Abstract
Background:
Immunophenotypic markers can play significant role in prognostic assessment for different cancers and leukocyte-associated Ig-like receptor (LAIR-1) is a recently identified inhibitory immuno-receptor.
Methods:
We measured LAIR-1 expression in paediatric ALL patients (n-42) and appropriate controls by flow cytometry. Median fluorescence intensities (MFIs) were calculated and correlated with demographic and clinical variables and early treatment outcome parameters.
Results:
The ALL cohort had an age range of 1 - 11 y and a M:F ratio of 2.5:1. 64% had WBC counts <50 x 109/L and 15 (36%) >50 x 109/L, 52% being standard risk and 48% high risk. There were 6 cases of T-ALL and 36 of B-ALL. AML1-TEL, E2A-PBX, BCR-ABL and MLL-AF4 transcripts were noted in 3, 6, 2 and 1 patient, respectively. Day 8 ABC was <1,000 in 31 and >1,000 in 8 cases, while 30 had low and 7 high MRD (both >0.01) at day 35 of treatment. The median MFI for LAIR-1 expression in control cases was 8.2 (range 7.76-11.69) and in ALL cases 4.02 (range 0.56 to 11.87), with 74% (n-31) of ALL cases showing reduced LAIR-1 expression. However, no significant correlations were found between standard ALL risk factors and LAIR-1 expression. Out of 42 patients, 4 died during induction treatment and one exited therapy, 60% (n-3/5) of these featuring low expression of LAIR-1. Also ALL patients with low LAIR-1 expression had t (12;21), t (1;19) and t (4;11) translocations in 2, 4 and 1 samples, respectively, but none had t (9;22). Of those with high LAIR-1 expression, 2 had t (9;22) (MFIs-14.43 and 11.87).
Conclusions:
This pilot study of LAIR-1expression in ALL suggests low expression of the inhibitory molecule in leukemic cells. However, the findings need to be confirmed with larger cohort, along with studies focusing on pathophysiological roles in leukemic clone survival and escape from the immune system.
Keywords: Acute lymphoblastic leukemia, minimal residual disease, LAIR-1
Introduction
B-cell acute lymphoblastic leukaemia (B-ALL) is the most common type of childhood leukaemia accounting for 80-85% of cases. With improving supportive and diagnostic care and optimized treatment protocols the overall survival of B-ALL cases has improved over the years and varies from 85% to 90% in developed countries (Pui et al., 2015) to 67-74% in developing countries (Panya et al., 2015; Trehan et al., 2017) . However there is constant ongoing research to look into newer prognostic markers in B-ALL that can be of utility for targeted therapies or predictors of disease relapse. Of these, Leukocyte-associated immunoglobulin-like receptor-1 (LAIR-1) has recently gained interest in acute leukemias as a possible immune evasion marker expressed on leukemic blasts (Florian et al., 2006; Meyaard et al., 1997; Poggi et al., 1995; Poggi et al., 1998; Verbrugge et al., 2006). LAIR-1 (CD305) is an ITIM associated inhibitory receptor and is expressed on most of the immune cells. It is a type I transmembrane glycoprotein containing one extracellular Ig-like domain and two ITIMs (Meyaard et al., 1997). LAIR-1 is known to be expressed on various lineage of leukemic cells and a number of human AML cell lines, such as MV4-1, THP-1 and U937 as well as on B-ALL cell lines 697, Kasumi 2 and RCH-ACV (Kang et al., 2015). Collagen is the major ligand of LAIR-1 and in vitro experiments have confirmed its role in inhibiting immune cell activation (Lebbink et al., 2006; Lebbink et al., 2007). Previous studies have demonstrated that LAIR-1 mediates its immune inhibitory function by cross linking itself on immune effector cells and initiating inhibitory signalling and thereby impeding the cell killing by NK cells or T cells and blocking the Ig and cytokine production by B cells (Meyaard et al., 1997; Poggi et al., 1995; Meyaard et al., 1999; Ouyang et al., 2003; Massho et al., 2005; Poggi et al., 1997; Merlo et al., 2005).
Few studies till date have been reported on the expression and role of LAIR-1 in leukaemia patients. In a study from a cohort of AML patients, it was demonstrated that haematopoiesis is unaffected on blocking the expression of LAIR-1 in normal cells but its inhibition suppresses the leukaemia progression since LAIR-1 helps in sustaining the survival and self-renewal properties of AML stem cells (Kang et al., 2015). Further in in-vitro and in-vivo (mouse model) experimentation by Chen et al., (2015), it was revealed that deletion of LAIR-1 can lead to cell death or remission of B-ALL respectively. However reports from cohorts of CLL patients suggests differently, showing that LAIR-1 is predominantly expressed in early stages of CLL and its expression is lower in patients with high-risk CLL (Perbellini et al., 2014). Thus the expression and role of LAIR-1 in different forms of leukaemia is still controversial. Considering above conflicting data on LAIR-1 expression and role in leukaemia progression, we prospectively analyzed the same in a cohort of paediatric ALL and correlated it with clinical variables and early treatment outcome parameters.
Materials and Methods
A total of 42 newly diagnosed pediatric cases (0-12 years) of acute lymphoblastic leukemia and ten children with normal hematological parameters as control were enrolled in the study. 1-2 ml of peripheral blood (blast count ≥80% in all patient samples) taken in EDTA coated vials was processed for flow cytometric analysis within 2-3 h by standard surface staining procedure. Briefly, APC conjugated CD45 (BD Biosciences) and FITC conjugated LAIR-1 (BD Biosciences) monoclonal antibodies were used to stain the cells followed by red blood cell lysis (ammonium chloride solution). The samples were washed twice with PBS and then acquired using Beckman Coulter Navios flow cytometer. Unstained cells were used as negative control for staining and compensation was done using appropriate isotope matched negative control for anti LAIR-1. Cells with dim-intermediate CD45 positivity on SSC vs CD45 plot were gated and percentage positivity and mean fluorescence intensity (MFI) of LAIR-1 on these cells was analyzed.
The leukemia cases are routinely classified as B-/T-ALL by standard multicolor flow cytometry at our institute using an extended panel for acute leukemia including myeloid markers and MPO. The patients were stratified according to NCI risk criteria and treated as per modified ICICLE protocol which is an Indian adaptation of UKMRC 2003 protocol. Demographic and clinical variables of patients were noted and correlated with expression level of LAIR-1. The data was analyzed using SPSS 2.0 software. The association between two categorical variables was done using the chi-square test and p value <0.05 was considered statistically significant. Moods median method was performed to analyze any difference in LAIR-1 expression in control and case group. The study was approved by Institutional Ethics Committee and Departmental review board. Informed consent of the inpatient/guardian was taken from all the cases enrolled in the study.
Results
LAIR-1 expression in control group (n-10)
LAIR-1 expression was first analyzed on lymphocytes of 10 pediatric control cases. The median of the MFIs of LAIR-1 expression on brightly positive CD45 lymphocytes was 8.2 (Figure 1) with lower and higher limits being 7.76 and 11.69 respectively (logarithmic scale). The expression of LAIR-1 on control samples was used to categorize the ALL cases into two groups (low or normal expression) based on the median MFI value.
Figure 1.

Flow Histograms of LAIR-1 MFI in Control and ALL Cases Showing Low and High Expression
LAIR-1 expression in ALL patients (n-42)
After setting the range of LAIR-1 expression in pediatric control samples, we analyzed LAIR-1 expression in a cohort of 42 children with ALL at the time of initial diagnosis. Seventy four percent (n-31) of patients showed lower expression of LAIR-1 while 26% (n-11) of cases had normal expression of LAIR-1 on lymphoblasts as compared to the control group (Figure 2). The ALL cases had MFI for LAIR-1 expression in the range of 0.56 to 11.87 (Figure 1) with median of 4.02, which was approximately 50% of control samples (8.2) and appeared to have significant difference however when Moods median test was applied (due to skewed data with uneven low observations in control group), the difference was not found to be statistically significant. Three cases in control group had outliers with their highest MFI being 16.91.
Figure 2.
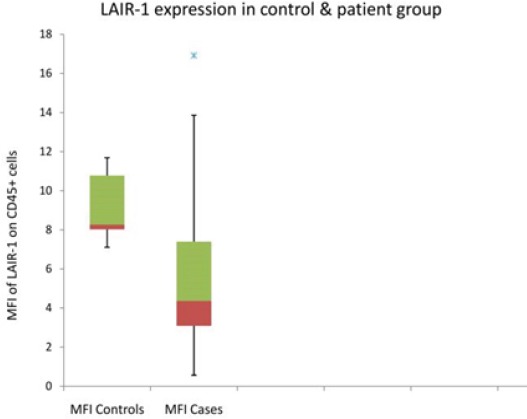
Box-Plot and Whisker Chart Demonstrating Difference in MFI of LAIR-1 Expression in Control and Case (ALL) Group
Biology of the ALL patient samples (n-42)
The patient group comprised of 30 male and 12 females with male to female ratio of 2.5:1. The age range of cases was 1 - 11 y (median- 5.5). At the time of initial diagnostic work up, 27 out of 42 patients had WBC count <50 x 109/L and 15 patients had WBC >50 x 109/L. According to NCI risk criterion, 22 patients were stratified into standard risk and 20 were at high risk. Six patients were identified as T-ALL and 36 were identified as B-ALL by immnuo-phenotyping. Common fusion transcripts of AML1-TEL, E2A-PBX, BCR-ABL and MLL-AF4 were identified in 3, 6, 2 and 1 patient respectively. Day 8 absolute blast count (ABC) was noted to be <1,000 in 31 patients and 8 patients had >1,000 day 8 ABC count. Thirty patients had low MRD (<0.01) and 7 patients had high MRD (>0.01) status at day 35 of the treatment regime.
Correlation of LAIR-1 expression in patients with diagnosis and treatment parameters
On correlation of the two groups with low and normal LAIR-1 expression in ALL cases, no statistically significant association was noted with ALL risk factors, early treatment parameters or MRD status (Table 1). Out of 42 patients from our study cohort, four patients died during induction treatment and one left therapy. Sixty percent of the patients (n-3/5) with above event had low expression of LAIR-1. We also looked at the common fusion transcript expression profile of the patients and found that ALL patients with low LAIR-1 expression had t (12;21), t (1;19) and t (4;11) translocations in 2, 4 and 1 samples respectively but none of the patients had t (9;22). While in the case of patients with high LAIR-1 expression, 2 patients had t (9;22) and their MFIs were noted to be 14.43 and 11.87. Out of 11 patients having normal LAIR-1 expression 1 and 2 patients also had t (12;21) and t (1;19) respectively.
Table 1.
Clinical Correlation of LAIR-1 Expression in Low and Normal MFI Groups in ALL Cases
| Variable/Category | Low LAIR-1 expression | Normal LAIR-1 expression | p | Total |
|---|---|---|---|---|
| Age (y) (n-42) | ||||
| < 1/>10 | 3 | 2 | 0.45 | 5 |
| >1/<10 | 28 | 9 | 37 | |
| Gender (n-42) | ||||
| Male | 22 | 8 | 0.91 | 30 |
| Female | 9 | 3 | 12 | |
| WBC (n-42) | ||||
| <50x109/L | 19 | 8 | 0.46 | 27 |
| >50x109/L | 12 | 3 | 15 | |
| NCI Risk (n-42) | ||||
| Standard | 16 | 6 | 0.86 | 22 |
| High | 15 | 5 | 20 | |
| Day 8 abc (n-9) | ||||
| <1,000 | 22 | 9 | 0.82 | 31 |
| >1,000 | 6 | 2 | 8 | |
| MRD (n-37) | ||||
| <0.01 | 22 | 8 | 0.49 | 30 |
| >0.01 | 6 | 1 | 7 | |
| Event (n-42) | ||||
| Yes | 3 | 2 | 0.45 | 5 |
| No | 28 | 9 | 36 | |
Discussion
LAIR-1 has recently been a subject of investigation as an immuno-phenotypic marker in different leukaemia’s. We at a tertiary care hospital and research centre in India examined the potential of this newly identified marker in our paediatric cohort of ALL patients. At this initial stage we looked at the expression level of LAIR-1 on immature lymphoid cells of newly diagnosed patients and tried to find any correlation with standard risk factors and early treatment outcome parameters. We noted that a significant number of ALL patients had lower level of LAIR-1 expression than control cases. Perbellini et al., (2014) have previously shown that lower expression of LAIR-1 is associated with high risk CLL patients that are expected to have worse clinical stage at diagnosis and poor biology. Similar observation was noted by Poggi et al., (2008) in a population of CLL patients. In our cohort as per NCI risk criterion 20 cases were at high risk, however we could not correlate our data statistically due to the limited sample size.
In a report by Mirkowoska et al., (2013) the MFI of LAIR-1 (CD305) expression on CD19+ B-cell population of leukemic and normal cells showed similar expression at the time of diagnosis and increased significantly in the MRD samples at day 15 of the treatment from the same sample. However, we in our study performed LAIR-1 expression analysis at a single time point only. In another study by Chen et al., (2015), LAIR-1 was found to be highly expressed in Philadelphia positive ALL and was associated with shorter overall and relapse-free survival. In our study we noted that two of the cases with BCR-ABL t (9;22) positivity that had higher expression of LAIR-1 with their MFIs (11.87 and 14.43) much higher than median of MFIs (4.2) of all the patients tested. No patient was reported to have BCR-ABL t (9;22) translocation in the group of low LAIR-1 expression. Although tested in the small cohort our data is in agreement with Chen et al., (2015)’s report.
Though significantly lower expression of LAIR-1 has been noted in pediatric ALL cases, the prognostic implication of same are limited since no correlation was observed between LAIR-1 expression and ALL risk and early treatment outcome parameters. It is hypothesized that normal to high LAIR-1 expression on lymphoblasts protects them from immune system, however this is mainly derived from in-vitro studies on AML cell lines and it needs to be seen how ALL clones behave. It is likely from our pilot study results that high risk sub-groups of ALL, like BCR-ABL positive clones may actually have high LAIR-1 expression and this may be a contributing factor in their evasion from immune cells to finally manifest as early or late relapse. However, larger prospective studies are still needed especially in cases with good and poor risk genetic abnormalities to better assess the significance of LAIR-1 as a prognostic marker or a potential therapeutic target.
Grant
Intramural Institute Grant (PGIMER, Chandigarh) for year 2015-2017; Grant No. 421.
Presentation
Poster presentation at ESMO Immunology Oncology Congress held inGeneva, Switzerland from 7-10th Dec 2017; Poster abstract No. 152 published as abstract in Supplement of Annals of Oncology No. 90P.
Disclosure of Interest
None.
Acknowledgments
The study was supported by Institutional research grant from PGIMER, Chandigarh. The authors declare no conflict of interest.
References
- 1.Chen Z, Shojaee S, Buchner M, et al. Signalling thresholds and negative B-cell selection in acute lymphoblastic leukaemia. Nature. 2015;521:357–61. doi: 10.1038/nature14231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Florian S, Sonneck K, Czerny M, et al. Detection of novel leukocyte differentiation antigens on basophils and mast cells by HLDA8 antibodies. Allergy. 2006;61:1054–62. doi: 10.1111/j.1398-9995.2006.01171.x. [DOI] [PubMed] [Google Scholar]
- 3.Kang X, Lu Z, Cui C, et al. The ITIM-containing receptor LAIR1 is essential for acute myeloid leukaemia development. Nat Cell Biol. 2015;17:665–77. doi: 10.1038/ncb3158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lebbink RJ, de Ruiter T, Adelmeijer J, et al. Collagens are functional, high affinity ligands for the inhibitory immune receptor LAIR-1. J Exp Med. 2006;203:1419–25. doi: 10.1084/jem.20052554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lebbink RJ, de Ruiter T, Kaptijn GJA, et al. Mouse leukocyte-associated Ig-like receptor-1 (mLAIR-1) functions as an inhibitory collagen-binding receptor on immune cells. Int Immunol. 2007;19:1011–9. doi: 10.1093/intimm/dxm071. [DOI] [PubMed] [Google Scholar]
- 6.Maasho K, Masilamani M, Valas R, et al. The inhibitory leukocyte-associated Ig-like receptor-1 (LAIR-1) is expressed at high levels by human naive T cells and inhibits TCR mediated activation. Mol Immunol. 2005;42:1521–30. doi: 10.1016/j.molimm.2005.01.004. [DOI] [PubMed] [Google Scholar]
- 7.Merlo A, Tenca C, Fais F, et al. Inhibitory receptors CD85j, LAIR-1, and CD152 down-regulate immunoglobulin and cytokine production by human B lymphocytes. Clin Diagn Lab Immunol. 2005;12:705–12. doi: 10.1128/CDLI.12.6.705-712.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Meyaard L, Adema GJ, Chang C, et al. LAIR-1, a novel inhibitory receptor expressed on human mononuclear leukocytes. Immunity. 1997;7:283–90. doi: 10.1016/s1074-7613(00)80530-0. [DOI] [PubMed] [Google Scholar]
- 9.Meyaard L, Hurenkamp J, Clevers H, Lanier LL, Phillips JH. Leukocyte-associated Ig-like receptor-1 functions as an inhibitory receptor on cytotoxic T cells. J Immunol. 1999;162:5800–4. [PubMed] [Google Scholar]
- 10.Mirkowska P, Hofmann A, Sedek L, et al. Leukemia surfaceome analysis reveals new disease-associated features. Blood. 2013;121:149–59. doi: 10.1182/blood-2012-11-468702. [DOI] [PubMed] [Google Scholar]
- 11.Ouyang W, Ma D, Lin D, et al. 9.1C3 is identical to LAIR-1, which is expressed on hematopoietic progenitors. Biochem Biophys Res Commun. 2003;310:1236–40. doi: 10.1016/j.bbrc.2003.09.152. [DOI] [PubMed] [Google Scholar]
- 12.Panya S, Surapon W, Gavivann V, et al. Outcome of childhood acute lymphoblastic leukemia treated using the Thai National Protocols. Asian Pac J Cancer Prev. 2015;16:4609–14. doi: 10.7314/apjcp.2015.16.11.4609. [DOI] [PubMed] [Google Scholar]
- 13.Perbellini O, Falisi E, Giaretta I, et al. Clinical significance of LAIR1 (CD305) as assessed by flow cytometry in a prospective series of patients with chronic lymphocytic leukemia. Haematologica. 2014;99:881–7. doi: 10.3324/haematol.2013.096362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Poggi A, Pella N, Morelli L, et al. p40, a novel surface molecule involved in the regulation of the non-major histocompatibility complex-restricted cytolytic activity in humans. Eur J Immunol. 1995;25:369–76. doi: 10.1002/eji.1830250210. [DOI] [PubMed] [Google Scholar]
- 15.Poggi A, Tomasello E, Revello V, et al. p40 molecule regulates NK cell activation mediated by NK receptors for HLA class I antigens and TCR-mediated triggering of T lymphocytes. Int Immunol. 1997;9:1271–9. doi: 10.1093/intimm/9.9.1271. [DOI] [PubMed] [Google Scholar]
- 16.Poggi A, Tomasello E, Ferrero E, Zocchi MR, Moretta L. p40/LAIR-1 regulates the differentiation of peripheral blood precursors to dendritic cells induced by granulocyte-monocyte colony-stimulating factor. Eur J Immunol. 1998;28:2086–91. doi: 10.1002/(SICI)1521-4141(199807)28:07<2086::AID-IMMU2086>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 17.Poggi A, Catellani S, Bruzzone A, et al. Lack of the leukocyte-associated Ig-like receptor-1 expression in high-risk chronic lymphocytic leukaemia results in the absence of a negative signal regulating kinase activation and cell division. Leukemia. 2008;22:980–8. doi: 10.1038/leu.2008.21. [DOI] [PubMed] [Google Scholar]
- 18.Pui C-H, Yang JJ, Hunger SP, et al. Childhood acute lymphoblastic leukemia:Progress through collaboration. J Clin Oncol. 2015;33:2938–48. doi: 10.1200/JCO.2014.59.1636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Trehan A, Bansal D, Varma N, et al. Improving outcome of acute lymphoblastic leukemia with a simplified protocol:report from a tertiary care center in north India. Pediatr Blood Cancer. 2017;64:e26281. doi: 10.1002/pbc.26281. [DOI] [PubMed] [Google Scholar]
- 20.Verbrugge A, de Ruiter T, Geest C, Coffer PJ, Meyaard L. Differential expression of leukocyte-associated Ig-like receptor-1 during neutrophil differentiation and activation. J Leukoc Biol. 2006;79:828–36. doi: 10.1189/jlb.0705370. [DOI] [PubMed] [Google Scholar]


