Abstract
The cross-talk between cellular lipid metabolism and the innate immune responses remains obscure. In addition to presenting lipid antigens to Natural Killer T-cells (NKT cells), the Cluster of Differentiation 1D Glycoprotein (CD1d) might mediate reverse signaling in antigen-presenting cells (APCs). Here we found CD1d deficiency attenuated Toll-like receptor (TLR)-triggered inflammatory innate responses in macrophages and dendritic cells, protecting mice from endotoxin shock. TLR activation in macrophages induced metabolic changes of glycosphingolipids (GSLs), among which glycolipid isoglobotrihexosylceramide (iGb3) was rapidly produced. The endogenously generated iGb3 bound CD1d in endosomal compartments and then synergized with the initially activated TLR signal to induce Tyr332 phosphorylation of CD1d intracellular domain. This led to the recruitment and activation of proline-rich tyrosine kinase 2 (Pyk2). Pyk2 interacted with IκB kinase β (IKKβ) and TANK-binding kinase 1 (TBK1), and enhanced tyrosine phosphorylation of Tyr188/199 of IKKβ and Tyr179 of TBK1 and thus, their activation to promote full activation of TLR signaling. Thus, intracellular CD1d reverse signaling, triggered by endogenous iGb3, amplifies inflammatory innate responses in APCs. Our findings identify a non-canonical function of CD1d reverse signaling activated by lipid metabolite in the innate immune response.
Subject terms: Toll-like receptors, Lipid signalling
Introduction
The close connection between immunity and metabolism has attracted attention in recent years.1–3 These two physiological systems display bidirectional communication and coordination. On the one hand, immune cells participate in the metabolism of the host.4 Multiple kinds of immune cells, including macrophages, dendritic cells (DCs), T cells and B cells, have been identified as critical effector cells in the initiation of chronic tissue inflammation and pathophysiology of many metabolic diseases that include pathological obesity and type 2 diabetes.5 On the other hand, cellular metabolic changes can regulate the function and fate of immune cells.6,7 Toll-like receptor (TLR) activation in DCs results in a remarkable metabolic transformation in glycolysis that is crucial for DC activation and survival.8,9 Activation of TLR signaling in macrophages also induces significant remodeling of the composition of lipids and their subcellular location.10,11 These bioactive lipid mediators participate in the inflammation and immune responses. For example, prostaglandin E2 and 25-hydroxycholesterol produced by macrophages inhibit or enhance anti-viral immunity respectively.12,13 Genetic evidence indicates that β-glucosylceramide-derived glycosphingolipids (GSLs) are natural lipid ligands that are critical for the function of NKT cells.14,15 Altered GSL profiles are found during TLR9-mediated maturation of DCs, also required for the activation of NKT cells.16,17 However, the cross-talk between the metabolic dynamics of cellular lipid and innate immune responses remains to be fully understood.
The CD1d molecule is a major histocompatibility complex (MHC) class I-like transmembrane glycoprotein.18 In contrast to the MHC class I and class II molecules that present peptide antigens, the CD1d molecule primarily presents lipid antigens to CD1d-restricted NKT cells.19 Lipid antigens presented by CD1d include both exogenous and endogenous glycolipid antigens. For example, isoglobotrihexosylceramide (iGb3), a neutral GSL, has been identified as a potential endogenous lipid antigen presented by CD1d to human and mouse NKT cells.14,15
Unlike other members of the CD1 family, which show an inducible expression pattern after activation by pathogens, cytokines or other stimuli, the CD1d protein is constitutively expressed in antigen-presenting cells (APCs) and therefore better meets the function of an innate immune receptor.20 Increasing evidence has shown that CD1d is involved in the pathogenesis of autoimmune diseases, as well as influencing the outcome of infections by microbes such as bacteria, viruses, fungi and parasites.21 Most of these studies focus on the classical role of CD1d to activate NKT cells. However, apart from the well-known classical function of lipid antigen presentation, CD1d also exerts some non-classical biological functions independent of presenting lipid antigen.22–25 The specific antibody cross-linking of CD1d on intestinal epithelial cells can induce STAT3 (Signal transducer and activator of transcription 3)-dependent IL-10 and HSP110 production to elicit the protective mucosal immunity.22,23 Direct ligation of CD1d by specific antibodies on APCs also stimulates IL-12 release both in vitro and in vivo upon infection, which is correlated with rapid phosphorylation of IκB and activation of NF-κB.24,25 These findings imply that CD1d may be able to initiate its intracellular reverse signaling upon ligation by specific antibody. Since multiple endogenous lipid antigens can be presented by CD1d, it is possible that certain kinds of endogenous natural lipids could be recognized by CD1d to trigger a physiological CD1d reverse pathway.
Toll-like receptors (TLRs), pattern recognition receptors expressed in APCs, have critical roles in detecting invading pathogens and initiating innate immune responses.26–28 Our previous studies have demonstrated the reverse signaling mediated by MHC class II or MHC class I promotes or inhibits TLR-triggered innate immune responses respectively.29,30 Nevertheless, the non-classical roles of lipid antigen-presenting molecule CD1d in innate immunity and inflammatory responses need to be explored.
Here we find TLR activation induces metabolic changes of GSLs in macrophages, among which generation of iGb3 is significant. Our data demonstrate that iGb3 acts as an innate lipid effector to bind CD1d in endosomes and activates intracellular CD1d reverse signaling to promote the full activation of TLR-triggered innate immune responses.
Results
CD1d deficiency attenuates TLR-triggered innate immune responses
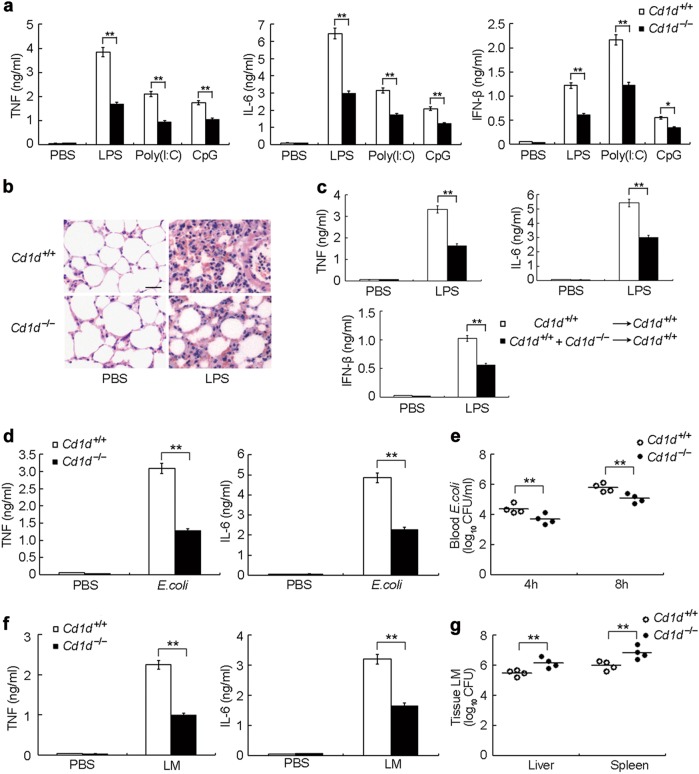
We first investigated the effect of CD1d deficiency on innate immunity utilizing Cd1d–/– mice. This revealed CD1d deficiency had no influence on myeloid development, macrophage differentiation and TLR expression in macrophages (Supplementary information, Fig. S1a-d). Macrophages from Cd1d+/+ or Cd1d–/– mice showed similar responses to IFN-α or IFN-γ stimulation (Supplementary information, Fig. S1e, f). We then challenged Cd1d–/– and control littermate Cd1d+/+ mice with different TLR ligands, including lipopolysaccharide (LPS), Poly(I:C) or CpG oligodeoxynucleotide (ODN). We found that Cd1d–/– mice produced significantly less TNF, IL-6 and IFN-β than Cd1d+/+ mice did (Fig. 1a). Less infiltration of inflammatory cells was found in the lungs of Cd1d–/– mice after LPS challenge (Fig. 1b). Given the deficiency of NKT cells in Cd1d−/− mice, we performed reconstitution experiments to exclude the impact of this deficiency. Lethally irradiated wild type mice were reconstituted with a 1:1 mixture of bone marrow cells from Cd1d+/+ and Cd1d–/– mice. These reconstituted chimeric mice developed NKT cells just as well as mice reconstituted with Cd1d+/+ bone marrow cells alone (Supplementary information, Fig. S1g) but comprising both Cd1d+/+ and Cd1d–/– APCs. Due to the loss of CD1d in part of the APC population, LPS-induced production of TNF, IL-6 and IFN-β was also lower in chimeric mice than in mice reconstituted with Cd1d+/+ bone marrow cells alone (Fig. 1c). We then challenged Cd1d–/– and Cd1d+/+ mice with Gram-negative E. coli or Gram-positive Listeria monocytogenes. Upon E. coli infection, CD1d-deficient mice produced significantly less TNF and IL-6 in their sera (Fig. 1d). Accordingly, the blood load of E. coli was reduced in CD1d-deficient mice (Fig. 1e). After infection with Listeria monocytogenes, the production of TNF and IL-6 was also lower in CD1d-deficient mice (Fig. 1f). Moreover, the CD1d deficiency resulted in higher bacterial loads in the liver and spleen (Fig. 1g). Similarly, Cd1d–/– peritoneal macrophages showed greater bacterial loads upon Listeria monocytogenes infection (Supplementary information, Fig. S1h). These data are consistent with the previous finding that proinflammatory cytokines promote the dissemination of E. coli and clearance of Listeria monocytogenes.30,31 Together, the above data suggest that CD1d has an important contribution to host innate immune responses.
Fig. 1.
CD1d deficiency protects mice from challenge with TLR ligands and pathogen infection. a ELISA of TNF, IL-6 and IFN-β in sera of Cd1d+/+ and Cd1d–/– mice (n = 5 per group) 2 h after intraperitoneal injection of LPS, Poly(I:C) or CpG-ODN (CpG). b HE staining of lungs from Cd1d+/+ and Cd1d–/– mice 8 h after intraperitoneal challenge with LPS. Scale bar, 20 μm. c Wild-type mice (n = 6 per group) were lethally irradiated and then adoptively transferred with bone marrow cells from Cd1d+/+ mice or with a 1:1 mixture of bone marrow cells from Cd1d+/+ and Cd1d–/– mice. Eight weeks later, ELISA of serum cytokines were performed 2 h after intraperitoneal injection with LPS. d ELISA of serum cytokines from Cd1d+/+ and Cd1d–/– mice (n = 4 per group) 4 h after intraperitoneal infection with 1 × 107 E. coli. e Blood bacteria load 4 h or 8 h after infection as in (d). f ELISA of serum cytokines from Cd1d+/+ and Cd1d–/– mice (n = 4 per group) 4 h after infected with 1 × 104 Listeria monocytogenes. g Bacteria loads in livers and spleens from Cd1d+/+ and Cd1d–/– mice 2 days after infection as in (f). Quantitative data are presented as the mean ± SEM. *P < 0.05 and **P < 0.01 (Student’s t-test)
Impaired cytokine production in TLR-triggered CD1d-deficient APCs
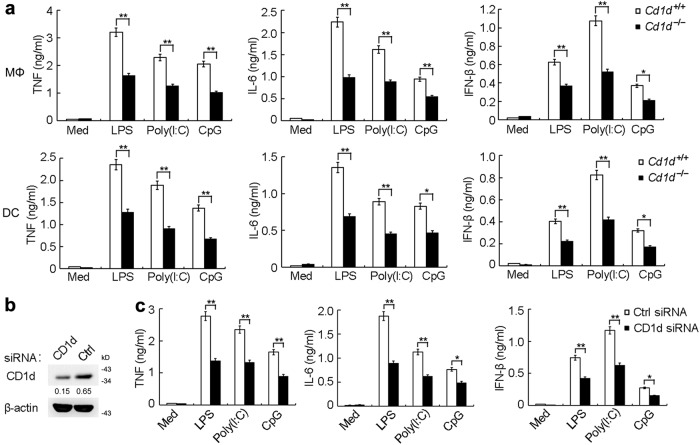
Consistent with the above in vivo observations, we found that the TLR-triggered production of TNF, IL-6 and IFN-β was markedly impaired in both peritoneal macrophages and bone marrow-derived DCs from Cd1d–/– mice (Fig. 2a). The silencing of CD1d also significantly inhibited cytokine production in peritoneal macrophages stimulated with TLR ligands (Fig. 2b, c). Furthermore, CD1d silencing also led to decreased cytokine production in human monocyte-derived DCs (Supplementary information, Fig. S2). These data suggest that the CD1d molecule can promote TLR-triggered production of proinflammatory cytokines and type I interferon (IFN).
Fig. 2.
CD1d deficiency attenuates TLR-triggered production of proinflammatory cytokines and type I IFN in macrophages and DCs. a ELISA of cytokines in supernatants of peritoneal macrophages or BMDCs from Cd1d+/+ and Cd1d–/– mice either unstimulated (Med) or stimulated with LPS (100 ng/mL), Poly(I:C) (10 μg/mL) or CpG (0.3 μM) for 6 h. b Immunoblot of CD1d expression in macrophages 48 h after transfection with control siRNA (Ctrl) or CD1d siRNA. c ELISA of cytokines in supernatants of macrophages transfected with siRNA as in b, followed by stimulation with LPS, Poly(I:C) or CpG for 6 h. Data are from three independent experiments (a, c; mean ± SEM) or are representative of three independent experiments with similar results (b). *P < 0.05 and **P < 0.01 (Student’s t-test)
TLR activation induces endogenous production of glycosphingolipid iGb3
Previous studies have shown that CD1d ligation by antibodies in monocytes and epithelial cells can activate intracellular signaling and elicit physiological responses.22,24 Given that the CD1d molecule promoted TLR-triggered cytokine production in APCs, we hypothesized whether CD1d reverse signaling could be activated by endogenously generated natural ligands induced by TLR ligation. Since GSLs have been reported to be the natural ligands for NKT cells that can be presented by CD1d, we assessed GSL profiles in macrophages with or without TLR ligand stimulation. To gain a clear and precise view of GSL profiles in macrophages, we used electrospray ionization linear-ion-trap mass spectrometry (ESI-LIT-MS) techniques. The major GSLs identified in cell lysates from mouse peritoneal macrophages were: GlcCer, LacCer, Gb3/iGb3, Lc3 and Gb4/iGb4/nlc4 (Supplementary information, Table S1). Representative ESI-LIT-MS profiles for major GSLs are presented in Supplementary information, Figure S3a. To study whether TLR activation would influence GSL profiles, mouse macrophages were stimulated with LPS or Poly(I:C) for different times and the ratio of GSLs in TLR-activated macrophages was compared to that in untreated macrophages. We found that the percentage of LacCer, Lc3 or Gb4/iGb4/nlc4 in total GSLs did not change after LPS stimulation. However, we found increased relative abundance of GlcCer (1.2 fold) or Gb3/iGb3 (1.6 fold) in LPS-stimulated macrophages (Supplementary information, Fig. S3a and Table S2).
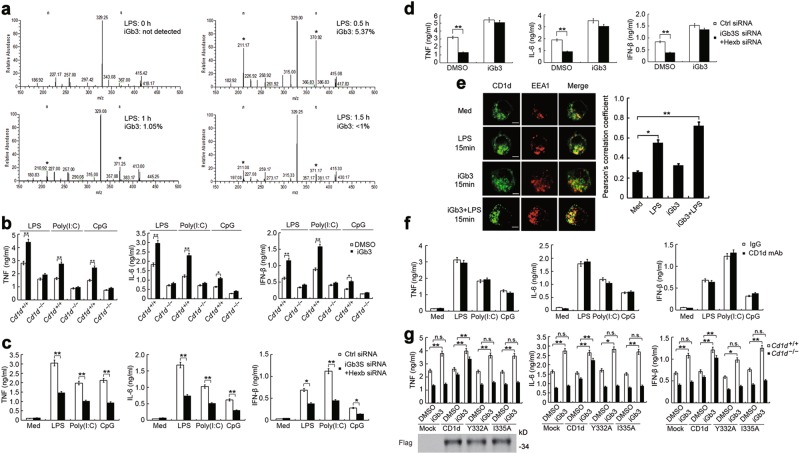
We then analyzed the expression pattern of individual GSLs with the more sensitive linear ion-trap mass spectrometry. Of the two molecular species of regioisomers of trihexosylceramide (iGb3 and Gb3) observed in MS1, iGb3 was present at less than 1% of the total level of trihexosylceramide ions32 and was difficult to detect. However, it is reported that expression of iGb3 can be detected and measured from characteristic ions produced in MS4 analysis of selected ions.16 After selecting m/z 1215 (representing regioisomers of iGb3/Gb3) for MS4, iGb3 (specific ions m/z 211 and m/z 371) was undetectable in untreated macrophages but significantly increased in macrophages stimulated with LPS. The latter cells showed rapid production of iGb3 within 15 minutes and the most significant increase after 30 minutes (Fig. 3a and Supplementary information, Fig. S3b). We made similar findings in Poly(I:C)-stimulated macrophages (Supplementary information, Fig. S3c). Together these results indicate that iGb3 is specially generated in macrophages after TLR activation.
Fig. 3.
Glycolipid iGb3 promotes TLR-triggered production of inflammatory cytokines via intracellular CD1d. a GSLs from unstimulated or LPS-stimulated macrophages were extracted, permethylated and analyzed by linear ion-trap MSn analysis. The positive ion ESI-LIT-MS4 spectras (m/z 1215 → 667 → 445 → ) of permethylated GSLs were shown. The relative quantities of iGb3 in the molecular precursor m/z 1215 in macrophages were determined. Signature ions (× 10) generated from iGb3 were indicated as ‘*’. b ELISA of cytokines in supernatants of macrophages from Cd1d+/+ and Cd1d–/– mice pretreated with iGb3 and then stimulated with LPS, Poly(I:C) or CpG for 6 h. c ELISA of cytokines in supernatants of macrophages transfected with Ctrl siRNA or iGb3S plus Hexb siRNA, and 48 h later, followed by stimulation with LPS, Poly(I:C) or CpG for 6 h. d ELISA of cytokines in supernatants of macrophages transfected with siRNAs as in (c), and 48 h later, pretreated with iGb3 and followed by stimulation with LPS for 6 h. e Confocal microscopy observation of peritoneal macrophages treated as indicated and labeled with CD1d and EEA1 antibodies. Scale bars, 5 μm. More than 30 macrophages from three independent experiments per group were observed and used to quantification analysis. f ELISA of cytokines in supernatants of macrophages blocked with CD1d neutralizing mAb (WTH-2) and then stimulated with LPS, Poly(I:C) or CpG for 6 h. g ELISA of cytokines in supernatants of Cd1d+/+ and Cd1d–/– macrophages transfected with wild-type CD1d or CD1d mutants, 36 h later, then pretreated with iGb3 and stimulated with LPS for 6 h. Data are from three independent experiments (a–d, f–g; mean ± SEM) or are representative of three independent experiments with similar results (e). *P < 0.05 and **P < 0.01 (Student’s t-test or ANOVA)
Glycosphingolipid iGb3-triggered CD1d reverse signaling promotes TLR-triggered innate responses
iGb3 has been identified as a potent CD1d-presented self-antigen for NKT cells that is crucial for antimicrobial and antitumor defense.33 Therefore, we explored the role of iGb3 in the activation of CD1d reverse signaling. Pure iGb3 was synthesized as previously described,34 which also excluded the possibility that this antigen was contaminated by α-glycosylceramides.35 We found that this synthesized iGb3 induced slight production of TNF and IL-6 in a dose dependent manner, but not IFN-β (Supplementary information, Fig. S4a). We then observed the effect of iGb3 on TLR-triggered cytokine production. Pretreating macrophages with iGb3 significantly promoted the TLR-triggered production of TNF, IL-6 and IFN-β (Supplementary information, Fig. S4b). Since iGb3 is an endogenous ligand for CD1d, we investigated whether iGb3 exerted effects through CD1d. iGb3 could not enhance TLR-triggered cytokine production in CD1d-deficient macrophages (Fig. 3b), indicating that CD1d is required for iGb3 function.
Intracellular iGb3 production is controlled by iGb3 synthase (iGb3S) and β-hexosaminidase (Hexb).32 We found that the mRNA expression of these two enzymes in macrophages was significantly upregulated after TLR ligand stimulation (Supplementary information, Fig. S5a). This was reversed by the treatment of ERK inhibitor, suggesting a necessary role of ERK signaling activation in the increased transcription of the genes encoding these two enzymes (Supplementary information, Fig. S5b). We further knocked down the expression of iGb3S or Hexb in macrophages by siRNA to reduce endogenous iGb3 production. This revealed that silencing of either iGb3S or Hexb inhibited TLR-initiated cytokine production (Supplementary information, Fig. S5c). Silencing of iGb3S and Hexb together exerted the more significant inhibitory effect on TLR-triggered cytokine production (Fig. 3c) but this could be restored by iGb3 treatment (Fig. 3d).
To test the specificity of iGb3 to initiate reverse signaling, we also explored whether other GSLs had similar effects. We found that Gb3, LacCer, β-GlucCer and β-GalCer, which all have similar structures to iGb3, could not activate macrophages to induce cytokine production. Moreover, the exogenous lipid antigen α-galactosylceramide (α-GalCer), which can be presented to NKT cells by CD1d, also failed to induce cytokine production (Supplementary information, Fig. S5d). Silencing of other enzymes in the metabolic pathway of GSLs in macrophages, including Gb3S and Gb4S, also had no influence on cytokine production induced by TLR ligands (Supplementary information, Fig. S5e), suggesting a requirement of iGb3 synthesis for TLR-induced cytokine production.
Endogenous iGb3 activates CD1d reverse signaling in endosomal compartments
We further investigated whether endogenous iGb3 mediated its effect by acting on plasma membrane-associated or intracellular CD1d. Observations by confocal microscopy showed that CD1d was present not only on the plasma membrane but also in endosomal compartments in resting peritoneal macrophages (Fig. 3e). Interestingly, there appeared to be an increase in CD1d located in endosomal compartments after LPS stimulation or co-treatment with LPS and iGb3 (Fig. 3e). The anti-CD1d monoclonal antibody WTH-2 has been show to effectively block ligand recognition by NKT cells.36 This was confirmed by our finding that treatment with WTH-2 neutralizing antibody could significantly inhibit IFN-γ production in NKT cells co-cultured with DCs in the presence of iGb3 (Supplementary information, Fig. S6a). When we used the WTH-2 antibody to block CD1d molecules located on plasma membranes, we found that TLR-triggered cytokine production was not influenced (Fig. 3f). These data exclude the possibility that TLR-induced endogenous iGb3 is secreted into the culture media to bind and activate CD1d on plasma membranes. Thus, this suggests that iGb3 may have its effect through intracellular CD1d in endosomal compartments.
Considering that CD1d is recycled between plasma membranes and endosomal compartments to facilitate lipid exchange and antigen presentation,14 we hypothesized that exogenously added iGb3 might be transported into endosomal compartments to exert its effect through CD1d. Intracellular trafficking of plasma membrane-associated CD1d through compartments of the endocytic pathway relies on the YXXZ motif (Y is tyrosine, X is any amino acid, and Z is bulky hydrophobic residue) in its cytoplasmic tail.19,37 We therefore constructed two CD1d mutants, Y332A and I335A as described previously,19,37 and found that indeed, these two CD1d mutants could not traffic to endosomal compartments in CD1d-deficient macrophages upon co-treatment with LPS and iGb3 (Supplementary information, Fig. S6b). Overexpression of wild type CD1d in CD1d-deficient macrophages restored TLR-induced cytokine production whereas overexpression of CD1d Y332A or CD1d I335A did not (Fig. 3g). Moreover, exogenous iGb3 failed to promote TLR-induced cytokine production in Cd1d–/– macrophages over-expressing CD1d Y332A or CD1d I335A (Fig. 3g). The above findings suggest that exogenously added iGb3 cannot activate the reverse signaling of CD1d on the plasma membrane but instead needs to be transported into endosomal compartments via CD1d to initiate CD1d reverse signaling. Together, these data indicate that TLR signaling upregulates the expression of iGb3S and Hexb to promote the generation of endogenous iGb3, and iGb3, which in turn facilitates TLR-triggered innate responses through CD1d in endosomal compartments.
Impaired TLR signaling in CD1d-deficient macrophages
We further explored the effect of CD1d deficiency on TLR signaling pathways in macrophages. We found that CD1d deficiency had no effect on the LPS-induced phosphorylation of MAPKs including ERK, JNK and p38 (Supplementary information, Fig. S7a). However, LPS-induced activation of NF-κB signaling (assessed by phosphorylation levels of IKKα/β, IκBα and p65) was significantly attenuated in CD1d-deficient macrophages (Supplementary information, Fig. S7a). We also found the phosphorylation of TANK-binding kinase 1 (TBK1) and interferon regulatory factor 3 (IRF3) were both impaired in CD1d-deficient macrophages (Supplementary information, Fig. S7a). Similar results were also obtained in Poly(I:C)- or CpG ODN-stimulated CD1d-deficient macrophages (Supplementary information, Fig. S7b). iGb3 treatment could promote the LPS-induced activation of NF-κB and IRF3 pathways in macrophages whereas the activation of MAPKs was not affected (Supplementary information, Fig S7c). Similar results were also obtained in macrophages treated with iGb3 following stimulation with Poly(I:C) or CpG ODN (Supplementary information, Fig. S7d). However, treatment with iGb3 alone did not activate MAPK and IRF3 pathways and only led to a weak increase in p65 phosphorylation (Supplementary information, Fig. S7e). These data indicate that iGb3-activated CD1d reverse signaling promotes TLR-triggered innate responses by enhancing the activation of NF-κB and IRF3 signaling.
CD1d interacts with Pyk2 via intracellular phosphorylated tyrosine
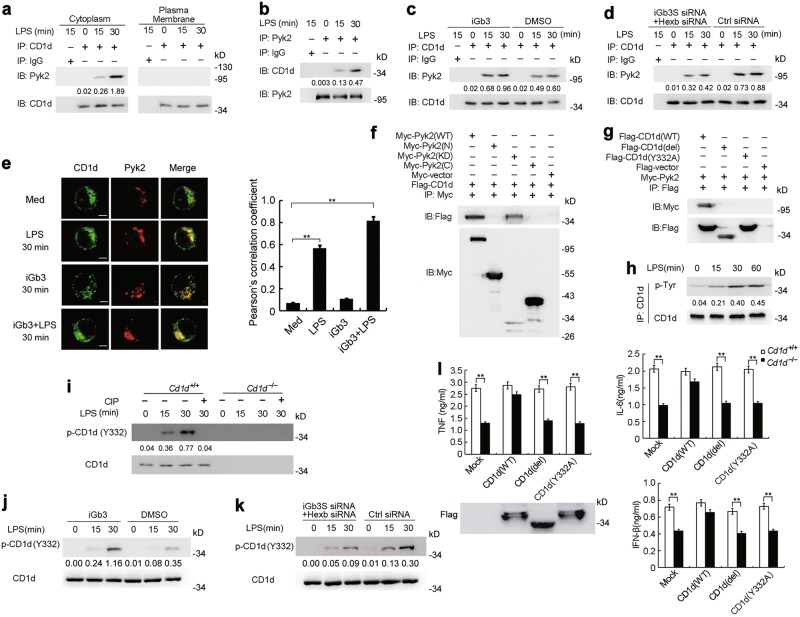
To investigate the underlying mechanisms by which CD1d reverse signaling was transduced, we subjected the protein complexes immunoprecipitated by anti-CD1d antibody from lysates of LPS-stimulated macrophages to mass spectrometry in order to identify CD1d-associated molecules (Supplementary information, Fig. S8a). Given that protein kinases are important regulators of TLR signaling, we focused on possible CD1d-interacting kinases. Among the CD1d-interacting protein kinases in the immune-precipitate we detected proline-rich tyrosine kinase 2 (Pyk2), a non-receptor protein tyrosine kinase that is highly expressed in hematopoietic cells and reported to function in inflammatory responses.38,39 Inhibition of Pyk2 can attenuate LPS-induced lung inflammation and injury in mice.40 Immunoprecipitation assays further showed that CD1d interacted with Pyk2 after LPS stimulation in macrophages (Fig. 4a, b). Specifically, only the cytoplasmic CD1d could associate with Pyk2, whereas CD1d located on plasma membrane could not (Fig. 4a and Supplementary information, Fig. S8b). Pretreatment of macrophages with iGb3 enhanced the interaction between CD1d and Pyk2 (Fig. 4c). Moreover, the ability of CD1d to recruit Pyk2 was attenuated when iGb3S and Hexb were silenced in macrophages (Fig. 4d). Confocal microscopy revealed that cytoplasmic CD1d co-localized with Pyk2 in LPS-stimulated macrophages. Moreover, pretreatment of macrophages with iGb3 further increased the LPS-induced co-localization of CD1d and Pyk2 whereas iGb3 treatment alone did not (Fig. 4e). These findings indicate that iGb3 preferentially activates CD1d in endosomal compartments but not CD1d on plasma membrane and then triggers the recruitment of Pyk2 upon TLR activation.
Fig. 4.
Intracellular CD1d interacts with Pyk2. a Immunoblot (IB) analysis of Pyk2 and CD1d immunoprecipitated with CD1d Ab from cytoplasmic and plasma membrane protein in lysates of macrophages stimulated with LPS for the indicated times. b IB analysis of CD1d and Pyk2 immunoprecipitated with Pyk2 Ab in lysates of macrophages stimulated with LPS for the indicated times. c IB analysis of Pyk2 and CD1d immunoprecipitated with CD1d Ab in lysates of macrophages pretreated with iGb3 and followed by stimulation with LPS. d IB analysis of Pyk2 and CD1d immunoprecipitated with CD1d Ab in lysates of macrophages transfected with control siRNA or iGb3S plus Hexb siRNA, and 48 h later, stimulated with LPS. e Confocal microscopy observation of peritoneal macrophages treated as indicated and labeled with CD1d and Pyk2 Abs. Scale bars, 5 μm. More than 30 macrophages from three independent experiments per group were observed and used to quantification analysis. f Macrophages were transfected with Myc-tagged wild type Pyk2, the N terminus, kinase domain or C terminus plus Flag-CD1d and then stimulated with LPS, followed by immunoprecipitation (IP) with Myc Ab and immunoblot with Flag or Myc Ab. g Macrophages were transfected with Flag-tagged wild type CD1d, CD1d lacking the intracellular domain (del) or CD1d Y332A mutant plus Myc-Pyk2 and then stimulated with LPS, followed by IP with Flag Ab and IB with Flag or Myc Ab. h IB analysis of tyrosine phosphorylated CD1d or total CD1d after IP with CD1d Ab in lysates of LPS-stimulated macrophages. i IB analysis of phosphorylated CD1d at Y332 or total CD1d in lysates of Cd1d+/+ and Cd1d–/– macrophages pretreated with or without CIP and then stimulated with LPS. j IB analysis of phosphorylated CD1d at Y332 or total CD1d in lysates of macrophages pretreated with iGb3 and then stimulated with LPS. k IB analysis of phosphorylated CD1d at Y332 or total CD1d in lysates of macrophages transfected with Ctrl siRNA or iGb3S plus Hexb siRNA and then stimulated with LPS. l ELISA of cytokines in supernatants of Cd1d+/+ and Cd1d–/– macrophages transfected with wild type CD1d or CD1d mutants as in g and then stimulated with LPS. Data are representative of three independent experiments with similar results (a–k) or are from three independent experiments (l; mean ± SEM). **P < 0.01 (Student’s t-test or ANOVA)
We then examined the domains required for the interaction between CD1d and Pyk2. We found that CD1d specifically associated with the kinase domain of Pyk2 (Fig. 4f). The CD1d molecule has a relatively short cytoplasmic tail, which contains an intracellular tyrosine (Tyr332). We found that only full length CD1d could interact with Pyk2 whereas mutants lacking the intracellular domain (CD1d del) or having a mutated tyrosine (Y332A) could not (Fig. 4g). In addition, we observed CD1d to be phosphorylated at tyrosine residues in TLR-activated macrophages (Fig. 4h). This led us to generate an antibody specific to the phosphorylated CD1d at Tyr332. We confirmed the specificity of this antibody by treating CD1d with calf intestinal alkaline phosphatase (CIP) (Fig. 4i). This allowed us to show that TLR activation could induce CD1d phosphorylation at Tyr332 (Fig. 4i and Supplementary information, Fig. S8c). Moreover, CD1d phosphorylation at Tyr332 was substantially impaired by the Src family tyrosine kinase inhibitor PP1 (Supplementary information, Fig. S8c) suggesting that a member of the Src tyrosine kinase family might be involved in Tyr332 phosphorylation of CD1d. Furthermore, iGb3 treatment enhanced TLR-triggered CD1d phosphorylation at Tyr332, while iGb3 treatment alone did not induce Tyr332 phosphorylation (Fig. 4J and Supplementary information, Fig. S8d). Moreover, TLR-induced CD1d Tyr332 phosphorylation could be inhibited by silencing iGb3S and Hexb in macrophages (Fig. 4k and Supplementary information, Fig. S8d). Furthermore, overexpression of wild type CD1d restored TLR-triggered cytokine production in CD1d-deficient macrophages whereas overexpression of mutants lacking the intracellular domain (CD1d del) or with the mutated tyrosine (Y332A) did not have this effect (Fig. 4l). These above findings indicate that endogenously generated iGb3 in TLR-activated macrophages induces phosphorylation at Tyr332 in CD1d’s cytoplasmic tail and that the phosphorylated CD1d, in turn, recruits Pyk2 to achieve full activation of TLR-triggered responses.
CD1d promotes TLR signaling by maintaining Pyk2 activation
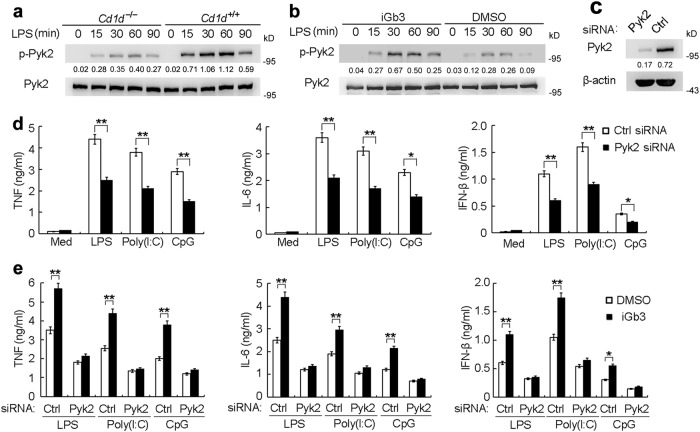
We next investigated whether CD1d reverse signaling enhanced TLR-triggered innate responses by activating Pyk2. TLR activation could induce Pyk2 phosphorylation in macrophages whereas Pyk2 phosphorylation was substantially impaired in CD1d-deficient macrophages (Fig. 5a and Supplementary information, Fig. S9a). Moreover, iGb3 treatment enhanced TLR-induced Pyk2 phosphorylation in macrophages (Fig. 5b and Supplementary information, Fig. S9b) indicatingd that CD1d contributes to the activation of TLR-triggered Pyk2. We then explored the role of Pyk2 in TLR signaling. Silencing of Pyk2 significantly impaired LPS-, Poly(I:C)- or CpG-induced production of TNF, IL-6 and IFN-β in macrophages (Fig. 5c, d). Furthermore, the promotion of TLR-triggered cytokine production by iGb3 treatment was reversed in Pyk2-silenced macrophages (Fig. 5e). This suggests that Pyk2 activation mediated by iGb3-CD1d reverse signaling positively regulates TLR-triggered innate responses.
Fig. 5.
CD1d promotes TLR-triggered innate responses by maintaining Pyk2 activation. a, b IB analysis of phosphorylated Pyk2 or total Pyk2 in lysates of Cd1d+/+ and Cd1d–/– macrophages (a) or macrophages pretreated with iGb3 (b), and stimulated with LPS for the indicated times. c IB analysis of Pyk2 in macrophages transfected with Ctrl siRNA or Pyk2 siRNA. d ELISA of cytokines in supernatants of macrophages transfected with siRNAs as in c, then stimulated with LPS, Poly(I:C) or CpG for 6 h. e ELISA of cytokines in supernatants of macrophages transfected with siRNAs as in c, and then pretreated with iGb3 followed by stimulation with LPS, Poly(I:C) or CpG. Data are representative of three independent experiments with similar results (a-c) or are from three independent experiments (d, e; mean ± SEM). *P < 0.05 and **P < 0.01 (Student’s t test or ANOVA)
Previous studies have shown that activating antibodies against CD1d can induce CD1d reverse signaling22–25 but the signaling mechanisms remain unclear. We therefore wondered whether antibody ligation-activated CD1d reverse signaling shared a similar pathway to the one suggested by our findings. We found that ligation of CD1d by the activating antibody (CD1d mAb 1B1) could increase TLR-induced cytokine production in macrophages (Supplementary information, Fig. S9c). TLR-induced Pyk2 recruitment to CD1d and Pyk2 phosphorylation was also enhanced after ligation of CD1d (Supplementary information, Fig. S9d, e). The above findings further demonstrate that CD1d reverse signaling promotes TLR responses by associating with Pyk2 and maintaining its activation.
Pyk2 interacts with IKKβ and TBK1 through its C-terminus
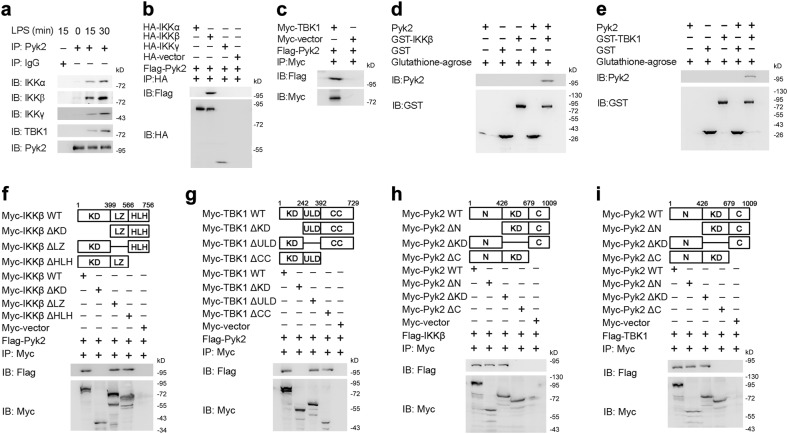
Next, we investigated the mechanism by which Pyk2 promotes TLR-triggered innate responses. We first asked whether Pyk2 interacted with components of the myeloid differentiation primary response gene 88 (MyD88)-dependent and TRIF-dependent pathways, such as interleukin 1 receptor associated kinase 1 (IRAK1), TNF receptor associated factor 6 (TRAF6), TGF-beta activated kinase 1 (TAK1), IKKs, TRAF3, TBK1 and IRF3. We found that only the IKK complex (IKKα, IKKβ and IKKγ) and TBK1 could be detected in anti-Pyk2 antibody-precipitated lysates from LPS-stimulated macrophages (Fig. 6a). However, the co-immunoprecipitation assay in HEK293 cells revealed that IKKβ and TBK1 could associate with Pyk2 whereas IKKα and IKKγ did not (Fig. 6b, c) indicating that Pyk2 specifically bound IKKβ and TBK1. Glutathione S-transferase pull-downs also revealed that Pyk2 directly interacted with IKKβ and TBK1 (Fig. 6d and e). The above findings suggest Pyk2 modulates TLR signaling by directly targeting IKKβ and TBK1.
Fig. 6.
Pyk2 interacts with IKKβ and TBK1. a Immunoblot analysis of IKKα, IKKβ, IKKγ, TBK1 and Pyk2 immunoprecipitated with Pyk2 Ab in lysates of LPS-stimulated macrophages. b HEK293 cells were cotransfected with HA-IKKα, HA-IKKβ or HA-IKKγ plus Flag-Pyk2 followed by IP with HA Ab and IB with Flag or HA Ab. c HEK293 cells were cotransfected with Myc-TBK1 and Flag-Pyk2 followed by IP with Myc Ab and IB with Flag or Myc Ab. d, e GST pull-down assay of proteins after incubating GST-IKKβ (d) or GST-TBK1 (e) with recombinant Pyk2 protein. f, g HEK293 cells were transfected with wild type IKKβ or IKKβ mutants (f), or wild type TBK1 or TBK1 mutants (g) plus Flag-Pyk2 followed by IP with Myc Ab and IB with Flag or Myc Ab. h, i HEK293 cells were transfected with wild type Pyk2, Pyk2 mutants plus Flag-IKKβ (h) or Flag-TBK1 (i) followed by IP with Myc Ab and IB with Flag or Myc Ab. Data are representative of three independent experiments
We then investigated the domains of IKKβ and TBK1 responsible for interaction with Pyk2. We found that both IKKβ and TBK1 having a deleted kinase domain could not bind Flag-tagged Pyk2 (Fig. 6f, g) indicating that IKKβ and TBK1 interact with Pyk2 through their amino-terminal kinase domain. Co-immunoprecipitation experiments also showed that Pyk2 lacking its C-terminus could not bind Flag-tagged IKKβ or TBK1 (Fig. 6h, i). Taken together, the above findings suggest that Pyk2 interacts with the kinase domain of IKKβ or TBK1 through its C-terminus.
Pyk2 promotes activation of IKKβ and TBK1 by enhancing their phosphorylation
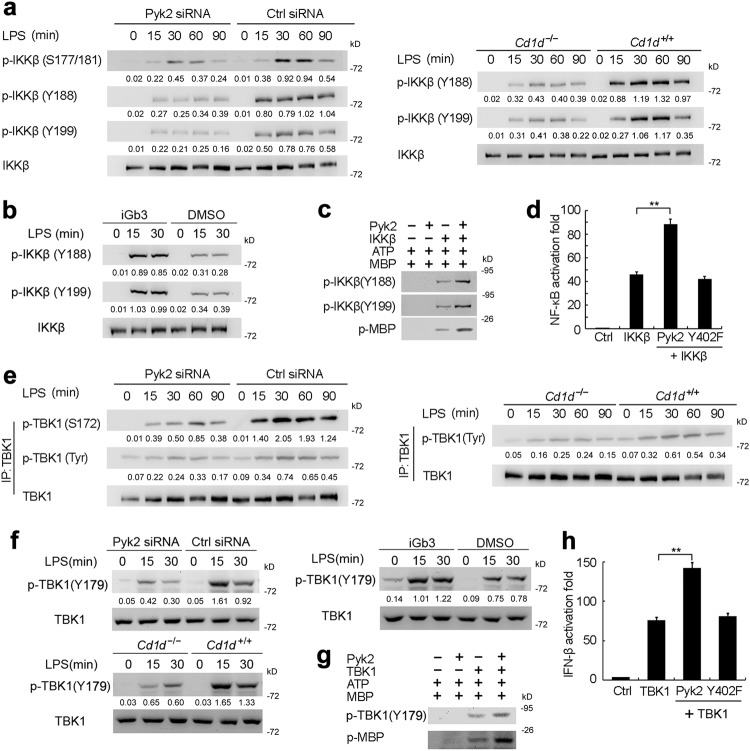
We further found that LPS-induced phosphorylation of IKKβ at Ser177/181 was significantly attenuated in Pyk2-silenced macrophages or CD1d-deficient macrophages (Fig. 7a and Supplementary information, Fig. S7a). Given that Pyk2 is a tyrosine kinase, we explored the effect of Pyk2 on tyrosine phosphorylation of IKKβ. Tyr188 and Tyr199 within the activation T loop of IKKβ, which have been reported to be important for its activation and the downstream activation of NF-κB.41 We found that tyrosine phosphorylation of IKKβ at Tyr188/199 induced by LPS was inhibited in Pyk2-silenced macrophages or CD1d-deficient macrophages (Fig. 7a); iGb3 treatment enhanced LPS-induced IKKβ Tyr188/199 phosphorylation; and iGb3 treatment alone did not lead to Tyr188/199 phosphorylation (Fig. 7b and Supplementary information, Fig. S10a). By carrying out kinase assays in vitro, we found that Pyk2 increased the tyrosine phosphorylation of IKKβ at Tyr188/199 and its ability to phosphorylate MBP (Fig. 7c). Reporter gene assays showed that Pyk2 promoted IKKβ-mediated NF-κB reporter activation whereas the kinase inactive mutant Pyk2-Y402F could not (Fig. 7d). This indicates that Pyk2 promotes IKKβ activation in a kinase activity-dependent manner.
Fig. 7.
Pyk2 promotes phosphorylation and activation of IKKβ and TBK1. a IB analysis of phosphorylated IKKβ or total IKKβ in lysates of Pyk2-silenced macrophages or Cd1d–/– macrophages stimulated with LPS. b IB analysis of phosphorylated IKKβ or total IKKβ in lysates of macrophages pretreated with iGb3 and then stimulated with LPS. c IB analysis of phosphorylated IKKβ and phosphorylated MBP with or without pre-incubating IKKβ with Pyk2 recombinant protein. d Luciferase assay in lysates of HEK293 cells transfected with NF-κB-luc reporter plasmids, plus IKKβ plasmid alone or together with wild type Pyk2 plasmid or Pyk2 kinase inactive mutant (Y402F). e IB analysis of phosphorylated TBK1 or total TBK1 in lysates of LPS-stimulated Pyk2-silenced macrophages or Cd1d–/– macrophages after IP with TBK1 Ab. f IB analysis of phosphorylated TBK1 or total TBK1 in lysates from Pyk2-silenced macrophages, Cd1d–/– macrophages or wild type macrophages treated with iGb3, followed by stimulation with LPS. g IB analysis of phosphorylated TBK1 and phosphorylated MBP with or without preincubation TBK1 with Pyk2 protein. h Luciferase assay in lysates of HEK293 cells transfected with IFN-β-luc reporter plasmids, plus TBK1 plasmid alone or together with Pyk2 plasmid or Pyk2 kinase inactive mutant (Y402F). Data are representative of three independent experiments with similar results (a–c, e–g) or are from three independent experiments (d, h; mean ± SEM). **P < 0.01 (ANOVA)
TBK1 activity is regulated by phosphorylation at Ser172 within its classical kinase activation loop.42 Silencing of Pyk2 or CD1d deficiency in macrophages significantly inhibited TBK1 phosphorylation at Ser172 (Fig. 7e and Supplementary information, Fig. S7a). TBK1 phosphorylation at tyrosine residues was also reduced in Pyk2-silenced macrophages and CD1d-deficient macrophages (Fig. 7e) indicating that Pyk2 might also promote tyrosine phosphorylation of TBK1. Recent studies have shown the phosphorylation of TBK1 at Tyr179 plays an important role in enhancing TBK1 activity by priming its autophosphorylation.43 We found that silencing of Pyk2 or CD1d deficiency attenuated the TLR-induced TBK1 phosphorylation at Tyr179 (Fig. 7f and Supplementary information, Fig. S10b, c). Moreover, iGb3 treatment could markedly enhance TLR-induced TBK1 phosphorylation at Tyr179 whereas iGb3 treatment alone did not trigger Tyr179 phosphorylation (Fig. 7f, Supplementary information Fig. S10a and d). In vitro kinase assays revealed that recombinant Pyk2 protein promoted not only the phosphorylation of TBK1 at Tyr179 but also the ability of TBK1 to phosphorylate MBP (Fig. 7g). Reporter gene assays also showed that Pyk2 promoted TBK1-mediated IFN-β reporter activation in a kinase activity-dependent manner (Fig. 7h).
Taken together, these data demonstrate that the TLR-induced generation of iGb3 can activate intracellular CD1d reverse signaling which in turn promotes TLR-triggered innate responses via Pyk2-mediated IKKβ and TBK1 activation (Supplementary information, Fig. S11).
Discussion
It is well-known that CD1d plays critical roles in antimicrobial responses, antitumor immunity, and in regulating the balance between tolerance and autoimmunity. Defects in CD1d are correlated with impaired host immunity as well as immune disorders.19,21 However, these classical functions of CD1d are reported to be exerted through CD1d-NKT cell axis. Here we demonstrate that endogenously generated iGb3 in macrophages, induced by TLR activation, can activate intracellular CD1d reverse signaling and thus amplify TLR-triggered innate immune responses. This sheds new light on the non-classical function of CD1d in immune responses mediated by APCs but not NKT cells. For the kinetics of iGb3 generation in macrophages, the enzymatic activity of iGb3S and Hexb that pre-exists in macrophages may be upregulated upon TLR ligation and contribute to the rapid iGb3 synthesis at the early phase (within 15 min) of TLR signaling. The TLR signal also significantly increases the expression levels of iGb3S and Hexb after 15 min, which then rapidly generates more iGb3. Thus, endogenous iGb3 generated at different time phases of the response acts to give important positive feedback for the full activation of TLR signaling.
Previous studies have indicated that the alteration in CD1d expression may be associated with autoimmune diseases such as rheumatoid arthritis and systemic lupus erythematosus.44,45 Since the inflammatory response induced by endogenous TLR ligands plays an important role in the development of autoimmune diseases, it is reasonable that the alteration of CD1d’s expression level and CD1d-mediated reverse signaling may lead to the imbalance of inflammatory immune responses and development of autoimmune disease. However, the role of CD1d in the pathogenesis of inflammatory or autoimmune diseases is not fully understood.
Lipids are not only the material basis for bioactivities but are also crucial mediators in the induction and resolution of inflammation. Lipid metabolism is under tight control to maintain homeostasis. Dysregulated lipid metabolism is closely related to the pathogenesis of various inflammatory and immune disorders.46,47 In recent years, the close relationship between lipid metabolism and immunity has attracted more and more attention. It has been found the activation of immune cells couples with enormous metabolic changes of lipids.10,11 More importantly, the fate and function of immune cells such as macrophages can be influenced by intracellular metabolic changes.1,48,49 GSLs are a class of glycolipid characterized by complex glycan structures linked to a ceramide backbone by a β-glycosidic bond.16 Little has been known about the relation between GSL metabolism and immune responses. In the present study, we describe substantial changes in metabolic profiles of GSLs after TLR activation. Importantly, one neutral GSL, iGb3, is found to be markedly induced in TLR-stimulated macrophages. Consistent with our results, Darmoise and colleagues have reported that TLR engagement in DCs may lead to lysosomal accumulation of GSL, amongst which the endogenous antigen iGb3 is also thought to accumulate most abundantly.50 Moreover, our data demonstrate that iGb3 is implicated to act as a lipid effector that promotes TLR-triggered inflammatory responses providing new evidence on the link between lipid metabolism and innate immunity. It is also intriguing to explore further the function of iGb3-triggered CD1d reverse signaling in metabolic disorders. In addition, there is a need to explore whether other GSLs, whose expression levels are significantly changed after TLR activation, may also be involved in inflammatory and metabolic diseases.
The self-lipid iGb3 is proposed to be a natural ligand for CD1d.15,33 Nevertheless, previous studies indicate the difficulty of detecting iGb3 in mouse tissues by HPLC.32 Our collaborator previously developed a specific and sensitive method based on ion trap mass spectrometry for the detection of iGb3 from small amounts of samples.16,51 They have developed a MS4 technique that can detect and quantify iGb3 in a mixture of Gb3 and iGb3 standards,52 and the presence of iGb3 in human and mouse thymus or DCs.16,52 Our study, and another previous report, found iGb3 to accumulate significantly in APCs after TLR ligand stimulation50 indicating that iGb3 could act as the endogenous antigen that is induced upon TLR activation. In addition, we cannot formally exclude the possibility that iGb3 is synthesized through an alternative pathway. For example, iGb3 can also be generated in lysosomes through cleavage of iGb4 by Hexb. Apart from iGb3, other endogenous ligands may also induce CD1d reverse signaling in APCs and this needs to be further investigated.
To explore the specificity of iGb3 to trigger CD1d reverse signaling, we also investigated whether other GSLs (Gb3, LacCer, β-GlucCer, β-GalCer, α-GalCer) had similar effects. Although the structures of the above GSLs are similar to iGb3, they failed to promote the activation of macrophages. The crystal structure of the mouse CD1d-iGb3 complex has been revealed previously.53 We speculate that the specificity of iGb3 to trigger CD1d reverse signaling may be due to its special structure and the induced expression pattern after TLR ligation. Furthermore, we found that iGb3 binding promoted TLR stimulation-induced phosphorylation of Y332 in the cytoplasmic tail of CD1d, which was required for the recruitment of downstream kinase Pyk2. It is also possible that the binding of iGb3 to CD1d might result in the conformational change of CD1d allowing it to be activated or phosphorylated. Indeed, our results showed that a member of the Src protein tyrosine kinase family might be involved in phosphorylating CD1d at Y332. CD1d has also been reported to be associated with the MHC class II molecule.54 CD1d ligation by antibody acted in synergy with CD40 to activate APCs.24 Thus, after iGb3 binding, the CD1d molecule possibly works in concert with MHC class II, CD40 or other unknown proteins to activate reverse signaling, a possibility that remains to be explored further. In addition, CD1d is so far identified as the only known receptor of iGb3. There is a remaining possibility to be investigated of whether other proteins can bind iGb3 to mediate reverse signaling.
In conclusion, our study demonstrates that the iGb3 that is endogenously generated after TLR activation can activate CD1d reverse signaling in endosomal compartments. This, in turn, enhances TLR-triggered innate immune responses to give positive feedback. Our findings implicate a non-classical role of CD1d in the inflammatory response and add a novel insight to the cross-talk between lipid metabolism and innate immune responses.
Materials and methods
Mice
Mice deficient in CD1d (C.129S2-Cd1tm1Gru/J; Mouse Genome Informatics accession code, 003814) were obtained from Jackson Laboratories and were bred in specific pathogen-free conditions. 6- to 8-week-old littermates, matched for body weight and sex, were used in all experiments. All animal experiments were in accordance with the National Institute of Health Guide for the Care and Use of Laboratory Animals, with the approval of the Scientific Investigation Board of the Second Military Medical University, Shanghai.
Antibodies and reagents
Antibodies to ERK phosphorylated at Thr202/Tyr204 (9106); JNK phosphorylated at Thr183/Tyr185 (9255); p38 phosphorylated at Thr180/Tyr182 (9211); NF-κB p65 phosphorylated at Ser536 (3033); IκBα phosphorylated at Ser32/36 (9246); IRF3 phosphorylated at Ser396 (4947); IKKα/β phosphorylated at Ser177/Ser181 (2697); TBK1 phosphorylated at Ser172 (5483); Pyk2 phosphorylated at Tyr402 (3291); Pyk2 (3292), IKKβ (8943), EEA1 (2411); Myc-tag (2272), hemagglutinin-tag (2367); and β-actin (3700) were obtained from Cell Signaling Technology. Antibodies to IKKβ phosphorylated at Tyr188 (ab194519) or Tyr199 (ab195923) were obtained from Abcam. Anti-CD1d (sc-373858) was obtained from Santa Cruz. Anti-CD1d mAb (WTH-2 and 1B1) were from BD Pharmingen. Anti-Flag (M2) was from Sigma. Rabbit polyclonal antibodies to CD1d phosphorylated at Tyr332 and TBK1 phosphorylated at Tyr179 were generated by Abmart (Shanghai, China). LPS (E.coli serotype 0111:B4) and Poly(I:C) were obtained from Sigma. CpG-ODN was from InvivoGen. E. coli serotype 0111:B4 was from the China Center for Type Culture Collection. Listeria monocytogenes was provided by Dr. H. Shen (University of Pennsylvania). CD1d tetramers used to identify NKT cells were from ProImmune (Oxford, UK). Inhibitors for ERK, JNK, p38 and NF-κB were from Merck Millipore.
Cell culture and transfection
Mouse bone marrow-derived DCs (BMDCs) were generated as described.54 Mouse BMDCs were prepared from bone marrow progenitors cultured in 25 ng/mL rmGM-CSF and 10 ng/mL rmIL-4 (R&D Systems). Nonadherent cells were gently washed out on the third day of culture; the remaining loosely adherent clusters were cultured to the 6th day. Thioglycollate-elicited mouse peritoneal macrophages were prepared and cultured in endotoxin-free RPMI-1640 medium (PAA) with 10% FCS (Gibco) as described.55 Mouse peritoneal macrophages were transfected with siRNA duplexes (Dharmacon) using Lipofectamine RNAiMAX reagent (ThermoFisher Scientific) according to standard protocols or transfected with plasmids through nucleofection by using a Macrophage Nucleofector kit (Lonza). The HEK293 cells (American Type Culture Collection) were transfected with JetPEI reagents (PolyPlus).
The culture of human monocyte-derived DCs was performed as described.56 Human peripheral blood mononuclear cells (PBMCs) were isolated from blood samples of healthy donors through Ficoll Paque Plus (GE healthcare) density gradient centrifugation. The experiments were approved by the Ethics Committee of the Second Military Medical University and informed donor consent was obtained. Monocytes were purified from PBMCs using anti-CD14 microbeads (Miltenyi Biotech) and then cultured in RPMI-1640 Medium (PAA) with 10% (vol/vol) FCS (Gibco) containing 100 ng/mL human GM-CSF and 20 ng/mL IL-4 (R&D Systems). Half of the medium was replaced with fresh medium containing GM-CSF and IL-4 at day 3. Human DCs were harvested on day 6 and then transfected with siRNA duplexes (Dharmacon) through nucleofection using a human DC Nucleofector kit (Lonza).
Cytokine detection
TNF, IL-6 and IFN-β in supernatants and sera were measured with ELISA kits (R&D Systems).
Lung histology
Lungs from LPS-challenged Cd1d+/+ and Cd1d−/− mice were dissected, fixed in 10% phosphate-buffered formaldehyde, embedded into paraffin, sectioned, stained with hematoxylin and eosin solution, and examined by light microscopy for histological changes.
Confocal microscopy
Macrophages plated on glass coverslips in six-well plates were left unstimulated or stimulated with LPS, iGb3 or LPS plus iGb3. Then, cells were fixed with 4% paraformaldehyde for 15 min and permeabilized with 0.2% Triton X-100 for 5 min. After blocking with 5% BSA, cells were labeled with anti-CD1d, anti-EEA1 or anti-Pyk2 antibody followed by staining with appropriate secondary antibodies. The immuno-stained cells were observed with a Leica TCS SP2 confocal laser microscope.
Assay of luciferase reporter gene expression
HEK293 cells were cotransfected with a mixture of the appropriate luciferase reporter plasmid, pRL-TK-renilla-luciferase plasmid and the additional constructs. Equivalent amounts of total plasmid DNA were used by adding empty control vector. Luciferase activities were measured using the Dual-Luciferase Reporter Assay system (Promega) according to the manufacturer’s instructions. Data were normalized for transfection efficiency by dividing firefly luciferase activity with that of renilla luciferase.
Membrane protein extraction
Macrophage membrane proteins were extracted using the Mem-PER Eukaryotic Membrane Protein Extraction Reagent Kit (Pierce) according to the manufacturer’s instructions. A second extraction was performed for more efficient enrichment of integral membrane proteins. The hydrophilic phase containing cytoplasmic proteins (the top layer) was separated from the hydrophobic phase containing plasma membrane proteins (the bottom layer).
Immunoprecipitation and immunoblot analysis
Cells were lysed with cell lysis buffer (Cell signaling Technology) supplemented with a protease inhibitor ‘cocktail’ (Merck Millipore). Protein concentrations in the extracts were measured by the BCA assay (Pierce). Immunoprecipitation and immunoblot analysis were performed as described.55 For immunoblot analysis, equal amounts of extracts were separated by SDS-PAGE and then transferred onto nitrocellulose membranes and detected with appropriate antibodies. For immunoprecipitation, proteins were immunoprecipitated from cell lysates with appropriate antibodies, separated by SDS-PAGE, and subjected to immunoblot analysis.
GST pull-down assay
GST-tagged IKKβ or TBK1 protein (Abcam) was incubated with recombinant Pyk2 protein (Creative Biomart) at 4 °C for 30 min, further incubated with glutathione-Sepharose 4B (Pierce) for 2 h in immunoprecipitation buffer. After washing 3 times, the precipitates were subjected to immunoblot analysis.
In vitro kinase assay
Purified active IKKβ or TBK1 protein (Abcam) was preincubated with active Pyk2 protein (Creative Biomart) in ice water for 15 min. Then MBP and a magnesium-ATP ‘cocktail’ (Upstate) were added, followed by incubation for 20 min at 30 °C. Samples were subjected to immunoblot analysis with antibody to phosphorylated IKKβ, TBK1 or MBP.
Bone marrow transplantation
A 1:1 mixture of bone marrow cells (1 × 107) from Cd1d+/+ and Cd1d–/– mice or bone marrow cells from Cd1d+/+ mice alone were transplanted into lethally irradiated wild type mice (cumulative dose, 10 Gy) by injection into the tail vein. Eight weeks later, NKT cells in liver were separated and analyzed by flow cytometry.
Electrospray ionization linear-ion-trap mass spectrometry (ESI-LIT-MS) of GSLs
GSLs from mouse peritoneal macrophages were extracted and permethylated. Mass spectrometric (MS and MSn) analyses were carried on a linear ion-trap mass spectrometer (LTQ, Thermo Finnigan, San Jose, CA) as described.16,51,52 The relative abundances of major product ions appearing in the MS4 spectra (1215 → 667 → 445 → ) were plotted with respect to the percentage of iGb3 in these mixtures. Specifically, plots were made using abundance ratios of the ions m/z 211, 371, 329, as follows: [A(iGb3)211 + A(iGb3)371]/[A(iGb3)211 + A(iGb3)371 + 2 × A(Gb3)329].
Nanospray liquid chromatography-tandem mass spectrometry
Immunoprecipitation was performed using anti-CD1d antibody upon lysates of LPS-stimulated macrophages (3 × 108). The immunoprecipitated proteins were eluted and digested, followed by analysis with reverse-phase nanospray liquid chromatography-tandem mass spectrometry. Data from liquid chromatography-tandem mass spectrometry were processed through ProteinLynx Global Serverversion 2.4 (PLGS 2.4); the resulting peak lists were used for searching the NCBI protein database with the Mascot search engine.
Establishment of endotoxin shock models and bacterial infection
Cd1d+/+ and Cd1d–/– mice were intraperitoneally injected with LPS, Poly(I:C) or CpG ODN at a dose of 15, 20 or 20 mg per kg body weight, respectively. For bacterial infection, Cd1d+/+ and Cd1d−/− mice were injected intraperitoneally with 1 × 107 E.coli (strain 0111.B4) or injected intravenously with 1 × 104 Listeria monocytogenes. Sera were then collected for measurement of cytokines by ELISA (or colony-forming units for E. coli). Spleens or livers were collected and lysed for measurement of colony-forming units (Listeria monocytogenes) as previously described.30
Statistical analysis
The statistical significance of comparisons between two groups was determined using an unpaired Student’s t test. One-way ANOVA was used for comparison of more than 2 groups. P values of less than 0.05 were considered statistically significant.
Electronic supplementary material
Acknowledgements
We thank Dr. H. Shen (University of Pennsylvania) for Listeria monocytogenes; and X. Sun and M. Jin for technical assistance. This work was supported by the National Key Basic Research Program of China (2015CB964403 to X.C.), the National Natural Science Foundation of China (81788101 to X.C., 31570871 to X.L., 31770970 to X.L., 81600182 to P.Z., 81571543 to Y.L.), CAMS Innovation Fund for Medical Sciences (2016-12M-1-003 to X.C.), and “Shuguang Program” of Shanghai Education Development Foundation and Shanghai Municipal Education Commission (18SG33 to X.L.).
Author contributions
X.C. and X.L. conceived this project and supervised the experiments. X.C., X.L., P.Z. and Y.Z. wrote the paper. X.L., P.Z., Y.Z., Z.W., S.X., Y.L., W.H., Q.Z., X.C., X.C., N.L. P.W. and Y.L. performed the experiments and analyzed the data.
Competing interests
The authors declare no competing interests.
Contributor Information
Xingguang Liu, Email: liuxg@immunol.org.
Xuetao Cao, Email: caoxt@immunol.org.
Electronic supplementary material
Supplementary Information accompanies this paper at 10.1038/s41422-018-0122-7.
References
- 1.Buck MD, Sowell RT, Kaech SM, Pearce EL. Metabolic instruction of immunity. Cell. 2017;169:570–586. doi: 10.1016/j.cell.2017.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ganeshan K, Chawla A. Metabolic regulation of immune responses. Annu. Rev. Immunol. 2014;32:609–634. doi: 10.1146/annurev-immunol-032713-120236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ghesquiere B, Wong BW, Kuchnio A, Carmeliet P. Metabolism of stromal and immune cells in health and disease. Nature. 2014;511:167–176. doi: 10.1038/nature13312. [DOI] [PubMed] [Google Scholar]
- 4.Brestoff JR, Artis D. Immune regulation of metabolic homeostasis in health and disease. Cell. 2015;161:146–160. doi: 10.1016/j.cell.2015.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McNelis JC, Olefsky JM. Macrophages, immunity, and metabolic disease. Immunity. 2014;41:36–48. doi: 10.1016/j.immuni.2014.05.010. [DOI] [PubMed] [Google Scholar]
- 6.O’Neill LA, Pearce EJ. Immunometabolism governs dendritic cell and macrophage function. J. Exp. Med. 2016;213:15–23. doi: 10.1084/jem.20151570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Phan AT, Goldrath AW, Glass CK. Metabolic and epigenetic coordination of T cell and macrophage immunity. Immunity. 2017;46:714–729. doi: 10.1016/j.immuni.2017.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Everts B, et al. TLR-driven early glycolytic reprogramming via the kinases TBK1-IKKε supports the anabolic demands of dendritic cell activation. Nat. Immunol. 2014;15:323–332. doi: 10.1038/ni.2833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pearce EJ, Everts B. Dendritic cell metabolism. Nat. Rev. Immunol. 2015;15:18–29. doi: 10.1038/nri3771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Andreyev AY, et al. Subcellular organelle lipidomics in TLR-4-activated macrophages. J. Lip. Res. 2010;51:2785–2797. doi: 10.1194/jlr.M008748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dennis EA, et al. A mouse macrophage lipidome. J. Biol. Chem. 2010;285:39976–39985. doi: 10.1074/jbc.M110.182915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Blanc M, et al. The transcription factor STAT-1 couples macrophage synthesis of 25-hydroxycholesterol to the interferon antiviral response. Immunity. 2013;38:106–118. doi: 10.1016/j.immuni.2012.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Coulombe F, et al. Targeted prostaglandin E2 inhibition enhances antiviral immunity through induction of type I interferon and apoptosis in macrophages. Immunity. 2014;40:554–568. doi: 10.1016/j.immuni.2014.02.013. [DOI] [PubMed] [Google Scholar]
- 14.McEwen-Smith RM, Salio M, Cerundolo V. CD1d-dependent endogenous and exogenous lipid antigen presentation. Curr. Opin. Immunol. 2015;34:116–125. doi: 10.1016/j.coi.2015.03.004. [DOI] [PubMed] [Google Scholar]
- 15.Zhou D, et al. Lysosomal glycosphingolipid recognition by NKT cells. Science. 2004;306:1786–1789. doi: 10.1126/science.1103440. [DOI] [PubMed] [Google Scholar]
- 16.Li Y, et al. Immunologic glycosphingolipidomics and NKT cell development in mouse thymus. J. Proteome Res. 2009;8:2740–2751. doi: 10.1021/pr801040h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Paget C, et al. Activation of invariant NKT cells by toll-like receptor 9-stimulated dendritic cells requires type I interferon and charged glycosphingolipids. Immunity. 2007;27:597–609. doi: 10.1016/j.immuni.2007.08.017. [DOI] [PubMed] [Google Scholar]
- 18.Salio M, Silk JD, Jones EY, Cerundolo V. Biology of CD1- and MR1-restricted T cells. Annu. Rev. Immunol. 2014;32:323–366. doi: 10.1146/annurev-immunol-032713-120243. [DOI] [PubMed] [Google Scholar]
- 19.Rossjohn J, Pellicci DG, Patel O, Gapin L, Godfrey DI. Recognition of CD1d-restricted antigens by natural killer T cells. Nat. Rev. Immunol. 2012;12:845–857. doi: 10.1038/nri3328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moody DB. TLR gateways to CD1 function. Nat. Immunol. 2006;7:811–817. doi: 10.1038/ni1368. [DOI] [PubMed] [Google Scholar]
- 21.Kasmar A, Van, Rhijn. I, Moody DB. The evolved functions of CD1 during infection. Curr. Opin. Immunol. 2009;21:397–403. doi: 10.1016/j.coi.2009.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Colgan SP, Hershberg RM, Furuta GT, Blumberg RS. Ligation of intestinal epithelial CD1d induces bioactive IL-10: critical role of the cytoplasmic tail in autocrine signaling. Proc. . Natl Acad. Sci. USA. 1999;96:13938–13943. doi: 10.1073/pnas.96.24.13938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Olszak T, et al. Protective mucosal immunity mediated by epithelial CD1d and IL-10. Nature. 2014;509:497–502. doi: 10.1038/nature13150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yue SC, Shaulov A, Wang R, Balk SP, Exley MA. CD1d ligation on human monocytes directly signals rapid NF-κB activation and production of bioactive IL-12. Proc. . Natl Acad. Sci. USA. 2005;102:11811–11816. doi: 10.1073/pnas.0503366102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yue SC, et al. Direct CD1d-mediated stimulation of APC IL-12 production and protective immune response to virus infection in vivo. J. Immunol. 2010;184:268–276. doi: 10.4049/jimmunol.0800924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brubaker SW, Bonham KS, Zanoni I, Kagan JC. Innate immune pattern recognition: a cell biological perspective. Annu. Rev. Immunol. 2015;33:257–290. doi: 10.1146/annurev-immunol-032414-112240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cao X. Self-regulation and cross-regulation of pattern-recognition receptor signalling in health and disease. Nat. Rev. Immunol. 2016;16:35–50. doi: 10.1038/nri.2015.8. [DOI] [PubMed] [Google Scholar]
- 28.O’Neill LA, Golenbock D, Bowie AG. The history of Toll-like receptors- redefining innate immunity. Nat. Rev. Immunol. 2013;13:453–460. doi: 10.1038/nri3446. [DOI] [PubMed] [Google Scholar]
- 29.Liu X, et al. Intracellular MHC class II molecules promote TLR-triggered innate immune responses by maintaining activation of the kinase Btk. Nat. Immunol. 2011;12:416–424. doi: 10.1038/ni.2015. [DOI] [PubMed] [Google Scholar]
- 30.Xu S, et al. Constitutive MHC class I molecules negatively regulate TLR-triggered inflammatory responses via the Fps-SHP-2 pathway. Nat. Immunol. 2012;13:551–559. doi: 10.1038/ni.2283. [DOI] [PubMed] [Google Scholar]
- 31.Haziot A, et al. Resistance to endotoxin shock and reduced dissemination of gram-negative bacteria in CD14-deficient mice. Immunity. 1996;4:407–414. doi: 10.1016/S1074-7613(00)80254-X. [DOI] [PubMed] [Google Scholar]
- 32.Speak AO, et al. Implications for invariant natural killer T cell ligands due to the restricted presence of isoglobotrihexosylceramide in mammals. Proc. . Natl Acad. Sci. USA. 2007;104:5971–5976. doi: 10.1073/pnas.0607285104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mattner J, et al. Exogenous and endogenous glycolipid antigens activate NKT cells during microbial infections. Nature. 2005;434:525–529. doi: 10.1038/nature03408. [DOI] [PubMed] [Google Scholar]
- 34.Xia C, et al. Synthesis and biological evaluation of alpha-galactosylceramide (KRN7000) and isoglobotrihexosylceramide (iGb3) Bioorg. Med. Chem. Lett. 2006;16:2195–2199. doi: 10.1016/j.bmcl.2006.01.040. [DOI] [PubMed] [Google Scholar]
- 35.Kain L, et al. The identification of the endogenous ligands of natural killer T cells reveals the presence of mammalian alpha-linked glycosylceramides. Immunity. 2014;41:543–554. doi: 10.1016/j.immuni.2014.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Monzon-Casanova E, et al. CD1d expression in paneth cells and rat exocrine pancreas revealed by novel monoclonal antibodies which differentially affect NKT cell activation. PLoS ONE. 2010;5:e13089. doi: 10.1371/journal.pone.0013089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rodionov DG, Nordeng TW, Pedersen K, Balk SP, Bakke O. A critical tyrosine residue in the cytoplasmic tail is important for CD1d internalization but not for its basolateral sorting in MDCK cells. J. Immunol. 1999;162:1488–1495. [PubMed] [Google Scholar]
- 38.Kamen LA, Schlessinger J, Lowell CA. Pyk2 is required for neutrophil degranulation and host defense responses to bacterial infection. J. Immunol. 2011;186:1656–1665. doi: 10.4049/jimmunol.1002093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Okigaki M, et al. Pyk2 regulates multiple signaling events crucial for macrophage morphology and migration. Proc. . Natl Acad. Sci. USA. 2003;100:10740–10745. doi: 10.1073/pnas.1834348100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Duan Y, Learoyd J, Meliton AY, Leff AR, Zhu X. Inhibition of Pyk2 blocks lung inflammation and injury in a mouse model of acute lung injury. Resp. Res. 2012;13:4. doi: 10.1186/1465-9921-13-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhang P, et al. Protein tyrosine phosphatase with proline-glutamine-serine-threonine-rich motifs negatively regulates TLR-triggered innate responses by selectively inhibiting IκB kinase β/NF-κB activation. J. Immunol. 2013;190:1685–1694. doi: 10.4049/jimmunol.1202384. [DOI] [PubMed] [Google Scholar]
- 42.Larabi A, et al. Crystal structure and mechanism of activation of TANK-binding kinase 1. Cell Rep. 2013;3:734–746. doi: 10.1016/j.celrep.2013.01.034. [DOI] [PubMed] [Google Scholar]
- 43.Li X, et al. The tyrosine kinase Src promotes phosphorylation of the kinase TBK1 to facilitate type I interferon production after viral infection. Sci. Signal. 2017;10:eaae0435. doi: 10.1126/scisignal.aae0435. [DOI] [PubMed] [Google Scholar]
- 44.Chaudhry MS, Karadimitris A. Role and regulation of CD1d in normal and pathological B cells. J. Immunol. 2014;193:4761–4768. doi: 10.4049/jimmunol.1401805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kojo S, Tsutsumi. A, Goto D, Sumida T. Low expression levels of soluble CD1d gene in patients with rheumatoid arthritis. J. Rheum. 2003;30:2524–2528. [PubMed] [Google Scholar]
- 46.Maceyka M, Spiegel S. Sphingolipid metabolites in inflammatory disease. Nature. 2014;510:58–67. doi: 10.1038/nature13475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Perry RJ, Samuel VT, Petersen KF, Shulman GI. The role of hepatic lipids in hepatic insulin resistance and type 2 diabetes. Nature. 2014;510:84–91. doi: 10.1038/nature13478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Buckley CD, Gilroy DW, Serhan CN. Proresolving lipid mediators and mechanisms in the resolution of acute inflammation. Immunity. 2014;40:315–327. doi: 10.1016/j.immuni.2014.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kelly B, O’Neill LA. Metabolic reprogramming in macrophages and dendritic cells in innate immunity. Cell Res. 2015;25:771–784. doi: 10.1038/cr.2015.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Darmoise A, et al. Lysosomal alpha-galactosidase controls the generation of self lipid antigens for natural killer T cells. Immunity. 2010;33:216–228. doi: 10.1016/j.immuni.2010.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Li Y, Zhou D, Xia C, Wang PG, Levery SB. Sensitive quantitation of isoglobotriaosylceramide in the presence of isobaric components using electrospray ionization-ion trap mass spectrometry. Glycobiology. 2008;18:166–176. doi: 10.1093/glycob/cwm127. [DOI] [PubMed] [Google Scholar]
- 52.Li Y, et al. Sensitive detection of isoglobo and globo series tetraglycosylceramides in human thymus by ion trap mass spectrometry. Glycobiology. 2008;18:158–165. doi: 10.1093/glycob/cwm129. [DOI] [PubMed] [Google Scholar]
- 53.Zajonc DM, Savage PB, Bendelac A, Wilson IA, Teyton L. Crystal structures of mouse CD1d-iGb3 complex and its cognate Valpha14 T cell receptor suggest a model for dual recognition of foreign and self glycolipids. J. Mol. Biol. 2008;377:1104–1116. doi: 10.1016/j.jmb.2008.01.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kang SJ, Cresswell P. Regulation of intracellular trafficking of human CD1d by association with MHC class II molecules. EMBO J. 2002;21:1650–1660. doi: 10.1093/emboj/21.7.1650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhang Q, et al. Tet2 is required to resolve inflammation by recruiting Hdac2 to specifically repress IL-6. Nature. 2015;525:389–393. doi: 10.1038/nature15252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wang P, et al. The STAT3-binding long noncoding RNA lnc-DC controls human dendritic cell differentiation. Science. 2014;344:310–313. doi: 10.1126/science.1251456. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.