Abstract
Poliovirus receptor (PVR, CD155) has recently been gaining scientific interest as a therapeutic target in the field of tumor immunology due to its prominent endogenous and immune functions. In contrast to healthy tissues, PVR is expressed at high levels in several human malignancies and seems to have protumorigenic and therapeutically attractive properties that are currently being investigated in the field of recombinant oncolytic virotherapy. More intriguingly, PVR participates in a considerable number of immunoregulatory functions through its interactions with activating and inhibitory immune cell receptors. These functions are often modified in the tumor microenvironment, contributing to tumor immunosuppression. Indeed, increasing evidence supports the rationale for developing strategies targeting these interactions, either in terms of checkpoint therapy (i.e., targeting inhibitory receptors) or in adoptive cell therapy, which targets PVR as a tumor marker.
Keywords: PVR, tumor, immunotherapy, checkpoint, TIGIT, poliovirus
Introduction
The discovery of new targets and the development of new approaches for anti-tumor therapy is one of the major tasks of current science and represents one of the broadest and fastest growing areas of research. Nevertheless, despite the exponential research growth, only a few approaches have translated from bench to bedside.
Poliovirus receptor (PVR, CD155), a member of the nectin-like family of proteins, is recently emerging as a promising target in immunotherapy that enhances the existing anti-tumor responses. Similar to other members of the family, PVR is involved in important cellular processes, such as adhesion, contact inhibition, migration, proliferation, and the immune response.1 PVR is dramatically overexpressed in several human malignancies, whereas its expression is low or absent in most healthy tissues.2–6 Consistent with PVR biology, its overexpression promotes tumor cell invasion, migration, and proliferation and is associated with a poor prognosis and enhanced tumor progression.7–10 Simultaneously, PVR has a considerable immunoregulatory potential. It interacts with the (co)stimulatory receptor DNAM-1 and the inhibitory receptors TIGIT and CD96, resulting in either immune cell activation or inhibition, respectively.11 In healthy individuals, the balance between activating and inhibitory signals maintains the normal function of immune cells. However, since this balance is often disturbed in the tumor microenvironment and accumulating data suggest that PVR overexpression induces the immune escape of tumor cells, strategies targeting PVR might direct the immune response toward the elimination of tumor cells.6,11–15
Based on these findings, several different approaches based on PVR and its interactions are being explored in the field of anti-tumor therapy and are described in this review.
The multiple faces of the PVR protein
PVR, which was originally described as a poliovirus entry receptor 30 years ago,16 has been a focus of scientific interest ever since. Research by several laboratories ultimately led to the discovery of a series of physiological processes in which this protein is involved (Fig. 1), which is also why PVR is known by many different names, such as CD155, TAGE4, or Necl-5. PVR is a member of an immunoglobulin superfamily defined by the presence of immunoglobulin domains V, a C1-like domain and a C2 domain in the extracellular region.1 PVR is also considered a member of the nectin-like family of adhesion molecules. This family shares three conserved motifs in the IgV domain with nectins,17 but it differs by a lack of binding to the F-actin-binding protein afadin through its intracellular domain.1
Fig. 1.
The multiple faces of the PVR protein. PVR was originally identified as a poliovirus entry receptor that facilitates the attachment and entry of poliovirus into susceptible cells. Replication and translation of the viral genome result in the production of virions that burst from the cell, causing its lysis (lower left panel). Endogenous functions of PVR include cellular adhesion, contact inhibition, cell motility, proliferation, and survival. Many of these PVR functions are achieved by the activation of Ras and RAP1 signaling pathways and require the interactions of PVR with other receptors, such as growth factor receptors (GFR) and integrins in cis, or Nectin-3 (Nec3) in trans (upper right panel). Of particular interest is the immunoregulatory role of PVR (lower right panel) that is accomplished through its interactions with activating and inhibitory immune cell receptors: DNAM-1, TIGIT, and CD96. These complex interactions impact the outcome of the immune response. In addition to transmembrane forms of PVR that perform the functions described above, PVR also exists as a soluble or secreted form (upper left panel). Although this form of PVR is present in many different body fluids and its levels are increased in patients with cancer, its role is still mostly unknown
In addition to being produced as a transmembrane glycoprotein, with two alternatively spliced isoforms denoted as α and δ, PVR is also produced as two splice forms lacking the transmembrane region, designated β and γ.18 PVR β and γ have been described as soluble or secreted isoforms (Fig. 1) that are present in many different body fluids, including blood, cerebrospinal fluid, and urine.19,20 Since the soluble PVR protein has been shown to resemble the extracellular part of transmembrane isoforms, this isoform was postulated to compete with membrane-anchored PVR for the binding of poliovirus to host cells.19 In addition, soluble PVR levels are increased in the sera of patients with various cancers and as such represent a potential biomarker of cancer development and progression.20 Because transmembrane PVR acts as a ligand for important immune cell receptors and considering the roles of other soluble immune cell ligands,21–23 researchers have hypothesized that soluble PVR might also have an important immune-related function. Nevertheless, the roles of soluble PVR in both healthy individuals and patients with cancer, as well as the potential differences between the two soluble isoforms have not been studied in detail.
Transmembrane isoforms of PVR, α and δ, are distinguishable by the last few amino acids, with PVR δ having a shorter C terminus and lacking the immunoreceptor tyrosine-based inhibitory motif (ITIM) that is engaged in maintaining PVR localization in polarized cells and intrinsic PVR signaling.24,25 Consequently, PVR δ localizes to both the apical and basolateral plasma membrane, whereas PVR α localizes to the basolateral membrane of polarized epithelial cells. Regarding the role of ITIM in intrinsic PVR α signaling, ITIM has been shown to recruit SH2-containing tyrosine phosphatase-2 (SHP-2)24,26 to initiate signal transduction by acting as a mediator of the important cellular functions of PVR, such as cellular adhesion,27 contact inhibition,1 cellular motility,24,28,29 proliferation, and survival.30 Many of these PVR functions are achieved through interactions with other members of the immunoglobulin superfamily. For example, PVR accumulates at the leading edge of moving cells and enhances PDGFR and integrin-αvβ3 signaling, resulting in the formation of membrane protrusions that facilitate cell movement toward chemoattractant gradient, such as lamellipodia and membrane ruffles.31,32 This interaction was also shown to be important for the regulation of cellular proliferation because PVR enhances the PDGF-induced Ras-Raf-MEK-ERK signaling pathway that is involved in the cell cycle,30,33 and subsequent studies showed that PVR also interacts with other growth factor receptors, such as IGF1R, VEGFR, and Met receptor, participating in cell proliferation.34,35 PVR is also required for cellular adhesion and contact inhibition. Namely, although dominant proteins mediating cell-to-cell adhesion are cadherins and nectins,36 the initial cell-to-cell contact starts with a heterophilic interaction between PVR on one cell and Nectin-3 on the adjacent cell. This interaction is transient and results in PVR internalization by endocytosis.37 PVR internalization and the resulting disruption of the GFR-integrin complex contributes to the contact inhibition of cell proliferation. Obviously, these important functions of PVR, i.e., cell proliferation, motility, and adhesion, are among the most important issues in cancer pathology. In contrast to normal tissues where PVR, although widely distributed, is expressed at low levels on the surface, PVR is overexpressed in many human malignant tumors.2–6 The overexpression of PVR interferes with all of the previously mentioned functions, as will be covered in more details in subsequent sections.
In addition to the aforementioned functions, most of the research in the past decade has focused on the immune effects of the PVR protein, resulting in the discovery of new findings that PVR might show great promise for the future development of cancer immunotherapies. PVR is one of the few ligands recognized by both activating and inhibitory receptors on immune cells, the so-called paired receptors.38 DNAM-1 (DNAX accessory molecule 1; CD226), an activating or co-stimulatory receptor that recognizes PVR and Nectin-2, is expressed on most immune cells, including T cells, B cells, NK cells, and monocytes.39–42 On the other hand, PVR is also recognized by TIGIT (VSIG9, WUCAM, and VSTM3), an inhibitory receptor expressed on NK cells, activated and memory T cells and Tregs;43,44 the inhibitory receptor TIGIT was discovered much later than the activating receptor DNAM-1: we have observed that protein named VSIG9 (V-set and immunoglobulin domain-containing protein 9) interacts with PVR and serves as a direct inhibitory receptor for NK cells, with its inhibitory activity depending on the ITIM in its intracellular domain.45, Yu et al. identified the same protein during the same time frame, showed that it was important in T cell function and named it TIGIT (T cell immunoglobulin and ITIM domain).46
Finally, PVR also binds to CD96, another receptor member of the immunoglobulin superfamily that was initially inconsistently characterized in different species using various assays,47–49 but has recently been described as an inhibitory receptor on Th9 and NK cells.40,50 In other words, PVR functions as an immunoregulatory molecule that impacts the outcome of the immune response by interacting with activating and inhibitory receptors.
Rationale for the development of strategies targeting PVR in tumors
PVR was first proposed as a target of anti-tumor therapy in 1990s following the discovery of high levels of PVR expression in tumors,2,3 although at that time, researchers had not clearly determined that the identified tumor antigen was the previously described poliovirus receptor.51,52 Since then, the number of tumors reported to express high levels of PVR on their surfaces has constantly increased. These tumors include colorectal cancer,3 glioma, neuroblastoma,2,4 myeloid leukemia,5 ovarian cancer,6 lung adenocarcinoma,53 pancreatic cancer,10 melanoma,54,55 cholangiocarcinoma,56 and other tumors. The increased expression of PVR in tumors might result from different mechanisms. First, the presence of the Ras oncogene increases PVR expression via the Raf-MEK-ERK signaling pathway.57 Second, activation of the Shh signaling pathway, which is often aberrantly activated in human tumors, increases PVR transcription.58 Third, the DNA damage response orchestrated by ATM/ATR pathways, which are commonly activated in precancerous cells,59 also leads to PVR induction.60,61 Therefore, PVR is considered a stress-induced ligand that should serve to alert the immune system of a potentially dangerous and malignant transformation in progress. Nevertheless, a large number of research studies have stipulated that PVR overexpression in tumors is a direct marker of tumor progression and correlates with a worse survival prognosis, suggesting that this protein has a role in tumor pathology.20,56,62 Accordingly, researchers have proposed that increased PVR expression promotes tumor cell invasion, migration, proliferation, and angiogenesis through a similar mechanism as used to exert its regular effects on these processes in healthy cells.7–10 For example, due to the involvement of PVR in cellular motility and leading-edge formation, PVR overexpression enhances tumor cell motility and PVR knockdown suppresses tumor cell invasiveness and decreases their migration in vitro.7,63,64 Another group of researchers showed that PVR overexpression in ras-transformed cells enhances growth factor-induced cell proliferation by modifying the expression profiles of cyclins and other key cell cycle molecules.8 Consistent with these findings, PVR overexpression was recently shown to exert an effect on tumor growth in vivo as well: PVR knockdown reduced both the tumor volume and weight in a colon cancer model, as well as the metastatic burden in several other mouse tumor models.13,65 These data support the proposed proto-oncogenic role of PVR and the potential utility of strategies targeting this protein in anti-tumor therapy (Fig. 2).
Fig. 2.
Rationale for the development of strategies targeting PVR in tumors. Under physiological conditions (left panel), PVR is expressed at low levels and limited to certain cell types; moreover, a balance between activating and inhibitory signals mediated by PVR maintains the normal function of immune cells. In the tumor microenvironment (right panel), PVR is dramatically overexpressed, suggesting that strategies targeting this protein might be highly selective for tumor cells. In addition, due to its endogenous functions, overexpressed PVR promotes tumor cell invasion, migration, proliferation, and angiogenesis, supporting its therapeutically attractive proto-oncogenic role. Finally, the balance between activating and inhibitory signals is often disturbed in the tumor microenvironment: inhibitory receptors are upregulated and the activating receptor is downregulated, which suggests a prevalence of inhibitory signaling. This phenomenon provides researchers an opportunity to reverse immunosuppression by PVR-targeted inhibition. APC antigen-presenting cell, TILs tumor-infiltrating lymphocytes
Notably, future discoveries regarding PVR expression on healthy tissues might place even higher value on PVR as candidate for tumor-targeting therapy. Although several groups have reported the ubiquitous expression of PVR transcripts in the majority of adult tissues,16,18 the distribution of the protein on healthy tissues remains uncertain and is mainly associated with the sites of poliovirus propagation. Currently, the PVR protein is expressed at low levels on healthy tissues and is limited to certain cells, such as vascular endothelial cells, spinal cord motor neurons, and some immune cell subsets.13,66–68 Therefore, strategies targeting PVR are potentially highly selective treatments for tumor cells that minimize eventual side effects caused by PVR expression on healthy cells.
Finally, the most interesting aspect of PVR targeting is probably related to the immune role of PVR. Several groups have reported that the interaction of PVR with receptors on immune cells exerts a significant effect on the immune response to tumors. For example, PVR overexpressed on tumor cells increases the activation of NK cells and elimination of tumor cells via its interaction with DNAM-1.4,69 On CD8 T cells, DNAM-1 also contributes to the response to tumors: it acts as co-stimulatory molecule that is required for stimulation by weakly immunogenic antigen-presenting cells.70 In addition, given the broad expression of DNAM-1 on different immune cell subsets,42 many studies have suggested that it is involved in many other immunological functions, such as T cell proliferation and differentiation,70,71 the maturation of CD8a+ dendritic cells (DCs), the killing of immature DCs,72–74 transendothelial leukocyte migration,28 and other functions.75–77 Hence, not surprisingly, some tumors have developed mechanisms to downregulate DNAM-1 expression.6,12,78 Nevertheless, research showing activating and co-stimulatory roles of DNAM-1 expressed on NK and T cells provided a strong rationale for the development of strategies to promote the DNAM-1-dependent anti-tumor response, such as the use of DNAM-1-positive cytokine-induced killer cells (CIK)79 or DNAM-1 chimeric antigen receptor (CAR) T cells,80 as discussed later in this review. Finally, considering the role of DNAM-1 in controlling some other non-physiological conditions, such as virus infections,81 researchers have not excluded the possibility that additional DNAM-1-related anti-tumor functions will be discovered in future.
In contrast to DNAM-1, TIGIT is an inhibitory receptor that is solely expressed on T and NK cells.43–45 TIGIT binds PVR with stronger affinity than DNAM-146 and acts as an immune checkpoint. Upon binding to its ligands, TIGIT inhibits immune cell activation. Several models have been proposed as mechanisms for TIGIT inhibition47: first, cell-intrinsic signaling (i.e., direct signaling to immune cells) caused by TIGIT stimulation and mediated by its ITIM domain44,45; second, cytokine-dependent inhibition with cytokines produced either by TIGIT-triggered PVR-expressing dendritic cells46 or by TIGIT-positive Tregs82; and third, indirect inhibition through the direct interaction of TIGIT and DNAM-1. Importantly, a crystal structure of the TIGIT–PVR interaction revealed that both molecules must form homodimers in cis to interact as heterotetramers in trans.17 Since DNAM-1 also forms homodimers and TIGIT interferes with its dimerization, this mechanism has been suggested to underlie the TIGIT-mediated interruption of DNAM signaling and subsequent inhibition of immune cells.83 Nevertheless, further research is needed to assess whether these mechanisms exclude each other or work in tandem. In addition to TIGIT-mediated immune cell inhibition, numerous studies have also shown increased TIGIT expression in the tumor microenvironment compared to the periphery.55,83,84 Since the dominance of inhibitory or activating pathway is postulated to depend on the relative levels of receptor and ligand expression, high levels of both PVR and TIGIT suggest a predominantly immunosuppressive role for this axis in the tumor microenvironment (Fig. 2) and opens a possibility of reversing the immunosuppression by targeting this inhibitory signaling pathway.
The least characterized and third PVR receptor is CD96 or Tactile (T cell activation increased late expression). Although initially described as an activating receptor that stimulates NK cell cytotoxicity,49 more recent data suggest that it has a predominant inhibitory function in both NK cells and T cell subsets.40,50 The discrepancy in these findings might be due to pronounced differences in CD96 between species.47,85 Nevertheless, CD96 has many similarities to TIGIT, indicating that it has an inhibitory role. For example, CD96 is also upregulated upon T cell activation,86 its expression is enriched in tumors83,87–89 and its binding affinity for PVR is stronger than DNAM-1.90 Moreover, similar to TIGIT, CD96 also possesses an ITIM-like domain that is putatively involved in inhibitory signaling.91 Finally, antibody-mediated blockade of CD96 in murine tumor models increases survival and reduces the metastatic burden,48,92 supporting the use of CD96 in checkpoint therapy, as discussed below.
In conclusion, PVR overexpression and its involvement in tumor pathology, together with its involvement in the immune response to tumors, particularly immune evasion, strongly support the rationale for the development of strategies targeting this protein.
Anti-tumor approaches targeting PVR and its interactions
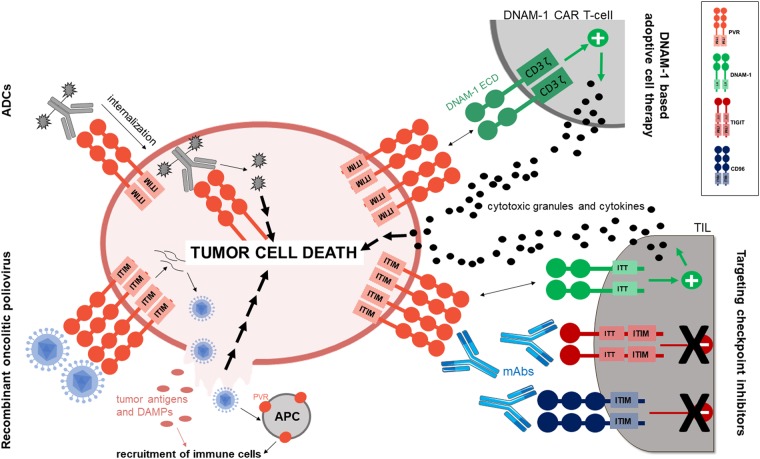
Currently, several different approaches for anti-tumor therapy based on PVR and its interactions are being investigated: direct targeting of tumor cells overexpressing PVR by recombinant oncolytic polioviruses; the use of monoclonal antibodies (mAbs) to block inhibitory PVR receptors, i.e., checkpoint therapy; and the use of genetically engineered or in vitro-induced effector cells that act via DNAM-1 (Fig. 3). Approaches that are being evaluated in clinical trials are summarized in Table 1.
Fig. 3.
Anti-tumor approaches targeting PVR and its receptors. Several different approaches of anti-tumor therapy based on PVR and its interactions are currently being investigated. One is the direct targeting of tumor cells overexpressing PVR via recombinant oncolytic polioviruses (lower left panel) that productively infect tumor cells, resulting in their lysis and cell death. In addition, the release of tumor antigens and DAMPs from lysed cells, as well as the infection of PVR-expressing antigen-presenting cells, results in the recruitment of other immune cell subsets, enhancing the anti-tumor effect of this approach. Major progress in anti-tumor therapy has also been obtained by targeting PVR checkpoint inhibitors using monoclonal antibodies (lower right panel). By blocking inhibitory interactions, the antibodies reverse immunosupression and increase TIL activation and cytotoxicity, ultimately resulting in the death of tumor cells. Based on accumulating evidence, the blockade of PVR with monoclonal antibodies might exert similar effects on immune cells and their effector capacities, as well as additional immune-independent, anti-tumor mechanisms. An additional potential therapeutic approach targeting PVR might be the use of antibody–drug conjugates (upper left panel), in which a highly potent cytotoxic molecule is complexed to an antibody and delivered to cells by receptor-mediated endocytosis, leading to cell death. The important and potent roles of DNAM-1 in PVR-dependent anti-tumor immune responses, together with the large number of tumors that overexpress PVR provide a strong rationale for the use of DNAM-1 as a chimeric antigen receptor in adoptive cell therapy (upper right panel) designed to enhance effector capacities of these cells and target multiple tumor types. ADCs antibody–drug conjugates, APC antigen-presenting cell, CAR chimeric antigen receptor, DAMP damage-associated molecular pattern, ECD extracellular domain, mAbs monoclonal antibodies, TIL tumor-infiltrating lymphocyte
Table 1.
Summary of ongoing clinical trials targeting PVR or its interactions
| Strategy | Agent(s) | Identifier | Phase | Condition |
|---|---|---|---|---|
| Recombinant oncolytic poliovirus | PVSRIPO | NCT01491893 | I | Recurrent grade IV malignant glioma |
| PVSRIPO | NCT03043391 | Ib | Recurrent grade III or IV malignant glioma; pediatric patients | |
| PVSRIPO with/without lomustine | NCT02986178 | II | Recurrent grade IV malignant glioma | |
| PVSRIPO | NCT03564782 | I | Triple negative breast cancer | |
| Monoclonal antibodies | Anti-TIGIT antibody (OMP-313M32) with/without nivolumab | NCT03119428 | I | Locally advanced or metastatic solid tumors |
| Anti-TIGIT antibody (BMS-986207) with/without nivolumab | NCT02913313 | I/IIa | Advanced solid tumors | |
| Anti-TIGIT antibody (MTIG7192A) with/without atezolizumab | NCT03563716 | II | Non-small cell lung cancer | |
| Anti-TIGIT antibody (MTIG7192A) with/without atezolizumab | NCT02794571 | I | Advanced/metastatic tumors |
Recombinant oncolytic polioviruses
One of the first approaches to be investigated in the field of anti-tumor therapy was the use of oncolytic viruses, an idea that was based on nearly a century-old discovery of naturally occurring viruses that are able to kill cancer cells.93 With the development of recombinant DNA technology, the number of research studies on potential strains with superior performance grew exponentially. In addition, a better understanding of virus–host interactions, which are as important as oncolytic capacity, ultimately led to the approval of recombinant oncolytic viruses as treatments. To date, two of these viruses have been approved as anti-tumor treatments: H101, a recombinant oncolytic adenovirus that was approved by the Chinese FDA in 2005 as a treatment for patients with head and neck carcinoma, and T-VEC, a genetically engineered HSV-1 virus that was approved in 2015 by the USA FDA as a treatment for melanoma.93 In addition, many other different viruses are currently being investigated or have already entered early phase clinical trials as vectors for oncolytic therapy.94 One is a recombinant oncolytic poliovirus named PVSRIPO, which is currently in a phase II clinical trial against glioma95 (Table 1). The main rationale for this approach is the tropism of poliovirus for a single host cell receptor, PVR, while the second reason lies in the neuro-attenuation of the recombinant variant. In general, the anti-tumor properties of oncolytic viruses are mediated by two mechanisms with equal contributions: selective replication in tumor cells and subsequent lysis; and the induction of an anti-tumor immune response.94 For poliovirus, productive infection is restricted to epithelial cells of the gastrointestinal tract and spinal cord motor neurons, with infection of the latter resulting in poliomyelitis, a severe neurological disease. In particular, the attachment of poliovirus capsid proteins to cellular PVR results in formation of pores in the cell membrane and the release of the viral RNA in the cytoplasm.96 The viral RNA is then replicated, and the translation of the genome is initiated by the viral internal ribosome entry site (IRES), ultimately resulting in the production of virions that burst from the cell, causing its lysis.97 Although researchers initially proposed that PVR expression coincides with the susceptibility of cells to infection,68,98,99 the detection of PVR in organs such as the kidneys in which poliovirus replication does not occur68,100,101 suggested that the viral tropism might depend on other mechanisms, such as organ-specific IRES-mediated translation differences102 or IFN α/β responses.103 Nevertheless, PVR expression is still considered the principal determinant of poliovirus tropism, and the discovery that PVR is not only broadly expressed but also upregulated in tumors opened the possibility of targeting this protein using a recombinant oncolytic poliovirus. Certainly, this therapeutic approach required the elimination or minimization of the effect of viral tropism on healthy cells, in this case, the prevention of viral neurotoxicity. The basis of poliovirus neuro-attenuation was discovered in the Sabin vaccine strain. Sequencing of the genome of the Sabin strain showed the presence of several single-point mutations in the viral IRES that when genetically reversed, resulted in vaccine-associated poliomyelitis, supporting the role of IRES in neurovirulence.104,105 These findings facilitated the construction of an even more neuro-attenuated and genetically stable virus, PVSRIPO. Specifically, in PVSRIPO, the entire poliovirus IRES is substituted with the IRES from human rhinovirus type 2 (HRV2) that precludes replication in normal cells but allows replication in tumor cells.106 This specificity is likely due to the IRES-binding protein DRBP76 that prevents ribosome binding and subsequent viral protein synthesis and replication in neuronal cells, while in tumor cells, the absence of this protein in the cytoplasm means that the IRES is not bound, resulting in the viral replication and lytic cycle.107–110 Numerous studies have confirmed the inability of this recombinant poliovirus to kill neuronal cells while retaining the ability to kill tumor cells,2,106,111–114 and the safety of PVSRIPO has also been confirmed in preclinical, human PVR transgenic mouse models.115
However, as mentioned above, the selective replication of oncolytic viruses in tumor cells is not the sole requirement for their efficacy and therapeutic applications. In particular, since many tumors suppress the immune response, oncolytic viruses are designed to be immunogenic, i.e., they can activate or enhance the immune response that persists even after virus clearance. In the case of poliovirus, the viral RNA, which is recognized by TLRs and RIG-like receptors, activates type I interferon response.116 Poliovirus engages different mechanisms to overcome the inhibitory effects of type I IFNs either by suppressing IFN induction in infected cells117,118 or by favoring selective viral translation that will induce cytotoxicity in the presence of the IFN response.119,120 The oncolytic variant PVSRIPO not only exploits this insensitivity to IFN, but also enhances the immune response. Namely, PVSRIPO infection and the lysis of tumor cells results in release of damage- and pattern-associated molecular patterns (DAMPs and PAMPs) that activate the pro-inflammatory response;121 this pro-inflammatory response involves the recruitment of innate immune cells such as neutrophils, which might act either directly by killing tumor cells or indirectly through cytokines.122 Cytokines recruit cells involved in adaptive immunity, such as T cells that have already been shown to be involved in the anti-tumor response induced by oncolytic poliovirus A133Gmono-crePV.123 In addition, a recent study by Brown et al.121 showed a role for another immune cell subset in the anti-tumor activity of PVSRIPO: dendritic cells (DCs). Since DCs express PVR, they are also infected by PVSRIPO, but similar to the effects observed in neurons, the infection is not productive and does not result in their death. In contrast, the infection of DCs results in a strong type I IFN response121 that, together with the release of tumor antigens resulting from cell lysis,120 primes T cells and induces a productive anti-tumor T cell response.121 Hence, several arms of the immune response seem to be involved in the anti-tumor immunity induced by PVSRIPO, thereby satisfying all desirable characteristics of the anti-tumor therapeutic construct: the induction of an immune response, selectivity, safety, and stability. Therefore, not surprisingly, this virus entered clinical trials. As mentioned above, current trials are being conducted on patients with glioma due to the findings supporting certain associations between PVR and central nervous system neoplasia. First, PVR is expressed at high levels in tumors from patients with glioblastoma multiforme (GBM), with ubiquitous PVR expression.67,99,124–126 The ubiquitous presence of PVR in GBM tumors might be related to the presumed involvement of PVR in CNS development, in particular the structures generating spinal anterior horn motor neurons, the site of poliovirus replication.127 Second, many experiments showing that PVR overexpression enhances tumor cell motility have been performed using a glioblastoma model, and the effects of PVR on this model have been particularly well studied.7,64 Additionally, GBM tumors are attractive targets for oncolytic poliovirus therapy, due to the lack of effective therapies and poor outcome of the disease. Finally, several studies on mice preceded clinical trials, establishing the basis for the clinical applications of the recombinant oncolytic poliovirus. In one study, immunocompetent hPVR transgenic mice were subcutaneously implanted with an hPVR-expressing neuroblastoma cell line and treated with an intratumor inoculation of attenuated poliovirus strain A133Gmono-crePV, resulting in complete eradication of the tumor.128 Intratumor inoculation was chosen based on a previous study showing the maximum efficacy of this administration route.2 Another study showed similar performance of PVSRIPO in a glioma xenograft model, with almost complete tumor regression observed by the end of the study.129 A more recent study using a PV variant that was selected to be able to replicate in murine cells, mRIPO, showed that this virus also substantially delays tumor growth and increases the survival of animals bearing hPVR-expressing murine cancer cell lines.121 Finally, the anti-tumor potential of PVSRIPO was also confirmed in models other than glioma. According to a study by Holl et al.,122 a single intratumor administration resulted in tumor regression in xenograft models of both prostate and breast tumors, providing a rationale for the initiation of clinical translation to other tumors.
In addition, recent results from a phase I clinical trial in patients with malignant glioma (Identifier: NCT01491893, Table 1) have been reported95 and seem promising. The survival rates of PVSRIPO-treated patients was increased at 24 and 36 months compared to historical controls, 8 of 35 patients who were treated for more than 24 months remained alive, and the virus did not display neurovirulence potential.
In summary, oncolytic poliovirus-based therapy has currently achieved substantial experimental success and anti-tumor potential. Nevertheless, some challenges remain for other oncolytic virotherapies and lingering questions must be addressed before oncolytic polioviruses are approved for patient use.130
Checkpoint inhibitors targeting PVR receptors
One of the most advanced anti-tumor approaches currently being investigated is certainly checkpoint immunotherapy, a therapy that enhances the anti-tumor response by targeting regulatory pathways of immune surveillance, most commonly T cell-related inhibitory pathways. These pathways are originally triggered upon T cell activation to regulate the immune response, i.e., maintain self-tolerance and prevent autoimmune reactions. However, in the tumor microenvironment, as mentioned above, the immune balance is shifted and tumors often overexpress checkpoint ligands to exploit checkpoints as one of their mechanisms of evading the immune system.131 Because checkpoint inhibition depends on interactions between these ligands and their respective receptors, antibody blockade of one or both has been shown to be an effective approach in reversing immunosuppression. In other words, “inhibition of inhibition” allows the re-activation and enhancement of the anti-tumor activity of immune cells. Consistent with this theory, several checkpoint inhibitor-targeting antibodies have already been approved as treatments for numerous immunogenic cancers:132,133 ipilimumab, which targets cytotoxic T-lymphocyte-associated antigen 4 (CTLA-4)134; nivolumab and pembrolizumab, which target programmed cell death protein-1 (PD-1)135; and atezolizumab, durvalumab, and avelumab, which target PD-L1, the ligand of the PD-1.136 Nevertheless, despite the great success of the indicated therapies, all types of cancer share a common feature: some patients do not respond to these therapies.137 Therefore, new potential targets are constantly being investigated.138 TIGIT has recently emerged as promising target.47 Early research on TIGIT has already suggested that its inhibition enhances the killing of tumor cells. For example, in the study by Stanietsky et al.,90 blockade of TIGIT with polyclonal antibodies enhanced the killing of B12 cells by mouse PBMCs in vitro. More recently, several ex vivo and murine studies suggested that blockade of TIGIT in the tumor microenvironment synergizes with the blockade of other checkpoint inhibitors and potentially results in the reversal of T cell exhaustion and subsequent tumor rejection. For instance, the inhibition of both TIGIT and PD-1 on CD8+ tumor-infiltrating lymphocytes from patients with melanoma enhances their proliferation, cytokine production, and degranulation.139 In a murine CT26 colon carcinoma model and syngeneic EMT6 breast carcinoma model, the inhibition of both TIGIT and PD-1 resulted in a striking reversal of tumor growth and the induction of a protective, antigen-specific memory response.83 Similarly, a more recent study also showed a synergistic and anti-tumor effect of anti-TIGIT and anti-PD-1 blocking antibodies on an MC38 colon carcinoma model, resulting in complete tumor regression.140 In addition, experiments performed on TIGIT knockout mice suggested a synergistic effect of TIGIT and another recently identified checkpoint target: TIM3.141 The critical role of TIGIT-mediated tumor suppression was suggested to be due to its function in Tregs rather than its function in effector CD8 T cells, which might explain its complementary effects. In particular, TIGIT signaling in Tregs in the tumor microenvironment drives a cell-intrinsic gene program that alters the Treg phenotype and confers superior suppressive functions to these cells. Thus, TIGIT-positive Tregs drive the formation of a dysfunctional phenotype in effector T cells by shifting the cytokine balance either intrinsically or in antigen-presenting cells via an interaction with PVR.43 Finally, a recent study has dissected the role of TIGIT in the regulation of NK cell-mediated anti-tumor immunity.142 A TIGIT deficiency or blockade in NK cells alone, independent of the adaptive immune system, was sufficient to delay tumor growth in vivo and improve host survival, indicating a critical role for NK cells in TIGIT-blocking strategies. The same study suggested the importance of TIGIT expressed on NK cells in human tumors, as TIGIT was expressed at higher levels on NK cells in intratumor regions than in peritumor regions. These data are consistent with the previously published role of TIGIT expressed on human NK cells, in which Stanietsky et al. showed that TIGIT directly inhibits human NK cytotoxicity toward target cells in vitro.45 Altogether, these data not only suggest the potency of strategies targeting TIGIT alone or in combination with well-characterized, already in use checkpoint inhibitors but also potentially broader effects of these treatments. Moreover, most published studies have shown increased expression of TIGIT on immune cells within the tumor microenvironment compared to the peripheral immune cells, suggesting an additional advantage of this therapy and potentially less systemic toxicities. Hence, not surprisingly, several ongoing clinical trials are investigating monoclonal antibody-based targeting of TIGIT, either as a single agent or in combination with other checkpoint inhibitors (Table 1).
Similar to TIGIT, CD96 has also recently attracted interest as a potential target for anti-tumor therapy.143 Namely, publications addressing the function of murine CD96 using knockout mice suggested a predominantly inhibitory function in NK cells. Consequently, mice lacking CD96 are more resistant to MCA-induced fibrosarcomas, and the authors proposed that this resistance is not due to direct NK cytotoxicity but increased production of cytokines, particularly IFNγ.40 These findings were confirmed by the use of a CD96 blocking antibody, and several other in vivo studies showed the anti-tumor properties of strategies targeting CD96. For example, according to Blake et al.,48 a mCD96 antibody suppresses experimental lung metastases in several models, B16 melanoma, 3LL lung carcinoma, LWT1 melanoma, and RM-1 prostate carcinoma, through an IFNγ- and NK cell-dependent mechanism. Barrow et al. recently confirmed these results in a B16 metastatic tumor model.144 In addition, in a study by Blake et al., combinations of anti-CD96 antibodies with anti-CTLA-4 or anti-PD-1 antibodies were more effective than anti-CD96 alone, both in terms of anti-metastatic potential and mouse survival, while Barrow et al. showed that CD96 blockade cooperated with PDGF-DD-NKp44-mediated NK cell control,144 revealing the superior therapeutic potential of the combination therapy. Similar findings were recently confirmed in double knockout mice lacking both PD-1 and CD96.145 Moreover, indirect evidence supporting the enhanced anti-tumor performance when both PVR receptors are targeted has been published. TIGIT knockout mice exhibit less metastasis when treated with an anti-CD96 mAb than equivalently treated wild-type mice, indicating that either distinct mechanism or different lymphocyte subsets are involved in this control,48 although this finding was not confirmed in double knockout mice.145 However, most of the studies did not adequately address the role of the CD96–PVR interaction and the mechanism of action of these antibodies. As shown in a recent study by Aquilera et al.,92 a specific anti-CD96 antibody that binds to second Ig domain of CD96 and does not prevent its interaction with PVR still retains NK cell-mediated anti-metastatic activity in several murine melanoma models. Although the authors did not investigate possible explanations for these phenomena, their data reveals that different options targeting CD96 might be exploited for their therapeutic benefits, even in PVR-negative tumors.
Nevertheless, despite the promising results obtained with strategies targeting CD96 in mouse models, a limited number of studies have translated this knowledge to humans. Actually, the reason supporting the inhibitory role of CD96 in humans partially relies on similarities between CD96 and TIGIT, as described above. However, human and mouse CD96 differ significantly: (i) in humans, unlike mice, CD96 exists as splice variants,146 (ii) mouse CD96, but not human CD96, interacts with Nectin-1,147 and (iii) human CD96, but not mouse CD96, has a potential activation sequence, the Tyr-X-X-Met box, in its C terminus.146 Furthermore, contradictory data regarding the function of hCD96 has been obtained in the studies published to date. The first study characterizing human CD96 implied that it has an activating role, as suggested by the enhanced lysis of FcR-positive mouse cell line P815 by polyclonal human NK cells in the presence of an anti-CD96 antibody.49 However, subsequent in vitro experiments failed to confirm this finding: the anti-CD96 antibody had no influence on NK cell-mediated killing of human ovarian carcinoma or myeloma cells.148,149 Obviously, the setup of the indicated experiments was different and not comparable, which might account for the discrepancy. Additionally, the knowledge obtained from the murine model suggesting a potential mechanism of action for CD96, i.e., cytokine production, might explain why in vitro efforts using purified NK cells had failed. A limited number of ex vivo studies have addressed the role of hCD96. As shown in a recent study by Peng et al., CD96 expression is decreased on NK cells from patients with pancreatic cancer and correlates with disease progression, supporting its activating role.150 However, more studies are needed to corroborate these results, particularly since other researchers have reported the upregulated expression of hCD96 on cells within the tumor microenvironment and hCD96 itself was identified as tumor marker.87–89,151 Finally, although CD96 was originally identified as a human T cell antigen,86 its function in T cells generally remains obscure, particularly in the field of tumor immunology. Hence, in addition to critical points regarding the inhibitory potential of hCD96, many other unresolved questions remain on this subject.
In conclusion, checkpoint therapies targeting inhibitory PVR receptors have already obtained substantial experimental support in preclinical cancer models, both as a monotherapy and in combination with other targets. In particular, TIGIT targeting is more advanced, although several questions remain. For example, PD-1 and CTLA-4 immunotherapy increase the development of immune-related adverse events, a consequence of augmented host immunity that must be assessed for strategies targeting TIGIT (and CD96) as well.152,153 In addition, better characterization of the dynamic regulation and pattern of receptor expression is required. In particular, the existence of paired receptors recognizing the same ligand implies that the magnitude and outcome of the immune response are probably dictated not only by the relative availability of ligands, but also by the context-dependent kinetics of receptor expression. Most studies analyzing the function of PVR receptors focus on a single receptor, although the majority of immune cells co-express these receptors. Because PVR receptors have been suggested to act on each other in cis,83 a better understanding of the cross-talk, dynamics, and context-dependent functions of PVR receptors is an absolute requirement for the development of more successful anti-tumor therapies.
DNAM-1-based adoptive cell therapy
In addition to checkpoint therapy, one of the fastest growing areas of immunotherapy is adoptive cell therapy, which is designed to fight cancer through the use of patient-derived, ex vivo boosted lymphocytes. In that sense, several different approaches are currently being investigated: non-specific effectors such as lymphokine-activated killer (LAK) and cytokine-induced killer (CIK) cells, and tumor-specific effectors such as tumor-infiltrating lymphocytes (TILs), CAR/TCR-engineered T cells, or endogenous T cells.154,155 Among these approaches, the most relevant cells for this review are CAR-engineered T cells. This approach employs the patient’s own cells, or more recently, even allogeneic cells that are genetically engineered to express transgenic chimeric antigen receptors recognizing tumor antigens. The expression of these receptors confers potent effector capacity to these cells, ultimately leading to their robust activation and killing of the target tumor cells.156–158 Usually, CARs are engineered to contain an extracellular antigen-recognizing region usually antibody-derived single-chain variable fragment linked to an intracellular signaling domain, usually TCR/CD3 ζ-chain and co-stimulatory domains.156 Since antigen recognition of these cells does not depend on MHC, CAR T cells are able to circumvent some of the MHC-targeted tumor immune evasion mechanisms. In addition, unlike TCR, CARs recognize structures other than protein epitopes, which increases the pool of potential target antigens. Finally, the fourth generation CAR T cell constructs contain additional genetic modifications that enhance their anti-tumor activity, such as cytokine production.159 Overall, CARs have already resulted in remarkable achievements in a number of clinical trials,160 and the first CAR T cell products, Yescarta and Kymriah, were recently approved by the FDA as treatments for B cell malignancies.161 Both therapies target the CD19 protein, whose expression is selective and restricted to most B-lineage lymphocytes, while it is not expressed on normal tissues.162 Thus, CD19 is high on the list of targets fulfilling the criteria for an immunotherapy to target antigens that are predominantly expressed on cancer cells. Nevertheless, only a few human antigens meet this criterion, particularly in solid malignancies where the situation is even more complicated. In addition to a lack of tumor-specific targets and the heterogeneity of solid tumors, the microenvironment is hostile and, as mentioned above, immunosuppressive for T cells.160 Hence, more recently, new CAR candidates have become the focus of active research, including candidates based on activating NK receptors such as DNAM-1 that facilitate the targeting of many different tumors.80,163,164 According to numerous studies, DNAM-1, via its interaction with PVR, enhances the activation and cytotoxic capacity of NK cells both in vitro and in vivo.4,69 For example, neuroblastoma cells expressing high levels of PVR are highly susceptible to NK cell/DNAM-1-mediated killing, with a positive correlation observed between the surface expression of PVR and the susceptibility of the cells to lysis.4 Similarly, NK cell-mediated lysis of leukemia cells in vitro depends on DNAM-1 and does not occur when target cells do not express DNAM-1 ligands.5 DNAM-1 is indispensable for NK cell-mediated suppression of metastases in vivo.9,69,70,165,166 In addition, accumulating evidence supports the potential of DNAM-1 in adoptive transfer approaches. For example, Pievani et al.79 and, more recently, Cappel et al.167 showed that DNAM-1 plays a role in CIK cell-mediated anti-tumor cytotoxicity in vitro, whereas Martinet et al.168 showed that adoptive transfer of purified DNAM-1-positive NK cells exerts a superior anti-metastatic effect to DNAM-1-negative NK cells on a B16F10 metastasis murine model in vivo. Similarly, Rosskopf et al.169 reported the enhanced sensitivity of TCR-transgenic Jurkat cells expressing DNAM-1 to tumor cells in vitro, suggesting that this strategy might effectively increase the response of TCR-transgenic cells in adoptive T cell therapy as well.
Nevertheless, numerous studies have reported reduced DNAM-1 expression in patients with tumors, directly affecting the cytotoxicity and effector capacities of immune cells.6,12,13,78,170 For example, in ovarian cancer, chronic exposure to the ligand (PVR) leads to decreased expression of DNAM-1 on NK cells, resulting in impaired anti-tumor control.6 The same group obtained similar findings from bone marrow NK cells from patients with myelodysplastic syndrome (MDS), where decreased expression of DNAM-1 correlated with suppressed NK cell function and elevated blast counts.12 Consistent with these findings, a lack of either host or tumor PVR was recently shown to increase DNAM-1 expression on various immune TIL (tumor-infiltrating lymphocyte) subsets, supporting the chronic antigen exposure theory.13 On the other hand, DNAM-1 downregulation might be a consequence of more complex immunosuppressive mechanisms, such as increased production of soluble factors in the tumor microenvironment, among others.78
Regardless of the causes of DNAM-1 downregulation, the findings clearly support the importance of DNAM-1 in anti-tumor immunity and strengthens the rationale for its use in chimeric antigen receptor-based adoptive cell therapy. Consistent with these results, a recent study by Wu et al.80 attempted to investigate the DNAM-1-based CAR T cell therapy. The authors designed different DNAM-1 CAR T cell constructs based on the full-length DNAM-1 protein and tested them for their capacity to kill tumor cells in vitro and in vivo. As anticipated, DNAM-1 CAR T cells showed high levels of cytotoxicity toward multiple tumor cell types in vitro and reduced tumor burden in a syngeneic melanoma model in vivo. Nevertheless, despite these promising results, one of the major drawbacks of CAR T cell therapy in general is cytokine release syndrome (CRS) caused by excessive cytokine release by activated effector cells, which is potentially fatal.161,171 Another research group analyzed the effect of DNAM-1 CAR T cells on overall health status and the amount of different cytokines in the sera of engrafted mice to investigate the drawbacks of DNAM-1 CAR T cell therapy.172 The injection of a high dose of DNAM-1 CAR T cells indeed resulted in CRS-like responses in mice, similar to those observed in patients. In addition, the severity of CRS depended on the quantity of the engrafted cells, specific CAR T cell effector mechanisms, and host immune cells, which were not specific for DNAM-1 CAR T cells, but were related to CAR T therapy in general. Based on these findings, although DNAM-1-based adoptive cell therapy might be a highly efficient anti-tumor treatment,173 further improvements in CAR T cell strategies and an understanding of the factors impacting clinical outcome are absolute prerequisites for any potential therapeutic applications.
Anti-PVR antibodies in anti-tumor therapy
Although several different approaches for treating cancer that focus on PVR are currently being investigated in clinical trials or preclinical models, approaches directly targeting PVR are still scarce, except for the oncolytic polioviruses. For example, no clinical trials are using anti-PVR antibodies, and the number of published preclinical studies employing this strategy is also limited, which is curious, given the advantages and enormous recent progress in the field of antibody-based immunotherapy.132,174
One of the possible reasons for the limited number of studies in this field might be the lack of information regarding PVR induction in healthy cells. Although PVR is expressed at low levels in healthy tissues, it is considered a stress-induced molecule. This function might be particularly important in patients receiving chemotherapy, since some chemotherapeutic agents have been shown to induce PVR expression.175 The induction of PVR expression might exert a synergistic cytotoxic effect on tumor cells, but it might also worsen the side effects on healthy cells. On the other hand, some chemotherapeutic agents exert the opposite effect on PVR and decrease its expression on tumor cells, leading to their lower susceptibility to NK cells and contributing to tumor evasion.176 This finding reveals the importance of studies aiming to perform a more thorough characterization of PVR expression patterns and kinetics. Adding to the complexity, PVR is also expressed on immune cells and is upregulated upon exposure to different stimuli, such as TLR activation or TCR stimulation.177,178 While PVR expressed on immune cells has been shown to regulate the development and function of these cells,46,66,76,177–179 the role of PVR in immune cells within the tumor microenvironment (TME) has remained undefined until recently, and thus researchers have not been able to easily predict the outcomes of strategies targeting this protein. In a recent publication by the Smyth group,13 the authors showed that: (i) hematopoietic cells in TME express PVR, (ii) PVR has an immunosuppressive role and contributes to tumor progression, and (iii) strategies targeting both host and tumor-derived PVR might have greater anti-tumor benefits. Finally, the fact that inhibitory PVR receptors were discovered much later than the activating receptor might explain why researchers did not consider PVR an immune checkpoint for many years. Additionally, TIGIT, DNAM-1, and CD96 share a common binding site on PVR.46 Hence, the targeting of PVR with mAbs, for example, might result in unwanted inhibition of activating interactions and potentially have weaker effect than direct blockade of inhibitory receptors. On the other hand, the concerns associated with the blockade of the PVR-DNAM-1 interaction might not be justified. First, DNAM-1 also binds to another member of the nectin family, Nectin-2 (CD112), which is also overexpressed on tumor cells and involved in immune response.39,180–182 This interaction would not be affected by PVR targeting; second, numerous studies proposing PVR-dependent DNAM-1 downregulation12,13,170,183 support the hypothesis that PVR blockade might even increase DNAM-1 expression and related immune cell functions. In addition, the higher binding affinity of PVR for inhibitory receptors suggests that strategies targeting this interaction would theoretically be sufficient to reverse immune inhibition. Consistent with these findings, in vitro antibody-mediated blockade of PVR on acute myeloid leukemia cells was recently shown to increase the anti-leukemic effects of immune cells.184 Similar findings were observed when melanoma cell lines were treated with anti-PVR antibody, leading to increased production of IFNγ by antigen-specific CTLs.55 In addition, the first study using an anti-PVR mAb in in vivo tumor model also reported the anti-tumor properties of this treatment, with the Ab-inhibiting cancer metastasis.185 According to another recent study, NSG mice that were reconstituted with human T cells and engrafted with tumor cells lacking both PVR and Nectin-2 exhibit prolonged survival compared to mice engrafted with WT (ligand expressing) tumor cells.184 Although the indicated studies attributed the observed anti-tumor effects to immune cells, i.e., to the immune cell-dependent role of PVR, strategies targeting PVR might also rely on additional mechanisms. For example, given the role of PVR in cellular proliferation and its association with growth factor receptors,34,35 researchers have hypothesized that the disruption of these complexes with antibodies might exert an anti-tumor effect. Similar findings have already been obtained in a study by Lee et al.,34 where the use of a biomimetic peptide disrupting complexes between IGF1R and Met receptor-binding proteins, including PVR, inhibited breast cancer growth and metastasis in vivo. Consistent with “immune-independent” PVR targeting approach, Li et al.13 showed that tumors lacking PVR, independent of immune cells, showed slower tumor growth and reduced metastases in vivo, as previously shown in vitro,1,7 again suggesting the possibility of interfering with these cell cycle- and proliferation-related roles of PVR. Finally, another potential therapeutic approach targeting PVR might be the use of antibody–drug conjugates (ADCs), one of the most promising strategies of targeted therapy.186 The strategy involves the construction of an antigen-specific monoclonal antibody conjugated to a highly potent cytotoxic molecule that will, upon binding of the ADCs to the specific targets on the surface of tumor cells, initiate the internalization of the complex, followed by lysosomal degradation and subsequent release of the cytotoxic component. This treatment will ultimately disrupt the cellular function, causing irreversible cell death. This approach has been already successfully applied to another protein of the same family, Nectin-4,187,188 and it is currently being investigated in a Phase II clinical trial of patients with urothelial cancers.
Concluding remarks
Although PVR was discovered ~30 years ago, this multifunctional molecule did not regain the interest of scientists until recently, mostly due to the relatively recent discovery of its complex interactions and related prominent roles in the immune response. PVR in the context of tumors has received particular interest, where it is often upregulated, in contrast to healthy tissues. This therapeutically attractive property of PVR is currently being investigated mostly in the field of recombinant oncolytic virotherapy that has already achieved significant experimental success and anti-tumor potential, particularly in patients with glioma. Most of the other currently investigated PVR-related anti-tumor strategies are focusing on targeting inhibitory PVR receptors, an approach known as checkpoint blockade, aiming to reverse the exhausted state of immune cells and induce the recovery of their effector functions. Although this approach clearly shows that these proteins (inhibitory PVR receptors) are promising immunotherapeutic targets, at least in in vivo mouse models, abundant clinical research on similar targets (e.g., PD-1 or CTLA-4) indicates that we are still far from achieving 100% success: clinical outcomes remain highly variable and only a small percentage of patients benefits from checkpoint blockade therapies. However, many preclinical studies show the superior potential of combinational therapy, i.e., strategies targeting multiple checkpoint inhibitors in parallel; therefore, future research will likely focus on investigating the efficacy of this approach in the context of PVR receptors and the discovery of new potential targets. Here, PVR might be high on the list of novel targets, particularly since accumulating evidence supports its important immune-related and proto-oncogenic functions in both host and tumor cells. Hence, PVR will likely attract even more interest for the development of new technologies and approaches, such as antibody–drug conjugates or gene editing strategies. Finally, because most of the enhanced anti-tumor properties described above are attributed to DNAM-1, adoptive cell transfer methods exploiting DNAM-1 might also be highly efficient and have major therapeutic benefits. In conclusion, although multiple strategies are currently being investigated that target PVR or its immune interactions, further progress must be achieved. With the development of new therapeutic approaches and increasing knowledge on the roles of PVR in the tumor microenvironment and in general, PVR will attract even more scientific and clinical attention.
Acknowledgements
S.J. is supported by the grant “Strengthening the capacity of CerVirVac for research in virus immunology and vaccinology”, KK.01.1.1.01.0006, awarded to the Scientific Centre of Excellence for Virus Immunology and Vaccines and co-financed by the European Regional Development Fund. P.K.B. and T.L.R. are supported by the Croatian Science Foundation (HRZZ) under project number 1533.
Competing interests
S.J., O.M. and P.T. are shareholders in Nectin Therapeutics Ltd. The remaining authors declare no competing interests.
Footnotes
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Paola Kučan Brlić, Email: paola.kucan@uniri.hr.
Stipan Jonjić, Email: stipan.jonjic@uniri.hr.
References
- 1.Takai Y, Miyoshi J, Ikeda W, Ogita H. Nectins and nectin-like molecules: roles in contact inhibition of cell movement and proliferation. Nat. Rev. Mol. Cell Biol. 2008;9:603–615. doi: 10.1038/nrm2457. [DOI] [PubMed] [Google Scholar]
- 2.Gromeier M, Lachmann S, Rosenfeld MR, Gutin PH, Wimmer E. Intergeneric poliovirus recombinants for the treatment of malignant glioma. Proc. Natl Acad. Sci. USA. 2000;97:6803–6808. doi: 10.1073/pnas.97.12.6803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Masson D, et al. Overexpression of the CD155 gene in human colorectal carcinoma. Gut. 2001;49:236–240. doi: 10.1136/gut.49.2.236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Castriconi R, et al. Natural killer cell-mediated killing of freshly isolated neuroblastoma cells: critical role of DNAX accessory molecule-1-poliovirus receptor interaction. Cancer Res. 2004;64:9180–9184. doi: 10.1158/0008-5472.CAN-04-2682. [DOI] [PubMed] [Google Scholar]
- 5.Pende D, et al. Analysis of the receptor-ligand interactions in the natural killer-mediated lysis of freshly isolated myeloid or lymphoblastic leukemias: evidence for the involvement of the Poliovirus receptor (CD155) and Nectin-2 (CD112) Blood. 2005;105:2066–2073. doi: 10.1182/blood-2004-09-3548. [DOI] [PubMed] [Google Scholar]
- 6.Carlsten M, et al. Primary human tumor cells expressing CD155 impair tumor targeting by down-regulating DNAM-1 on NK cells. J. Immunol. 2009;183:4921–4930. doi: 10.4049/jimmunol.0901226. [DOI] [PubMed] [Google Scholar]
- 7.Sloan KE, Stewart JK, Treloar AF, Matthews RT, Jay DG. CD155/PVR enhances glioma cell dispersal by regulating adhesion signaling and focal adhesion dynamics. Cancer Res. 2005;65:10930–10937. doi: 10.1158/0008-5472.CAN-05-1890. [DOI] [PubMed] [Google Scholar]
- 8.Kono T, et al. The CD155/poliovirus receptor enhances the proliferation of ras-mutated cells. Int. J. Cancer. 2008;122:317–324. doi: 10.1002/ijc.23080. [DOI] [PubMed] [Google Scholar]
- 9.Martinet L, Smyth MJ. Balancing natural killer cell activation through paired receptors. Nat. Rev. Immunol. 2015;15:243–254. doi: 10.1038/nri3799. [DOI] [PubMed] [Google Scholar]
- 10.Nishiwada S, et al. Clinical significance of CD155 expression in human pancreatic cancer. Anticancer Res. 2015;35:2287–2297. [PubMed] [Google Scholar]
- 11.Bowers JR, Readler JM, Sharma P, Excoffon K. Poliovirus receptor: more than a simple viral receptor. Virus Res. 2017;242:1–6. doi: 10.1016/j.virusres.2017.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Carlsten M, et al. Reduced DNAM-1 expression on bone marrow NK cells associated with impaired killing of CD34+ blasts in myelodysplastic syndrome. Leukemia. 2010;24:1607–1616. doi: 10.1038/leu.2010.149. [DOI] [PubMed] [Google Scholar]
- 13.Li XY, et al. CD155 loss enhances tumor suppression via combined host and tumor-intrinsic mechanisms. J. Clin. Invest. 2018;128:2613–2625. doi: 10.1172/JCI98769. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 14.Bronte V. The expanding constellation of immune checkpoints: a DNAMic control by CD155. J. Clin. Invest. 2018;128:2199–2201. doi: 10.1172/JCI121229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gao J, Zheng Q, Xin N, Wang W, Zhao C. CD155, an onco-immunologic molecule in human tumors. Cancer Sci. 2017;108:1934–1938. doi: 10.1111/cas.13324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mendelsohn CL, Wimmer E, Racaniello VR. Cellular receptor for poliovirus: molecular cloning, nucleotide sequence, and expression of a new member of the immunoglobulin superfamily. Cell. 1989;56:855–865. doi: 10.1016/0092-8674(89)90690-9. [DOI] [PubMed] [Google Scholar]
- 17.Stengel KF, et al. Structure of TIGIT immunoreceptor bound to poliovirus receptor reveals a cell-cell adhesion and signaling mechanism that requires cis-trans receptor clustering. Proc. Natl Acad. Sci. USA. 2012;109:5399–5404. doi: 10.1073/pnas.1120606109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Koike S, et al. The poliovirus receptor protein is produced both as membrane-bound and secreted forms. EMBO J. 1990;9:3217–3224. doi: 10.1002/j.1460-2075.1990.tb07520.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Baury B, et al. Identification of secreted CD155 isoforms. Biochem. Biophys. Res. Commun. 2003;309:175–182. doi: 10.1016/S0006-291X(03)01560-2. [DOI] [PubMed] [Google Scholar]
- 20.Iguchi-Manaka A, et al. Increased soluble CD155 in the serum of cancer patients. PLoS ONE. 2016;11:e0152982. doi: 10.1371/journal.pone.0152982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Deng W, et al. Antitumor immunity. A shed NKG2D ligand that promotes natural killer cell activation and tumor rejection. Science. 2015;348:136–139. doi: 10.1126/science.1258867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Groh V, Wu J, Yee C, Spies T. Tumour-derived soluble MIC ligands impair expression of NKG2D and T cell activation. Nature. 2002;419:734–738. doi: 10.1038/nature01112. [DOI] [PubMed] [Google Scholar]
- 23.Zhu X, Lang J. Soluble PD-1 and PD-L1: predictive and prognostic significance in cancer. Oncotarget. 2017;8:97671–97682. doi: 10.18632/oncotarget.18311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Oda T, Ohka S, Nomoto A. Ligand stimulation of CD155alpha inhibits cell adhesion and enhances cell migration in fibroblasts. Biochem. Biophys. Res. Commun. 2004;319:1253–1264. doi: 10.1016/j.bbrc.2004.05.111. [DOI] [PubMed] [Google Scholar]
- 25.Ohka S, Ohno H, Tohyama K, Nomoto A. Basolateral sorting of human poliovirus receptor alpha involves an interaction with the mu1B subunit of the clathrin adaptor complex in polarized epithelial cells. Biochem. Biophys. Res. Commun. 2001;287:941–948. doi: 10.1006/bbrc.2001.5660. [DOI] [PubMed] [Google Scholar]
- 26.Yusa S, Catina TL, Campbell KS. SHP-1- and phosphotyrosine-independent inhibitory signaling by a killer cell Ig-like receptor cytoplasmic domain in human NK cells. J. Immunol. 2002;168:5047–5057. doi: 10.4049/jimmunol.168.10.5047. [DOI] [PubMed] [Google Scholar]
- 27.Lange R, Peng X, Wimmer E, Lipp M, Bernhardt G. The poliovirus receptor CD155 mediates cell-to-matrix contacts by specifically binding to vitronectin. Virology. 2001;285:218–227. doi: 10.1006/viro.2001.0943. [DOI] [PubMed] [Google Scholar]
- 28.Reymond N, et al. DNAM-1 and PVR regulate monocyte migration through endothelial junctions. J. Exp. Med. 2004;199:1331–1341. doi: 10.1084/jem.20032206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sullivan DP, Seidman MA, Muller WA. Poliovirus receptor (CD155) regulates a step in transendothelial migration between PECAM and CD99. Am. J. Pathol. 2013;182:1031–1042. doi: 10.1016/j.ajpath.2012.11.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kakunaga S, et al. Enhancement of serum- and platelet-derived growth factor-induced cell proliferation by Necl-5/Tage4/poliovirus receptor/CD155 through the Ras-Raf-MEK-ERK signaling. J. Biol. Chem. 2004;279:36419–36425. doi: 10.1074/jbc.M406340200. [DOI] [PubMed] [Google Scholar]
- 31.Ikeda W, et al. Nectin-like molecule-5/Tage4 enhances cell migration in an integrin-dependent, Nectin-3-independent manner. J. Biol. Chem. 2004;279:18015–18025. doi: 10.1074/jbc.M312969200. [DOI] [PubMed] [Google Scholar]
- 32.Minami Y, et al. Necl-5/poliovirus receptor interacts in cis with integrin alphaVbeta3 and regulates its clustering and focal complex formation. J. Biol. Chem. 2007;282:18481–18496. doi: 10.1074/jbc.M611330200. [DOI] [PubMed] [Google Scholar]
- 33.Kajita M, Ikeda W, Tamaru Y, Takai Y. Regulation of platelet-derived growth factor-induced Ras signaling by poliovirus receptor Necl-5 and negative growth regulator Sprouty2. Genes Cells. 2007;12:345–357. doi: 10.1111/j.1365-2443.2007.01062.x. [DOI] [PubMed] [Google Scholar]
- 34.Lee E, et al. Inhibition of breast cancer growth and metastasis by a biomimetic peptide. Sci. Rep. 2014;4:7139. doi: 10.1038/srep07139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kinugasa M, et al. Necl-5/poliovirus receptor interacts with VEGFR2 and regulates VEGF-induced angiogenesis. Circ. Res. 2012;110:716–726. doi: 10.1161/CIRCRESAHA.111.256834. [DOI] [PubMed] [Google Scholar]
- 36.Campbell HK, Maiers JL, DeMali KA. Interplay between tight junctions & adherens junctions. Exp. Cell Res. 2017;358:39–44. doi: 10.1016/j.yexcr.2017.03.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fujito T, et al. Inhibition of cell movement and proliferation by cell-cell contact-induced interaction of Necl-5 with nectin-3. J. Cell Biol. 2005;171:165–173. doi: 10.1083/jcb.200501090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stanietsky N, Mandelboim O. Paired NK cell receptors controlling NK cytotoxicity. FEBS Lett. 2010;584:4895–4900. doi: 10.1016/j.febslet.2010.08.047. [DOI] [PubMed] [Google Scholar]
- 39.Bottino C, et al. Identification of PVR (CD155) and Nectin-2 (CD112) as cell surface ligands for the human DNAM-1 (CD226) activating molecule. J. Exp. Med. 2003;198:557–567. doi: 10.1084/jem.20030788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chan CJ, et al. The receptors CD96 and CD226 oppose each other in the regulation of natural killer cell functions. Nat. Immunol. 2014;15:431–438. doi: 10.1038/ni.2850. [DOI] [PubMed] [Google Scholar]
- 41.de Andrade LF, Smyth MJ, Martinet L. DNAM-1 control of natural killer cells functions through nectin and nectin-like proteins. Immunol. Cell Biol. 2014;92:237–244. doi: 10.1038/icb.2013.95. [DOI] [PubMed] [Google Scholar]
- 42.Shibuya A, et al. DNAM-1, a novel adhesion molecule involved in the cytolytic function of T lymphocytes. Immunity. 1996;4:573–581. doi: 10.1016/S1074-7613(00)70060-4. [DOI] [PubMed] [Google Scholar]
- 43.Anderson AC, Joller N, Kuchroo VK. Lag-3, Tim-3, and TIGIT: co-inhibitory receptors with specialized functions in immune regulation. Immunity. 2016;44:989–1004. doi: 10.1016/j.immuni.2016.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Joller N, et al. Cutting edge: TIGIT has T cell-intrinsic inhibitory functions. J. Immunol. 2011;186:1338–1342. doi: 10.4049/jimmunol.1003081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Stanietsky N, et al. The interaction of TIGIT with PVR and PVRL2 inhibits human NK cell cytotoxicity. Proc. Natl Acad. Sci. USA. 2009;106:17858–17863. doi: 10.1073/pnas.0903474106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yu X, et al. The surface protein TIGIT suppresses T cell activation by promoting the generation of mature immunoregulatory dendritic cells. Nat. Immunol. 2009;10:48–57. doi: 10.1038/ni.1674. [DOI] [PubMed] [Google Scholar]
- 47.Dougall WC, Kurtulus S, Smyth MJ, Anderson AC. TIGIT and CD96: new checkpoint receptor targets for cancer immunotherapy. Immunol. Rev. 2017;276:112–120. doi: 10.1111/imr.12518. [DOI] [PubMed] [Google Scholar]
- 48.Blake SJ, et al. Suppression of metastases using a new lymphocyte checkpoint target for cancer immunotherapy. Cancer Discov. 2016;6:446–459. doi: 10.1158/2159-8290.CD-15-0944. [DOI] [PubMed] [Google Scholar]
- 49.Fuchs A, Cella M, Giurisato E, Shaw AS, Colonna M. Cutting edge: CD96 (tactile) promotes NK cell-target cell adhesion by interacting with the poliovirus receptor (CD155) J. Immunol. 2004;172:3994–3998. doi: 10.4049/jimmunol.172.7.3994. [DOI] [PubMed] [Google Scholar]
- 50.Stanko K, et al. CD96 expression determines the inflammatory potential of IL-9-producing Th9 cells. Proc. Natl Acad. Sci. USA. 2018;115:E2940–E2949. doi: 10.1073/pnas.1708329115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Denis MG. Characterization, cloning and expression of the Tage4 gene, a member of the immunoglobulin superfamily. Int. J. Oncol. 1998;12:997–1005. doi: 10.3892/ijo.12.5.997. [DOI] [PubMed] [Google Scholar]
- 52.Lim YP, Fowler LC, Hixson DC, Wehbe T, Thompson NL. TuAg.1 is the liver isoform of the rat colon tumor-associated antigen pE4 and a member of the immunoglobulin-like supergene family. Cancer Res. 1996;56:3934–3940. [PubMed] [Google Scholar]
- 53.Nakai R, et al. Overexpression of Necl-5 correlates with unfavorable prognosis in patients with lung adenocarcinoma. Cancer Sci. 2010;101:1326–1330. doi: 10.1111/j.1349-7006.2010.01530.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bevelacqua V, et al. Nectin like-5 overexpression correlates with the malignant phenotype in cutaneous melanoma. Oncotarget. 2012;3:882–892. doi: 10.18632/oncotarget.594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Inozume T, et al. Melanoma cells control antimelanoma CTL responses via interaction between TIGIT and CD155 in the effector phase. J. Invest. Dermatol. 2016;136:255–263. doi: 10.1038/JID.2015.404. [DOI] [PubMed] [Google Scholar]
- 56.Huang DW, Huang M, Lin XS, Huang Q. CD155 expression and its correlation with clinicopathologic characteristics, angiogenesis, and prognosis in human cholangiocarcinoma. Onco. Targets Ther. 2017;10:3817–3825. doi: 10.2147/OTT.S141476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hirota T, Irie K, Okamoto R, Ikeda W, Takai Y. Transcriptional activation of the mouse Necl-5/Tage4/PVR/CD155 gene by fibroblast growth factor or oncogenic Ras through the Raf-MEK-ERK-AP-1 pathway. Oncogene. 2005;24:2229–2235. doi: 10.1038/sj.onc.1208409. [DOI] [PubMed] [Google Scholar]
- 58.Solecki DJ, Gromeier M, Mueller S, Bernhardt G, Wimmer E. Expression of the human poliovirus receptor/CD155 gene is activated by sonic hedgehog. J. Biol. Chem. 2002;277:25697–25702. doi: 10.1074/jbc.M201378200. [DOI] [PubMed] [Google Scholar]
- 59.Gorgoulis VG, et al. Activation of the DNA damage checkpoint and genomic instability in human precancerous lesions. Nature. 2005;434:907–913. doi: 10.1038/nature03485. [DOI] [PubMed] [Google Scholar]
- 60.Soriani A, et al. ATM-ATR-dependent up-regulation of DNAM-1 and NKG2D ligands on multiple myeloma cells by therapeutic agents results in enhanced NK-cell susceptibility and is associated with a senescent phenotype. Blood. 2009;113:3503–3511. doi: 10.1182/blood-2008-08-173914. [DOI] [PubMed] [Google Scholar]
- 61.Soriani A, et al. Chemotherapy-elicited upregulation of NKG2D and DNAM-1 ligands as a therapeutic target in multiple myeloma. Oncoimmunology. 2013;2:e26663. doi: 10.4161/onci.26663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Atsumi S, et al. Prognostic significance of CD155 mRNA expression in soft tissue sarcomas. Oncol. Lett. 2013;5:1771–1776. doi: 10.3892/ol.2013.1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tane S, et al. The role of Necl-5 in the invasive activity of lung adenocarcinoma. Exp. Mol. Pathol. 2013;94:330–335. doi: 10.1016/j.yexmp.2012.12.003. [DOI] [PubMed] [Google Scholar]
- 64.Sloan KE, et al. CD155/PVR plays a key role in cell motility during tumor cell invasion and migration. BMC Cancer. 2004;4:73. doi: 10.1186/1471-2407-4-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zheng Q, et al. CD155 knockdown promotes apoptosis via AKT/Bcl-2/Bax in colon cancer cells. J. Cell. Mol. Med. 2018;22:131–140. doi: 10.1111/jcmm.13301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Escalante NK, von Rossum A, Lee M, Choy JC. CD155 on human vascular endothelial cells attenuates the acquisition of effector functions in CD8 T cells. Arterioscler. Thromb. Vasc. Biol. 2011;31:1177–1184. doi: 10.1161/ATVBAHA.111.224162. [DOI] [PubMed] [Google Scholar]
- 67.Merrill MK, et al. Poliovirus receptor CD155-targeted oncolysis of glioma. Neuro. Oncol. 2004;6:208–217. doi: 10.1215/S1152851703000577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Iwasaki A, et al. Immunofluorescence analysis of poliovirus receptor expression in Peyer’s patches of humans, primates, and CD155 transgenic mice: implications for poliovirus infection. J. Infect. Dis. 2002;186:585–592. doi: 10.1086/342682. [DOI] [PubMed] [Google Scholar]
- 69.Iguchi-Manaka A, et al. Accelerated tumor growth in mice deficient in DNAM-1 receptor. J. Exp. Med. 2008;205:2959–2964. doi: 10.1084/jem.20081611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Gilfillan S, et al. DNAM-1 promotes activation of cytotoxic lymphocytes by nonprofessional antigen-presenting cells and tumors. J. Exp. Med. 2008;205:2965–2973. doi: 10.1084/jem.20081752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Shibuya K, et al. CD226 (DNAM-1) is involved in lymphocyte function-associated antigen 1 costimulatory signal for naive T cell differentiation and proliferation. J. Exp. Med. 2003;198:1829–1839. doi: 10.1084/jem.20030958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Tahara-Hanaoka S, et al. Tumor rejection by the poliovirus receptor family ligands of the DNAM-1 (CD226) receptor. Blood. 2006;107:1491–1496. doi: 10.1182/blood-2005-04-1684. [DOI] [PubMed] [Google Scholar]
- 73.Seth S, et al. Heterogeneous expression of the adhesion receptor CD226 on murine NK and T cells and its function in NK-mediated killing of immature dendritic cells. J. Leukoc. Biol. 2009;86:91–101. doi: 10.1189/jlb.1208745. [DOI] [PubMed] [Google Scholar]
- 74.Smith LE, et al. Sensitivity of dendritic cells to NK-mediated lysis depends on the inflammatory environment and is modulated by CD54/CD226-driven interactions. J. Leukoc. Biol. 2016;100:781–789. doi: 10.1189/jlb.3A0615-271RR. [DOI] [PubMed] [Google Scholar]
- 75.Bachelet I, Munitz A, Mankutad D, Levi-Schaffer F. Mast cell costimulation by CD226/CD112 (DNAM-1/Nectin-2): a novel interface in the allergic process. J. Biol. Chem. 2006;281:27190–27196. doi: 10.1074/jbc.M602359200. [DOI] [PubMed] [Google Scholar]
- 76.Pende D, et al. Expression of the DNAM-1 ligands, Nectin-2 (CD112) and poliovirus receptor (CD155), on dendritic cells: relevance for natural killer-dendritic cell interaction. Blood. 2006;107:2030–2036. doi: 10.1182/blood-2005-07-2696. [DOI] [PubMed] [Google Scholar]
- 77.Kojima H, et al. CD226 mediates platelet and megakaryocytic cell adhesion to vascular endothelial cells. J. Biol. Chem. 2003;278:36748–36753. doi: 10.1074/jbc.M300702200. [DOI] [PubMed] [Google Scholar]
- 78.Mamessier E, et al. Human breast cancer cells enhance self tolerance by promoting evasion from NK cell antitumor immunity. J. Clin. Invest. 2011;121:3609–3622. doi: 10.1172/JCI45816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Pievani A, et al. Dual-functional capability of CD3+ CD56 + CIK cells, a T-cell subset that acquires NK function and retains TCR-mediated specific cytotoxicity. Blood. 2011;118:3301–3310. doi: 10.1182/blood-2011-02-336321. [DOI] [PubMed] [Google Scholar]
- 80.Wu MR, Zhang T, Alcon A, Sentman CL. DNAM-1-based chimeric antigen receptors enhance T cell effector function and exhibit in vivo efficacy against melanoma. Cancer Immunol. Immunother. 2015;64:409–418. doi: 10.1007/s00262-014-1648-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lenac Rovis T, et al. Inflammatory monocytes and NK cells play a crucial role in DNAM-1-dependent control of cytomegalovirus infection. J. Exp. Med. 2016;213:1835–1850. doi: 10.1084/jem.20151899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Joller N, et al. Treg cells expressing the coinhibitory molecule TIGIT selectively inhibit proinflammatory Th1 and Th17 cell responses. Immunity. 2014;40:569–581. doi: 10.1016/j.immuni.2014.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Johnston RJ, et al. The immunoreceptor TIGIT regulates antitumor and antiviral CD8(+) T cell effector function. Cancer Cell. 2014;26:923–937. doi: 10.1016/j.ccell.2014.10.018. [DOI] [PubMed] [Google Scholar]
- 84.Pauken KE, Wherry EJ. TIGIT and CD226: tipping the balance between costimulatory and coinhibitory molecules to augment the cancer immunotherapy toolkit. Cancer Cell. 2014;26:785–787. doi: 10.1016/j.ccell.2014.11.016. [DOI] [PubMed] [Google Scholar]
- 85.Blake SJ, Dougall WC, Miles JJ, Teng MW, Smyth MJ. Molecular pathways: targeting CD96 and TIGIT for cancer immunotherapy. Clin. Cancer Res. 2016;22:5183–5188. doi: 10.1158/1078-0432.CCR-16-0933. [DOI] [PubMed] [Google Scholar]
- 86.Wang PL, O’Farrell S, Clayberger C, Krensky AM. Identification and molecular cloning of tactile. A novel human T cell activation antigen that is a member of the Ig gene superfamily. J. Immunol. 1992;148:2600–2608. [PubMed] [Google Scholar]
- 87.Zhang W, et al. Expressions of CD96 and CD123 in bone marrow cells of patients with myelodysplastic syndromes. Clin. Lab. 2015;61:1429–1434. doi: 10.7754/clin.lab.2015.141240. [DOI] [PubMed] [Google Scholar]
- 88.Hosen N, et al. CD96 is a leukemic stem cell-specific marker in human acute myeloid leukemia. Proc. Natl Acad. Sci. USA. 2007;104:11008–11013. doi: 10.1073/pnas.0704271104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Gramatzki M, et al. Antibodies TC-12 (“unique”) and TH-111 (CD96) characterize T-cell acute lymphoblastic leukemia and a subgroup of acute myeloid leukemia. Exp. Hematol. 1998;26:1209–1214. [PubMed] [Google Scholar]
- 90.Stanietsky N, et al. Mouse TIGIT inhibits NK-cell cytotoxicity upon interaction with PVR. Eur. J. Immunol. 2013;43:2138–2150. doi: 10.1002/eji.201243072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.McVicar DW, Burshtyn DN. Intracellular signaling by the killer immunoglobulin-like receptors and Ly49. Sci. STKE. 2001;2001:re1. doi: 10.1126/stke.2001.75.re1. [DOI] [PubMed] [Google Scholar]
- 92.Roman Aguilera A, et al. CD96 targeted antibodies need not block CD96-CD155 interactions to promote NK cell anti-metastatic activity. Oncoimmunology. 2018;7:e1424677. doi: 10.1080/2162402X.2018.1424677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Fukuhara H, Ino Y, Todo T. Oncolytic virus therapy: a new era of cancer treatment at dawn. Cancer Sci. 2016;107:1373–1379. doi: 10.1111/cas.13027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kaufman HL, Kohlhapp FJ, Zloza A. Oncolytic viruses: a new class of immunotherapy drugs. Nat. Rev. Drug Discov. 2015;14:642–662. doi: 10.1038/nrd4663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Desjardins A, et al. Recurrent glioblastoma treated with recombinant poliovirus. N. Engl. J. Med. 2018;379:150–161. doi: 10.1056/NEJMoa1716435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Hogle JM. Poliovirus cell entry: common structural themes in viral cell entry pathways. Annu. Rev. Microbiol. 2002;56:677–702. doi: 10.1146/annurev.micro.56.012302.160757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Lwoff A, Dulbecco R, Vogt M, Lwoff M. Kinetics of the release of poliomyelitis virus from single cells. Ann. N. Y. Acad. Sci. 1955;61:801–805. doi: 10.1111/j.1749-6632.1955.tb42536.x. [DOI] [PubMed] [Google Scholar]
- 98.Daley JK, Gechman LA, Skipworth J, Rall GF. Poliovirus replication and spread in primary neuron cultures. Virology. 2005;340:10–20. doi: 10.1016/j.virol.2005.05.032. [DOI] [PubMed] [Google Scholar]
- 99.Chandramohan V, et al. Validation of an immunohistochemistry assay for detection of CD155, the poliovirus receptor, in malignant gliomas. Arch. Pathol. Lab. Med. 2017;141:1697–1704. doi: 10.5858/arpa.2016-0580-OA. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Bodian D. Emerging concept of poliomyelitis infection. Science. 1955;122:105–108. doi: 10.1126/science.122.3159.105. [DOI] [PubMed] [Google Scholar]
- 101.Sabin AB. Pathogenesis of poliomyelitis; reappraisal in the light of new data. Science. 1956;123:1151–1157. doi: 10.1126/science.123.3209.1151. [DOI] [PubMed] [Google Scholar]
- 102.Kauder SE, Racaniello VR. Poliovirus tropism and attenuation are determined after internal ribosome entry. J. Clin. Invest. 2004;113:1743–1753. doi: 10.1172/JCI200421323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Ida-Hosonuma M, et al. The alpha/beta interferon response controls tissue tropism and pathogenicity of poliovirus. J. Virol. 2005;79:4460–4469. doi: 10.1128/JVI.79.7.4460-4469.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Georgescu MM, et al. Evolution of the Sabin type 1 poliovirus in humans: characterization of strains isolated from patients with vaccine-associated paralytic poliomyelitis. J. Virol. 1997;71:7758–7768. doi: 10.1128/jvi.71.10.7758-7768.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Wimmer E, Hellen CU, Cao X. Genetics of poliovirus. Annu. Rev. Genet. 1993;27:353–436. doi: 10.1146/annurev.ge.27.120193.002033. [DOI] [PubMed] [Google Scholar]
- 106.Gromeier M, Alexander L, Wimmer E. Internal ribosomal entry site substitution eliminates neurovirulence in intergeneric poliovirus recombinants. Proc. Natl Acad. Sci. USA. 1996;93:2370–2375. doi: 10.1073/pnas.93.6.2370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Merrill MK, Dobrikova EY, Gromeier M. Cell-type-specific repression of internal ribosome entry site activity by double-stranded RNA-binding protein 76. J. Virol. 2006;80:3147–3156. doi: 10.1128/JVI.80.7.3147-3156.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Merrill MK, Gromeier M. The double-stranded RNA binding protein 76:NF45 heterodimer inhibits translation initiation at the rhinovirus type 2 internal ribosome entry site. J. Virol. 2006;80:6936–6942. doi: 10.1128/JVI.00243-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Neplioueva V, Dobrikova EY, Mukherjee N, Keene JD, Gromeier M. Tissue type-specific expression of the dsRNA-binding protein 76 and genome-wide elucidation of its target mRNAs. PLoS ONE. 2010;5:e11710. doi: 10.1371/journal.pone.0011710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Brown MC, Gromeier M. Cytotoxic and immunogenic mechanisms of recombinant oncolytic poliovirus. Curr. Opin. Virol. 2015;13:81–85. doi: 10.1016/j.coviro.2015.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Gromeier M, Bossert B, Arita M, Nomoto A, Wimmer E. Dual stem loops within the poliovirus internal ribosomal entry site control neurovirulence. J. Virol. 1999;73:958–964. doi: 10.1128/jvi.73.2.958-964.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Campbell SA, Lin J, Dobrikova EY, Gromeier M. Genetic determinants of cell type-specific poliovirus propagation in HEK 293 cells. J. Virol. 2005;79:6281–6290. doi: 10.1128/JVI.79.10.6281-6290.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Goetz C, Dobrikova E, Shveygert M, Dobrikov M, Gromeier M. Oncolytic poliovirus against malignant glioma. Future Virol. 2011;6:1045–1058. doi: 10.2217/fvl.11.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Thompson EM, et al. Poliovirus receptor (CD155) expression in pediatric brain tumors mediates oncolysis of medulloblastoma and pleomorphic xanthoastrocytoma. J. Neuropathol. Exp. Neurol. 2018;77:696–702. doi: 10.1093/jnen/nly045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Cello J, et al. Growth phenotypes and biosafety profiles in poliovirus-receptor transgenic mice of recombinant oncolytic polio/human rhinoviruses. J. Med. Virol. 2008;80:352–359. doi: 10.1002/jmv.21063. [DOI] [PubMed] [Google Scholar]
- 116.Abe Y, et al. The toll-like receptor 3-mediated antiviral response is important for protection against poliovirus infection in poliovirus receptor transgenic mice. J. Virol. 2012;86:185–194. doi: 10.1128/JVI.05245-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Kotla S, Gustin KE. Proteolysis of MDA5 and IPS-1 is not required for inhibition of the type I IFN response by poliovirus. Virol. J. 2015;12:158. doi: 10.1186/s12985-015-0393-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Dodd DA, Giddings TH, Jr, Kirkegaard K. Poliovirus 3A protein limits interleukin-6 (IL-6), IL-8, and beta interferon secretion during viral infection. J. Virol. 2001;75:8158–8165. doi: 10.1128/JVI.75.17.8158-8165.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Morrison JM, Racaniello VR. Proteinase 2Apro is essential for enterovirus replication in type I interferon-treated cells. J. Virol. 2009;83:4412–4422. doi: 10.1128/JVI.02177-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Brown MC, et al. Oncolytic polio virotherapy of cancer. Cancer. 2014;120:3277–3286. doi: 10.1002/cncr.28862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Brown, M. C. et al. Cancer immunotherapy with recombinant poliovirus induces IFN-dominant activation of dendritic cells and tumor antigen-specific CTLs. Sci. Transl. Med.9, eaan4220 (2017). [DOI] [PMC free article] [PubMed]
- 122.Holl EK, et al. Recombinant oncolytic poliovirus, PVSRIPO, has potent cytotoxic and innate inflammatory effects, mediating therapy in human breast and prostate cancer xenograft models. Oncotarget. 2016;7:79828–79841. doi: 10.18632/oncotarget.12975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Toyoda H, Wimmer E, Cello J. Oncolytic poliovirus therapy and immunization with poliovirus-infected cell lysate induces potent antitumor immunity against neuroblastoma in vivo. Int. J. Oncol. 2011;38:81–87. [PubMed] [Google Scholar]
- 124.Castriconi R, et al. NK cells recognize and kill human glioblastoma cells with stem cell-like properties. J. Immunol. 2009;182:3530–3539. doi: 10.4049/jimmunol.0802845. [DOI] [PubMed] [Google Scholar]
- 125.Maherally Z, Smith JR, An Q, Pilkington GJ. Receptors for hyaluronic acid and poliovirus: a combinatorial role in glioma invasion? PLoS ONE. 2012;7:e30691. doi: 10.1371/journal.pone.0030691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Enloe BM, Jay DG. Inhibition of Necl-5 (CD155/PVR) reduces glioblastoma dispersal and decreases MMP-2 expression and activity. J. Neurooncol. 2011;102:225–235. doi: 10.1007/s11060-010-0323-5. [DOI] [PubMed] [Google Scholar]
- 127.Gromeier M, Solecki D, Patel DD, Wimmer E. Expression of the human poliovirus receptor/CD155 gene during development of the central nervous system: implications for the pathogenesis of poliomyelitis. Virology. 2000;273:248–257. doi: 10.1006/viro.2000.0418. [DOI] [PubMed] [Google Scholar]
- 128.Toyoda H, Yin J, Mueller S, Wimmer E, Cello J. Oncolytic treatment and cure of neuroblastoma by a novel attenuated poliovirus in a novel poliovirus-susceptible animal model. Cancer Res. 2007;67:2857–2864. doi: 10.1158/0008-5472.CAN-06-3713. [DOI] [PubMed] [Google Scholar]
- 129.Dobrikova EY, et al. Recombinant oncolytic poliovirus eliminates glioma in vivo without genetic adaptation to a pathogenic phenotype. Mol. Ther. 2008;16:1865–1872. doi: 10.1038/mt.2008.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Denniston E, et al. The practical consideration of poliovirus as an oncolytic virotherapy. Am. J. Virol. 2016;5:1–7. doi: 10.21092/jav.v5i1.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Drake CG, Jaffee E, Pardoll DM. Mechanisms of immune evasion by tumors. Adv. Immunol. 2006;90:51–81. doi: 10.1016/S0065-2776(06)90002-9. [DOI] [PubMed] [Google Scholar]
- 132.Strohl WR. Current progress in innovative engineered antibodies. Protein Cell. 2018;9:86–120. doi: 10.1007/s13238-017-0457-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Mahmoudi M, Farokhzad OC. Cancer immunotherapy: wound-bound checkpoint blockade. Nat. Biomed. Eng. 2017;1:0031. doi: 10.1038/s41551-017-0031. [DOI] [Google Scholar]
- 134.Leach DR, Krummel MF, Allison JP. Enhancement of antitumor immunity by CTLA-4 blockade. Science. 1996;271:1734–1736. doi: 10.1126/science.271.5256.1734. [DOI] [PubMed] [Google Scholar]
- 135.Freeman GJ, et al. Engagement of the PD-1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation. J. Exp. Med. 2000;192:1027–1034. doi: 10.1084/jem.192.7.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Chen L, Han X. Anti-PD-1/PD-L1 therapy of human cancer: past, present, and future. J. Clin. Invest. 2015;125:3384–3391. doi: 10.1172/JCI80011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Callahan MK, Wolchok JD. At the bedside: CTLA-4- and PD-1-blocking antibodies in cancer immunotherapy. J. Leukoc. Biol. 2013;94:41–53. doi: 10.1189/jlb.1212631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Burugu, S., Dancsok, A. R. & Nielsen, T. O. Emerging targets in cancer immunotherapy. Semin Cancer Biol. (2017). 10.1016/j.semcancer.2017.10.001. [DOI] [PubMed]
- 139.Chauvin JM, et al. TIGIT and PD-1 impair tumor antigen-specific CD8(+) T cells in melanoma patients. J. Clin. Invest. 2015;125:2046–2058. doi: 10.1172/JCI80445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Dixon KO, et al. Functional anti-TIGIT antibodies regulate development of autoimmunity and antitumor immunity. J. Immunol. 2018;200:3000–3007. doi: 10.4049/jimmunol.1700407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Kurtulus S, et al. TIGIT predominantly regulates the immune response via regulatory T cells. J. Clin. Invest. 2015;125:4053–4062. doi: 10.1172/JCI81187. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 142.Zhang, Q. et al. Blockade of the checkpoint receptor TIGIT prevents NK cell exhaustion and elicits potent anti-tumor immunity. Nat. Immunol. (2018). [DOI] [PubMed]
- 143.Georgiev H, Ravens I, Papadogianni G, Bernhardt G. Coming of age: CD96 emerges as modulator of immune responses. Front. Immunol. 2018;9:1072. doi: 10.3389/fimmu.2018.01072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Barrow AD, et al. Natural killer cells control tumor growth by sensing a growth factor. Cell. 2018;172:534–48 e19. doi: 10.1016/j.cell.2017.11.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Harjunpaa H, et al. Deficiency of host CD96 and PD-1 or TIGIT enhances tumor immunity without significantly compromising immune homeostasis. Oncoimmunology. 2018;7:e1445949. doi: 10.1080/2162402X.2018.1445949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Meyer D, et al. CD96 interaction with CD155 via its first Ig-like domain is modulated by alternative splicing or mutations in distal Ig-like domains. J. Biol. Chem. 2009;284:2235–2244. doi: 10.1074/jbc.M807698200. [DOI] [PubMed] [Google Scholar]
- 147.Seth S, et al. The murine pan T cell marker CD96 is an adhesion receptor for CD155 and nectin-1. Biochem. Biophys. Res. Commun. 2007;364:959–965. doi: 10.1016/j.bbrc.2007.10.102. [DOI] [PubMed] [Google Scholar]
- 148.Carlsten M, et al. DNAX accessory molecule-1 mediated recognition of freshly isolated ovarian carcinoma by resting natural killer cells. Cancer Res. 2007;67:1317–1325. doi: 10.1158/0008-5472.CAN-06-2264. [DOI] [PubMed] [Google Scholar]
- 149.El-Sherbiny YM, et al. The requirement for DNAM-1, NKG2D, and NKp46 in the natural killer cell-mediated killing of myeloma cells. Cancer Res. 2007;67:8444–8449. doi: 10.1158/0008-5472.CAN-06-4230. [DOI] [PubMed] [Google Scholar]
- 150.Peng YP, et al. Altered expression of CD226 and CD96 on natural killer cells in patients with pancreatic cancer. Oncotarget. 2016;7:66586–66594. doi: 10.18632/oncotarget.11953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Coustan-Smith, E. et al. Universal monitoring of minimal residual disease in acute myeloid leukemia. JCI Insight3, 98561 (2018). [DOI] [PMC free article] [PubMed]
- 152.Michot JM, et al. Immune-related adverse events with immune checkpoint blockade: a comprehensive review. Eur. J. Cancer. 2016;54:139–148. doi: 10.1016/j.ejca.2015.11.016. [DOI] [PubMed] [Google Scholar]
- 153.Kourie HR, Klastersky J. Immune checkpoint inhibitors side effects and management. Immunotherapy. 2016;8:799–807. doi: 10.2217/imt-2016-0029. [DOI] [PubMed] [Google Scholar]
- 154.Zang YW, Gu XD, Xiang JB, Chen ZY. Clinical application of adoptive T cell therapy in solid tumors. Med. Sci. Monit. 2014;20:953–959. doi: 10.12659/MSM.890496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Kunert A, Debets R. Engineering T cells for adoptive therapy: outsmarting the tumor. Curr. Opin. Immunol. 2018;51:133–139. doi: 10.1016/j.coi.2018.03.014. [DOI] [PubMed] [Google Scholar]
- 156.Dai, H., Wang, Y., Lu, X. & Han, W. Chimeric antigen receptors modified T-cells for cancer therapy. J. Natl Cancer Inst. 108, djv439 (2016). [DOI] [PMC free article] [PubMed]
- 157.Iyer RK, Bowles PA, Kim H, Dulgar-Tulloch A. Industrializing autologous adoptive immunotherapies: manufacturing advances and challenges. Front. Med. 2018;5:150. doi: 10.3389/fmed.2018.00150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Kosti P, Maher J, Arnold JN. Perspectives on chimeric antigen receptor T-cell immunotherapy for solid tumors. Front. Immunol. 2018;9:1104. doi: 10.3389/fimmu.2018.01104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Chmielewski M, Abken H. TRUCKs: the fourth generation of CARs. Expert Opin. Biol. Ther. 2015;15:1145–1154. doi: 10.1517/14712598.2015.1046430. [DOI] [PubMed] [Google Scholar]
- 160.Pang Y, Hou X, Yang C, Liu Y, Jiang G. Advances on chimeric antigen receptor-modified T-cell therapy for oncotherapy. Mol. Cancer. 2018;17:91. doi: 10.1186/s12943-018-0840-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Salmikangas P, Kinsella N, Chamberlain P. Chimeric antigen receptor T-cells (CAR T-Cells) for cancer immunotherapy - moving target for industry? Pharm. Res. 2018;35:152. doi: 10.1007/s11095-018-2436-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Scheuermann RH, Racila E. CD19 antigen in leukemia and lymphoma diagnosis and immunotherapy. Leuk. Lymphoma. 1995;18:385–397. doi: 10.3109/10428199509059636. [DOI] [PubMed] [Google Scholar]
- 163.Morgan RA, et al. Case report of a serious adverse event following the administration of T cells transduced with a chimeric antigen receptor recognizing ERBB2. Mol. Ther. 2010;18:843–851. doi: 10.1038/mt.2010.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Gacerez AT, Arellano B, Sentman CL. How chimeric antigen receptor design affects adoptive T cell therapy. J. Cell. Physiol. 2016;231:2590–2598. doi: 10.1002/jcp.25419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Chan CJ, et al. DNAM-1/CD155 interactions promote cytokine and NK cell-mediated suppression of poorly immunogenic melanoma metastases. J. Immunol. 2010;184:902–911. doi: 10.4049/jimmunol.0903225. [DOI] [PubMed] [Google Scholar]
- 166.Kim JS, et al. Cd226(-/-) natural killer cells fail to establish stable contacts with cancer cells and show impaired control of tumor metastasis in vivo. Oncoimmunology. 2017;6:e1338994. doi: 10.1080/2162402X.2017.1338994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Cappel C, et al. Cytotoxic potential of IL-15-activated cytokine-induced killer cells against human neuroblastoma cells. Pediatr. Blood Cancer. 2016;63:2230–2239. doi: 10.1002/pbc.26147. [DOI] [PubMed] [Google Scholar]
- 168.Martinet L, et al. DNAM-1 expression marks an alternative program of NK cell maturation. Cell Rep. 2015;11:85–97. doi: 10.1016/j.celrep.2015.03.006. [DOI] [PubMed] [Google Scholar]
- 169.Rosskopf S, et al. A Jurkat 76 based triple parameter reporter system to evaluate TCR functions and adoptive T cell strategies. Oncotarget. 2018;9:17608–17619. doi: 10.18632/oncotarget.24807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Sanchez-Correa B, et al. Decreased expression of DNAM-1 on NK cells from acute myeloid leukemia patients. Immunol. Cell Biol. 2012;90:109–115. doi: 10.1038/icb.2011.15. [DOI] [PubMed] [Google Scholar]
- 171.Sun S, Hao H, Yang G, Zhang Y, Fu Y. Immunotherapy with CAR-modified T cells: toxicities and overcoming strategies. J. Immunol. Res. 2018;2018:2386187. doi: 10.1155/2018/2386187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Sentman ML, et al. Mechanisms of acute toxicity in NKG2D chimeric antigen receptor T cell-treated mice. J. Immunol. 2016;197:4674–4685. doi: 10.4049/jimmunol.1600769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Morisaki T, Onishi H, Katano M. Cancer immunotherapy using NKG2D and DNAM-1 systems. Anticancer Res. 2012;32:2241–2247. [PubMed] [Google Scholar]
- 174.Lee A, Sun S, Sandler A, Hoang T. Recent progress in therapeutic antibodies for cancer immunotherapy. Curr. Opin. Chem. Biol. 2018;44:56–65. doi: 10.1016/j.cbpa.2018.05.006. [DOI] [PubMed] [Google Scholar]
- 175.Niu C, et al. Low-dose bortezomib increases the expression of NKG2D and DNAM-1 ligands and enhances induced NK and gammadelta T cell-mediated lysis in multiple myeloma. Oncotarget. 2017;8:5954–5964. doi: 10.18632/oncotarget.13979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Lopez-Cobo S, et al. Impaired NK cell recognition of vemurafenib-treated melanoma cells is overcome by simultaneous application of histone deacetylase inhibitors. Oncoimmunology. 2018;7:e1392426. doi: 10.1080/2162402X.2017.1392426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Kamran N, et al. Toll-like receptor ligands induce expression of the costimulatory molecule CD155 on antigen-presenting cells. PLoS ONE. 2013;8:e54406. doi: 10.1371/journal.pone.0054406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Yamashita-Kanemaru Y, et al. CD155 (PVR/Necl-5) mediates a costimulatory signal in CD4+T cells and regulates allergic inflammation. J. Immunol. 2015;194:5644–5653. doi: 10.4049/jimmunol.1401942. [DOI] [PubMed] [Google Scholar]
- 179.Maier MK, et al. The adhesion receptor CD155 determines the magnitude of humoral immune responses against orally ingested antigens. Eur. J. Immunol. 2007;37:2214–2225. doi: 10.1002/eji.200737072. [DOI] [PubMed] [Google Scholar]
- 180.Tahara-Hanaoka S, et al. Functional characterization of DNAM-1 (CD226) interaction with its ligands PVR (CD155) and nectin-2 (PRR-2/CD112) Int. Immunol. 2004;16:533–538. doi: 10.1093/intimm/dxh059. [DOI] [PubMed] [Google Scholar]
- 181.Oshima T, et al. Nectin-2 is a potential target for antibody therapy of breast and ovarian cancers. Mol. Cancer. 2013;12:60. doi: 10.1186/1476-4598-12-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Liu J, et al. Crystal structure of cell adhesion molecule nectin-2/CD112 and its binding to immune receptor DNAM-1/CD226. J. Immunol. 2012;188:5511–5520. doi: 10.4049/jimmunol.1200324. [DOI] [PubMed] [Google Scholar]
- 183.Seth S, et al. Intranodal interaction with dendritic cells dynamically regulates surface expression of the co-stimulatory receptor CD226 protein on murine T cells. J. Biol. Chem. 2011;286:39153–39163. doi: 10.1074/jbc.M111.264697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Stamm, H. et al. Immune checkpoints PVR and PVRL2 are prognostic markers in AML and their blockade represents a new therapeutic option. Oncogene (2018). 10.1038/s41388-018-0288-y. [DOI] [PMC free article] [PubMed]
- 185.Morimoto K, et al. Interaction of cancer cells with platelets mediated by Necl-5/poliovirus receptor enhances cancer cell metastasis to the lungs. Oncogene. 2008;27:264–273. doi: 10.1038/sj.onc.1210645. [DOI] [PubMed] [Google Scholar]
- 186.Nasiri H, Valedkarimi Z, Aghebati-Maleki L, Majidi J. Antibody-drug conjugates: promising and efficient tools for targeted cancer therapy. J. Cell. Physiol. 2018;233:6441–6457. doi: 10.1002/jcp.26435. [DOI] [PubMed] [Google Scholar]
- 187.American Association for Cancer Research. Targeting Nectin-4 in bladder cancer. Cancer Discov. 7, OF3 (2017). 10.1158/2159-8290.CD-NB2017-095. [DOI] [PubMed]
- 188.Challita-Eid PM, et al. Enfortumab Vedotin antibody-drug conjugate targeting Nectin-4 is a highly potent therapeutic agent in multiple preclinical cancer models. Cancer Res. 2016;76:3003–3013. doi: 10.1158/0008-5472.CAN-15-1313. [DOI] [PubMed] [Google Scholar]