Abstract
The disturbance of maternal immune tolerance to a semiallogeneic fetus is recognized as one of the key pathologies of preeclampsia (PE), in which an imbalance between the inflammation-limiting regulatory T cells (Tregs) and the inflammation-mediating Th17 cells plays an essential role. Previously, we reported that the abnormal upregulation of tetraspannin CD81 in trophoblast cells (fetal component) participated in the pathogenesis of PE. However, as one of the potential immune regulatory molecules, whether CD81 induces PE by interfering with the balance of the maternal immune system has not yet been clarified. Thus, we investigated the relationship between the upregulation of CD81 in trophoblast cells and the imbalance of Treg and Th17 cells in mothers. Here, we demonstrated that upregulation of CD81 in trophoblast cells was accompanied by a decrease in Treg cells and an increase in Th17 cells in both the basal plate (placental maternal side) and peripheral blood of patients with PE. In vitro culture of naïve T cells with medium from the CD81-overexpressing trophoblast cell line HTR-8 resulted in enhanced differentiation of T cells into Th17 cells and decreased the formation of Tregs, which was dependent on the paracrine signaling of IL-6 in trophocytes, induced by CD81. In a CD81-induced PE rat model, we found a significant shift of T cell differentiation towards Th17 cells, and administration of IL-6 antibody mitigated the PE phenotype and the imbalance of the Treg/Th17 cells. These results define a vital regulatory cascade involving trophocyte-derived CD81, IL-6, and maternal Treg/Th17 cells in the pathogenesis of PE and suggests new therapeutic approaches based on CD81 and IL-6 downregulation to prevent human PE.
Keywords: CD81; preeclampsia; Treg; Th17; trophoblasts; immune tolerance; IL-6
Subject terms: Immunology, Signal transduction
Introduction
Preeclampsia (PE) is a pregnancy-specific syndrome that affects approximately 3–5% of pregnant women and is a main cause of maternal, fetal, and neonatal morbidity and mortality worldwide.1 Although efforts have been made, the mechanisms underlying PE remain elusive.1–4 Increasing evidence has indicated that a disturbed tolerance at the maternal–fetal interface plays an important role in the pathogenesis of PE.5 In response to stimulation from the semiallogeneic conceptus, naïve T cells readily differentiate into Treg cells in normal pregnancy. By creating a “tolerant” microenvironment, Treg cells at the maternal–fetal interface have been shown to prevent fetus rejection.6 Another lineage of CD4+ T cells are Th17 cells, which mediate the inflammatory response against pathogenic infections.7 Th17 cells increase significantly in allogeneic pregnant CBA/J × DBA/2 mice compared with syngeneic pregnant CBA/J × CBA/J mice and have a potential role in spontaneous fetus loss.8 In PE, there are implications that an imbalance in Treg and Th17 cell differentiation may be an important factor in the disease development.9,10 However, the factors that cause the Treg/Th17 imbalance in PE remain to be clarified.
It is well known that trophoblasts play an important role in instructing maternal T cell activation and differentiation to maintain maternal–fetal tolerance.11,12 Trophoblast abnormalities could contribute to immune deregulation. Previously, we reported that CD81 was significantly increased in both cytotrophoblast and syncytiotrophoblast cells of patients with early-onset severe PE (sPE), and injecting AdCD81 into rats could induce a PE-like model. These results indicate that CD81 is pathologically related to PE.13 In the current study, we demonstrated that CD81 upregulation in trophoblasts promoted a shift of T cell differentiation to Th17 and inhibited Treg differentiation. This effect was mediated by the paracrine signal of interlekin-6 (IL-6) from trophoblasts. Our results highlight CD81 as a pivotal molecule in the regulation of Treg/Th17 imbalance in the pathogenesis of PE.
Materials and methods
Patients and sample collection
This study was reviewed and approved by the Ethics Committee of Drum Tower Hospital, Nanjing University Medical School. Written informed consent was obtained from all participants. The placental tissues used in this study were obtained from the Jiangsu Biobank of Clinical Resources.13 To study the correlation between CD81 and Treg/Th17 in early-onset sPE, 40 basal plates of placenta from sPE (n = 20) and nPTB (non-infected preterm birth, n = 20) were collected for RT-PCR and immunohistochemistry analyses. sPE was diagnosed according to the 23rd edition of Williams Obstetrics.14 Patients with chronic hypertension, gestational diabetes mellitus, renal disease, autoimmune diseases, and hepatitis with HCV infection were excluded from this study. The clinical characteristics of sPE and nPTB are summarized in Table 1.
Table 1.
Clinical characteristics of patients collected for the placentas (values are mean ± SD)
| Analyzed items | nPTB, n = 20 | sPE, n = 20 | P value |
|---|---|---|---|
| Maternal age, years | 30.25 ± 1.23 | 30.35 ± 1.26 | >0.05 |
| Gestational age, weeks | 31.36 ± 0.53 | 31.68 ± 0.56 | >0.05 |
| BMI, kg/m2 | 26.83 ± 0.26 | 27.54 ± 0.72 | >0.05 |
| Systolic BP, mmHg | 115.70 ± 2.25 | 152.80 ± 6.31 | <0.05 |
| Diastolic BP, mmHg | 69.95 ± 1.71 | 101.10 ± 4.15 | <0.05 |
| Proteinuria | − | + to ++++ | <0.05 |
| Fetal weight, g | 1827.00 ± 105.60 | 1552.00 ± 100.30 | >0.05 |
| Placental weight, g | 437.10 ± 21.40 | 433.30 ± 43.86 | >0.05 |
BP blood pressure, nPTB non-infected preterm birth
For evaluating the correlation between CD81 and Treg/Th17 in peripheral circulation, fresh whole-blood samples were collected from pregnant women with early-onset sPE (n = 10) at the time of disease diagnosis and from normal pregnant (NP) women (n = 10) at the time they were receiving routine blood examination during approximately 32 weeks of antenatal care. The clinical characteristics of the two groups are summarized in Table 2.
Table 2.
Clinical characteristics of patients collected for fresh whole blood (values are mean ± SD)
| Analyzed items | NP, n = 10 | sPE, n = 10 | P value |
|---|---|---|---|
| Maternal age, years | 29.80 ± 1.65 | 29.90 ± 1.40 | >0.05 |
| Gestational age, weeks | 32.07 ± 0.64 | 31.64 ± 0.87 | >0.05 |
| BMI, kg/m2 | 26.27 ± 1.36 | 27.90 ± 1.06 | >0.05 |
| Systolic BP, mmHg | 117.90 ± 2.42 | 159.40 ± 11.28 | <0.05 |
| Diastolic BP, mmHg | 75.20 ± 2.63 | 104.80 ± 7.09 | <0.05 |
| Proteinuria | − | + to ++++ | <0.05 |
BP blood pressure, NP normal pregnancy
Antibodies and cytokines
The antibodies used in this study are listed in Table 3. Recombinant human IL-2 and transforming growth factor-β (TGFβ) were purchased from PeproTech (Rocky Hill, NJ, USA).
Table 3.
Antibodies used for flow cytometry (FC), immunohistochemistry (IHC), immunofluorescence (IF), western blotting (WB), and cell culture
| Antigen | Label | Reactivity | Clone | Manufacturer | Application |
|---|---|---|---|---|---|
| CD4 | FITC | Human | SK-3 | eBioscience | FC |
| CD25 | APC | Human | BC96 | eBioscience | FC |
| FOXP3 | PE | Human | 236A/E7 | eBioscience | FC |
| IL-17A | APC | Human | eBio64DEC17 | eBioscience | FC |
| CD4 | FITC | Rat | OX35 | eBioscience | FC |
| CD25 | APC | Rat | OX39 | eBioscience | FC |
| FOXP3 | PE | Rat | FJK-16s | eBioscience | FC |
| IL-17A | APC | Rat | eBio17B7 | eBioscience | FC |
| CD81 | – | Human | 1.3.3.22 | Santa Cruz | IHC/IF |
| FOXP3 | – | Human | 236A/E7 | Abcam | IHC |
| IL-17A | – | Human | ab9565 | Abcam | IHC |
| P-P65 | – | Human/rat | 93H1 | Cell Signaling Technology | WB/IF |
| P65 | – | Human/rat | L8F6 | Cell Signaling Technology | WB |
| FLAG | – | Proteintech | WB | ||
| P-STAT3 | – | Human/rat | Y705 | Cell Signaling Technology | WB |
| STAT3 | – | Human/rat | 124H6 | Cell Signaling Technology | WB |
| GAPDH | – | Human/rat | 6C5 | Santa Cruz | WB |
| General secondary antibody | – | – | – | Typing | IHC |
| Goat anti-mouse | HRP | – | – | Cell Signaling Technology | WB |
| Goat anti-rabbit | HRP | – | – | Cell Signaling Technology | WB |
| Goat anti-mouse | Rhodamine | – | – | Jackson ImmunoResearch | IF |
| Goat anti-rabbit | Alexa Fluor 488 | – | – | Jackson ImmunoResearch | IF |
| CD3 | – | Human | OKT3 | eBioscience | Cell culture |
| CD28 | – | Human | CD28.2 | eBioscience | Cell culture |
| IL-6 | – | Human | 6708 | R&D Systems | Neutralization |
| IL-6 | – | Rat | P20607 | R&D Systems | Neutralization |
| FVS780 | – | 565388 | BD | Viability staining in FC | |
| CD3 | PE | Human | UCHT1 | eBioscience | FC |
| Mouse IgG1, k FITC | FITC | 11-4714-42 | eBioscience | FC | |
| Mouse IgG1, k APC | APC | 17-4714-81 | eBioscience | FC | |
| Mouse IgG1, k PE | PE | 12-4714-81 | eBioscience | ||
| Cell Stimulation Cocktail (plus protein transport inhibitors) | 00-4975-93 | Invitrogen | FC |
FC flow cytometry, IHC immunohistochemistry, IF immunofluorescence, WB western blotting
Isolation of peripheral blood mononuclear cells
Whole peripheral mononuclear cells were isolated from fresh blood drawn in EDTA tubes by standard Ficoll Hypaque (Axis Shield, Dundee, Scotland, UK) gradient centrifugation. The obtained mononuclear cell-rich ring was washed twice with phosphate buffer solution (PBS) at 500 × g for 5 min. The isolated peripheral blood mononuclear cells (PBMCs) were used for CD4+ naïve T cell isolation and flow cytometry analysis.
Cells isolation and culture
HTR-8/SV neo cells, derived from human first trimester extravillous trophoblast cells, were maintained in a 5% CO2 incubator at 37 °C. RPMI 1640 medium (Thermo Fisher, Waltham, MA, USA) was supplemented with 10% heat-inactivated fetal bovine serum (FBS) (Thermo Fisher, Waltham, MA, USA), 100 U/mL penicillin, and 100 μg/mL streptomycin.
CD4+ naïve T cells were isolated from human PBMCs by magnetic-activated cell sorting (Stem Cell Technologies, New York, NY, USA) in accordance with the manufacturer’s protocol. The sorted and purified naïve T cells were cultured in 96-well plates coated with anti-CD3 (5 μg/mL) and anti-CD28 (5 μg/mL) in RPMI 1640 supplemented with 10% FBS, 100 U/mL penicillin, and 100 μg/mL streptomycin. For Treg cell polarization, the cultures were supplemented with IL-2 (10 ng/mL) and TGF-β (20 ng/mL). For Th17 cell polarization, a low dose of TGF-β (2 ng/mL) alone was added to the medium.15 IL-6 neutralizing antibody (5 μg/mL) and recombinant human CD81 protein (Sino Biological Inc., Beijing, China) were used to investigate the role of IL-6 and soluble CD81 in the differentiation of T cells. After 84 h of culture, the cells were harvested for flow cytometry analysis.
Preparation of conditional culture medium and exosome isolation
HTR-8/SV neo cells were infected with a CD81 overexpression adenovirus (AdCD81) or AdCTL [200 multiplicity of infection (MOI)] for 48 h. Next, the culture media was centrifuged at 2000 × g for 5 min to remove the dead cells and was stored for coculturing with naïve T cells.
Exosomes were separated with differential centrifugal forces: 2000 × g for 5 min (to remove the dead cells), 10,000 × g for 30 min (to remove the cell debris), and 100,000 × g for 1 h. After the last centrifugation, the supernatant was collected as exosome-free culture medium, and the pellet was supplemented with RPMI 1640 as exosomes for naïve T cell coculture.
Animal model
Sprague–Dawley rats from the Animal Center of Nanjing Medical University, aged 8–12 weeks and weighing 240–260 g, were used. Pregnancy was achieved by housing female and male rats together for one night. Day 0 of pregnancy (GD0) was determined by the presence of vaginal spermatozoa. The pregnant rats on GD5 were injected with either AdCD81 or AdCTL (3 × 109 plaque-forming units) through the tail vein. The blood pressure and proteinuria (n = 8 in each group) were examined as described in a previous study.16 Fresh blood samples for evaluating T cell subsets were obtained when the rats (n = 3 in each group) were sacrificed at different time points (GD6, GD9, GD12, GD15, and GD18). The placentas and sera were collected. To block the effect of IL-6, rat IL-6 antibodies (R&D Systems) were administered at a dose of 16.6 μg/kg by intraperitoneal injection on GD6 following blood pressure measurement.17 The manipulation of rats used in this study was approved by the Ethics Review Board for Animal Studies of Drum Tower Hospital.
Quantitative real-time PCR
Briefly, total RNA (1 μg), extracted from tissues or cells with TRIzol (Invitrogen, Carlsbad, CA, USA), was reverse-transcribed into cDNA in a 20 μL reaction. Quantitative real-time PCR (RT-PCR) was performed as previously described.18 The data from three independent experiments were used for analyzing the relative gene expression. The primers used in this study are summarized in Table 4.
Table 4.
Oligonucleotide primer sequences for quantitative real-time PCR
| Genes | Forward primer | Reverse primer |
|---|---|---|
| Homo-CD81 | GCGCCCAACACCTTCTATGTA | CCAGGAAGCCAACGAACATCA |
| Homo-FOXP3 | GTGGCCCGGATGTGAGAAG | GGAGCCCTTGTCGGATGATG |
| Homo-RORC | CTGGGCATGTCCCGAGATG | GAGGGGTCTTGACCACTGG |
| Homo-GAPDH | CTGGGCTACACTGAGCACC | AAGTGGTCGTTGAGGGCAATG |
| Homo-IL1β | ATGATGGCTTATTACAGTGGCAA | GTCGGAGATTCGTAGCTGGA |
| Homo-IL6 | ACTCACCTCTTCAGAACGAATTG | CATCTTTGGAAGGTTCAGGTTG |
| Homo-IL21 | TAGAGACAAACTGTGAGTGGTCA | GGGCATGTTAGTCTGTGTTTCTG |
| Homo-IL23 | CTCAGGGACAACAGTCAGTTC | ACAGGGCTATCAGGGAGCA |
| Rat-FOXP3 | ACTTCTCAGGCTCCCTCTTC | CCGCACAGCAAACAAGC |
| Rat-RORC | GCATCCACTACGGGGTTAT | GCGGCTGGTTCGGTCAA |
| Rat-IL6 | CCAGCCAGTTGCCTTCTT | TCTGTTGTGGGTGGTATCCT |
| Rat-GAPDH | ATGGGAAGCTGGTCATCAAC | GGATGCAGGGATGATGTTCT |
Immunohistochemistry
Placental tissues were fixed with 10% formalin, dehydrated, and embedded in paraffin. Paraffin sections (2 μm) were prepared, dewaxed, hydrated, and endogenous peroxides were quenched with 0.3% H2O2. After heat-induced antigen retrieval, the sections were incubated with the primary antibodies anti-CD81 (1:500), anti-FOXP3 (1:100), and anti-IL-17 (1:500), followed by incubation with horseradish peroxidase-conjugated secondary antibody. After incubation with diaminobenzidine, the sections were viewed using a Leica DM6B microscope.
Flow cytometry and gating
For the analysis of Treg cells, PBMCs were stained for surface markers by incubation with anti-CD4 and anti-CD25 for 20 min. Intracellular staining of FOXP3 was performed following fixation and permeabilization according to the manufacturer’s instructions. For the analysis of Th17 cells, PBMCs were stimulated for 4 h with a cell stimulation cocktail containing protein transport inhibitors (Invitrogen, Carlsbad, CA, USA) in 24-well plates. The cells were then harvested into 5 mL sterile tubes, washed once in PBS, and stained for viability (FVS780, Fixable Viability Stain 780) and surface markers (anti-CD3 and anti-CD4) for 20 min. Subsequently, the cells were stained with anti-IL-17A after fixation and permeabilization. The samples were analyzed using a BD Aria II fluorescence-activated cell sorting (FACS) analyzer.
Enzyme-linked immunosorbent assay
The human CD81 kit (detection range, 0.5−15 ng/mL), human IL-6 kit (detection range, 0.4–9 pg/mL), and rat IL-6 kit (detection range, 8–150 pg/mL) were purchased from SenBeiJia Biological Technology (Nanjing, JS, China). Enzyme-linked immunosorbent assay (ELISA) tests were carried out strictly following the manufacturer’s instructions.
Western blotting
Proteins were separated by SDS-PAGE. Primary antibodies against FLAG (1:5000), p-P65 (1:1000), P65 (1:1000), P-STAT3 (1:1000), STAT3 (1:1000), and GAPDH (1:1000) were used for immunoblotting. Immunodetection was accomplished by goat anti-rabbit (1:2000) or goat anti-mouse (1:2000) HRP-conjugated secondary antibody and an enhanced chemiluminescence detection kit (Bio-Rad, Berkeley, California, USA).
Immunofluorescence for nuclear translocation of NF-κB p65
HTR-8 cells infected with AdCD81 or AdCTL were fixed in 4% formaldehyde for 20 min at room temperature and permeabilized with 0.1% Triton X-100. The cells were incubated with antibodies against CD81 (1:100) and p-P65 (1:100) overnight and were then incubated with rhodamine-conjugated goat anti-mouse (1:200) and Alexa Fluor 488-conjugated goat anti-rabbit (1:200) for 1 h. For nuclear staining, cells were further incubated with DAPI (Abcam, Cambridge, MA, USA). A Leica DM6B microscope was used for observing the images.
Statistical analysis
Normally distributed data are presented as the mean ± SD. Differences between two groups were analyzed by a two-tailed Student’s t-test. Multiple comparisons were assessed by ANOVA. For all statistical tests, P values < 0.05 were considered to be significantly different.
Results
Upregulation of CD81 is accompanied by the imbalance of Treg/Th17 cells in patients with sPE
To test whether the upregulation of CD81 in trophocytes of patients with PE was associated with an imbalance of Treg/Th17 cells, we first evaluated the expression level of CD81 and the number of Treg and Th17 cells in basal plates of placenta from patients with early-onset sPE. Compared with nPTB, we confirmed a significant upregulation of CD81 in the placental basal plates of sPE patients, which was accompanied by a significant decrease in FOXP3 (a specific nuclear transcription factor of Tregs) and an increase in RORC (a specific nuclear transcription factor of Th17 cells) (Fig. 1a). The ratio of FOXP3/RORC was much lower in sPE than in nPTB. Immunolocalization analysis demonstrated that the upregulation of CD81 in both villous trophoblasts and extravillous trophoblasts simultaneously existed with decreased numbers of FOXP3+ cells and increased numbers of IL-17+ cells in maternal decidua immunocytes of sPE (Fig. 1b).
Fig. 1.
Upregulation of CD81 accompanied by the imbalance of Treg/Th17 is observed in women with sPE. a The expression of CD81, FOXP3, and RORC in the basal plates of placenta from early-onset sPE (n = 20) and gestational age-matched nPTB (n = 20) were detected by RT-PCR. The FOXP3/RORC ratio was analyzed. b The localization and expression of CD81 (in FV and BP), FOXP3, and IL-17A (in BP) were analyzed by immunohistology. c The expression of CD81 in maternal plasma (sPE, 10; gestational age-matched NP, 10) was detected by ELISA. d The proportions of Treg and Th17 cells in peripheral blood from early-onset sPE (n = 10) and gestational age-matched NP (n = 10) were assessed by flow cytometry analysis. sPE severe preeclampsia, nPTB noninfective preterm birth, NP normal pregnancy, FV free villous, BP basal plate. The data are presented as the mean ± SD. *P < 0.05, **P < 0.01, ***P < 0.001; ns, no significance. Scale bars, 50 μm
Considering that CD81 from syncytiotrophoblasts could be released into the maternal peripheral circulation,13 we explored whether the level of CD81 was related to the imbalance of Treg and Th17 cells in maternal peripheral blood. Fresh blood samples from sPE (n = 10) and gestational age-matched NP (n = 10) women were collected. Compared with gestational age-matched NP, the concentration of CD81 increased significantly in the plasma of sPE (Fig. 1c) patients. Simultaneously, Tregs decreased and Th17 cells increased significantly in the peripheral blood of sPE patients (Fig. 1d) (gating strategies in Supplementary Fig 1). These results showed an association between abnormally upregulated CD81 in trophocytes and a shift towards Th17 differentiation in the mothers.
Overexpression of CD81 in trophoblast cells inhibits Tregs and promotes Th17 cell differentiation through a paracrine-dependent mode in vitro
To further clarify the correlation between CD81 and the imbalance of Treg and Th17 cells in PE, naïve CD4+ T cells were treated with cell-conditioned medium from AdCD81- or AdCTL-infected HTR-8/SV neo cells under Treg or Th17 polarization conditions (as described in the Methods). After 84 h, the proportions of Treg and Th17 cells were detected by FACS (Fig. 2a, b). The cell-conditioned medium from AdCD81-infected HTR-8/SV neo cells inhibited the differentiation of Tregs (73.77 ± 3.92 vs. 44.48 ± 6.73, P < 0.001) and promoted the differentiation of Th17 cells (2.48 ± 0.64 vs. 4.21 ± 1.09, P < 0.01). We also quantified the relative expression of FOXP3 (cells under Treg polarizing conditions) and RORC (cells under Th17 polarizing conditions) by RT-PCR (Fig. 2c, d). The results showed that the expression of FOXP3 decreased markedly, while the expression of RORC increased significantly.
Fig. 2.
CD81 overexpression inhibits the differentiation of Tregs and promotes the differentiation of Th17 cells in vitro. Naïve CD4+ T cells were separated from healthy donors and then cocultured in conditional medium from AdCD81- or AdCTL-infected HTR-8/SV neo cells. a The proportion of Tregs was analyzed by flow cytometry after 84 h of coculture under Treg cell polarizing conditions. b The proportion of Th17 cells was analyzed after 84 h of coculture under Th17 cell polarizing conditions. c, d FOXP3 and RORC mRNA levels of differentiated T cells were quantified by RT-PCR. The results were from at least three independent experiments (a–d). *P < 0.05, **P < 0.01, ***P < 0.001
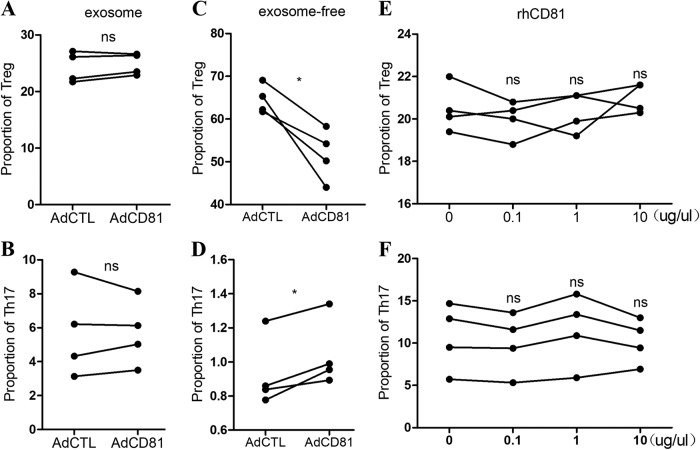
To identify the mediator of this phenomenon, we first tested whether CD81 regulates the differentiation of naïve T cells through exosomes secreted by trophoblasts, as it is widely known that CD81 is a main component of exosomes. We separated the exosomes from the culture media of AdCD81- or AdCTL-infected HTR-8 cells by differential centrifugation, as described in the Methods. The exosome or exosome-free medium was then used to culture the naïve CD4+ T cells. Compared with the AdCTL group, the exosome-free medium in the AdCD81 group significantly inhibited Tregs and promoted Th17 cell differentiation (Fig. 3c, d), but the exosomes did not show any obvious effects on the differentiation of Treg or Th17 cells (Fig. 3a, b). Because CD81 also exists in serum as a soluble form that is free of exosomes,13 we treated naïve CD4+ T cells with the recombinant large extracellular loop of CD81 under the polarizing condition of Treg or Th17 cells. The results showed that there was no obvious change in the differentiation of Treg or Th17 cells in response to the soluble CD81 protein (Fig. 3e, f), which indicated that CD81 did not directly regulate T cell differentiation.
Fig. 3.
CD81 inhibits Tregs and promotes Th17 cell differentiation through a paracrine-dependent mode. The culture media from AdCD81- or AdCTL-infected HTR-8/SV neo cells were separated into exosome and exosome-free media by differential centrifugation. Exosomes (a, b) and exosome-free media (c, d) were added into naïve T cell culture medium under Treg or Th17 polarizing conditions, respectively. The proportions of Treg cells (a, c) and Th17 cells (b, d) were analyzed by flow cytometry. The large extracellular loop of recombinant human CD81 (rhCD81) was added to the naïve T cell culture medium under Treg or Th17 polarizing conditions at different concentrations (e, f). The proportion of Treg cells (e) and Th17 cells (f) was then analyzed by flow cytometry. The results were from at least three independent experiments. *P < 0.05; ns, no significance
IL-6 mediates the imbalance of maternal Treg/Th17 cells triggered by CD81 in trophoblasts
Since cytokines serve as the important regulator of naïve T cell differentiation,19 we next measured the cytokines that have been reported to regulate T cell differentiation, including IL-1β, IL-6, IL-21, and IL-23, in AdCTL- and AdCD81-infected HTR-8 cells by RT-PCR. Of these cytokines, only IL-6 increased significantly in AdCD81-infected HTR-8 cells (Fig. 4a). ELISA assays confirmed that IL-6 protein increased significantly in the supernatant from AdCD81-infected HTR-8 cells (Fig. 4b). To further investigate the function of IL-6, naïve CD4+ T cells were treated with culture medium from AdCD81-infected HTR-8 cells with or without IL-6 neutralizing antibody (anti-IL-6). As the data showed, the imbalance of Treg and Th17 cells triggered by the CD81-upregulated trophoblast culture medium was alleviated by anti-IL-6 (Fig. 4c–f).
Fig. 4.
CD81 regulates the differentiation of Tregs and Th17 cells via IL-6. a The transcription levels of IL-1β, IL-6, IL-21, and IL-23 in HTR-8 cells from AdCTL- or AdCD81-infected HTR-8/SV neo cells were detected by RT-PCR. b The level of IL-6 in the supernatant was measured by ELISA. c, d Naïve T cells were cultured in the presence of conditioned medium from infected HTR-8 cells with/without anti-IL-6 under Treg cell-polarizing conditions for 3 days. The proportion of Treg cells was analyzed. e, f Naïve CD4+ T cells were separated and treated as in c, except for under Th17 cell-polarizing conditions. The proportion of Th17 cells was analyzed. The results were from at least three independent experiments. *P < 0.05, **P < 0.01; ns, no significance
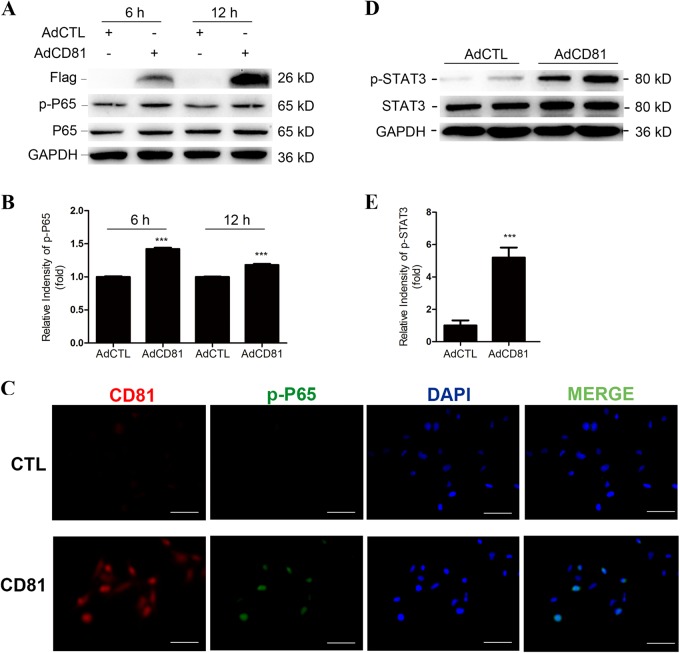
Next, we detected the upstream transcriptional factor of IL-6, phosphorylated NF-κB/P65, in HTR-8 neo cells after AdCD81 or AdCTL infection (Fig. 5a). The results showed that phosphorylated NF-κB/P65 was increased in AdCD81-infected cells compared with AdCTL-infected cells (Fig. 5b). Immunofluorescence analysis confirmed that CD81 promotes p65 nuclear translocation (Fig. 5c). Meanwhile, we detected the classical downstream transcription factor of IL-6, phosphorylated-STAT3, in PBMCs after coculture with the conditioned culture medium from AdCD81-infected HTR-8 cells, as the activation of STAT3 could promote the expression of RORC and inhibit the expression of FOXP3. The results showed that phosphorylated-STAT3 increased significantly in PBMCs cocultured with AdCD81-conditioned culture medium (Fig. 5d, e). All of the above results indicate that IL-6 plays a central role in the inhibition of Tregs and the promotion of Th17 cell differentiation via CD81.
Fig. 5.
CD81 promotes the secretion of IL-6 through the NF-κB pathway. a CD81-FLAG, p-P65, and P65 levels were determined by western blot analysis in HTR-8 after transduction with AdCTL or AdCD81 for 6 and 12 h. b Relative expression of p-P65/P65 in HTR-8 was analyzed. c Nuclear translocation of p65 NF-κB was confirmed by immunofluorescence microscopy. d p-STAT3 and STAT3 levels were determined by western blot analysis in PBMCs cultured with conditioned media from AdCTL- or AdCD81-infected HTR-8 cells. e Relative expression of p-STAT3/STAT3 in PBMCs was analyzed. The relative intensity of protein levels was assessed by ImageJ. The results were from at least three independent experiments. ***P < 0.001. Scale bars, 25 μm
The imbalance of Treg/Th17 cells is present in the AdCD81-induced PE-like rats and is reversed by anti-IL-6
A CD81-induced PE-like rat model was successfully constructed, as previously described.13 We investigated the effects of CD81 on the balance of Treg/Th17 cells in vivo. We separately collected the maternal side of the placenta in the AdCD81 and AdCTL groups from GD6 to GD18 (n = 3 at each time point) and detected the expression of FOXP3 and RORC by RT-PCR. As shown in Fig. 6a, b, the expression of FOXP3 decreased significantly after injection of AdCD81, and this tendency was maintained until GD12. The expression of RORC increased sharply in the AdCD81 group, and the increase could be observed from GD6 to GD18. The proportions of Treg and Th17 cells in the peripheral blood of the two groups were tested by FACS. The change of Treg and Th17 cells in the peripheral blood was similar to that in placenta (Fig. 6c, d). Furthermore, the NF-κB pathway was activated in the placenta of the AdCD81 group, which was consistent with the in vitro experiments (Fig. 6e). Additionally, IL-6 significantly increased in the serum of CD81-overexpressing rats (Fig. 6f), while the administration of anti-IL-6 antibody to the PE rat model on GD6 following CD81 induction successfully reversed the decreased Treg and increased Th17 proportions (Fig. 7a, b). In addition, PE-like symptoms, including high blood pressure, elevated urinary protein, and fetal growth retardation (Fig. 7c–e), were alleviated after anti-IL-6 administration.
Fig. 6.
The imbalance of Treg and Th17 cells is observed in the AdCD81-induced PE-like rats. a, b Pregnant rats were infected with either AdCD81 or AdCTL on the fifth day of gestation (GD5). The transcriptional levels of FOXP3 and RORC in the placenta from GD6 to GD18 were detected by RT-PCR. c, d The proportions of Treg and Th17 cells were analyzed every 3 days from GD6 to GD18 by FACS (n = 3 at each time point). e The expression of p-P65 and P65 in rat placentas was detected by WB. GAPDH served as an internal control. The relative expression of p-P65/P65 was assessed by ImageJ. f The protein level of IL-6 in rat serum was detected by ELISA. GD gestational day. *P < 0.05, **P < 0.01, ***P < 0.001; ns, no significance
Fig. 7.
Anti-IL-6 blocks CD81-induced PE-like symptoms and reverses the imbalance of Treg/Th17 cells. Pregnant rats were infected with AdCD81 or AdCTL on GD5. IL-6-blocking antibodies were administered by intraperitoneal injection on GD6 after blood pressure measurement. The proportions of Tregs (a) and Th17 cells (b) were analyzed after sacrifice on GD9 (AdCTL, n = 3; AdCD81, n = 6; AdCTL + anti-IL-6, n = 4; AdCD81 + anti-IL-6, n = 7). c Systolic blood pressure and d Urinary protein concentration were increased in AdCD81-infected rats and were alleviated by anti-IL6. AdCD81 (n = 5) vs. AdCTL (n = 3), P < 0.05; AdCD81 + anti-IL-6 (n = 5) vs. AdCD81 (n = 5), P < 0.05; AdCTL + anti-IL-6 (n = 3) vs. AdCTL (n = 3), P > 0.05. e Fetal weights were recorded on GD19 when the pregnant rats were sacrificed (AdCTL, n = 3, litter size = 47; AdCD81, n = 5, litter size = 65; AdCTL + anti-IL-6, n = 3, litter size = 48; AdCD81 + anti-IL-6, n = 5, litter size = 71). The significant differences in a, b, e were analyzed by t-test. The significant differences in c, d were determined by repeated measurement ANOVA. *P < 0.05, **P < 0.01, ***P < 0.001; ns, no significance
Discussion
The balance between Treg and Th17 cells is critical for maintaining homeostasis.20 At the maternal–fetal interface, immunity is a double-edged sword. It protects local tissues against infection while also tolerating the semiallogeneic fetus.11 Treg cells are one of the most important groups of immune regulatory cells at the maternal–fetal interface. The Treg cells dampen the excessive immune response and thus are thought to be necessary for a successful pregnancy. Counterfunctional to Tregs is the Th17 cell group, which mediates the inflammatory response upon recognition of foreign antigens in pregnancy.7,21
At the implantation site, the outer surface of the placenta is embedded within the maternal decidua. This is where placental trophoblasts and uterine leukocytes most obviously come into contact and where any frontal immunological assault on the placenta would originate.11,22 T cells comprise approximately 10–20% of the total leukocytes at this maternal–fetal interface, among which Tregs help the conceptus evade maternal immune rejection.11 Although many studies have reported that a deficiency of Tregs in the decidua leads to adverse pregnancy outcomes, most of them focused on abortion,23,24 but in studies of PE, few reports have referred to the deficiency at this interface.25 As such, we are the first group to detect the number of Tregs and counterfunctional Th17 cells in the basal plates by RT-PCR and immunolocalization, and we have verified a decrease of Tregs and an increase of Th17 cells in PE at this maternal–fetal interface.
The adaptation of maternal immunity occurs not only locally at the site of implantation but also globally in the circulation, as the second maternal–fetal interface formed by syncytiotrophoblast cells is surrounded by maternal blood.26,27 Evidence that pregnancy can ameliorate the course of autoimmune diseases, which often relapse after delivery,28 also suggests a systemic change of maternal immune function during pregnancy. Many studies have reported an association between PE and reduced Treg cells in the circulation, while the results regarding the proportion of Tregs in PE are somewhat inconsistent due to the different markers that have been used to identify Tregs.25 Researchers have used CD4+CD25+, CD4+FOXP3+, or CD4+CD25brightCTLA-4+ to identify Tregs,29,30 which could lead to overestimation because although FOXP3 is widely used as the marker to identify Treg cells, it is also evidenced to be transiently expressed in activated CD4+CD25-T cells after stimulation.31,32 In the present study, we combined three makers (CD4+CD25+FOXP3+) to detect Treg cells in flow cytometry and showed that the proportion of Tregs in sPE is significantly lower than that in gestational age-matched controls.
We have previously reported that CD81 was significantly upregulated in trophoblasts in sPE.13 It is widely known that trophoblasts play a central part in the development and maintenance of a successful pregnancy, as they modulate maternal immune cells to ensure toleration of the fetus.12,33 Whether abnormally expressed CD81 caused PE by triggering the disturbance of immune regulatory function in trophoblasts has not been clarified. Over the past decades, CD81 has been reported to participate in the activation of T cells and B cells34; it controls sustained T cell activation signaling and preferentially costimulates naïve T cells.35 However, none of the studies have evaluated the contribution of CD81 in the differentiation of naïve T cells.
In this study, we first proved that trophoblast-derived CD81 inhibits Tregs but promotes Th17 cell differentiation through the upregulation of IL-6. To ascertain the mediator of this regulation, we assessed the effect of exosomes, exosome-free medium, and recombinant human CD81 protein, and only exosome-free medium functioned like whole conditioned medium, which suggests that CD81 regulates T cell differentiation by a paracrine effect. In T cell differentiation, cytokines in the environment determine the cell fate.36 In the current study, we found that IL-6 was the important mediator in this process. Neutralizing IL-6 not only alleviated CD81-induced Th17 bias in vitro and in vivo but also improved the clinical symptoms, including hypertension and proteinuria, in PE rats. Thus, we elucidated an important regulatory axis: trophoblast-derived CD81, IL-6, and maternal T cell differentiation.
Taken together, we verified that the upregulation of CD81 in trophoblast cells inhibits Tregs and promotes Th17 cell differentiation in mothers and damages the maternal immune tolerance environment, which participates in PE development. Furthermore, IL-6 was the mediator of this regulation, which could be a potential target in the treatment of PE. Our findings provide new insights into the function of CD81 in PE and advance our understanding of the crosstalk between fetuses and mothers.
Electronic supplementary material
Acknowledgements
This work was supported by the National Natural Science Foundation of China (81571462, 81600353, and 81701472), Jiangsu Provincial Key Medical Center (YXZXB2016004), Jiangsu Biobank of Clinical Resources (BM2015004), and Jiangsu Province Grant for Science and Technology (BK20161106). We would like to thank our patients and our colleagues at the Biobank of Nanjing for the donation and collection of placental tissues and blood samples in this study.
Author contributions
H.D. and Y.D designed and performed the experiments, data analysis, and manuscript drafting. Y.L. and Z.W. helped to construct the PE rat model and performed part of the flow cytometry analysis. D.L. and N.G. analyzed data and drew the graphs. R.L. and M.Z. summarized the data and wrote the manuscript. L.S. and X.Z designed the study and collected clinical samples. G.Z. and Y.H. planned and supervised this study. All authors have approved the final version.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally to this work: Hailin Ding, Yimin Dai.
Contributor Information
Guangfeng Zhao, Email: guangfeng_zhao@126.com.
Yali Hu, Email: yalihu@nju.edu.cn.
Electronic supplementary material
The online version of this article (10.1038/s41423-018-0186-9) contains supplementary material.
References
- 1.Mol BWJ, et al. Pre-eclampsia. Lancet. 2016;387:999–1011. doi: 10.1016/S0140-6736(15)00070-7. [DOI] [PubMed] [Google Scholar]
- 2.Buurma AJ, et al. Genetic variants in pre-eclampsia: a meta-analysis. Hum. Reprod. Update. 2013;19:289–303. doi: 10.1093/humupd/dms060. [DOI] [PubMed] [Google Scholar]
- 3.Rana S, Karumanchi SA, Lindheimer MD. Angiogenic factors in diagnosis, management, and research in preeclampsia. Hypertension. 2014;63:198–202. doi: 10.1161/HYPERTENSIONAHA.113.02293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McGinnis R, Steinthorsdottir V, Williams NO, Thorleifsson G, Shooter S. Variants in the fetal genome near FLT1 are associated with risk of preeclampsia. Nat. Genet. 2017;49:1255–1260. doi: 10.1038/ng.3895. [DOI] [PubMed] [Google Scholar]
- 5.Redman CW, Sargent IL. Immunology of pre-eclampsia. Am. J. Reprod. Immunol. 2010;63:534–543. doi: 10.1111/j.1600-0897.2010.00831.x. [DOI] [PubMed] [Google Scholar]
- 6.Gobert M, Lafaille J. Maternal-fetal immune tolerance, block by block. Cell. 2012;150:7–9. doi: 10.1016/j.cell.2012.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kimura A, Kishimoto T. IL-6: regulator of Treg/Th17 balance. Eur. J. Immunol. 2010;40:1830–1835. doi: 10.1002/eji.201040391. [DOI] [PubMed] [Google Scholar]
- 8.Fu B, et al. Natural killer cells promote immune tolerance by regulating inflammatory TH17 cells at the human maternal-fetal interface. Proc. Natl. Acad. Sci. USA. 2013;110:E231–E240. doi: 10.1073/pnas.1206322110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jianjun Z, Yali H, Zhiqun W, Mingming Z, Xia Z. Imbalance of T-cell transcription factors contributes to the Th1 type immunity predominant in pre-eclampsia. Am. J. Reprod. Immunol. 2010;63:38–45. doi: 10.1111/j.1600-0897.2009.00763.x. [DOI] [PubMed] [Google Scholar]
- 10.Zhang Y, et al. The altered PD-1/PD-L1 pathway delivers the ‘one-two punch’ effects to promote the Treg/Th17 imbalance in pre-eclampsia. Cell. Mol. Immunol. 2018;15:710–723. doi: 10.1038/cmi.2017.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Erlebacher A. Mechanisms of T cell tolerance towards the allogeneic fetus. Nat. Rev. Immunol. 2013;13:23–33. doi: 10.1038/nri3361. [DOI] [PubMed] [Google Scholar]
- 12.Du M, et al. Embryonic trophoblasts induce decidual regulatory T cell differentiation and maternal-fetal tolerance through thymic stromal lymphopoietin instructing dendritic cells. J. Immunol. 2014;192:1502–1511. doi: 10.4049/jimmunol.1203425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shen L, et al. Up-regulation of CD81 inhibits cytotrophoblast invasion and mediates maternal endothelial cell dysfunction in preeclampsia. Proc. Natl. Acad. Sci. USA. 2017;114:1940–1945. doi: 10.1073/pnas.1617601114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cunningham, F., Leveno, K., Bloom, S., Hauth, J., Rouse, D. & Spong, C. Williams Obstetrics 23rd edn (McGraw-Hill Education, New York, USA, 2009).
- 15.Laurence A, et al. Interleukin-2 signaling via STAT5 constrains T helper 17 cell generation. Immunity. 2007;26:371–381. doi: 10.1016/j.immuni.2007.02.009. [DOI] [PubMed] [Google Scholar]
- 16.Gong P, et al. Curcumin improves LPS-induced preeclampsia-like phenotype in rat by inhibiting the TLR4 signaling pathway. Placenta. 2016;41:45–52. doi: 10.1016/j.placenta.2016.03.002. [DOI] [PubMed] [Google Scholar]
- 17.Nozu T, Miyagishi S, Nozu R, Takakusaki K, Okumura T. Repeated water avoidance stress induces visceral hypersensitivity: Role of interleukin-1, interleukin-6, and peripheral corticotropin-releasing factor. J. Gastroenterol. Hepatol. 2017;32:1958–1965. doi: 10.1111/jgh.13787. [DOI] [PubMed] [Google Scholar]
- 18.Zhang Y, et al. MicroRNA-155 contributes to preeclampsia by down-regulating CYR61. Am. J. Obstet. Gynecol. 2010;202:466.e461–467. doi: 10.1016/j.ajog.2009.10.889. [DOI] [PubMed] [Google Scholar]
- 19.Littman DR, Rudensky AY. Th17 and regulatory T cells in mediating and restraining inflammation. Cell. 2010;140:845–858. doi: 10.1016/j.cell.2010.02.021. [DOI] [PubMed] [Google Scholar]
- 20.Knochelmann HM, et al. When worlds collide: Th17 and Treg cells in cancer and autoimmunity. Cell Mol. Immunol. 2018;15:458–469. doi: 10.1038/s41423-018-0004-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fu B, Tian Z, Wei H. TH17 cells in human recurrent pregnancy loss and pre-eclampsia. Cell Mol. Immunol. 2014;11:564–570. doi: 10.1038/cmi.2014.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Moffett A, Loke C. Immunology of placentation in eutherian mammals. Nat. Rev. Immunol. 2006;6:584–594. doi: 10.1038/nri1897. [DOI] [PubMed] [Google Scholar]
- 23.Li L, Tu J, Jiang Y, Zhou J, Schust DJ. Regulatory T cells decrease invariant natural killer T cell-mediated pregnancy loss in mice. Mucosal Immunol. 2017;10:613–623. doi: 10.1038/mi.2016.84. [DOI] [PubMed] [Google Scholar]
- 24.Wu L, et al. IL-7/IL-7R signaling pathway might play a role in recurrent pregnancy losses by increasing inflammatory Th17 cells and decreasing Treg cells. Am. J. Reprod. Immunol. 2016;76:454–464. doi: 10.1111/aji.12588. [DOI] [PubMed] [Google Scholar]
- 25.Rahimzadeh M, Norouzian M, Arabpour F, Naderi N. Regulatory T-cells and preeclampsia: an overview of literature. Expert Rev. Clin. Immunol. 2016;12:209–227. doi: 10.1586/1744666X.2016.1105740. [DOI] [PubMed] [Google Scholar]
- 26.Trowsdale J, Betz AG. Mother’s little helpers: mechanisms of maternal-fetal tolerance. Nat. Immunol. 2006;7:241–246. doi: 10.1038/ni1317. [DOI] [PubMed] [Google Scholar]
- 27.Arck PC, Hecher K. Fetomaternal immune cross-talk and its consequences for maternal and offspring’s health. Nat. Med. 2013;19:548–556. doi: 10.1038/nm.3160. [DOI] [PubMed] [Google Scholar]
- 28.Varghese S, Crocker I, Bruce IN, Tower C. Systemic lupus erythematosus, regulatory T cells and pregnancy. Expert Rev. Clin. Immunol. 2011;7:635–648. doi: 10.1586/eci.11.59. [DOI] [PubMed] [Google Scholar]
- 29.Toldi G, et al. Decreased number of FoxP3+ regulatory T cells in preeclampsia. Acta Obstet. Gynecol. Scand. 2008;87:1229–1233. doi: 10.1080/00016340802389470. [DOI] [PubMed] [Google Scholar]
- 30.Prins J, et al. Preeclampsia is associated with lower percentages of regulatory T cells in maternal blood. Hypertens. Pregnancy. 2009;28:300–311. doi: 10.1080/10641950802601237. [DOI] [PubMed] [Google Scholar]
- 31.Wang J, Ioan-Facsinay A, van der Voort EI, Huizinga TW, Toes RE. Transient expression of FOXP3 in human activated nonregulatory CD4+ T cells. Eur. J. Immunol. 2007;37:129–138. doi: 10.1002/eji.200636435. [DOI] [PubMed] [Google Scholar]
- 32.Miyao T, et al. Plasticity of Foxp3(+) T cells reflects promiscuous Foxp3 expression in conventional T cells but not reprogramming of regulatory T cells. Immunity. 2012;36:262–275. doi: 10.1016/j.immuni.2011.12.012. [DOI] [PubMed] [Google Scholar]
- 33.Zhang Y, et al. Human trophoblast cells induced MDSCs from peripheral blood CD14(+) myelomonocytic cells via elevated levels of CCL2. Cell Mol. Immunol. 2016;13:615–627. doi: 10.1038/cmi.2015.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Levy S. Function of the tetraspanin molecule CD81 in B and T cells. Immunol. Res. 2014;58:179–185. doi: 10.1007/s12026-014-8490-7. [DOI] [PubMed] [Google Scholar]
- 35.Sagi Y, Landrigan A, Levy R, Levy S. Complementary costimulation of human T-cell subpopulations by cluster of differentiation 28 (CD28) and CD81. Proc. Natl. Acad. Sci. USA. 2012;109:1613–1618. doi: 10.1073/pnas.1121307109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li P, Spolski R, Liao W, Leonard WJ. Complex interactions of transcription factors in mediating cytokine biology in T cells. Immunol. Rev. 2014;261:141–156. doi: 10.1111/imr.12199. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.