Abstract
Reward plays a crucial role in enhancing response inhibition. While it is generally assumed that the process of response inhibition involves attentional capture and the stopping of action, it is unclear whether this reflects a direct impact of reward on response inhibition or rather an indirect mediation via attentional capture. Here, we employed a revised stop-signal task (SST) that separated these two cognitive elements, by including a continue signal that required the same motor response as in go trials, but also attention to a cue, as in stop trials. We first confirmed the engagement of the right inferior frontal gyrus (IFG) during stop and continue trials, both of which required the attentional capture of the task-relevant cue, but only one of which required motor inhibition. The pre-supplementary motor area (pre-SMA) was specifically activated by the contrast of the stop trials with the continue trials. The results indicated that the IFG played an important role in attentional capture by unexpected stimuli, while the pre-SMA was responsible for the direct control of motor inhibition. Behavioral performance of the SST was improved by reward, and moreover, reward induced an increase in IFG activity. In addition, this advantageous reward effect was associated with enhanced connectivity between the anterior cingulate cortex and the IFG. These results indicated that the reward facilitation effect on response inhibition was indirect, occurring via a change in attentional processing. The present data confirm the specific function of the IFG and pre-SMA in response inhibition and provide straightforward evidence that reward can increase attentional capture-related activation in the IFG, which in turn improves the performance of response inhibition.
Keywords: response inhibition, reward, attentional capture, inferior frontal cortex
Introduction
The ability to inhibit inappropriate actions is a fundamental aspect of optimal goal-directed behavior, which has been widely investigated using the stop-signal task (SST), as it provides an assessment of the time course of inhibitory processes. Reward is known to be a strong driving force for goal-directed behavior (Krebs et al., 2011; Botvinick and Braver, 2015; Etzel et al., 2016). For instance, stimulus–reward associations consistently result in improved behavioral performance, that is, faster reaction time (RT) and decreased error rates (Boehler et al., 2012, 2014; Freeman et al., 2014; Freeman and Aron, 2016).
A limitation of the previous work is that it primarily focused on the enhancement of response inhibition affected by reward, but it did not directly investigate how reward affects the component task processes that are involved in response inhibition. In the SST, the participant responds to the go signal but cancels a previously initiated response when an unexpected and highly salient stop signal appears briefly after the go signal. Accordingly, the SST involves attending to when a cue stops, which may confound a role in attentional capture with a role in response inhibition (Chikazoe et al., 2009; Sharp et al., 2010; Dodds et al., 2011; Hampshire and Sharp, 2015; Lee et al., 2016; Xu et al., 2017). Recent studies indicated that reward was an effective motivator of behavior and facilitated response inhibition (Boehler et al., 2012, 2014). However, it is unclear whether this reflects a direct impact of reward on response inhibition or an effect mediated via attentional capture by an unexpected stimulus.
Existing work has failed to separate the distinct cognitive processes that are involved in response inhibition or to demonstrate whether the reward effect is due to the response inhibition itself or due to the attentional capture of unexpected stimuli. Recent findings support the plausibility of independently assessing the changes in attentional capture and changes in response inhibition because the brain systems underlying each are distinct from one another. More specifically, the right inferior frontal gyrus (IFG) and the pre-supplementary motor area (pre-SMA) are frequently identified as important for a range of tasks requiring response inhibition (Sharp et al., 2010; Coxon et al., 2012; Rae et al., 2015; Watanabe et al., 2015; Cai et al., 2016; Coxon et al., 2016; Lee et al., 2016). Sharp et al. (2010) employed a novel variation of the SST that contained not only infrequent stop trials but also infrequent continue trials. These continue trials instructed participants to execute the go response and to ignore a visual cue, which provides a baseline that controls for attentional capture by an infrequent stimulus. They found that the IFG did not differentiate between the stop and the continue trials, both of which required attending to an infrequent signal but only one of which required motor inhibition, and the pre-SMA was more activated during the stop trials than during the continue trials. These results suggest that studies of response inhibition link attentional capture processing to the IFG, whereas the stopping motor action is associated with the pre-SMA (Sharp et al., 2010; Dodds et al., 2011; Hampshire and Sharp, 2015; Lee et al., 2016). However, no studies have demonstrated the neural mechanisms that support the enhancement of response inhibition affected by reward.
Furthermore, recent evidence suggests that the anterior cingulate cortex (ACC) is crucial for the processing of inhibition control and reward (Camille et al., 2011; Hayden et al., 2011; Alexander and Brown, 2012; Shenhav et al., 2013; Kim, 2014; David et al., 2015; Duverne and Koechlin, 2017). Particularly, the ACC has been reported to be connected with the pre-SMA, and it is implicated in cognitive control (Duverne and Koechlin, 2017). The ACC and the IFG, both engaged as part of the ventral attentional system, are also involved in the detection of cue targets (Sharp et al., 2010; Kim, 2014). Meanwhile, the ACC is thought to play an important role in linking reward expectations to actions (Camille et al., 2011; Alexander and Brown, 2012; Duverne and Koechlin, 2017). It has been suggested that the ACC encodes the reward advantage to inhibit the ongoing task set (Boorman et al., 2013). Nevertheless, it is unknown how the functional connectivity between these brain regions specifically contributes to the improvement of inhibition control.
In this study, we administered a modified SST that incorporates a go signal, reward-related (RR) stop signal, reward-unrelated (RU) stop signal, RR continue signal and RU continue signal in one comprehensive experimental paradigm. This design not only allowed us to confirm the inhibitory processing- and attentional processing-related brain activity but also provided conditions in which the correct response to the target did in fact lead to reward, even for non-inhibitory trials, allowing us to dissociate the impact of reward on attentional capture from its impact on response inhibition. Specifically, we hypothesized that IFG activation is correlated with the attentional processing of unexpected stimuli and that the pre-SMA is responsible for the direct control of motor inhibition. This is based on the recent finding that the IFG is recruited during a range of attention-demanding conditions, especially in response to the detection of unexpected, behaviorally relevant targets (Bledowski et al., 2004; Corbetta et al., 2008; Dodds et al., 2011; Erika-Florence et al., 2014; Hampshire, 2015). Furthermore, a growing body of evidence has shown that the disruption of pre-SMA activity behaviorally impairs response inhibition (Neubert et al., 2010; Nettekoven et al., 2014; Watanabe et al., 2015; Lee et al., 2016), while stimulation of the pre-SMA often improves the motor response (Scangos and Stuphorn, 2010). We also hypothesized that reward improves response inhibition by modulating attentional capture via enhanced processing in attention-relevant regions, including the IFG. This hypothesis derives from the recent literature finding that stimulus–reward associations can influence sensory processing at an early stage, which can strongly modulate attention processing to optimize goal-directed behavior (Pessoa and Engelmann, 2010; Krebs et al., 2011, 2013).
Materials and methods
Participants
Thirty healthy, right-handed volunteers were recruited for this study and financially compensated for their participation. All of the subjects had normal color perception and normal or corrected-to-normal vision. Informed written consent was obtained from all of the participants before the experiment; the study protocol was approved by the Ethics Committee of Southwest University, China. Three subjects were excluded from further analysis because they had excessive head movement (>2.5 mm) during the scanning in each direction. The final sample consisted of 27 subjects (9 males, 18–23 years old).
Experimental task
The task contained three types of trials: go trials, stop trials and continue trials (Figure 1). Each trial started with a white cross-shaped fixation for 1000 ms. This was followed by a left- or right-pointing arrow stimulus with equal probability, and the subject responded as fast as possible with the index finger of each hand. The arrow vanished after 1000 ms, and the trial was then terminated. The trials were separated by jittered intertrial intervals ranging from 2 to 3 s in uniformly distributed steps of 200 ms, during which a blank screen was shown. For the stop trials, after a short variable delay (stop-signal delay; SSD), an upward-pointing triangle was presented above the location of the arrow stimulus (20% of the trials), and subjects were instructed to withhold their response. The SSD was determined by staircase tracking algorithms, which modified the delay between the go and stop signals in increments or decrements of 34 ms, to ensure an ~50% stopping accuracy in all subjects. To reduce participants’ anticipation, the initial value of SSD was sampled from one of the four staircases (100, 150, 200 and 250 ms). Similarly, a downward-pointing triangle appeared above the location of the arrow stimulus for the continue trials. Participants were instructed to execute the go response and to ignore the triangle signal (20% of the trials). To ensure that the manipulation of the continue signal is consistent with that of the stop signal, the continue signal was presented shortly after the arrow stimulus (continue signal delay); the interval between the onset of the continue signal and the onset of the arrow stimulus was varied randomly from 159 to 335 ms. This offset was chosen according to previous studies where a similar range to that was reported for the SSD (Erika-Florence et al., 2014; Hampshire, 2015). In addition, two colors were associated with different reward amounts. Before the experiment properly started, participants were explicitly informed of the relationship between each color stimuli and its associated outcome. In the continue trials, where a yellow triangle appeared above the arrow and fast and correct responses were given, a ¥0.2 gain (¥1 ≈ $0.15) (RR) resulted, whereas incorrect or slow responses resulted in the loss of ¥0.2. Similarly, in the stop trials, participants could win ¥0.2 for each correct RR trial, but they could lose that same amount for incorrect RR trials. Meanwhile, responses to the blue triangle resulted in a ¥0 gain or a penalty (RU). The payment for the experiment was the sum of the base bonus, ¥60, and the additional bonus based on the subject’s performance in the experiment.
Fig. 1.
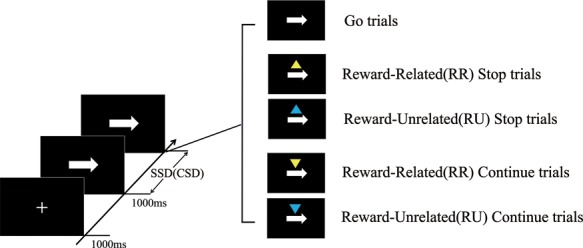
STT. Participants were required to quickly press in response to a left- or right-pointing arrow. Occasionally, an upward-pointing triangle was presented above the location of the arrow stimulus, and participants must withhold the button press (stop trials). Similarly, when a downward-pointing triangle appeared above the location of the arrow stimulus, participants should execute the go response and ignore the triangle signal (continue trials). When a yellow triangle appeared, fast and correct responses resulted in a ¥0.2 gain (RR); incorrect or slow responses incurred a loss of ¥0.2. Responses to the blue triangle resulted in a ¥0 gain or penalty (RU).
In addition, to avoid strategic slowing for the purpose of achieving a higher success rate in stopping, participants would receive visual feedback in the form of the words “Speed up” if their go or continue RT was 2 standard deviations greater than their respective mean RT. The starting point for the mean go and continue RTs was 600 ms. Subsequently, the mean go and continue RTs were separately recalculated and updated on each go and continue trial.
Participants completed 360 go trials, 120 stop trials (60 RR trials and 60 RU trials) and 120 continue trials (60 RR trials and 60 RU trials) that were divided into five blocks with a rest break of 1 min between blocks. Both blocks lasted ~9.5 min each, for a total scanning time of 51.5 min. Before the start of the experiments, participants performed a training session including 48 go trials, 24 stop trials (12 RR trials and 12 RU trials) and 24 continue trials (12 RR trials and 12 RU trials).
Behavioral analysis
The behavioral performance of the SST was quantified by the stop-signal reaction time (SSRT) obtained for each condition. A lower SSRT reflects individuals who require less time to inhibit a response. SSRTs were estimated using the integration method (Verbruggen and Logan, 2009; Verbruggen et al., 2013; Watanabe et al., 2015). In this method, the RT for the go trials were sorted from fast to slow, and the Nth RT was selected, where N was estimated by multiplying the probability of mistakes in the stop trials by the number of stop trials. The average SSD was then subtracted from Nth RT value to obtain the SSRT.
Functional magnetic resonance imaging (fMRI) image acquisition and pre-processing
Images were obtained with a 3.0-T Siemens scanner (Siemens Magnetom Trio TIM, Erlangen, Germany). Functional images were collected parallel to the anterior commissure–posterior commissure line with a T2-weighted echoplanar imaging sequence of 32 axial slices (time repetition [TR] = 2000 ms, time echo [TE] = 30 ms, flip angle = 85°, field of view [FoV] = 224 mm, matrix size = 64×64) of 3 mm thickness. T1-weighted images were recorded with a total of 176 slices at a thickness of 1 mm (TR = 1900 ms, TE = 2.52 ms, flip angle = 90°, matrix size = 256×256).
All pre-processing and statistical analyses were conducted using SPM8 (http://www.fil.ion.ucl.ac.uk/spm/). The first five volumes of each run were discarded to mitigate non-equilibrium effects. Functional data were slice-timing corrected, motion corrected, coregistered to the structural image, normalized to the Montreal Neurological Institute (MNI) template and smoothed with a full-width half-maximum Gaussian kernel of 6 × 6 × 6 mm3.
Statistical analysis
A general-linear model (GLM) approach was used to estimate the parametric maps of brain activation. The model regressors included temporal onsets for (i) RR successful stop trials, (ii) RU successful stop trials, (iii) RR failed stop trials, (iv) RU failed stop trials, (v) RR continue trials, (vi) RU continue trials, (vii) go trials and (viii) errors (incorrect continue and go trials). All regressors were convolved with the canonical hemodynamic response function, and the model contained the six head motion parameters as regressors of non-interest.
In the random effects group analyses, we performed paired t-tests for voxel-wise comparisons with four goals: (i) to identify response inhibition-related brain activity for successful stop trials (combined RR and RU) vs go trials, (ii) to identify continue trials-related brain activity for successful continue trials (combined RR and RU) vs go trials, (iii) to identify the outright stopping of a motor response-related brain activity for successful stop trials (combined RR and RU) vs successful continue trials (combined RR and RU) and (iv) to identify brain activity reflecting the effects of reward for RR successful stop trials vs RU successful stop trials and RR successful continue trials vs RU successful continue trials.
To further verify the observed differential effects within the pre-SMA and right IFG, we performed a region of interest (ROI) analysis using the MarsBar analysis toolbox (Brett et al., 2002). For the right IFG, we defined ROIs based on group peak coordinates of activation in successful stop trials (combined RR and RU) contrasted with go trials (x = 45, y = 9, z = 24). For the pre-SMA, we used the group peak coordinates of activation, found by contrasting successful stop trials (combined RR and RU) with continue trials (combined RR and RU) (x = 6, y = 12, z = 51). The ROIs were defined as a sphere with a 6 mm radius, and the blood oxygenation level dependent signal change was extracted for each condition of interest.
Sharp et al.(2010) argued that, compared with go trials, the appearance of the infrequent continue signals trigger an incomplete inhibitory processing result in slowing in continue trials. If the pre-SMA is associated with outright stopping, the high degree of slowing in the continue trials would increase this region’s activation. The slowing degree was defined by the discrepancy between the mean RT for RU continue trials and go trials. Individuals with a high degree of slowing (i.e. above the average) were classified as high-slowing participants, and those with a low degree of slowing (below the average) were classified as low-slowing participants. Using these ROIs, we calculated the different activities between the high-slowing and low-slowing groups in the right IFG and pre-SMA during the RU condition.
The psychophysiological interaction (PPI) was conducted to probe whether activity in the ACC was differentially correlated with the IFG or pre-SMA responses in RR trials compared with those in RU trials. Specifically, we were interested in seeing whether there were potentially different connectivity patterns in the reward conditions for continue trials, which reflect attentional capture, relative to stop trials, which are involved in attentional capture and outright stopping. A conjunction analysis was conducted to identify the ACC that uniquely contributed to the response inhibition and reward effect. Specifically, the contrasts RR stop trials > RU stop trials, RR continue trials > RU continue trials, RU stop trials > go trials and RU continue trials > go trials. The ACC (x = 3, y = 36, z = 21) were submitted to the conjunction analysis, by which the ROI for the PPI was defined. We extracted the time course of the ACC as the physiological variable and defined the contrast vector representing the task effect (RR > RU) as the psychological variable. The PPI term was created with the physiological variable and the psychological variable. Then, the physiological variable, the psychological variable and their interaction term as well as six motion regressors were entered into a new GLM on the first level. In the next step, the results were taken into a regression analysis at the second level with the effect size of the reward effect as the explanatory variable. All of the group-level results were thresholded at P < 0.05 and corrected for multiple comparisons (familywise error; FWE) with a cluster extent of k = 10 voxels.
Results
Behavioral results
The proportions of correct responses and RTs are summarized in Table 1. In the continue trials, the current study used the staircasing procedure; the stop performance was ~50% correct during both conditions. However, paired t-tests revealed that the participants also committed less errors in the RR trials compared to the RU trials [t(26) = 3.08, P = 0.005]. Critically, the SSRT was shorter during the RR trials than during the RU trials [t(26) = 3.01, P = 0.006], revealing that it was easier to inhibit the behavioral response during the former condition. In the continue trials, participants responded faster to the RU condition than to the RR condition [t(26) = 2.94, P = 0.007]. However, accuracy was not significantly different between the two conditions [t(26) = 0.64, P = 0.53]. Finally, when comparing the RU and RR continue trials with the go trials separately, the RT was significantly slower in the continue trials [t(26) = 6.76, P < 0.001; t(26) = 7.43, P < 0.001].
Table 1.
Behavioral performance during SST (average ± SEM)
| Trial type | RU | RR |
|---|---|---|
| Go RT (ms) | 478 ± 10 | – |
| Go ACC | 0.98 ± 0.01 | – |
| SSD | 189 ± 10 | 206 ± 9 |
| SSRT | 281 ± 10 | 251 ± 8 |
| Stop trials ACC | 0.48 ± 0.03 | 0.53 ± 0.02 |
| Continue trials RT (ms) | 544 ± 18 | 557 ± 19 |
| Continue trials ACC | 0.95 ± 0.01 | 0.95 ± 0.01 |
Neural activity during the stop and continue trials
To verify that the previously described ‘stop networks’, including the brain regions involved in response inhibition and attentional processing of an unexpected event, were recruited, we contrasted activity between the stop trials and the go trials. As shown previously (Aron and Poldrack, 2006; Li et al., 2006; Sharp et al., 2010; Rae et al., 2015; Coxon et al., 2016), stop trials are associated with significant activation in the medial frontal cortex, including the ACC and pre-SMA, bilateral insula, bilateral IFG and thalamus (Table 2A and Figure 2A).
Table 2.
Brain regions active for contrast stop > go, continue > go and stop > continue
| MNI coordinates | |||||||
|---|---|---|---|---|---|---|---|
| Region | Hemisphere | x | y | z | t | P | Extent (voxels) |
| A. Stop trials > go trials | |||||||
| Insula/IFG | R | 30 | 21 | 3 | 15.96 | <0.001 | 5201 |
| Pre-SMA | R | 3 | 15 | 48 | 11.26 | <0.001 | |
| Middle frontal gyrus | R | 45 | 9 | 33 | 15.29 | <0.001 | |
| Inferior parietal lobule | L | −39 | −48 | 45 | 15.14 | <0.001 | 4130 |
| IFG | L | −45 | 3 | 30 | 12.56 | <0.001 | |
| Insula | L | −30 | 24 | 3 | 12.45 | <0.001 | |
| Precuneus | R | 15 | −66 | 36 | 14.77 | <0.001 | |
| L | −51 | −42 | 48 | 14.38 | <0.001 | ||
| Posterior cingulate | R | 21 | −63 | 9 | 9.04 | <0.001 | 105 |
| R | 6 | −69 | 9 | 7.23 | 0.003 | ||
| L | −21 | −60 | 3 | 7.81 | <0.001 | 62 | |
| Caudate | L | −12 | −69 | 6 | 7.67 | <0.001 | |
| Thalamus | L | −18 | −30 | −3 | 8.75 | <0.001 | 51 |
| L | −9 | −9 | 9 | 7.17 | 0.003 | 26 | |
| L | −9 | −21 | 9 | 6.63 | 0.01 | ||
| Caudate | L | −15 | 18 | −15 | 8.73 | <0.001 | 89 |
| L | −12 | 12 | 3 | 7.72 | <0.001 | ||
| Temporal Lobe | L | −39 | −6 | −15 | 7.55 | 0.001 | 20 |
| L | −36 | −12 | −9 | 7.47 | 0.001 | ||
| B. Continue trials > go trials | |||||||
| Parietal lobe | R | 36 | −54 | 51 | 17.32 | <0.001 | 5790 |
| Occipital lobe | L | −48 | −63 | −9 | 16.71 | <0.001 | |
| Parietal lobe | L | −33 | −60 | 54 | 16.19 | <0.001 | |
| Thalamus | R | 24 | −33 | −3 | 15.65 | <0.001 | 5183 |
| IFG | L | −48 | 3 | 12 | 12.30 | <0.001 | |
| R | 33 | 18 | −3 | 12.87 | <0.001 | ||
| R | 48 | 12 | 33 | 12.11 | <0.001 | ||
| Insula | L | −27 | 24 | 3 | 15.61 | <0.001 | |
| Pre-SMA | R | −6 | 12 | 45 | 13.86 | <0.001 | |
| Cingulate gyrus | R | 6 | −30 | 27 | 14.90 | <0.001 | |
| Temporal lobe | R | 33 | −9 | −9 | 9.12 | <0.001 | 25 |
| C. Stop trials > continue trials | |||||||
| Middle frontal gyrus (pre-SMA) | R | 18 | 0 | 66 | 8.08 | <0.001 | 24 |
| Parietal lobe | L | −60 | −48 | 36 | 7.83 | <0.001 | 21 |
| Inferior parietal lobule | R | 66 | −36 | 27 | 8.14 | <0.001 | 68 |
| R | 60 | −30 | 36 | 7.55 | 0.001 | ||
All clusters reliable at P < 0.05, corrected. Coordinates are the center of mass in MNI.
Fig. 2.
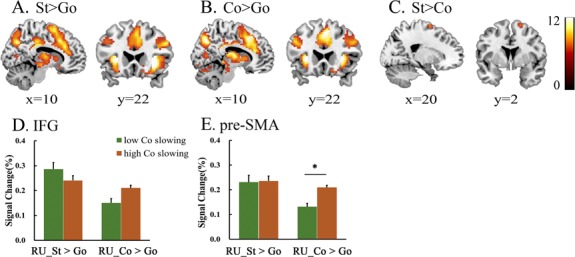
Group-level activations for A. Stop trials (combined RR and RU) vs go trials (St > Go); B. Continue trials (combined RR and RU) vs go trials (Co > Go); C. Stop trials (combined RR and RU) vs continue trials (combined RR and RU) (St > Co). D and E. Different activity between the high-slowing and low-slowing groups in the IFG and pre-SMA for the two contrasts stop > go and continue > go during the RU condition. *P < 0.05 in activation between the two groups in the pre-SMA activation for continue vs go trials. Note: color bar shows a scale of the t values and all results were corrected at P < 0.05 by FWE.
Similarly, to identify the brain activation network specifically induced by the continue signal, a comparison between the continue trials and the go trials showed activation within the medial frontal cortex, including the ACC and pre-SMA, bilateral IFG, bilateral insula and thalamus (Table 2B and Figure 2B).
Neural activity in the contrast of stop and continue trials
One of the current main goals was to disentangle the effects related to the outright stopping of a motor response from the processing of the stop signal, which conflates processing associated with attentional capture with that associated with response inhibition. To isolate the neural response that supports action stopping, we directly compared the stop trials and continue trials, which provided a baseline that controls for the attentional processing of unexpected stimuli. The analysis revealed a higher activation of the medial frontal, including parts of the pre-SMA, in the stop trials (Table 2C and Figure 2C). There was no significant difference in the activation of the IFG in this contrast, indicating that this region did not specifically support the outright stopping of a motor response.
Moreover, the ROI analysis shows that the pre-SMA showed greater activation when participants slowed their responses [t(25) = 2.35, P = 0.027] (Figure 2E), and it also demonstrates the motor response function in this region. In contrast, no significant difference in the activation of the IFG was observed in the continue trials with different slowing groups (Figure 2D).
Neural activity associated with a reward effect
The foregoing analyses yielded clear evidence of the specific function of pre-SMA and IFG activity in stopping. We further evaluated reward effects on regional activity, and a whole brain contrast revealed differential functional recruitment during the execution of RR stop and continue trials relative to RU conditions. Activation was observed within the ACC, bilateral insula, bilateral IFG and caudate in RR stop trials. We also found activation in the pre-SMA, bilateral IFG, bilateral insula, ACC and caudate in RR continue trials. Note that the pre-SMA only increased activity in the continue trials (Table 3 and Figure 3A).
Table 3.
Brain regions associated with reward effect on stop and continue trials
| MNI coordinates | |||||||
|---|---|---|---|---|---|---|---|
| Region | Hemisphere | x | y | z | t | P | Extent (voxels) |
| A. RR stop trials > RU stop trials | |||||||
| Anterior cingulate | R | 9 | 36 | 21 | 9.12 | <0.001 | 199 |
| Thalamus | R | 3 | 48 | −3 | 7.92 | <0.001 | |
| Occipital lobe | R | 3 | 42 | 3 | 8.57 | <0.001 | |
| Parahippocampa gyrus | L | 6 | −3 | 12 | 8.04 | <0.001 | 21 |
| Hippocampus | L | −12 | −39 | 0 | 7.87 | <0.001 | |
| Cingulate gyrus | L | −24 | −30 | −6 | 7.66 | <0.001 | |
| Anterior cingulate | R | 9 | 21 | −6 | 11.64 | <0.001 | 246 |
| IFG | R | 27 | 21 | −18 | 10.38 | <0.001 | |
| R | 30 | 27 | −3 | 9.08 | <0.001 | ||
| Fusiform gyrus | L | −36 | −66 | −18 | 10.90 | <0.001 | 130 |
| R | 33 | −54 | −18 | 7.54 | 0.001 | 75 | |
| Caudate | L | −12 | 18 | −6 | 10.15 | <0.001 | 137 |
| L | −15 | 3 | −15 | 8.46 | <0.001 | ||
| IFG | L | −27 | 18 | −15 | 7.82 | <0.001 | |
| Hippocampus | R | 21 | −33 | 0 | 9.70 | <0.001 | 39 |
| R | 6 | −33 | 0 | 8.50 | <0.001 | ||
| R | 27 | −24 | −9 | 7.11 | 0.003 | ||
| Occipital lobe | R | 30 | −63 | −15 | 9.55 | <0.001 | 75 |
| Cingulate gyrus | R | 0 | −30 | 27 | 7.83 | <0.001 | 29 |
| Inferior parietal lobule | R | 6 | −18 | 30 | 7.12 | 0.003 | |
| R | 42 | −48 | 42 | 7.02 | 0.004 | 27 | |
| R | 33 | −45 | 42 | 7.01 | 0.004 | ||
| R | 42 | −57 | 42 | 6.87 | 0.006 | ||
| B. RR continue trials > RU continue trials | |||||||
| Medial frontal gyrus (pre-SMA) | R | 6 | 27 | 39 | 9.64 | <0.001 | 368 |
| Anterior cingulate | R | 3 | 45 | 12 | 9.16 | <0.001 | |
| Inferior occipital gyrus | R | 30 | −84 | −9 | 7.44 | 0.001 | 21 |
| Insula | R | 36 | 18 | −3 | 9.31 | <0.001 | 135 |
| L | −36 | 15 | −6 | 9.03 | <0.001 | 68 | |
| Occipital lobe | L | −9 | −99 | 3 | 11.88 | <0.001 | 405 |
| L | −27 | −93 | 3 | 8.84 | <0.001 | ||
| R | 21 | −96 | 3 | 9.57 | <0.001 | ||
| Inferior parietal lobule | R | 51 | −48 | 51 | 10.22 | <0.001 | 200 |
| R | 36 | −63 | 48 | 8.25 | <0.001 | ||
| Caudate | R | 9 | 12 | −3 | 9.71 | <0.001 | 67 |
| R | 15 | 21 | −15 | 7.08 | 0.003 | ||
| L | −9 | 12 | −12 | 7.37 | 0.002 | 24 | |
| L | −9 | 18 | −3 | 7.21 | 0.003 | ||
| IFG | R | 24 | 18 | −18 | 7.31 | 0.002 | 135 |
| L | −27 | 24 | −9 | 9.16 | <0.001 | 68 | |
| L | −15 | 21 | −18 | 7.24 | 0.002 | 24 | |
| Fusiform gyrus | L | −33 | −72 | −15 | 8.80 | <0.001 | 92 |
| L | −30 | −81 | −18 | 7.28 | 0.002 | ||
| R | 27 | −75 | −12 | 6.64 | 0.011 | 21 | |
| R | 33 | −69 | −21 | 8.22 | <0.001 | 68 | |
| R | 33 | −60 | −12 | 7.64 | <0.001 | ||
| Cingulate gyrus | R | 0 | −33 | 24 | 8.61 | <0.001 | 151 |
| R | 6 | 33 | 30 | 9.62 | <0.001 | 368 | |
| R | 6 | −15 | 30 | 8.40 | <0.001 | 151 | |
| L | 0 | −21 | 33 | 8.41 | <0.001 | ||
| Cerebellum posterior lobe | R | 36 | −57 | −21 | 7.15 | 0.003 | 68 |
All clusters reliable at P < 0.05, corrected. Coordinates are the center of mass in MNI.
Fig. 3.
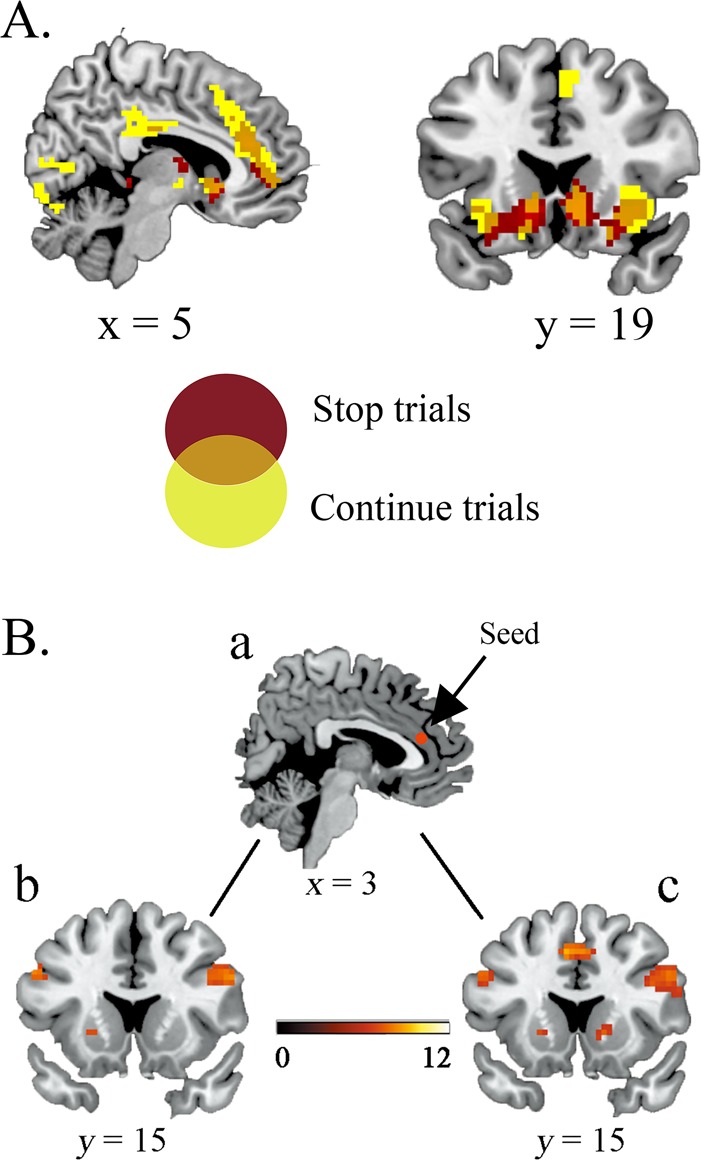
A. Overlap of stop trials and continue trials for RR vs RU contrast. Regions informative about the reward effect on stop trials are shown in red; regions informative about the reward effect on continue trials are shown in yellow. Overlapping regions informative about the reward effect on both stop trials and continue trials are shown in orange and indicate overlap in the right IFG/insula and striatum. The pre-SMA is only activated in continue trials. B. Distinct ACC–IFG and ACC–pre-SMA functional pathways in stop trials and continue trials for contrast RR vs RU. a. The ACC seed (x = 3, y = 36, z = 21). b. Increased ACC functional connectivity with the right IFG during stop trials. c. Increased ACC functional connectivity with the right IFG and pre-SMA during continue trials. Note: color bar shows a scale of the t values and all results were corrected at P < 0.05 by FWE.
Furthermore, a PPI analysis showed that increased ACC activity during the stop trials functionally coupled the bilateral IFG activity to a greater degree in the RR vs the RU condition (Table 4A and Figure 3Bb). The ACC also exhibited increased positive coupling with the bilateral IFG and pre-SMA in the continue trials (Table 4B and Figure 3Bc). Note that there was no significant covariation of ACC and pre-SMA observed during the stop trials at a corrected FWE P < 0.05 threshold.
Table 4.
Regions in which functional connectivity strengths with ACC were significantly related to reward effect
| MNI coordinates | |||||||
|---|---|---|---|---|---|---|---|
| Region | Hemisphere | x | y | z | t | P | Extent (voxels) |
| A. Stop trials | |||||||
| Occipital lobe | R | 45 | −69 | −9 | 11.12 | <0.001 | 384 |
| L | −45 | −69 | −12 | 9.82 | <0.001 | 468 | |
| Fusiform gyrus | R | 51 | −57 | −18 | 8.64 | <0.001 | 384 |
| R | 48 | −39 | −18 | 8.33 | <0.001 | ||
| Parietal lobe | L | −27 | −63 | 42 | 9.65 | <0.001 | 161 |
| R | 33 | −69 | 27 | 9.02 | <0.001 | 273 | |
| IFG | L | −57 | 9 | 12 | 8.47 | <0.001 | 75 |
| L | −27 | 24 | 0 | 8.06 | <0.001 | 31 | |
| L | −42 | 36 | 15 | 8.03 | <0.001 | ||
| R | 54 | 18 | 30 | 7.98 | <0.001 | 67 | |
| B. Continue trials | |||||||
| Medial frontal gyrus (pre-SMA) | L | −6 | 15 | 45 | 9.04 | <0.001 | 100 |
| Caudate | L | −15 | 9 | 6 | 8.55 | <0.001 | 35 |
| Occipital lobe | L | −42 | −75 | −9 | 12.72 | <0.001 | 1093 |
| R | 45 | −69 | −9 | 12.25 | <0.001 | 996 | |
| IFG | L | −57 | 9 | 12 | 9.44 | <0.001 | 109 |
| L | −42 | 39 | 15 | 8.19 | <0.001 | 33 | |
| R | 51 | 15 | 33 | 7.96 | <0.001 | 98 | |
| Middle frontal gyrus | L | −30 | −6 | 63 | 7.88 | <0.001 | 70 |
| R | 36 | −3 | 63 | 7.56 | <0.001 | 30 | |
All clusters reliable at P < 0.05, corrected. Coordinates are the center of mass in MNI.
Discussion
Response inhibition not only involves motor inhibition but also requires attending to task-relevant signals and planning and executing appropriate actions (Sharp et al., 2010; Xu et al., 2017). In the present study, we introduced the continue trials to control attentional capture and investigated the effect of reward on response inhibition directly or on response inhibition mediated by the attentional capture. The results support two main conclusions. First, they provide further evidence of brain activity related to two distinct cognitive processes that are involved in response inhibition. Successful inhibition was associated with a simultaneous increase in the activity of the IFG and pre-SMA. Importantly, only the increased pre-SMA activation reflected an outright stopping of a motor response from a high-level baseline that controlled for attentional capture. Second, the results provide strong evidence that the behavioral performance improvements observed in the stop trials with reward were attributable to enhanced attentional capture during the SST.
A series of lesion and functional neuroimaging studies demonstrate that the pre-SMA is necessary for stopping actions (Neubert et al., 2010; Aron, 2011; Coxon et al., 2012; Nettekoven et al., 2014; Rae et al., 2015; Watanabe et al., 2015). In line with previous studies, we observed a significant increase in pre-SMA activation in the stop trials compared to the continue trials. Thus, the pre-SMA appears to be critical for response inhibition. The introduction of the continue trials also allowed for an exploration of the neural activity associated with the slowing of a motor response. The RT for the continue trials was longer than that for the go trials. This result is concordant with the notion that the infrequent and unexpected continue signal triggered a degree of inhibitory processing that delayed the motor response (Sharp et al., 2010; Lee et al., 2016). The activation of the pre-SMA differentiated between the low-slowing and high-slowing groups, indicating that the pre-SMA is sensitive to an increase in response control demands. Crucially, attentional capture is distinct from stopping motor actions in response inhibition paradigms (Sharp et al., 2010; Erika-Florence et al., 2014; Meffert et al., 2016; Duverne and Koechlin, 2017; Xu et al., 2017). The observation that both the stop and continue trials preferentially activate IFG demonstrates a role for this region in attentional capture. Likewise, recent studies have reported that the IFG is recruited during a range of attentionally demanding conditions that have no obvious requirement for response inhibition (Hampshire et al., 2010; Erika-Florence et al., 2014; Hampshire, 2015; Hampshire and Sharp, 2015). The researchers account for this pattern by postulating that an increased right IFG reflects the attentional capture of task-relevant stimuli rather than the response inhibition per se. Moreover, we extend these results by demonstrating that reward further increases activation in this region and enhances behavioral performance.
Behavioral data revealed a significantly shorter SSRT in RR stop trials compared to RU stop trials. This result implies a reward facilitation effect on response inhibition. Considering the processing of stop trials, motor activity is triggered first by the go signal, and the inhibition reaction is triggered subsequently by the presentation of the stop signal. Successful response inhibition depends on whether the infrequent stop signal can be detected in a stream of frequent stimuli so that it can enable individuals to rapidly implement the stop process and withhold the already initiated motor activity. In this process, attentional processing is crucial because the attentional capture of the stop signal must occur before any hypothetical inhibitory mechanism can conceivably have an impact (Salinas and Stanford, 2013). Accordingly, it is important to acknowledge that reward exerts a powerful influence on attentional processing. When stimuli associated with reward become salient and attention drawing (Anderson et al., 2011; Hickey et al., 2015; Barbaro et al., 2017), participants paid increased attention to the RR stop signal, which was thereby better detected and showed further improved inhibitory performance. Indeed, contrasting the RR and RU stop trials revealed activation in the IFG, which reflects a higher attention in the RR stop signals. Similarly, reward also increased the ACC–IFG connection in which the ACC and IFG have both been implicated in the attentional processing of task-relevant visual information. With this result, it seems plausible that the reward facilitation effect was indirect in that it involved attentional processing. Notably, the stop process is modeled as a race, with the independent processes of ‘Go’ and ‘Stop’ competing for earlier completion. The results of magnetoencephalography experiments suggest that the race is influenced at an early stage of the sensory processing of go and stop signal (Boehler et al., 2009). Additionally, recent computational work has shown that the outcome of rapid sensory detection determines whether the completion of the stop process is a success (Salinas and Stanford, 2013). These studies propose the notion that the attentional processing of task-relevant stimuli, rather than the stop process per se, may be a critical factor in the improvement of response inhibition (Boehler et al., 2009; Salinas and Stanford, 2013). RR stop signals capture attention more rapidly; furthermore, the RR stop process is initiated and completed faster, and the response is successfully inhibited. The present results support the notion that reward improves response inhibition by enhancing attentional capture.
Moreover, we found a significantly longer RT in RR than in RU continue trials. For the purpose of achieving a higher success rate in RR stop trials, we consider that participants would become more cautious to discriminate the signal and to delay the response when facing an RR signal. Importantly, this delay induced the increase in IFG activation, pre-SMA activation and the ACC–pre-SMA connection. As mentioned above, the RR signal was more salient and attention drawing, and so the RR continue signal increased attentional capture-related activation in the IFG. Activation of the pre-SMA associated with behavioral delay had the same result as that in the preceding analysis, which found that this region differentiated between the low-slowing and high-slowing groups. These results support our hypothesis that the pre-SMA is efficient for the direct control of action stopping and for delaying a response. In addition, the ACC has been shown to predict action outcomes and to determine task-set selection (Alexander and Brown, 2012). For instance, a number of studies suggest that the ACC contributes to cognitive control by tracking the reward benefit to switch away rather than to stay on the ongoing task set (Boorman et al., 2013; Duverne and Koechlin, 2017). In the current study, the ACC along with the pre-SMA regulates the behavioral selection in which the ACC encodes the reward and selects the stimulus-related behavior and then signals the pre-SMA to maintain, initiate and implement appropriate goal-directed actions.
Taken together, our findings highlight the processing of the stop signal, which is involved in attentional capture, motor control and the functional distinction between the IFG and the pre-SMA, with the IFG demonstrating more activation related to attentional capture and the pre-SMA playing a more direct role in motor control. Specifically, reward increases attentional capture-related activation in the IFG, which further improves the performance of response inhibition. In brief, attention processing is a crucial factor in inhibition control, and the RR stimulus captures more attention, thus indirectly contributing to the enhancement of response inhibition. Based on this finding, we might conjecture that a dysfunction of inhibitory control may be compounded by other factors such as attentional capture rather than a dysfunction in motor inhibition per se (O'Connor et al., 2015). Indeed, recent computational work revealed that deficits characterized by response inhibition could be explained by factors such as motivation and attentional processing and their corresponding neural mechanisms (Wiecki and Frank, 2013). Our investigation not only provides useful insights into the underlying processes and mechanisms of response inhibition but also enhances our knowledge of the reward facilitation effect on response inhibition.
Funding
This work was supported by grants from the National Natural Science Foundation of China (61431013, 31771254) and the Fundamental Research Funds for the Central Universities of PR China (SWU1609106, SWU1709107).
References
- Alexander W.H., Brown J.W. (2012). Medial prefrontal cortex as an action-outcome predictor. Nature Neuroscience, 14(10), 1338–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson B.A., Laurent P.A., Yantis S. (2011). Value-driven attentional capture. Proceedings of the National Academy of Sciences of the United States of America, 108(25), 10367–10371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aron A.R. (2011). From reactive to proactive and selective control: developing a richer model for stopping inappropriate responses. Biological Psychiatry ,69(12), e55–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aron A.R., Poldrack R.A. (2006). Cortical and subcortical contributions to stop signal response inhibition: role of the subthalamic nucleus. Journal of Neuroscience, 26(9), 2424–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbaro L., Peelen M.V., Hickey C. (2017). Valence, not utility, underlies reward-driven prioritization in human vision. Journal of Neuroscience, 37(43), 1128–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bledowski C., Prvulovic D., Goebel R., Zanella F.E., Linden D.E. (2004). Attentional systems in target and distractor processing: a combined ERP and fMRI study. NeuroImage, 22(2), 530–40. [DOI] [PubMed] [Google Scholar]
- Boehler C.N., Hopf J.-M., Stoppel C.M., Krebs R.M. (2012). Motivating inhibition—reward prospect speeds up response cancellation. Cognition, 125(3), 498–503. [DOI] [PubMed] [Google Scholar]
- Boehler C.N., Muente T.F., Krebs R.M., Heinze H.J., Schoenfeld M.A., Hopf J.M. (2009). Sensory MEG responses predict successful and failed inhibition in a stop-signal task. Cerebral Cortex, 19(1), 134–45. [DOI] [PubMed] [Google Scholar]
- Boehler C.N., Schevernels H., Hopf J.M., Stoppel C.M., Krebs R.M. (2014). Reward prospect rapidly speeds up response inhibition via reactive control. Cognitive, Affective & Behavioral Neuroscience, 14(2), 593–609. [DOI] [PubMed] [Google Scholar]
- Boorman E.D., Rushworth M.F., Behrens T.E. (2013). Ventromedial prefrontal and anterior cingulate cortex adopt choice and default reference frames during sequential multi-alternative choice. Journal of Neuroscience, 33(6), 2242–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botvinick M., Braver T. (2015). Motivation and cognitive control: from behavior to neural mechanism. Annual Review of Psychology, 66(1), 83–113. [DOI] [PubMed] [Google Scholar]
- Brett M., Anton J., Valabregue R., Poline J. (2002). Region of interest analysis using an SPM toolbox. NeuroImage, 16(2), 210–7. [Google Scholar]
- Cai Y., Li S., Liu J., et al. (2016). The role of the frontal and parietal cortex in proactive and reactive inhibitory control: a transcranial direct current stimulation study. Journal of Cognitive Neuroscience, 28(1), 177–86. [DOI] [PubMed] [Google Scholar]
- Camille N., Tsuchida A., Fellows L.K. (2011). Double dissociation of stimulus-value and action-value learning in humans with orbitofrontal or anterior cingulate cortex damage. Journal of Neuroscience, 31(42), 15048–15052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chikazoe J., Jimura K., Asari T., et al. (2009). Functional dissociation in right inferior frontal cortex during performance of go/no-go task. Cerebral Cortex, 19(1), 146–52. [DOI] [PubMed] [Google Scholar]
- Corbetta M., Patel G., Shulman G.L. (2008). The reorienting system of the human brain: from environment to theory of mind. Neuron, 58(3), 306–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coxon J.P., Goble D.J., Leunissen I., Van Impe A., Wenderoth N., Swinnen S.P. (2016). Functional brain activation associated with inhibitory control deficits in older adults. Cerebral Cortex, 26(1), 12–22. [DOI] [PubMed] [Google Scholar]
- Coxon J.P., Van Impe A., Wenderoth N., Swinnen S.P. (2012). Aging and inhibitory control of action: cortico-subthalamic connection strength predicts stopping performance. Journal of Neuroscience, 32(24), 8401–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David W., Carlo R., Ida M., Thorsten K., John-Dylan H. (2015). The role of the parietal cortex in the representation of task-reward associations. Journal of Neuroscience, 35(36), 12355–12365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodds C.M., Morein-Zamir S., Robbins T.W. (2011). Dissociating inhibition, attention, and response control in the frontoparietal network using functional magnetic resonance imaging. Cerebral Cortex, 21(5), 1155–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duverne S., Koechlin E. (2017). Rewards and cognitive control in the human prefrontal cortex. Cerebral Cortex, 27(10), 5024–39. [DOI] [PubMed] [Google Scholar]
- Erika-Florence M., Leech R., Hampshire A. (2014). A functional network perspective on response inhibition and attentional control. Nature Communications, 5(5), 4073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etzel J.A., Cole M.W., Zacks J.M., Kay K.N., Braver T.S. (2016). Reward motivation enhances task coding in frontoparietal cortex. Cerebral Cortex, 26(4), 1647–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman S.M., Aron A.R. (2016). Withholding a reward-driven action: studies of the rise and fall of motor activation and the effect of cognitive depletion. Journal of Cognitive Neuroscience, 28(2), 237–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman S.M., Razhas I., Aron A.R. (2014). Top–down response suppression mitigates action tendencies triggered by a motivating stimulus. Current Biology, 24(2), 212–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampshire A. (2015). Putting the brakes on inhibitory models of frontal lobe function. NeuroImage, 113, 340–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampshire A., Chamberlain S.R., Monti M.M., Duncan J., Owen A.M. (2010). The role of the right inferior frontal gyrus: inhibition and attentional control. NeuroImage, 50(3), 1313–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampshire A., Sharp D.J. (2015). Contrasting network and modular perspectives on inhibitory control. Trends in Cognitive Sciences, 19(8), 445–52. [DOI] [PubMed] [Google Scholar]
- Hayden B.Y., Heilbronner S.R., Pearson J.M., Platt M.L. (2011). Surprise signals in anterior cingulate cortex: neuronal encoding of unsigned reward prediction errors driving adjustment in behavior. Journal of Neuroscience, 31(11), 4178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickey C., Kaiser D., Peelen M. (2015). Neural mechanisms of incentive salience in naturalistic human vision. Neuron, 15(12), 512–8. [DOI] [PubMed] [Google Scholar]
- Kim H. (2014). Involvement of the dorsal and ventral attention networks in oddball stimulus processing: a meta-analysis. Human Brain Mapping, 35(5), 2265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krebs R.M., Boehler C.N., Appelbaum L.G., Woldorff M.G. (2013). Reward associations reduce behavioral interference by changing the temporal dynamics of conflict processing. PLoS One, 8(1), e53894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krebs R.M., Boehler C.N., Egner T., Woldorff M.G. (2011). The neural underpinnings of how reward associations can both guide and misguide attention. Journal of Neuroscience, 31(26), 9752–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H.W., Lu M.S., Chen C.Y., Muggleton N.G., Hsu T.Y., Juan C.H. (2016). Roles of the pre-SMA and rIFG in conditional stopping revealed by transcranial magnetic stimulation. Behavioural Brain Research, 296(1), 459–67. [DOI] [PubMed] [Google Scholar]
- Li C.S., Huang C., Constable R.T., Sinha R. (2006). Imaging response inhibition in a stop-signal task: neural correlates independent of signal monitoring and post-response processing. Journal of Neuroscience, 26(1), 186–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meffert H., Hwang S., Nolan Z.T., Chen G., Blair J.R. (2016). Segregating attention from response control when performing a motor inhibition task: segregating attention from response control. NeuroImage, 126, 27–38. [DOI] [PubMed] [Google Scholar]
- Nettekoven C., Volz L.J., Kutscha M., et al. (2014). Dose-dependent effects of theta burst rTMS on cortical excitability and resting-state connectivity of the human motor system. Journal of Neuroscience, 34(20), 6849–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neubert F.X., Mars R.B., Buch E.R., Olivier E., Rushworth M.F. (2010). Cortical and subcortical interactions during action reprogramming and their related white matter pathways. Proceedings of the National Academy of Sciences of the United States of America, 107(30), 13240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Connor D.A., Upton D.J., Moore J., Hester R. (2015). Motivationally significant self-control: enhanced action withholding involves the right inferior frontal junction. Journal of Cognitive Neuroscience, 27(1), 112–23. [DOI] [PubMed] [Google Scholar]
- Pessoa L., Engelmann J.B. (2010). Embedding reward signals into perception and cognition. Frontiers in Neuroscience, 4(17), 4–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rae C.L., Hughes L.E., Anderson M.C., Rowe J.B. (2015). The prefrontal cortex achieves inhibitory control by facilitating subcortical motor pathway connectivity. Journal of Neuroscience, 35(2), 786–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salinas E., Stanford T.R. (2013). The countermanding task revisited: fast stimulus detection is a key determinant of psychophysical performance. Journal of Neuroscience, 33(13), 5668–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scangos K.W., Stuphorn V. (2010). Medial frontal cortex motivates but does not control movement initiation in the countermanding task. Journal of Neuroscience, 30(5), 1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp D.J., Bonnelle V., De Boissezon X., et al. (2010). Distinct frontal systems for response inhibition, attentional capture, and error processing. Proceedings of the National Academy of Sciences of the United States of America, 107(13), 6106–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shenhav A., Botvinick M., Cohen J. (2013). The expected value of control: an integrative theory of anterior cingulate cortex function. Neuron, 79(2), 217–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verbruggen F., Chambers C.D., Logan G.D. (2013). Fictitious inhibitory differences: how skewness and slowing distort the estimation of stopping latencies. Psychological Science, 24(3), 352–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verbruggen F., Logan G.D. (2009). Models of response inhibition in the stop-signal and stop-change paradigms. Neuroscience Behavioral Review, 33(5), 647–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe T., Hanajima R., Shirota Y., et al. (2015). Effects of rTMS of pre-supplementary motor area on fronto basal ganglia network activity during stop-signal task. Journal of Neuroscience, 35(12), 4813–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiecki T.V., Frank M.J. (2013). A computational model of inhibitory control in frontal cortex and basal ganglia. Psychological Review, 120(2), 329–55. [DOI] [PubMed] [Google Scholar]
- Xu K.Z., Anderson B.A., Emeric E.E., et al. (2017). Neural basis of cognitive control over movement inhibition: human fMRI and primate electrophysiology evidence. Neuron, 96(6), 1447–58. [DOI] [PMC free article] [PubMed] [Google Scholar]


