Abstract
REV-ERBα, the NR1D1 (nuclear receptor subfamily 1, group D, member 1) gene product, is a dominant transcriptional silencer that represses the expression of genes involved in numerous physiological functions, including circadian rhythm, inflammation, and metabolism, and plays a crucial role in maintaining immune functions. Microglia-mediated neuroinflammation is tightly associated with various neurodegenerative diseases and psychiatric disorders. However, the role of REV-ERBα in neuroinflammation is largely unclear. In this study, we investigated whether and how pharmacological activation of REV-ERBα affected lipopolysaccharide (LPS)-induced neuroinflammation in mouse microglia in vitro and in vivo. In BV2 cells or primary mouse cultured microglia, application of REV-ERBα agonist GSK4112 or SR9011 dose-dependently suppressed LPS-induced microglial activation through the nuclear factor kappa B (NF-κB) pathway. In BV2 cells, pretreatment with GSK4112 inhibited LPS-induced phosphorylation of the inhibitor of NF-κB alpha (IκBα) kinase (IκK), thus restraining the phosphorylation and degradation of IκBα, and blocked the nuclear translocation of p65, a NF-κB subunit, thereby suppressing the expression and secretion of the proinflammatory cytokines, such as interleukin 6 (IL-6) and tumor necrosis factor α (TNFα). Moreover, REV-ERBα agonist-induced inhibition on neuroinflammation protected neurons from microglial activation-induced damage, which were also demonstrated in mice with their ventral midbrain microinjected with GSK4112, and then stimulated with LPS. Our results reveal that enhanced REV-ERBα activity suppresses microglial activation through the NF-κB pathway in the central nervous system.
Keywords: REV-ERBα, GSK4112, SR9011, LPS, microglia, neuroinflammation, NF-κB
Introduction
Microglia, a type of macrophage in the central nervous system (CNS), play vital roles in neuroinflammation, a process associated with numerous neurological and psychiatric diseases, including neurodegenerative diseases and psychiatric disorders[1]. When microglia are activated by environmental stimulation, such as microbial components or viral nucleic acids, they produce cytotoxic factors, such as superoxide and nitric oxide, that cause neuronal damage and contribute to brain diseases[2]. Therefore, inhibition of microglial over-activation could be an effective therapeutic strategy for numerous brain diseases. Toll-like receptor 4 (TLR4), a type of pattern recognition receptor, can recognize microbial components, such as lipopolysaccharide (LPS); activate the NF-κB signaling pathway; and subsequently induce expression of inflammatory gene products, such as interleukin 6 (IL-6) and tumor necrosis factor α (TNFα), which play important roles in the innate immune response[3]. Under normal conditions, NF-κB inhibitory protein (IκB) masks the nuclear translocation signal of NF-κB and retains the NF-κB complex in the cytoplasm. Upon inflammatory stimulation, IκB is rapidly phosphorylated and degraded. Then, the released NF-κB translocates into the nucleus and induces transcription of inflammatory genes[4].
REV-ERBα, the NR1D1 (nuclear receptor subfamily 1, group D, member 1) gene product, is a dominant transcriptional silencer that represses the expression of genes that are involved in numerous physiological functions, including circadian rhythm, inflammation, and metabolism[5]. REV-ERBα represses gene expression by directly binding and recruiting nuclear receptor corepressor (NCoR) and histone deacetylase 3 (HDAC3) complexes to the REV-ERBα response element (RRE) in its target gene promoters and enhancers, such as brain and muscle aryl hydrocarbon receptor nuclear translocator-like protein 1 (BMAL1), glucose-6-phosphatase catalytic subunit (G6PC), and matrix metallopeptidase 9 (MMP9)[6]. GSK4112 is the first synthetic REV-ERBα-targeting ligand[7]. GSK4112 or other agonists that activate REV-ERBα can inhibit the expression of proinflammatory gene products, such as MMP9, C-X3-C motif chemokine receptor 1 (CX3CR1) and C–C motif chemokine ligand 2 (CCL2) in macrophages, probably via regulation of p38 and ERK pathways[8, 9]. Thus, evidence suggests that activation of REV-ERBα plays a vital role in the peripheral immune system. However, the mechanism by which REV-ERBα regulates neuroinflammation and the role of REV-ERBα in the CNS remains unclear.
In the present study, we demonstrated that pharmacological activation of REV-ERBα suppresses LPS-induced microglial activation both in vitro and in vivo, which is mediated by the NF-κB pathway. In addition, neuroinflammation inhibition via a REV-ERBα agonist protects neurons from LPS-mediated damage.
Materials and methods
Cell culture and drugs
Primary microglia were extracted from the newborn mouse cortex and prepared as previously described[10]. In brief, the cortex of newborn C57BL/6 mice was chopped and dissected. Cortex tissues were digested by 0.25% Trypsin (Gibco, Grand Island, NY, USA) and plated on poly-D-lysine-coated bottles (Corning, Tewksbury, MA, USA). Mixed glial cells were cultured in Dulbecco’s modified Eagle’s media (DMEM)/F12 (Gibco) containing 10% heat-inactivated fetal bovine serum (FBS) (Gibco) and penicillin/streptomycin (100 μg/mL). After 2 weeks, microglia were separated by shaking the bottles at 150 revolutions per min for 2 h. Separated microglia were plated on poly-D-lysine-coated 24-well plates (Corning) for the next assay. Ethical approval for the harvesting of primary microglia from newborn mice was obtained from the ethical committee of Soochow University. BV2 cells were maintained in DMEM containing 10% heat-inactivated FBS and penicillin/streptomycin (100 μg/mL). SH-SY5Y cells were cultured in DF12 media containing 10% FBS and penicillin/streptomycin (100 μg/mL). GSK4112 (Sigma, St. Louis, MO, USA), SR9011 (MCE, NJ, USA) and BAY 11–7082 (Beyotime Biotechnology, Shanghai, China) were dissolved with DMSO. LPS (Sigma, St. Louis, MO, USA) was dissolved in saline.
Immunoblot analysis and antibodies
Immunoblot analysis was performed with the following primary antibodies: anti-COX-2, anti-H2B, anti-IκK and anti-iNOS antibodies (Abcam, Cambridge, UK); anti-glyceraldehyde-3-phosphate dehydrogenase (GAPDH) antibody (Millipore, Billerica, MA, USA); anti-cleaved caspase-3, anti-IκBα, anti-p-IκBα, anti-p-IκKα/β (176/180) and anti-p-p65 (S536) antibodies (Cell Signaling Technology, Danvers, MA, USA); and anti-p65 antibodies (Santa Cruz, CA, USA). The secondary antibodies, horseradish peroxidase (HRP)-conjugated sheep anti-mouse or anti-rabbit antibodies (Thermo Fisher, Waltham, MA, USA), were used. The proteins were visualized using an ECL detection kit (Thermo Fisher).
Animal study
Male C57BL/6 mice (6–8 weeks of age) were purchased from SLACCAL Lab Animal Ltd. (Shanghai, China). The mice were housed under a 12-h light/dark cycle. All animal protocols were approved by the Animal Committee of Soochow University. Mice were anesthetized and mounted in a stereotaxic apparatus (RWD Life Science Co, Shenzhen, China) that was described previously[11]. GSK4112 was dissolved in DMSO to a concentration of 40 μM and loaded into a 5-µL Hamilton syringe, which is fixed on stereotaxic apparatus. GSK4112 was microinfused into the ventral midbrain (VMB) (AP-3.3 mm, ML ± 1.2 mm, DV-4.6 mm) (2 μL /mouse) at a rate of 0.1 µL/min. Two days later, LPS (1 mg/mL) (2 μL/mouse) was microinfused into VMB (AP-3.3 mm, ML ± 1.2 mm, DV-4.6 mm) in the same manner. Seven days after LPS injection, animals were killed, and brain slices were prepared for immunohistochemistry. All animal experimental operations were performed simultaneously according to the Institutional Guidelines for Animal Use and Care, and all procedures were approved by the Ethical Committee of Soochow University.
Immunohistochemistry
Mouse slices were stained with the anti-IBA1 antibody (Wako, Japan) for microglia or anti-TH (AB152, Millipore) antibodies for dopaminergic neurons followed by an incubation with the rhodamine (red)-conjugated secondary antibody (Invitrogen, Carlsbad, CA, USA) and subsequently observed with an inverted system microscope IX71 (Olympus, Tokyo, Japan).
Subcellular fractionation assay for nuclear extraction
The procedure was performed as previously proposed[12]. BV2 cells were lysed in a fractionation buffer (320 mmol/L sucrose, 3 mmol/L CaCl2, 0.1 mmol/L EDTA, 2 mmol/L MgAc, 1 mmol/L DTT, 0.5 mmol/L PMSF, and 0.5% NP-40) for 20 min on ice and centrifuged at 600×g for 15 min at 4 °C. The supernatant included the cytoplasmic fraction, and the precipitate included the nuclear fraction that was then washed with a fractionation buffer without NP-40 and lysed in CLB. Histone 2B (H2B) served as a nuclear marker, and GAPDH served as a cytoplasmic marker.
Immunofluorescence
The immunofluorescent analysis was performed as previously described[13]. Primary microglia or BV2 cells were labeled with the primary anti-IBA1 (Wako, Japan) or anti-p65 antibodies and secondary Alexa Fluor 594 donkey anti-rabbit antibodies (Jackson Immuno Research, PA, USA). The cells were then stained with DAPI (Invitrogen) and were visualized under a fluorescence microscope IX71 (Olympus, Tokyo, Japan) or laser confocal microscope (Zeiss, LSM710, Germany).
MTT assay
MTT assays were performed according to the standard protocol from the Cell Growth Determination Kit MTT Based (Sigma, St. Louis, MO, USA). Briefly, after the culture media were removed, the cells were incubated with an amount equal to the original culture volume of MTT dissolved in warmed PBS (0.5 mg/mL) for 3 to 4 h at 37 °C in an incubator. Then, the MTT solution was discarded, and an equal volume of DMSO was added to dissolve the formazan. The absorbance was measured at a wavelength of 570 nm by subtracting background absorbance measured at 690 nm.
qRT-PCR
Total RNA was isolated from BV2 cells using TRIzol reagent (Invitrogen). cDNA was reversely transcribed from total RNA using PrimeScript RT Master Mix (Takara, Shiga, Japan). qRT-PCR analysis was performed using the 2−ΔΔCT method. qRT-PCR primers were designed as follows: mouse iNOS: 5′-TCCCAGCCTGCCCCTTCAAT-3′ and 5′-CGGATCTCTCTCCTCCTGGG-3′; COX-2: 5′-CAGGCTGAACTTCGAAACA-3′ and 5′-GCTCACGAGGCCACTGATACCTA-3′; TNFα: 5′-CATCTTCTCAAAATTCGAGTGACAA-3′ and 5′-TGGGAGTAGACAAGGTACAACCC-3′; IL-6: 5′-GAGGATACCACTCCCAACAGACC-3′ and 5′-AAGTGCATCATCGTTGTTCATACA-3′; Bmal1: 5′-CAAGCACCTTCCTTCCAATG-3′ and 5′-GATTGCAGTCCACACCACTG-3′; β-actin: 5′-GACCTGACTGACTACCTC-3′ and 5′-GACAGCGAGGCCAGGATG-3′.
ELISA
Cells were pretreated with or without GSK4112 for 4 h and were then cultured for 12 h in the absence or presence of LPS. The supernatants were collected for detections of IL-6 and TNFα using an ELISA kit (BOSTER, Wuhan, China) according to manufacturer’s instructions.
Luciferase reporter assay
HEK293 cells were co-transfected with a Bmal1-reporter plasmid and Renilla (Promega) using Lipofectamine 2000. 24 h after transfection, the cells were assessed using a dual luciferase assay kit (Promega) and measured using the microplate reader Infinite M1000 Pro (Tecan, Austria GmbH, Groedig, Austria) according to the manufacturer’s instructions. Data from three independent transfection experiments are presented. BV2 cells harboring the NF-κB reporter plasmid (pGL6-NF-κB luc, Beyotime, Shanghai, China) were pretreated with DMSO or GSK4112 (40 μM) for 4 h. Cells were then exposed to LPS (100 ng/mL) for 12 h. Then, luciferase activity was measured using an Infinite M1000 Pro (Tecan) microplate reader.
Conditioned media (CM)
BV2 cells were pretreated with or without GSK4112 (40 μM) for 4 h and then exposed to LPS (100 ng/mL) for 12 h. The media were then removed, and the cells were cultured in fresh DMEM for 24 h. Then, CM were filtered through 0.45-μm filters and used to culture SH-SY5Y cells for 24 h. After culture, SH-SY5Y cells were harvested for further experiments.
Statistical analysis
Quantitative analysis of immunoblots from three independent experiments was performed using Photoshop 7.0 (Adobe, San Jose, CA, USA). Data were analyzed using GraphPad Prism 6 (GraphPad Software Inc., San Diego, CA, USA). Statistical significance was evaluated by t-tests for comparing two groups and one-way ANOVA for all groups. The criterion of significance was set as P < 0.05. Values are presented as the mean ± SEM.
Results
Pharmacological activation of REV-ERBα suppresses BV2 cell activation
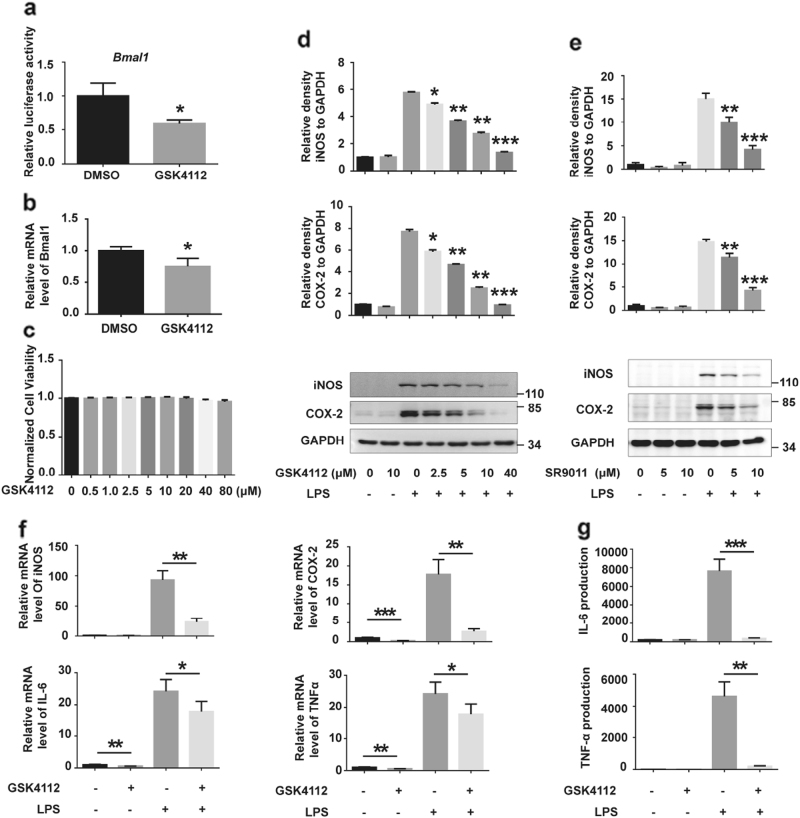
REV-ERBα represses transcriptional activity of the core clock gene Bmal1 through binding to the Bmal1 promoter[14]. We detected Bmal1 luciferase activity in HEK 293 cells and Bmal1 mRNA levels in BV2 cells to identify the pharmacological activity of the REV-ERBα agonist GSK4112. In cells treated with GSK4112, Bmal1 luciferase activity and its mRNA levels were significantly decreased (Fig. 1a, b), suggesting that GSK4112 activates REV-ERBα activity. In addition, GSK4112 itself did not influence BV2 cell viability up to a concentration of 80 μM (Fig. 1c). To identify the role of GSK4112 on microglial activation, we examined the effects of GSK4112 on BV2 cells that were treated with LPS. Pretreatment of BV2 cells with GSK4112 significantly inhibited LPS-induced iNOS and COX-2 protein expression levels in a dose-dependent manner (Fig. 1d). We also utilized SR9011, another agonist of REV-ERBα, to verify the role of REV-ERBα in microglial activation. Similar to GSK4112, SR9011 also inhibited LPS-induced iNOS and COX-2 protein levels (Fig. 1e). Given the significant anti-inflammatory effects and lack of cytotoxicity noted for GSK4112 at a concentration of 40 μM, we used this concentration for the following study. In the GSK4112 pretreatment group, iNOS and COX-2 mRNA levels were significantly reduced compared to those in control, suggesting that GSK4112 influences iNOS and COX-2 at the transcriptional level (Fig. 1f). Proinflammatory cytokines are secreted from microglia and play vital roles in mediating the innate immune response in CNS[15]. GSK4112 pretreatment also reduced mRNA levels of proinflammatory cytokines, such as IL-6 and TNFα, in LPS-treated BV2 cells (Fig. 1f). Moreover, IL-6 and TNFα secretion by BV2 cells was significantly increased after LPS treatment, whereas GSK4112 pretreatment inhibited their secretions (Fig. 1g).
Fig. 1.
GSK4112 inhibited LPS-induced BV2 cell activation. a HEK293 cells were transfected with Bmal1 and Renilla luciferase reporter and then treated with DMSO or GSK4112 (40 μM) for 16 h. The value of the group without drug (dissolvent only) was set to 1. *P < 0.05, n = 3. b BV2 cells were pretreated with DMSO or GSK4112 (40 μM) for 16 h. Cell lysates were collected to measure Bmal1 mRNA levels by qRT-PCR assay. *P < 0.05, n = 3. c Normalized BV2 cell viability influenced by different dose of GSK4112 (0–80 μM) for 16 h as assessed by MTT assay. d BV2 cells were pretreated with different doses of GSK4112 (2.5–40 μM) for 4 h and then exposed to LPS (100 ng/mL) for 12 h. Cell lysates were analyzed by Western blot for iNOS, COX-2, and GAPDH. The relative band intensity of iNOS and COX-2 to GAPDH were analyzed. *P < 0.05, **P < 0.01, ***P < 0.001, n = 3. e BV2 cells were pretreated with different doses of SR9011 (0–10 μM) for 4 h and then exposed to LPS (100 ng/mL) for 12 h. Cell lysates were analyzed by Western blot for iNOS, COX-2, and GAPDH. The relative band intensities of iNOS and COX-2 to GAPDH were analyzed. **P < 0.01, ***P < 0.001, n = 3. f, g BV2 cells were pretreated with DMSO or GSK4112 (40 μM) for 4 h and then exposed to LPS (100 ng/mL) for 12 h. Cell lysates were collected to measure iNOS, COX-2, IL-6, and TNFα mRNA levels by qRT-PCR assays. Cultured media were collected to measure IL-6 and TNFα levels by ELISA assays. *P < 0.05, **P < 0.01, ***P < 0.001, n = 3
Pharmacological activation of REV-ERBα suppresses microglial activation in vivo
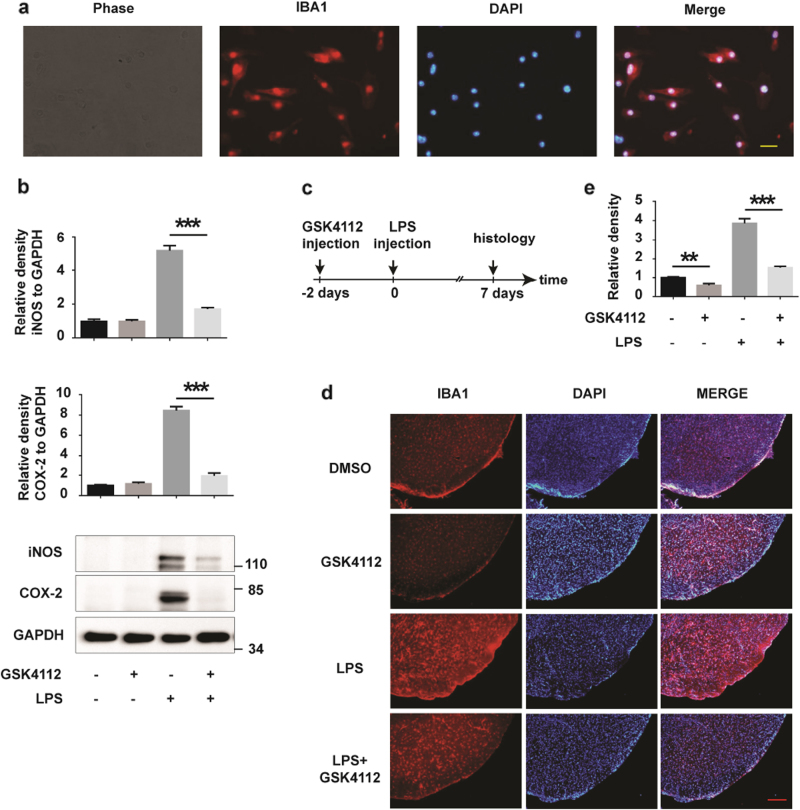
To further identify the role of GSK4112 in microglial activation, we examined the effects of GSK4112 on inflammation using primary cultured microglia and LPS-treated mice. The purity of primary microglia was examined with the microglial marker ionized calcium-binding adapter molecule-1 (IBA1) (Fig. 2a). GSK4112 dramatically reduced iNOS and COX-2 expression in LPS-stimulated primary microglia (Fig. 2b). Moreover, microglia in mouse midbrains were significantly activated by microinfusion of LPS; however, GSK4112 pretreatment significantly inhibited LPS-induced microglial activation in vivo (Fig. 2c–e). Together, these findings suggest that GSK4112 inhibits LPS-induced microglial activation in vitro and in vivo.
Fig. 2.
GSK4112 inhibited LPS-induced microglial activation in vivo. a Primary cultured microglia were stained using an IBA1 (red) antibody. The nuclei were stained with DAPI (1 μg/mL) (blue). Scale bars, 20 μm. b Primary cultured microglia were treated as described in Fig. 1f. Cell lysates were analyzed by Western blot for iNOS, COX-2, and GAPDH. The relative band intensities of iNOS and COX-2 to GAPDH were analyzed. ***P < 0.001, n = 3. c, d Mice received stereotaxic injection of DMSO or GSK4112 for 2 days and were then stimulated with PBS or LPS for 7 days. Mouse brains were cut into slices, and microglia were stained with an IBA1 antibody. Representative images of IBA1 (red) immunofluorescence staining in VTA are presented at AP −3.3 mm. The nuclei were stained with DAPI (1 μg/mL) (blue). Scale bar, 100 μm. e Intensity of IBA1 immunofluorescence signals in d was analyzed. **P < 0.01, ***P < 0.001, n = 3
Pharmacological activation of REV-ERBα suppresses p65 nuclear translocation
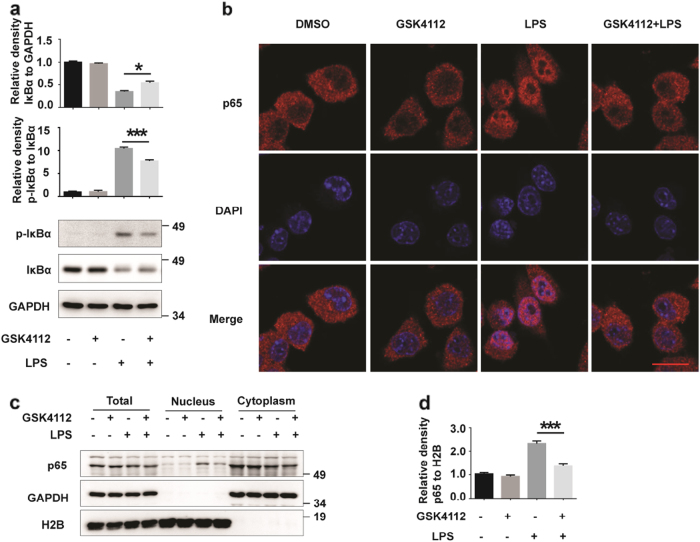
The NF-κB signaling pathway is a classic inflammatory pathway[4]. We assessed whether the anti-inflammatory effects of GSK4112 are mediated by NF-κB pathway. NF-κB nuclear translocation is a critical step for NF-κB pathway activation. Under normal conditions, NF-κB is located in the cytoplasm. After LPS stimulation, IκB was phosphorylated and degraded. Thus, the NF-κB nuclear translocation signal was subsequently exposed[16]. We therefore examined the levels of total and phosphorylated forms of IκBα. The level of phosphorylated IκBα (p-IκBα) increased, and the level of IκBα decreased in response to LPS stimulation (Fig. 3a). However, GSK4112 pretreatment significantly reduced LPS-induced phosphorylation of IκBα and IκBα degradation (Fig. 3a). Through immunocytochemical assays, we monitored the subcellular distribution of p65, a subunit of NF-κB, and found that LPS stimulated p65 translocation into the nucleus. However, GSK4112 pretreatment blocked this process (Fig. 3b). To further assess the inhibition of NF-κB nuclear translocation by GSK4112, we performed subcellular fractionation assays to verify the effects of GSK4112 on p65 nuclear translocation. Consistent with immunofluorescence data, GSK4112 inhibited LPS-induced p65 nuclear translocation (Fig. 3c, d).
Fig. 3.
GSK4112 suppressed p65 nuclear translocation. BV2 cells were pretreated with DMSO or GSK4112 (40 μM) for 4 h and then exposed to LPS (100 ng/mL) for 15 min. a The protein levels of total and phosphorylated IκBα and GAPDH were detected using immunoblot analysis. The relative levels of indicated proteins were analyzed. *P < 0.05, ***P < 0.001, n = 3. b Immunofluorescence was performed to measure nuclear accumulation of the NF-κB p65 subunit in BV2 cells. The cells were labeled with the anti-p65 (red) antibody. The nuclei were stained with DAPI (1 μg/mL) (blue). Scale bars, 5 μm. c Subcellular fractionation assay for nuclear extraction was performed. The nuclear and cytoplasmic fractions of BV2 cells were immunoblotted with anti-p65, anti-H2B and anti-GAPDH antibodies. d The intensities of p65 (nuclear fraction) relative to H2B in c were analyzed. ***P < 0.001, n = 3
Pharmacological activation of REV-ERBα suppresses p65 phosphorylation
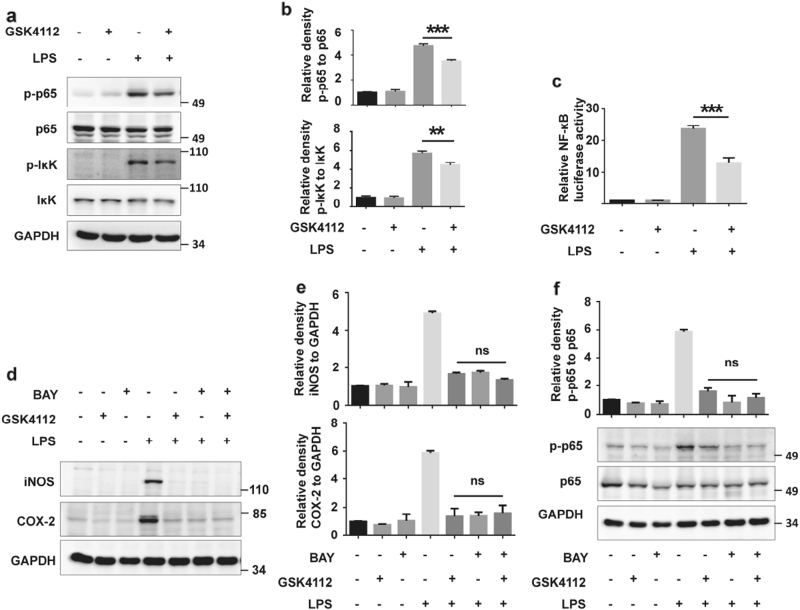
p65 phosphorylation is a critical indicator of NF-κB pathway activation. IκBα kinase (IκK) is phosphorylated upon LPS stimulation and subsequently phosphorylates p65[17]. The phosphorylation of serine 536 in p65 represents an active mark for canonical NF-κB activation[18]. In LPS-treated BV2 cells, IκK and p65 phosphorylation increased, whereas GSK4112 pretreatment inhibited this phosphorylation (Fig. 4a, b). Moreover, we performed luciferase report assays to further identify the effects of GSK4112 on NF-κB transcriptional activity. LPS dramatically enhanced NF-κB transcriptional activity, whereas GSK4112 pretreatment significantly inhibited its transcriptional activity induced by LPS (Fig. 4c). BAY 11–7082 is an NF-κB inhibitor that inhibits IκBα phosphorylation[19]. We used BAY 11–7082 to investigate whether GSK4112 inhibits microglial activation through the NF-κB pathway. BAY 11–7082 and GSK4112 pretreatment displayed similar anti-inflammatory effects, demonstrating that both inhibited iNOS expression and p65 phosphorylation induced by LPS in BV2 cells (Fig. 4d–f). However, GSK4112 in combination with BAY 11–7082 could not further enhance its anti-inflammatory effects (Fig. 4d–f), suggesting that the NF-κB pathway is closely involved in GSK4112-mediated microglial inhibition.
Fig. 4.
GSK4112 suppressed p65 phosphorylation. a BV2 cells were treated as described in Fig. 3. The protein levels of total and phosphorylated IκK and p65 were detected using immunoblot analysis. b The relative levels of indicated proteins in a were analyzed. **P < 0.01, ***P < 0.001, n = 3. c NF-κB luciferase plasmid was transfected into BV2 cells with AAV. Then, cells were subjected to a luciferase reporter assay. ***P < 0.001, n = 3. d BV2 cells were pretreated with BAY 11–7082 (10 μM) or GSK4112 (40 μM) alone or in combination for 4 h and then stimulated with LPS (100 ng/mL) for 12 h. The protein levels of iNOS, COX 2, and GAPDH were detected using immunoblot analysis. e The relative band intensities of iNOS and COX 2 to GAPDH in d were analyzed. ns, no statistical significance, n = 3. f BV2 cells were pretreated with BAY 11–7082 (10 μM) or GSK4112 (40 μM) alone or in combination for 16 h and then stimulated with LPS (100 ng/mL) for 15 min. The indicated protein levels were detected using immunoblot analysis. The relative levels of indicated proteins were analyzed. ns, no statistical significance, n = 3
Pharmacological activation of REV-ERBα inhibits microglial-mediated neurotoxicity
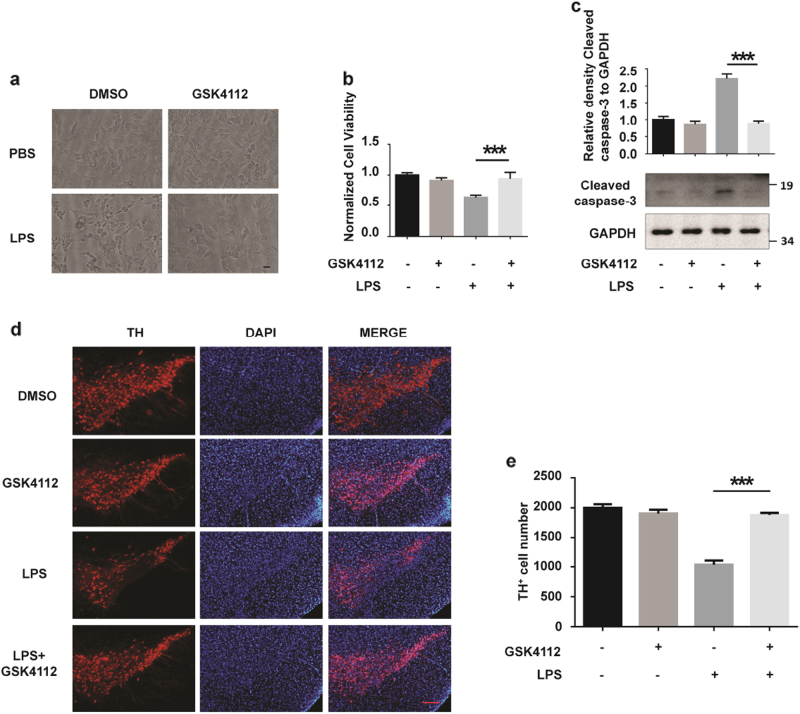
We further tested whether REV-ERBα-mediated microglial activation has effects on neuronal survival. The CM from LPS-treated BV2 cells induced cellular damage of SH-SY5Y cells, a neuroblastoma cell line. CM from LPS-treated BV2 cells pretreated with GSK4112 reduced cellular toxicity in SH-SY5Y cells (Fig. 5a). Consistently, MTT assays also demonstrated that GSK4112 decreased the cellular toxicity of CM from LPS-treated BV2 cells (Fig. 5b). Cleaved caspase-3, an activated form of caspase-3, is a key mediator of programmed cell death[20, 21]. GSK4112 pretreatment of BV2 cells inhibited the activation of caspase-3 in SH-SH5Y cells treated with CM of LPS-stimulated BV2 cells (Fig. 5c). In mice administered LPS, the number of tyrosine hydroxylase (TH)-immunoreactive neurons in the midbrain was significantly reduced (Fig. 5d, e). However, GSK4112 pretreatment significantly attenuated TH-immunoreactive neuronal loss induced by LPS, suggesting that GSK4112 protects neurons from microglial activation-induced neuronal death in vivo (Fig. 5d, e).
Fig. 5.
GSK4112 inhibited microglial-mediated neurotoxicity. a SH-SY5Y were cultured in different BV2 CM for 24 h and then assessed by microscopy. Scale bar, 20 μm. b Normalized SH-SY5Y cell viability influenced by different BV2 CM as assessed using the MTT assay. ***P < 0.001, n = 3. c Cleaved caspase-3 and GAPDH protein levels were detected via immunoblot analysis. The intensity of cleaved caspase-3 relative to GAPDH was analyzed. ***P < 0.001, n = 3. d Fluorescence-TH (red) staining of a representative brain section of mice is presented at AP −3.3 mm. The nuclei were stained with DAPI (1 μg/mL) (blue). Scale bar, 50 μm. e Quantification of TH-positive cell numbers in midbrain in d was analyzed. Scale bar, 100 µm. ***P < 0.001, n = 3
Discussion
Circadian rhythms play crucial roles in physiological functions and pathological processes, including neurodegeneration, cancer, and immunity[22–24]. In addition to functioning in rhythm, circadian proteins have roles in physiological functions independent of rhythm regulation. In our study, we found that pharmacological activation of REV-ERBα suppresses LPS-induced microglia activation through inhibiting the NF-κB signaling pathway.
The immune system was disrupted by loss of circadian clock function[24]. The absence of CRY, a protein that is expressed by the activation of its transcription factor BMAL1/CLOCK but inhibits BMAL1/CLOCK by a negative-feedback loop, leads to the elevation of proinflammatory cytokines[25]. Bmal1 or Rev-erbα knockout (KO) mice exhibit aberrant cytokine responses and an increased inflammatory response[26]. Thus, circadian rhythm or circadian proteins that function in a circadian-independent manner may facilitate the inflammatory response.
REV-ERBα functions as a transcriptional repressor in a ligand-dependent manner. Reduction of intracellular heme (an endogenous ligand for REV-ERBα) levels decreased REV-ERBα-mediated repression of target genes BMAL1, G6PC, and MMP9, impaired the recruitment of NCOR to REV-ERBα target gene promoters and decreased the interactions between REV-ERBα and the NCOR-HDAC3 co-repressor complex in cells[6, 27]. GSK4112 increases the binding of NCOR to REV-ERBα in a concentration-dependent manner [28]. and promotes the recruitment of HDAC3 to the G6PC promoter or NCOR to the BMAL1 promoter [29, 30], which is involved in the repressive effects of the compound on REV-ERBα target genes. Activation of REV-ERBα by GSK4112 blocks TNF-induced astrocyte activation, as evidenced by reductions in TNF-induced MMP-9 and CCL2[31]. Thus, data suggest that REV-ERBα is involved in the regulation of the expression of circadian genes, such as BMAL1, and the activation of astrocytes via multiple pathways. In our study, REV-ERBα activation by GSK4112 inhibited iNOS and COX-2 expression as well as IL-6 and TNFα production in LPS-treated microglia. This finding further suggests a role of REV-ERBα in the regulation of immune cells in response to stimulation.
The NF-κB signal has been documented to be regulated by circadian clockworks. NF-κB signaling is activated in Cry1- or Cry2-deficient cells[25]. Interestingly, CLOCK increases NF-κB activity and the role of TNF in the absence of BMAL1, suggesting a circadian rhythm-independent role of CLOCK in the regulation of NF-κB activity[32]. In peritoneal cells lacking BMAL1 (transcriptional factor of REV-ERBα), phosphorylation of p65 after LPS treatment was increased to levels greater than those noted in control cells[33]. Knockout of the retinoid-related orphan receptor-alpha (RORα), which is a potent competitive repressor of REV-ERBα, decreases cytokine production, and RORα inhibition decreases the inflammatory profile and suppresses atherosclerosis[34]. In contrast, REV-ERBα activation by SR9009 decreases cytokine production via inhibition of the NF-κB pathway and promotes survival in myocardial inaction animals [35], suggesting inhibitory effects of REV-ERBα on the NF-κB pathway. In our study, GSK4112 pretreatment inhibited the phosphorylation and nuclear translocation of p65 in microglia, suggesting a role of REV-ERBα in the regulation of microglial activation. In addition, GSK4112 decreased LPS-induced neuroinflammation and dopaminergic (DA) neuronal loss in the VMB. In cultured primary microglia, GSK4112 inhibited inflammatory factor production, thus reducing microglial CM toxicity to neurons. Our study provides a potential therapeutic method by targeting REV-ERBα for anti-neuroinflammation.
Acknowledgements
This work was supported by the National Key Scientific R&D Program of China (No. 2016YFC1306000), the National Natural Science Foundation of China (No's. 31471012, 81761148024, and 31330030), Suzhou Clinical Research Center of Neurological Disease (No. Szzx201503) and a Project Funded by the Priority Academic Program Development of Jiangsu Higher Education Institutions.
Author contribution
Dong-kai Guo performed most of the experiments and analyzed the data as well as drafted and revised manuscript; Yao Zhu supplemented experiments and revised the manuscript; Hong-yang Sun performed animal experiments; Xing-yun Xu performed immunoblot assays; Shun Zhang performed immunoblot assays; Zong-bing Hao performed immunoblot assays; Guang-hui Wang revised the manuscript; Chen-chen Mu performed immunoblot analyses; Hai-gang Ren analyzed the data, drafted the manuscript, and discussed the experiments.
Competing interests
The authors declare no competing interests.
References
- 1.Glass CK, Saijo K, Winner B, Marchetto MC, Gage FH. Mechanisms underlying inflammation in neurodegeneration. Cell. 2010;140:918–34. doi: 10.1016/j.cell.2010.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Prinz M, Priller J. Microglia and brain macrophages in the molecular age: from origin to neuropsychiatric disease. Nat Rev Neurosci. 2014;15:300–12. doi: 10.1038/nrn3722. [DOI] [PubMed] [Google Scholar]
- 3.Lin JR, Fang SC, Tang SS, Hu M, Long Y, Ghosh A, et al. Hippocampal CysLT1R knockdown or blockade represses LPS-induced depressive behaviors and neuroinflammatory response in mice. Acta Pharmacol Sin. 2017;38:477–87. doi: 10.1038/aps.2016.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hoesel B, Schmid JA. The complexity of NF-kappaB signaling in inflammation and cancer. Mol Cancer. 2013;12:86. doi: 10.1186/1476-4598-12-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Musiek ES, Holtzman DM. Mechanisms linking circadian clocks, sleep, and neurodegeneration. Science. 2016;354:1004–8. doi: 10.1126/science.aah4968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yin L, Wu N, Curtin JC, Qatanani M, Szwergold NR, Reid RA, et al. Rev-erbalpha, a heme sensor that coordinates metabolic and circadian pathways. Science. 2007;318:1786–9. doi: 10.1126/science.1150179. [DOI] [PubMed] [Google Scholar]
- 7.Meng QJ, McMaster A, Beesley S, Lu WQ, Gibbs J, Parks D, et al. Ligand modulation of REV-ERBalpha function resets the peripheral circadian clock in a phasic manner. J Cell Sci. 2008;121:3629–35. doi: 10.1242/jcs.035048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lam MT, Cho H, Lesch HP, Gosselin D, Heinz S, Tanaka-Oishi Y, et al. Rev-Erbs repress macrophage gene expression by inhibiting enhancer-directed transcription. Nature. 2013;498:511–5. doi: 10.1038/nature12209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sato S, Sakurai T, Ogasawara J, Takahashi M, Izawa T, Imaizumi K, et al. A circadian clock gene, Rev-erbalpha, modulates the inflammatory function of macrophages through the negative regulation of Ccl2 expression. J Immunol. 2014;192:407–17. doi: 10.4049/jimmunol.1301982. [DOI] [PubMed] [Google Scholar]
- 10.Xia Q, Hu Q, Wang H, Yang H, Gao F, Ren H, et al. Induction of COX-2-PGE2 synthesis by activation of the MAPK/ERK pathway contributes to neuronal death triggered by TDP-43-depleted microglia. Cell Death Dis. 2015;6:e1702. doi: 10.1038/cddis.2015.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guo D, Zhang S, Sun H, Xu X, Hao Z, Mu C, et al. Tyrosine hydroxylase down-regulation after loss of Abelson helper integration site 1 (AHI1) promotes depression via the circadian clock pathway in mice. J Biol Chem. 2018;293:5090–101. doi: 10.1074/jbc.RA117.000618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yu YX, Li YP, Gao F, Hu QS, Zhang Y, Chen D, et al. Vitamin K2 suppresses rotenone-induced microglial activation in vitro. Acta Pharmacol Sin. 2016;37:1178–89. doi: 10.1038/aps.2016.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fu K, Wang Y, Guo D, Wang G, Ren H. Familial Parkinson’s disease-associated L166P mutant DJ-1 is cleaved by mitochondrial serine protease Omi/HtrA2. Neurosci Bull. 2017;33:685–94. doi: 10.1007/s12264-017-0196-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yin L, Lazar MA. The orphan nuclear receptor Rev-erbalpha recruits the N-CoR/histone deacetylase 3 corepressor to regulate the circadian Bmal1 gene. Mol Endocrinol. 2005;19:1452–9. doi: 10.1210/me.2005-0057. [DOI] [PubMed] [Google Scholar]
- 15.Norden DM, Trojanowski PJ, Villanueva E, Navarro E, Godbout JP. Sequential activation of microglia and astrocyte cytokine expression precedes increased Iba-1 or GFAP immunoreactivity following systemic immune challenge. Glia. 2016;64:300–16. doi: 10.1002/glia.22930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li ST, Dai Q, Zhang SX, Liu YJ, Yu QQ, Tan F, et al. Ulinastatin attenuates LPS-induced inflammation in mouse macrophage RAW264.7 cells by inhibiting the JNK/NF-kappaB signaling pathway and activating the PI3K/Akt/Nrf2 pathway. Acta Pharmacol Sin. 2018 Jan 11. 10.1038/aps.2017.143. [DOI] [PMC free article] [PubMed]
- 17.Huang Q, Wang T, Wang HY. Ginsenoside Rb2 enhances the anti-inflammatory effect of omega-3 fatty acid in LPS-stimulated RAW264.7 macrophages by upregulating GPR120 expression. Acta Pharmacol Sin. 2017;38:192–200. doi: 10.1038/aps.2016.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sandur SK, Ichikawa H, Sethi G, Ahn KS, Aggarwal BB. Plumbagin (5-hydroxy-2-methyl-1,4-naphthoquinone) suppresses NF-kappaB activation and NF-kappaB-regulated gene products through modulation of p65 and IkappaBalpha kinase activation, leading to potentiation of apoptosis induced by cytokine and chemotherapeutic agents. J Biol Chem. 2006;281:17023–33. doi: 10.1074/jbc.M601595200. [DOI] [PubMed] [Google Scholar]
- 19.Sun X, Li L, Ma HG, Sun P, Wang QL, Zhang TT, et al. Bisindolylmaleimide alkaloid BMA-155Cl induces autophagy and apoptosis in human hepatocarcinoma HepG-2 cells through the NF-kappaB p65 pathway. Acta Pharmacol Sin. 2017;38:524–38. doi: 10.1038/aps.2016.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ren H, Fu K, Wang D, Mu C, Wang G. Oxidized DJ-1 interacts with the mitochondrial protein BCL-XL. J Biol Chem. 2011;286:35308–17. doi: 10.1074/jbc.M110.207134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zaman F, Chrysis D, Huntjens K, Chagin A, Takigawa M, Fadeel B, et al. Dexamethasone differentially regulates Bcl-2 family proteins in human proliferative chondrocytes: role of pro-apoptotic Bid. Toxicol Lett. 2014;224:196–200. doi: 10.1016/j.toxlet.2013.10.020. [DOI] [PubMed] [Google Scholar]
- 22.Hampton T. Circadian clock boosts stress-response genes during aging. JAMA. 2017;317:1307. doi: 10.1001/jama.2017.3108. [DOI] [PubMed] [Google Scholar]
- 23.Sulli G, Rommel A, Wang X, Kolar MJ, Puca F, Saghatelian A, et al. Pharmacological activation of REV-ERBs is lethal in cancer and oncogene-induced senescence. Nature. 2018;553:351–5. doi: 10.1038/nature25170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Scheiermann C, Kunisaki Y, Frenette PS. Circadian control of the immune system. Nat Rev Immunol. 2013;13:190–8. doi: 10.1038/nri3386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Narasimamurthy R, Hatori M, Nayak SK, Liu F, Panda S, Verma IM. Circadian clock protein cryptochrome regulates the expression of proinflammatory cytokines. Proc Natl Acad Sci USA. 2012;109:12662–7. doi: 10.1073/pnas.1209965109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gibbs JE, Blaikley J, Beesley S, Matthews L, Simpson KD, Boyce SH, et al. The nuclear receptor REV-ERBalpha mediates circadian regulation of innate immunity through selective regulation of inflammatory cytokines. Proc Natl Acad Sci USA. 2012;109:582–7. doi: 10.1073/pnas.1106750109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Raghuram S, Stayrook KR, Huang P, Rogers PM, Nosie AK, McClure DB, et al. Identification of heme as the ligand for the orphan nuclear receptors REV-ERBalpha and REV-ERBbeta. Nat Struct Mol Biol. 2007;14:1207–13. doi: 10.1038/nsmb1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Burris TP, Busby SA, Griffin PR. Targeting orphan nuclear receptors for treatment of metabolic diseases and autoimmunity. Chem Biol. 2012;19:51–9. doi: 10.1016/j.chembiol.2011.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Grant D, Yin L, Collins JL, Parks DJ, Orband-Miller LA, Wisely GB, et al. GSK4112, a small molecule chemical probe for the cell biology of the nuclear heme receptor Rev-erbalpha. ACS Chem Biol. 2010;5:925–32. doi: 10.1021/cb100141y. [DOI] [PubMed] [Google Scholar]
- 30.Kumar N, Solt LA, Wang Y, Rogers PM, Bhattacharyya G, Kamenecka TM, et al. Regulation of adipogenesis by natural and synthetic REV-ERB ligands. Endocrinology. 2010;151:3015–25. doi: 10.1210/en.2009-0800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Morioka N, Tomori M, Zhang FF, Saeki M, Hisaoka-Nakashima K, Nakata Y. Stimulation of nuclear receptor REV-ERBs regulates tumor necrosis factor-induced expression of proinflammatory molecules in C6 astroglial cells. Biochem Biophys Res Commun. 2016;469:151–7. doi: 10.1016/j.bbrc.2015.11.086. [DOI] [PubMed] [Google Scholar]
- 32.Spengler ML, Kuropatwinski KK, Comas M, Gasparian AV, Fedtsova N, Gleiberman AS, et al. Core circadian protein CLOCK is a positive regulator of NF-kappaB-mediated transcription. Proc Natl Acad Sci USA. 2012;109:E2457–65. doi: 10.1073/pnas.1206274109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Curtis AM, Fagundes CT, Yang G, Palsson-McDermott EM, Wochal P, McGettrick AF, et al. Circadian control of innate immunity in macrophages by miR-155 targeting Bmal1. Proc Natl Acad Sci USA. 2015;112:7231–6. doi: 10.1073/pnas.1501327112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Billon C, Sitaula S, Burris TP. Inhibition of RORalpha/gamma suppresses atherosclerosis via inhibition of both cholesterol absorption and inflammation. Mol Metabol. 2016;5:997–1005. doi: 10.1016/j.molmet.2016.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stujanna EN, Murakoshi N, Tajiri K, Xu D, Kimura T, Qin R, et al. Rev-erb agonist improves adverse cardiac remodeling and survival in myocardial infarction through an anti-inflammatory mechanism. PLoS ONE. 2017;12:e0189330. doi: 10.1371/journal.pone.0189330. [DOI] [PMC free article] [PubMed] [Google Scholar]