Abstract
Objective:
This study aimed to identify micro-satellite instability (MSI) based on the expression of MMRp (MSH2 and MSH6) and to evaluate the association of MSI and with clinicopathological features in patients with colorectal cancer (CRC).
Methods:
MMRp expression in 80 tissue samples from patients with adenocarcinoma CRC were evaluated by using anti-MSH2 and -MSH6 antibodies. Loss of MSH2 and/or MSH6 expression was stated as MSI. The association between MSI status and clinicopathological features were analyzed by using binary logistic regression (p<0.05).
Results:
The frequency of MSI in patients with CRC varied, corresponding to 8.3% (6/72) MSH2 MSI, 36.1% (26/72) MSH6 MSI and 6.9% (5/72) MSH2-MSH6 MSI. Male patients (OR=1.98), with tumor located in colon (OR=1.47) and late stage tumor (OR=1.48) have a tendency of having MSH2 MSI. Male patients (OR=1.4), with tumor located in colon (OR=2.53) and poor tumor differentiation (OR=3.02) have a tendency to encounter MSH6 MSI. Male patients (OR=4.93) with late stage tumor (OR=1.69) have a tendency of having MSH2-MSH6 MSI.
Conclusion:
Patients more likely to have MSH2 MSI are males, and/or having tumor located in colon, and /or having late stage tumor. Patients more likely to have MSH6 MSI are males, and/or having tumor located in colon, and/or having tumor with poor differentiation. Patients who have greater tendency to have MSH2 and MSH6 MSI are males, and/or having late stage tumor.
Keywords: Colorectal cancer, microsatellite instability, MSH2, MSH6
Introduction
Colorectal cancer (CRC) is the third highest malignancy in the world involving 1.36 million cases after lung cancer (1.8 million) and breast cancer (1.6 million) (Ferlay et al., 2015). The incidence in Indonesia is 17.2 per 100,000 population per year and there is a trending tendency to increase every year (American Cancer Society 2013; Kimman et al., 2012). This high incidence rate of CRC is also followed by an increasing mortality rate (Kementerian Kesehatan, 2015).
CRC develops through two distinct pathways, namely chromosomal instability (CIN) and microsatellite instability (MSI). Chromosomal instability occurs in 85% and MSI occurs in 15% of CRC cases (Roper and Hung, 2013; Zhang and Li, 2013). Microsatellite is a repetitive short DNA sequence that is particularly susceptible to mutations. Alteration in the number of nucleotides in microsatellite region is referred to as MSI (Sandeep et al., 2010). This alteration should be detected and repaired by heterodimers mismatch repair protein (MMRp) complex such as MSH2 in pairs with MSH6 (MutSα). This complex plays a role in the recognition of DNA strand damage and accommodates formation of repair complex (MutLα) in reforming the new DNA strands (Vilar and Gruber, 2012). Inactivation of one or more MMRp can cause MSI (de la Chapelle and Hampel, 2010). While MSI is only detected in 15% of CRC, it provides a significant clinical contribution regarding the proper management of patients with CRC and MSI. Patients with CRC and MSI show better prognosis when not being treated with 5-FU chemotherapy after surgery (Gryfe et al., 2000; Sargent et al., 2010). Therefore, MSI detection can provide appropriate information concerning therapeutic predictions to avoid drug toxicity effects in patients with CRC and MSI (Benson et al., 2013; Schmoll et al., 2012).
The gold standard of MSI detection is the polymerase chain reaction (PCR) method with MSI markers (BAT25, BAT26, NR21, NR24 and NR27), but this method is relatively expensive, especially when being used as a routine test in developing countries. Hence, alternatively MMRp detection with immunohistochemistry (IHC) can be used for MSI detection. The sensitivity and specificity of MMRp IHC testing compared to PCR method in detecting MSI are 77%-100% and 98%-100%, respectively (Lindor et al., 2002).
In Indonesia, research on CRC with MSI is very limited. Some previous studies were done to evaluate the MLH1 and MSH2 expression in old and young CRC patients, and the result showed that there is no significant differences between the variables (Sudoyo et al., 2010). Other studies examined the expression of MMR protein expression (MLH1, MSH2, PMS2, MSH6) and evaluated the correlation with sites of the tumor. The results showed that the frequency of MSH6 expression was 11 times higher at the distal colon than at the proximal (Effendi-ys et al., 2013). This study aimed to identify MSI based on the expression of MMRp markers (MSH2 and MSH6) that play key roles in recognition of DNA defects and to evaluate the association of MSI with clinicopathological features (age, sex, size, location, stage, and differentiation of tumor) in patients with CRC.
Materials and Methods
Samples and clinicopathological data
Consecutive sampling was done to obtain eighty FFPE (formalin fixed paraffin embedded) tissue samples and clinicopathological data were used from patients with adenocarcinoma CRC from the Anatomical Pathology Laboratory, of Dr. Sardjito General Hospital and several clinical laboratories in Yogyakarta between 2010-2016. The tissue samples used in this study are the ones with adenocarcinoma and accompanied by clinicopathological data. The study was approved by the Ethics Committee of Faculty of Medicine Universitas Gadjah Mada, ref. no. KE/FK/901/EC/2016.
Immunohistochemistry
Tissues (4 μm thickness) were stained using antibody anti-MSH2 (Biocare Medical CM219 AK, BK, CK) and anti-MSH6 (Biocare Medical CM265 AK, BK, CK). The IHC kit was the Star Trek Universal HRP Detection System (STUHRP700 H, L10) from Biocare. Positive controls were commercial slides from Sigma Aldrich Cell Marque cat. no. 286S for MSH2 and cat.no. 287S for MSH6. In-house made positive controls derived from normal colon tissue from Anatomical Pathology Laboratory, Dr. Sardjito General Hospital were also used. These provided results equal with the commercial ones. Human tonsil tissues were used as negative control.
Interpretation of IHC staining
MMRp, i.e. MSH2 and MSH6 expression scoring was done by calculating the average of positive cells (in percentage) on 5 fields of view. It was evaluated in strong magnification (400x) by 2 blind observers. The MMRp expressions were determined as positive when at least 1% of nuclei of tumor cells were brown stained (Karahan et al. 2015). When the tumor did not express MMRp or only expressed less than 1% MMRp, then this was labeled as MSI.
Statistical analysis
Statistical analysis was performed with the SPSS statistics 19 (SPSS Inc., Chicago, II., U.S.A.). Binary logistic regression test (CI 95%) was used to analyze the association between MSI and clinicopathological features of the patients with CRC (p>0.05).
Results
MMRp IHC Staining
Expression of MSH2 and MSH6 protein can be observed as the presence of brown nuclei in the tumor cells. Lack of MMRp expressions was defined as MSI, which was characterized by the absence of nuclear staining within tumor cells (Figure 1).
Figure 1.
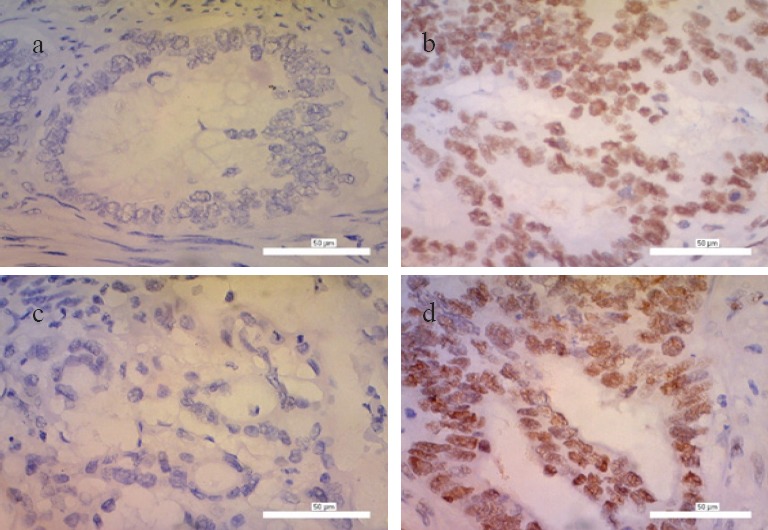
Immunohistochemistry Staining with Anti-MSH2 and Anti-MSH6 Antibody in CRC Tissue, 400x (a) Negative Expression of MSH2 or Called as MSH2 MSI Positive; (b) Positive Expression of MSH2 or Called as MSH2 MSI Negative; (c) Negative Expression of MSH6 or Called as MSH6 MSI Positive; (d) Positive Expression of MSH6 or Called as MSH6 MSI Negative.
Clinicopathological features of CRC patients
Eighty CRC samples were obtained from the Anatomical Pathology Laboratory of Dr. Sardjito General Hospital Yogyakarta between 2010-2016. After IHC staining, 8 samples were excluded due to the absence of tumor cells or non-colonic tissue. Therefore, only 72 samples were analyzed. Tumor size data were found for 61 patients and tumor stage data for 71 patients. Age was categorized into <50 and ≥50 years old, sex: male and female, tumor size: T1-T2 and T3-T4, tumor location: colon and rectum. Tumor stage was grouped into early stage (stage I and II) and late stage (stage III and IV). Tumor differentiation was categorized into well and poor differentiation, while moderate differentiation was in the well category.
In this study, patients with CRC in age ≥50 years are predominant, corresponding to 68.06% (49/72). Males were also predominant, corresponding to 58.33% (42/72). Most of the patients (91.80%; 56/61) had T3-T4 tumor size. Tumor in colon was found in 65.28% of patients (47/72). Late stage tumor was found in 67.61% of patients (48/71) and 87.50% (63/72) had well-differentiated tumor (Table 1).
Table 1.
Clinicopathological Features of Patients with CRC
| Parameter | Total | % |
|---|---|---|
| Age | n = 72 | |
| <50 y.o | 23 | 31.94 |
| ≥50 y.o | 49 | 68.06 |
| Sex | n = 72 | |
| Male | 42 | 58.33 |
| Female | 30 | 41.67 |
| Tumor Size | n = 61 | |
| T1-T2 | 5 | 8.20 |
| T3-T4 | 56 | 91.80 |
| Tumor Location | n = 72 | |
| Colon | 47 | 65.28 |
| Rectum | 25 | 34.72 |
| Tumor Stage | n = 71 | |
| Early | 23 | 32.39 |
| Late | 48 | 67.61 |
| Tumor Differentiation | n = 72 | |
| Well | 63 | 87.50 |
| Poor | 9 | 12.50 |
Frequency of MSI (MSH2, MSH6 and combination of MSH2-MSH6) in patients with CRC
The MSH2 or MSH6 negative expression either single or in conjunction was called as MSI (Zhang and Li, 2013). In this study, 8.3% (6/72) patients with CRC had MSH2 MSI, 36.1% (26/72) had MSH6 MSI and 6.9% (5/72) had MSH2-MSH6 MSI combination (Table 2).
Table 2.
Frequency of MSH2 MSI, MSH6 MSI and MSH2 and MSH6 MSI in Patient with CRC
| MSH2 Number (%) | MSH6 Number (%) | MSH2 and MSH6* Number (%) | |
|---|---|---|---|
| Positive MSI | 6 (8.3) | 26 (36.1) | 5 (6.9) |
| Negative MSI | 66 (91.7) | 46 (63.9) | 67 (93.1) |
| Total | 72 (100) | 72 (100) | 72 (100) |
If both MMR proteins were not expressed
Association between MSI with clinicopathologcal features of patients with CRC
The association between MSI and several clinicopathological features was analyzed by binary logistic regression. Patients at age ≥50 years old were 0.41 times more likely to have MSI than MSH2 MSI, 0.88 times for MSH6 MSI and 0.59 times for MSH2-MSH6 MSI. Men were 1.98 times more likely to have MSH2 MSI, 1.40 times for MSH6 MSI and 4.93 times for MSH2-MSH6 MSI. The association of MSH2 and/or MSH6 MSI with tumor size could not be analyzed because none of the samples were categorized into T1-T2 tumor size. Patients with tumor located in the colon were 1.47 times more likely to have MSH2 MSI and 2.53 times for MSH6 MSI, while none of the patients with tumor located in the rectum had MSH2-MSH6 MSI. Patients having late stage tumor were 1.48 times more likely to have MSH2 MSI, 0.49 times for MSH6 MSI and 1.69 times for MSH2-MSH6 MSI. Patients with poor tumor differentiation were 0.52 times more likely to have MSH2 MSI, 3.02 times for MSH6 MSI and 0.29 times for MSH2-MSH6 MSI (Table 3).
Table 3.
Association between MSH2 MSI, MSH6 MSI and MSH2 MSI- MSH6 MSI with Clinicopathological Features of CRC
| Clinicopath-ological Features | CI 95% | CI 95% | CI 95% | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MSH2 MSI | OR | Lower | Upper | P value | MSH6 MSI | OR | Lower | Upper | P Value | MSH2- MSH6 MSI | OR | Lower | Upper | P Value | |
| Age | 0.41 | 0.04 | 4.10 | 0.444 | 0.88 | 0.25 | 3.08 | 0.844 | 0.59 | 0.05 | 7.01 | 0.676 | |||
| <50 y.o | 1 (1.4%) | 6 (8.3%) | 1 (1.4%) | ||||||||||||
| ≥50 y.o * | 5 (6.9%) | 20 (27.8%) | 4 (5.6%) | ||||||||||||
| Sex | 1.98 | 0.31 | 12.63 | 0.471 | 1.40 | 0.46 | 4.31 | 0.553 | 4.93 | 0.45 | 53.80 | 0.190 | |||
| Male* | 4 (5.6%) | 17 (23.6%) | 4 (5.6%) | ||||||||||||
| Female | 2 (2.8%) | 9 (12.5%) | 1 (1.4%) | ||||||||||||
| Tumor Size | - | - | - | - | - | - | - | - | - | - | - | - | |||
| T1-T2 | 0 (0%) | 0 (0%) | 0 (0.0%) | ||||||||||||
| T3-T4* | 6 (9.8%) | 22 (36.1%) | 5 (8.2%) | ||||||||||||
| Tumor Location | 1.47 | 0.10 | 20.99 | 0.776 | 2.53 | 0.51 | 12.58 | 0.256 | - | - | - | - | |||
| Colon* | 5 (6.9%) | 18 (25.0%) | 5 (6.9%) | ||||||||||||
| Rectum | 1 (1.4%) | 8 (11.1%) | 0 (0.0%) | ||||||||||||
| Tumor Stage | 1.48 | 0.16 | 13.75 | 0.732 | 0.49 | 0.11 | 2.10 | 0.336 | 1.69 | 0.14 | 20.49 | 0.681 | |||
| Early | 3 (4.2%) | 9 (12.7%) | 3 (4.2%) | ||||||||||||
| Late* | 3 (4.2%) | 17 (23.9%) | 2 (2.8%) | ||||||||||||
| Tumor Differenti-ation | 0.52 | 0.03 | 8.05 | 0.639 | 3.02 | 0.44 | 20.66 | 0.260 | 0.29 | 0.01 | 7.33 | 0.449 | |||
| Non-Poor | 5 (6.9%) | 23 (31.9%) | 4 (5.6%) | ||||||||||||
| Poor* | 1 (1.4%) | 1 (1.4%) | |||||||||||||
references
Discussion
Colorectal cancer (CRC) is one of the most common gastrointestinal cancers (Desen and Zhizhong, 2008). The MSI profile in CRC provides information for prognosis and predicting the treatment response. It allows the possibility to modify the chemotherapy protocols offered to patients in the future (Ribic et al., 2003). Generally, MSI detection was done by the Immunohistochemical (IHC) method using 4 MMRp including MSH2, MSH6, MLH1 and PMS2 (Boland and Goel, 2010; Kim, 2009). In this study, the MSI detection was performed by evaluating MSH2 and MSH6 expression, since both proteins have a corresponding role as the recognition complex of mismatch base sequence (Vilar and Gruber, 2012). Patients with CRC showed that the frequency of having MSH6 MSI (36.1%) were more frequent than MSH2 MSI (8.3%). Studies in Turkey and Ohio, USA also showed that MSH6 MSI (3.7% in Turkey; 17.5% in Ohio) was higher than MSH2 MSI (1.6% in Turkey; 0% in Ohio) (Karahan et al., 2015; South et al., 2009). However, the occurrence of MSI varies in different countries. In this study, the frequency of MSH6 MSI was almost 10 times higher than the findings in Turkey. This difference may be due to population heterogeneity, different environmental exposures and etiology. Furthermore, biological conditions might play a role in this process. The risk of CRC is related to the environmental factors, such as high dietary intakes of animal products, smoking and alcohol consumption and endogenous factor such as bacterial toxic hydrogen and secondary bile salts (Ashktorab et al., 2016; Haggar et al., 2009).
MSH2 protein performs its function by forming a heterodimer complex with MSH6 (MutSα) or with another alternative pair MSH3 (MutSβ). Lack of MSH2 expression can occur due to mutations of MSH2 and EpCAM (epithelial cellular adhesion molecule) genes. Meanwhile, MSH6 can only be expressed when it forms a pair with MSH2 because MSH6 has a special intrinsic ATPase activity for binding to MSH2 (Mukherjee and Feig, 2009). In addition, MSH2 mutations also cause weak or unbinding to MSH6 that result in the degradation of MSH6. The negative expression of MSH6 by itself in IHC indicates the MSH6 germline mutation (Marginean and Melosky, 2017).
CRC is one of the most common causes of cancer morbidity both in men and women. However, women at age >65 years old showed higher mortality and lower 5-year survival rate of CRC compared to men at the same age (Kim et al., 2015). In this study, men had a tendency of having MSH2 and/or MSH6 MSI. Murphy et al., (2011) found that risk factors such as smoking, alcohol and red meat consumption in men play a role in this tendency. Furthermore, Slattery et al., (2001) indicated that estrogen exposure is a protective factor against MSI, while a lack of estrogen in older women may increase the risk of MSI-H.
Tumor location can be found in proximal (cecum, ascending colon, transverse colon), distal (descending colon, sigmoid colon) areas and rectum. However, in some cases, the discrimination of the proximal and distal regions are difficult (Willet, 2001). In this study, tumor location was divided into colon and rectum. Patients with tumor location in the colon had a tendency to have MSH2 MSI or MSH6 MSI. Another study also showed that mutations are more frequent in the colonic region than in the rectum (Slattery et al., 2009). Charara et al., (2004) also reported that MSI-H cases were more common in patients with colon cancer (15-20%) than rectal cancer (<10%). Moreover, proximal colon is more likely to express MSI and hyper-mutation of MMR, KRAS, BRAF and PIK3Ca proteins. Environmental factors such as bacterial toxins or CYP450 metabolites can allegedly increase mutation rates in this region (Missiaglia et al., 2014).
The WHO recommends a 2 tiered histological grading system, namely low grade for well and moderate differentiated adenocarcinomas (50%-100% gland formation) and high grade for poor differentiated adenocarcinomas (0%-49% gland formation) (Hamilton et al., 2010). In this study, most of the patients had the well-differentiated tumor. The result was similar to the findings of Fleming et al., (2012) that showed approximately 70% of CRC patients with adenocarcinoma have a moderate differentiation and belong to the well-differentiated category. However, CRC cases that have a tendency to have MSI-H usually involve the poor differentiated tumor (Greenson et al., 2009; Xiao et al., 2013). Our result was in concordance with this finding, since patients with poor tumor differentiation were more likely to have MSH6 MSI (OR>3). Xiao et al., (2013) stated that patients with MSI and poor tumor differentiation exhibit better disease free survival (DFS) approximately 4 years longer than patients with MSS and poor tumor differentiation. The patients with MSI with poor tumor differentiation generally have a lower incidence of lymph node metastasis than the MSS and poor tumor differentiation.
Tumor staging is the most important prognosis factor and the best guidance in determining the therapy in patients with CRC (Schischmanoff et al., 2009). In this study, patients with late stage tumor had the tendency to have MSH2 MSI or MSH2-MSH6 MSI. Yashiro et al., (2010) suggest that hMSH3, hMSH6, TGFβRII and BAX frameshift mutations might play an important role in tumor progression from early to late stage tumors in sporadic CRC with MSI. A study by Mohan et al., (2016) showed that MSI was associated with a reduced risk of nodal and distant metastases, which improved DFS in patients with stage I and II CRC. However, patients with CRC and MSI in stage III have worse outcomes that are indicated by high rates of lymphovascular and perineural invasion.
The American Cancer Society (2013) found that CRC diagnosis increases for patients ≥50 years of age. The incidence rate of CRC is 15 times higher in patients at age ≥50 years than those at age 20 – 49 years old (Wei et al., 2011). Previous research showed that CRC with MSI in elderly patients (60-70 years and >87 years) were associated with MLH1 inactivation and MLH1 promoter methylation, while tumors in the young group (<45 years) were associated with MSH2 inactivation (Yiu et al., 2005). This study showed that patients at ages ≥50 years old had less tendency to have MSH2 and/or MSH6 MSI (OR <1). One of the common characteristics of MSH2 MSI is the occurrence of CRC at a young age (Coggins et al., 2005), since mutations of MSH2 or MLH1 are often associated with hereditary non polyposis colorectal cancer/HNPCC (Wei et al., 2011).
Tumor size is one of the important parameters that underscore the determination of the CRC stages, which can be used to predict the prognosis of the patient. Tumor size is determined by the tumor diameter and distance of invasion to the surrounding tissues (Takeuchi et al., 2004). In this study, patients with MSH2 and/or MSH6 MSI had T3-T4 tumor size and none were with T1-T2. This finding was possibly due to most of the patients being diagnosed when the symptoms clearly appeared and the carcinoma had entered the late stage (Cappell, 2005). Tumor at T3-T4 shows penetration into sub-serosa and other visceral organs. This finding was associated with the ability of the tumor to undergo metastases (UICC, 2010). Study by Balta et al. (2014) and showed a significant association between tumor size and metastatic state. TGF-βRII mutations found in >80% MSI cases are thought to have an important role in metastatic conditions. Colussi et al., (2013) also stated that TGF-βRII mutation found in MSI-H patients also plays a role in CRC development from dysplasia to metastasis.
In conclusion, we found patients with CRC showed 8.3% MSH2 MSI, 36.1% MSH6 MSI and 6.9% MSH2-MSH6 MSI. Male patients with tumor located in the colon and late stage tumor have higher chance to encounter MSH2 MSI. Male patients with tumor located in colon and poor differentiation tumor tended to have MSH6 MSI. Male patients with late stage tumor also have higher tendency to have MSH2-MSH6 MSI.
Funding Statement
This study was supported by grant from the Ministry of Research, Technology and Higher Education of the Republic of Indonesia (KEMENRISTEKDIKTI) through PUPT project and Dana Masyarakat from Faculty of Medicine, Public Health and Nursing, Universitas Gadjah Mada.
Acknowledgements
The researcher would like to thank Y. Suhardi as laboratory staff in Histology and Cell Biology Department and Prita Anindita as clinical data manager of Tulip Oncology, Dr. Sardjito General Hospital for technical assistance.
References
- 1.American Cancer Society. Colorectal Cancer Facts and Figures, 2011-2013. Atlanta: 2013. [Google Scholar]
- 2.Ashktorab H, Ahuja S, Kannan L, et al. A meta-analysis of MSI frequency and race in colorectal cancer. Oncotarget. 2016;7:34546–57. doi: 10.18632/oncotarget.8945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Balta AZ, Özdemir Y, Sücüllü I, et al. Can horizontal diameter of colorectal tumor help predict prognosis? Ulusal Cerrahi Dergisi. 2014;30:115–9. doi: 10.5152/UCD.2014.2701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Benson AB, Bekaii-saab T, Chan E, et al. Localized colon cancer , version 3. 2013 featured updates to the NCCN guidelines. J Natl Compr Canc Netw. 2013;11:519–28. doi: 10.6004/jnccn.2013.0069. [DOI] [PubMed] [Google Scholar]
- 5.Boland CR, Goel A. Microsatellite Instability in Colorectal Cancer. Gastroenterology. 2010;138:2073–87. doi: 10.1053/j.gastro.2009.12.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cappell MS. The pathophysiology, clinical presentation and diagnosis of colon cancer and adenomatous polyps. Med Clin North Am. 2005;89:1–42. doi: 10.1016/j.mcna.2004.08.011. [DOI] [PubMed] [Google Scholar]
- 7.Charara M, Edmonston TB, Burkholder S, et al. Microsatellite status and cell cycle associated markers in rectal cancer patients undergoing a combined regimen of 5-FU and CPT-11 chemotherapy and radiotherapy. Anticancer Res. 2004;24:3161–8. [PubMed] [Google Scholar]
- 8.Coggins RP, Cawkwell L, Bell SM, et al. Association between family history and mismatch repair in colorectal cancer. Gut. 2005;54:636–42. doi: 10.1136/gut.2003.017517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Colussi D, Brandi G, Bazzoli F, et al. Molecular pathways involved in colorectal cancer:implications for disease behavior and prevention. Int J Mol Sci. 2013;14:16365–85. doi: 10.3390/ijms140816365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.de la Chapelle A, Hampel H. Clinical relevance of microsatellite instability in colorectal cancer. J Clin Oncol. 2010;28:3380–7. doi: 10.1200/JCO.2009.27.0652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Desen W, Zhizhong . Kanker Usus Besar. In: W Desen., editor. Buku Ajar Onkologis Klinis. Jakarta: Balai Penerbit Fakultas Kedokteran Universitas Indonesia; 2008. [Google Scholar]
- 12.Effendi-ys R, Zain LH, Siregar GA, et al. Adenomatous polyposis coli, mismatch repair, and microsatellite instability in colorectal cancer based on different locations. Acta Med Indonesiana. 2013;45:275–83. [PubMed] [Google Scholar]
- 13.Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide:sources, methods and major patterns in GLOBOCAN. Int J Cancer 2012. 2015;136:359–86. doi: 10.1002/ijc.29210. [DOI] [PubMed] [Google Scholar]
- 14.Fleming M, Ravula S, Tatishchev SF, et al. Colorectal carcinoma:pathologic aspects. J Gastrointest Cancer. 2012;3:153–73. doi: 10.3978/j.issn.2078-6891.2012.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Greenson JK, Huang SC, Herron C, et al. Pathologic predictors of microsatellite instability in colorectal cancer. Am JSurg Pathol. 2009;33:126–33. doi: 10.1097/PAS.0b013e31817ec2b1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gryfe R, Kim H, Hsieh ETK, et al. Tumor microsatellite instability and clinical outcome in young patients with colorectal cancer. N Engl J Med. 2000;342:69–77. doi: 10.1056/NEJM200001133420201. [DOI] [PubMed] [Google Scholar]
- 17.Haggar F, Boushey R. Colorectal cancer epidemiology:incidence, mortality, survival, and risk factors. Clin Colon Rectal Surg. 2009;22:191–7. doi: 10.1055/s-0029-1242458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hamilton S, Bosman F, Boffeta P, et al. In World Health Organization Classification of Tumours of the Digestive System. Lyon, France: International Agency for Research in Cancer (IARC); 2010. Carcinoma of the colon and rectum; pp. 134–46. [Google Scholar]
- 19.Karahan B, Argon A, Yıldırım M, et al. MSH-6 expression and clinicopathological features in colorectal cancer. Int J Clin Exp Pathol. 2015;8:4044–53. [PMC free article] [PubMed] [Google Scholar]
- 20.Kementerian Kesehatan RI. P. data dan I, Situasi penyakit kanker 4, Jakarta. 2015 [Google Scholar]
- 21.Kim KE. Early detection and prevention of colorectal cancer. Chicago: Slack Incorporated; 2009. pp. 34–46. [Google Scholar]
- 22.Kim S, Paik HY, Yoon H, et al. Sex- and gender-specific disparities in colorectal cancer risk. World J Gastroenterol. 2015;21:5167–75. doi: 10.3748/wjg.v21.i17.5167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kimman M, Norman R, Jan S, et al. The burden of cancer in member countries of the Association of Southeast Asian Nations (ASEAN) Asian Pac J Cancer Prev. 2012;13:411–20. doi: 10.7314/apjcp.2012.13.2.411. [DOI] [PubMed] [Google Scholar]
- 24.Lindor BNM, Burgart L, Leontovich O, et al. Testing in phenotyping colorectal tumors. J Clin Oncol. 2002;20:1043–8. doi: 10.1200/JCO.2002.20.4.1043. [DOI] [PubMed] [Google Scholar]
- 25.Marginean EC, Melosky B. Is there a role for programmed death ligand-1 testing and immunotherapy in colorectal cancer with microsatellite instability?part i, colorectal cancer:microsatellite instability, testing, and clinical implications. Arch Pathol Lab Med. 2017;142:17–25. doi: 10.5858/arpa.2017-0040-RA. [DOI] [PubMed] [Google Scholar]
- 26.Missiaglia E, Jacobs B, D'Ario G, et al. Distal and proximal colon cancers differ in terms of molecular, pathological, and clinical features. Ann Oncol. 2014;25:1995–2001. doi: 10.1093/annonc/mdu275. [DOI] [PubMed] [Google Scholar]
- 27.Mohan HM, Ryan E, Balasubramanian I, et al. Microsatellite instability is associated with reduced disease specific survival in stage III colon cancer. Eur J Surg Oncol. 2016;42:1680–86. doi: 10.1016/j.ejso.2016.05.013. [DOI] [PubMed] [Google Scholar]
- 28.Mukherjee S, Feig M. Conformational change in MSH2 and MSH6 upon binding DNA coupled to ATPase activity. Biophys J. 2009;96:63–5. doi: 10.1016/j.bpj.2009.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Murphy G, Devesa S, Cross A, et al. Sex disparities in colorectal cancer incidence by anatomic subsite, race and age. Int J Cancer. 2011;128:1668–75. doi: 10.1002/ijc.25481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ribic CM, Sargent D, Moore M, et al. Tumor microsatellite-instability status as a predictor of benefit from fluorouracil-based adjuvant chemotherapy for colon cancer. N Engl J Med. 2003;349:247–57. doi: 10.1056/NEJMoa022289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Roper J, Hung KE. Molecular mechanisms of colorectal carcinogenesis. USA: Springer; 2013. pp. 25–45. [Google Scholar]
- 32.Sandeep NS, Suzanne EH, Kristin AE. Defective mismatch repair, microsatellite mutation bias, and variability in clinical cancer phenotypes. Cancer Res. 2010;70:431–5. doi: 10.1158/0008-5472.CAN-09-3049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sargent DJ, Marsoni S, Monges G, et al. Defective mismatch repair as a predictive marker for lack of efficacy of fluorouracil-based adjuvant therapy in colon cancer. J Clin Oncol. 2010;28:3219–26. doi: 10.1200/JCO.2009.27.1825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schischmanoff O, Nicolas P, Uzzan B. Does microsatellite instability predict the efficacy of adjuvant chemotherapy in colorectal cancer?A systematic review with. Eur J Cancer. 2009;45:1890–6. doi: 10.1016/j.ejca.2009.04.018. [DOI] [PubMed] [Google Scholar]
- 35.Schmoll HJ, Cutsem E, Stein A, et al. ESMO Consensus Guidelines for management of patients with colon and rectal cancer. A personalized approach to clinical decision making. Ann Oncol. 2012;23:2479–516. doi: 10.1093/annonc/mds236. [DOI] [PubMed] [Google Scholar]
- 36.Slattery ML, Curtin K, Wolff R, et al. A comparison of colon and rectal somatic DNA alterations. Dis Colon Rectum. 2009;52:1304–11. doi: 10.1007/DCR.0b013e3181a0e5df. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Slattery ML, Potter J, Curtin K, et al. Estrogens reduce and withdrawal of estrogens increases risk of microsatellite. Cancer Res. 2001;61:126–30. [PubMed] [Google Scholar]
- 38.South CD, Yearsley M, Martin E, et al. Immunohistochemistry staining for the mismatch repair proteins in the clinical care of patients with colorectal cancer. Genet Med. 2009;11:812–7. doi: 10.1097/GIM.0b013e3181b99b75. [DOI] [PubMed] [Google Scholar]
- 39.Sudoyo AW, Hernowo B, Krisnuhoni E, et al. Colorectal cancer among young native Indonesians:a clinicopathological and molecular assessment on microsatellite instability. Med J Indonesia. 2010;19:245–51. [Google Scholar]
- 40.Takeuchi K, Kuwano H, Tsuzuki Y, et al. Clinicopathological characteristics of poorly differentiated adenocarcinoma of the colon and rectum. Hepatogastroenterology. 2004;51:1698–702. [PubMed] [Google Scholar]
- 41.Gospodarowicz M, Wittekind C, Sobin L, editors. UICC. TNM classification of malignant tumours. 7th ed. Wiley Blackwell; 2010. pp. 122–7. [Google Scholar]
- 42.Vilar E, Gruber SB. Microsatellite instability in colorectal cancer - the stable evidence. Nat Rev Clin Oncol. 2012;7:153–62. doi: 10.1038/nrclinonc.2009.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wei W, Liu F, Liu L, et al. Distinct mutations in MLH1 and MSH2 genes in hereditary non-polyposis colorectal cancer (HNPCC) families from China. BMB Rep. 2011;44:317–22. doi: 10.5483/BMBRep.2011.44.5.317. [DOI] [PubMed] [Google Scholar]
- 44.Willet CG. Cancer of the lower gastrointestinal tract. London: B.C Decker Inc; 2001. pp. 53–7. [Google Scholar]
- 45.Xiao H, Yoon Y, Hong S, et al. Poorly differentiated colorectal cancers:correlation of microsatellite instability with clinicopathologic features and survival. Am J Clin Pathol. 2013;140:341–7. doi: 10.1309/AJCP8P2DYNKGRBVI. [DOI] [PubMed] [Google Scholar]
- 46.Yashiro M, Hirakawa K, Boland CR. Mutations in TGFbeta-RII and BAX mediate tumor progression in the later stages of colorectal cancer with microsatellite instability. BMC Cancer. 2010;10:1–6. doi: 10.1186/1471-2407-10-303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yiu R, Qiu H, Lee S, et al. No title mechanisms of microsatellite instability in colorectal cancer patients in different age groups. Dis Colon Rectum. 2005;48:2061–69. doi: 10.1007/s10350-005-0171-0. [DOI] [PubMed] [Google Scholar]
- 48.Zhang X, Li J. Era of universal testing of microsatellite instability in colorectal cancer. World J Gastrointest Oncol. 2013;5:12–19. doi: 10.4251/wjgo.v5.i2.12. [DOI] [PMC free article] [PubMed] [Google Scholar]


