Abstract
ILC2s are implicated in asthma pathogenesis, but little is known about the mechanisms underlying their accumulation in airways. We investigated the time course of ILC2 accumulation in different tissues in murine models of asthma induced by a serial per-nasal challenge with ovalbumin (OVA), house dust mice (HDM), IL-25 and IL-33 and explored the potential roles of ILC2-attracting chemokines in this phenomenon. Flow cytometry was used to enumerate ILC2s at various time points. The effects of cytokines and chemokines on ILC2 migration were measured in vitro using a chemotaxis assay and in vivo using small animal imaging. Compared with saline and OVA challenge, both IL-25 and IL-33 challenge alone induced significant accumulation of ILC2s in the mediastinal lymph nodes, lung tissue and bronchoalveolar lavage fluid of challenged animals, but with a distinct potency and kinetics. In vitro, IL-33 and CXCL16, but not IL-25 or CCL25, directly induced ILC2 migration. Small animal in vivo imaging further confirmed that a single intranasal provocation with IL-33 or CXCL16 was sufficient to induce the accumulation of ILC2s in the lungs following injection via the tail vein. Moreover, IL-33-induced ILC2 migration involved the activation of ERK1/2, p38, Akt, JNK and NF-κB, while CXCL16-induced ILC2 migration involved the activation of ERK1/2, p38 and Akt. These data support the hypothesis that epithelium-derived IL-25 and IL-33 induce lung accumulation of ILC2s, while IL-33 exerts a direct chemotactic effect in this process. Although ILC2s express the chemokine receptors CXCR6 and CCR9, only CXCL16, the ligand of CXCR6, exhibits a direct chemoattractant effect.
Key words: ILC2s, Asthma, Accumulation, IL-33, IL-25, Chemokine
Subject terms: Chemokines, Innate lymphoid cells, Interleukins
Introduction
Currently, three groups (1-3) of innate lymphoid cells (ILCs) have been delineated based on their transcription factor requirements, cytokine production profiles and roles in immunity.1–3 Among these subsets of ILCs, some have the potential to play a prominent role in parasitic immunity and allergy.1–3 In particular, ILC2s express the transcription factor GATA-3 and produce copious amounts of the “type 2” cytokines IL-5, IL-9 and IL-13 as well IL-6, IL-10, GM-CSF and small quantities of IL-4,4,5 which likely participate in mucosal defense against parasites but also potentially contribute to airway allergic inflammation. ILC2s develop from common lymphoid progenitors (CLPs) and lack classic hematopoietic lineage markers and are thus defined as lineage-negative. These cells express Thy-1 (CD90), T-cell co-stimulator (ICOS), Sca-1, IL-7Rα, CD25, CD117, IL-25 receptor (IL17BR), and IL-33 receptor (T1/ST2), leading to the frequent use of these markers to identify and isolate ILC2s.4–6
Since the discovery of ILCs, their presence has been reported in the murine and human respiratory tracts.7–9 In particular, ILC2s have been reported to accumulate in the lung tissue and airway mucosa and contribute to type 2 inflammatory responses in mice following infection with influenza virus and challenge with multiple allergens, including Alternaria, papain and ovalbumin (OVA),10–13 as well as in the sputum of human patients with asthma, with numbers increasing in response to specific allergen inhalation challenge.9
Although the importance of the potential contribution of ILC2s to airway inflammation in asthma and rhinosinusitis is increasingly appreciated, relatively little is known about their biology, including where and how they are activated and whether and how they are attracted to sites of mucosal inflammation. Respiratory tract mucosal cells can produce multiple cytokines in response to a variety of environmental stimuli, including allergens, particulate pollution and sources of oxidative stress. Principal among these are IL-25, IL-33, and possibly thymic stromal lymphopoietin (TSLP), which amplify T cell responses, “type 2” inflammation and eosinophilic infiltration. Intranasal administration of IL-25 or IL-33 to mice induced the accumulation of IL-13-producing ILC2s in the lung and the BAL fluid.12–14 Conversely, administration of an anti-IL-33R (anti-T1/ST2) blocking antibody to wild-type mice following influenza virus infection significantly reduced the numbers of ILC2s in their lungs relative to PBS-treated controls.7 IL-33 appears to be more potent than IL-25in this regard, and IL-33-primed ILC2s are also more potent producers of IL-5 and IL-13.12,14 These data suggest that airway mucosal epithelial cytokines such as IL-25 and IL-33 may induce, directly or indirectly, the accumulation of ILC2s in the lung and airways. In addition to these potential effects of IL-25 and IL-33, it has also been reported that ILC precursors express the chemokine receptors CXCR6 and CCR9, further raising the possibility that their ligands might also participate in the homing of ILCs to tissues, perhaps within a certain time window during embryogenesis and later during the course of inflammation. Currently, there is little data to support this hypothesis.15 Some of these effects may also be tissue- specific since CCR9 deficiency considerably impaired the homing of ILC2 precursors to the intestinal lamina propria but did not apparently affect their homing to bone marrow, lungs and mesenteric lymph nodes.16
In the present study, using the lineage-CD45+ICOS+ST2+GATA3+ phenotype to identify and isolate mouse ILC2s, we addressed the hypothesis that IL-25 and IL-33, which we have shown17,18 induce pathophysiological features of asthma in naïve animals, applied directly to the airway mucosa of laboratory mice induce the infiltration of ILC2s into the mediastinal lymph nodes, lung parenchyma and airway lumen with distinct kinetics and potency. We compared the effects of direct application of these cytokines with those of conventional, IgE-mediated “allergen” sensitization using OVA and HDM. We further hypothesized that the chemokine ligands of CXCR6 and CCR9, i.e., CXCL16 and CCL25, induce ILC2 chemotaxis both in vitro and in vivo, and we addressed the latter using small animal imaging technology with fluorochrome-tagged ILC2s.
Methods
Murine surrogates of asthma
Female BALB/c mice (8–10 weeks old) were purchased from Vital River Laboratories (Beijing, China) and kept in a specific pathogen-free mouse facility located in the Department of Laboratory Animal Sciences, Capital Medical University, Beijing, China. All experiments were undertaken with the approval of the Institutional Animal Care and Use Committee (IACUC).
To compare the differences between IL-33-induced and IL-25-induced accumulation of ILC2 cells, mice were assigned to 4 groups challenged with OVA, IL-25, IL-33, and saline control, as previously described.17,18 Briefly, the mice in the OVA-challenged group were first sensitized by intraperitoneal injection of OVA (Sigma-Aldrich, Beijing, China, 100 μg emulsified in AL[OH]3/dose) on day-18 and day-6, then further challenged daily per-nasally on days 1 to 6 with 50 μg of OVA in 50 μL of saline/dose. Mice in the IL-33-challenged and IL-25-challenged groups were not sensitized with OVA but were treated daily per-nasally from days 1 to 6 with recombinant mouse IL-33 [mIL-33, R&D Systems, Minneapolis MN, USA, 100 ng in 50 μL of saline] or IL-25 [mIL-25, R&D Systems, 2 μg in 50 μL of saline].17 Following this, the mice in the relevant groups were further challenged intranasally with OVA, IL-33, or IL-25 every 2 days for an additional 30 days. Some mice in each group were observed for a further 17 days after stopping the challenge. Mice in the saline control challenge group were injected intraperitoneally with the same amount of AL[OH]3 and then nasally challenged with saline at time points corresponding to those in the active challenge groups (Fig. 1a). Groups of 5 mice from each challenge group were euthanized at various time points during and following OVA/saline or cytokine challenge (Fig. 1a), and cells and tissues were isolated for further analysis as detailed below.
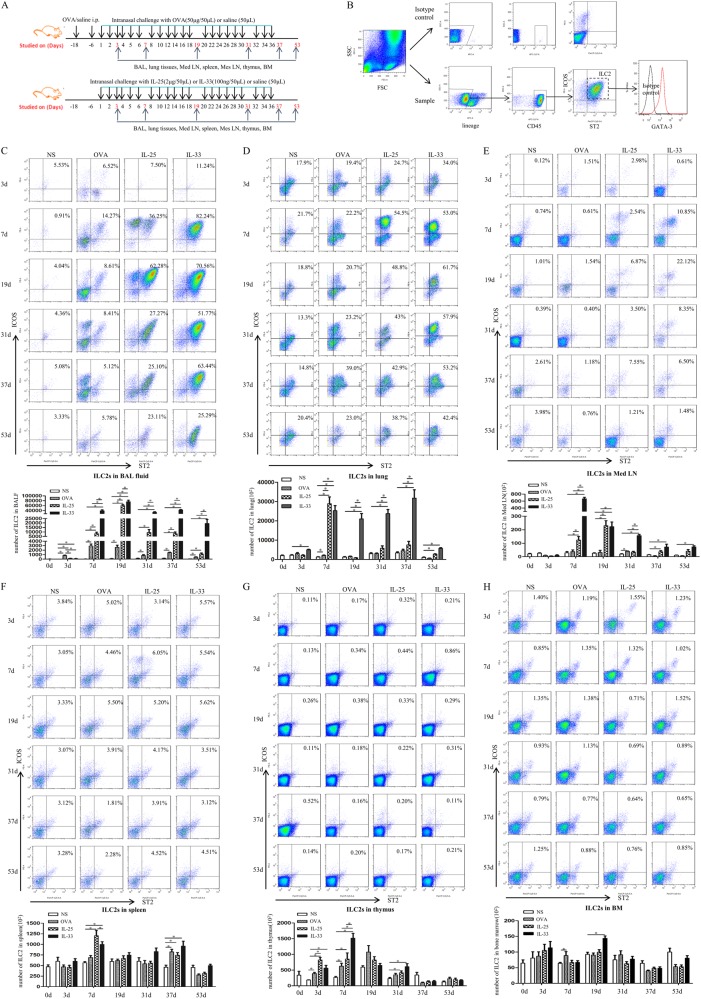
Fig. 1.
a Schedule of murine challenge. b ILC2s were gated by the protocol. (C-H) Flow cytometric identification of ILC2s in single-cell suspensions of the BAL fluid (BALF, c), lung parenchyma (d), mediastinal lymph nodes (Med LN, e), spleen (f), thymus (g) and bone marrow, (h) of treated mice. Top panels: plots showing expression of the indicated markers on lineage-negative cells (samples from 5 mice/group pooled for ILC2 staining). Bottom panels: quantification of ILC2s in treated groups. Bars show the mean ± SEM (n = 5 in each group at each time-point). *p < 0.05
Preparation of single-cell suspensions and flow cytometric analysis
Single-cell suspensions were prepared from the bone marrow, spleen, thymus, mediastinal lymph nodes, lungs and BAL fluid from groups of 5 mice at each time point shown in Fig. 1a. Spleen, thymus, lymph nodes and lungs were mechanically disrupted using a 100-μM cell strainer (Corning, NY, USA). Bone marrow cells were obtained by flushing the marrow out of each femur with PBS. Red blood cells from bone marrow, spleen and lung were lysed using FACS lysing solution (Beijing Solarbio Technology Co Ltd, Beijing, China).
To identify tissue ILC2s, cells were first incubated with a monoclonal antibody to CD16/CD32 (2.4G2, BD Pharmingen, San Jose, CA, USA) for 10 min to block Fc receptors. Cells were then stained with PE-conjugated anti-ICOS (7E.17G9, BD Pharmingen), PerCP-eFluor-710-conjugated anti-ST2 (RMST2-33, eBioscience, Santa Clara, CA, USA), APC-Cy7-conjugated anti-CD45 (30-F11, BD Pharmingen), an APC-conjugated anti-lineage cocktail (BD Pharmingen) and PE-CF594-conjugated anti-GATA3 (L50-823, BD Pharmingen). ILC2s were identified as lineage-CD45+ICOS+ST2+, as previously described.13,14,19 Isotype and single-stain controls were included. The samples were acquired using LSRFortessa (BD Biosciences, San Jose, CA, USA) or FACSAria (BD Biosciences) flow cytometers and analyzed with Flow Jo software (version7.6, Tree Star, Inc., Ashland OR, USA).
Isolation of lung ILC2s for in vitro studies
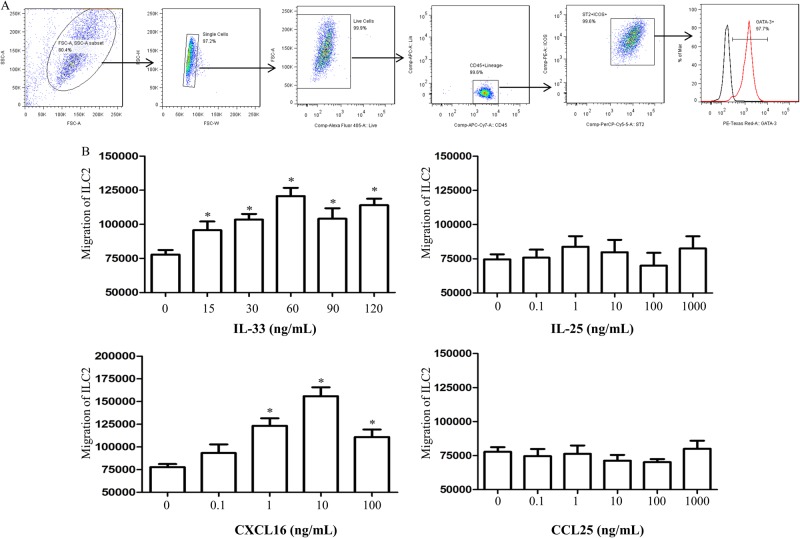
ILC2s were isolated from the lung tissues of the group of 5 mice euthanized 24 h after the final per-nasal challenge with rmIL-33 (Fig. 1a). Cells were stained and sorted as lineage-CD45+ICOS+ST2+ cells using a MoFlo XDP cell sorter (Beckman Coulter, Brea CA, USA) at a maximum concentration of 5 × 107cells/ml. Sorted ILC2s were cultured for up to 10 days in RPMI 1640 containing 20% FBS, 1% penicillin/streptomycin, 0.1% β-mercaptoethanol, 10 ng/ml rmIL-2 (R&D Systems, Minneapolis MN, USA), and 10 ng/ml rmIL-7 (R&D Systems).13,20 The purity of the resulting ILC2 population (lineage-CD45+ICOS+ST2+GATA-3+) exceeded 95% (Fig. 3a).
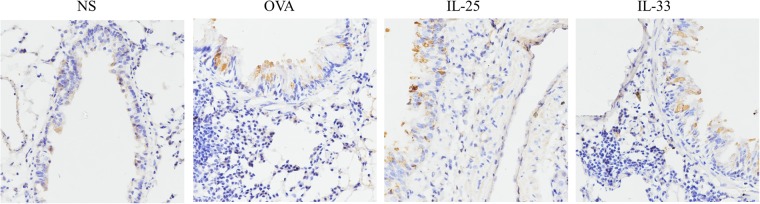
Fig. 3.
Representative photomicrographs of CXCL16+ cells in lung sections from IL-33-challenged, IL-25-challenged, OVA-challenged and saline (NS)-challenged mice at day 19 (original magnification ×40)
In vitro chemotaxis assays
To measure cellular migration, ILC2s were resuspended at a concentration of 2.5 × 106/ml in RPMI1640 medium, and 100 μL of the cell suspension was placed in the upper chamber of a 5-μm pore-size, 24-transwell plate (Corning, NY, USA). Five hundred microliters of test chemoattractants consisting of various concentrations of rmIL-33, rmIL-25, rmCXCL16, and rmCCL25 (R&D Systems, Minneapolis MN, USA) diluted in RPMI 1640 were applied to the lower chambers of the transwell plate. Following incubation (37 °C, 3 h), cells migrating to the lower chambers were counted.
In vivo imaging of DiR-stained ILC2s
ILC2s isolated from the lung tissues of mice nasally challenged with rmIL-33 as described above were incubated with 320 μg/ml XenoLight DiR fluorescent dye (Caliper Life Sciences, Inc., Hopkinton MA, USA). After 30 min of incubation, cells were centrifuged (5 min, 1000 rpm, 4 °C), washed twice in PBS and then injected intravenously into naïve, syngeneic mice (5 × 105cells/mouse). The mice were then immediately challenged per-nasally with a single dosage of recombinant, murine IL-25, IL-33, CXCL16, or CCL25 (100 nmol/L in 50 μL of saline in each case), and whole-body imaging was performed using an IVIS Spectrum machine (PerkinElmer, Inc., Waltham MA, USA) at 0, 2, 4, 6, 24, 26, 28, and 30 h following per-nasal challenge. The animals were then euthanized, and the thymus, lungs, heart, liver, spleen, kidneys and bones were harvested for ex vivo imaging at the 30-h time point.
Flow cytometric analysis of the signaling pathways mediating IL-33 or CXCL16 function
To determine the signaling pathways mediating IL-33 or CXCL16 function, isolated ILC2s were pre-treated with 10 μM U0126-EtOH (ERK-selective inhibitor), 10 μM SB203580 (p38 MAP kinase-selective inhibitor), 10 μM SP600125 (JNK- selective inhibitor), 30 μM LY294002 (selective inhibitor of phosphatidylinositol 3-kinase) or 20 μM BAY 11-7082 (NF-κB-selective inhibitor) (all from Selleckchem, USA) overnight. Then, the cells were stimulated with IL-33 (60 ng/ml) or CXCL16 (10 ng/ml) at 37 °C for 10 min. Cells were fixed in 1 × BD PhosflowTM Lyse/Fix Buffer at 37 °C for 10 min and permeabilized in BD PhosflowTM Perm Buffer III on ice for 30 min. Cells were then stained with anti-mouse phosphor-ERK1/2 (T202/Y204) Alexa Fluor 488 (MILAN8R, eBioscience), phospho-p38 MAPK (Thr180,Tyr182) PerCP-eFluor710 (4NIT4KK, eBioscience), phospho-AKT (Ser473) PE (SDRNR, eBioscience), phospho-JNK (T183,Y185) Alexa Fluor647 (N9-66, BD Pharmingen) and phospho-NF-κB p65 (Ser536) (93H1) Rabbit mAb (Alexa Fluor647 Conjugate) (Cell Signaling Technology, Inc., USA). Isotype and single-stain controls were included. The samples were acquired using LSRFortessa (BD Biosciences) and analyzed with Flow Jo software (version7.6, Tree Star, Inc.).
Immunohistochemistry
Immunohistochemistry was used to detect lung CXCL16 expression.21 The mouse CXCL16 antibody was purchased from R&D Systems.
Statistics
For comparisons between two groups, Student’s unpaired t-test was used. The statistical significance of differences between more than two groups was calculated using one-way ANOVA followed by the Bonferroni post-test. Data were analyzed and graphs were prepared using Prism (GraphPad software, San Diego, CA, USA).
Results
Kinetics of ILC2 accumulation in IL-25-induced and IL-33-induced murine asthma models
Flow cytometry showed that lineage-CD45+ICOS+ST2+ ILC2s express the transcription factor GATA-3 (Fig. 1b).
Flow cytometry showed that per-nasal, direct airway challenge of naïve mice with IL-33 and IL-25 and OVA-sensitized mice with OVA caused robust elevation of ILC2 numbers in bronchoalveolar lavage (BAL) fluid from the challenged animals starting from day 3 (Fig. 1c). The mean number of ILC2s in the BAL fluid of the OVA-challenged mice peaked at day 7, while the mean number of ILC2s in the IL-25-challenged mice peaked at day 19 and then rapidly declined. In contrast, the mean number of ILC2s in the IL-33-challenged mice peaked at day 19 and then declined more slowly, remaining significantly elevated relative to the saline control-challenged animals up to day 53, 17 days after the cessation of challenge. From day 7 onwards, the mean number of ILC2s in the BAL fluid of the IL-33-challenged mice was statistically greater than the mean numbers of these cells in the BAL fluid of OVA- and IL-25-challenged animals (Fig. 1c).
The mean number of ILC2s was also elevated relative to saline control-challenged animals in the lung tissue of IL-33-challenged (days 3-53), IL-25-challenged (days 7 and 53) and OVA-challenged (day 7) mice. In addition, the mean total numbers of ILC2s in the lungs of the IL-33-challenged mice at days 19, 31, and 37 were significantly greater than those observed in the IL-25-challenged animals (Fig. 1d).
The mean number of ILC2s in cells from the mediastinal lymph nodes (Med LN) of the IL-33-challenged mice was significantly greater than those observed in the saline-challenged control mice at all time points from day 7 onwards, again with a peak at day 7 (Fig. 1e). In contrast, the mean number of ILC2s in the IL-25-challenged mice was also elevated at day 7 and 19. At all time points from day 7 onwards, the mean total numbers of ILC2s among cells from the Med LN of the IL-33-challenged mice were significantly greater than those observed in the OVA-challenged and IL-25-challenged animals.
We then analyzed the mean numbers of ILC2s in the spleen, thymus and bone marrow of the experimental animals. The mean number of ILC2s in splenic cells from the OVA-challenged animals was significantly greater than that observed in the saline-challenged animals at day 37. At days 7 and 37, the mean numbers of ILC2s at this site in the IL-25- and IL-33-challenged mice were significantly elevated relative to the saline challenged control-animals (Fig. 1f). The mean number of ILC2s in thymic cells from the OVA-challenged mice was significantly elevated at days 3 and 7 relative to the saline-challenged control animals, while the mean numbers of ILC2s at this site following IL-33 challenge and IL-25 challenge were significantly greater than those observed in saline-challenged control animals at days 3, 7, and 31 (Fig. 1g). Significantly elevated mean numbers of ILC2s were observed in bone marrow cells in IL-33-challenged and OVA-challenged mice at days 19 and 7, respectively, relative to control saline-challenged animals (Fig. 1h). IL-25 challenge had no significant effect at this site.
Expression of the chemokines CXCL16 and CCL25 and their receptors CXCR6 and CCR9 in asthma models
ELISA analysis of lung homogenates showed that per-nasal challenge of the mice with IL-25, IL-33, or OVA was associated with a significantly elevated mean concentration of the chemokine CXCL16 in extracts of the lung parenchyma relative to control saline challenge. The peaks of IL-33-induced and IL-25-induced CXCL16 expression were observed at day 31, whereas the peak of OVA-induced expression was observed slightly earlier at day 19 (Fig. 2a). IL-33-induced CXCL16 expression was earlier (day 7) and more persistent (up to day 53) than IL-25-induced expression.
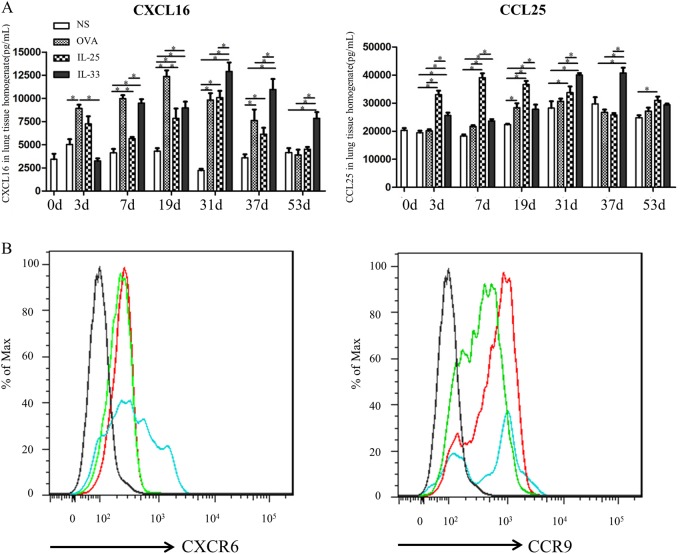
Fig. 2.
a Concentrations of the chemokines CXCL16 and CCL25 in lung homogenates from IL-33-challenged, IL-25-challenged, OVA-challenged and saline (NS)-challenged mice at various time points. Bars show the mean ± SEM (n = 5 in each group at each time-point). *p < 0.05. b Flow cytometric analysis showing that ILC2s express the surface chemokine receptors CXCR6 and CCR9 following OVA, IL-25 and IL-33 challenge at day 19 (lung tissues from 5 mice/group pooled for staining). Black line: isotype control, red line: IL-25, green line: IL-33, blue line: OVA
Per-nasal challenge of the animals with IL-25 and IL-33, but not OVA, was also associated with a significantly elevated mean concentration of the chemokine CCL25 in the lung homogenates. The peak of IL-25-induced CCL25 expression (day 7) was earlier than that of IL-33-induced expression (day 37) (Fig. 2a).
In addition, flow cytometric analysis confirmed that ILC2s isolated from the lung parenchyma of the mice challenged with IL-25, IL-33, and OVA at day 19 robustly expressed the surface chemokine receptors CXCR6 and CCR9 (Fig. 2b).
Expression of CXCL16 by immunohistology
As shown in Fig. 3, in sections of lung tissue from the challenged mice, CXCL16 was immunolocalized principally in the epithelial cells of the airway and also in some submucosal mononuclear cells and vascular endothelial cells.22 OVA, IL-25, and IL-33 challenge induced elevated expression of CXCL16 at day 19, consistent with the ELISA data above.
IL-33 and CXCL16 induced ILC2 chemotaxis in vitro
To further explore potential mechanisms of ILC2 recruitment to the lungs, we investigated possible direct effects of IL-33, CXCL16, IL-25, and CCL25 on ILC2 migration using an in vitro transwell assay. For this purpose, lineage−/CD45+/ICOS+/ST2+ ILC2s were isolated from the lung tissue of mice subjected to IL-33 challenge for 18 days (equivalent to day 19 shown on the schedule in Fig. 1a) (Fig. 4a). Both IL-33 and CXCL16, but not IL-25 or CCL25, induced the migration of ILC2s in a concentration-dependent fashion (Fig. 4b).
Fig. 4.
a Isolation protocol and purity of ILC2 cells. b Migration of ILC2s in response to IL-33, IL-25, CXCL16, and CCL25 alone. *p < 0.05 compared with medium control
IL-33 and CXCL16 alone independently induced the migration of DiR-stained ILC2s from the circulation to the lung parenchyma in vivo
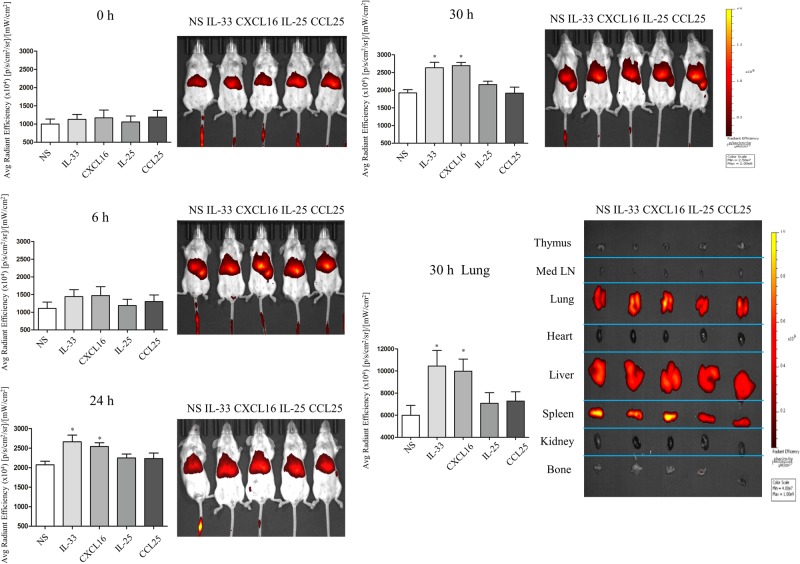
We employed non-invasive, in vivo imaging to track the migration of intravenously injected ILC2s into the lungs of experimental animals in real time. Per-nasal challenge with both IL-33 and CXCL16 independently induced the migration of DiR-stained ILC2s into the lung tissue of the challenged animals (Fig. 5). Quantitative analysis of the average radiant efficiency demonstrated that this elevated migration was first detectable at 6 h and significantly greater than that observed in saline-challenged control animals by 24 h after transfusion. In contrast, no elevated ILC2 migration to this site was detectable in IL-25-challenged or CCL25-challenged animals throughout the duration of the experiment. Thymus, Med LN, lungs, heart, liver, spleen, kidney and bone were harvested from the challenged mice 30 h after the administration of labeled ILC2s (at the end of the in vivo tracking period). Quantitative analysis of these organs by ex vivo imaging further confirmed that ILC2s selectively migrated into the lung tissues of the IL-33-challenged and CXCL16-challenged animals (Fig. 5).
Fig. 5.
Mice were injected with XenoLight DiR-stained ILC2s intravenously, then intranasally challenged with IL-25, IL-33, CXCL16 or CCL25 (all 100 nmoL/L) and imaged ventrally with an IVIS Spectrum machine at 0, 6, 24 and 30 h. Thymus, Med LN, lungs, heart, liver, spleen, kidney and bone were harvested for ex vivo imaging at the 30-h time point. *p < 0.05 (n = 3 for each group)
Effects of IL-33 or CXCL16 on the activation of the ERK, p38, JNK, Akt, and NF-κB pathways in ILC2s
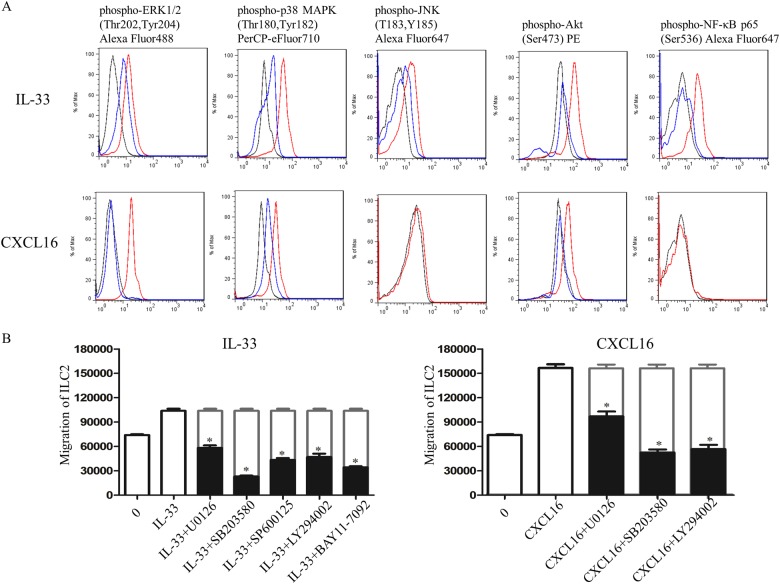
IL-33 binds to ST2 and initiates the recruitment of Mal or MyD88 signaling adaptor molecules, leading to the activation of downstream pathways (including NF-κB and MAPKs).23,24 In murine peritoneal macrophages, human aortic smooth muscle cells and human umbilical vein endothelial cells, CXCL16 activated ERK1/2, p38, and PI3K/Akt, respectively.21,25,26 To determine whether IL-33 or CXCL16 can activate these signaling pathways in ILC2s, we treated ILC2s with IL-33 (60 ng/ml) or CXCL16 (10 ng/ml) (the optimal concentrations of IL-33 and CXCL16 in vitro in the transwell assay) for 10 min. Flow cytometric analysis revealed that IL-33 markedly increased the phosphorylation of ERK1/2, p38, Akt, and NF-κB and, to a lesser extent, that of JNK. CXCL16 induced marked phosphorylation of ERK1/2 and p38 and, to a lesser extent, that of Akt (Fig. 6a). Furthermore, we observed that the specific inhibitors U0126-EtOH, SB203580, SP600125, LY294002, and BAY 11-7082 markedly reduced the phosphorylation of their specific targets in the ILC2s (Fig. 6a).
Fig. 6.
a Flow cytometric analysis showing IL-33-induced and CXCL16-induced phosphorylation of Erk1/2, p38, JNK, Akt, and NF-κB. Red lines: IL-33 or CXCL16 stimulation; black lines: PBS stimulation; blue lines: IL-33 or CXCL16 stimulation in the presence of inhibitors of Erk1/2 (U0126-EtOH), p38 (SB203580), JNK (SP600125), Akt (LY294002), and NF-κB (BAY 11-7082). b Inhibitors of Erk1/2 (U0126-EtOH), p38 (SB203580), JNK (SP600125), Akt (LY294002), and NF-κB (BAY 11-7082) significantly blocked the migration of ILC2s in response to IL-33 and CXCL16 alone. *p < 0.05 compared with positive control
To further analyze the relevance of these signaling pathways for ILC2 migration, migration assays were performed with IL-33 or CXCL16 in the presence of various signaling pathway inhibitors. As shown in Fig. 6b, IL-33-induced ILC2 migration was significantly blocked by U0126-EtOH, SB203580, SP600125, LY294002, and BAY 11-7082, suggesting the involvement of both the MAPK and NF-κB pathways in ILC2 chemotaxis toward IL-33 in vitro. CXCL16-induced ILC2 migration was significantly blocked by U0126-EtOH, SB203580 and LY294002, suggesting the involvement of ERK, p38 and Akt signaling in CXCL16-induced ILC2 migration.
Pulmonary ILC2s in CXCL16-challenged and CCL25-challenged mice
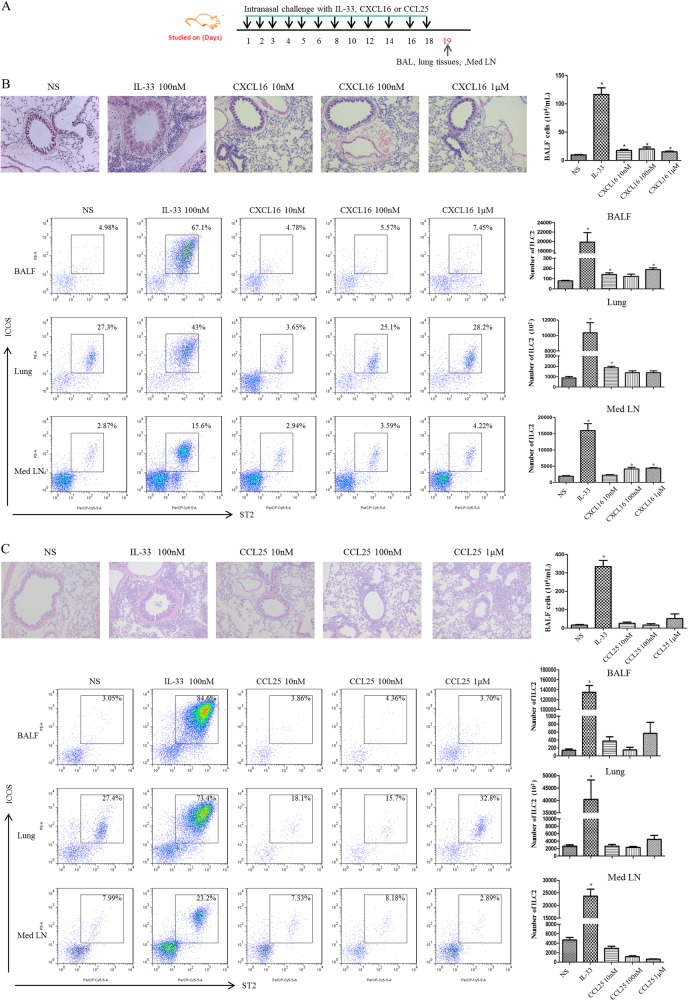
Having shown that IL-33 and CXCL16, but not IL-25 or CCL25, applied to the airway mucosa can attract ILC2s from the peripheral circulation to the lung parenchyma, we next investigated whether these mediators applied locally to the airway mucosa were able to influence the infiltration of ILC2s into the airway mucosa and lumen in vivo. To accomplish this, we set up challenge protocols with these mediators, as shown in the schedule in Fig. 7a. Briefly, 8 to10-week-old BALB/c mice were anesthetized and challenged intranasally daily for 6 days and then every 2 days for a further 12 days with recombinant murine CXCL16 (mCXCL16) or CCL25 (mCCL25) (R&D Systems). Mice challenged with saline or IL-33 were used as negative and positive controls, respectively.
Fig. 7.
a Schedule of murine challenge. b, c Top panels show representative photomicrographs of hematoxylin & eosin-stained lung sections (original magnification×20) from CXCL16-challenged (b) and CCL25-challenged (c) mice. Bottom panels show the flow cytometric identification of ILC2s in single-cell suspensions of BAL fluid (BALF), lung parenchyma (Lung) and mediastinal lymph nodes (Med LN) of challenged animals. Left: plots showing the expression of the indicated markers on lineage-negative cells (tissues from 4 mice/group pooled for ILC2 staining). Right: quantification of ILC2s in lung, BALF and Med LN in the treated groups. Bars show the mean ± SEM (n = 4 in each group). *p < 0.05 compared with saline control
Under these conditions, CXCL16 challenge resulted in a small but significant increase in the mean numbers of total cells in BAL fluid (Fig. 7b) and ILC2s in the lung tissue, BAL fluid and mediastinal lymph nodes (Fig. 7b) of the challenged animals relative to the saline-challenged, negative control animals, although IL-33 was considerably more efficacious in this regard. In contrast, challenge with equivalent concentrations of CCL25 exerted no consistent effects on the numbers of total or ILC2 cells in any of these compartments (Fig. 7c).
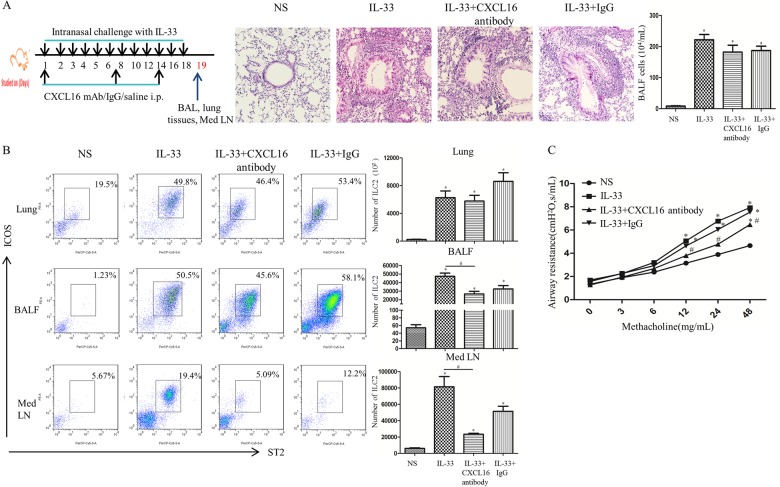
Anti-CXCL16 antibody reduced ILC2 accumulation in the lung tissue of IL-33-challenged mice
To further confirm that CXCL16 exerts a chemotactic effect on ILC2s in vivo, we set up challenge protocols with anti-mouse CXCL16 antibody as shown in the schedule in Fig. 8a. To block CXCL16, IL-33-challenged mice received 100 μg of monoclonal rat anti-mouse CXCL16 neutralizing antibody (R&D Systems) or rat IgG control antibody by weekly intraperitoneal injection.27 Lung function was assessed 24 h after the last intranasal challenge. Airway responsiveness was determined by changes in lung resistance in anesthetized and tracheostomized mice in response to increasing concentrations of aerosolized methacholine (0–48 mg/mL) using the FlexiVent system (Scireq, Inc., Montreal, Canada) as described previously.17,18,28
Fig. 8.
a Top left panel shows the schedule of murine challenge. Top right panel shows representative photomicrographs of hematoxylin & eosin-stained lung sections (original magnification ×20). b Left: flow cytometric identification of ILC2s in single-cell suspensions of the lung parenchyma (Lung), BAL fluid (BALF) and mediastinal lymph nodes (Med LN) of challenged animals. Right: quantification of ILC2s in lung, BALF and Med LN in the treated groups. Bars show the mean ± SEM ((n = 4 in each group. * p < 0.05 vs. NS-challenged mice, #p < 0.05 vs. IL-33-challenged mice). c Airway resistance of IL-33-challenged, IL-33 + CXCL16 antibody-challenged, IL-33 + IgG-challenged and saline (NS)-challenged mice. Data are presented as the mean ± SEM (n = 4 in each group. *p < 0.05 vs. NS-challenged mice, # p < 0.05 vs. IL-33-challenged mice)
Under these conditions, anti-mouse CXCL16 antibody-treated mice showed a marked reduction in ILC2s in the BAL fluid and mediastinal lymph nodes (Fig. 8b) relative to IL-33-challenged, positive control animals.
Intranasal challenge with IL-33 significantly increased airway hyperresponsiveness (AHR) to methacholine, while concurrent treatment with anti-CXCL16 antibody abrogated this increase (Fig. 8c).
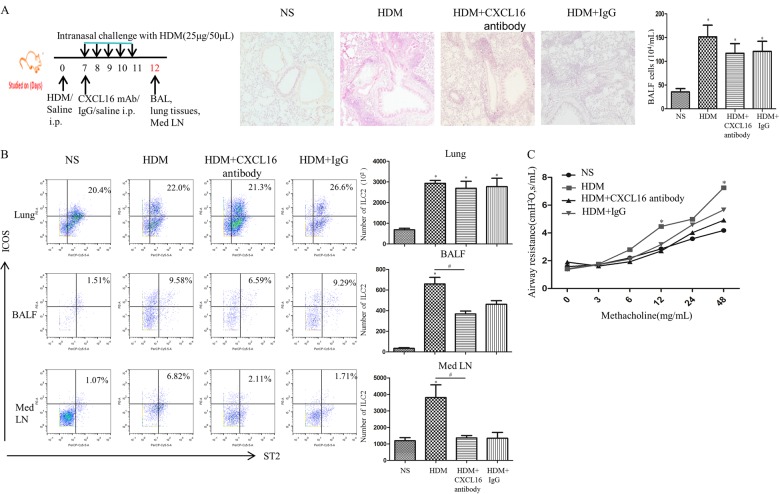
Anti-CXCL16 antibody reduced ILC2 accumulation in the lung tissue of an HDM-induced murine asthma model
To further confirm that CXCL16 regulates ILC2 accumulation in a house dust mite (HDM)-induced murine asthma model, we set up challenge protocols with anti-mouse CXCL16 antibody as shown in the schedule in Fig. 9a. Briefly, the mice in the HDM-challenged group were first sensitized by intraperitoneal injection of HDM (Cosmo Bio Co LTD, Japan, 100 μg emulsified in AL[OH]3/dose) on day 0. Seven days later, mice were challenged daily for five consecutive days with 25 μg of HDM intranasally.29 To block CXCL16, the HDM-challenged mice received 100 μg of monoclonal rat anti-mouse CXCL16 neutralizing antibody (R&D Systems) or rat IgG control antibody on day 7 (concomitantly with the first intranasal HDM challenge) by intraperitoneal injection. Lung function was assessed 24 h after the last intranasal challenge. Airway responsiveness was determined as described previously.17,18,28
Fig. 9.
a Top left panel shows the schedule of murine challenge. Top right panel shows representative photomicrographs of hematoxylin and eosin-stained lung sections (original magnification ×20). b Left: flow cytometric identification of ILC2s in single-cell suspensions of the lung parenchyma (Lung), BAL fluid (BALF) and mediastinal lymph nodes (Med LN) of challenged animals. Right: quantification of ILC2s in the lung, BALF and Med LN in treated groups. Bars show the mean ± SEM ((n = 4 in each group. *p < 0.05 vs. NS-challenged mice, #p < 0.05 vs. HDM-challenged mice). c Airway resistance of HDM-challenged, HDM + CXCL16 antibody-challenged, HDM + IgG-challenged and saline (NS)-challenged mice. Data are presented as the mean ± SEM (n = 4 in each group. *p < 0.05 vs. NS-challenged mice, # p < 0.05 vs. HDM-challenged mice)
Under these conditions, anti-mouse CXCL16 antibody-treated mice showed a slight reduction in the mean number of ILC2s in the lung parenchyma and a marked and significant reduction in the mean numbers of ILC2s in the BAL fluid and mediastinal lymph nodes (Fig. 9b) relative to HDM-challenged, positive control animals. Intranasal challenge with HDM also significantly increased airway hyperresponsiveness (AHR) to methacholine, while concurrent treatment with anti-CXCL16 antibody significantly abrogated this increase (Fig. 9c).
Interestingly, some slightly reduced effects were also noticed in the IgG control group, possibly because IgG control antibody binds to inhibitory Fc receptors such as FcγRIIB on these cells, leading to general biological attenuation.
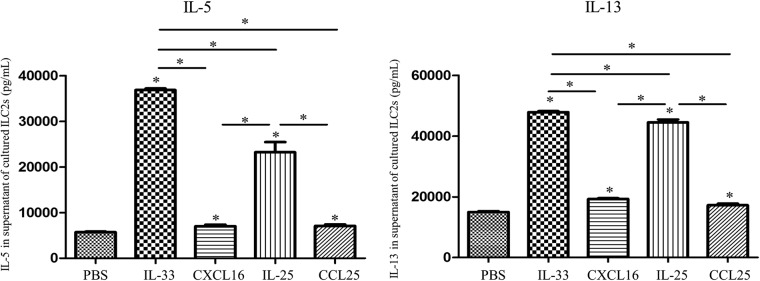
Activation of ILC2s induced type 2 cytokine production
Murine ILC2s were separately stimulated with IL-33, CXCL16, IL-25, and CCL25 (0.5 nM in each case) for 48 h. ELISA analysis of the culture supernatants showed that exposure to both IL-33 and IL-25 markedly and significantly increased the mean supernatant concentrations of both IL-5 and IL-13 (Fig. 10). While both CXCL16 and CCL25 increased IL-5 and IL-13 production in a detectable and statistically significant manner, these effects were much less marked than those of IL-33 and IL-25 at particular concentrations (Fig. 10).
Fig. 10.
Concentrations of the cytokines IL-5 and IL-13 in culture supernatants of ILC2s following incubation with the indicated stimuli (0.5 nM in each case). Bars show the mean ± SEM (n = 3). *p < 0.05
Discussion
Although studies increasingly highlight the epithelium-derived cytokines IL-25 and IL-33 as critical activators of ILC2-mediated innate immunity,21 there is a paucity of parallel studies systematically investigating the kinetics of the local accumulation of ILC2s in “type 2” cytokine-mediated diseases (such as asthma) and potential mechanisms of accumulation of ILCs in these diseases. Our regimen offers the opportunity to pursue the dynamics of accumulation of ILC2s in various organs and tissues in the context of IL-25-induced and IL-33-induced, as well as IgE-dependent, murine models of asthma in the experimental acute, subacute, chronic and convalescent phases. Our data clearly show that local production of both IL-25 and IL-33 in the airways has the propensity to induce significant accumulation of ILC2s in the lung parenchyma and airway lumen over the course of asthma development, with distinct kinetics and independent of IgE-mediated mechanisms. Further, these effects were significantly more marked with IL-33 than with IL-25, suggesting that IL-33 might play a more significant role in the recruitment and activation of ILC2s early in the development of asthma, as has been suggested in another recent study,14 although we accept that these conclusions may be tempered by the relative potency of these mediators in activating their specific receptors at the particular concentrations employed and the possibility of their differential rates of metabolism within the airways. In addition, a very recent study showed that IL-33 induces neutrophil polarization to selectively produce Th2-type cytokines, which might also contribute to pathological changes in asthma.24
It is also of great interest that, in the present study, per-nasal airway administration of IL-25 and IL-33 induced the accumulation of ILC2s not only in the lung parenchyma, airway lumen and mediastinal lymph nodes but also, at least transiently, in the spleen, thymus and bone marrow of the challenged animals. A recent study also showed that IL-25 and IL-33 administered into the peritoneal cavity of mice increased the number of ILC2s in the lung.13 These findings are compatible with the hypothesis that local administration or elevated production of IL-25 and IL-33 at any site may result in systemic accumulation of ILC2s through effects on the local environment, which may include the induction of ILC2 chemotaxis.
All innate lymphoid cells appear to be derived from a common bone marrow precursor expressing the Id2 transcription factor and the common γ chain of the IL-2 receptor.30 With regard to the present study, although we are unable to rule out the possibility that some or all of the ILC2s appearing in the airways in response to local IL-33 or IL-25 provocation were generated locally, the distinct kinetics of their accumulation in both local and remote tissues strongly suggests that these cells may at least partly have been derived from the bone marrow. On the other hand, accumulation of these cells was much more florid in the airway parenchyma and lumen, while their elevation in the bone marrow was much less conspicuous and more transient. One possible way to reconcile these observations is to hypothesize that ILC development is initiated in the bone marrow, but later stages of ILC differentiation devolve to other elements of the reticuloendothelial system. In support of this, it has been suggested that ILC precursors are unable to upregulate RORγt in the bone marrow, and later stages of ILC3 differentiation might devolve to other organs such as the spleen.31 Another possibility is that increased numbers of ILC2s in particular tissues in response to local environmental conditions are at least partially derived from the local proliferation of resident cells.
One possible reason why IL-33 caused more marked accumulation of ILC2s than IL-25 in our experiments is that IL-33, but not IL-25, exerted a direct chemotactic effect on these cells, as shown by our in vitro migration assays and in vivo small animal imaging studies, in addition to promoting the release of other ILC2 chemoattractants such as CXCL16 and CCL25, whereas IL-25 acted solely by the latter mechanism (Fig. 2). Similarly, Salimi and colleagues showed that while IL-33 and prostaglandin D2 elicited the migration of human skin-derived ILC2s in vitro,32 the chemotactic effects of thymic stromal lymphopoietin and IL-25, while detectable at high concentrations, were relatively poor.32,33 Despite this knowledge, it seems clear that there is still much to be learned about the mechanisms of ILC accumulation at sites of inflammation, infection or in response to environmental insults. For example, it has been shown that the ability of IL-33 to induce large numbers of ILC2s in the lung tissue of wild-type mice is abolished in RAGE (receptor for advanced glycation end-products) gene-deleted animals,28 suggesting that IL-33-mediated accumulation of ILC2s in the lung might require RAGE and perhaps other intermediate interactions.
Notwithstanding these many uncertainties, it is well recognized that chemokines play key roles in cellular adhesion and migration and direct the homing of specific populations or sub-populations of cells to both normal and inflamed tissues depending on their expression of chemokine receptors.34 Previous studies have implicated integrin α4β7, CCR9, CCR6, and CXCR6 in directing the homing of specific ILCs to tissues, putatively within a certain time window during embryogenesis.15 For example, it has been suggested that CCR9 may be involved in directing the homing of precursors of ILC2s to the intestinal lamina propria,16 while CXCR6 may be required for the circulation of ILC precursors.30 There remains, however, a lack of clarity in this area. In the present study we show that murine ILC2s express CXCR6 and CCR9, which are receptors for the chemokines CXCL16 and CCL25, respectively. We also demonstrate that expression of CXCL16 was elevated early in the lungs of our OVA-challenged mice and later in the lungs of our IL-25-challenged and IL-33-challenged mice, suggesting that CXCL16 might also contribute to the accumulation of ILC2s in these murine asthma surrogates. Meanwhile, CXCL16 was immunolocalized in the airway mucosa of mice, and its local expression might favor ILC2 migration into BAL fluid.
In contrast, expression of CCL25 was elevated only in the lungs of the IL-25 and IL-33-treated mice. Although previous studies have suggested that ILC precursors in the bone marrow express CCR9 and that this directs their migration to the intestines,15,16 our data from both in vivo and in vitro experiments suggest that CXCL16, but not CCL25, is capable of inducing ILC2 migration. Even then, direct per-nasal challenge with CXCL16 barely increased the migration of ILC2s into the airway lumen, suggesting that this might not be a key facet of the action of IL-33 and IL-25 in recruiting ILC2s to the airways and again underlining the possibility that other factors may be involved. Certainly, CXCL16 might not be the only chemoattractant induced by IL-33 and IL-25 that is capable of increasing the accumulation of ILC2 cells in the airways, the numbers of which might also, at least in theory, be altered by local proliferation in response to cytokines such as IL-33 and IL-25.35 Having set out these reservations, it appears indisputable that, following direct, per-nasal application of IL-33 or HDM allergen in our murine model of IL-33-induced or HDM allergen-induced asthma (Figs. 8 and 9), blockade of CXCL16 abrogates, albeit not completely, the accumulation of ILC2 cells in the airway lumen and local lymph nodes in real time, suggesting a direct, if partial, role for CXCL16 in this phenomenon.
So far, little is known about the mechanisms of intracellular signaling in ILC2s. In previous studies, phosphorylation of p38, JNK kinases and Akt has been shown to be a feature of chemokine receptor-dependent signaling.21,36–38 In particular, CXCL16 predominantly activates the MEK-ERK MAP kinase signaling pathway which has also been shown to be a major signaling pathway of other chemokine receptors such as CCR6, CXCR4, and CX3CR1, whereas other chemokines such as CXCR6 predominantly phosphorylate Akt, with less prominent phosphorylation of the JNK and p38 MAP kinases.21,36–39 Our studies focusing on these signaling pathways demonstrated that stimulation of ILC2s with IL-33 increased phosphorylation of the signaling molecules ERK1/2, p38, Akt, NF-κB, and JNK, while CXCL16 induced marked phosphorylation of ERK1/2 and p38 and weaker Akt phosphorylation. This finding correlates with the data from our in vitro ILC2 migration assays. For example, in the presence of U0126-EtOH (inhibitor of the ERK pathway), IL-33-induced or CXCL16-induced ILC2 migration was significantly abrogated.
Despite these effects of CXCL16 on ILC2 migration, the effects of both CXCL16 and CCL25 on type 2 cytokine production by ILC2s were marginal at the concentrations employed, in contrast to the direct effects of IL-33 and IL-25.
In summary, our data clearly show that airway epithelium-derived IL-25 and IL-33 are able to induce the accumulation of ILC2s in the lung tissue, local lymph nodes and airway lumen, and IL-33 directly induces ILC2 chemotaxis. Moreover, IL-33-induced ILC2 migration involves the activation of ERK1/2, p38, Akt, JNK, and NF-κB. Although ILC2s expressed the chemokine receptors CXCR6 and CCR9, only the CXCR6 ligand CXCL16 directly induced ILC2 chemotaxis. CXCL16 induced ILC2 migration involving activation of the ERK1/2, p38 and Akt pathways.
Acknowledgements
We acknowledge financial support from the National Natural Science Foundation of China (81373177, 81471594, and 81700026).
Footnotes
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Wei Wang, Email: wy_robin@ccmu.edu.cn.
Sun Ying, Email: ying.sun@ccmu.edu.cn.
References
- 1.Spits H, et al. Innate lymphoid cells--a proposal for uniform nomenclature. Nat. Rev. Immunol. 2013;13:145–149. doi: 10.1038/nri3365. [DOI] [PubMed] [Google Scholar]
- 2.Klose CS, et al. Differentiation of type 1 ILCs from a common progenitor to all helper-like innate lymphoid cell lineages. Cell. 2014;157:340–356. doi: 10.1016/j.cell.2014.03.030. [DOI] [PubMed] [Google Scholar]
- 3.Spits H, Di Santo JP. The expanding family of innate lymphoid cells: regulators and effectors of immunity and tissue remodeling. Nat. Immunol. 2011;12:21–27. doi: 10.1038/ni.1962. [DOI] [PubMed] [Google Scholar]
- 4.Walker JA, McKenzie AN. Development and function of group 2 innate lymphoid cells. Curr. Opin. Immunol. 2013;25:148–155. doi: 10.1016/j.coi.2013.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barlow JL, McKenzie AN. Type-2 innate lymphoid cells in human allergic disease. Curr. Opin. Allergy Clin. Immunol. 2014;14:397–403. doi: 10.1097/ACI.0000000000000090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lund S, Walford HH, Doherty TA. Type 2 Innate Lymphoid Cells in Allergic Disease. Curr. Immunol. Rev. 2013;9:214–221. doi: 10.2174/1573395510666140304235916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Monticelli L, et al. Innate lymphoid cells promote lung-tissue homeostasis after infection with influenza virus. Nat. Immunol. 2011;12:1045–1054. doi: 10.1038/ni.2131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mjosberg JM, et al. Human IL-25- and IL-33-responsive type 2 innate lymphoid cells are defined by expression of CRTH2 and CD161. Nat. Immunol. 2011;12:1055–1062. doi: 10.1038/ni.2104. [DOI] [PubMed] [Google Scholar]
- 9.Allakhverdi Z, et al. CD34 + hemopoietic progenitor cells are potent effectors of allergic inflammation. J. Allergy Clin. Immunol. 2009;123:472–478. doi: 10.1016/j.jaci.2008.10.022. [DOI] [PubMed] [Google Scholar]
- 10.Chang YJ, et al. Innate lymphoid cells mediate influenza-induced airway hyper-reactivity independently of adaptive immunity. Nat. Immunol. 2011;12:631–638. doi: 10.1038/ni.2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim HK, et al. Innate type 2 response to Alternaria extract enhances ryegrass-induced lung inflammation. Int. Arch. Allergy Immunol. 2014;163:92–105. doi: 10.1159/000356341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wilhelm C, et al. An IL-9 fate reporter demonstrates the induction of an innate IL-9 response in lung inflammation. Nat. Immunol. 2011;12:1071–1077. doi: 10.1038/ni.2133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Barlow JL, et al. Innate IL-13-producing nuocytes arise during allergic lung inflammation and contribute to airways hyperreactivity. J. Allergy Clin. Immunol. 2012;129:191–198. doi: 10.1016/j.jaci.2011.09.041. [DOI] [PubMed] [Google Scholar]
- 14.Barlow JL, et al. IL-33 is more potent than IL-25 in provoking IL-13-producing nuocytes (type 2 innate lymphoid cells) and airway contraction. J. Allergy Clin. Immunol. 2013;132:933–941. doi: 10.1016/j.jaci.2013.05.012. [DOI] [PubMed] [Google Scholar]
- 15.Klose CS, Artis D. Innate lymphoid cells as regulators of immunity, inflammation and tissue homeostasis. Nat. Immunol. 2016;17:765–774. doi: 10.1038/ni.3489. [DOI] [PubMed] [Google Scholar]
- 16.Hoyler T, et al. The transcription factor GATA-3 controls cell fate and maintenance of type 2 innate lymphoid cells. Immunity. 2012;37:634–648. doi: 10.1016/j.immuni.2012.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yao XJ, et al. Direct comparison of the dynamics of IL-25- and ‘allergen’-induced airways inflammation, remodelling and hypersensitivity in a murine asthma model. Clin. Exp. Allergy. 2014;44:765–777. doi: 10.1111/cea.12298. [DOI] [PubMed] [Google Scholar]
- 18.Li Y, et al. Distinct sustained structural and functional effects of interleukin-33 and interleukin-25 on the airways in a murine asthma surrogate. Immunology. 2015;145:508–518. doi: 10.1111/imm.12465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Neill DR, et al. Nuocytes represent a new innate effector leukocyte that mediates type-2 immunity. Nature. 2010;464:1367–1370. doi: 10.1038/nature08900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Drake LY, Iijima K, Kita H. Group 2 innate lymphoid cells and CD4 + T cells cooperate to mediate type 2 immune response in mice. Allergy. 2014;69:1300–1307. doi: 10.1111/all.12446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Diegelmann J, et al. Expression and regulation of the chemokine CXCL16 in Crohn’s disease and models of intestinal inflammation. Inflamm. Bowel Dis. 2010;16:1871–1881. doi: 10.1002/ibd.21306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Marques P, et al. Cigarette smoke increases endothelial CXCL16-leukocyte CXCR6 adhesion in Vitro and in vivo. Potential consequences in chronic obstructive pulmonary disease. Front. Immunol. 2017;13:1766. doi: 10.3389/fimmu.2017.01766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Grotenboer NS, Ketelaar ME, Koppelman GH, Nawijn MC. Decoding asthma: translating genetic variation in IL33 and IL1RL1 into disease pathophysiology. J. Allergy Clin. Immunol. 2013;131:856–865. doi: 10.1016/j.jaci.2012.11.028. [DOI] [PubMed] [Google Scholar]
- 24.Sun, B. et al. Characterization and allergic role of IL-33-induced neutrophil polarization. Cell Mol. Immunol. 15,782–793 (2018). [DOI] [PMC free article] [PubMed]
- 25.Kwon KH, Ohigashi H, Murakami A. Dextran sulfate sodium enhances interleukin-1 beta release via activation of p38 MAPK and ERK1/2 pathways in murine peritoneal macrophages. Life. Sci. 2007;81:362–371. doi: 10.1016/j.lfs.2007.05.022. [DOI] [PubMed] [Google Scholar]
- 26.Yu X, et al. CXCL16 induces angiogenesis in autocrine signaling pathway involving hypoxia-inducible factor 1alpha in human umbilical vein endothelial cells. Oncol. Rep. 2016;35:1557–1565. doi: 10.3892/or.2015.4520. [DOI] [PubMed] [Google Scholar]
- 27.Wehr A, et al. Pharmacological inhibition of the chemokine CXCL16 diminishes liver macrophage infiltration and steatohepatitis in chronic hepatic injury. PLoS One. 2014;9:e112327. doi: 10.1371/journal.pone.0112327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Riesenfeld E, et al. The Temporal Evolution of Airways Hyperresponsiveness and Inflammation. J. Allergy Ther. 2012;1:1–7. doi: 10.4172/2155-6121.S1-005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li BW, et al. T cells are necessary for ILC2 activation in house dust mite-induced allergic airway inflammation in mice. Eur. J. Immunol. 2016;46:1392–1403. doi: 10.1002/eji.201546119. [DOI] [PubMed] [Google Scholar]
- 30.Chea S, et al. CXCR6 Expression Is Important for Retention and Circulation of ILC Precursors. Mediat. Inflamm. 2015;2015:368427. doi: 10.1155/2015/368427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Possot C, et al. Notch signaling is necessary for adult, but not fetal, development of RORgammat( + ) innate lymphoid cells. Nat. Immunol. 2011;12:949–958. doi: 10.1038/ni.2105. [DOI] [PubMed] [Google Scholar]
- 32.Salimi M, et al. A role for IL-25 and IL-33-driven type-2 innate lymphoid cells in atopic dermatitis. J. Exp. Med. 2013;210:2939–2950. doi: 10.1084/jem.20130351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xue L, et al. Prostaglandin D2 activates group 2 innate lymphoid cells through chemoattractant receptor-homologous molecule expressed on TH2 cells. J. Allergy Clin. Immunol. 2014;133:1184–1194. doi: 10.1016/j.jaci.2013.10.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Johnston B, Kim CH, Soler D, Emoto M, Butcher EC. Differential chemokine responses and homing patterns of murine TCR alpha beta NKT cell subsets. J. Immunol. 2003;171:2960–2969. doi: 10.4049/jimmunol.171.6.2960. [DOI] [PubMed] [Google Scholar]
- 35.Rajput C, et al. RORα-dependent type 2 innate lymphoid cells are required and sufficient for mucous metaplasia in immature mice. Am. J. Physiol. Lung Cell. Mol. Physiol. 2017;312:L983–L993. doi: 10.1152/ajplung.00368.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Brand S, Sakaguchi T, Gu X, Colgan SP, Reinecker HC. Fractalkine-mediated signals regulate cell-survival and immune-modulatory responses in intestinal epithelial cells. Gastroenterology. 2002;122:166–177. doi: 10.1053/gast.2002.30329. [DOI] [PubMed] [Google Scholar]
- 37.Brand S, et al. CXCR4 and CXCL12 are inversely expressed in colorectal cancer cells and modulate cancer cell migration, invasion and MMP-9 activation. Exp. Cell Res. 2005;310:117–130. doi: 10.1016/j.yexcr.2005.07.006. [DOI] [PubMed] [Google Scholar]
- 38.Brand S, et al. Cell differentiation dependent expressed CCR6 mediates ERK-1/2, SAPK/JNK, and Akt signaling resulting in proliferation and migration of colorectal cancer cells. J. Cell. Biochem. 2006;97:709–723. doi: 10.1002/jcb.20672. [DOI] [PubMed] [Google Scholar]
- 39.Tilton B, et al. Signal transduction by CXC chemokine receptor 4. Stromal cell-derived factor 1 stimulates prolonged protein kinase B and extracellular signal-regulated kinase 2 activation in T lymphocytes. J. Exp. Med. 2000;192:313–324. doi: 10.1084/jem.192.3.313. [DOI] [PMC free article] [PubMed] [Google Scholar]