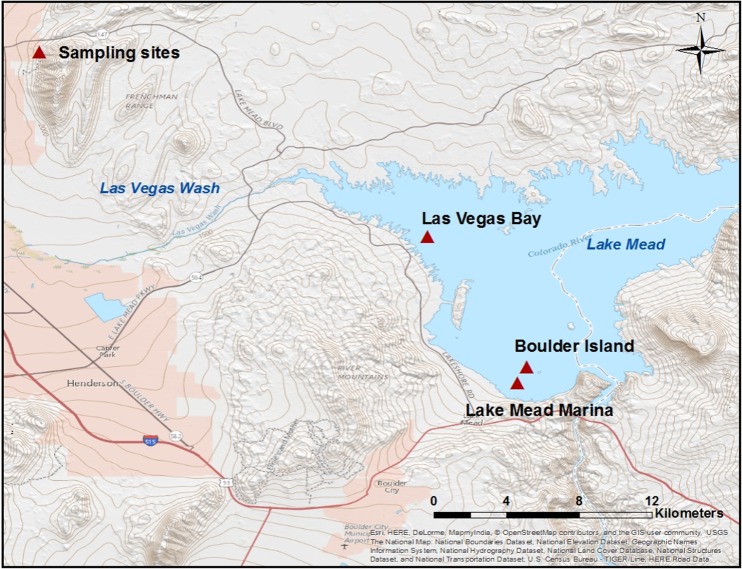
Quagga mussels were collected from three locations in the Lake Mead area: Lake Mead Marina, Las Vegas Bay, and Boulder Island (Table 1 and Fig. 1). Mussels were collected at a 1-m depth from Lake Mead Marina and a 12-m depth from Las Vegas Bay and Boulder Island based on their availability at the different locations during the sampling season. The collected mussels were rinsed with lake water to remove debris, placed in ventilated containers filled with lake water, and then transported to the laboratory immediately. In the laboratory, adult mussels from each sampling site were rinsed with deionized (DI) water several times and deshelled immediately. Mussels larger than 12 mm were considered adults (Thaw 2013), and only adult mussels were selected for the following studies. Eventually, approximately 10 g (wet weight) of mussel tissue from each sampling site was obtained and kept frozen in a wide-mouth glass jar at − 20 °C. The mussel tissue was then shipped overnight on ice to the EPA certified Weck Laboratories Inc. (City of Industry, CA) for trace organic chemical analysis. An additional batch of mussels was collected from Lake Mead Marina only and kept in an aquarium tank filled with lake water to acclimate to the laboratory conditions at room temperature for a week. The aquarium was aerated and exposed to 12 h of light and 12 h of darkness to maintain normal algal growth. These mussels were used for the following bench-scale exposure experiments.
Table 1.
Site description for water and quagga mussel sampling in Lake Mead
| Site | Latitude (N) | Longitude (W) | Sample Date | Depth (m) |
|---|---|---|---|---|
| Lake Mead Marina | 36.029306 | − 114.772056 | January 9, 2017 | 1 |
| Boulder Island | 36.037153 | − 114.767529 | February 14, 2017 | 12 |
| Las Vegas Bay | 36.101688 | − 114.816596 | February 14, 2017 | 12 |
Fig. 1.
Map of sampling sites in Lake Mead, Nevada
Water was collected in duplicate simultaneously from the same sampling sites using 1-L amber glass bottles preserved with sodium azide (1 g/L) and ascorbic acid (50 mg/L). Water samples were kept on ice and transported to the laboratory immediately after collection. Water samples were kept at 4 °C in the laboratory until further chemical analysis performed. Additionally, bulk lake water was collected from Lake Mead Marina in 10 gal carboys and filtered using a 35-μm mesh filter to remove plankton, sediments, and large pieces of algae (Thaw 2013). The filtered water was stored in aerated buckets in the dark at room temperature for further experiments.
Bench-scale exposure experiments
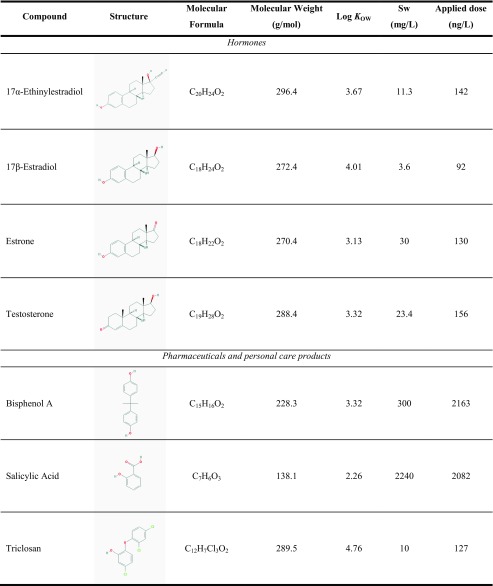
According to the field observations, the EDCs that were detected in the mussel tissue and all steroidal hormones were studied using a series of laboratory-based exposure experiments. Steroidal hormones are the most potent EDCs that accumulate in the mussel tissue and they can also cause adverse effects to other species within the food web. Although steroidal hormones were not frequently detected in the surface water or biomass, they are known as the most potent EDCs so far. For the exposure study, 30 adult mussels (size between 12 and 20 mm) were transferred from the aquarium tank to a 2-L beaker filled with the filtered, aerated lake water. Each beaker with 30 mussels was aerated and lightly covered with foil throughout the duration of the experiment (i.e., 7, 21, and 42 days) and kept at room temperature (~ 23 °C). During the experimental period, two studies were used to evaluate two potential exposure pathways: direct water exposure and food chain exposure (i.e., via algae feeding). For the direct water exposure treatment, the lake water was spiked with 50-μL EDC stock solution to reach the desired initial concentrations (Table 2) in the beginning of the experiment. To keep the water fresh and clean in each beaker, 1 L of water with mussel feces was disposed twice a week, and 1 L of fresh lake water spiked with the EDCs at the initial doses was added to refill the beaker. A freshwater green alga species, Nannochloris sp., was used as a food source to keep the mussels alive and healthy throughout the experiments. Algal cultivation followed previous methods (Bai and Acharya 2016, 2017), and approximately 0.01 g (dry weight) of the alga was applied to feed the mussels on a daily basis.
Table 2.
Properties of target EDCs used for laboratory-based experiments
For the food chain exposure treatment, every day before feeding, 5 μL of the stock EDC solution was added to the algae, which allowed binding of EDCs to the algal biomass, and the algae were then used to feed the mussels. Our previous findings showed that biosorption to algal cells was a major pathway for the uptake of hydrophobic compounds (Bai and Acharya 2016, 2017). The water changing process for this treatment followed the same process as the direct water exposure treatment, but no EDCs were spiked to the water to ensure that the EDCs applied were associated with the algal cells only. Five beakers were used for each treatment and for each experimental duration. For each treatment, at the end of the experiment (i.e., 7, 21, and 42 days), all 30 mussels from each beaker were collected and deshelled, and the tissue sampled from all five beakers was mixed together as one composite sample to obtain enough mass and to reduce the sample size. Each tissue sample (~ 10 g wet weight) was kept frozen at − 20 °C in a wide-mouth glass jar until further analysis could be conducted. A control using filtered lake water without EDC spiking was used to identify the effects of the laboratory conditions and the EDCs on the mortality of the mussels. The results showed no mortality throughout the study period even with EDC spiking.
Chemical analysis
Standard chemicals of 17α-ethinylestradiol, 17β-estradiol, estrone, testosterone, bisphenol A, salicylic acid, and triclosan were purchased from Sigma-Aldrich (St. Louis, MO) with purity > 98%. The initial concentrations of the target EDCs were determined based on their reported levels in wastewater effluents and surface waters. The selected doses are approximately five times greater than the reported levels in wastewater effluents to ensure detection as well as avoid toxic effects on the mussels (Lubliner et al. 2010). The concentrations of the stock solution (in methanol) ranged from 3.7 to 86.5 mg/L. The physicochemical properties and initial doses of the compounds used in the study are summarized in Table 2. All analyses of the target compounds in the mussel and water samples were performed by the Weck Laboratories Inc. as described elsewhere (Bai and Acharya 2017). Briefly, the analytical method for water samples followed the EPA standard method for pharmaceuticals and personal care products (i.e., EPA 1694 M ESI-) and hormones (i.e., EPA 1694 M-APCI) using liquid chromatography tandem mass spectrometry (LC-MS/MS) (US EPA 2007), and an isotope spike was applied before sample pretreatment and extraction that was subject to the same analytical procedure. For the mussel tissue, the analytes were extracted using a quick, easy, cheap, effective, rugged, and safe (QuEChERS) method, which has been successfully used to determine pharmaceuticals in vegetables (Chuang et al. 2015) and fish (Lopes et al. 2012). Briefly, the mussel tissue was mixed with Na2EDTA solution and then added with acetonitrile and methanol. After adding anhydrous Na2SO4 and NaCl, the mixture was centrifuged at 2990 rpm for 10 min. The supernatant was collected, to which the d-solid-phase extraction sorbents (consisting of MgSO4, primary–secondary amine, C18, and graphitized carbon black) were added and centrifuged at 9240 rpm for 10 min. The mussel extracts were then analyzed for the target compounds using identical LC-MS/MS procedures to those used for the water samples.
Results and discussion
EDCs in water samples
The EDC concentrations in the water samples collected at the three study locations can be found in Table 3. Las Vegas Bay is the receiving waterway of municipal wastewater effluents. Boulder Island and Lake Mead Marina are further downstream of the Las Vegas Bay and expected to be less affected by wastewater discharge. However, these two locations are two of the most popular recreation sites at the lake, and recreational activities are considered the primary route for human exposure. Additionally, the nearby fisheries may also be influenced by these contaminants. Monitoring the contaminants using quagga mussels at these locations indicates the potential risks to other aquatic organisms, and even humans. The screening results showed that steroidal hormones were not found in any water samples. All of the PPCPs analyzed were detected in at least one of the sampling locations, except for iopromide. Bisphenol A, salicylic acid, and triclosan were found to be more abundant in the water samples compared with other analytes. The authors previously monitored over 200 wastewater organic contaminants in the Upper Colorado River watershed in Denver, Colorado, and reported that diclofenac, gemfibrozil, bisphenol A, and triclosan had median concentrations of 26.1, 32.3, 139, and 92.4 ng/L, respectively (Bai et al. 2018). The frequency of detection for diclofenac, gemfibrozil, bisphenol A, and triclosan was 40.1%, 42.5%, 50.9%, and 11.4%, respectively (Bai et al. 2018). Steroidal hormones were also found at low frequencies but relatively high concentrations at selected sampling sites in the Denver watershed: 17β-estradiol at 393 ng/L (11.4%), estrone at 112 ng/L (1.2%), and 17α-ethinylestradiol at 228 ng/L (9.6%). Additionally, triclosan has been detected at 2.6 and 8.0 ng/L in Lake Mead Marina and upstream of Las Vegas Bay, respectively (Bai and Acharya 2017). Similar monitoring studies have been done in Taihu Lake, China, where ibuprofen and diclofenac were detected at levels up to 65.3 ng/L (Xie et al. 2015).
Table 3.
Concentrations of EDCs measured in water (ng/L) and mussel (ng/g dry weight) samples in Lake Mead
| Analyte | Lake Mead Marinaa | Boulder Islandb | Las Vegas Bayb | MDL |
|---|---|---|---|---|
| Water (ng/L) | ||||
| Hormones | ||||
| 17α-Ethinylestradiol | ND | ND | ND | 0.56 |
| 17β-Estradiol | ND | ND | ND | 0.31 |
| Estrone | ND | ND | ND | 0.20 |
| Progesterone | ND | ND | ND | 0.17 |
| Testosterone | ND | ND | ND | 0.14 |
| Pharmaceuticals and personal care products | ||||
| Bisphenol A | 1.2 | 30 | 22 | 0.27 |
| Diclofenac | 0.49 | ND | 1.5 | 0.26 |
| Gemfibrozil | ND | ND | 0.91 | 0.080 |
| Ibuprofen | ND | 0.55 | 1.5 | 0.39 |
| Iopromide | ND | ND | ND | 1.8 |
| Naproxen | ND | ND | 1.1 | 0.25 |
| Salicylic Acid | 17 | 28 | 36 | 0.86 |
| Triclosan | ND | 6.1 | 2.4 | 1.2 |
| Mussel tissue (ng/g) | ||||
| Hormones | ||||
| 17α-Ethinylestradiol | ND | ND | ND | 5.0 |
| 17β-Estradiol | ND | ND | ND | 5.0 |
| Estrone | ND | ND | ND | 5.0 |
| Progesterone | ND | ND | ND | 5.0 |
| Testosterone | 6.3 | 12 | 20 | 5.0 |
| Pharmaceuticals and personal care products | ||||
| Bisphenol A | 47 | ND | ND | 5.0 |
| Diclofenac | ND | ND | ND | 5.0 |
| Gemfibrozil | ND | ND | ND | 5.0 |
| Ibuprofen | ND | ND | ND | 5.0 |
| Iopromide | ND | ND | ND | 25 |
| Naproxen | ND | ND | ND | 5.0 |
| Salicylic Acid | 430 | ND | ND | 250 |
| Triclosan | 24 | 28 | ND | 10 |
aSample collected at 1 m
bSample collected at 12 m
MDL, method detection limit; ND, not detected
When comparing Lake Mead Marina and Boulder Island—which are the two adjacent sampling sites—the measured PPCPs appear to have higher concentrations in Boulder Island at 12-m depth (Table 3). This indicates that the contaminants are more persistent in the deeper water column, which may be because of low light exposure, low oxygen, and low microbial activities that inhibit the degradation of the contaminants. The persistence of EDCs in the deeper horizon may also cause a potential exposure risk for bottom-dwelling aquatic organisms. When comparing the Boulder Island and Las Vegas Bay sites sampled at the same depth (i.e., 12 m), more PPCPs were detected in the Las Vegas Bay water. Las Vegas Bay receives municipal wastewater effluent, surface runoff, and periodical flood water, all of which may be sources of the contaminants measured (Bai et al. 2018; Boyd and Furlong 2002; Rosen et al. 2010). Conversely, the contaminants can undergo natural attenuation and dilution as water flows into Lake Mead, resulting in lower detections (Bai and Acharya 2017). In a previous study performed at the same locations by the U.S. Geological Survey (Rosen et al. 2010), the highest concentrations of hydrophobic organic contaminants were found at a depth of 8 m in Las Vegas Bay. Rosen et al. (2010) also suggested that contaminants were generally confined to within 6 m of the lake bottom during the winter and spring, when Las Vegas Wash water sank to the bottom because of temperature and density contrasts between the Las Vegas Wash and Lake Mead water. The results of this study further demonstrate that many EDCs are present in the southern Nevada watershed. Therefore, the ecological impacts of these contaminants need to be fully evaluated despite the trace levels detected, and the vertical gradient of the contaminants needs to be monitored at different depths in the lake to assess the potential risks.
Uptake of EDCs by quagga mussels
Steroids
For the quagga mussels collected from the field sites, testosterone was the only steroidal hormone that was detected (Table 3). Testosterone was not found in the water samples, but it was detected in the mussel tissue from all the three sampling sites at various levels listed in order from high to low: Las Vegas Bay > Boulder Island > Lake Mead Marina (Table 3). Many studies have reported the occurrence of steroidal hormones in mollusks—mainly testosterone and 17β-estradiol—but the origins are still unknown. Fernandes et al. (2010) found that the naturally occurring testosterone along the mussel reproductive cycle ranges from 0.1 to 1.4 ng/g, which is much lower than the concentrations measured in this study (i.e., 6.3 to 20 ng/g in Table 3). Scott (2012) reviewed most of the existing studies on vertebrate sex steroids found in mollusks so far, and he developed three hypotheses: (a) vertebrate steroids are found in mollusks because of the limitations of analytical procedures; (b) mollusks biosynthesize steroids themselves; and (c) mollusks accumulate steroids from the environment. Steroidal hormones in aquatic environments can be from wastewater effluents and animal feeding operations. Moreover, species such as fish are demonstrated to release steroids in urine and feces. Therefore, mussels are continuously exposed to exogenous steroids throughout their life cycle, and it is challenging to identify the origins of the steroids found in the mussel tissue. Furthermore, humans and animals usually release estrogens in biologically inactive conjugated forms (i.e., sulfate and glucuronide), which are also commonly found in aquatic environments (Bai et al. 2015; Bai et al. 2013). Interestingly, mollusks are known to be rich in the enzymes that hydrolyze the steroid conjugates, and therefore they are capable of taking up steroid conjugates from the environment and converting them to free steroids. All of these may be explanations for the occurrence of steroidal hormones in invertebrates. Although free steroids were not detected in the water samples in this study, it is speculated that free steroids and their conjugates might accumulate and transfer within the food web to cause adverse effects.
Testosterone was no longer detected in the mussels after they were exposed to EDCs under laboratory conditions for several weeks (Table 4). This is likely because the mussels may have released testosterone back to the water or esterified with fatty acids to stabilize the exogenous steroid. Similarly, Fernandes et al. (2010) stated that exposure to exogenous testosterone in the laboratory did not increase testosterone levels in the mussels and that the mussels likely excreted testosterone into the water. Mussels tend to esterify steroids with fatty acids and transform them into a more stable form that is resistant to metabolism (i.e., esterified steroids) (Scott 2012). Fatty acid esterification is a key mechanism that allows mussels to maintain their normal steroid levels following environmental exposure (Scott 2012). This pathway might have occurred in this study, but esterified steroids were not measured in the mussel tissue.
Table 4.
Concentrations of selected EDCs measured in mussel tissue from laboratory-based exposure experiments (ng/g dry weight)
| Analyte | 7 days | 21 days | 42 days | |||
|---|---|---|---|---|---|---|
| Result | MDL | Result | MDL | Result | MDL | |
| Direct water exposure | ||||||
| Hormones | ||||||
| 17α-Ethinylestradiol | ND | 0.67 | ND | 1.0 | ND | 0.55 |
| 17β-Estradiol | ND | 0.67 | ND | 1.0 | ND | 0.55 |
| Estrone | ND | 0.67 | ND | 1.0 | ND | 0.55 |
| Testosterone | ND | 0.67 | ND | 1.0 | ND | 0.55 |
| Pharmaceuticals and personal care products | ||||||
| Bisphenol A | ND | 0.56 | ND | 1.0 | 6.5 | 0.55 |
| Salicylic Acid | ND | 28 | ND | 51 | ND | 28 |
| Triclosan | 4.0 | 1.1 | ND | 2 | ND | 1.1 |
| Food chain exposure | ||||||
| Hormones | ||||||
| 17α-Ethinylestradiol | ND | 0.75 | ND | 0.62 | 7.4 | 0.65 |
| 17β-Estradiol | ND | 0.75 | ND | 0.62 | ND | 0.65 |
| Estrone | ND | 0.75 | ND | 0.62 | ND | 0.65 |
| Testosterone | ND | 0.75 | ND | 0.62 | ND | 0.65 |
| Pharmaceuticals and personal care products | ||||||
| Bisphenol A | 26 | 0.89 | ND | 0.62 | 6.5 | 0.65 |
| Salicylic Acid | 830 | 44 | ND | 31 | ND | 33 |
| Triclosan | ND | 1.8 | ND | 1.2 | ND | 1.3 |
MDL, method detection limit; ND, not detected
Under laboratory conditions, the synthetic estrogen 17α-ethinylestradiol was detected at 7.4 ng/g after 42 days of exposure from the food chain (Table 4). 17α-Ethinylestradiol detection in the mussel tissue suggests that exogenous estrogens can be taken up by aquatic invertebrate species from the environment because 17α-ethinylestradiol is a synthetic estrogen that cannot form endogenously. 17α-Ethinylestradiol has been found in wild-caught mollusks at levels up to 80–130 ng/g of dry tissue weight (Liu et al. 2009; Lu et al. 2001). Naturally occurring estrogens were not detected in the mussel after 42 days of exposure, and therefore it appears that they either did not accumulate in the mussel or the estrogens were esterified and stabilized in the tissue. Of the naturally occurring estrogens, 17β-estradiol is the most frequently found in invertebrates, even though it was not detected in this study. Peck et al. (2007) reported that zebra mussels (Dreissena polymorpha)—which are freshwater bivalves—were susceptible to estrogen exposure, and when they were exposed to 5.5 ng/L of 17β-estradiol for 13 days, 17β-estradiol could accumulate in the mussel tissue at 840- and 580-fold in males and females, respectively. Additionally, testosterone and 17β-estradiol concentrations were found to increase dramatically over time in mollusks that were caged downstream of sewage treatment plants (Gust et al. 2010a; Gust et al. 2010b).
PPCPs
Bisphenol A, salicylic acid, and triclosan were found at relatively high levels in the mussel tissue based on the field observations (Table 3). Bisphenol A, salicylic acid, and triclosan can act as xenoestrogens because they have similar chemical structures to estrogens, and they may interfere with the endocrine systems of aquatic organisms. All of the contaminants detected are hydrophobic with log Kow values ranging from 2.26 to 4.76 (Table 2), so they tend to be associated with the biomass in the aquatic ecosystem. According to the field monitoring study in Taihu Lake, China, roxithromycin, propranolol, diclofenac, and 17β-estradiol were found in mussels (Anodonta) with bioaccumulation factors of 406, 234, 70, and 59 L/Kg, respectively (Xie et al. 2015). All these findings demonstrated the bioaccumulation potential of selected wastewater contaminants in mussels in aquatic environments.
After being exposed to EDCs for 7, 21, and 42 days in the laboratory, the mussels were able to accumulate selected EDCs at different levels. For both the direct water exposure and food chain exposure studies, bisphenol A and triclosan were found in the mussel tissue at levels lower than the field observations, and salicylic acid from the food chain exposure study was measured at a higher concentration compared with the field observation (Tables 3 and 4). Moreover, bisphenol A and salicylic acid were measured at higher levels in the mussel tissue from the food chain exposure study compared with the direct water exposure study (Table 4), indicating that mussels may take up these compounds from the food source rather than directly from the water. Some mollusks are reported to be particularly susceptible to exposure to xenoestrogens such as bisphenol A, which can result in superfeminization of prosobranch snails (Jobling et al. 2003; Oehlmann et al. 2000). Triclosan was only detected after 7 days of exposure in the direct water exposure study (Table 4). This may be because triclosan is highly susceptible to light and it tends to photodegrade, and algae can increase its photodegradation rates (Bai and Acharya 2016, 2017). This results in the rapid dissipation of this compound in the aquatic environment and lower accumulation in the mussels. However, other studies have found triclosan in male common carp (C. carpio) from Las Vegas Bay to decrease sperm counts and induce vitellogenin (Jenkins et al. 2018; Leiker et al. 2009). Nonetheless, xenoestrogens may play a role in the reproductive physiology of aquatic organisms, but the adverse effects to aquatic invertebrates following exposure to xenoestrogens and exogenous steroids need to be further evaluated.
Conclusions
This study evaluated the occurrence and uptake of a suite of steroidal hormones and other EDCs in quagga mussels, which are an invasive species found in the freshwater systems of the southwestern United States. The field sampling results showed that steroidal hormones were not detected in the Lake Mead water, and testosterone was found at relatively high levels in the mussel tissue from all of the sampling locations. Many other synthetic chemicals were detected in the lake water, but only triclosan, bisphenol A, and salicylic acid were found to be abundant in the mussels. Based on the laboratory exposure experiments, testosterone seemed to have the capability to be released back to water or esterified, so free testosterone was not found in the mussels after exposure to EDCs. Bisphenol A, salicylic acid, and triclosan were found to have accumulated at different levels in the tissue after exposure to EDCs. These results demonstrate that naturally occurring steroids and synthetic chemicals can accumulate in quagga mussel, and uptake via food chain is likely to be a primary pathway. This research provides useful information for understanding the potential of the widespread invasive species to accumulate wastewater organic contaminants, and has potential to be used as a biomonitor for endocrine disruption.
Acknowledgements
The authors would like to thank Brian Moore, Ben Smith, and Theresa Thom from the Lake Mead National Recreation Area headquarters for quagga mussel and water collection.
Funding information
This study was supported by the U.S. Geological Survey Nevada Water Resources Research Institute.
References
- Bai X, Acharya K. Removal of trimethoprim, sulfamethoxazole, and triclosan by the green alga Nannochloris sp. J Hazard Mater. 2016;315:70–75. doi: 10.1016/j.jhazmat.2016.04.067. [DOI] [PubMed] [Google Scholar]
- Bai X, Acharya K. Algae-mediated removal of selected pharmaceutical and personal care products (PPCPs) from Lake Mead water. Sci Total Environ. 2017;581:734–740. doi: 10.1016/j.scitotenv.2016.12.192. [DOI] [PubMed] [Google Scholar]
- Bai X, Casey FXM, Hakk H, DeSutter TM, Oduor PG, Khan E. Dissipation and transformation of 17β-estradiol-17-sulfate in soil-water systems. J Hazard Mater. 2013;260:733–739. doi: 10.1016/j.jhazmat.2013.06.036. [DOI] [PubMed] [Google Scholar]
- Bai X, Casey FX, Hakk H, DeSutter TM, Oduor PG, Khan E. Sorption and degradation of 17beta-estradiol-17-sulfate in sterilized soil-water systems. Chemosphere. 2015;119:1322–1328. doi: 10.1016/j.chemosphere.2014.02.016. [DOI] [PubMed] [Google Scholar]
- Bai X, Lutz A, Carroll R, Keteles K, Dahlin K, Murphy M, Nguyen D. Occurrence, distribution, and seasonality of emerging contaminants in urban watersheds. Chemosphere. 2018;200:133–142. doi: 10.1016/j.chemosphere.2018.02.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bevans HE, Goodbred S, Miesner J, Watkins S, Gross T, Denslow N, Choeb T (1996) Synthetic organic compounds and carp endocrinology and histology in Las Vegas Wash and Las Vegas and Callville Bays of Lake Mead, Nevada, 1992 and 1995, US Dept. of the Interior, US Geological Survey
- Boyd RA, Furlong ET. Human-health pharmaceutical compounds in Lake Mead, Nevada and Arizona, and Las Vegas Wash, Nevada, October 2000–August 2001. Carson City: U.S. Geological Survey; 2002. [Google Scholar]
- Brian JV, Harris CA, Scholze M, Backhaus T, Booy P, Lamoree M, Pojana G, Jonkers N, Runnalls T, Bonfa A, Marcomini A, Sumpter JP. Accurate prediction of the response of freshwater fish to a mixture of estrogenic chemicals. Environ Health Perspect. 2005;113:721–728. doi: 10.1289/ehp.7598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brian JV, Harris CA, Scholze M, Kortenkamp A, Booy P, Lamoree M, Pojana G, Jonkers N, Marcomini A, Sumpter JP. Evidence of estrogenic mixture effects on the reproductive performance of fish. Environ Sci Technol. 2007;41:337–344. doi: 10.1021/es0617439. [DOI] [PubMed] [Google Scholar]
- Chuang YH, Zhang YJ, Zhang W, Boyd SA, Li H. Comparison of accelerated solvent extraction and quick, easy, cheap, effective, rugged and safe method for extraction and determination of pharmaceuticals in vegetables. J Chromatogr A. 2015;1404:1–9. doi: 10.1016/j.chroma.2015.05.022. [DOI] [PubMed] [Google Scholar]
- Ellis JB. Pharmaceutical and personal care products (PPCPs) in urban receiving waters. Environ Pollut. 2006;144:184–189. doi: 10.1016/j.envpol.2005.12.018. [DOI] [PubMed] [Google Scholar]
- U.S. EPA (2007) Method 1694: Pharmaceuticals and personal care products in water, soil, sediment, and biosolids by HPLC/MS/MS. EPA-821-R-08-002
- Fernandes D, Navarro JC, Riva C, Bordonali S, Porte C. Does exposure to testosterone significantly alter endogenous metabolism in the marine mussel Mytilus galloprovincialis? Aquat Toxicol. 2010;100:313–320. doi: 10.1016/j.aquatox.2010.08.003. [DOI] [PubMed] [Google Scholar]
- Fernandes D, Loi B, Porte C. Biosynthesis and metabolism of steroids in molluscs. J Steroid Biochem Mol Biol. 2011;127:189–195. doi: 10.1016/j.jsbmb.2010.12.009. [DOI] [PubMed] [Google Scholar]
- Giusti A, Joaquim-Justo C. Esterification of vertebrate like steroids in molluscs: a target of endocrine disruptors? Comp Biochem Physiol C Toxicol Pharmacol. 2013;158:187–198. doi: 10.1016/j.cbpc.2013.08.003. [DOI] [PubMed] [Google Scholar]
- Goodbred SL, Patino R, Torres L, Echols KR, Jenkins JA, Rosen MR, Orsak E. Are endocrine and reproductive biomarkers altered in contaminant-exposed wild male Largemouth Bass (Micropterus salmoides) of Lake Mead, Nevada/Arizona, USA? Gen Comp Endocrinol. 2015;219:125–135. doi: 10.1016/j.ygcen.2015.02.015. [DOI] [PubMed] [Google Scholar]
- Guillette LJ, Crain DA, Rooney AA, Pickford DB. Organization versus activation - the role of endocrine-disrupting contaminants (EDCs) during embryonic-development in wildlife. Environ Health Perspect. 1995;103:157–164. doi: 10.1289/ehp.95103s7157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gust M, Buronfosse T, Geffard O, Mons R, Queau H, Mouthon J, Garric J. In situ biomonitoring of freshwater quality using the New Zealand mudsnail Potamopyrgus antipodarum (gray) exposed to waste water treatment plant (WWTP) effluent discharges. Water Res. 2010;44:4517–4528. doi: 10.1016/j.watres.2010.06.019. [DOI] [PubMed] [Google Scholar]
- Gust M, Vulliet E, Giroud B, Garnier F, Couturier S, Garric J, Buronfosse T. Development, validation and comparison of LC-MS/MS and RIA methods for quantification of vertebrates-like sex-steroids in prosobranch molluscs. J Chromatogr B Anal Technol Biomed Life Sci. 2010;878:1487–1492. doi: 10.1016/j.jchromb.2010.03.046. [DOI] [PubMed] [Google Scholar]
- Hallmann A, Smolarz K, Konieczna L, Zabrzafiska S, Belka M, Baczek T. LC-MS measurement of free steroids in mussels (Mytilus trossulus) from the southern Baltic Sea. J Pharm Biomed Anal. 2016;117:311–315. doi: 10.1016/j.jpba.2015.09.013. [DOI] [PubMed] [Google Scholar]
- Janer G, Porte C. Sex steroids and potential mechanisms of non-genomic endocrine disruption in invertebrates. Ecotoxicology. 2007;16:145–160. doi: 10.1007/s10646-006-0110-4. [DOI] [PubMed] [Google Scholar]
- Jenkins JA, Rosen MR, Draugelis-Dale RO, Echols KR, Torres L, Wieser CM, Kersten CA, Goodbred SL. Sperm quality biomarkers complement reproductive and endocrine parameters in investigating environmental contaminants in common carp (Cyprinus carpio) from the Lake Mead National Recreation Area. Environ Res. 2018;163:149–164. doi: 10.1016/j.envres.2018.01.041. [DOI] [PubMed] [Google Scholar]
- Jobling S, Casey D, Rodgers-Gray T, Oehlmann J, Schulte-Oehlmann U, Pawlowski S, Baunbeck T, Turner AP, Tyler CR. Comparative responses of molluscs and fish to environmental estrogens and an estrogenic effluent. Aquat Toxicol. 2003;65:205–220. doi: 10.1016/S0166-445X(03)00134-6. [DOI] [PubMed] [Google Scholar]
- Jones OAH, Voulvoulis N, Lester JN. Human pharmaceuticals in wastewater treatment processes. Crit Rev Environ Sci Technol. 2005;35:401–427. doi: 10.1080/10643380590956966. [DOI] [Google Scholar]
- Jones-Lepp TL, Sanchez C, Alvarez DA, Wilson DC, Taniguchi-Fu RL. Point sources of emerging contaminants along the Colorado River Basin: source water for the arid Southwestern United States. Sci Total Environ. 2012;430:237–245. doi: 10.1016/j.scitotenv.2012.04.053. [DOI] [PubMed] [Google Scholar]
- Kolpin DW, Furlong ET, Meyer MT, Thurman EM, Zaugg SD, Barber LB, Buxton HT. Pharmaceuticals, hormones, and other organic wastewater contaminants in US streams, 1999–2000: a national reconnaissance. Environ Sci Technol. 2002;36:1202–1211. doi: 10.1021/es011055j. [DOI] [PubMed] [Google Scholar]
- Leiker TJ, Abney SR, Goodbred SL, Rosen MR. Identification of methyl triclosan and halogenated analogues in male common carp (Cyprinus carpio) from Las Vegas Bay and semipermeable membrane devices from Las Vegas Wash, Nevada. Sci Total Environ. 2009;407:2102–2114. doi: 10.1016/j.scitotenv.2008.11.009. [DOI] [PubMed] [Google Scholar]
- Lin AYC, Reinhard M. Photodegradation of common environmental pharmaceuticals and estrogens in river water. Environ Toxicol Chem. 2005;24:1303–1309. doi: 10.1897/04-236R.1. [DOI] [PubMed] [Google Scholar]
- Liu Y, Guan YT, Mizuno T, Tsuno H, Zhu WP. A pretreatment method for GC-MS determination of endocrine disrupting chemicals in mollusk tissues. Chromatographia. 2009;69:65–71. doi: 10.1365/s10337-008-0852-7. [DOI] [Google Scholar]
- Lopes RP, Reyes RC, Romero-Gonzalez R, Vidal JLM, Frenich AG. Multiresidue determination of veterinary drugs in aquaculture fish samples by ultra high performance liquid chromatography coupled to tandem mass spectrometry. J Chromatogr B Anal Technol Biomed Life Sci. 2012;895:39–47. doi: 10.1016/j.jchromb.2012.03.011. [DOI] [PubMed] [Google Scholar]
- Lu M, Horiguchi T, Shiraishi H, Shibata Y, Abo M, Okubo A, Yamazaki S. Identification and quantitation of steroid hormones in marine gastropods by GC/MS. Bunseki Kagaku. 2001;50:247–255. doi: 10.2116/bunsekikagaku.50.247. [DOI] [Google Scholar]
- Lubliner B, Redding M, Ragsdale D (2010) Pharmaceuticals and personal care products in municipal wastewater and their removal by nutrient treatment technologies. Washington State Department of Ecology, Olympia, WA. Publication number 10-03-004. http://www.ecy.wa.gov/biblio/1003004.html
- Maes HM, Maletz SX, Ratte HT, Hollender J, Schaeffer A. Uptake, elimination, and biotransformation of 17alpha-ethinylestradiol by the freshwater alga Desmodesmus subspicatus. Environ Sci Technol. 2014;48:12354–12361. doi: 10.1021/es503574z. [DOI] [PubMed] [Google Scholar]
- McLachlan JA. Environmental signaling: what embryos and evolution teach us about endocrine disrupting chemicals. Endocr Rev. 2001;22:319–341. doi: 10.1210/edrv.22.3.0432. [DOI] [PubMed] [Google Scholar]
- Miao XS, Koenig BG, Metcalfe CD. Analysis of acidic drugs in the effluents of sewage treatment plants using liquid chromatography-electrospray ionization tandem mass spectrometry. J Chromatogr A. 2002;952:139–147. doi: 10.1016/S0021-9673(02)00088-2. [DOI] [PubMed] [Google Scholar]
- Miao XS, Bishay F, Chen M, Metcalfe CD. Occurrence of antimicrobials in the final effluents of wastewater treatment plants in Canada. Environ Sci Technol. 2004;38:3533–3541. doi: 10.1021/es030653q. [DOI] [PubMed] [Google Scholar]
- Oehlmann J, Schulte-Oehlmann U, Tillmann M, Markert B. Effects of endocrine disruptors on prosobranch snails (Mollusca: Gastropoda) in the laboratory. Part I: bisphenol A and octylphenol as xeno-estrogens. Ecotoxicology. 2000;9:383–397. doi: 10.1023/A:1008972518019. [DOI] [PubMed] [Google Scholar]
- Patino R, Goodbred SL, Draugelis-Dale R, Barry CE, Foott JS, Wainscott MR, Gross TS, Covay KJ. Morphometric and histopathological parameters of gonadal development in adult common carp from contaminated and reference sites in Lake Mead, Nevada. J Aquat Anim Health. 2003;15:55–68. doi: 10.1577/1548-8667(2003)015<0055:MAHPOG>2.0.CO;2. [DOI] [Google Scholar]
- Patino R, VanLandeghern MM, Goodbred SL, Orsak E, Jenkins JA, Echols K, Rosen MR, Torres L. Novel associations between contaminant body burdens and biomarkers of reproductive condition in male Common Carp along multiple gradients of contaminant exposure in Lake Mead National Recreation Area, USA. Gen Comp Endocrinol. 2015;219:112–124. doi: 10.1016/j.ygcen.2014.12.013. [DOI] [PubMed] [Google Scholar]
- Peck MR, Labadie P, Minier C, Hill EM. Profiles of environmental and endogenous estrogens in the zebra mussel Dreissena polymorpha. Chemosphere. 2007;69:1–8. doi: 10.1016/j.chemosphere.2007.04.082. [DOI] [PubMed] [Google Scholar]
- Richman L, Somers K. Can we use zebra and quagga mussels for biomonitoring contaminants in the Niagara River? Water Air Soil Pollut. 2005;167:155–178. doi: 10.1007/s11270-005-0083-6. [DOI] [Google Scholar]
- Rosen MR, Alvarez DA, Goodbred SL, Leiker TJ, Patiño R. Sources and distribution of organic compounds using passive samplers in Lake Mead National Recreation Area, Nevada and Arizona, and their implications for potential effects on aquatic biota. J Environ Qual. 2010;39:1161. doi: 10.2134/jeq2009.0095. [DOI] [PubMed] [Google Scholar]
- Schwarz TI, Katsiadaki I, Maskrey BH, Scott AP. Mussels (Mytilus spp.) display an ability for rapid and high capacity uptake of the vertebrate steroid, estradiol-17 beta from water. J Steroid Biochem Mol Biol. 2017;165:407–420. doi: 10.1016/j.jsbmb.2016.08.007. [DOI] [PubMed] [Google Scholar]
- Scott AP. Do mollusks use vertebrate sex steroids as reproductive hormones? Part I: critical appraisal of the evidence for the presence, biosynthesis and uptake of steroids. Steroids. 2012;77:1450–1468. doi: 10.1016/j.steroids.2012.08.009. [DOI] [PubMed] [Google Scholar]
- Shi W, Wang L, Rousseau DP, Lens PN. Removal of estrone, 17alpha-ethinylestradiol, and 17beta-estradiol in algae and duckweed-based wastewater treatment systems. Environ Sci Pollut Res Int. 2010;17:824–833. doi: 10.1007/s11356-010-0301-7. [DOI] [PubMed] [Google Scholar]
- Snyder SA, Benotti MJ. Endocrine disruptors and pharmaceuticals: implications for water sustainability. Water Sci Technol. 2010;61:145–154. doi: 10.2166/wst.2010.791. [DOI] [PubMed] [Google Scholar]
- Soulet B, Tauxe A, Tarradellas J. Analysis of acidic drugs in Swiss wastewaters. Int J Environ Anal Chem. 2002;82:659–667. doi: 10.1080/0306731021000075384. [DOI] [Google Scholar]
- Stan HJ, Heberer T. Pharmaceuticals in the aquatic environment. Analusis. 1997;25:M20–M23. [Google Scholar]
- Ternes TA, Meisenheimer M, McDowell D, Sacher F, Brauch HJ, Gulde BH, Preuss G, Wilme U, Seibert NZ. Removal of pharmaceuticals during drinking water treatment. Environ Sci Technol. 2002;36:3855–3863. doi: 10.1021/es015757k. [DOI] [PubMed] [Google Scholar]
- Thaw MN. Understanding basin specific life history characteristics of Lake Mead quagga mussels (Dreissena bugensis) and a potential treatment using UV radiation in laboratory studies. Las Vegas: University of Nevada; 2013. [Google Scholar]
- Vajda AM, Barber LB, Gray JL, Lopez EM, Woodling JD, Norris DO. Reproductive disruption in fish downstream from an estrogenic wastewater effluent. Environ Sci Technol. 2008;42:3407–3414. doi: 10.1021/es0720661. [DOI] [PubMed] [Google Scholar]
- Xie ZX, Lu GH, Liu JC, Yan ZH, Ma BN, Zhang ZH, Chen W. Occurrence, bioaccumulation, and trophic magnification of pharmaceutically active compounds in Taihu Lake, China. Chemosphere. 2015;138:140–147. doi: 10.1016/j.chemosphere.2015.05.086. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Habteselassie MY, Resurreccion EP, Mantripragada V, Peng S, Bauer S, Colosi LM. Evaluating removal of steroid estrogens by a model alga as a possible sustainability benefit of hypothetical integrated algae cultivation and wastewater treatment systems. ACS Sustain Chem Eng. 2014;2:2544–2553. doi: 10.1021/sc5004538. [DOI] [Google Scholar]