Abstract
The metabolic control of immune cell development and function has been shown to be critical for the maintenance of immune homeostasis and is also involved in the pathogenesis of immune disorders. Pathogenic infections or cancers may induce metabolic reprogramming through different pathways to meet the energy and metabolite demands for pathogen propagation or cancer progression. In addition, some deregulated metabolites could trigger or regulate immune responses, thus causing chronic inflammation or immune disorders, such as viral infection, cancer and obesity. Therefore, the methods through which metabolism is regulated and the role of metabolic regulation in inflammation and immunity attract much attention. Epigenetic regulation of inflammation and immunity is an emerging field. Long noncoding RNAs (lncRNAs) have been well documented to play crucial roles in many biological processes through diverse mechanisms, including immune regulation and metabolic alternation. Here, we review the functions and mechanisms of lncRNAs in the metabolic regulation of inflammatory immune disorders, aiming to deepen our understanding of the epigenetic regulation of inflammation and immunity.
Introduction
Immunity and metabolism are two of the most fundamental programs in life science. Metabolism is composed of a series of enzymatic biological processes for metabolite transformation that fuel all cellular activities through energy production and providing materials for the synthesis of proteins and nucleic acids. Generally, the metabolic system includes the metabolism of glucose, glutamine, lipids, and hormones (e.g., insulin and glucagon).1 The immune system is responsible for a host sensing dangerous non-self signals and defending against environmental threats, such as invading pathogens, and intrinsic harmful stresses, such as tissue damage-induced sterile inflammation.
Growing evidence shows the vital role of metabolic regulation in immune development and function. For instance, metabolites may interact with epigenetic enzymes to regulate T-cell fates, as was reported recently.2 HIF1α-dependent glycolysis is reduced by glucocorticoid receptor signaling to promote the immune suppressive activity of myeloid-derived suppressor cells (MDSCs) in inflammation-driven hepatic injury.3 Moreover, it has been reported that metabolic alterations are crucial for the regulation of infection-related immune responses and T-cell exhaustion.4, 5 However, metabolism disorders in regional tissues are often associated with or lead to chronic inflammatory states, such as obesity, type 2 diabetes (T2D) and atherosclerosis (AS), which are serious health threats and prevalent public clinical issues.6 Thus, immune responses are extensively correlated with metabolic regulation, and the unbalanced interaction between them could result in immunometabolic dysfunction, leading to the pathogenesis of immune disorders, such as cancers, T2D, and chronic inflammatory autoimmune diseases.7
With advances in a new generation of sequencing techniques and genome-wide transcriptome studies, such as the ENCODE project, more than tens of thousands of noncoding RNAs have been discovered that function as RNA molecules without evident protein-coding capacities. Long noncoding RNAs (lncRNAs) constitute a large catalog of noncoding RNAs with lengths of more than 200 nucleotides and are distinct from other smaller ncRNAs, such as microRNAs (miRNAs), and piwi-interacting RNAs (piRNAs). Similar to mRNAs, lncRNAs are transcribed by RNA polymerase II/III and are primarily modified with a 5’ cap and 3’ polyadenylation. lncRNAs are generally expressed in a cell-specific manner and are highly regulated in response to physiological or pathological signals. lncRNAs have been demonstrated to be indispensable during the development of immune cells and in the regulation of the immune response.8–10 Here, we review the known functional lncRNAs on a case-by-case basis to clarify their functions and the mechanisms through which they are involved in the metabolic regulation of inflammatory responses and immune disorders.
lncRNAs in metabolic regulation during viral infection
The immune system could hardly function without the proper regulation of metabolism for cytokine production, phagocytosis, and pathogen elimination. In contrast, pathogens, such as viruses, also require the host cell metabolism for proliferation and survival. Interestingly, pathogens have developed several strategies to manipulate the host cell metabolism to escape the immune system and to spread. Emerging studies have revealed the underlying mechanisms through which viruses regulate the host cell glutamine metabolism11–13 and glycolysis.14–18 Nevertheless, whether lncRNAs participate in this metabolic regulation for viral survival has been elusive until a recent work demonstrating that a novel lncRNA, lncRNA-ACOD1, was involved in the metabolic regulation of viral infection.19 This study showed that lncRNA-ACOD1 could be induced by viral infection in an interferon regulatory factor 3 (IRF3)/ type I IFN (IFN-I) signaling-independent but NF-κB-dependent pathway. An in vitro or in vivo deficiency in lncRNA-ACOD1 significantly reduces the viral load in macrophages and in immune organs through an IRF3/IFN-I independent pathway. Moreover, lncRNA-ACOD1 directly interacts with glutamic-oxaloacetic transaminase 2 (GOT2), an enzyme involved in amino acid metabolism and tricarboxylic acid (TCA) cycles during viral infection. Consequently, GOT2 catalytic activity is enhanced, along with the increased production of metabolites, to facilitate virus replication and escape from the innate immune response. lncRNA-ACOD1 is suggested to be a potential target for the control of viral infections, as a deficiency in lncRNA-ACOD1 has no influence on cell viability. GOT2 has been demonstrated to be a key regulator of metabolism and viral-induced inflammation through its interaction with lncRNA, indicating that the direct interaction between cytoplasmic lncRNA and proteins is important for the pathogenic invasion and escape from the immune response.
In another study, focusing on chronic hepatic infection with hepatitis C virus (HCV), the lncRNA HOTAIR was suggested to be induced by the HCV core protein, followed by the decreased expression of the Silent information regulator 1 (Sirt1), a histone deacetylase that modulate the glucose- and lipid metabolism-related gene profiles, resulting in metabolic disorders in hepatocytes.20, 21
These studies reveal an important role for lncRNAs, which are utilized by pathogens to alter metabolic pathways, and demonstrate that lncRNAs are actively involved in the interactions between the pathogens and the host. With the Yin–Yang balance theory, we speculate that there must be some host lncRNAs that fight the invading pathogens by regulating the metabolic pathways in immune cells, which remain to be further identified. More recently, the cytoplasmic lnc-Lsm3b was found to be induced by IFN-I during the late stage of the innate immune response. Feedback then disables the innate receptor RIG-I, which senses pathogenic RNA, and consequently terminates the production of IFN-I during the late innate response.22 It is well known that IFN-I can induce metabolic changes in the innate immune cells, determining the outcome of the innate response. Therefore, we hypothesize that other previously unidentified lncRNAs may interact with the major innate receptors and pathways to regulate the pathogenic infection-induced metabolic changes and inflammatory responses.
lncRNAs in metabolic regulation during immunometabolic disorders
As mentioned above, the proper maintenance of the delicate balance between the immune response and metabolism is essential for physiological homeostasis, and a dysfunction of this balance could cause chronic metabolic disorders, such as obesity, T2D, and inflammatory cardiovascular diseases, or autoimmune diseases, such as type 1 diabetes.5, 23 Many studies have shown that lncRNAs play important roles in the metabolic regulation of obesity and T2D, as described below.
lncRNAs in the metabolic regulation of lipid metabolism in obesity and nonalcoholic fatty liver disease
Obesity, a low-grade chronic inflammatory stress, is considered to be the excessive intracellular lipid accumulation that causes the infiltration of immune cells into adipose tissues and the production of proinflammatory cytokines by adipocytes.6 The regulation of lipid metabolism in adipose tissue is necessary to maintain the balance between energy accumulation (white adipose tissue) and energy expenditure (brown adipose tissue).24 Nonalcoholic fatty liver disease (NAFLD) is also highly associated with lipid metabolism and obesity.24 There are several reports focusing on lncRNA-mediated regulation of lipid metabolism, attempting to understand the pathogenesis of these diseases.
Adiponectin (AdipoQ), the hormone expressed in adipocytes, positively regulates glucose and lipid metabolism.24, 25 A recent study revealed that AdipoQ antisense lncRNA (AdipoQ AS), expressed in adipocytes and base paired with the AdipoQ mRNA, attenuates the AdipoQ translation, resulting in the negative regulation of adipogenesis. Therefore, a dysfunction in AdipoQ AS lncRNA increases the obesity threat.26
lncRNA H19 is highly expressed in human chronic liver disease but expressed to a lesser degree in healthy adult livers.27 H19 enhances lipid accumulation in NAFLD by functioning as a fatty acid sensor and reprogramming hepatic metabolism.28 Mechanistically, in NAFLD, H19 RNA interacts with polypyrimidine tract-binding protein 1 (PTBP1), an RNA-binding protein that regulates mRNA stability and splicing,29 and facilitates PTBP1 binding to sterol regulatory element-binding protein 1c (SREBP1c) mRNA and protein, promoting the higher protein expression and increased activity of SREBP1c, which further enhances lipogenic activity.
Another lncRNA, uc.417, transcribed from an ultraconserved region in rodents, impairs thermogenic activity and adipogenesis in brown adipose tissue by inhibiting the phosphorylation of p38 mitogen-activated protein kinase (p38 MAPK).30
From the above cases, it can be observed that lncRNAs are often associated with the functions of different proteins. However, there is less remarkable progress regarding lncRNAs regulation for lipid metabolism, such as lncRNAs in adipocyte differentiation or high-density lipoprotein biogenesis. Therefore, more efforts should be made to explore the function of lncRNAs in obesity or NAFLD.
lncRNAs in the regulation of metabolism in T2D
Obesity is a very strong risk factor for T2D development.31 The clinical symptoms of T2D are glucose and lipid metabolism disorders.32 In addition, diabetic nephropathy (DN) is the most common microvascular complication of diabetes.
Insulin, the hormone produced by pancreatic β cells, controls the glucose level in blood by regulating anabolic metabolism. In addition to lipid metabolism, insulin signaling also has been suggested to be regulated by lncRNA H19.33 Mechanically, in the muscles of humans with T2D, the downregulated expression of H19 results in an increase in the expression of its sponge target, miRNA let-7, followed by a cascade effect in which let-7 represses targets related to glucose metabolism. Therefore, the depletion of H19 in muscle results in impaired insulin signaling and glucose uptake.
In another study, βlinc1 (β-cell long intergenic noncoding RNA 1) was demonstrated be necessary for the function of insulin-producing β cells.34 Knockout of βlinc1 causes glucose intolerance and islet developmental defects in mice, due to βlinc1 regulating a set of related transcription factors (TFs).
In DN, the expression level of peroxisome proliferator-activated receptor γ (PPARγ) coactivator α (PGC-1α, encoded by Ppargc1a) is decreased, and PGC-1α plays an indispensable role in mitochondrial bioenergetics.35, 36 Recently, PGC-1α was found to be rescued by the overexpression of the lncRNA taurine-upregulated gene 1 (Tug1), with improvements in mitochondrial bioenergetics in the podocytes of DN mice.37 tug1 binds to the promoter of Ppargc1a and enhances Ppargc1a transcriptional activity to regulate energy metabolism in the mitochondria of podocytes.37
Taken together, the expression levels of lncRNAs are closely associated with insulin resistance and pancreatic β-cell dysfunction. In perspective, insulin or glucagon may control the metabolic pathways by modulating the expression patterns of lncRNAs. Therefore, studying hormone-regulated lncRNAs would be beneficial.
lncRNAs in the metabolic regulation of cancer
Cancer is characterized by rapid and uncontrolled proliferation, with increasing energy demands. Thus, a reprogrammed metabolic system is necessary to meet the energy demands for cancer cell survival and proliferation. A series of lncRNAs have been shown to be involved in metabolic reprogramming in cancers.
SAMMSON, a lncRNA located near the melanoma-specific oncogene microphthalmia-associated transcription factor (MITF), can be detected in >90% of both human primary and metastatic melanomas. Knockdown of SAMMSON robustly reduced the melanoma cell viability and mitochondrial activity.38 Mechanically, SAMMSON interacts with p32 and promotes its mitochondrial localization. In turn, p32 is involved in the maturation of 16S rRNA and the bioenergetics in mitochondria necessary to survive the melanoma.39–41
Under energy stress conditions, AMP-activated protein kinase (AMPK) primarily functions as a metabolic switch to reprogram metabolism, reducing anabolic activities and enhancing catabolic activities in cells.42, 43 Therefore, this results in AMPK activation serving to inhibit tumor development. In addition, the lncRNA NBR2 (neighbor of BRCA1 gene 2) is induced by energy stress through the liver kinase B1 (LKB1)-AMPK pathway and interacts with AMPK to promote AMPK kinase activity, forming a feed-forward loop. Consequently, a deficiency in the lncRNA NBR2 weakens the activation of AMPK resulting in enhanced tumor development in vivo.44 The low expression level of NBR2 could be a biomarker for poor tumor prognosis in some human cancers.
As mentioned above, glutamine metabolism is believed to be an essential energy-producing metabolic pathway. Glutamine is deaminated by glutaminase (GLS) to produce glutamate, the substrate for the TCA cycle and other metabolic pathways that generate ATP. However, glutamine metabolism is often altered in cancers. A lncRNA, named colon cancer-associated transcripts 2 (CCAT2), has been reported to be associated with high risk for multiple types of cancer.45, 46 CCAT2 has been demonstrated to regulate glutamine metabolism in an allele-specific manner.47 The CCAT2 alleles interact with the cleavage factor I (CFIm) complex with distinct affinities to regulate the alternative splicing of GLS. Briefly, the CCAT2 G allele promotes the production of GAC (glutaminase isoform C), a GLS isoform with higher catalytic activity, to supply the production of more glutamate for the TCA cycle. Conversely, the production of KGA (glutaminase kidney isoform) is dominant when the CCAT2 T allele interacts with the CFIm complex. It is very interesting that lncRNA alleles could differentially regulate metabolism in cancers.
There are many other lncRNAs that have been reported to have functions in cancer metabolism, such as FLINC1 (FoxO-induced lncRNA 1), which inhibits c-Myc-mediated energy metabolism to restrain tumor development in renal cancer,48 PCGEM1 (prostate cancer gene expression marker 1), which serves as a coactivator for c-Myc to promote the reprogramming of tumor metabolism by affecting multiple metabolic pathways in prostate cancer,49 lncRNA IDH1-AS1, through which c-Myc activates the Warburg effect (aerobic glycolysis in tumors) in cancer cells,50 and lnc-IGFBP4-1, whose overexpression could enhance the aerobic glycolysis rate in lung cancer.51
Together, these examples indicate the crucial roles of lncRNAs in the regulation of cancer metabolism. It is worth mentioning that the case of CCAT2 alleles implies that lncRNAs with different alleles may exert different functions in diverse states. In addition, the mechanisms through which lncRNAs participate in the regulation of the chronic inflammation that contributes to carcinogenesis and cancer metastasis requires more investigation.
Concluding remarks
The development and function of the immune system is regulated by metabolic factors. In turn, the immune molecules could also alter the metabolism of tissues and cells during the inflammatory response to affect the progression of metabolic disorders. Here, we have summarized the progress on the functional lncRNAs in the immunometabolism and immunometabolic disorders, emphasizing the roles of lncRNAs in the metabolic regulation of inflammation and immune disorders (Table 1). As shown above, lncRNAs can regulate inflammation and innate immunity by targeting various metabolic pathways in different manners (Fig. 1), functioning through cis-regulation (e.g., βlinc134) antisense inhibition (e.g., AdipoQ AS26 and IDH1-AS150) interaction with proteins (e.g., lncRNA-ACOD1,20 SAMMSON,37 and NBR243) or miRNA sponges (e.g., H19.33) Furthermore, changes in lncRNAs expression are extremely valuable as potential biomarkers for predicting the prognosis of related diseases, and data on the genome-wide analysis of lncRNAs expression is essential for functional screening. For mechanistic studies of functional lncRNAs, the interactions between lncRNAs and proteins (especially RNA-binding proteins) should receive more attention, in addition to the well-known models involving sequence-based mechanisms, such as miRNA sponges and antisense inhibition. Hopefully, more functional lncRNAs will be identified in the regulation of metabolism, inflammation and immunity, contributing to a better understanding of health and disease by serving as potential biomarkers and targets for the control of immunometabolic dysfunction.
Table 1.
lncRNAs in metabolic and inflammatory diseases
| Disease | lncRNA | Biological function | Target | Reference |
|---|---|---|---|---|
| Viral infection | lncRNA-ACOD1 | Promotes viral replication by enhancing GOT2 activity. | GOT2 | 19 |
| HOTAIR | Promotes glucose and lipid metabolism in hepatocytes | Sirt | 21 | |
| Obesity | AdipoQ AS | Down-regulates adipogenesis by attenuating AdipoQ translation. | AdipoQ | 26 |
| H19 | Enhances lipid accumulation in NAFLD. | PTBP1 | 28 | |
| uc.417 | Impairs thermogenesis and adipogenesis in brown adipose tissue. | p38 MAPK | 30 | |
| T2D | H19 | Promotes insulin signaling and glucose uptake. | let-7 | 31 |
| βlinc1 | Necessary for the function of insulin-producing β cells. | A set of TFs | 34 | |
| Tug1 | Promotes Ppargc1a transcription to regulate energy metabolism. | Ppargc1a | 35 | |
| Gm10768 | Activates hepatic gluconeogenesis. | miR-214 | 52 | |
| Lethe | Inhibits the production of reactive oxygen species (ROS). | p65-NF- κB | 53 | |
| AS | MeXis | Promotes macrophage cholesterol efflux by enhancing Abca1 transcription. | DDX17 | 54 |
| Cancer | SAMMSON | Enhances the bioenergetics in mitochondria to survive melanoma. | p32 | 38 |
| NBR2 | Promotes AMPK kinase activity to inhibit tumor development. | AMPK | 44 | |
| CCAT2 | Regulates glutamine metabolism in an allele-specific manner. | CFIm | 47 | |
| FLINC1 | Inhibits c-Myc-mediated energy metabolism to restrain tumor development in renal cancer. | AUF1 | 48 | |
| PCGEM1 | Serves as a coactivator of c-Myc to promote multiple metabolic pathways in prostate cancer. | c-Myc | 49 | |
| IDH1-AS1 | Inhibits the Warburg effect. | IDH1 | 50 | |
| lnc-IGFBP4-1 | Enhances aerobic glycolysis in lung cancer. | IGFBP4 | 51 | |
| UCA1 | Promotes mitochondrial function in bladder cancer. | ARL2 | 55 |
Fig. 1. lncRNAs mediate the metabolic regulation of inflammation and immune disorders by targeting different metabolic pathways.
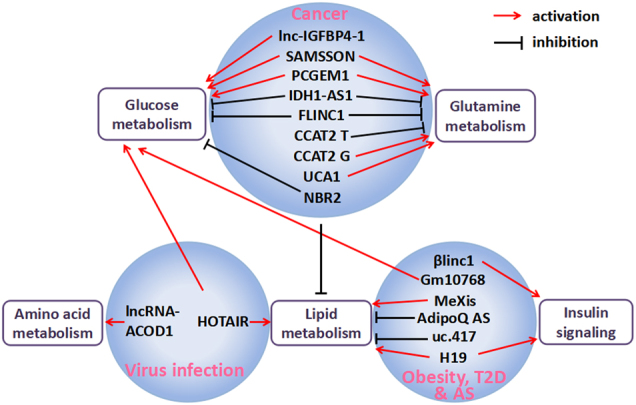
lncRNAs discovered in viral infections (e.g., lncRNA-ACOD1 and HOTAIR), obesity, T2D, AS (e.g., βlinc1, Gm10768, MeXis, AdipoQ AS, uc.417, and H19), and cancer (e.g., lnc-IGFBP4-1, SAMSSON, PCGEM1, IDH1-AS1, FLINC1, CCAT2, UCA1, and NBR2) target different metabolic pathways, promoting (labeled with a red arrow) or inhibiting (labeled with a black bar) biological processes
Acknowledgements
This work was supported by grants from the National Natural Science Foundation of China (81788101, 91542000) and CAMS Innovation Fund for Medical Science (2016-I2M-1-003).
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Redis RS, Calin GA. SnapShot: non-coding RNAs and Metabolism. Cell Metab. 2017;25:220–220. doi: 10.1016/j.cmet.2016.12.012. [DOI] [PubMed] [Google Scholar]
- 2.Zhang X, Liu J, Cao X. Metabolic control of T-cell immunity via epigenetic mechanisms. Cell Mol. Immunol. 2018;15:203–205. doi: 10.1038/cmi.2017.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lu, Y. et al. Glucocorticoid receptor promotes the function of myeloid-derived suppressor cells by suppressing HIF1alpha-dependent glycolysis. Cell Mol. Immunol. (2017) [Epub ahead of print]. [DOI] [PMC free article] [PubMed]
- 4.Pearce EL, Pearce EJ. Metabolic pathways in immune cell activation and quiescence. Immunity. 2013;38:633–643. doi: 10.1016/j.immuni.2013.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McKinney EF, Smith KGC. Metabolic exhaustion in infection, cancer and autoimmunity. Nat. Immunol. 2018;19:213–221. doi: 10.1038/s41590-018-0045-y. [DOI] [PubMed] [Google Scholar]
- 6.Hotamisligil GS. Inflammation and metabolic disorders. Nature. 2006;444:860–867. doi: 10.1038/nature05485. [DOI] [PubMed] [Google Scholar]
- 7.Cairns RA, Harris IS, Mak TW. Regulation of cancer cell metabolism. Nat. Rev. Cancer. 2011;11:85–95. doi: 10.1038/nrc2981. [DOI] [PubMed] [Google Scholar]
- 8.Atianand MK, et al. A long noncoding RNA lincRNA-EPS acts as a transcriptional brake to restrain inflammation. Cell. 2016;165:1672–1685. doi: 10.1016/j.cell.2016.05.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Guo CJ, Zhang W, Gershwin ME. Long noncoding RNA lncKdm2b: a critical player in the maintenance of group 3 innate lymphoid cells. Cell. Mol. Immunol. 2018;15:5–7. doi: 10.1038/cmi.2017.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang P, et al. The STAT3-binding long noncoding RNA lnc-DC controls human dendritic cell differentiation. Science. 2014;344:310–313. doi: 10.1126/science.1251456. [DOI] [PubMed] [Google Scholar]
- 11.Mazzon M, Castro C, Roberts LD, Griffin JL, Smith GL. A role for vaccinia virus protein C16 in reprogramming cellular energy metabolism. J. Gen. Virol. 2015;96:395–407. doi: 10.1099/vir.0.069591-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fontaine KA, Camarda R, Lagunoff M. Vaccinia virus requires glutamine but not glucose for efficient replication. J. Virol. 2014;88:4366–4374. doi: 10.1128/JVI.03134-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Thai M, et al. MYC-induced reprogramming of glutamine catabolism supports optimal virus replication. Nat. Commun. 2015;6:8873. doi: 10.1038/ncomms9873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fontaine KA, Sanchez EL, Camarda R, Lagunoff M. Dengue virus induces and requires glycolysis for optimal replication. J. Virol. 2015;89:2358–2366. doi: 10.1128/JVI.02309-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ripoli M, et al. Hepatitis C virus-linked mitochondrial dysfunction promotes hypoxia-inducible factor 1 alpha-mediated glycolytic adaptation. J. Virol. 2010;84:647–660. doi: 10.1128/JVI.00769-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Thai M, et al. Adenovirus E4ORF1-induced MYC activation promotes host cell anabolic glucose metabolism and virus replication. Cell Metab. 2014;19:694–701. doi: 10.1016/j.cmet.2014.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vastag L, Koyuncu E, Grady SL, Shenk TE, Rabinowitz JD. Divergent effects of human cytomegalovirus and herpes simplex virus-1 on cellular metabolism. PLoS. Pathog. 2011;7:e1002124. doi: 10.1371/journal.ppat.1002124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mazzon M, et al. Alphavirus-induced hyperactivation of PI3K/AKT directs pro-viral metabolic changes. PLoS Pathog. 2018;14:e1006835. doi: 10.1371/journal.ppat.1006835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang P, Xu J. An interferon-independent lncRNA promotes viral replication by modulating cellular metabolism. Science. 2017;358:1051–1055. doi: 10.1126/science.aao0409. [DOI] [PubMed] [Google Scholar]
- 20.Perlemuter G, et al. Hepatitis C virus core protein inhibits microsomal triglyceride transfer protein activity and very low density lipoprotein secretion: a model of viral-related steatosis. FASEB J. 2002;16:185–194. doi: 10.1096/fj.01-0396com. [DOI] [PubMed] [Google Scholar]
- 21.Li, Z. Q. et al. Hepatitis C virus core protein impairs metabolic disorder of liver cell via HOTAIR-Sirt1 signalling. Biosci. Rep.36, e00336 (2016). [DOI] [PMC free article] [PubMed]
- 22.Jiang M, Zhang S, et al. Self-recognition of an inducible host lncRNA by RIG-I feedback restricts innate immune response. Cell. 2018;173:906–919. doi: 10.1016/j.cell.2018.03.064. [DOI] [PubMed] [Google Scholar]
- 23.Sorini C, Cosorich I, Falcone M. New therapeutic perspectives in Type 1 Diabetes: dietary interventions prevent beta cell-autoimmunity by modifying the gut metabolic environment. Cell. Mol. Immunol. 2017;14:951–953. doi: 10.1038/cmi.2017.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Losko M, Kotlinowski J, Jura J. Long noncoding RNAs in metabolic syndrome related disorders. Mediat. Inflamm. 2016;2016:5365209. doi: 10.1155/2016/5365209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Marchesini G, et al. Nonalcoholic fatty liver disease: a feature of the metabolic syndrome. Diabetes. 2001;50:1844–1850. doi: 10.2337/diabetes.50.8.1844. [DOI] [PubMed] [Google Scholar]
- 26.Cai R, et al. Adiponectin AS lncRNA inhibits adipogenesis by transferring from nucleus to cytoplasm and attenuating Adiponectin mRNA translation. Biochim. Biophys. Acta. 2018;1863:420–432. doi: 10.1016/j.bbalip.2018.01.005. [DOI] [PubMed] [Google Scholar]
- 27.Zhang Y, et al. Bcl2 is a critical regulator of bile acid homeostasis by dictating Shp and lncRNA H19 function. Sci. Rep. 2016;6:20559. doi: 10.1038/srep20559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu C, et al. lncRNA H19 interacts with polypyrimidine tract-binding protein 1 to reprogram hepatic lipid homeostasis. Hepatology. 2018;67:1768–1783. doi: 10.1002/hep.29654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rotman Y, Sanyal AJ. Current and upcoming pharmacotherapy for non-alcoholic fatty liver disease. Gut. 2017;66:180–190. doi: 10.1136/gutjnl-2016-312431. [DOI] [PubMed] [Google Scholar]
- 30.Cui X, et al. A transcribed ultraconserved noncoding RNA, uc.417, serves as a negative regulator of brown adipose tissue thermogenesis. FASEB J. 2016;30:4301–4312. doi: 10.1096/fj.201600694R. [DOI] [PubMed] [Google Scholar]
- 31.Kahn SE, Hull RL, Utzschneider KM. Mechanisms linking obesity to insulin resistance and type 2 diabetes. Nature. 2006;444:840–846. doi: 10.1038/nature05482. [DOI] [PubMed] [Google Scholar]
- 32.Goyal N, Kesharwani D, Datta M. Lnc-ing non-coding RNAs with metabolism and diabetes: Roles of lncRNAs. Cell Mol. Life Sci. 2018;75:1827–1837. doi: 10.1007/s00018-018-2760-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gao Y, et al. The H19/let-7 double-negative feedback loop contributes to glucose metabolism in muscle cells. Nucleic Acids Res. 2014;42:13799–13811. doi: 10.1093/nar/gku1160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Arnes L, Akerman I, Balderes DA, Ferrer J, Sussel L. βlinc1 encodes a long noncoding RNA that regulates islet β-cell formation and function. Genes Dev. 2016;30:502–507. doi: 10.1101/gad.273821.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang W, et al. Mitochondrial fission triggered by hyperglycemia is mediated by ROCK1 activation in podocytes and endothelial cells. Cell Metab. 2012;15:186–200. doi: 10.1016/j.cmet.2012.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Guo K, et al. Protective role of PGC-1αin diabetic nephropathy is associated with the inhibition of ROS through mitochondrial dynamic remodeling. PLoS ONE. 2015;10:e0125176. doi: 10.1371/journal.pone.0125176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Long J, et al. Long noncoding RNA Tug1 regulates mitochondrial bioenergetics in diabetic nephropathy. J. Clin. Invest. 2016;126:4205–4218. doi: 10.1172/JCI87927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Leucci E, et al. Melanoma addiction to the long non-coding RNA SAMMSON. Nature. 2016;531:518–522. doi: 10.1038/nature17161. [DOI] [PubMed] [Google Scholar]
- 39.Fogal V, et al. Mitochondrial p32 protein is a critical regulator of tumor metabolism via maintenance of oxidative phosphorylation. Mol. Cell Biol. 2010;30:1303–1318. doi: 10.1128/MCB.01101-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yagi M, et al. p32/gC1qR is indispensable for fetal development and mitochondrial translation: importance of its RNA-binding ability. Nucleic Acids Res. 2012;40:9717–9737. doi: 10.1093/nar/gks774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Muta T, Kang D, Kitajima S, Fujiwara T, Hamasaki N. p32 protein, a splicing factor 2-associated protein, is localized in mitochondrial matrix and is functionally important in maintaining oxidative phosphorylation. J. Biol. Chem. 1997;272:24363–24370. doi: 10.1074/jbc.272.39.24363. [DOI] [PubMed] [Google Scholar]
- 42.Hardie DG, Schaffer BE, Brunet A. AMPK: An energy-sensing pathway with multiple inputs and outputs. Trends Cell Biol. 2016;26:190–201. doi: 10.1016/j.tcb.2015.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shackelford DB, Shaw RJ. The LKB1-AMPK pathway: metabolism and growth control in tumour suppression. Nat. Rev. Cancer. 2009;9:563–575. doi: 10.1038/nrc2676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Liu X, et al. LncRNA NBR2 engages a metabolic checkpoint by regulating AMPK under energy stress. Nat. Cell Biol. 2016;18:431–442. doi: 10.1038/ncb3328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tomlinson I, et al. A genome-wide association scan of tag SNPs identifies a susceptibility variant for colorectal cancer at 8q24.21. Nat. Genet. 2007;39:984–988. doi: 10.1038/ng2085. [DOI] [PubMed] [Google Scholar]
- 46.Tuupanen S, et al. The common colorectal cancer predisposition SNP rs6983267 at chromosome 8q24 confers potential to enhanced Wnt signaling. Nat. Genet. 2009;41:885–890. doi: 10.1038/ng.406. [DOI] [PubMed] [Google Scholar]
- 47.Redis RS, et al. Allele-specific reprogramming of cancer metabolism by the Long non-coding RNA CCAT2. Mol. Cell. 2016;61:640. doi: 10.1016/j.molcel.2016.02.006. [DOI] [PubMed] [Google Scholar]
- 48.Xiao ZD, et al. Energy stress-induced lncRNA FILNC1 represses c-Myc-mediated energy metabolism and inhibits renal tumor development. Nat. Commun. 2017;8:783. doi: 10.1038/s41467-017-00902-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hung CL, et al. A long noncoding RNA connects c-Myc to tumor metabolism. Proc. Natl Acad. Sci. USA. 2014;111:18697–18702. doi: 10.1073/pnas.1415669112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Xiang S, et al. LncRNA IDH1-AS1 links the functions of c-Myc and HIF1α via IDH1 to regulate the Warburg effect. Proc. Natl Acad. Sci. USA. 2018;115:E1465–E1474. doi: 10.1073/pnas.1711257115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yang B, et al. Overexpression of lncRNA IGFBP4-1 reprograms energy metabolism to promote lung cancer progression. Mol. Cancer. 2017;16:154. doi: 10.1186/s12943-017-0722-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cui X, et al. The long non-coding RNA Gm10768 activates hepatic gluconeogenesis by sequestering microRNA-214 in mice. J. Biol. Chem. 2018;293:4097–4109. doi: 10.1074/jbc.M117.812818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zgheib C, Hodges MM, Hu J, Liechty KW, Xu J. Long non-coding RNA Lethe regulates hyperglycemia-induced reactive oxygen species production in macrophages. PLoS ONE. 2017;12:e0177453. doi: 10.1371/journal.pone.0177453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sallam T, Jones M, Thomas BJ, et al. Transcriptional regulation of macrophage cholesterol efflux and atherogenesis by a long noncoding RNA. Nat. Med. 2018;24:304–312. doi: 10.1038/nm.4479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Li HJ, et al. LncRNA UCA1 promotes mitochondrial function of bladder cancer via the MiR-195/ARL2 signaling pathway. Cell. Physiol. Biochem. 2017;43:2548–2561. doi: 10.1159/000484507. [DOI] [PubMed] [Google Scholar]


