Abstract
The safe treatment of patients with chronic obstructive pulmonary disease (COPD) in dental office–based settings can be quite complex without a current understanding of the etiology, course, severity, and treatment modalities of the disease. The additional concerns of providing sedation and/or general anesthesia to patients with COPD in settings outside of a hospital demand thorough investigation of individual patient presentation and realistic development of planned treatment that patients suffering from this respiratory condition can tolerate. Along with other comorbidities, such as advanced age and potential significant cardiovascular compromise, the dental practitioner providing sedation or general anesthesia must tailor any treatment plan to address multiple organ systems and mitigate risks of precipitating acute respiratory failure from inadequate pain and/or anxiety control. Part I of this article will cover the epidemiology, etiology, and pathophysiology of COPD. Patient evaluation in the preoperative period will also be reviewed. Part II will cover which patients are acceptable for sedation/general anesthesia in the dental office–based setting as well as sedation/general anesthesia techniques that may be considered.
Key Words: COPD, Sedation, General anesthesia, Office-based
Chronic obstructive pulmonary disease (COPD) is a progressive inflammatory condition characterized by persistent, irreversible airflow limitation and respiratory symptoms often related to noxious particle or gas exposure. Depending on the severity of the disease and the duration of symptoms, these patients typically have lower airway abnormalities caused by a combination of conducting airway disease and parenchymal destruction. The etiology, clinical manifestations, and disease prognosis vary greatly, making definition and diagnosis challenging.
Although the definition of COPD has evolved to the point where the terms chronic bronchitis and emphysema are no longer included in the definition, understanding of these conditions is crucial to understanding of the disease process and its prognosis. Emphysema refers specifically to destruction of the alveolar septa, leading to alveolar coalescence and a decreased cross-sectional surface area for alveolar gas exchange. It is a pathological term that is often used as a clinical term, describing only one of the structural changes present in patients with COPD. On the other hand, chronic bronchitis is a clinical term defined as cough and sputum production occurring for at least 3 months in 2 consecutive years, in which other potential etiologies have been eliminated. Currently, patients with asthma are also considered to have COPD when the airflow obstruction that is characteristic of that disease is not completely reversible. Emphysema, chronic bronchitis. and asthma may present independently, consecutively, or simultaneously. Regardless, a patient with any of these conditions is not considered to have COPD unless the patient has irreversible airflow obstruction.
EPIDEMIOLOGY
COPD is a common condition, affecting an estimated 384 million people worldwide.1 In the United States alone, 15.7 million people have been diagnosed, accounting for 6.4% of the population.2 However- this estimate is likely deceptively low, as it is based on patient report of a doctor's diagnosis and the disease is often underrecognized and underdiagnosed.3 In fact, more than 50% of adults with reduced pulmonary function may be unaware of their disease status, and the actual number of COPD cases in the United States may be closer to 24 million.3,4 COPD contributes $32 billion annually directly to national health care costs and an additional $20.4 billion in indirect costs. These costs are both expected to rise.5 Despite efforts towards increased awareness and prevention, COPD remains the third leading cause of death among adults in the United States and is expected to become the third leading cause of death worldwide by 2020.6 Given the continued popularity of smoking and increasing life expectancy, the number of deaths may rise as high as 4.5 million per year worldwide.7,8
Multiple epidemiologic studies have shown that exposure to tobacco smoke is the single greatest risk factor for developing COPD, leading to higher prevalence in smokers and former smokers.9 Risk is also increased with exposure to air pollution, occupational dusts, and fumes, as well as heredity factors, a history of childhood respiratory infection, and lower socioeconomic status.10 Although the incidence of disease increases with age, it is unclear if this is due to increased exposure to risk factors or physical aging. Historically, men were more commonly afflicted than women, but as it has become more common for women to smoke tobacco in developed nations, as well as being exposed to indoor air pollution in lower-income countries, women and men are affected almost equally. Interestingly, some studies have shown that women may be more susceptible to developing COPD and emphysema.11,12 Risk is also increased in patients with genetic conditions such as alpha-1 antitrypsin deficiency, in which patients lack a major inhibitor of serine proteases,13 which may lead to destruction of alveolar tissue.
PATHOLOGY
Pathological changes associated with COPD are present in the airways, the lung parenchyma, and the pulmonary vasculature. Patient presentation depends on the underlying mechanism of the disease, disease severity, and individual susceptibility.
Inflammation is the normal response to respiratory irritants such as cigarette smoke or pollution that leads to COPD in genetically susceptible individuals, although the pathogenesis is not fully elucidated. Evidence of chronic inflammation and structural changes from repeated injury and repair within the airways are common findings in all cases of COPD.14 Examples of structural changes include parenchymal fibrosis, coalescence of alveoli, and both narrowing and reduction in the number of small conducting airways present.14 Patients with emphysema and chronic bronchitis typically have increased CD8+ T lymphocytes, neutrophils, and CD68+ monocytes/macrophages. This is contrast to asthma, in which inflammation is associated with increased CD4+ T lymphocytes, eosinophils, IL-4, and IL-5.15 Evidence of oxidative stress (eg, peroxides) is increased in COPD patients and likely contributes to the inappropriate inflammatory response to noxious particles characteristic of COPD.16 The body's ability to manage this influx of oxidizing agents is reduced because of a decrease in endogenous antioxidants in COPD patients.17 Features more specific to chronic bronchitis include increased numbers of goblet cells, enlarged mucous glands, and mucociliary dysfunction.15 Clinical evidence of excess mucus production and fibrosis related to chronic inflammation contribute to increased airway resistance.
Inflammatory cells release proteases such as elastase and matrix metalloproteinases that damage the connective tissue of the alveolar walls and septa of the lung parenchyma. Patients with COPD show a greater number of the protease enzymes that damage connective tissue compared to the antiproteases that typically counteract this action.18 The enlargement and destruction of the distal air spaces causes a loss of elastic recoil, which ultimately leads to diminished expiratory flow rates, air trapping, and airway collapse.19 The pattern in which the lung parenchyma is remodeled depends on the etiology. When this occurs in the central portion of the acinus, the multilobed sacs containing groups of alveoli, it is called proximal acinar or centrilobular emphysema.20 This is most commonly associated with smoking. In panacinar emphysema, the destruction occurs throughout the acinus. This pattern is typically seen in patients with alpha-1 antitrypsin deficiency, although it may present in a subset of patients with proximal acinar emphysema characteristic of smokers. Distal acinar or paraseptal emphysema primarily affects the alveolar ducts.
The pulmonary vasculature is also affected. Early changes include intimal hyperplasia and endothelial dysfunction. As the condition progresses, smooth muscle hypertrophy, collagen deposition, and destruction of the capillary beds eventually occur.20 These factors and resultant global chronic hypoxic vasoconstriction of the pulmonary arteries/arterioles lead to pulmonary hypertension. Chronic pulmonary hypertension increases right ventricular afterload, leading to compromised right heart systolic function. This can lead to right-sided heart enlargement/failure secondary to pulmonary hypertension, also known as cor pulmonale.21
PATHOPHYSIOLOGY
As the disease progresses, airflow limitation increases because of a number of mechanisms. Inflammation with resultant narrowing and loss of small airways leads to increased airway resistance. The extent of this damage is measured by the reduction in forced expiratory volume in 1 second (FEV1) and the FEV1 to forced vital capacity (FVC) ratio.22 The FEV1/FVC ratio represents the proportion of vital capacity that an individual can exhale in the first second of forced expiration. In a patient without obstructive disease, at least 80% of expiration takes place in the first second (FEV1/FVC ≥ 0.8). An irreversible FEV1/FVC ratio less than 0.8 indicates the presence of COPD, with a ratio less than 0.3 indicative of severe COPD. Clinically, this means that exhalation may not be complete by the time the next breath is initiated, leading to air trapping and dynamic hyperinflation.
Emphysema
The loss of the elastic recoil of the lungs that accompanies emphysema contributes to decreased expiratory flow and leads to air trapping and airway collapse. The progressive trapping of gas during expiration causes static hyperinflation, reducing inspiratory capacity and contributing to severe dyspnea and limited exercise capacity.23 Patients with emphysema will often purse their lips during expiration (auto pulmonary end-expiration pressure) to slow collapse of the small airways, hence the term “pink puffers.” On pulmonary function tests these patients typically have increased residual volume (RV), functional residual capacity, total lung capacity (TLC), and RV/TLC ratio (see Figure 1). Although the TLC and functional residual capacity are increased, the area of increased volume, particularly the RV, does not participate in gas exchange. However, these patients are generally only mildly hypoxic, and significant hypercarbia is rare.
Figure 1.
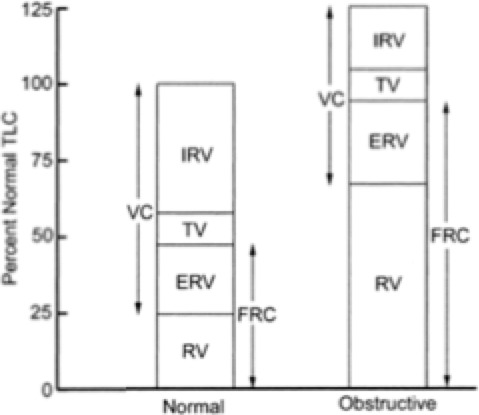
Lung volumes and capacities, normal and COPD.
Destruction of the alveolar structures reduces the diffusing capacity of the lungs, creating a low ventilation/perfusion (V˙/Q˙) mismatch, which impairs gas exchange. Effective gas exchange relies on a balance of blood flow and ventilation; the alveoli must be well ventilated and perfused via the pulmonary capillaries. In emphysema, this V˙/Q˙ mismatch may present relatively early and progress as the disease becomes more severe.
Chronic Bronchitis
The source of the airway obstruction in cases of chronic bronchitis is decreased conducting airway lumen size due to mucus hypersecretion, edema, and smooth muscle hypertrophy from persistent inflammation of the airways. It is an immune reaction to chronic airway irritation by smoke, particulate, or other noxious agents. This manifests clinically as a chronic productive cough along with recurrent viral and bacterial pulmonary infections. Ventilation is severely limited in these patients, resulting in intrapulmonary shunting (low V˙/Q˙ ratio), chronic hypoxemia, and hypercarbia. For this reason, patients with chronic bronchitis may be referred to as “blue bloaters.” Pulmonary function tests will show an increased RV, but TLC is typically maintained.
Eventually, chronic hypoxemia will lead to polycythemia, significant pulmonary hypertension, and eventually cor pulmonale. Chronic hypercapnia and respiratory acidosis contribute to pulmonary arterial vasoconstriction. The long-term pulmonary arteriolar effects include intimal hyperplasia followed by smooth muscle hypertrophy.24 These changes also occur in the microvasculature along with evidence of endothelial cell dysfunction that occurs throughout the lungs. Pulmonary hypertension can be life threatening, leading to right-sided heart failure.
COPD exacerbations may be triggered by bacterial or viral respiratory viruses, environmental toxins, or sometimes unknown factors. Exacerbations are characterized by hypoxemia and increased outflow obstruction as well as hyperinflation and gas trapping, presenting as aggravated dyspnea.24 V˙/Q˙ mismatch will worsen and may result in severe hypoxemia requiring supplemental oxygen administration. In respiratory failure, intubation and prolonged mechanical ventilation may be necessary with poor prognosis.
Supplemental oxygen–induced hypercapnia and decreased ventilation may also occur in patients with COPD. It was previously thought that the primary etiology was due to a blunted response to CO2 in chronically hypercarbic COPD patients, who then relied on hypoxic ventilatory drive to stimulate ventilation.25 Therefore, in the presence of adequate oxygen levels, the patient would not be stimulated to breathe and CO2 levels would continue to rise, which could lead to apnea, unconsciousness, and hypoxia. This classical thinking has been updated to involve a more complex understanding of the mechanisms of hypercarbia induced by increases in supplemental oxygen administration to patients with significant and severe COPD. A minimal decrease in minute ventilation with a corresponding rise in arteriolar partial pressure of carbon dioxide may instead be due to peripheral chemoreceptor response to the increase in fraction of inspired oxygen, but this does not fully account for the hypercarbia. Further investigation and current understanding of the disease process have shown that the principal cause of oxygen-induced hypercapnia in patients with COPD is largely due to a worsening V˙/Q˙ mismatch and the associated increase in dead space.25 Increased fraction of inspired oxygen indirectly redistributes blood flow from well-ventilated alveoli to poorly ventilated areas because of simultaneous loss of hypoxic pulmonary vasoconstriction and CO2-mediated bronchodilation.26 The Haldane effect, the rightward shift of the oxyhemoglobin dissociation curve in the presence of increased PaO2, is another contributing mechanism. In hypoxic patients given high levels of supplemental oxygen, oxyhemoglobin levels increase, resulting in the dissociation of CO2 from hemoglobin, and thereby increasing arteriolar partial pressure of carbon dioxide.
Many patients with COPD also present with multiple comorbidities related to overlapping risk factors, namely smoking, aging, and poor exercise tolerance. Hallmarks of COPD such as airflow obstruction and hyperinflation can have serious cardiac implications in addition to the respiratory symptoms.24 Further, the chronic presence of circulating inflammatory cells may contribute to the development of skeletal muscle wasting, osteoporosis, ischemic heart disease, anemia, diabetes, and metabolic symptoms.27
SEDATION AND GENERAL ANESTHESIA CONCERNS IN COPD PATIENTS: PREOPERATIVE PERIOD
COPD is a common condition that presents multiple challenges for dental sedation and general anesthesia (GA) providers. Increased risk of perioperative pulmonary complications (PPCs) is compounded by the fact that many dental anesthetics are administered in the office-based setting. It is the responsibility of the sedation/GA provider to carefully evaluate these patients preoperatively to determine treatment modification protocols or referral to the hospital setting, if needed. This review focuses on specific considerations for COPD patients undergoing sedation/GA in an office-based setting.
The preoperative period is critical to determine if a COPD patient is a suitable candidate for sedation or GA in the office setting. The 3 key aspects to be evaluated in the preoperative history and physical evaluation specific to COPD itself include the following: (a) identify risk factors for COPD, (b) evaluate the severity of the diminished respiratory function, and (c) determine whether the patient's medical condition can be improved or optimized prior to the procedure. These 3 steps will determine the final anesthetic plan and appropriate venue for sedation/GA. Throughout the preanesthetic evaluation, 2 key questions must be considered: “Is this patient fit for surgery/anesthesia in the office-based setting and, if so, is there anything that can be done to improve the patient's condition that will improve outcome?”28
Identify Signs, Symptoms, and Risk Factors for COPD
COPD is known to lead to a significant increase in PPCs such as lung infection, atelectasis, and airflow limitation, potentially leading to respiratory failure.29 Although some patients are aware of their disease status and are actively treated for COPD, many go undiagnosed and untreated every year. It is the responsibility of the sedation/GA provider to perform a thorough history and physical examination to ensure each patient is properly managed throughout the perioperative period. In addition, because COPD is commonly associated with multiple comorbidities, including cardiovascular disease, skeletal muscle disease, lung cancer, infections, osteoporosis, diabetes, and a constellation of comorbidities known as metabolic syndrome, the presence and severity of these diseases, if present, must also be independently assessed.29
The most common risk factor for COPD is cigarette or tobacco smoking. Smoking history measured in pack-years should be obtained for all patients regardless of presenting symptoms. Other risk factors include occupational exposure, air pollution, and alpha-1 antitrypsin deficiency due to genetic factors. Signs and symptoms of COPD include hyperreactivity to exogenous stimuli, prolonged expiration, and an audible wheeze.30,31 According to the literature, adverse pulmonary events in the hospital setting are increased in patients over 60 years of age, duration of anesthesia over 2.5 hours, a positive cough test (defined by having the patient take a deep inspiration and cough, which leads to another cough), smoking over 40 pack-years, and significant changes in spirometry (FEV1 <1 L; normal is variable from ∼2.5 to 5 L based on age, sex, and body mass).28,32
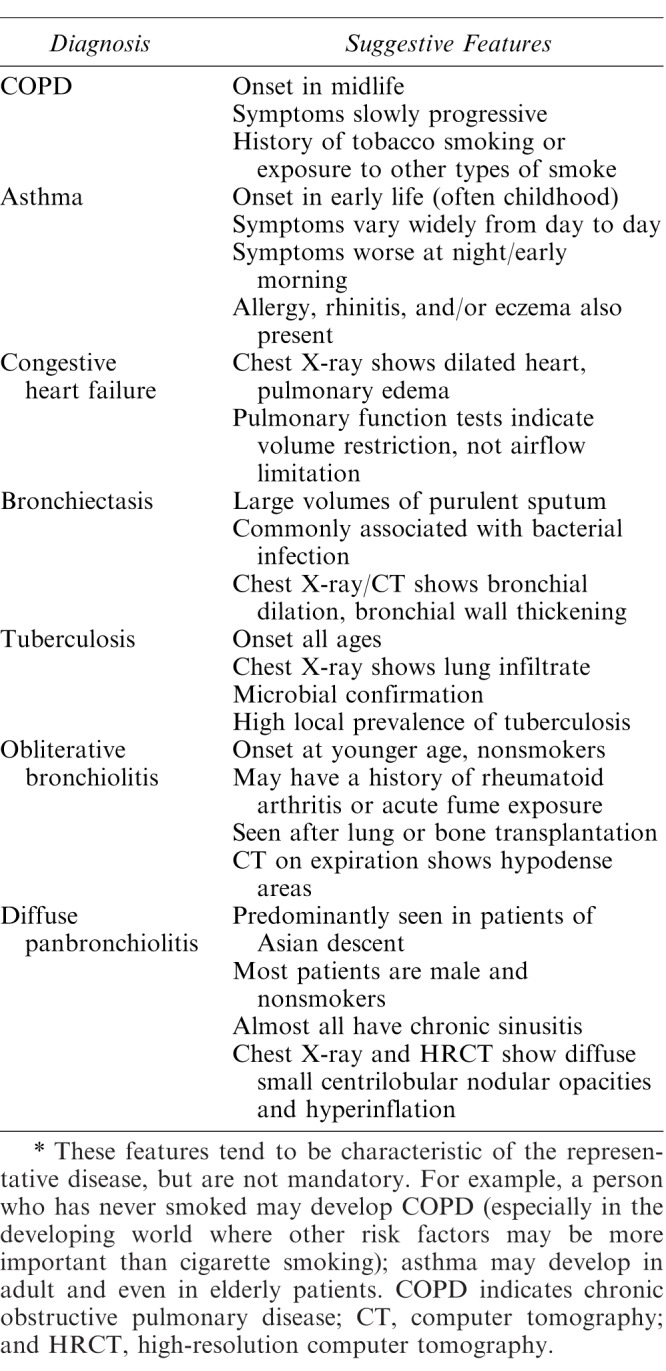
Even without a known diagnosis of COPD, patients may present with overt symptoms such as shortness of breath, wheezing, chronic cough, cyanosis, frequent respiratory infections, and a lack of energy. The differential diagnosis associated with pulmonary dysfunction is vast. Some common conditions and their presentations are listed in Table 1. Physical findings suggestive of severe COPD disease may include a barrel chest, clubbing of the fingers, and jugular venous distension/lower extremity edema. Patients with any of these findings are not appropriate for office-based GA, and even moderate sedation may be inappropriate. Local anesthesia alone, possibly with minimal sedation, may be preferable if the patient's pain and anxiety level allow, and as long as cardiovascular precautions with vasoconstrictors are taken. If any of these presenting signs or symptoms of severe disease are present, the patient should be referred for medical consultation and medically optimized, if possible, by the patient's pulmonologist or primary care provider prior to local anesthesia with possible minimal sedation.
Table 1.
COPD and Its Differential Diagnosis
Evaluate the Severity of Diminished Respiratory Function
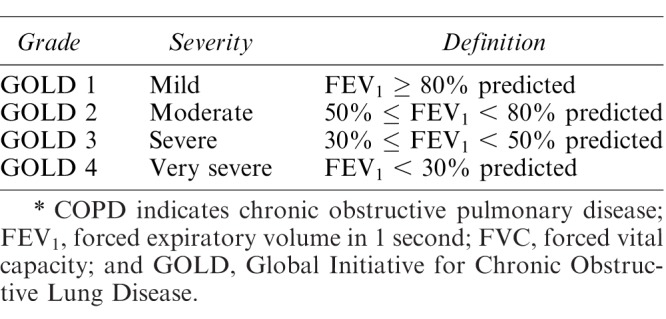
There are multiple ways to assess the severity of COPD. Pulmonary function tests are most frequently used to assess severity and diagnose COPD. Additional testing is generally not indicated for stable patients.33 The Global Initiative for Chronic Obstructive Lung Disease (GOLD) measures respiratory impairment via postbronchodilator airway treatment. GOLD grading, from 1 = mild to 4 = very severe, is defined as mild (FEV1 ≥ 80%), moderate (50% ≤ FEV1 < 80%), severe (30% ≤ FEV1 <50%), and very severe (FEV1 < 30%), which correlates with the incidence of PPCs (Table 2).29
Table 2.
GOLD Severity of Airflow Limitation in COPD, Based on Postbronchodilator FEV1.
Further pulmonary testing that can help determine if a patient is fit for dental/oral surgery may include objective exercise testing and exercise tolerance, such as walking, or interval exercise testing. Metabolic equivalents can be used to evaluate exercise tolerance, although more advanced GOLD 2 or greater patients will likely not be able to achieve 4 metabolic equivalents. Lung volume and diffusing capacity measurements can be useful to assess the severity of COPD but are rarely needed for patient management.29 A positive cough test and smoking over 40 pack-years have been shown to be good predictors of PPCs, and even without a diagnosis of COPD, most of these patients' pulmonary function should ideally be investigated prior to surgery.28 Increased body mass index is another factor that may lead to diminished respiratory function and should be considered in COPD patients.
A 12-lead electrocardiogram is a useful tool to evaluate the presence of right ventricular hypertrophy and conduction disturbances and to rule out evidence of ischemic heart disease. Right ventricular hypertrophy may present as right axis deviation on the electrocardiogram. This manifests as a positive QRS complex in aVF and a negative QRS complex in lead I with an R/S ratio >1 in lead V1. ST-T changes in the right precordial leads and other findings may be present. Right atrial enlargement, seen with severe pulmonary disease and commonly with right ventricular hypertrophy, may lead to a P wave with an amplitude over 2.5 mm in leads II, III, and aVF. Additionally, an echocardiogram should be performed for patients with significant COPD or any previous cardiac output dysfunction history such as pulmonary edema, ischemic or valvular heart disease, or poor exercise tolerance (metabolic equivalents <4).34 COPD patients who develop pulmonary hypertension have a higher likelihood of right ventricular failure and hemodynamic collapse, worsening their prognosis throughout the perioperative period28 through to recovery. Patients with significant pulmonary and cardiovascular compromise should be referred to a hospital for further care if sedation/GA is deemed necessary.
Particularly in the office-based setting, initial pulse oximetry measurements can be useful to assess a patient's room air oxygen saturation, especially with moderate to severe disease.29 If room air pulse oximeter saturation is less than 92%, these patients are at higher risk for intraoperative and postoperative complications, particularly in the dental office setting. It should also be appreciated that elevated carboxyhemoglobin levels seen in chronic smokers may produce an artificially high oxygen saturation reading by pulse oximetry up to 15% higher than the actual reading, as carboxyhemoglobin and oxyhemoglobin have similar absorption spectra. Therefore, even chronic smokers with an oxygen saturation of 95% are likely to be at least mildly hypoxic (PaO2 < 80 mm Hg).
Although not commonly implemented to assess stable pulmonary function, chest radiographs can be used to rule out lower respiratory infections and other possible pathologies associated with acute changes in pulmonary status. Laboratory studies for patients taking medications for COPD, such as direct-acting beta-agonists and inhaled corticosteroids, may reveal electrolyte abnormalities including hyperglycemia, hypokalemia, and hypomagnesemia.35
Optimize a Patient's Medical Condition Prior to Sedation/GA
Patients with COPD who are deemed appropriate for the dental office–based setting should be medically optimized prior to office-based sedation with the goal of decreasing symptoms and risk of perioperative complications.29 If there are any recent changes in health, activity level, dyspnea, increase in mucus production, active infection, recent hospitalizations, or signs of unstable respiratory function, the dental sedation provider or anesthesiologist should consult with the patient's pulmonologist in order to achieve optimization prior to sedation or GA. Multiple pharmacological and nonpharmacological techniques can be used to optimize patients prior to sedation or GA, but may require substantial lead-in time that may impact timely dental care or surgery.
One of the most beneficial interventions prior to sedation/GA is smoking cessation.36 Cigarette smoking alone is a risk factor for multiple pulmonary complications including bronchospasm, laryngospasm, cough, and hypoxemia.28 This increased risk is due to increased mucus production, decreased mucus clearance, increased airway sensitivity, lower surfactant production, and depressed immune system function.28 Although there may be some benefit to smoking cessation for 48 hours prior to sedation/GA, such as decreased carboxyhemoglobin and nicotine levels, evidence shows that maximum benefit occurs when patients quit at least 6 weeks prior to GA.37 Cessation therapy generally requires behavioral support and medication adjuncts such as nicotinic receptor partial agonists (eg, varenicline [Chantix]) to help control nicotine withdrawal symptoms. For patients with known COPD who are still smoking, smoking cessation counseling by the dentist is challenging.
Patients with COPD will likely be on medications to improve respiratory function and decrease symptoms. Anticholinergics (ipratropium, tiotropium), beta-2 agonists (salmeterol, formoterol, arformoterol, olodaterol, albuterol), and corticosteroids (budesonide, fluticasone, triamcinolone, etc) are all inhaled medications often prescribed for patients with COPD. Nonspecific phosphodiesterase inhibitors, such as theophylline, are rarely prescribed today because of adverse cardiovascular effects, but a newer specific phosphodiesterase-4 inhibitor, roflumilast (Daliresp), is effective in reducing the number of COPD exacerbations not controlled by bronchodilators. Intravenous aminophylline is very rarely used today as a rescue medication. Recent studies have shown that inhaled anticholinergics are more effective than beta-2 agonists for long-term therapy of COPD.23 Oxygen therapy is indicated in severe disease. It is important to continue these medications through the perioperative period in order to optimize the patient's respiratory function.
Although less studied preoperatively, breathing and muscle training exercises may also be useful to improve outcomes for COPD patients.38 Techniques such as deep breathing and spirometry measured breathing are shown to decrease hospital stay length and postoperative pulmonary complications by up to 50%.39
Part II of this series will cover clinical sedation/GA management for these patients in the office-based setting.
CONTINUING EDUCATION QUESTIONS
This continuing education (CE) program is designed for dentists who desire to advance their understanding of pain and anxiety control in clinical practice. After reading the designated article, the participant should be able to evaluate and utilize the information appropriately in providing patient care.
The American Dental Society of Anesthesiology (ADSA) is accredited by the American Dental Association and Academy of General Dentistry to sponsor CE for dentists and will award CE credit for each article completed. You must answer 3 of the 4 questions correctly to receive credit.
Submit your answers online at www.adsahome.org. Click on “On Demand CE.”
CE questions must be completed within 3 months and prior to the next issue.
-
1.
Chronic obstructive pulmonary disease (COPD) requires that the respiratory impairment be irreversible.
True
False
-
2.
What is the most common cause of COPD?
Air pollution
Alpha-1 antitrypsin deficiency
Cigarette smoking
Occupational exposure to respiratory irritants
-
3.
In a chronic, 1-pack-per-day cigarette smoker, room air pulse oximetry may overestimate the actual arteriolar oxygen concentration because of the presence of carboxyhemoglobin. The overestimation may be as high as:
5%
15%
25%
35%
-
4.
Which lung volume/capacity is most increased in COPD?
Expiratory reserve volume
Residual volume
Tidal volume
Vital capacity
REFERENCES
- 1.Adeloye D, Chua S, Lee C, et al. Global and regional estimates of COPD prevalence: systematic review and meta-analysis. J Glob Health. 2015;5:020415. doi: 10.7189/jogh.05-020415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wheaton AG, Cunningham TJ, Ford ES, Croft JB. Employment and activity limitations among adults with chronic obstructive pulmonary disease—United States, 2013. MMWR Morb Mortal Wkly Rep. 2015;64:290–295. [PMC free article] [PubMed] [Google Scholar]
- 3.Centers for Disease Control and Prevention. Chronic obstructive pulmonary disease surveillance—United States, 1971–2000. MMWR Morb Mortal Wkly Rep. 2002;51(SS06):1–16. [Google Scholar]
- 4.Mannino DM, Gagnon RC, Petty TL, Lydick E. Obstructive lung disease and low lung function in adults in the United States: data from the National Health Care and Nutrition Examination Survey 1988–1994. Arch Intern Med. 2000;160:1683–1689. doi: 10.1001/archinte.160.11.1683. [DOI] [PubMed] [Google Scholar]
- 5.Guarascio AJ, Ray SM, Finch CK, Self TH. The clinical and economic burden of chronic obstructive pulmonary disease in the USA. Clinicoecon Outcomes Res. 2013;5:235–245. doi: 10.2147/CEOR.S34321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lopez AD, Shibuya K, Rao C, et al. Chronic obstructive pulmonary disease: current burden and future projections. Eur Respir J. 2006;27:397. doi: 10.1183/09031936.06.00025805. [DOI] [PubMed] [Google Scholar]
- 7.WHO. Projections and causes of death, 2015 and 2030. Available at: www.who.int/healthinfor/global_burden_disease/projections/en/ Accessed May 9, 2017.
- 8.Mathers CD, Loncar D. Projections of global mortality and burden of disease from 2002 to 2030. PLoS Med. 2006;3:e442. doi: 10.1371/journal.pmed.0030442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Global strategy for the diagnosis, management and prevention of COPD, Global Initiative for Chronic Obstructive Lung Disease (GOLD) 2016 doi: 10.3760/cma.j.issn.0376-2491.2016.34.001. Available at: www.goldcopd.org Accessed May 9, 2017. [DOI] [PubMed]
- 10.Postma DS, Bush A, van den Berge M. Risk factors and early origins of chronic obstructive pulmonary disease. Lancet. 2015;385:899. doi: 10.1016/S0140-6736(14)60446-3. [DOI] [PubMed] [Google Scholar]
- 11.Kamil F, Ppinson I, Foreman MG. Sex and race factors in early-onset COPD. Curr Opin Pulm Med. 2013;19:140. doi: 10.1097/MCP.0b013e32835d903b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hardin M, Foreman MG, Dransfield MT, et al. Sex-specific features of emphysema among current and former smokers with COPD. Eur Respir J. 2016;47:104. doi: 10.1183/13993003.00996-2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stoller JK, Aboussouan LS. Alpha-1-antitrypsin deficiency. Lancet. 2005;365(9478):2225–2236. doi: 10.1016/S0140-6736(05)66781-5. [DOI] [PubMed] [Google Scholar]
- 14.McDonough JE, Yuan R, Suzuki M, et al. Small-airway obstruction and emphysema in chronic obstructive pulmonary disease. N Engl J Med. 2011;365:1567. doi: 10.1056/NEJMoa1106955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cosio MG, Saetta M, Agusti A. Immunologic aspects of chronic obstructive pulmonary disease. N Engl J Med. 2009;360:2445. doi: 10.1056/NEJMra0804752. [DOI] [PubMed] [Google Scholar]
- 16.Barnes PJ. Inflammatory mechanisms in patients with chronic obstructive pulmonary disease. J Allergy Clin Immunol. 2016;138:16–27. doi: 10.1016/j.jaci.2016.05.011. [DOI] [PubMed] [Google Scholar]
- 17.Domej W, Oettl K, Renner W. Oxidative stress and free radicals in COPD—implications and relevance for treatment. Int J Chron Obstruct Pulmon Dis. 2014;9:1207–1224. doi: 10.2147/COPD.S51226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stockley RA. Neutrophils and protease/antiprotease imbalance. Am J Respir Crit Care Med. 1999;160(5, pt 2):S49–S52. doi: 10.1164/ajrccm.160.supplement_1.13. [DOI] [PubMed] [Google Scholar]
- 19.Shapiro SD, Ingenito EP. The pathogenesis of chronic obstructive pulmonary disease: advances in the past 100 years. Am J Respir Cell Mol Biol. 2005;32:367–372. doi: 10.1165/rcmb.F296. [DOI] [PubMed] [Google Scholar]
- 20.MacNee W. Pathology, pathogenesis and pathophysiology. BMJ. 2006;332:1202–1204. [Google Scholar]
- 21.Harkness LM, Kanabar V, Sharma HS, et al. Pulmonary vascular changes in asthma and COPD. Pulm Pharmacol Ther. 2014;29:144. doi: 10.1016/j.pupt.2014.09.003. [DOI] [PubMed] [Google Scholar]
- 22.Hogg JC, Chu F, Utokaparch S, et al. The nature of small-airway obstruction in chronic obstructive pulmonary disease. N Engl J Med. 2004;350:2645–2653. doi: 10.1056/NEJMoa032158. [DOI] [PubMed] [Google Scholar]
- 23.Elbehairy AF, Ciavaglia CE, Webb KA, et al. Pulmonary gas exchange abnormalities in mild chronic obstructive pulmonary disease. Implications for dyspnea and exercise intolerance. Am J Respir Crit Care Med. 2015;191:1384–1394. doi: 10.1164/rccm.201501-0157OC. [DOI] [PubMed] [Google Scholar]
- 24.Parker CM, Voduc N, Aaron SD, Webb KA, O'Donnell DE. Physiological changes during symptom recovery from moderate exacerbations of COPD. Eur Respir J. 2005;26:420–428. doi: 10.1183/09031936.05.00136304. [DOI] [PubMed] [Google Scholar]
- 25.Aubier M, Murciano D, Milic-Emili J, et al. Effects of the administration of O2 on ventilation and blood gases in patients with chronic obstructive pulmonary disease during acute respirator failure. Am Rev Respir Dis. 1980;122:747–754. doi: 10.1164/arrd.1980.122.5.747. [DOI] [PubMed] [Google Scholar]
- 26.Robinson TD, Freiberg DB, Regnis JA, Young IH. The role of hypoventilation and ventilation-perfusion redistribution in oxygen-induced hypercapnia during acute exacerbations of chronic obstructive pulmonary disease. Am J Respir Crit Care Med 200; 161:1524. doi: 10.1164/ajrccm.161.5.9904119. [DOI] [PubMed] [Google Scholar]
- 27.Miller J, Edwards LD, Agusti A, et al. Comorbidity, systemic inflammation and outcomes in the ECLIPSE cohort. Respir Med. 2013;107:1376–1384. doi: 10.1016/j.rmed.2013.05.001. [DOI] [PubMed] [Google Scholar]
- 28.Licker M, Schweizer A, Ellenberger C, Tschopp JM, Diaper J, Clergue F. Perioperative medical management of patients with COPD. Int J Chron Obstruct Pulmon Dis. 2007;2:493–515. [PMC free article] [PubMed] [Google Scholar]
- 29.Vestob J, Hurd SS, Agustí AG, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2013;187:347–365. doi: 10.1164/rccm.201204-0596PP. [DOI] [PubMed] [Google Scholar]
- 30.Edrich T, Sadovnikoff N. Anesthesia for patients with severe chronic obstructive pulmonary disease. Curr Opin Anaesthesiol. 2010;23:18–24. doi: 10.1097/ACO.0b013e328331ea5b. [DOI] [PubMed] [Google Scholar]
- 31.Smetana GW, Lawrence VA, Cornell JE. American College of Physicians. Preoperative pulmonary risk stratification for noncardiothoracic surgery: systematic review for the American College of Physicians. Ann Intern Med. 2006;144:581–595. doi: 10.7326/0003-4819-144-8-200604180-00009. [DOI] [PubMed] [Google Scholar]
- 32.Degani-Costa LH, Faresin SM. dos Reis Falcão LF. Preoperative evaluation of the patient with pulmonary disease. Braz J Anesthesiol. 2014;64:22–34. doi: 10.1016/j.bjane.2012.11.002. [DOI] [PubMed] [Google Scholar]
- 33.Qaseem A, Snow V, Fitterman N, et al. Risk assessment for and strategies to reduce perioperative pulmonary complications for patients undergoing noncardiothoracic surgery: a guideline from the American College of Physicians. Ann Intern Med. 2006;144:575. doi: 10.7326/0003-4819-144-8-200604180-00008. [DOI] [PubMed] [Google Scholar]
- 34.Silvanus MT, Groeben H, Peters J. Corticosteroids and inhaled salbutamol in patients with reversible airway obstruction markedly decrease the incidence of bronchospasm after tracheal intubation. Anesthesiology. 2004;100:1052–1057. doi: 10.1097/00000542-200405000-00004. [DOI] [PubMed] [Google Scholar]
- 35.Bodenhamer J, Bergstrom R, Brown D, Gabow P, Marx JA, Lowenstein SR. Frequently nebulized beta-agonists for asthma: effects on serum electrolytes. Ann Emerg Med. 1992;21:1337–1342. doi: 10.1016/s0196-0644(05)81898-0. [DOI] [PubMed] [Google Scholar]
- 36.Berry CE, Wise RA. Mortality in COPD: causes, risk factors, and prevention. COPD. 2010;7:375–382. doi: 10.3109/15412555.2010.510160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Warner MA, Offord KP, Warner ME, et al. Role of preoperative cessation of smoking and other factors in postoperative pulmonary complications: a blinded prospective study of coronary artery bypass patients. Mayo Clin Proc. 1989;64:609–616. doi: 10.1016/s0025-6196(12)65337-3. [DOI] [PubMed] [Google Scholar]
- 38.Valkenet K, van de Port IG, Dronkers JJ, et al. The effects of preoperative exercise therapy on postoperative outcome: a systematic review. Clin Rehabil. 2011;25:99. doi: 10.1177/0269215510380830. [DOI] [PubMed] [Google Scholar]
- 39.Thomas JA, McIntosh JM. Are incentive spirometry, intermittent positive pressure breathing, and deep breathing exercises effective in the prevention of postoperative pulmonary complications after upper abdominal surgery? A systematic overview and meta-analysis. Phys Ther. 1994;74:3–10. doi: 10.1093/ptj/74.1.3. [DOI] [PubMed] [Google Scholar]


