Abstract
Objectives
This paper aims to evaluate the extremity function and vascular outcome after limb-sparing surgery for extremity musculoskeletal tumors invading vascular structure required reconstruction.
Methods
Of the 507 patients with musculoskeletal tumors, who underwent surgery between 2004 and 2007, 17 (3,3%) patients with major vessel involvement were included in the study. The mean age was 37.8 ± 14.5, with a female/male ratio of 8/9. Thirteen (76.4%) patients had Stage IIb disease, and 2 (11,7%) patients had Stage III disease. In 2 (11,7%) patients have locally aggressive tumor that had Stage 3. Fifteen (88.2%) of the cases involved lower extremity, whilst 2 (11.8%) of them involved upper extremity. An arterial reconstruction was carried out in all patients. Wide tumor resection and endoprosthetic reconstruction were performed in 6 (35.2%) patients. Other 11 (65.8%) patients were treated with wide resection and soft tissue reconstruction. Postoperative data included; perioperative morbidities such as bleeding, infection, graft thrombosis, rupture, metastatic local recurrence and mortality. Ankle brachial index (ABI) and color-flow-duplex-scan (CFDS) were done at the final follow-up of the study, in order to prove the efficacy of reconstruction. Functional outcome was evaluated with International Society of Limb Salvage (ISOLS) criteria.
Results
The mean follow-up was of 39 months (range 3–120). Perioperative complications were arterial graft thrombosis occurred in 3 (17.6%) patients treated acutely with thrombectomy, uncontrolled deep wound infection occurred in 2 patients whom extremities were amputated.
The most frequent complication after surgery was limb edema according to possibly venous and lymphatic obstruction, staged as C1, C2 and C3 disease was established in 6 patients (two patients in each group), and 1 patient was classified as C6 disease. Three (17.6%) patients had local recurrence (1/3 patient died and 2/3 (11.7%) patients underwent transfemoral amputation). At the last follow-up, 9 (52.9%) patients were alive without evidence of disease, 8 (47.1%) patients were died due to primary disease. There were 8 (47.1%) patients alive with an intact limb. Although functional outcome scores were satisfactory, emotional acceptance scores were low. The limb salvage probability was 74.0%.
Conclusion
Limb-sparing oncological surgery in musculoskeletal tumors with vascular invasion provides a satisfactory limb function, which may lead to an improved life quality. Arterial reconstruction has a high rate of patency in the long term. The surgeon should be aware of early perioperative complication related to vascular reconstruction and infection that effect on the rate of extremity survival.
Level of evidence
Level IV, Therapeutic study
Keywords: Musculoskeletal tumor, Extremity sarcomas, Limb salvage, Vascular reconstruction, Vascular invasion
Introduction
The first vascular reconstruction in a patient with sarcoma of an extremity was reported in 1977.1 Thereafter, progress in surgical procedures and multidisciplinary approach, which includes an orthopedic, vascular and a plastic surgeon has allowed limb-preserving treatment in this cohort of patients.2, 3, 4, 5, 6, 7 Hence, in the vast majority of cases, limb salvage has replaced amputation as the standard treatment for lower and upper extremity sarcomas, which involve major vascular structures. Limb salvage is recommended only if the ability to achieve adequate margins is not compromised and the salvaged limb has a superior function when compared to a prosthetic limb.8 Arterial reconstruction, which can be performed with synthetic or autologous vein grafts, is almost always inevitable to preserve the limb in most of the cases because the risk of ischemia and limb loss is very high unless an arterial reconstruction is carried out.2, 7 However, there is not enough scientific data concerning the need of a venous repair regarding either the need to do or not to do it.9, 10 Moreover limb-sparing surgery has the opportunity to provide good or excellent limb function after the intervention.11, 12
This paper aims to evaluate the results of a multidisciplinary approach to patients with extremity sarcomas, and review our current results concerning extremity function and vascular outcome after limb-sparing surgery for extremity sarcomas with vascular invasion.
Material and method
Five hundred and seven patients with primary or recurrent musculoskeletal tumors, which were treated in the Department of Orthopedics and Traumatology, Istanbul Medical Faculty, Turkey, between 2004 and 2007, were reviewed retrospectively. Seventeen patients (3,35%), who were operated for musculoskeletal tumors that involved major vessels were included in the study. Fifteen (88.2%) of the cases involved lower extremity, whilst 2 (11.8%) of them involved upper extremity. Nine (52.9%) patients were men and 8 (47.1%) patients were women. The mean age of the patients was 37.8 ± 14.5 years. Patients' characteristics, with regards to age, gender, tumor location, diagnosis, involved vessels, cancer stage (According to the Musculoskeletal Tumour Society Staging System,13 13 (76.4%) patients had Stage IIb disease, and 2 (11,7%) patients had Stage III disease. In 2 (11,7%) patients have locally aggressive tumor that had Stage 3.) and outcomes are shown in Table 1.
Table 1.
Table shows the preoperative demographics of the patients, perioperative features and the survival during the follow-up.
| Pt | Ag | Gen. | Diagnosis | Site | Enk. | Orthopedic treatment | Vessels reconstructed | Graft type | Complication | Vascular invasion | Limb salvage | Current status | Follow-up Survival |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 21 | F | Fibrosarcoma recur. | thigh | IIb | resection | femoral artery | saphenous | _ | arterial + venous | Yes | DOD | 37 |
| 2 | 45 | M | Ewing's sarcoma recur. | thigh | III | resection | femoral artery | saphenous | recurrence | arterial + venous | No | DOD | 36 |
| 3 | 34 | F | Rhabdomyosarcoma recur. | shoulder | IIb | resection | axillary artery | primary anastomosis | thrombosis and wound infection | arterial | No | DOD | 4 |
| 4 | 47 | M | Chondrosarcoma | proximal tibia | IIb | resection | tibialis posterior | saphenous | _ | arterial | Yes | NED | 50 |
| 5 | 35 | M | Osteosarcoma recur. | distal femur | IIb | endoprosthetic replacement after resection | popliteal artery | saphenous | graft thrombosis wound infection (latissimus dorsi flap) | arterial + venous | Yes | NED | 35 |
| 6 | 14 | M | Desmoid Tumor recur. | popliteal fossa | stg3 | resection + siatic nerve sacr. | popliteal artery | saphenous | wound infection, foot d5 metatarsal osteomyelitis | arterial + venous | Yes | NED | 120 |
| 7 | 39 | F | Osteosarcoma | proximal tibia | III | endoprosthetic replacement after resection | popliteal artery | saphenous | _ | arterial + venous | Yes | NED | 88 |
| 8 | 43 | M | Chondrosarcoma | distal femur | IIb | endoprosthetic replacement after resection | popliteal artery + popliteal vein | A:saphenous V:synthetic | synthetic graft occlusion | arterial + venous | Yes | NED | 27 |
| 9 | 50 | F | Fusiform cell sarcoma | popliteal fossa | IIb | resection | popliteal artery | saphenous | _ | arterial + venous | Yes | DOD | 9 |
| 10 | 50 | M | Fusiform cell sarcoma | popliteal fossa | IIb | resection + siatic sacr. | popliteal artery | A:saphenous V:synthetic | wound infection and graft occlusion | arterial + venous | Yes | NED | 26 |
| 11 | 40 | F | Ewing's sarcoma | popliteal fossa | IIb | resection | tibialis posterior | saphenous | _ | arterial + venous | Yes | DOD | 39 |
| 12 | 35 | F | Osteosarcoma recur. | proximal tibia | IIb | endoprosthetic replacement after resection | popliteal artery | saphenous | recurrence | arterial | No | NED | 111 |
| 13 | 30 | M | Pleomorphic sarcoma recur. | thigh | IIb | resection | femoral artery | saphenous | _ | arterial | Yes | DOD | 3 |
| 14 | 74 | M | Giant cell tendon tm | wrist | stg3 | wrist artrodesis after resection | Radial + ulnar artery | primary anastomosis | _ | arterial + venous | Yes | NED | 31 |
| 15 | 22 | F | Ewing's sarcoma | politeal fossa | IIb | resection | popliteal artery | saphenous | _ | arterial + venous | Yes | NED | 32 |
| 16 | 46 | F | Synovial sarcoma recur. | popliteal fossa | IIb | resection | popliteal artery | saphenous | recurrence | arterial | Yes | DOD | 3 |
| 17 | 19 | M | Osteosarcoma | distal femur | IIb | endoprosthetic replacement after resection | popliteal artery | saphenous | thrombosis and deep wound infection | arterial + venous | No | DOD | 12 |
Pt: Patient, Ag: Age, F: Female, M: Male, Recur: Recurrence, Sacr: Sacrifice, Enk: Enneking, DOD: Dead of disease, NED: No evidence of disease, A: Arteria, V: Vein, Stg:stage, tm: tumor.
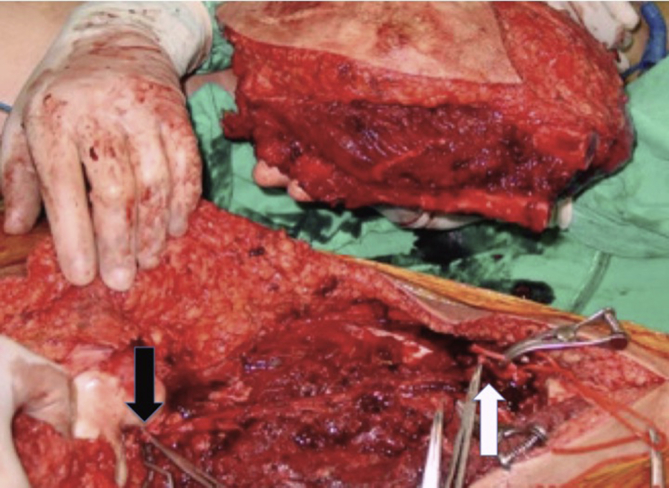
All patients were examined by an orthopedic and a single vascular surgeon (MA) before the operation and underwent nuclear magnetic resonance imaging of the affected extremity and chest computerized tomography, bone nuclear scintigraphy in order to evaluate the presence of a metastasis. Preoperative evaluation included physical examination and ankle brachial indices (ABI) in terms of vascular assessment. Abnormal physical examination findings were defined as the absence of peripheral pulses and an ankle-brachial index of less than 1.0 and findings suggestive of venous disease. The patients with abnormal physical findings underwent a vascular imaging technique such as color-flow-duplex scan, digital subtraction angiography or computerized tomography angiography either for arterial or venous pathology or both. The decision of a possible vascular invasion by the tumor was taken by the orthopedic and vascular surgeon during the operation and en-bloc resection of the vessels involved by the tumor was carried out (Fig. 1).
Fig. 1.
En-bloc resection of a musculoskeletal tumor, The Picture shows the proximal (black arrow-superficial femoral artery) and distal (white arrow-popliteal artery) site of the artery to be reconstructed. The dotted arrow shows the distal end of the femoral bone.
Antibiotic prophylaxis was covered with ampicillin and sulbactam in all patients.14 All patients were prepared as a candidate for a construction with the greater saphenous vein. After the resection of the tumor and decision of a vascular reconstruction (if necessary), ipsilateral extremity was covered with sterile waterproof dressings and contralateral extremity for recovery of the greater saphenous vein was prepped. All devices those were used during the tumor resection were banned in order to avoid any contamination. Following the recovery of the conduit, the skin was closed primarily and dressed. Before the vascular intervention, 70 units/kg unfractionated heparin was administered intravenously. Patients clinical follow up checking capillary filling and distal artery palpation is the most important indicator for determination pathology. Interrupted distal vascular supply is indicated for revision surgery. In revision surgery thrombectomy, reanastomosis could be performed according to reason of occlusion.
During surgery, solely arterial involvement and both arterial and vein involvement were identified on 5 (29,4%) and 12 (70,6%) patients, respectively. An arterial reconstruction was carried out on all patients. Arterial reconstructions were performed with contralateral greater saphenous vein on 15 (88,2%) patients, whilst remaining 2 (11,7%) patients underwent an arterial reconstruction in an end-to-end anastomosis manner. There were 2 (11,7%) patients, who underwent a venous reconstruction with ringed expanded polytetrafluoroethylene prosthesis. All other veins, which had tumor involvement, were ligated.
Wide tumor resection and reconstruction with endoprosthetic reconstruction was performed on 6 (35.2%) patients. Local rotational flap coverage on endoprosthesis was carried out on 3 (17.6%) patients. Other 11 (65.8%) patients were treated with wide resection and soft tissue reconstruction.
Postoperative data included; perioperative morbidities such as bleeding, infection, graft thrombosis, rupture, metastatic local recurrence and mortality. Patients with arterial reconstruction were discharged on 100 mg of acetylsalicylic acid daily.15 Patients with venous repair underwent a color flow duplex scan on the following day and on the days, when the daily findings were suggestive of deep vein thrombosis. Patients with a deep vein thrombosis (DVT), were received parenteral anticoagulants for at least five days. It is recommended to start Vitamin K antagonist (VKA) on the first treatment day because of the slow onset of action. And VKA therapy continued for 3 months.16 The patients were followed up in the first month, sixth month, twelfth month and annually afterward. The success of the arterial reconstruction or venous insufficiency were evaluated by clinical examination regularly. ABI and color-flow-duplex-scan (CFDS) were done at the final follow-up for the study, in order to prove the efficacy of reconstruction.
The follow up physical examination included ABI and color-flow-duplex-scan (CFDS) in order assess the patency of the grafts. During the follow-up, postoperative edema was monitored for venous insufficiency of the operated extremity. Leg circumference measurements were not used because they might not be reliable because of wide resection, soft tissue reconstruction and prosthetic replacement. Venous insufficiency was evaluated with CEAP chronic venous insufficiency classification system.17
The patients were examined postoperatively by an orthopedic surgeon (TA), who was not aware of the patients' diagnosis and managements using International Society of Limb Salvage (ISOLS) and Musculoskeletal tumor society (MSTS) criteria.18 ISOLS includes joint range of motion, pain, stability, deformity, muscle strength, functional activity and emotional acceptance in terms of functional outcome. It was rated on a 4-point scale: excellent, good, fair and poor. Also MSTS includes pain, function, emotional acceptance of the treatment outcome, need for walking aids, walking and gait. It was rated on numeric value as bad to very good with parallel awarding of points (0–5).18
The Kaplan–Meier analysis was performed to evaluate the survival of the arterial reconstruction.
Results
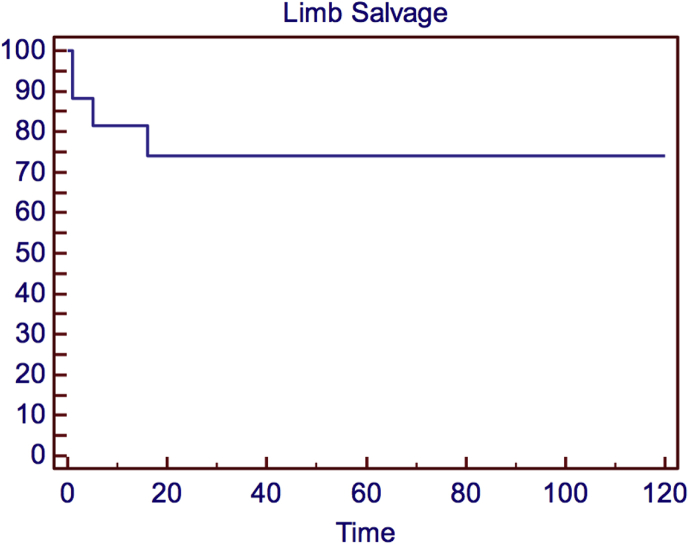
Perioperative arterial graft thrombosis occurred in 3 (17.6%) patients. Of these 3 patients, 2 (11.7%) had popliteal artery and one (5.8%) had axillary artery thrombosis within the first 48-hours after the intervention. Acute arterial thrombosis was treated with surgical thrombectomy immediately. Although the patency of grafts was achieved in all three patients, 2 (11.7%) major amputations were carried out due to uncontrolled infection and arterial rupture. During the follow-up, one arterial graft thrombosis occurred five months after surgery. This patient presented with subacute ischemia of the lower limb. A femoro-posterior tibial by pass with autologous vein was performed in this patient. The Kaplan–Meier analysis of the patency of arterial grafts is shown on Fig. 2.
Fig. 2.
The Kaplan–Meier analysis of the probability of patients alive with an intact limb during the follow-up.
One synthetic venous-graft thrombosis occurred within first 48 h after the intervention. Thrombosis was treated with oral anticoagulation for 3 months. The remaining venous reconstruction with prosthesis occluded at 6 months after surgery. Neither of them required any surgical intervention. The most frequent complication after surgery was limb edema according to possibly venous and lymphatic obstruction. However, no fasciotomies had to be performed on any patients. During the follow-up period, stage C1, C2 and C3 disease was established in 6 patients (two patients in each group), and 1 patient was classified as C6 disease, according to the CEAP classification.
The sciatic nerve was sacrificed in 2 (11.7%) patients. An ankle triple arthrodesis and tibial lengthening for dropped foot and shortening were carried out for the patient with sciatic palsy. This patient had chronic wound problems and chronic osteomyelitis at the foot region. The other patient who had sciatic nerve sacrifice was followed with an ankle-foot orthosis (AFO).
Wound infection developed in 5 (29.4%) patients. Two of them underwent a major amputation in the perioperative period because of intractable infection whereas; a free flap reconstruction and local debridement had to be carried out in 2 and 1 patients respectively.
During the follow-up, there were 3 (17.6%) patients, who developed pulmonary metastasis and two of them died of disease. Three (17.6%) patients had a local recurrence. One of these patients died of disease without any further treatment and major amputation while other 2 (11.7%) patients underwent transfemoral amputation. One of them had synchronous pulmonary metastasis and was treated with chemotherapy. At the last follow-up visit, the patient did not show any evidence of disease. The last patient, who underwent amputation, died of disease.
At the mean follow-up of 39 months (range 3–120), 9 (52.9%) patients were alive without evidence of disease, 8 (47.1%) patients were dead of disease. There were 8 (47.1%) patients alive with an intact limb. One of these patients (no. 12) did not accept to participate in the functional outcome assessment and another patient with wrist arthrodesis was removed from the function evaluation. Five patients had good or excellent motion in regards to ISOLS grading. Two patients had poor or fair extremity function. One patient, who needs non-narcotic analgesics, had fair pain control. Two patients, who had prosthesis loosening, had poor stability according to radiological criteria. Two patients had poor or fair deformity because of shortening. Two patients had neurologic complications according to sciatic nerve sacrifice. In terms of emotion acceptance, 3, 1, 2 and 1 patient had excellent good, fair or poor activity scores, respectively. In terms of functional activity, 2, 1 and 4 patients had excellent, good or fair activity scores, respectively (Table 2). The overall rate of the MSTS score is 70% in the lower limb. The pain, function, emotional acceptance, support, walking and gait scores were 3,8; 3,2; 3; 3,7; 3,9 and 3,9 retrospectively.
Table 2.
Results of Functional Activity Scores according to ISOLS* criteria. *International Society of Limb Salvage.
| Patient | Motion | Pain | Stability | Deformity | Muscle strength | Function | Emotional acceptance |
|---|---|---|---|---|---|---|---|
| 4 | 1 | 2 | 1 | 1 | 1 | 2 | 1 |
| 5 | 4 | 3 | 1 | 4 | 1 | 3 | 4 |
| 6 | 1 | 1 | 1 | 1 | 4 | 3 | 3 |
| 7 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| 8 | 3 | 1 | 4 | 3 | 2 | 3 | 2 |
| 10 | 2 | 2 | 1 | 1 | 4 | 3 | 3 |
| 15 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
Excellent: 1, good: 2, fair: 3, poor: 4.
Discussion
Although amputation was the treatment of choice in patients with extremity musculoskeletal tumors, which involve the vascular bundle of the extremity, the current approach is mainly focused on limb salvage in order to maintain a better quality of life.1, 2, 3, 4, 5, 6, 7 However, the involvement of major vascular bundle by the tumor requires an en-bloc resection and vascular reconstruction. The present data show that there is no difference concerning survival rates between major amputation and limb salvage.19, 20 Therefore, it seems that such an approach is justified in this group of patients. The present paper, which aims to evaluate the results of limb-sparing surgery, shows that functional outcome of the limb is satisfactory when limb salvage is achieved. In addition to that, our results show that the perioperative period is not complication-free and wound problems are major complications, which may lead to failure of revascularization and subsequently major amputation in the early postoperative period.
In order to maintain a disease-free survival, it is essential to carry out an en-bloc resection accompanied with resection of the vascular bundle. The main indication for vascular resection was providing R0 resection. However, the risk of acute limb ischemia is considerably high after ligature of the main arterial trunk of the limb. Therefore, an arterial reconstruction is mandatory in limb salvage. Arterial reconstruction can be performed by end-to-end anastomosis but this is rarely the case because en-bloc resection usually necessitates the resection of a long segment of the artery. Hence, use of an autologous substitute is mostly required. The saphenous vein is usually the conduit of choice. The patency rates are high for reconstruction with saphenous vein and management of infections is more successful with this approach.2, 21 We prefer to use the contralateral saphenous vein because venous return may be critically jeopardized in cases, which may require the sacrifice of the ipsilateral vein. In cases, which lack saphenous vein of adequate diameter, arm veins can be preferred. Synthetic grafts are the last choices.22, 23 In our series, there were three cases, which presented with acute limb ischemia within 48 h after surgery. Although they were salvaged with thrombectomy, ensuing infections with highly virulent bacteria led to inevitable amputations in two of the cases. It is possible that if a secondary intervention of thrombectomy had not been required, there would be no infections. In the current series, the perioperative period was not free of other complications. Hematoma and local infections are common complications in these procedures.5, 6, 7 In accordance with previous reports, we believe that these complications occurred mainly because of large wounds, prolonged operation time, and a large amount of synthetic and metallic material used.4 Likewise, recent papers report similar rates of infection in limb-sparing oncological surgery. The infection rate in skeletal sarcoma intervention is reported to range from 2.6 to 7.6%. When the intervention involves mainly soft tissue sarcomas, this rate may reach 30%.24, 25 The infection rate does not seem to rise when a vascular intervention is required. Therefore, we may emphasize that it is mainly the soft tissue component of surgery that relates to higher rates of infection.26, 27
When the tumor invades the neurovascular bundle, the vein is in danger as well the artery. In trauma, complex venous injuries are frequently managed with ligation and the overall results are satisfactory. The long-term results of vein ligation in our trauma patients did not reveal any significant squeal of chronic venous insufficiency, and venous ligation had no detrimental effect on associated arterial repair.28 The alternative routes of venous return possibly cover the role of the injured vein. Although a wide resection of muscle and skin is frequently required during oncological interventions and this may jeopardize the alternate venous routes, it seems that vein ligature is tolerated by these patients too.29 Although the most frequent postoperative complication in our series was lower limb edema, they did not require any further intervention. Moreover, patients who had a venous ligature did not present with an advanced venous disease during the follow-up. There was only one patient, who presented with an active venous ulcer (C6). It is also shown that the patency rates for venous reconstruction are lower than for arterial ones. Moreover, the advantage of a venous reconstruction is mostly valid for the early period. Similar reports show that surgery both with venous reconstruction and without is safe and effective.30, 31, 32 We do not prefer to perform a routine venous reconstruction because it extends the duration of surgery and its advantages are not clear. In case of large tumor resection and flap reconstruction, venous reconstruction was maintained to supply a better venous return and to avoid edema and congestion in the early postoperative period. In this series, two patients underwent vein reconstruction with synthetic graft because of the lack of an eligible autologous vein graft. However, one patient presented with thrombosis on the second postoperative day and finally infection. Although this infection did not lead to major amputation it prompted another intervention and removal of the infected synthetic graft.
Patients with extremity sarcomas, which involve the main vessels, are at a higher risk for amputation, and not all patients are eligible for limb salvage procedures.3 Nevertheless, recent papers concerning limb-sparing oncological surgery report a low rate of recurrence after surgery.33 In our series, there are three patients (3/17) (%17.6), who underwent a limb salvage surgery and had a local recurrence. During the follow-up, two of these three patients underwent a major amputation because of local recurrence. Moreover, extensive vascular involvement by extremity sarcoma raises a major concern regarding not only the risk of local recurrence after limb salvage surgery, but also an increased risk for metastatic disease.2 The rate of metastasis was 17.6% in our series. Therefore a limb salvage procedure is more helpful in maintaining an acceptable life quality because the expected survival of these patients is low complicated with a remarkable recurrence rate.
However, patency and limb salvage rates are physician-oriented outcome measures and may not necessarily reflect the contentment of the patient. Therefore, patient-oriented outcome measures such as quality of life assessments or functional outcome measures are required. In our study, we used ISOLS Criteria to evaluate the functional outcome of the salvaged limb. During the follow-up period, we have seen that 43% of our patients maintained an excellent or good function of the limb. The assessments show that motion and stability scores are mostly excellent or good. Nevertheless, the emotional acceptance scores are not as high as they are for other issues. It is possible that the burden of the disease is more evident in this category. This paper focuses on the functional outcome of a spared limb rather than the quality of life, therefore, we can only assume that maintaining a better functional outcome may be related to a better quality of life.
This study has some limitation as; the patient group is heterogeneous in means of their diagnosis, a limited number of patients and relatively short follow-up period. The heterogeneous follow-up time could effect our results due to including the patients with short follow-up. The timing the evaluation patient clinical functional results at the final follow-up. A patient with a high functional score in the early postoperative period may not maintain his/her overall functional status in a long time due to several potential complications that might cause a negative affect on the scores in the long postoperative period.
In conclusion, limb-sparing oncological surgery in musculoskeletal tumors with vascular invasion provides a satisfactory limb function, which may lead to an improved life quality. Arterial reconstruction, which is a major component of this approach, has a high rate of patency in the long term. The indication for a subsequent major amputation is the recurrence not ischemia in the long-term The surgeon should be aware of early perioperative complication related to vascular reconstruction and infection that effect on the rate of extremity survival.
Conflict of interest/funding
None.
Footnotes
Peer review under responsibility of Turkish Association of Orthopaedics and Traumatology.
Contributor Information
Turgut Akgül, Email: trgtakgul@gmail.com.
İsmail Cem Sormaz, Email: icsormaz@gmail.com.
Murat Aksoy, Email: maksoy@tnn.net.
Adem Uçar, Email: ucaradem@yahoo.com.
Harzem Özger, Email: harzemo@yahoo.com.
Levent Eralp, Email: drleventeralp@gmail.com.
References
- 1.Fortner J.G., Kim D.K., Shiu M.H. Limb-preserving vascular surgery for malignant tumours of the lower extremity. Arch Surg. 1977;112(4):391–394. doi: 10.1001/archsurg.1977.01370040043007. [DOI] [PubMed] [Google Scholar]
- 2.Ghert M.A., Davis A.M., Griffin A.M., Alyami A.A., White L. The surgical and functional outcome of limb salvage surgery with vascular reconstruction for soft tissue sarcomas of extremity. Ann Surg Oncol. 2005;12(12):1102–1110. doi: 10.1245/ASO.2005.06.036. [DOI] [PubMed] [Google Scholar]
- 3.Ghert M.A., Abudu A., Driver N. The indications for and the prognostic significance of amputation as the primary surgical procedure for localized soft tissue sarcoma of the extremity. Ann Surg Oncol. 2005;12(1):10–17. doi: 10.1007/s10434-004-1171-3. [DOI] [PubMed] [Google Scholar]
- 4.Koperna T., Teleky B., Vogl S., Windhager R., Kainberg F. Vascular reconstruction for limb salvage in sarcoma of the lower extremity. Arch Surg. 1999;131(10):1103–1107. doi: 10.1001/archsurg.1996.01430220097023. [DOI] [PubMed] [Google Scholar]
- 5.Teleky B., Ritschl P., Kotz R., Polterauer P. Gefasschrurgische rekonstruktionen bei orthopadischen Tumoroperationen. Chirurg. 1988;59(3):159–164. [PubMed] [Google Scholar]
- 6.Karakousis C.P., Emrich L.J., Vesper D.S. Soft tissue sarcomas of the proximal lower extremity. Arch Surg. 1989;124(11):1297–1300. doi: 10.1001/archsurg.1989.01410110055011. [DOI] [PubMed] [Google Scholar]
- 7.Nambisan R.N., Karakousis C.P. Vascular reconstruction for limb salvage in soft tissue sarcomas. Surgery. 1987;101(6):668–677. [PubMed] [Google Scholar]
- 8.Puri A. Limb salvage: when, where and how? Indian J Orthop. 2015;49(1):46–55. doi: 10.4103/0019-5413.143912. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 9.Hohenberger P., Allenberg J.R., Schlag P.M., Reichardt P. Results of surgery and multimodal therapy for patients with soft tissue sarcoma invading to vascular structures. Cancer. 1999;85(2):396–408. [PubMed] [Google Scholar]
- 10.Gooding G.A.W., Perez S., Rapp J.H., Krupski W.C. Lower- extremity vascular grafts placed for peripheral vascular disease: prospective evaluation with duplex Doppler sonography. Radiology. 1991;180(2):379–386. doi: 10.1148/radiology.180.2.2068299. [DOI] [PubMed] [Google Scholar]
- 11.Tsubuyama T., Windhager R., Dock W., Bochdansky T., Yamamuro T., Kotz R. Knee function after operation for malignancy of the distal femur. Acta Orthop Scand. 1993;64(6):673–677. doi: 10.3109/17453679308994596. [DOI] [PubMed] [Google Scholar]
- 12.Tsubuyama T., Windhager R., Bochdansky T., Yamamuro T., Kotz R. Gait after knee arthroplasty for femoral tumor. Acta Orthop Scand. 1994;65(1):51–54. doi: 10.3109/17453679408993718. [DOI] [PubMed] [Google Scholar]
- 13.Enneking W.F., Spanier S.S., Goodman M.A. Current concepts review. The surgical staging of musculoskeletal sarcoma. J Bone Joint Surg Am. 1980;62(6):1027–1030. [PubMed] [Google Scholar]
- 14.Kato D., Maezawa K., Yonezawa I. Randomized prospective study on prophylactic antibiotics in clean orthopedic surgery in oneward for 1 year. J Orthop Sci. 2006 Jan;11(1):20–27. doi: 10.1007/s00776-005-0970-0. [DOI] [PubMed] [Google Scholar]
- 15.Clagett G.P., Sobel M., Jackson M.R., Lip G.Y., Tangelder M., Verhaeghe R. Antithrombotic therapy in peripheral arterial occlusive disease: the seventh ACCP conference on antithrombotic and thrombolytic therapy. Chest. 2004;126(3):609–626. doi: 10.1378/chest.126.3_suppl.609S. [DOI] [PubMed] [Google Scholar]
- 16.Agnelli G., Gitt A.K., Bauersachs R., PREFER in VTE investigators The management of acute venous thromboembolism in clinical practice – study rationale and protocol of the European PREFER in VTE Registry. Thromb J. 2015;21(13):13–41. doi: 10.1186/s12959-015-0071-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Padberg F.T., Jr. CEAP classification for chronic venous disease. Dis Mon. 2005;51(2–3):176–182. doi: 10.1016/j.disamonth.2005.03.013. [DOI] [PubMed] [Google Scholar]
- 18.Enneking W.F., Dunham W., Gebhardt M.C., Malawar M., Pritchard D.J. A system for the functional evaluation of reconstructive procedures after surgical treatment of tumors of the musculoskeletal system. Clin Orthop Relat Res. 1993;286:241–246. [PubMed] [Google Scholar]
- 19.Spiro I.J., Rosenboerg A.E., Springfield D., Suıt H. Combined surgery and radiation therapy for limb preservation in soft tissue sarcoma of the extremity. Cancer Invest. 1995;13(1):137–138. doi: 10.3109/07357909509024899. [DOI] [PubMed] [Google Scholar]
- 20.Malawer M.M., Link M.P., Donaldson S.S. Sarcomas of bone. In: De Vita V.T., Hellman S., Rosenberg S.A., editors. Cancer: Principles and Practice of Oncology. 4th ed. JB Lippincott; Philadelphia Pa: 1993. pp. 1509–1566. [Google Scholar]
- 21.Leggon R.E., Huber T.S., Scarborough M.T. Limb salvage surgery with vascular reconstruction. Clin Orthop Relat Res. 2001;387(387):207–216. doi: 10.1097/00003086-200106000-00028. [DOI] [PubMed] [Google Scholar]
- 22.Diperna C.A., Bowdish M.E., Weaver F.A. Concominant vascular procedures for malignancies with vascular invasion. Arc Surg. 2002;137(8):901–907. doi: 10.1001/archsurg.137.8.901. [DOI] [PubMed] [Google Scholar]
- 23.Fadel E., Chalepier A., Bacha E. Subclavian artery resection and reconstruction for thoracic inlet cancers. J Vasc Surg. 1999;29(4):581–588. doi: 10.1016/s0741-5214(99)70301-0. [DOI] [PubMed] [Google Scholar]
- 24.Xin Li M.D., Vincent M., Moretti M.D. Perioperative infection rate in patients with osteosarcomas treated with resection and prosthetic reconstruction. Clin Orthop Relat Res. 2011;469(10):2889–2894. doi: 10.1007/s11999-011-1877-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Myers G.J.C., Abudu A.T., Carter S.R., Tillman R.M., Grimer R.J. The long-term results of endoprosthetic replacement of the proximal tibia for bone tumours. J Bone Joint Surg Br. 2007;89(12):1632–1637. doi: 10.1302/0301-620X.89B12.19481. [DOI] [PubMed] [Google Scholar]
- 26.Muramatsu K., Ihara K., Miyoshi T., Yoshida K., Taguchi T. Clinical outcome of limb-salvage surgery after wide resection of sarcoma and femoral vessels reconstruction. Ann Vasc Surg. 2011;25(8):1070–1077. doi: 10.1016/j.avsg.2011.05.009. [DOI] [PubMed] [Google Scholar]
- 27.Emori M., Hamada K., Omori S. Surgery with vascular reconstruction for soft tissue sarcomas in the inguinal region: oncological and functional outcomes. Ann Vasc Surg. 2012;26(5):693–699. doi: 10.1016/j.avsg.2011.12.003. [DOI] [PubMed] [Google Scholar]
- 28.Kurtoglu M., Yanar H., Taviloglu K., Sivrikoz E., Plevin R., Aksoy M. Serious lower extremity venous injury management with ligation: prospective overview of 63 patients. Am Surg. 2007;73(10):1039–1043. [PubMed] [Google Scholar]
- 29.Oktar G.L. Iatrogenic major venous injuries incurred during cancer surgery. Surg Today. 2007;37(5):366–369. doi: 10.1007/s00595-006-3416-1. [DOI] [PubMed] [Google Scholar]
- 30.Adelani A.M., Holt G., Dittus R., Passman M., Schwartz H. Revascularization after segmental resection of lower extremity soft tissue sarcomas. J Surg Oncol. 2007;95(6):455–460. doi: 10.1002/jso.20679. [DOI] [PubMed] [Google Scholar]
- 31.Satoshi T., Yoshihiro N., Hideshi S., Hitoatsu N., Naoki I. Results of Limb-Salvage surgery with vascular reconstruction for soft tissue sarcoma in the lower extremity: comparison between only arterial and arterovenous reconstruction. J Surg Oncol. 2008;97(3):216–220. doi: 10.1002/jso.20945. [DOI] [PubMed] [Google Scholar]
- 32.Nishinari K., Wolosker N., Yazbek G., Zerati A.E., Nishimoto I.N. Venous reconstruction in lower limbs associated with resection of malignancies. J Vasc Surg. 2006;44(5):1046–1050. doi: 10.1016/j.jvs.2006.07.033. [DOI] [PubMed] [Google Scholar]
- 33.Ayerza M.A., Farfalli G.L., Aponte-Tinao L., Muscolo D.L. Does increased rate of limb-sparing surgery affect survival in osteosarcoma? Clin Orthop Relat Res. 2010;468(11):2854–2859. doi: 10.1007/s11999-010-1423-4. [DOI] [PMC free article] [PubMed] [Google Scholar]