Our previous studies have documented the unique enrichment of activated CD4 T cells in the liver. Blocking de novo T cell activation did not interfere with their function in ischemia reperfusion injury (IRI).1 We also demonstrated that the CD154-CD40 pathway, but not IFN-γ, was critical for IRI in the liver. Interestingly, alloimmune-primed hosts had significantly more severe IRI in their own livers, which was mediated mainly by CD4 T cells.2 These data prompted us to propose that pre-existing activated CD4 T cells in a host participate in the liver innate immune response against IRI. However, whether these activated/memory T cells require Ag stimulation to function in liver IRI remains unclear. In this report, we addressed this question by utilizing OT II (RAG −/−) T cell receptor transgenic CD4 T cells in a murine liver IRI model.
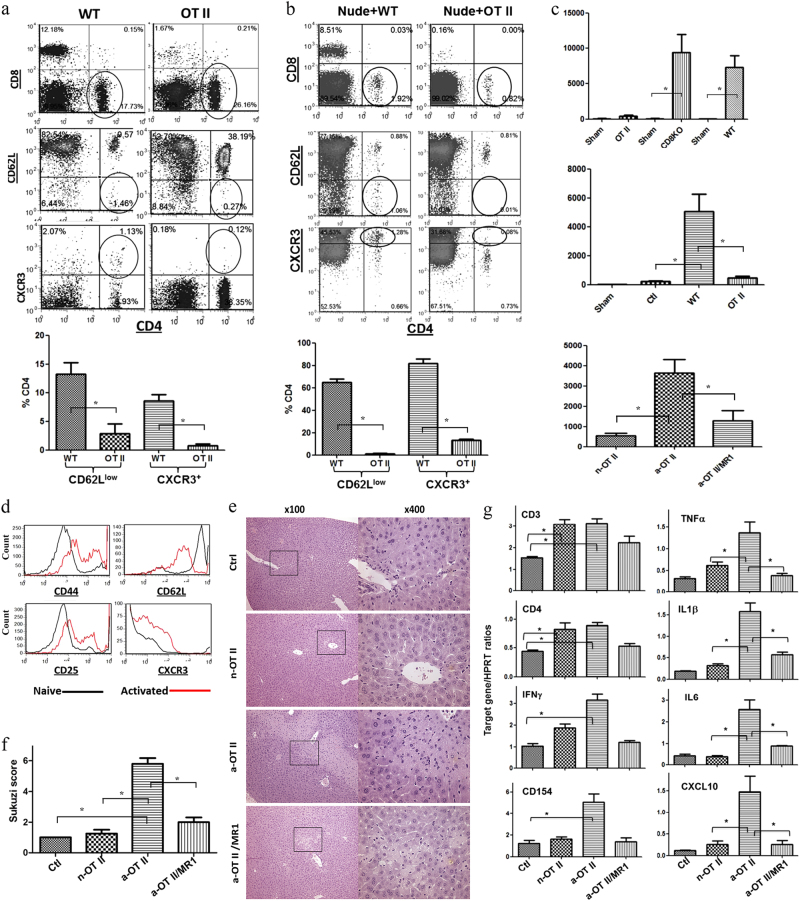
Rag (−/−) OT II TCR transgenic mice have a homogenous CD4 T cell population recognizing a chicken ovalbumin peptide, without apparent cross-reactivity with murine Ags. Based on FACS analysis of peripheral blood leukocytes, more than 95% of these CD4 T cells showed the naïve CD62Llow or CXCR3−phenotype, whereas ~10% of non-Tg CD4 T cells in naïve B6 mice showed the activated or memory phenotype (Fig. 1a). Importantly, these OT II cells maintained their naïve phenotype upon adoptive transfer into nude mice in marked contrast to WT CD4 T cells. Despite a slight increase in CXCR3+OTII (13.1 ± 1.2%), the frequency of the CD62Llow subset, representing activated/memory cells, remained low (1.3 ± 0.3%, Fig. 1b). To test the function of OT II T cells in our model, separate groups of OT II mice and nude mice reconstituted with OT II T cells were subjected to partial liver warm ischemia (90 min). By 6 h of reperfusion, OT II mice, unlike WT or CD8 KO mice, had suffered minimal liver IRI (Fig. 1c). In addition, naïve OT II T cells, but not WT T cells, failed to recreate liver IRI in nude mice (Fig. 1c). To determine whether activated T cells could facilitate liver IRI, OT II cells were stimulated in vitro with their cognate OVA peptide for 48 h prior to transfer. FACS analysis confirmed the activated cell phenotype, with increased CD44, CD25, and CXCR3 but reduced CD62L levels (Fig. 1d). Adoptive transfer of activated OT II T cells (5 × 106/mouse), but not their naïve counterparts, recreated liver IRI in nude mice (Fig. 1c). Interestingly, concomitant CD154 blockade (MR1 mAb) partially protected livers against IRI in nude mice reconstituted with activated OT II cells (p < 0.05). IR-associated liver damage was quantitated by Suzuki’s histological scores (Fig. 1f). Minimal visible areas of injured liver parenchyma were observed in nude mice that were untreated or reconstituted with naïve OT II cells. Liver necrotic areas, however, became prominent in nude mice reconstituted with activated OT II cells alone, but not in those given adjunctive anti-CD154 mAb. To determine whether OT II T cells promoted the liver inflammatory response against IR, we analyzed the intrahepatic inflammatory gene induction profile. CD3 and CD4 transcripts were increased in the livers of nude mice reconstituted with either naïve or activated OT II cells (Fig. 1g). However, IFN-γ and CD154 levels were significantly increased similarly to TNF-α, IL-1, IL-6, and CXCL10 levels exclusively in the livers conditioned with activated OT II cells. The pro-inflammatory gene induction profile was reduced after concomitant CD154 blockade (Fig. 1g).
Fig. 1.
Ag-specific CD4 T cells exacerbate liver IRI. a Cells from spleens and LNs of either OT II or WT mice were stained with fluorochrome-labeled Abs and subjected to FACS. b WT or OT II T cells were adaptively transferred into congenic nude mice at 50 × 106/mouse. FACS analysis of PBLs from these reconstituted nude mice was performed on day 6 post-reconstitution. c Serum ALT measured after 90 m of ischemia and 6 h of reperfusion in OTII, CD8 KO, or WT mice, nude mice reconstituted with OT II or WT T cells, nude mice with liver injuries (Ctl), or nude mice reconstituted with naïve (n-OT II) or activated OT II (a-OT II) with or without treatment with MR1 (a-OT II/MR1). d In vitro stimulation of OT II T cells with their cognate Ag, i.e., OVA peptide, activated these cells and FACS histograms show the levels of T cell activation markers. e Liver histological images of different nude mouse groups after IRI (H/E staining). f Suzuki scores. g Liver inflammatory gene expression levels were measured by qRT-PCR, and Target gene/HPRT ratios were plotted. n = 5–6/group, *p < 0.05
The current series of experiments provided the first direct evidence that only activated/memory T cells are capable of promoting the liver innate immune response in an Ag-independent and CD154-dependent manner. Combined with our previous studies,1 IR-triggered TLR4 upregulates CD40 on KCs, which then engages CD154 on activated/memory CD4 T cells, leading to reversed co-stimulatory signaling in KCs. Activation of both TLR and CD40 is required for full-scale activation of the liver inflammatory immune response against IRI.
Why is CD40 activation required for innate immune activation against liver IRI? Potentially, CD40 may help KCs overcome endotoxin tolerance induced by routine exposure to gut commensal bacterial products3 or counteract negative regulation due to interactions with other tissue-resident immune cells. KCs have been shown to produce IL-10 in response to TLR ligands and play a protective role in a total hepatic IRI model.4, 5 Although the precise role of regulatory T cells in liver IRI has yet to be established, their innate immune suppressive effects have been observed in kidney injury models.6 Regarding Ag-non-specific CD4 T cell function, activated CD4-mediated bystander injury has been shown to require tissue Fas expression and was inhibited with anti-IFN-gamma Ab treatment,7 which is very different from our observations in our model where IFN-γ seemed irrelevant, while TNF-a, but not Fas, was critical for IR-triggered inflammatory hepatocellular injury.8 T cells can also negatively regulate the innate immune network as a feedback mechanism to prevent excessive tissue inflammation in an Ag-specific manner. Natural regulatory T cells suppress innate immune-mediated pathology via immune-regulatory cytokines, such as IL-10, TGF-b, IL-4, and IL-13.9 T cells have been found to restrict innate immune activation in an Ag-non-specific manner by an MHC-dependent cell–cell contact mechanism.10 Therefore, our study provides evidence for a novel regulatory role of activated/memory CD4 T cells in the pro-inflammatory innate immune response in the IR-stressed liver.
Acknowledgements
This study was supported by the Foundation of Jiangsu Collaborative Innovation Center of Biomedical Functional Materials, Six talent peaks project in Jiangsu Province (WSW-019).
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Shen X, et al. CD4 T cells promote tissue inflammation via CD40 signaling without de novo activation in a murine model of liver ischemia/reperfusion injury. Hepatology. 2009;50:1537–1546. doi: 10.1002/hep.23153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shen X, et al. Alloimmune activation enhances innate tissue inflammation/injury in a mouse model of liver ischemia/reperfusion injury. Am. J. Transplant. 2010;10:1729–1737. doi: 10.1111/j.1600-6143.2010.03205.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Janelsins BM, Lu M, Datta SK. Altered inactivation of commensal LPS due to acyloxyacyl hydrolase deficiency in colonic dendritic cells impairs mucosal Th17 immunity. Proc. Natl Acad. Sci. USA. 2014;111:373–378. doi: 10.1073/pnas.1311987111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Abe T, et al. Kupffer cell-derived interleukin 10 is responsible for impaired bacterial clearance in bile duct-ligated mice. Hepatology. 2004;40:414–423. doi: 10.1002/hep.20301. [DOI] [PubMed] [Google Scholar]
- 5.Zhang M, Xu S, Han Y, Cao X. Apoptotic cells attenuate fulminant hepatitis by priming Kupffer cells to produce interleukin-10 through membrane-bound TGF-β. Hepatology. 2011;53:306–316. doi: 10.1002/hep.24029. [DOI] [PubMed] [Google Scholar]
- 6.Kinsey G, et al. Autocrine adenosine signaling promotes regulatory T cell-mediated renal protection. J. Am. Soc. Nephrol. 2012;23:1528–1537. doi: 10.1681/ASN.2012010070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Thangavelu G, Gill R, Boon L, Ellestad K, Anderson C. Control of in vivo collateral damage generated by T cell immunity. J. Immunol. 2013;191:1686–1691. doi: 10.4049/jimmunol.1203240. [DOI] [PubMed] [Google Scholar]
- 8.Zhai Y, et al. Type I, but not type II, interferon is critical in liver injury induced after ischemia and reperfusion. Hepatology. 2008;47:199–206. doi: 10.1002/hep.21970. [DOI] [PubMed] [Google Scholar]
- 9.Singer BD, King LS, D’Alessio FR. Regulatory T cells as immunotherapy. Front. Immunol. 2014;5:46. doi: 10.3389/fimmu.2014.00046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ma Z, Zhang E, Yang D, Lu M. Contribution of Toll-like receptors to the control of hepatitis B virus infection by initiating antiviral innate responses and promoting specific adaptive immune responses. Cell Mol. Immunol. 2015;12:273–282. doi: 10.1038/cmi.2014.112. [DOI] [PMC free article] [PubMed] [Google Scholar]