Abstract
Introduction
Neoadjuvant therapy prior to oesophagogastric resection is the gold standard of care for patients with T2 and/or nodal disease. Despite this, studies have taught us that chemotherapy decreases patients’ functional capacity as assessed by cardiopulmonary exercise (CPX) testing. We aim to show that a multimodal prehabilitation programme, comprising supervised exercise, psychological coaching and nutritional support, will physically, psychologically and metabolically optimise these patients prior to oesophagogastric cancer surgery so they may better withstand the immense physical and metabolic stress placed on them by radical curative major surgery.
Methods and analysis
This will be a prospective, randomised, controlled, parallel, single-centre superiority trial comparing a multimodal ‘prehabilitation’ intervention with ‘standard care’ in patients with oesophagogastric malignancy who are treated with neoadjuvant therapy prior to surgical resection. The primary aim is to demonstrate an improvement in baseline cardiopulmonary function as assessed by anaerobic threshold during CPX testing in an interventional (prehab) group following a 15-week preoperative exercise programme, throughout and following neoadjuvant treatment, when compared with those that undergo standard care (control group). Secondary objectives include changes in peak oxygen uptake and work rate (total watts achieved) at CPX testing, insulin resistance, quality of life, chemotherapy-related toxicity and completion, nutritional assessment, postoperative complication rate, length of stay and overall mortality.
Ethics and dissemination
This study has been approved by the London-Bromley Research Ethics Committee and registered on ClinicalTrials.gov. The results will be disseminated in a peer-reviewed journal.
Trial registration number
NCT02950324; Pre-results.
Keywords: prehabilitation, oesophageal, cancer, neoadjuvant, cardiopulmonary exercise test
Strengths and limitations of this study.
To our knowledge, no studies assessing the feasibility of a supervised exercise programme during chemotherapy before surgery for oesophagogastric cancer have been published.
This is a prospective, parallel, randomised controlled trial with patients randomised in a 1:1 manner and subjects analysed on an intention-to-treat basis.
The exercise component of the prehabilitation programme is supervised by a clinical exercise scientist who will construct a rigorous, tailored, individual, exercise programme for each patient based on their baseline functional capacity as assessed by cardiopulmonary exercise (CPX) testing.
CPX is established, non-invasive and safe and may be considered the ‘gold standard’ method of assessing patients’ cardiopulmonary reserve prior to surgery. CPX outcome measures will be objectively measured by an experienced consultant anaesthetist, external to the trial study group.
This unblinded, single-centre trial has a relatively small sample size is powered for anaerobic threshold and not clinical outcomes.
Introduction
As a result of the Medical Research Council Adjuvant Gastric Infusional Chemotherapy (MAGIC)1 and Medical Research Council (OEO2)2 trials, neoadjuvant therapy followed by surgery gives the best chance of cure for patients diagnosed with locally advanced oesophagogastric cancer. It aims to increase the chance of curative resection by eliminating micrometastases, downsizing the tumour and increasing the R0 resection rate.1 3 4 The ongoing open-label, phase III Neo-AEGIS trial,5 compares preoperative and postoperative chemotherapy and neoadjuvant chemoradiotherapy as per the MAGIC and CROSS6 protocols, respectively.
Adequate cardiopulmonary function is of great importance to patients undergoing oesophagogastric cancer surgery such as Ivor Lewis oesophagectomy, as this major two-stage, two-field elective operation is associated with a large metabolic stress response and significant morbidity.7 Reported side effects of chemotherapy are a reduction in functional capacity, which can be objectively measured using cardiopulmonary exercise testing (CPX). CPX is an established, non-invasive and safe method of assessing patients’ cardiopulmonary reserve prior to surgery. Both anaerobic threshold (AT) and peak oxygen uptake (peak VO2) have consistently been associated with morbidity and functional outcomes in patients undergoing major elective surgery,8–11 with a reported average decrease in AT of 2 mL/kg/min in patients undergoing neoadjuvant chemotherapy (NAC) prior to oesophagectomy. Furthermore, this decrease in fitness has been associated with diminished 1-year survival in these patients.12
The emerging concept of ‘prehabilitation’ is the process of enhancing an individual’s functional capacity to enable them to withstand a stressful event such as major elective surgery. A key component of prehabilitation, physical exercise training, has led to improvements in AT.13 14 When initiated in the neoadjuvant setting, prehabilitation may have important implications as exercise training can stimulate skeletal muscle (SM) adaptations such as increased mitochondrial content and improve oxygen uptake capacity.15 Both West et al 16 and Heldens et al 17 have demonstrated that an exercise programme during neoadjuvant therapy for cancer is feasible, with minimal patient drop-out.
Another key component of prehabilitation is a psychological intervention—‘Medical Coaching’. Anxiety and depression are commonplace in patients receiving cancer treatment and depression and may be associated with reduced treatment compliance.18 Psychological support aims to reduce anxiety and depression prior to surgery.19 20 It has been suggested that improvement in exercise capacity during the preoperative period may be a result of the belief of patients that fitness levels aid recovery.21 Using Bandura’s Social Cognitive Theory, it is proposed that psychological coaching can lead to an increase in self-belief to carry out a particular task and that it will empower patients to proactively take control of their behaviour preoperatively, leading to improved engagement with the exercise aspect of the intervention.22
In addition to cardiopulmonary function and anxiety, the physiological stress of surgery is associated with various metabolic derangements, central to which is the development of insulin resistance (IR). The degree of IR appears to be related to the magnitude of the ‘surgical stress’. IR may be one of the key mechanisms triggering major inflammatory complications following surgery.23 24
Sarcopenia, the involuntary loss of muscle mass, is readily induced as a result of chemotherapy. Oesophagogastric cancer patients with signs of sarcopenia have been shown to have high rates of treatment drop-out, higher postoperative complication rates and reduced overall survival.25 26
To our knowledge, there are no published studies assessing the feasibility of a supervised exercise programme during chemotherapy before surgery for oesophagogastric cancer. The primary aim is to demonstrate an improvement in baseline cardiopulmonary function as assessed by AT during CPX testing in an interventional (prehab) group following a 15-week preoperative exercise programme, throughout and following neoadjuvant treatment, when compared with those that undergo standard care (control group).
Methods and analysis
Study setting
This study is a prospective, randomised, controlled, parallel, open single-centre superiority trial that will compare ‘prehabilitation’ with ‘standard care’ in patients with oesophagogastric cancer who are treated with NAC or chemoradiotherapy (as part of the Neo-AEGIS trial) prior to surgical resection. The trial and treatment will be conducted at the Royal Surrey County Hospital (UK), a tertiary referral centre for oesophagogastric malignancy.
The research team attended the Oesophageal Patient Association Support Group where they were able to learn and understand about patients' previous experiences of cancer treatment. Patients’ experiences and views were taken into account when writing the study protocol to include the content of the prehab programme and mode of intervention delivery. The team were able to engage and empower the patient to contribute to the construction of a patient-centred trial.
Study objectives
In the intervention (prehab) group, the primary objective is to demonstrate an improvement in baseline AT following a 15-week preoperative exercise programme that will take place throughout NAC and during the 6-week period of recovery prior to surgical resection. AT will be compared with those that undergo standard care (the control group).
Secondary objectives will include assessment of the protocol feasibility (as determined by subject drop-out, and both attendance, and adherence to prehab exercise sessions). Alternative measures of functional reserve will be evaluated, in particular change in peak VO2 and work rate (total watts achieved) during CPX testing. The effect of a prehab programme on IR will be assessed by the Homeostasis Model Assessment (HOMA2) calculation. Further secondary objectives include the effect of the prehab programme on chemotherapy-related toxicities, tolerance and completion rates, the impact of preoperative psychological coaching on validated quality of life (QoL) scores (EORTC QLQ-C30, EORTC QLQ-OG25, Beck Anxiety Inventory (BAI)) and Beck Depression Inventory (BDI II)) and the effect of prehabilitation on nutritional status as assessed using handgrip strength (HGS) and sarcopenia. Postoperative complications will be assessed using the Clavien-Dindo classification and as agreed per the Esophagectomy Complications Consensus Group.27 Length of intensive care and hospital stay, 30-day, 90-day, 1-year and 5-year mortality will also be analysed.
Inclusion and exclusion criteria
Patients with T2 and/or N1 resectable oesophagogastric carcinoma being considered for neoadjuvant therapy prior to oesophagogastrectomy or extended total gastrectomy will be included. Patients will be excluded if they fulfil one or more of the following criteria: <18 years of age, a known contraindication to CPX testing (eg, unstable cardiac disease), a physical inability to perform CPX testing or undertake a prehabilitation exercise programme (eg, lower limb dysfunction), pregnancy (or those planning to become pregnant) or a lack of capacity to give informed consent.
Guidelines to cessation of participation in the study will include withdrawal of patient consent, serious adverse event and non-compliance. Decision for patient withdrawal will be made by the chief investigator (CI) in conjunction with the trial sponsor. In the case of withdrawal, the patient will continue standard treatment within the dedicated oesophagogastric and oncological departments.
Interventions
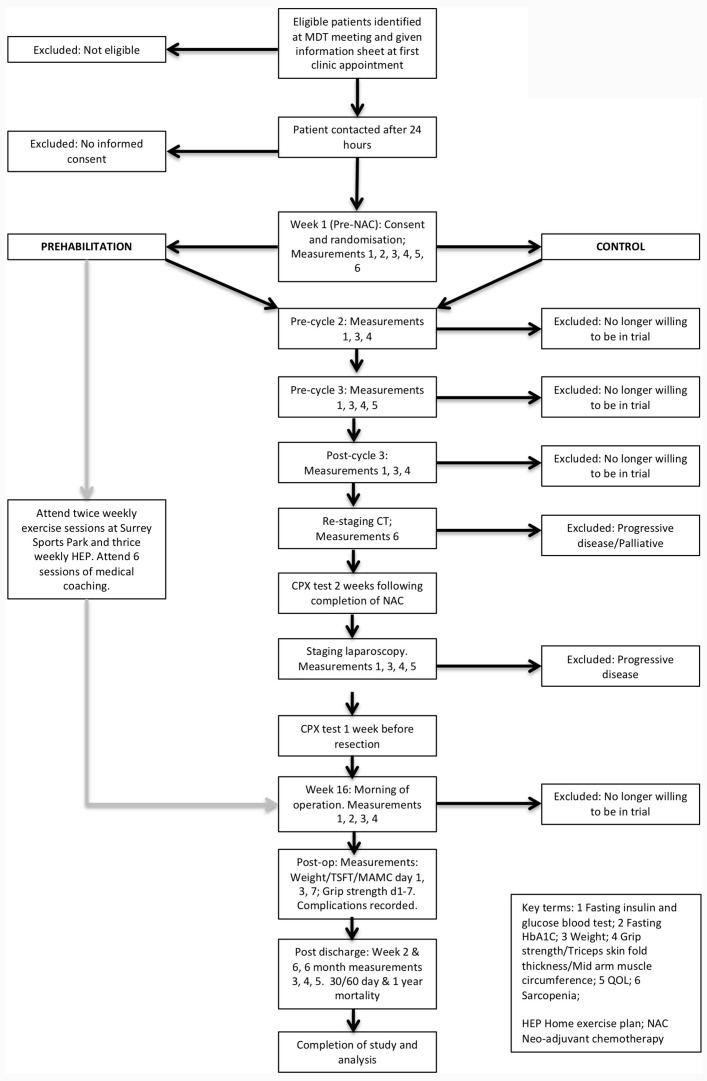
Following a dedicated oesophagogastric staging pathway, including anaesthetic and cancer multidisciplinary team (MDT) discussions, all patients whose proposed treatment includes neoadjuvant therapy and surgery will undergo a baseline CPX test as part of standard care. Here, eligibility will be assessed. At the next consultation (surgical or oncological outpatient clinic appointment), eligible patients will be approached by the CI or clinical supervisor (CS) in order to confirm inclusion and exclusion criteria. Patients will at this stage be invited to participate in the study (online supplementary appendix 1). If interested, one of the above research team members will explain the study to the patient and give them a copy of the patient information sheet to review. The patient will be given the opportunity to ask any questions they may have about the study and will be given at least 24 hours to consider participation. The research team will emphasise that non-participation will not adversely affect any aspects of their care. The patient will attend for prechemotherapy blood tests as part of their standard care pathway. At this time, the patient will be invited to give written consent to the trial. Patients will be informed that they are free to withdraw at any time without giving a reason and again that this will not adversely affect any aspects of their care. If the patient is willing to provide informed consent, they will be asked to sign the patient consent form. Consent will be obtained by a suitably qualified person in accordance with international Good Clinical Practice (GCP) guidelines. The patient will be randomised to the intervention (prehab) or control group by the consenting clinician (see ‘Methodology and Study Design’ below).
bmjopen-2018-023190supp001.jpg (990.3KB, jpg)
Study group
Prehab group
Exercise intervention
Over a 15-week period, patients will attend the Human Performance Institute at Surrey Sports Park for twice weekly 1-hour exercise sessions (30 sessions in total) supervised directly by a clinical exercise scientist with expertise in cancer care.
The exercise programme will consist of cardiorespiratory, resistance and flexibility training in accordance with the American College of Sports Medicine guidelines.28
At the first supervised exercise session, patients will be counselled by the trainer and issued with FitBit Flex2 (FitBit, USA) physical activity monitor. The trainer will construct a tailored programme for each patient based on their baseline (prechemotherapy) cardiopulmonary exercise test performance and calculated heart rate reserve (HRR).
During the aerobic training component, the intensity of cardiorespiratory exercise will be monitored every 5 min using the BORG rating of perceived exertion scale (RPE) (Borg G. Borg’s Perceived Exertion and Pain Scales. Human Kinetics 1998; pp. 46), with power output on the cycle ergometer (Ergoline, Lovemedical, UK) controlled within the ranges of 11 (‘Fairly light’) and 14 (‘Somewhat hard/Hard’) on the BORG scale. Heart rate will be recorded every 5 min using a Polar HR monitor (Polar FT1, Polar, UK). The trainer will aim for the patient to complete 20 min of cycling at an incremental increase from 40% HRR to 60% HRR over the duration of the course.
The resistance exercises performed will provide stimulus to each of the major muscle groups. Resistance training will be of a sufficient intensity tailored to each individual patient to enhance strength, muscular endurance and maintain fat-free mass with a progressive approach to exercise training over the 15 weeks. Two sets of 12 repetitions of each exercise will be performed. Flexibility exercises will be incorporated into the overall fitness programme sufficient to develop and maintain range of motion including appropriate static and/or dynamic stretches. Resistance exercises will be scored on a rating of perceived exertion scale; when the score drops below 12 for a given exercise, the intensity of resistance will be increased.
Patients will also undergo a Home Exercise Plan (HEP) for 1 hour, three times a week. The HEP will focus on resistance and core stability exercises and will be monitored via a patient-maintained diary.
Throughout the duration of the prehabilitation programme, all patients will be asked to wear a Fitbit Flex2 physical activity monitor on their non-dominant wrist as an objective measure of background activity. The clinical exercise scientist will record weekly steps at their supervised exercise sessions. They will also monitor session compliance.
Psychological (medical coaching) intervention
In conjunction with The Fountain Centre (St Luke’s Cancer Centre, Guildford, UK), patients will undergo six medical coaching sessions during their neoadjuvant treatment. The team consists of professional medical coaches with over 200 hours’ experience in coaching individuals with medical conditions. They are accredited with the international and UK coaching bodies, International Coaching Federation and National Council of Psychotherapists. Sessions will take the following form: discussion of medical and health status; strengths recognition; resilience profiling and development; social and support systems; emotional management; and goal setting. The medical coach will provide suggestions on how to enhance and reinforce patients’ motivation to comply with the exercise aspect of the intervention.
Nutritional support
Nutrition is of great importance to this cohort of patients as they are often malnourished and cachexic at presentation. The Trust employs 2.4 equivalent specialist dieticians per 60 cancer resections who are highly trained in the field of oesophagogastric surgery and have extensive experience in the management of complex nutritional problems related to the disease. All patients will receive frequent, tailored dietetic input, with calorie and protein intake increase where appropriate.
In order to minimise the number of appointments required to attend by the prehab group, where possible, supervised exercise sessions will be scheduled for the day of a preexisting oncology or surgical appointment. Following a face-to-face meeting with the medical coach, meetings will take place according to the patient’s preference, either in person (following on from their supervised exercise session) or via teleconference (eg, Skype).
Control group
The control group will not receive a prehabilitation intervention but will be treated according to the standard oesophagogastric (OG) care pathway. As part of usual care, all patients will be fully informed to improve fitness levels and to maintain a healthy lifestyle prior to surgery in order to obtain the best outcomes from high-risk surgery. Patients will continue to be offered standard dietetic and Clinical Nurse Specialist (CNS)-led psychological support as per the hospital’s current cancer pathway and standard of care. Patients will be asked to wear a Fitbit Flex2 physical activity monitor throughout their preoperative treatment. As an objective measure of background activity, weekly steps will be recorded by a member of the study’s delegation log. Nutritional support will be as per the standard pathway with regular telephone calls and specialist oesophagogastric dietetic consultations.
The control group will not be required to attend any extra appointments as outcome measures will be performed at the time of a prescheduled routine appointment (with the oncologist or surgeon).
Study outcomes
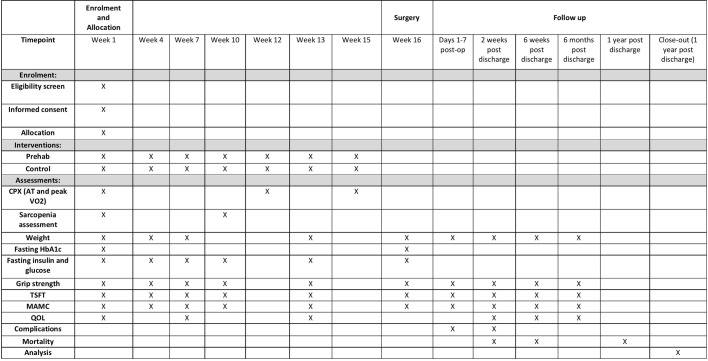
The primary outcome (change in AT) will be measured by an incremental symptom-limited CPX test performed by an experienced consultant anaesthetist. All patients will undergo CPX testing at baseline (before the start of neoadjuvant therapy), 2 weeks following completion of NAC and 1 week prior to surgery. Other CPX outcomes (peak VO2 and total work rate) will also be analysed.
Feasibility will be assessed by monitoring patient attendance at exercise and medical coaching sessions and adherence to the supervised exercise programme, as well as patient drop-out. Adherence to home exercise sessions will be monitored by a patient-reported diary. Patients will be deemed compliant to the intervention if they complete >75% of scheduled prehabilitation sessions. Weekly steps recorded via a Fitbit Flex2 physical activity monitor.
IR will be measured using the HOMA2 calculation. All patients will undergo fasting paired insulin and glucose tests at five stages along the protocol pathway: (1) before NAC; (2) after cycle 1 of NAC; (3) after cycle 2 of NAC (or if having chemoradiotherapy, midway through chemoradiotherapy); (4) following completion of cycle 3 (or at the end of chemoradiotherapy); (5) at restaging laparoscopy; and (6) on the morning of surgical resection. In addition, glycated haemoglobin will be measured at baseline and on the day of oesophagectomy/total gastrectomy.
Completion of neoadjuvant therapy will be recorded in conjunction with the patient’s consultant oncologist who will be a member of the trial delegation log. Toxicity will be monitored between cycles and after completion of chemotherapy and will be graded according to the Common Terminology Criteria for Adverse Events V.5.0: mild (grade 1), moderate (grade 2), severe (grade 3) or life threatening (grade 4), with specific parameters according to the organ system involved.
QoL will be assessed at specific time points: (1) before commencement of NAC (baseline); (2) midway through NAC; (3) following NAC completion; (4) 2 weeks posthospital discharge; (5) 6 weeks postdischarge; and (6) 6 months following discharge. Validated questionnaires will include EORTC QLQ-C30, EORTC QLQ –OG25, BAI and BDI II.
Nutritional assessment will take the form of HGS, midarm muscle circumference (MAMC), triceps skin-fold thickness (TSFT) and sarcopenia. HGS, MAMC and TSFT will be measured at the same time points that preoperative blood tests are taken, and HGS will be measured twice daily on postoperative days 1–3 and once daily on days 4–7. HGS, MAMC and TSFT will be measured postoperatively at 2 weeks, 6 weeks and 6 months following hospital discharge. As part of standard care, patients undergo staging CT imaging at baseline and following neoadjuvant therapy. Sarcopenia will be measured using SliceOmatic software (Tomovision, Magog, Canada) at these two time points. At the L3 level, total SM, subcutaneous fat and visceral fat will be measured. Skeletal muscle index will be calculated as follows: SM/height(m)2. Measurements will be recorded by two individuals, one of whom will be external to the trial group.
Surgery will be performed in a standard manner by three experienced oesophagogastric consultants. All patients will spend a period of time on intensive care postoperatively and will follow a dedicated oesophagogastric Enhanced Recovery After Surgery pathway. Length of intensive care and hospital stay will be recorded, as will postoperative complications which will be measured using the Clavien-Dindo classification and as per the Esophagectomy Complications Consensus Group.23 Mortality will be assessed at 30 days, 90 days and 1 year postoperatively. Figures 1 and 2 demonstrate the flow of patients (figure 1) and study schedule (figure 2).
Figure 1.
Consort diagram. CPX, cardiopulmonary exercise; HEP, Home Exercise Plan; MDT, multidisciplinary team; MAMC, midarm muscle circumference; NAC, neoadjuvant chemotherapy; QoL, quality of life; TSFT, triceps skin-fold thickness.
Figure 2.
Study diagram.
Methodology and study design
This trial will be conducted in a single tertiary referral centre for oesophagogastric cancer, with all patients treated and followed up at the Royal Surrey County Hospital, Guildford UK, in conjunction with St Luke’s Cancer Centre. Full disease staging, a dedicated oesophagogastric cancer MDT meeting and assessment of eligibility will take place prior to patients being approached by the CI or CS. Patients will be informed of the trial protocol via face-to-face discussion and a written patient information leaflet. On inclusion and formal consent to the trial, patients will be randomised to receive the intervention (prehab) or standard care (control). Randomisation will be carried out by a designated member of staff who is not directly involved in the study. In order to yield 1:1 groups, he or she will use computer-generated variable block randomisation, with the group name (‘prehab’ or ‘control’) placed in sequentially numbered brown opaque envelopes. The envelopes will be kept in a locked drawer. On consent of a patient to the trial, the next envelope in sequence will be handed to the CI who will open the envelope in front of the patient. Due to the nature of the intervention, the research team and trial participants will not be blinded to the assigned arm of the trial. Outcome measures are described in detail above.
Statistical considerations
Estimation of sample size
It has been shown that AT improves following NAC as a result of a prehabilitation programme compared with standard care, with an AT difference of 2.12 mL/kg/min between prehab and control groups.16
To achieve a power of 80% and a significance level of 5% and to allow for confounding factors in a postchemotherapy population, we calculate that 48 patients (24 per group) need to be studied in order to detect an AT difference of 2 mL/kg/min between prehab and control group subjects. To allow for a 20% patient drop-out rate (due to non-compliance or side effects from chemotherapy), 24 patients will be required for each treatment group resulting in a total accrual of 58.
Statistical analysis
Data will be analysed on an intention to treat basis using SPSS software (V.24). With the exception of interim analysis, a p value of <0.05 will be considered significant. Normality of data will be determined by using the Shapiro-Wilk test. Baseline characteristics for the two groups will be compared and demonstrated using mean (±SD) or the median (with IQR) for continuous data. A mixed-measure analysis of variance will be employed for the primary outcome of AT as this will be recorded at three times points (baseline, 2 weeks following neoadjuvant therapy and 1 week prior to surgery). An unpaired Student’s t-test will compare AT and peak VO2 between the intervention (prehab) and control groups. A subgroup analysis will be performed, categorising patients into ‘low risk’ (AT >11 mL/kg/min, peak VO2 >800 mL/min/m2) and ‘high risk’ (AT <11 mL/kg/min; peak VO2 <800 mL/min/m2).29 30 Secondary outcomes including length of hospital stay, grip strength, QoL, Fitbit data and so on, will also be analysed using a Student’s t-test or Mann-Whitney U test. Survival data will be determined using the Kaplan-Meier curve. Interim analysis will be performed once primary outcome data is available for 26 subjects.
Patient and public involvement
The CI attended the Oesophageal Patient Association Support Group to engage and empower patients to help decide on the programme from previous experiences. All members were fully engaged and enthusiastic. Patient experience helped shape the study design, in particular regarding the frequency of researcher and patient interaction, and number of scheduled exercise sessions.
At the time of consent, all patients will be asked whether they would like to receive a copy of the trial results. If this box is initialled, they will be emailed or posted (as per the patient’s preference) a copy of the completed manuscript.
Once the patient has completed the programme, the burden of the intervention will be assessed by patients themselves through the use of a questionnaire.
Discussion
Neoadjuvant therapy prior to oesophagogastric resection is the gold standard of care for patients with T2 and/or nodal disease. Despite this, studies have taught us that chemotherapy decreases a patients’ functional capacity. We aim to show that a multimodal prehabilitation programme will physically and psychologically optimise these patients, during and after neoadjuvant therapy, prior to major elective OG cancer surgery so they may better withstand the immense physical and metabolic stress placed on them by radical surgery.
Ethics and dissemination
Approval
Authorisation was obtained from the NHS Health Research Authority on 16 November 2016. Any substantial amendment to the protocol or consent form will be presented to the local research and development team and independent research ethics committee. Likewise, all serious adverse events will be reported to the local research and development team as well as the independent research ethics committee. The study is registered on the Clinical Trials website, ClinicalTrials.gov, under the number NCT02950324. The study is sponsored by The Royal Surrey County Hospital NHS Foundation Trust and funded by Macmillan Cancer Support. The sponsorship from Macmillan Cancer Support will fund the following: exercise sessions at Surrey Sports Park, psychological support in the form of medical coaching, fasting blood tests and the Fitbit Flex2 physical activity monitors.
Patient informed consent
As per international principles, written informed consent (online supplementary appendix 2) will be obtained from patients prior to their participation in the trial once they voluntarily confirm their understanding and willingness to participate in the trial at least 24 hours after verbal and written information has been provided and questions answered. Consent will be obtained by a suitably qualified person in accordance with international GCP guidelines. Patients will be informed that they are free to withdraw from the trial at any time without giving a reason, and they will be informed that this will not adversely affect any aspects of their care.
bmjopen-2018-023190supp002.jpg (924.8KB, jpg)
Data collection and quality management
All data will be collected, handled and stored securely in the Trial Site File only by experienced persons who have been suitably trained in GCP and who are a member of the trial Delegation Log. At the time of patient contact, data will be acquired using a paper case report form (CRF). All study data will be anonymised by using a using a unique study number assigned to each subject sequentially. CRFs will be stored in a locked cabinet within a locked drawer of the secure (card-access only) research department. Collated data will be maintained on a predefined confidentially stored and password-protected electronic spreadsheet with access granted only to the CI, CS and sponsor. Data will be kept for 5 years following recruitment of the final patient. The trial does not warrant a Data Monitoring Committee due to its short interventional duration and minimal associated risks; however, trial data will be regularly monitored and audited at regular intervals by the sponsor and local Research and Development (R&D) department in accordance with the University of Surrey Research Department and GCP policies.
Access to data and dissemination of results
The CI and CS will have full access to the completed data set, as will the trial’s sponsor. Final data will be summarised on ClinicalTrials.gov, published in a peer-reviewed journal and presented at international conferences.
Trial status
Authorisation was obtained from the NHS Health Research Authority on 16 November 2016. Recruitment started on 15 December 2016. To date, 43 patients have been recruited. Six patients have been lost to follow-up. Interim analysis will be performed once primary outcome data (change AT) is available for 26 subjects (13 per group). Recruitment will be completed by 1 June 2018.
Supplementary Material
Acknowledgments
The authors would like to thank the following for their contributions to this study protocol: Mohamad Atie, Lucy-Ann Bett, Sebastian Cummins, Shirley Edghill, Lizzie Elam, Annabelle Emery, Alice Extance, Madeleine Hewish, Emily Hodge, Fiona Huddy, Julie Hunt, Marianne Illsley, Alice Kidd, David King, Aga Kehinde, Anna McGuire, Ajay Mehta, Sarah Oakes, Anne Pike, Nick Porter, Lyn Pugsley, Natalie Silverdale, David Timbrell, Mary Townsend, Lizzie Underhill, Joe Wainwright, Naomi Westran, Martin Whyte, Minimal Access Therapy Training Unit (MATTU) Guildford, The Oesophageal Patient Association Support Group, The Royal Surrey County Hospital NHS Foundation Trust, Frimley Park Hospital, Human Performance Institute, Surrey Sports Park, Macmillan Cancer Support, The Fountain Centre, St Lukes Cancer Centre and The University of Surrey.
Footnotes
Contributors: SA, VB, MS, PP, TR, SP and JS have all made substantial contributions to conception and design, or acquisition of data, or analysis and interpretation of data, have been involved in drafting the manuscript or revising it critically for important intellectual content, have given final approval of the version to be published and have participated sufficiently in the work to take public responsibility for appropriate portions of the content and have agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The trial management committee (SKA and JS) will be responsible for the following: organisation of steering committee meetings; the trial site file; randomisation (performed by a person external to the trial); budget administration and liasing with the funding source and sponsor; reporting of adverse events; completion of case report forms; identification and recruitment of patients; adherence to the study protocol; and publication of study results. The steering committee (SKA/VB/PP/SRP/TR/JS) were in agreement of the final protocol and will review the progress of the study, liasing with the CI to ensure the study runs smoothly.
Funding: This work is supported by Macmillan Cancer Support, Grant number 5227431 (received 25/6/15) and Grant number 5635161 (received 25/10/16). The funding body are responsible for funding participant participation at Surrey Sports Park, all Medical Coaching sessions, the use of Fitbit physical activity monitors, the cost of fasting glucose and insulin blood tests and the CPX test.
Disclaimer: This funding source had no role in the design of this study and will not be involved in analysis of the results, interpretation of data or decision to submit results.
Competing interests: None declared.
Patient consent: Not required.
Ethics approval: In accordance with the Declaration of Helsinki, the trial was presented to an independent Research Ethics Committee—the London-Bromley Research Ethics Committee.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: Data have not yet been analysed.
References
- 1. Cunningham D, Allum WH, Stenning SP, et al. Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N Engl J Med 2006;355:11–20. 10.1056/NEJMoa055531 [DOI] [PubMed] [Google Scholar]
- 2. Allum WH, Stenning SP, Bancewicz J, et al. Long-term results of a randomized trial of surgery with or without preoperative chemotherapy in esophageal cancer. J Clin Oncol 2009;27:5062–7. 10.1200/JCO.2009.22.2083 [DOI] [PubMed] [Google Scholar]
- 3. Ychou M, Boige V, Pignon JP, et al. Perioperative chemotherapy compared with surgery alone for resectable gastroesophageal adenocarcinoma: an FNCLCC and FFCD multicenter phase III trial. J Clin Oncol 2011;29:1715–21. 10.1200/JCO.2010.33.0597 [DOI] [PubMed] [Google Scholar]
- 4. Schuhmacher C, Gretschel S, Lordick F, et al. Neoadjuvant chemotherapy compared with surgery alone for locally advanced cancer of the stomach and cardia: european organisation for research and treatment of cancer randomized trial 40954. J Clin Oncol 2010;28:5210–8. 10.1200/JCO.2009.26.6114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Keegan N, Keane F, Cuffe S, et al. ICORG 10-14: Neo-AEGIS: A randomized clinical trial of neoadjuvat and adjuvant chemotherapy [modified MAGIC regimen] versus neoadjuvant chemoradiation [CROSS protocol] in adenocarcinoma of the esophagus and esophagogastric junction. J Clin Oncol 2014;32(5s). [Google Scholar]
- 6. Shapiro J, van Lanschot JJB, Hulshof M, et al. Neoadjuvant chemoradiotherapy plus surgery versus surgery alone for oesophageal or junctional cancer (CROSS): long-term results of a randomised controlled trial. Lancet Oncol 2015;16:1090–8. 10.1016/S1470-2045(15)00040-6 [DOI] [PubMed] [Google Scholar]
- 7. Blencowe NS, Strong S, McNair AGK, et al. Reporting of short-term clinical outcomes after esophagectomy. Ann Surg 2012;255:658–66. 10.1097/SLA.0b013e3182480a6a [DOI] [PubMed] [Google Scholar]
- 8. West MA, Lythgoe D, Barben CP, et al. Cardiopulmonary exercise variables are associated with postoperative morbidity after major colonic surgery: a prospective blinded observational study. Br J Anaesth 2014;112:665–71. 10.1093/bja/aet408 [DOI] [PubMed] [Google Scholar]
- 9. West MA, Parry MG, Lythgoe D, et al. Cardiopulmonary exercise testing for the prediction of morbidity risk after rectal cancer surgery. Br J Surg 2014;101:1166–72. 10.1002/bjs.9551 [DOI] [PubMed] [Google Scholar]
- 10. Moran J, Wilson F, Guinan E, et al. Role of cardiopulmonary exercise testing as a risk-assessment method in patients undergoing intra-abdominal surgery: a systematic review. Br J Anaesth 2016;116:177–91. 10.1093/bja/aev454 [DOI] [PubMed] [Google Scholar]
- 11. West MA, Asher R, Browning M, et al. Validation of preoperative cardiopulmonary exercise testing-derived variables to predict in-hospital morbidity after major colorectal surgery. Br J Surg 2016;103:744–52. 10.1002/bjs.10112 [DOI] [PubMed] [Google Scholar]
- 12. Jack S, West MA, Raw D, et al. The effect of neoadjuvant chemotherapy on physical fitness and survival in patients undergoing oesophagogastric cancer surgery. Eur J Surg Oncol 2014;40:1313–20. 10.1016/j.ejso.2014.03.010 [DOI] [PubMed] [Google Scholar]
- 13. O’Doherty AF, West M, Jack S, et al. Preoperative aerobic exercise training in elective intra-cavity surgery: a systematic review. Br J Anaesth 2013;110:679–89. 10.1093/bja/aes514 [DOI] [PubMed] [Google Scholar]
- 14. Dunne DF, Jack S, Jones RP, et al. Randomized clinical trial of prehabilitation before planned liver resection. Br J Surg 2016;103:504–12. 10.1002/bjs.10096 [DOI] [PubMed] [Google Scholar]
- 15. Holloszy JO, Coyle EF. Adaptations of skeletal muscle to endurance exercise and their metabolic consequences. J Appl Physiol Respir Environ Exerc Physiol 1984;56:831–8. 10.1152/jappl.1984.56.4.831 [DOI] [PubMed] [Google Scholar]
- 16. West MA, Loughney L, Lythgoe D, et al. Effect of prehabilitation on objectively measured physical fitness after neoadjuvant treatment in preoperative rectal cancer patients: a blinded interventional pilot study. Br J Anaesth 2015;114:244–51. 10.1093/bja/aeu318 [DOI] [PubMed] [Google Scholar]
- 17. Heldens AF, Bongers BC, de Vos-Geelen J, et al. Feasibility and preliminary effectiveness of a physical exercise training program during neoadjuvant chemoradiotherapy in individual patients with rectal cancer prior to major elective surgery. Eur J Surg Oncol 2016;42:1322–30. 10.1016/j.ejso.2016.03.021 [DOI] [PubMed] [Google Scholar]
- 18. DiMatteo MR, Lepper HS, Croghan TW. Depression is a risk factor for noncompliance with medical treatment: meta-analysis of the effects of anxiety and depression on patient adherence. Arch Intern Med 2000;160:2101–7. [DOI] [PubMed] [Google Scholar]
- 19. Garssen B, Boomsma MF, Meezenbroek EJ, et al. Stress management training for breast cancer surgery patients. Psychooncology 2013;22:572–80. 10.1002/pon.3034 [DOI] [PubMed] [Google Scholar]
- 20. Parker PA, Pettaway CA, Babaian RJ, et al. The effects of a presurgical stress management intervention for men with prostate cancer undergoing radical prostatectomy. J Clin Oncol 2009;27:3169–76. 10.1200/JCO.2007.16.0036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Mayo NE, Feldman L, Scott S, et al. Impact of preoperative change in physical function on postoperative recovery: argument supporting prehabilitation for colorectal surgery. Surgery 2011;150:505–14. 10.1016/j.surg.2011.07.045 [DOI] [PubMed] [Google Scholar]
- 22. Tsimopoulou I, Pasquali S, Howard R, et al. Psychological Prehabilitation Before Cancer Surgery: a systematic review. Ann Surg Oncol 2015;22:4117–23. 10.1245/s10434-015-4550-z [DOI] [PubMed] [Google Scholar]
- 23. Sato H, Carvalho G, Sato T, et al. The association of preoperative glycemic control, intraoperative insulin sensitivity, and outcomes after cardiac surgery. J Clin Endocrinol Metab 2010;95:4338–44. 10.1210/jc.2010-0135 [DOI] [PubMed] [Google Scholar]
- 24. Ljungqvist O, Nygren J, Soop M, et al. Metabolic perioperative management: novel concepts. Curr Opin Crit Care 2005;11:295–9. 10.1097/01.ccx.0000166395.65764.71 [DOI] [PubMed] [Google Scholar]
- 25. Yip C, Goh V, Davies A, et al. Assessment of sarcopenia and changes in body composition after neoadjuvant chemotherapy and associations with clinical outcomes in oesophageal cancer. Eur Radiol 2014;24:998–1005. 10.1007/s00330-014-3110-4 [DOI] [PubMed] [Google Scholar]
- 26. Paireder M, Asari R, Kristo I, et al. Impact of sarcopenia on outcome in patients with esophageal resection following neoadjuvant chemotherapy for esophageal cancer. Eur J Surg Oncol 2017;43:478–84. 10.1016/j.ejso.2016.11.015 [DOI] [PubMed] [Google Scholar]
- 27. Low DE, Alderson D, Cecconello I, et al. International consensus on standardization of data collection for complications associated with esophagectomy: Esophagectomy Complications Consensus Group (ECCG). Ann Surg 2015;262:286–94. 10.1097/SLA.0000000000001098 [DOI] [PubMed] [Google Scholar]
- 28. Nelson ME, Rejeski WJ, Blair SN, et al. Physical activity and public health in older adults: recommendation from the American college of sports medicine and the American heart association. Circulation 2007;116:1094–105. 10.1161/CIRCULATIONAHA.107.185650 [DOI] [PubMed] [Google Scholar]
- 29. Older P, Hall A, Hader R. Cardiopulmonary exercise testing as a screening test for perioperative management of major surgery in the elderly. Chest 1999;116:355–62. 10.1378/chest.116.2.355 [DOI] [PubMed] [Google Scholar]
- 30. Nagamatsu Y, Shima I, Yamana H, et al. Preoperative evaluation of cardiopulmonary reserve with the use of expired gas analysis during exercise testing in patients with squamous cell carcinoma of the thoracic esophagus. J Thorac Cardiovasc Surg 2001;121:1064–8. 10.1067/mtc.2001.113596 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2018-023190supp001.jpg (990.3KB, jpg)
bmjopen-2018-023190supp002.jpg (924.8KB, jpg)