Memory traces are modified during several hours following learning, to produce behaviorally adaptive long-term memories. In a recent paper published in Cell Research, Zhang et al. provide evidence that N 6 -methyladenosine modification of mRNAs augments the strength of weak memories.
The relative strength of memories has a major role in cognition. Thus, memories not only need to be sufficiently strong for effective behavioral retrieval, but also to be adaptive for the individual, their strength needs to be adjusted to confidence level and relative importance. Remarkably, although mechanisms of learning and memory have been investigated in great detail1, very little is known about the mechanisms that regulate memory strength. This has inherent reasons, which include the fact that the strength of memories is ultimately evaluated at the time of behavioral retrieval, and that the processes involved in the consolidation of memories play out off-line, during several hours after the initial event of acquisition. As a consequence, investigations of the mechanisms underlying memory strength face challenges of definition (how to untangle strength of memory trace versus effectiveness of memory retrieval) and of experimental accessibility (how to untangle causal relationships when relevant cellular and molecular processes in subsets of involved neurons coexist with experimentally inaccessible network activity related to memory). To overcome these challenges, there has been a need for identification of relevant endogenous molecular processes that modulate memory strength after acquisition. The paper by Zhang et al.2 makes an important contribution towards this objective.
Studies of long-term memory consolidation have identified two time windows, when the strength of memories can be modified after acquisition through dopamine signaling and network activity3–5. Both time windows are critically dependent on de novo mRNA translation and its regulation of immediate early genes (IEGs) such as cFos and Arc1,3–5. How such experience-dependent de novo mRNA translation might be precisely controlled to fine-tune memories over time has remained unclear.
Recently, mRNA transcript stability and translation was shown to critically depend on N6-methyladenosine (m6A) modification6–8, a process bidirectionally regulated upon learning by the m6A methylase METTL3 and the demethylase FTO6–8. In the current study, Zhang et al.2 investigated whether m6A modification of mRNA might influence translation of IEGs and memory strength. The authors knocked out METTL3 in postnatal forebrain, including hippocampus, using mice expressing a conditional allele of METTL3 in a CaMK2-Cre background. Using a weak contextual fear conditioning (cFC) protocol involving only one foot shock (1US), they show that long-term memories assessed as freezing in the conditioning context 24 h after cFC are strongly reduced in the mutants. Notably, short-term memories assayed as freezing to context 30 min after cFC, were not affected by the absence of METTL3. Furthermore, mice lacking forebrain METTL3 were impaired during the first few days of water maze learning, consistent with reduced efficiency of long-term memory retrieval during the learning process. Notably, the same mice were not detectably affected when cFC was induced using a stronger Pavlovian associative protocol involving three (3US) instead of one foot shock, or when the effectiveness of water maze training was assessed towards the saturation phase of the learning curve. When the authors reintroduced wild-type or methyltransferase-deficient METTL3 in the dorsal hippocampus (dH) of conditional mutant mice, they found that functional METTL3 completely restores long-term memory function in the 1US cFC assay and during water maze training. In further experiments, the m6A methylome was dynamically regulated during memory consolidation, METTL3 promoted m6A modification of IEG mRNAs induced upon learning, and levels of IEG proteins upon learning were substantially reduced in the absence of METTL3, although the corresponding transcripts were not. Finally, overexpression of functional METTL3 in dH of wild-type mice enhanced behavioral performance in the water maze and upon 1US cFC. Taken together, the outcome of these loss-of-function and gain-of-function experiments provides convincing evidence that m6A modification of mRNAs upon learning is necessary and sufficient to enhance the strength of weak memories as determined upon behavioral retrieval. The findings are consistent with those of a study focusing on the demethylation process and the protein FTO9.
This study has several important strengths. First, conditional knockout of METTL3 in postnatal forebrain bypasses confounds related to key roles of this enzyme during development7,8. Second, the authors focus on a simple and well-controlled setting in which the behavioral strength of Pavlovian fear memories is influenced by the number of foot shocks (1US versus 3US) delivered to the animal in a defined context. Furthermore, the authors use AAV-mediated local expression of wild-type and methyltransferase-deficient versions of METTL3 in conditional knockout and wild-type mice to probe the notion that the enzymatic METTL3 domain is important to modulate memory strength. Importantly, they provide evidence that m6A modification of mRNA impacts memory strength modulation, but not memory formation or retrieval per se. However, the link between METTL3-dependent IEG expression and memory strength is mostly correlative, and could have been strengthened by analyzing IEG expression upon 3US cFC in the absence and presence of METTL3. Furthermore, although translation of IEG mRNA is the likely relevant process, the study does not address the possibility that regulation of IEG protein stability might be relevant in these experiments. Finally, because of the importance of neuronal assemblies in learning and memory1, it might have been informative to evaluate whether METTL3 mainly modulates IEG levels in expressing neurons, or whether it also affects the fraction of neurons that express detectable levels of IEGs upon learning.
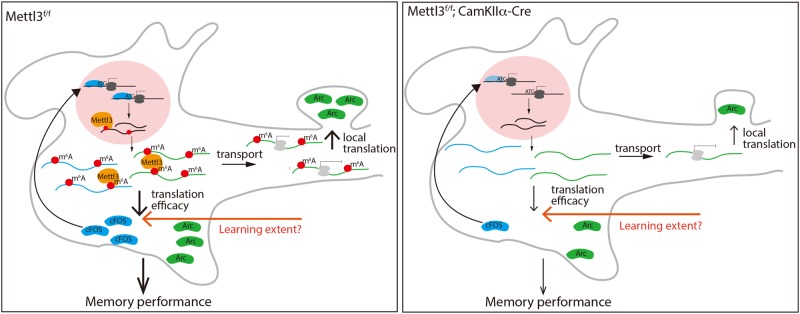
In conclusion, by identifying an endogenous learning-related molecular process with a role in modulating memory strength, the study by Zhang et al. makes an important contribution to molecular studies of learning and memory. Follow-up studies might address the molecular and cellular mechanisms that link learning processes, network activity, neuromodulatory signaling, neuronal excitability and trafficking of genes involved in memory to m6A metabolism, both at the time of initial acquisition and during time windows for long-term memory consolidation (Fig. 1).
Fig. 1.
Regulation of memory strength by METTL3. Left panel: METTL3-mediated m6A modification might influence translation efficacy, mRNA transport and local translation of IEGs by m6A readers and writers to modulate learning-induced plasticity in neurons. Right panel: Lack of m6A modification in METTL3-deficient animals prevents additional mRNA processing and leads to decreased levels of IEGs, and decreased memory performance. The decrease in IEG translation might be overcome by m6A modification-independent mechanisms induced through increased learning
References
- 1.Holtmaat A, Caroni P. Nat. Neurosci. 2016;19:1553–1562. doi: 10.1038/nn.4418. [DOI] [PubMed] [Google Scholar]
- 2.Zhang Z, et al. Cell Res. 2018;28:1050–1061. doi: 10.1038/s41422-018-0092-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cai DJ, et al. Nature. 2016;534:115–118. doi: 10.1038/nature17955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Karunakaran S, et al. Nat. Neurosci. 2016;19:454–464. doi: 10.1038/nn.4231. [DOI] [PubMed] [Google Scholar]
- 5.Kruttner S, et al. Cell Rep. 2015;11:1953–1965. doi: 10.1016/j.celrep.2015.05.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Knuckles P, Buhler M. FEBS Lett. 2018;592:2845–2859. doi: 10.1002/1873-3468.13107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Widagdo J, Anggono V. J. Neurochem. 2018;147:137–152. doi: 10.1111/jnc.14481. [DOI] [PubMed] [Google Scholar]
- 8.Widagdo J, et al. J. Neurosci. 2016;36:6771–6777. doi: 10.1523/JNEUROSCI.4053-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Walters BJ, et al. Neuropsychopharmacology. 2017;42:1502–1510. doi: 10.1038/npp.2017.31. [DOI] [PMC free article] [PubMed] [Google Scholar]