Abstract
Gender differences in aggression viewed from an evolutionary and sociocultural perspective have traditionally explained why men engage in more direct and physical aggression, and women engage in more indirect and relational aggression. However, psychological and behavioral studies offer inconsistent support for this theory due to personal or social factors, and little is known about the gender-based neurobiological mechanisms of aggression. This study investigates gender differences in aggression through an analysis of electroencephalography (EEG) and electrocardiography (ECG) based neurobiological responses to commonly encountered stimuli, as well as psychological approaches in healthy Korean youth. Our results from self-reports indicate that overall aggression indices, including physical and reactive/overt aggression, were stronger in men. This agrees with the results of previous studies. Furthermore, our study reveals prominent gender-related patterns in γ signals from the right ventrolateral frontal cortex and changes in heart rate through stimulation by aggressive videos. In particular, gender differences in EEG and ECG responses were observed in response to different scenes, as simple aversion and situation-dependent aggression, respectively. In addition, we discovered decisive gender-distinct EEG signals during stimulation of the situation-dependent aggression regions within the right ventromedial prefrontal and ventrolateral frontal regions. Our findings provide evidence of a psychological propensity for aggression and neurobiological mechanisms of oscillation underlying gender differences in aggression. Further studies of oscillatory responses to aggression and provocation will expand the objective understanding of the different emotional worlds between men and women.
Keywords: Gender, Aggression, EEG, ECG, CHAID
Graphical Abstract
INTRODUCTION
Aggression, here defined as feelings of anger or antipathy resulting in hostile or violent behavior, is a complex social phenomenon with many causes and manifestations. For many years, researchers have attempted to explain gender-based differences in aggression. In fact, aggression has traditionally been studied as a masculine characteristic only, and the theory that men are more aggressive than women has been widely accepted [1]. However, aggression exists in various forms. It can be divided into direct or indirect aggression, in addition to physical or verbal aggression depending on the action, hostile or instrumental aggression depending on the purpose, reactive or proactive aggression depending on the trigger, and overt or relational aggression depending on the target [2,3]. It has been reported widely that while men tend to express physical, overt, and direct aggression, women tend to express relational and indirect aggression more often [4,5,6,7]. Studies based on extensive behavioral research with an aggression paradigm (i.e., TAP, Taylor aggression paradigm [8]; PSAP, point subtraction aggression paradigm [9]) and self-report questionnaires (i.e., BDHI, Buss-Durkee Hostility Inventory [10]; BPAQ, Buss-Perry Aggression Questionnaire [11]) in men and/or women have authoritatively supported this conclusion [12,13,14,15]. Nonetheless, this theory proves unstable across developmental stages and differing cultural environments of the study participants [16,17,18,19]. Moreover, in several studies of aggression paradigms, men showed higher aggression than women under relatively neutral conditions; however, provocation rather weakens the difference in aggressive behavior between men and women [3,13,20]. Therefore, to investigate differences in aggression by gender, studies must consider provocation stimuli and the use of a multifaceted approach that involves behavioral, psychological, and neurobiological methods.
In recent decades, efforts to explore the neurobiological mechanism behind aggression have used neurophysiology or neuroimaging techniques such as functional magnetic resonance imaging (fMRI), event-related potential (ERP), electroencephalography (EEG), and electrocardiography (ECG). Studies using fMRI have shown that the neural activation of the prefrontal cortex, anterior cingulate cortex, and insula are strongly associated with aggression [21,22,23,24], and the amygdala seems to play a crucial role in impulsive aggression [23,25]. Some results of aggression paradigms suggest that men have higher amygdala activation during provocation than do women [26,27]. Moreover, studies using ERP have demonstrated that individuals displaying highly impulsive aggression and hostility have a decreased parietal or central P300 amplitude [28,29,30]. One ERP study using the TAP did not identify any gender differences in neurophysiological responses (i.e., frontal negativity) [31]. It also agreed with the fMRI study which reported no gender differences in the influence of alcohol on reactive aggression [32]. A number of studies using EEG and ECG also provide evidence of the neurobiology of human aggression. EEG research has identified that frontal α asymmetry during resting states and the increase in slow wave activity during emotional states are related to an individual's antisocial or aggressive tendencies [33,34,35,36,37]. ECG research has suggested that a low heart rate (HR) during resting states is a marker of aggression [38,39,40,41]. Despite abundant studies of aggression using such methods, few ECG studies have addressed gender differences in aggression-related responses. In particular, only one EEG study reported that hostile women, but not hostile men, show stronger θ synchronization, a marker of emotional response, and exert widespread α synchronization, which is tentatively explained as inhibitory control when provoked by emotional facial expressions [42]. Therefore, there is still a lack of neurobiological research on the differences between men and women. Most research investigating the nervous system's aggression mechanisms included only men and thus failed to consider the differences between the genders [21,22,31,35,37,43].
Our study aimed to investigate the influence of gender on aggression by analyzing the self-reports of aggression in young Korean men and women, as well as by measuring the neurobiological responses to aggression-related stimuli that are common in daily life using EEG and ECG. In particular, EEG and ECG allow real-time response measurements and are highly accessible; as an EEG provides fine-grained resolution signals across the spectrum range and an ECG provides evidence of direct autonomic reactivity, they are useful tools for studying functional differences in neural activation. Moreover, previous studies have established that EEG signals from the right frontal or prefrontal cortex, and ECG signals, are related to specific types of aggression (i.e., impulsivity, hostility, and reactive aggression) [44,45,46,47,48,49,50,51]. These results will enable a delicate analysis of aggression across gender in a pool of healthy adult individuals. Nevertheless, the sparse knowledge of the gender effect of aggression on oscillatory responses to stimuli or provocation encouraged us to research this topic. In this study, we verified that men and women exhibit a difference in the various types of aggression using three self-reports and their subscales. In addition, we provided information on the neurobiological characteristics of gender through our analyses of frequency-specific EEG responses and ECG responses to particular stimuli. We suggest that different pathways and features of neurobiological reactions, both in autonomic reactivity and cognition processes, contribute to gender-based distinctions in the experience and expression of aggression.
MATERIALS AND METHODS
Study participants
In the present study, 334 college (age: 18.3±1.2 (mean±SD)) and high school students (169 males, age: 18.8±0.8 and 165 females, age: 17.7±1.3) in Korea were recruited and surveyed. All participants were free of any history of pathological behavior or crime. We only used subjects who completed the required fields of the self-report questionnaires. Ninety-four of these participants (age: 18.9±0.7) from the same college year were randomly selected to partake in the measurement of EEG and ECG signals. Subjects participating in the EEG and ECG experiments were screened to confirm they had no history of psychiatric disorders, using the social records from the institution's Center for Student Counseling and Career Development. There also was little difference between men and women in demographic characteristics, including age, as detected by independent t-tests. The EEG results of 84 participants (36 males, age: 18.9±0.7 and 48 females, age: 18.7±0.7) and ECG results of 70 participants (30 males, age: 18.8±0.8 and 40 females, age: 18.7±0.7) were used as valid data for the analysis. We excluded EEG samples from left-handed subjects and in occurrences of poor electrode attachment or subject movement; we excluded ECG samples that were not identifiable with PQRST waveforms. The study was approved by the Institutional Review Board of DGIST [Number: DGIST_170614-HR-009-04]. All subjects gave written informed consent in accordance with the Declaration of Helsinki.
Self-report questionnaires
This study used three types of self-report questionnaires adapted in Korean. K-BDHI is an adaptation of the Buss-Durkee Hostility Inventory [10,52]. K-BPAQ is an adaptation of the Buss-Perry Aggression Questionnaire and includes a failure scenario [11,53,54,55]. It also contains four subcategories—anger, physical aggression, hostility, and verbal aggression. K-PCS is an adaptation of the Peer Conflict Scale (PCS) and consists of four categories comprising overt-relational aggression and reactive-proactive aggression [56,57]. All adapted self-report questionnaires have been verified in Korean populations by other studies [58,59,60].
Stimuli
The participants' neurobiological signals were measured while the participants were watching a 118 s-long video comprising the following scenes: fixation cross - black - neutral scene - black - scene 1 - black - scene 2 - black - scene 3 - black - end. The baseline scene consisted of a fixation cross on a white background and four black screens between the other scenes. The neutral scene depicted an empty school classroom that was familiar to the participants. The three scenes used to trigger aggression are described below. Scene 1 shows a passenger in a bus condescendingly shouting to the bus driver. The abuse of power is currently a major social issue in Korean society; it has been generating anger and hostility in people, and it has already been reported that verbal abuse is related to verbal aggression [61]. Scene 2 shows conflict between students working in a group, in which one student is intentionally excluded and burdened with all the work. Social exclusion has been reported to induce proactive and reactive aggression [62,63]. Scene 3 shows someone scratching the blackboard with their nails and this has been reported to induce irritability [64,65].
Data acquisition and processing of EEG and ECG results
Neurobiological signals were measured via four channels using MP36 (BIOPAC), which is useful for quick and simple measurements in large populations (sampling rate: 1.000 kHz, bandpass filtering rate: 0.5~100 Hz). EEG signals were measured via two channels according to the BIOPAC EEG electrode placement guidelines and International 10~20 EEG system, and the EOG and ECG signals (Einthoven's leads) were simultaneously measured [66]. Theories of frontal α asymmetry in aggression suggest that relatively greater resting neural activity in the left frontal cortex correlates with approach motivation, while greater resting neural activity in the right frontal cortex correlates with avoidance motivation [43,45]. Reportedly, differences in aggression-related EEG responses between men and women are also associated with inhibitory control, rather than emotional outbursts [42]. Moreover, EEG studies of healthy Korean men showed differences in α, β, and γ power values between emotion-related sounds, especially in the right frontal region (Fp2 and F8 channels) rather than the left frontal region (F3, Fp1, F7) [67]. Therefore, we decided to analyze the EEG signals concentrated in the right frontal regions, Fp2 (right ventromedial prefrontal region) and F8 (right ventrolateral frontal region). These two channels are also accessible for electrode attachment. EEG artifacts that occur from eye blinking or eye movement were excluded using an independent component analysis based on EOG data. The fast Fourier transform was used to calculate the power spectrum for each experimenter's signals in the Fp2 and F8 channels, and the data were processed by dividing the 0~90 Hz values by the integral values of the 0~90 Hz range. The average value of all subjects was visualized as a heat map according to time duration and frequencies. Power ratios were indicated by dividing the average value of the power spectrum by the average value at the baseline at each frequency interval (0.5 and 4 (δ), 4 and 8 (θ), 8 and 13 (α), 13 and 30 (β), and 30 and 90 (γ)). The ECG signals were converted into HR (unit: BPM) through 60,000/(R-R intervals) after detrending. The standard deviation of HR was identified as a variance of HR, and the difference between the maximum and minimum values was identified as ΔHR to be used in the analysis.
Statistical analyses
To examine whether the questionnaires and EEG and ECG results showed statistically significant differences according to gender, Prism 5.0 was used to conduct a two-tailed independent t-test with a 0.05 level of significance. To identify significant differences in the scores of questionnaire subscales between genders and in the EEG and ECG results between neutral and aggressive scenes, we used a two-way ANOVA. The chi-square automatic interaction detector (CHAID) was used to investigate the gender distinction according to the EEG and ECG signal variables. The standard p value of split and merge was identically set to 0.1, and the minimum number of cases was set to a parent node of 10 and child node of 5.
RESULTS
Questionnaire scores analysis
Aggression was measured using three self-report questionnaires—BDHI, BPAQ, and PCS—in men and women. The average BDHI score of men (40.86±8.69, mean±SD) was significantly higher than that of women (38.82±7.91) (Table 1). On the other hand, while the average total scores of BPAQ and PCS were not significantly different, a few subscales indicated statistically significant differences between genders. Our analysis of the anger and physical aggression subscales of BPAQ revealed that the average scores of men (17.29±4.95, 19.83±4.31, respectively) were significantly higher than those of women (15.96±4.43, 18.03±5.47, respectively). Similarly, men scored a significantly higher average (2.58±3.11) than women (1.81±2.26) in the reactive and overt aggression subscale of PCS. Therefore, self-report questionnaires measured higher levels of aggression in men than women, and the particular types of aggression including anger, physical aggression, and reactive and overt aggression, showed differences according to gender.
Table 1. Gender differences in the questionnaire scores.
| Questionnaires | Male | Female | t | df | Sig. | |||
|---|---|---|---|---|---|---|---|---|
| Score (mean±SD) | N | Score (mean±SD) | N | |||||
| BDHI | Total | 40.86±8.69 | 160 | 38.82±7.91 | 152 | 2.11 | 310 | * |
| BPAQ | Total | 73.87±14.68 | 164 | 70.42±18.02 | 157 | 1.88 | 319 | ns |
| Anger | 17.29±4.95 | 15.96±4.43 | 2.56 | * | ||||
| Physical Aggression | 19.83±4.31 | 18.03±5.47 | 2.60 | * | ||||
| Hostility | 23.14±4.10 | 22.93±5.15 | 0.40 | ns | ||||
| Verbal Aggression | 14.06±4.35 | 13.50±4.89 | 1.08 | ns | ||||
| PCS | Total | 9.21±7.89 | 165 | 7.83±6.13 | 162 | 1.76 | 325 | ns |
| Reactive&Overt | 2.58±3.11 | 1.81±2.26 | 3.03 | * | ||||
| Proactive&Overt | 1.13±1.79 | 0.70±1.20 | 1.69 | ns | ||||
| Reactive&Relational | 3.19±2.76 | 3.23±2.75 | 0.16 | ns | ||||
| Proactive&Relational | 2.32±2.15 | 2.08±1.77 | 0.94 | ns | ||||
The average scores, standard deviations, sample sizes, and values for t, df, and statistical significance (sig.) are shown.
*indicates statistically significant differences between genders at p<0.05 by the two-tailed independent t-test in total scores for BDHI, BPAQ, and PCS and by two-way ANOVA in subscales for BPAQ and PCS.
Aggression-related EEG responses
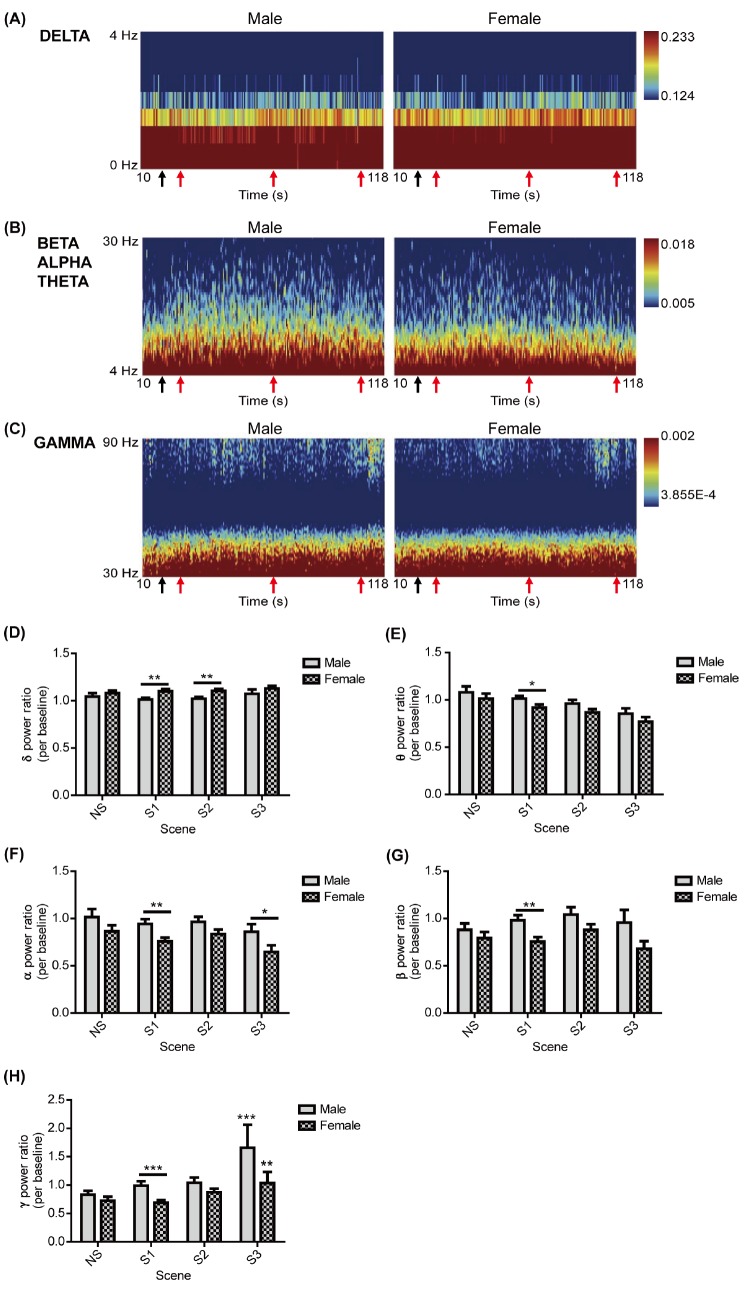
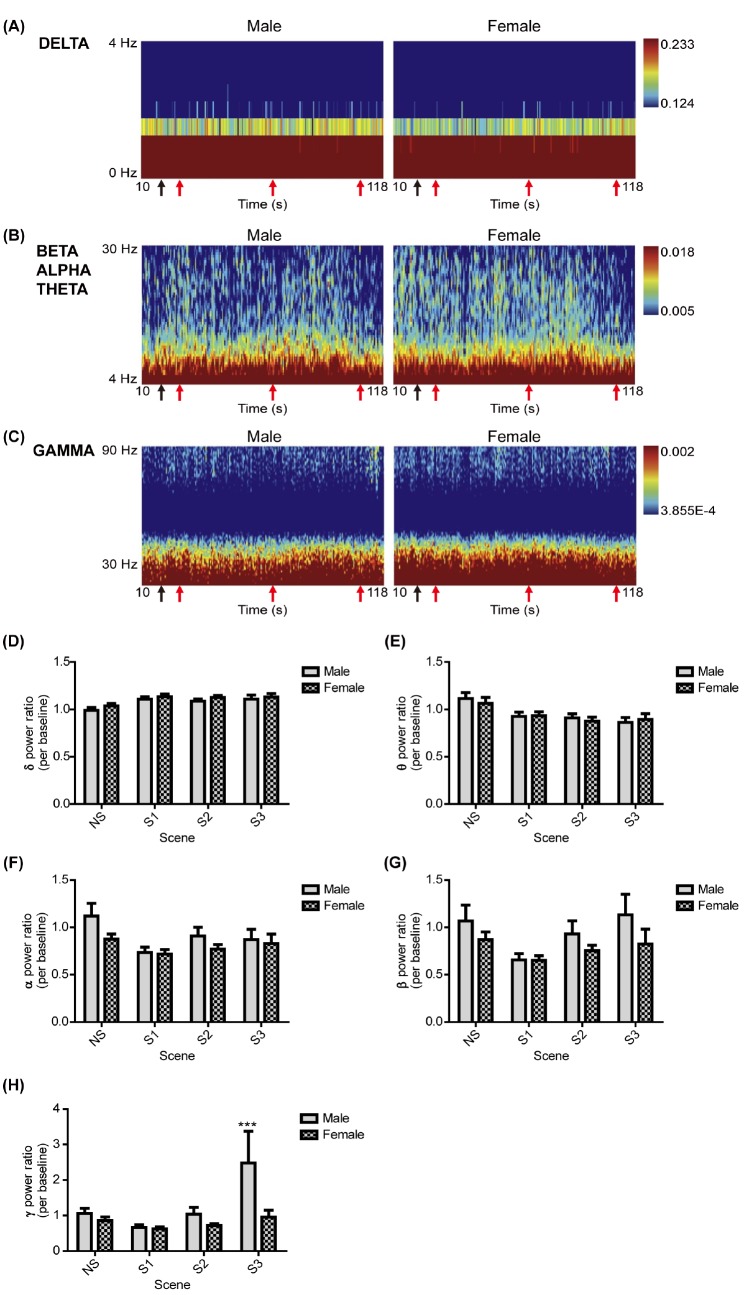
The EEG responses to the aggression-inducing video stimuli in Fp2 and F8 channels were analyzed by gender. The power spectrum of frequency intervals (0~4 Hz, δ signal; 4~30 Hz, θ, α, and β signals; 30~90 Hz, γ signal) according to time in the two channels was displayed as a heat map (Figs. 1A~C and 2A~C). When the power spectrum was quantified into power ratios according to the frequency intervals (α, β, γ, θ, and δ signals) and video stimuli (neutral scene, scene 1, scene 2, and scene 3), men and women showed a statistically significant higher γ power ratio in the F8 channel compared to the neutral scene responses only in response to scene 3 (Fig. 1H). There was a greater increase in the γ power ratio in men than in women (Fig. 1H). Although all of the power ratios in response to scene 1, the δ power ratio in response to scene 2, and the α power ratio in response to scene 3 showed significant differences between genders, the difference was not statistically significant between the neutral scene and aggressive scenes (Figs. 1D~H). For the power ratios measured in the Fp2 channel, only the γ power ratio response to scene 3 in men showed a significant difference to responses to the neutral scene, and the difference was not significantly different for women (Fig. 2H). Furthermore, another power ratio in the Fp2 channel did not show any significant difference between neutral scenes and aggression scenes in either men or women (Figs. 2D~H). Therefore, during scene 3, the γ signals measured in F8 were different from the neutral scene, and men showed a greater response than women.
Fig. 1. Comparisons of EEGs in the F8 channel between genders. (A), (B), and (C) represent the power spectrum by time (10~118 s) and frequency intervals, the δ (0~4 Hz), θ-α-β (4~8 Hz, 8~13 Hz, 13~30 Hz) signals, and the γ signals (30~90 Hz) in the F8 channel, respectively, according to gender. At the bottom of each panel, the first black arrow represents the start of the neutral scene (NS, 19 s), and the second, third, and fourth red arrows represent the starts of scene 1, scene 2, and scene 3 (S1, 28 s; S2, 70 s; S3, 108 s), respectively. (D~H) represent the power ratio of the δ, θ, α, β, and γ signals in the F8 channel, respectively, in response to the NS and aggressive scenes across genders. All values are represented as the mean±SEM. The sample size for EEG measurement was 84 individuals, of which 36 were men, and 48 were women. *, **, and *** above the horizontal lines indicate statistically significant differences between men and women at p<0.05, 0.01, and 0.001, respectively, as determined by the two-tailed independent t-test. Therefore, *, **, and *** above the graph bar indicate statistically significant differences between the NS and aggression scenes (S1 or S2 or S3) at p<0.05, 0.01, and 0.001, respectively, as determined by the twoway ANOVA.
Fig. 2. Comparisons of EEGs in the Fp2 channel between genders. (A), (B), and (C) represent the power spectrum across time (10~118 s) and frequency intervals, the δ (0~4 Hz), θ-α-β (4~8 Hz, 8~13 Hz, 13~30 Hz) signal, and γ signal (30~90 Hz) in the Fp2 channel, respectively, according to gender. At the bottom of each panel, the first black arrow represents the start of the NS (19 s); the second, third, and fourth red arrows represent the start of scene 1, scene 2, and scene 3 (S1, 28 s; S2, 70 s; S3, 108 s), respectively. (D~H) represent the power ratios of the δ, θ, α, β, and γ signals in the Fp2 channel, respectively, in response to the NS and aggression scenes, between sexes. All values are represented as the mean±SEM. The sample size for EEG measurement was 84 individuals, of whom 36 were men and 48 were women. None of the values was significantly different between genders, as determined by the two-tailed independent t-test. The symbol *** above the graph bar indicates statistically significant differences between the NS and the aggression scenes (S1 or S2 or S3) at p<0.001 according to the two-way ANOVA.
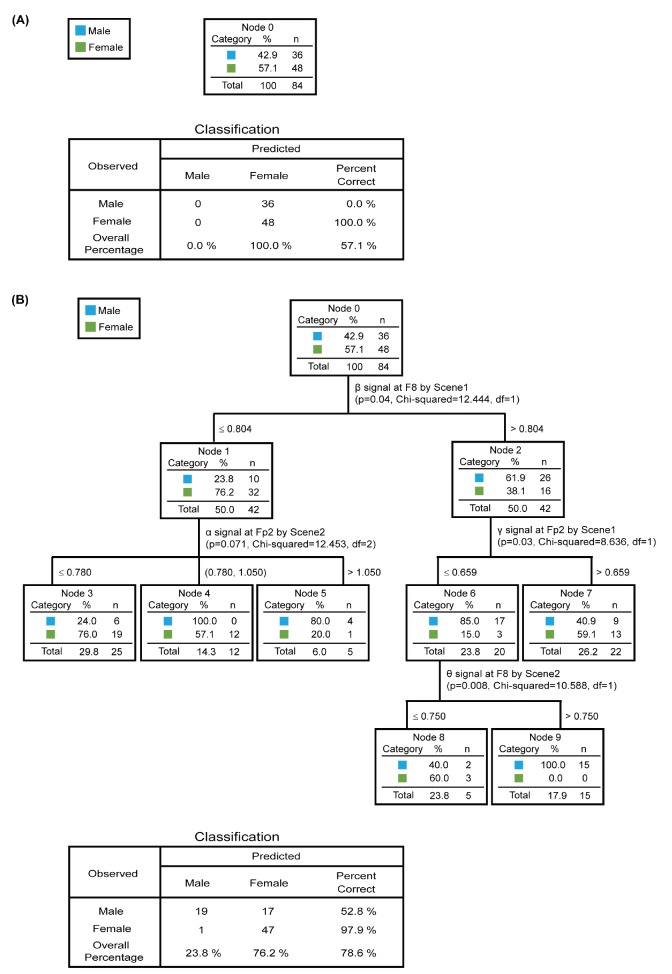
To investigate the relationship between gender and EEG responses, we performed a CHAID analysis. The power ratios of the frequency intervals for each video scene in the Fp2 and F8 channels were variables in determining the gender distinction. During exposure to the neutral scene, none of the power ratios in either channel predicted any variables that distinguished the genders (Fig. 3A). In contrast, in the power ratios of the frequency intervals in response to scenes 1, 2, and 3; the β signal in the F8 channel and the γ signal in the Fp2 channel in response to scene 1, the θ signal in the F8 channel, and the α signal in the Fp2 channel in response to scene 2 distinguished the genders with a 78.6% prediction value (Fig. 3B). Among these, the most critical value that differentiated men and women was the β signal in the F8 channel in response to scene 1, which represents the parent node. Moreover, the γ signal in the Fp2 channel in response to scene 1 represents the child node, and the α signal in the Fp2 channel and θ signal in the F8 channel in response to scene 2 represent the terminal nodes that act as variables to differentiate the sexes. Therefore, the EEG signals of α, β, γ, and θ in two channels are involved in gender-distinct responses to the video stimuli depicting an argument and conflict between peers.
Fig. 3. EEGs involved in gender distinction. The CHAID decision classification tree analysis was applied to identify gender distinctions in EEG signals from two channels. (A) EEG signals in response to the NS were target variables, but these were not included in the decision tree model. (B) EEG signals in response to scene 1, scene 2, and scene 3 (S1, S2, and S3, respectively) were target variables. The following four variables were used for grouping the decision tree model: the β signal in the F8 channel and the γ signal in the Fp2 channel in response to scene 1, and the α signal in the Fp2 channel, and the θ signal in the F8 channel, in response to scene 2. The model included a total of 10 nodes with six terminal nodes (number 3, 4, 5, 7, 8, and 9). Values for p, chi-squared, and the df are shown in each node. The sample size comprised 84 participants, of whom 36 were men and 48 were women. Variables reaching a significance value of p<0.1 were included in the model.
Aggression-related ECG responses
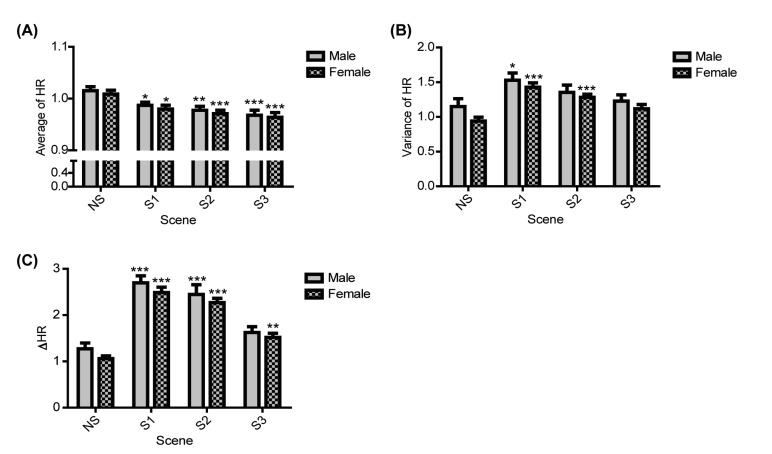
The HRs from the ECG responses to the aggression-inducing videos were analyzed by gender. The average HR was significantly different for all the aggressive scenes compared to the neutral scene; the decrease in HR in response to aggressive scenes rather than the neutral scene was no different between men and women (NS-S1, 0.0283 and 0.0288; NS-S2, 0.0380 and 0.0380; NS-S3, 0.0472 and 0.0450, difference values for men and women, respectively) (Fig. 4A). The analysis of HR variance between men and women showed a statically significant HR increase in response to scene 1 compared to the NS. The increase was higher in women than men (NS-S1, 0.381 and 0.486, difference values for men and women, respectively) (Fig. 4B). Meanwhile, for ΔHR, men and women both showed a statistically significance increase in response to scene 1 and scene 2 compared to the neutral scene, but there was little difference in the increase of ΔHR between genders (NS-S1, 1.423 and 1.428; NS-S2, 1.173 and 1.210, difference values for men and women, respectively) (Fig. 4C). The CHAID analysis showed that such HR values did not act as gender distinction variables (data not shown). Therefore, during response to aggression-inducing stimuli compared to neutral stimuli, our results showed that women's HR increases more than do men's.
Fig. 4. Comparison of ECGs between genders. The ECG signal was used to calculate HR (unit: BPM) and was converted the average, variance, and rate of change between maximum and minimum values. (A) represents the average HR, (B) represents the variance in HR, and (C) represents ΔHR in response to the NS and aggression scenes (S1, S2, and S3) in men and women. All values are represented as the mean±SEM. The ECG sample size comprised 70 participants, of whom 30 were men and 40 were women. None of the values showed a statistically significant difference between genders, as determined by the two-tailed independent t-test. The symbols *, **, and *** above the graph bar indicate statistically significant differences between the NS and aggression scenes (S1 or S2 or S3) at p<0.05, 0.01, and 0.001, respectively, as determined by the two-way ANOVA.
DISCUSSION
In psychology and the social sciences, gender difference in aggression are not only evolutionarily determined, and directly related to survival in terms of food, spouses, and territory competition, but it also results from the socialized roles of both genders and the associated customs accompanying industrial development that have interacted with their physical characteristics [68]. Men are commonly viewed as more aggressive than women [69]. Our results, which are based upon self-report questionnaires, whose long research history and widespread use ensure statistical relevance, also support this gender stereotype. The most widespread questionnaire, BDHI [70], identified that the average score was higher for men than for women. More specific differences between genders were identified with the BPAQ, which is an analytical improvement with subscales, and PCS, which includes subscales on questions about relational and indirect aggression. Men received higher scores than women in openly expressed anger, including physical, and reactive and overt aggression, which supports previous study results based upon self-report questionnaires and analyzing behavior [12,13,14,15]. However, our study found no differences between the genders in relational and indirect aggression, which are the dominant types of aggression experienced and expressed by women [4,5,6]. Gender differences in self-reports of indirect aggression were also not found for adults in other studies [6,71]. This indicates that direct aggression is more easily detected in these self-report questionnaires. Likewise, these results suggest that self-reports can be important variables for indirect aggression in both men and women. Therefore, there is a need to supplement the analysis of passive categories of aggression to further enhance our understanding of gender-based differences in aggression using psychological approaches.
When investigating neurobiological differences in experimentally induced aggression by gender, the EEG and ECG results of our study found gender-related patterns. We discovered that the difference in γ signals in the right ventrolateral frontal region between aggression-inducing stimuli and neutral stimuli was greater in men than in women. Previous studies have reported that EEG responses in those particular regions are related to aggressive behavior in people with mental illnesses [72]. In particular, right frontal γ signals show increased activity in response to unpleasant pictures [73] and are also involved in impulse control governing addiction [48]. While this is somewhat contrary to previous research showing that women have a higher emotional inhibition-related oscillation in response to aggressive situations [42], our result reveals that oscillational responses to aggression-inducing stimuli that are related to unpleasantness or emotional control are stronger in men. Furthermore, we also discovered greater ECG responses to aggression-inducing stimuli in women than in men. Although a low resting state HR is a well-established physiological characteristic of aggression [38,40,41,74], HR variabilities have been used to measure emotional regulation capacity under provocation rather than simple HR reactivity [75,76,77]. Our results showed that women experience a greater increase in HR in response to aggression-inducing stimuli than men. This suggests the possibility of a greater capacity for autonomic emotional control in women. Meanwhile, EEG and ECG are attributed to different conditions during emotional processing by the nervous system. EEG contributes to understanding cognitive processing by measuring electrocortical activation, while ECG provides evidence of autonomic reactivity by analyzing interactions between vagal and sympathetic systems [75,78]. We found that men have higher EEG responses and women have higher ECG responses to provocation. Our findings suggest that the areas of the nervous system that is more actively involved in aggression processes may differ depending on the gender.
One interesting result is that gender differences in ECG response appear to be determined by situational provocations, including verbal abuse, while the gender difference in EEG response appear to be determined by simple aversion, such as scratching a blackboard with fingernails. This is particularly noteworthy from a meta-analysis perspective that emphasizes provocation [12,13]. These analyses reveal that under relatively neutral conditions, men were more aggressive than women; however, this difference is attenuated by increasing provocations such as insults, physical attack, and negative feedback on the experimental paradigm [13]. Since we used visual stimuli, including provocations that reflect the real world rather than the experimental paradigm of aggression, a direct comparison with previous results is difficult. Our results suggest there is no constant direction of attenuation or augmentation of gender differences between aggression-related responses and neutral responses. Therefore, an analysis of provocations that correspond to the complexity of aggression are required when interpreting gender differences in neurophysiological phenomena in response to aggression-related stimuli.
The general model of aggression hypothesizes that personality factors such as aggression determine the response to provocations through cognitive and emotional pathways and induce impulsive actions [79]. While a mere observation of responses cannot cover all cognitive processes, our study determined that at least some aggression traits could be inferred based on these responses. As such, we conducted a CHAID analysis to verify that the EEG responses of the right ventromedial prefrontal α and γ signals and right ventrolateral frontal β and θ signals in response to situational aggression stimuli predict gender distinction. In particular, right frontal asymmetric activity of the α signal is an indicator of avoidance motivation related to aggression [43,45] while the right frontal β signal is also reported to reflect emotional avoidance [46,47]. Moreover, the right frontal γ signal is known to reflect impulse control [48], and another study has reported that the right frontal θ signal is involved in controlling reactive aggression [49]. Although research about the physiological functions of right frontal EEG activities remains uncertain, these results support the hypothesis that there is a functional difference between men and women in the cognition processes controlling aggression. In addition, the difference between the stimulus (scene 3) that induced gender differences and the stimuli (scene 1 and scene 2) involved in gender distinction also raises the importance of provocation mentioned above.
Our analyses of aggression by gender suggest that women show weaker psychological and EEG responses to provocation, but further research is necessary to solidify such claims. Aggression-related EEG and ECG responses leave room for interpretation; therefore, research investigating a wider variety of brain regional options, levels of provocation, and using different types of experimental paradigms is required, to more clearly identify the gender difference in aggression [80]. Despite such research limitations, our study provides novel evidence supporting the functional differences in cortical oscillational activity and autonomic reactivity between men and women in relation to aggression. This provides deeper insights into how gender affects cognitive and emotional neurobiological mechanisms that will improve predictions of behavior. In addition, improving the interpretation of oscillational activities in the nervous system will enhance our objective understanding of the different emotional realms of men and women.
ACKNOWLEDGEMENTS
We thank Mr. Won-Seok Kang for technical advice on the physiological experiments. This study was supported by DGIST funds from the Korean government; Laboratory Expenses of Academic Support and Infrastructure for Undergraduate Studies of Academic Infrastructure Establishment; Education Innovation Activity Fund [grant number 2018010154]; Undergraduate Research Program 2017 grant from the Korea Foundation for the Advancement of Science & Creativity; Undergraduate Group Research Program 2018 grant from DGIST; and the Ministry of Science, ICT and Future Planning & DGIST [grant number 18-BD-0402].
References
- 1.Tardiff K, Sweillam A. Assault, suicide, and mental illness. Arch Gen Psychiatry. 1980;37:164–169. doi: 10.1001/archpsyc.1980.01780150054005. [DOI] [PubMed] [Google Scholar]
- 2.Hartup WW. Aggression in childhood. Developmental perspectives. Am Psychol. 1974;29:336–341. doi: 10.1037/h0037622. [DOI] [PubMed] [Google Scholar]
- 3.Frodi A, Macaulay J, Thome PR. Are women always less aggressive than men? A review of the experimental literature. Psychol Bull. 1977;84:634–660. [PubMed] [Google Scholar]
- 4.Björkqvist K, Österman K, Lagerspetz KM. Sex differences in covert aggression among adults. Aggress Behav. 1994;20:27–33. [Google Scholar]
- 5.Österman K, Björkqvist K, Lagerspetz KM, Kaukiainen A, Landau SF, Frączek A, Caprara GV. Cross-cultural evidence of female indirect aggression. Aggress Behav. 1998;24:1–8. [Google Scholar]
- 6.Archer J, Coyne SM. An integrated review of indirect, relational, and social aggression. Pers Soc Psychol Rev. 2005;9:212–230. doi: 10.1207/s15327957pspr0903_2. [DOI] [PubMed] [Google Scholar]
- 7.Gregoski M, Malone WA, Richardson DS. Measuring direct and indirect aggression: behavior of is there a response bias? Psychol Rep. 2005;97:563–566. doi: 10.2466/pr0.97.2.563-566. [DOI] [PubMed] [Google Scholar]
- 8.Taylor SP. Aggressive behavior and physiological arousal as a function of provocation and the tendency to inhibit aggression. J Pers. 1967;35:297–310. doi: 10.1111/j.1467-6494.1967.tb01430.x. [DOI] [PubMed] [Google Scholar]
- 9.Cherek DR. Effects of smoking different doses of nicotine on human aggressive behavior. Psychopharmacology (Berl) 1981;75:339–345. doi: 10.1007/BF00435849. [DOI] [PubMed] [Google Scholar]
- 10.Buss AH, Durkee A. An inventory for assessing different kinds of hostility. J Consult Psychol. 1957;21:343–349. doi: 10.1037/h0046900. [DOI] [PubMed] [Google Scholar]
- 11.Buss AH, Perry M. The aggression questionnaire. J Pers Soc Psychol. 1992;63:452–459. doi: 10.1037//0022-3514.63.3.452. [DOI] [PubMed] [Google Scholar]
- 12.Bettencourt BA, Kernahan C. A meta-analysis of aggression in the presence of violent cues: effects of gender differences and aversive provocation. Aggress Behav. 1997;23:447–456. [Google Scholar]
- 13.Bettencourt BA, Miller N. Gender differences in aggression as a function of provocation: a meta-analysis. Psychol Bull. 1996;119:422–447. doi: 10.1037/0033-2909.119.3.422. [DOI] [PubMed] [Google Scholar]
- 14.Salmivalli C, Lagerspetz K, Björkqvist K, Österman K, Kaukiainen A. Bullying as a group process: participant roles and their relations to social status within the group. Aggress Behav. 1996;22:1–15. [Google Scholar]
- 15.Crick NR, Dodge KA. A review and reformulation of social information-processing mechanisms in children’s social adjustmen. Psychol Bull. 1994;115:74–101. [Google Scholar]
- 16.Crick NR, Werner NE, Casas JF, O’Brien KM, Nelson DA, Grotpeter JK, Markon K. Childhood aggression and gender: a new look at an old problem. Nebr Symp Motiv. 1998;45:75–141. [PubMed] [Google Scholar]
- 17.Rys GS, Bear GG. Relational aggression and peer relations: gender and developmental issues. Merrill-Palmer Q. 1997;43:87–106. [Google Scholar]
- 18.Henington C, Hughes JN, Cavell TA, Thompson B. The role of relational aggression in identifying aggressive boys and girls. J Sch Psychol. 1998;36:457–477. [Google Scholar]
- 19.Oh I, Hazler RJ. Contributions of personal and situational factors to bystanders’ reactions to school bullying. Sch Psychol Int. 2009;30:291–310. [Google Scholar]
- 20.Ahmed SM. Factors affecting frustrating and aggression relationships. J Soc Psychol. 1982;116:173–177. doi: 10.1080/00224545.1982.9922769. [DOI] [PubMed] [Google Scholar]
- 21.Krämer UM, Jansma H, Tempelmann C, Münte TF. Tit-for-tat: the neural basis of reactive aggression. Neuroimage. 2007;38:203–211. doi: 10.1016/j.neuroimage.2007.07.029. [DOI] [PubMed] [Google Scholar]
- 22.Skibsted AP, Cunha-Bang SD, Carré JM, Hansen AE, Beliveau V, Knudsen GM, Fisher PM. Aggression-related brain function assessed with the Point Subtraction Aggression Paradigm in fMRI. Aggress Behav. 2017;43:601–610. doi: 10.1002/ab.21718. [DOI] [PubMed] [Google Scholar]
- 23.Lotze M, Veit R, Anders S, Birbaumer N. Evidence for a different role of the ventral and dorsal medial prefrontal cortex for social reactive aggression: an interactive fMRI study. Neuroimage. 2007;34:470–478. doi: 10.1016/j.neuroimage.2006.09.028. [DOI] [PubMed] [Google Scholar]
- 24.Chester DS, DeWall CN. Sound the alarm: the effect of narcissism on retaliatory aggression is moderated by dACC reactivity to rejection. J Pers. 2016;84:361–368. doi: 10.1111/jopy.12164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Martinelli I, Lensing AW, Middeldorp S, Levi M, Beyer-Westendorf J, van Bellen B, Bounameaux H, Brighton TA, Cohen AT, Trajanovic M, Gebel M, Lam P, Wells PS, Prins MH. Recurrent venous thromboembolism and abnormal uterine bleeding with anticoagulant and hormone therapy use. Blood. 2016;127:1417–1425. doi: 10.1182/blood-2015-08-665927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Repple J, Habel U, Wagels L, Pawliczek CM, Schneider F, Kohn N. Sex differences in the neural correlates of aggression. Brain Struct Funct. 2018;223 doi: 10.1007/s00429-018-1739-5. [DOI] [PubMed] [Google Scholar]
- 27.Herpertz SC, Nagy K, Ueltzhöffer K, Schmitt R, Mancke F, Schmahl C, Bertsch K. Brain mechanisms underlying reactive aggression in borderline personality disorder-sex matters. Biol Psychiatry. 2017;82:257–266. doi: 10.1016/j.biopsych.2017.02.1175. [DOI] [PubMed] [Google Scholar]
- 28.Harmon-Jones E, Barratt ES, Wigg C. Impulsiveness, aggression, reading, and the P300 of the event-related potential. Pers Individ Dif. 1997;22:439–445. [Google Scholar]
- 29.Gerstle JE, Mathias CW, Stanford MS. Auditory P300 and self-reported impulsive aggression. Prog Neuropsychopharmacol Biol Psychiatry. 1998;22:575–583. doi: 10.1016/s0278-5846(98)00027-x. [DOI] [PubMed] [Google Scholar]
- 30.Mathias CW, Stanford MS. P300 under standard and surprise conditions in self-reported impulsive aggression. Prog Neuropsychopharmacol Biol Psychiatry. 1999;23:1037–1051. doi: 10.1016/s0278-5846(99)00053-6. [DOI] [PubMed] [Google Scholar]
- 31.Krämer UM, Büttner S, Roth G, Münte TF. Trait aggressiveness modulates neurophysiological correlates of laboratory-induced reactive aggression in humans. J Cogn Neurosci. 2008;20:1464–1477. doi: 10.1162/jocn.2008.20103. [DOI] [PubMed] [Google Scholar]
- 32.Green S, Egaña M, Baldi JC, Lamberts R, Regensteiner JG. Cardiovascular control during exercise in type 2 diabetes mellitus. J Diabetes Res. 2015;2015:654204. doi: 10.1155/2015/654204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Volavka J. Aggression, electroencephalography, and evoked potentials: a critical review. Neuropsychiatry Neuropsychol Behav Neurol. 1990;3:249–259. [Google Scholar]
- 34.Raine A, Venables PH, Williams M. Relationships between central and autonomic measures of arousal at age 15 years and criminality at age 24 years. Arch Gen Psychiatry. 1990;47:1003–1007. doi: 10.1001/archpsyc.1990.01810230019003. [DOI] [PubMed] [Google Scholar]
- 35.Harmon-Jones E, Allen JJ. Anger and frontal brain activity: EEG asymmetry consistent with approach motivation despite negative affective valence. J Pers Soc Psychol. 1998;74:1310–1316. doi: 10.1037//0022-3514.74.5.1310. [DOI] [PubMed] [Google Scholar]
- 36.Hewig J, Hagemann D, Seifert J, Naumann E, Bartussek D. On the selective relation of frontal cortical asymmetry and anger-out versus anger-control. J Pers Soc Psychol. 2004;87:926–939. doi: 10.1037/0022-3514.87.6.926. [DOI] [PubMed] [Google Scholar]
- 37.Stewart JL, Levin-Silton R, Sass SM, Heller W, Miller GA. Anger style, psychopathology, and regional brain activity. Emotion. 2008;8:701–713. doi: 10.1037/a0013447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Suls J, Wan CK. The relationship between trait hostility and cardiovascular reactivity: a quantitative review and analysis. Psychophysiology. 1993;30:615–626. doi: 10.1111/j.1469-8986.1993.tb02087.x. [DOI] [PubMed] [Google Scholar]
- 39.Katz LF, Gottman JM. Vagal tone protects children from marital conflict. Dev Psychopathol. 2009;7:83–92. [Google Scholar]
- 40.Scarpa A, Raine A. Psychophysiology of anger and violent behavior. Psychiatr Clin North Am. 1997;20:375–394. doi: 10.1016/s0193-953x(05)70318-x. [DOI] [PubMed] [Google Scholar]
- 41.Lorber MF. Psychophysiology of aggression, psychopathy, and conduct problems: a meta-analysis. Psychol Bull. 2004;130:531–552. doi: 10.1037/0033-2909.130.4.531. [DOI] [PubMed] [Google Scholar]
- 42.Knyazev GG, Bocharov AV, Slobodskoj-Plusnin JY. Hostility- and gender-related differences in oscillatory responses to emotional facial expressions. Aggress Behav. 2009;35:502–513. doi: 10.1002/ab.20318. [DOI] [PubMed] [Google Scholar]
- 43.Denson TF, O’Dean SM, Blake KR, Beames JR. Aggression in women: behavior, brain and hormones. Front Behav Neurosci. 2018;12:81. doi: 10.3389/fnbeh.2018.00081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Coyiuto C. Student library research awards. Wellesley, MA: Wellesley College Digital Scholarship and Archive; 2016. Resting EEG asymmetries and levels of irritability; p. 16. [Google Scholar]
- 45.Harmon-Jones E, Gable PA, Peterson CK. The role of asymmetric frontal cortical activity in emotion-related phenomena: a review and update. Biol Psychol. 2010;84:451–462. doi: 10.1016/j.biopsycho.2009.08.010. [DOI] [PubMed] [Google Scholar]
- 46.Jensen O, Lisman JE. Hippocampal sequence-encoding driven by a cortical multi-item working memory buffer. Trends Neurosci. 2005;28:67–72. doi: 10.1016/j.tins.2004.12.001. [DOI] [PubMed] [Google Scholar]
- 47.Schutter DJ, de Weijer AD, Meuwese JD, Morgan B, van Honk J. Interrelations between motivational stance, cortical excitability, and the frontal electroencephalogram asymmetry of emotion: a transcranial magnetic stimulation study. Hum Brain Mapp. 2008;29:574–580. doi: 10.1002/hbm.20417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Choi JS, Park SM, Lee J, Hwang JY, Jung HY, Choi SW, Kim DJ, Oh S, Lee JY. Resting-state beta and gamma activity in Internet addiction. Int J Psychophysiol. 2013;89:328–333. doi: 10.1016/j.ijpsycho.2013.06.007. [DOI] [PubMed] [Google Scholar]
- 49.Krämer UM, Kopyciok RP, Richter S, Münte TF. Oscillatory brain activity related to control mechanisms during laboratory-induced reactive aggression. Front Behav Neurosci. 2009;3:46. doi: 10.3389/neuro.08.046.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Suls J, Wan CK. The relationship between trait hostility and cardiovascular reactivity: a quantitative review and analysis. Psychophysiology. 1993;30:615–626. doi: 10.1111/j.1469-8986.1993.tb02087.x. [DOI] [PubMed] [Google Scholar]
- 51.Perach-Barzilay N, Tauber A, Klein E, Chistyakov A, Ne’eman R, Shamay-Tsoory SG. Asymmetry in the dorsolateral prefrontal cortex and aggressive behavior: a continuous thetaburst magnetic stimulation study. Soc Neurosci. 2013;8:178–188. doi: 10.1080/17470919.2012.720602. [DOI] [PubMed] [Google Scholar]
- 52.Hong KJ, Rho AY. The effects of assertive training on the reduction of aggression and anxiety in juvenile delinquents. Korean J Clin Psychol. 1983;4:19–31. [Google Scholar]
- 53.Jeong D. A buffering effects of social support on children’s school stress and maladjustments. Seoul: Korea University; 1995. [Google Scholar]
- 54.Lee S. Relations of psychopathy and narcissism with aggression: focusing on differential triggers. Seoul: Sungkyunkwan University; 2015. [Google Scholar]
- 55.Kuppens P, Van Mechelen I. Interactional appraisal models for the anger appraisals of threatened self-esteem, other-blame, and frustration. Cogn Emot. 2007;21:56–77. [Google Scholar]
- 56.Marsee MA, Barry CT, Childs KK, Frick PJ, Kimonis ER, Muñoz LC, Aucoin KJ, Fassnacht GM, Kunimatsu MM, Lau KS. Assessing the forms and functions of aggression using self-report: factor structure and invariance of the Peer Conflict Scale in youths. Psychol Assess. 2011;23:792–804. doi: 10.1037/a0023369. [DOI] [PubMed] [Google Scholar]
- 57.Han Y. The relation of psychological variables to relational aggression in early adolescence. Suwon: Ajou University; 2008. [Google Scholar]
- 58.Ha M, Kim J. The validation of the Korean Peer Conflict Scale(K-PCS) for use with young adolescents. Stud Korean Youth. 2013;24:71–101. [Google Scholar]
- 59.Kim Y. The relationship among the parent’s verbal control type the aggression of children and the self-esteems of children. Seoul: Ewha Womans University; 1997. [Google Scholar]
- 60.Seo SG, Kwon SM. Development and validation study of the Anger Thought Scale: primary/secondary anger-thought. Korean J Clin Psychol. 2005;24:187–206. [Google Scholar]
- 61.Infante DA, Rancer AS, Wigley CJ., III In defense of the argumentativeness and verbal aggressiveness scales. Commun Q. 2011;59:145–154. [Google Scholar]
- 62.Salmivalli C, Nieminen E. Proactive and reactive aggression among school bullies, victims, and bully-victims. Aggress Behav. 2002;28:30–44. [Google Scholar]
- 63.Jara N, Casas JA, Ortega-Ruiz R. Proactive and reactive aggressive behavior in bullying: the role of values. Int J Educ Psychol. 2017;6:1–24. [Google Scholar]
- 64.Ely DJ. Aversiveness without pain: potentiation of imaginai and auditory effects of blackboard screeches. Bull Psychon Soc. 1975;6:295–296. [Google Scholar]
- 65.Halpern DL, Blake R, Hillenbrand J. Psychoacoustics of a chilling sound. Percept Psychophys. 1986;39:77–80. doi: 10.3758/bf03211488. [DOI] [PubMed] [Google Scholar]
- 66.Torres G, Cinelli MP, Hynes AT, Kaplan IS, Leheste JR. Electroencephalogram mapping of brain states. J Neurosci Neuroeng. 2014;3:73–77. [Google Scholar]
- 67.Du R, Lee HJ. Power spectral performance analysis of EEG during emotional auditory experiment; Proceeding of the 2014 International Conference on Audio, Language and Image Processing; Shanghai. 2014. [Google Scholar]
- 68.Wood W, Eagly AH. A cross-cultural analysis of the behavior of women and men: implications for the origins of sex differences. Psychol Bull. 2002;128:699–727. doi: 10.1037/0033-2909.128.5.699. [DOI] [PubMed] [Google Scholar]
- 69.Scarduzio JA, Carlyle KE, Harris KL, Savage MW. “Maybe she was provoked”: Exploring gender stereotypes about male and female perpetrators of intimate partner violence. Violence Against Women. 2017;23:89–113. doi: 10.1177/1077801216636240. [DOI] [PubMed] [Google Scholar]
- 70.Biaggio MK. Assessment of anger arousal. J Pers Assess. 1980;44:289–298. doi: 10.1207/s15327752jpa4403_12. [DOI] [PubMed] [Google Scholar]
- 71.Forrest S, Eatough V, Shevlin M. Measuring adult indirect aggression: the development and psychometric assessment of the indirect aggression scales. Aggress Behav. 2005;31:84–97. [Google Scholar]
- 72.Li C, Wang XP, Zhang DK, Zhou JS, Guo M. An EEG study that may improve the violence risk assessment in male schizophrenic patients. Aust J Forensic Sci. 2015;47:104–115. [Google Scholar]
- 73.Martini N, Menicucci D, Sebastiani L, Bedini R, Pingitore A, Vanello N, Milanesi M, Landini L, Gemignani A. The dynamics of EEG gamma responses to unpleasant visual stimuli: from local activity to functional connectivity. Neuroimage. 2012;60:922–932. doi: 10.1016/j.neuroimage.2012.01.060. [DOI] [PubMed] [Google Scholar]
- 74.Ortiz J, Raine A. Heart rate level and antisocial behavior in children and adolescents: a meta-analysis. J Am Acad Child Adolesc Psychiatry. 2004;43:154–162. doi: 10.1097/00004583-200402000-00010. [DOI] [PubMed] [Google Scholar]
- 75.Patrick CJ. Psychophysiological correlates of aggression and violence: an integrative review. Philos Trans R Soc Lond B Biol Sci. 2008;363:2543–2555. doi: 10.1098/rstb.2008.0028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Juujärvi P, Kaartinen J, Pulkkinen L, Vanninen E, Laitinen T. Controlling reactive aggression through cognitive evaluation of proactive aggression cues. Cogn Emot. 2006;20:759–784. [Google Scholar]
- 77.Posthumus JA, Böcker KB, Raaijmakers MA, Van Engeland H, Matthys W. Heart rate and skin conductance in four-year-old children with aggressive behavior. Biol Psychol. 2009;82:164–168. doi: 10.1016/j.biopsycho.2009.07.003. [DOI] [PubMed] [Google Scholar]
- 78.Susman EJ. Psychobiology of persistent antisocial behavior: stress, early vulnerabilities and the attenuation hypothesis. Neurosci Biobehav Rev. 2006;30:376–389. doi: 10.1016/j.neubiorev.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 79.Anderson CA, Bushman BJ. Human aggression. Annu Rev Psychol. 2002;53:27–51. doi: 10.1146/annurev.psych.53.100901.135231. [DOI] [PubMed] [Google Scholar]
- 80.Richardson DR, Green LR. Social sanction and threat explanations of gender effects on direct and indirect aggression. Aggress Behav. 1999;25:425–434. [Google Scholar]