Abstract
Amyotrophic lateral sclerosis (ALS) is a neurodegenerative disease selectively targeting motor neurons in the brain and spinal cord. The reasons for differential motor neuron susceptibility remain elusive. We developed a stem cell-based motor neuron assay to study cell-autonomous mechanisms causing motor neuron degeneration, with implications for ALS. A small-molecule screen identified cyclopiazonic acid (CPA) as a stressor to which stem cell-derived motor neurons were more sensitive than interneurons. CPA induced endoplasmic reticulum stress and the unfolded protein response. Furthermore, CPA resulted in an accelerated degeneration of motor neurons expressing human superoxide dismutase 1 (hSOD1) carrying the ALS-causing G93A mutation, compared to motor neurons expressing wild-type hSOD1. A secondary screen identified compounds that alleviated CPA-mediated motor neuron degeneration: three kinase inhibitors and tauroursodeoxycholic acid (TUDCA), a bile acid derivative. The neuroprotective effects of these compounds were validated in human stem cell-derived motor neurons carrying a mutated SOD1 allele (hSOD1A4V). Moreover, we found that the administration of TUDCA in an hSOD1G93A mouse model of ALS reduced muscle denervation. Jointly, these results provide insights into the mechanisms contributing to the preferential susceptibility of ALS motor neurons, and they demonstrate the utility of stem cell-derived motor neurons for the discovery of new neuroprotective compounds.
Keywords: motor neuron, stem cell, ER stress, ALS, cyclopiazonic acid
In this study, Thams and colleagues present a new stem cell-based motor neuron screening platform, which was used to detect stressor compounds of relevance for studying neurodegeneration. The authors used one of the hit compounds, which induced ER stress, in a subsequent rescue screen to identify neuroprotective compounds.
Introduction
Amyotrophic lateral sclerosis (ALS) is a late-onset neurodegenerative disease that preferentially targets motor neurons in the cortex, brain stem, and spinal cord. Despite the discovery of >40 ALS-related genes,1, 2 the pathological processes leading to motor neuron degeneration and the reasons for differential neuronal subtype susceptibility to broadly expressed mutant proteins remain poorly understood. Even though many different cell types are involved in ALS pathogenesis,3 cell-autonomous factors are believed to play a critical role during the early stages of the disease.4, 5, 6, 7, 8
Effective modeling of motor neuron degeneration is hindered by the limited accessibility of motor neurons in patients and animal models and by the fact that ALS is a late-onset disorder. The development of stem cell technologies that facilitate large-scale production of motor neurons carrying disease-causing mutations has circumvented the first challenge, and it has enabled biochemical analysis and drug screening in a relevant cellular context.9, 10, 11, 12, 13 However, stem cell-derived motor neurons are transcriptionally and electrophysiologically immature, resembling embryonic or early post-natal motor neurons.14, 15, 16, 17 Most importantly, stem cell-derived motor neurons carrying ALS-causing mutations do not exhibit key hallmarks of motor neuron disease, such as aggregates of mutant proteins or p62-immunoreactive inclusions.18, 19, 20
Despite their relatively immature state, several studies have reported differences in the survival, physiology, and biochemistry of cultured human and mouse stem cell-derived ALS motor neurons.5, 6, 19, 21, 22, 23, 24, 25, 26, 27, 28 It has been suggested that many of these phenotypes result from a stressful in vitro environment that elicits premature or aberrant manifestations of pathological processes in cultured cells, yet the nature of these culture-related stressors remains ill defined. Understanding which specific stressors potentiate disease-relevant motor neuron pathology would enable the development of more faithful and reproducible models of ALS and, in turn, better tools to understand disease onset and progression. Ultimately, such models can be used to screen for neuroprotective drugs.
Here we describe the development of a highly sensitive motor neuron survival assay and how it was used to screen a library of bioactive compounds for stressors that accelerate the degeneration of mouse motor neurons carrying an ALS-causing human superoxide dismutase 1 (hSOD1)G93A transgene.29 The screen identified cyclopiazonic acid (CPA), an inhibitor of a calcium ATPase expressed in the endoplasmic reticulum (sarcoendoplasmic reticulum-associated calcium ATPase [SERCA]),30 as a compound to which motor neurons are highly sensitive, particularly those expressing hSOD1G93A.
In accordance with the literature, we demonstrate that CPA induces endoplasmic reticulum (ER) stress and activates the downstream cascades referred to as the unfolded protein response (UPR).31 This cellular stress response is induced by unfolded and/or misfolded proteins in the ER lumen, and it is mediated by three ER sensors: IRE1α (Ern1), PERK (Eif2ak3), and ATF6. In turn, these sensors activate separate signaling cascades aiming to alleviate protein misfolding. Despite the initial adaptive response, prolonged activation of ER stress leads to the activation of apoptotic pathways and cell death.32
The accumulation of misfolded proteins is a hallmark of many neurodegenerative diseases, and it has been described in conjunction with the activation of ER stress in animal and stem cell-based models of ALS,19, 33, 34, 35, 36 as well as in post mortem spinal cord samples from ALS patients.33, 37
Studies in animal models of ALS show that certain motor neuron populations degenerate early during the course of the disease while others remain unaffected up until end stage.38, 39 Even though the underlying causes for this vulnerability are not fully understood, it was suggested that protein misfolding and ER stress in vulnerable motor neurons are early and crucial events that distinguish vulnerable from more resistant motor neurons.34, 40
Based on our observation that CPA was selectively toxic to motor neurons, we developed an accelerated neurodegeneration assay, and we used it to screen for compounds that could attenuate the effects of ER stress. We demonstrate that kenpaullone, a protein kinase inhibitor that was recently shown to protect motor neurons from a neurotrophic factor withdrawal and to increase survival of human ALS motor neurons,13, 41 also protects motor neurons from ER stress. In addition to kenpaullone, we identified several other protective compounds, including additional kinase inhibitors and a bile acid derivative, tauroursodeoxycholic acid (TUDCA). In summary, we developed a novel, scalable, stem cell-based discovery platform that can be used for the evaluation of existing drugs and for the discovery of new compounds that protect motor neurons from ER stress-induced degeneration.
Results
A Screen for Stressors Inducing Preferential Degeneration of Stem Cell-Derived hSOD1G93A Motor Neurons
To gain insight into the cell-autonomous pathological mechanisms contributing to the onset of motor neuron degeneration in cells expressing mutant SOD1 protein, we developed a dual-color motor neuron survival in vitro assay. This robust, sensitive, and scalable system is ideal for the discovery of cell-autonomous motor neuron phenotypes.
To minimize well-to-well variation and to increase scalability, we designed an assay in which hSOD1WT and hSOD1G93A motor neurons (referred to hereafter as wild-type [WT] and ALS, respectively) expressing different fluorescent reporters were mixed in the same well (Figure 1A). For this purpose, we derived a set of new embryonic stem cell (ESC) lines by crossing mice carrying hSOD1WT (WT control) or hSOD1G93A (ALS mutant) transgenes29 with mice expressing EGFP12 or tagRFP under the control of a motor neuron-specific Hb9 (Mnx1) promoter (Figure S1A). Immunostaining with antibodies against Hb9 and the motor neuron transcription factor Islet1 confirmed that the new cell lines differentiated into motor neurons with comparable efficiency (Figures S1B–S1E), and immunoprecipitation confirmed the presence of misfolded SOD1 protein in mutant motor neurons (Figures S1F and S1G). RFP-expressing WT motor neurons were mixed with GFP-expressing ALS motor neurons in equal proportions, and they were cultured in 96-well plates (Figure 1A) in the presence of glial cell-derived neurotrophic factor (GDNF; G) and the cyclic AMP (cAMP)-elevating compounds IBMX (I) and forskolin (F).42 Under these basal conditions, we observed a small decrease (∼15%) in ALS motor neuron survival compared to WT controls (Figure S1H).
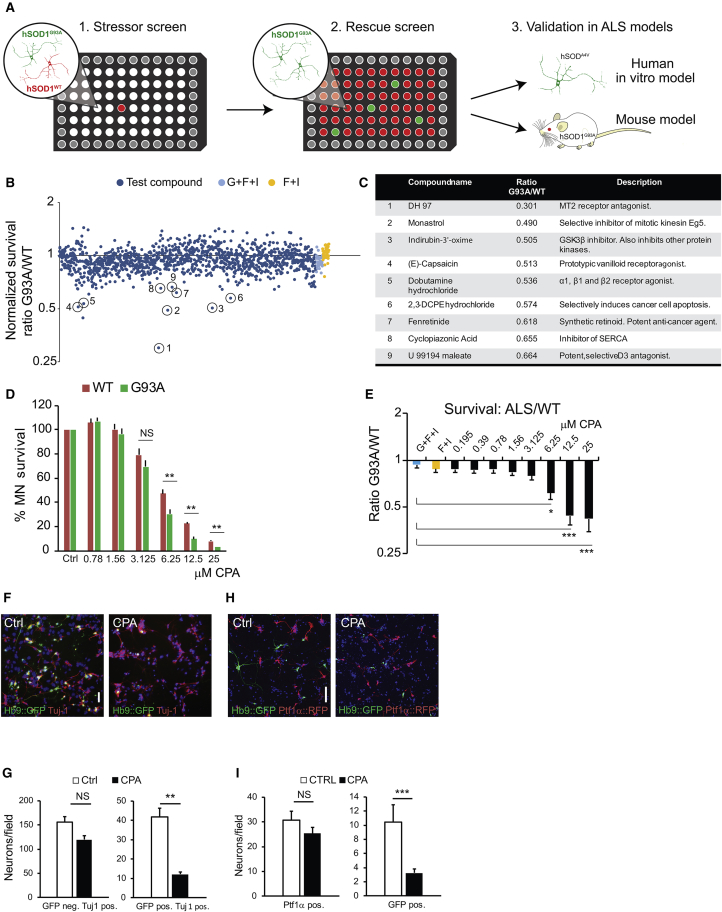
Figure 1.
Results from a Dual-Color Motor Neuron Stressor Screen: Dose-Respone Characterization of a Lead Compound and Subtype-Dependent Neuronal Survival
(A) Overview of the experimental design from primary stressor screen to secondary rescue screen and subsequent validation models. (B) Results of small molecule screen at 48 hr of exposure. Dark blue data points denote normalized G93A:WT survival ratio for well exposed to compounds, and light blue (negative control, GDNF + forskolin + IBMX + vehicle) and yellow data points (positive control, forskolin + IBMX + vehicle) denote G93A:WT survival ratio for the controls. (C) Circles mark confirmed lead compounds after secondary screening. (D and E) Dose-response curve (D) for cyclopiazonic acid (CPA), showing survival of Hb9::RFP hSOD1WT and Hb9::GFP hSOD1G93A motor neurons and normalized G93A:WT survival ratio (E). Results were compiled using two independent pairs of WT-G93A cell lines; individual results are shown in Figures S2A and S2B. Bars denote average, and error bars indicate SEM; *p < 0.05, **p < 0.01, and ***p < 0.001 (n = 9, one-way ANOVA, post hoc Dunnett’s multiple comparison). (F and G) Light microscope micrographs (F) showing control and CPA-treated motor neuron cultures. Scale bar, 50 μm. Survival of ctrl and CPA-exposed hSOD1G93A GFP+ motor neurons and Tuj-1+ GFP− neurons was quantified (G) at 48 hr (n = 3). (H and I) Light microscope micrographs (H) showing control and CPA-treated non-purified motor neuron-interneuron co-cultures. Survival of GFP+ hSOD1WT motor neurons and Ptf1α+ interneurons was quantified (I) at 48 hr (n = 3). Scale bar, 50 μm. Survival of GFP+ hSOD1WT motor neurons and Ptf1α+ interneurons was quantified at 48 hr (n = 3 for both analyses). Bars denote average, and error bars indicate SEM; **p < 0.01 and ***p < 0.001 (n = 3, unpaired two-tailed Student’s t test).
To identify stressors that potentiate ALS pathology, plated motor neurons were treated with a library of 1,275 biologically active small molecules (Tocris Screen Mini and Custom Collection, Tocris Bioscience). Compounds were added 24 hr after motor neuron plating at a final concentration of 10 μM using an automated robot-assisted liquid-handling platform. The ratio of surviving GFP (ALS):RFP (WT) motor neurons was determined 48 hr later, using whole-well imaging in conjunction with automated image analysis software (Figures S1I and S1J). The screen identified several compounds that preferentially decreased the survival of mutant motor neurons. These compounds included agonists and antagonists of membrane receptors, ion pump and channel inhibitors, an anti-mitotic drug, and general pro-apoptotic agents (Figures 1B and 1C).
One of the selective stressors identified in the screen was CPA, a mycotoxin that reversibly blocks SERCA. SERCA is responsible for sequestering calcium from the cytoplasm into the ER.30 Since calcium is an essential co-factor for protein-folding chaperones, SERCA blockade with subsequent depletion of calcium from the ER leads to the accumulation of misfolded proteins and activation of the UPR, ER stress, and apoptotic pathways.31 We titrated CPA using two independent pairs of ALS-WT cell lines (Figures S2A and S2B) to establish the effective concentration range (6.25–12.5 μM; unless stated otherwise, all subsequent experiments were performed with 7.5 μM CPA) at which motor neurons show a reproducible cell death response. We found that ALS motor neurons exhibited reduced survival compared to WT motor neurons (Figures 1D and 1E).
To further investigate the effects of CPA, we examined whether it acts directly on motor neurons. We found that motor neurons purified by fluorescence-activated cell sorting (FACS) (Figures S2C–S2F) were as sensitive to CPA as motor neurons in mixed cultures, indicating that CPA acts directly on motor neurons rather than on other cell types that then produce secondary toxins.5, 6, 8, 43 These findings also suggest that the other cell types present in mixed cultures do not provide significant protection to CPA-treated motor neurons.
Preferential degeneration of motor neurons in the spinal cord, brain stem, and motor cortex is a hallmark of ALS.44 To determine whether stem cell-derived motor neurons of spinal identity are more sensitive to CPA treatment than other spinal neurons, we immunostained surviving cells for pan-neuronal marker Tuj-1. Quantitative analysis of immunostained cultures revealed that, while the survival of GFP-expressing ALS motor neurons was reduced by ∼71%, the survival of GFP− Tuj-1+ non-motor neurons of the same genotype was reduced only by ∼23% (Figures 1F and 1G). The increased sensitivity of ALS motor neurons to CPA prompted us to ask whether even WT motor neurons are more sensitive to CPA than other nerve cells.
For this analysis, we generated a new ESC line that expresses tdTomato in dorsal spinal inhibitory interneurons derived from Ptf1α-expressing progenitors.45 Following differentiation of this cell line under conditions that promote the specification of dorsal interneuron identity, tdTomato-expressing interneurons were co-cultured with GFP-expressing stem cell-derived motor neurons of spinal identity (Figures 1H and 1I). Quantification of RFP- versus GFP-positive neurons revealed that CPA treatment reduced dorsal spinal interneuron survival by only ∼17%, compared to a ∼70% decrease in the survival of co-cultured motor neurons. Together these data demonstrate that motor neurons expressing WT SOD1 too are significantly more sensitive to CPA than other spinal neurons of the same regional identity.
Effects of CPA on Cytosolic Calcium Levels
CPA is a reversible inhibitor of the SERCA pump, which is important for sequestration of cytosolic calcium into the ER. Indeed, CPA treatment resulted in an attenuated clearance of cytosolic calcium following motor neuron depolarization with kainic acid (Figures S3A–S3E). Elevated cytosolic calcium may activate multiple intracellular signaling processes, including cell death pathways.46, 47, 48 Moreover, calcium dysregulation has been implicated in many neurodegenerative conditions, including ALS.19, 49, 50, 51, 52, 53, 54, 55, 56, 57 To determine whether motor neuron degeneration following CPA treatment is primarily caused by increased cytosolic calcium, we evaluated a panel of compounds with known effects on cytosolic calcium handling and/or signaling. These included BAPTA-am, a cell-permeable calcium chelator; dantrolene, an inhibitor of the ryanodine receptor that releases calcium from ER stores into the cytoplasm; three inhibitors of calpains, a family of calcium-dependent cysteine proteases; and three inhibitors of the calcium-activated kinase CaMKK/II. Notably, none of these treatments improved motor neuron degeneration or neurite retraction elicited by CPA exposure (Figures S4A and S4B). These data suggested that a cytosolic calcium overload is unlikely to be the primary cause of CPA-induced motor neuron death.
Stem Cell-Derived Motor Neurons Are Sensitive to the Activation of ER Stress Pathways
In addition to its effects on cytosolic calcium, CPA treatment has been shown to decrease calcium levels in the ER, leading to the activation of ER stress pathways.31, 58 These pathways are initiated by the binding immunoglobulin protein (BiP; HSPA5; GRP-78), an ER-resident chaperone, which translocates from its binding site on ER membrane-bound stress sensors upon detection of unfolded proteins in the ER lumen. Unbound BiP is involved in the activation of three separate signaling pathways associated with the UPR: the PERK, ATF-6, and IRE1α pathways.59, 60, 61, 62
To assess the activation of these pathways in motor neurons exposed to CPA, we used RT-PCR to examine the expression levels of 15 stress-associated genes at three time points following CPA treatment in hSOD1G93A motor neurons (Figure 2A). We observed a rapid increase in the expression of Bip and the key downstream effector Chop (Ddit3). Bip increased 2-fold after 1 hr of CPA exposure, and it continued to increase to approximately 5-fold by 8 hr. Chop was induced 4-fold after 1 hr of CPA treatment, and it reached 16-fold induction after 4 and 8 hr. Other genes with >2-fold induction included the following: p58IPK (6.5-fold at 8 hr), an ER stress-induced protein kinase; Growth arrest and DNA damage-inducible protein 45 alpha (Gadd45a, 5-fold at 4 hr), which has been shown to be upregulated in the spinal cord of presymptomatic SOD1G93A mice;34 Erdj4, a Bip cofactor with involvement in ER-associated protein degradation (ERAD) (5-fold at 4–8 hr); Atf4, a downstream mediator of the PERK axis of the UPR (3-fold at 8 hr); Calreticulin, an ER-associated chaperone (3-fold at 8 hr), which was linked to nitric oxide (NO)-mediated motor neuron degeneration in hSOD1G93A mice;49 and Nrf2, a PERK substrate (2-fold at 8 hr). Taken together, these expression changes pointed to strong activation of multiple axes of the UPR in motor neurons exposed to CPA.
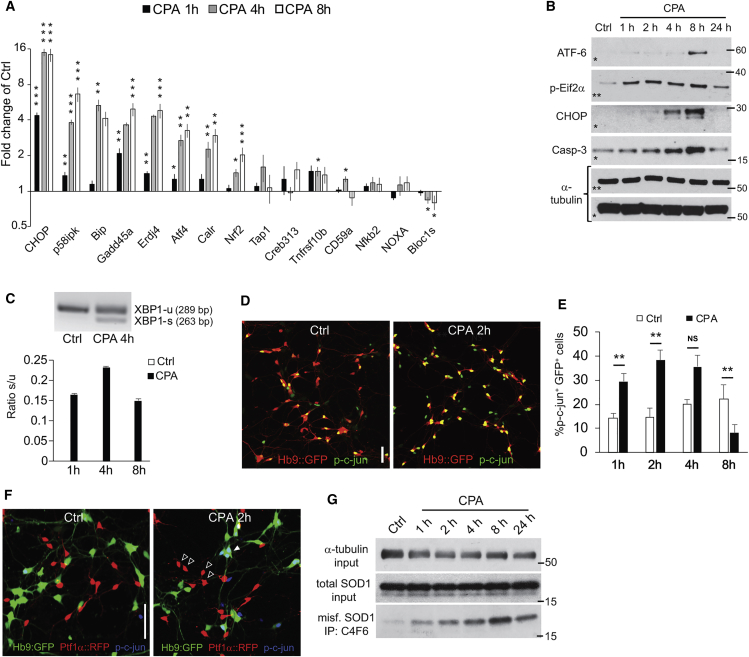
Figure 2.
Characterization of ER Stress Markers in Motor Neuron Cultures Treated with Cyclopiazonic Acid
(A) Histogram showing qPCR results points to genes of particular interest at an earlier time after CPA exposure. RNA was extracted from unpurified hSOD1G93A motor neurons (n = 3, independent culture dishes). Bars denote average, and error bars indicate SEM. CPA was compared to control for each gene and time point; *p < 0.05, **p < 0.01, and ***p < 0.001 (unpaired two-tailed Student’s t test). (B) Immunoblots showing expression of ER stress-related proteins and their loading controls at different time points after CPA exposure (asterisks denote lanes originating from the same gel). (C) Histogram and inverted gel image showing XBP1 splicing in vehicle and CPA-treated hSOD1G93A motor neurons at different time points after CPA exposure (n = 3). Bars denote average ratio s/u, and error bars indicate SEM. Note that no XBP1 splicing was detected in the vehicle-treated group (ctrl). (D and E) Confocal micrographs (D) and histograms (E) showing phospho-c-jun+ motor neurons in ctrls and CPA-treated motor neuron cultures (n = 5). Scale bar, 50 μm. Bars denote average, and error bars indicate SEM; **p < 0.01 (one-way ANOVA, post hoc Dunnett’s multiple comparison test). (F) Confocal micrograph showing phospho-c-jun staining (blue) in co-cultures of ALS motor neurons and dorsal interneurons (Ptf1α+). Empty arrowheads indicate CPA-treated interneurons negative for phospho-c-jun, and filled arrowheads indicate motor neurons with strong nuclear staining for phospho-c-jun. Scale bar, 50 μm. (G) Immunoblots showing SOD1 expression and input loading control protein (α-tubulin) in lysates from CPA-treated hSOD1G93A cells. Middle lanes show panSOD1 expression. Lower lanes show immunoprecipitated lysates using antibodies specific for misfolded hSOD1 species (C4F6 clone).
Western blot analysis of protein extracts from control and mutant motor neurons exposed to CPA for 1, 2, 4, 8, and 24 hr confirmed the early activation of the PERK pathway: an increase in Eif2α phosphorylation was already detectable after only 1 hr of CPA exposure, followed by the induction of CHOP (Figure 2B; Figures S6A–S6C). An accumulation of the active cleaved form of ATF-6 was detectable at 8 hr (Figure 2B). Activation of the IRE1α branch was assessed by qPCR analysis of X-box-binding protein 1 (XBP1) splicing, which was already induced by 1 hr of CPA treatment and persisted at 4 and 8 hr of exposure. Splicing of XBP1 was not detected in vehicle-treated controls (Figure 2C; Figure S6D). We further evaluated activation of the IRE1α branch by immunocytochemical analysis of c-jun phosphorylation,63 which peaked after 2 hr of CPA treatment (Figures 2D and 2E). Notably, reactive c-jun phosphorylation was absent in Ptf1α-expressing interneurons exposed to CPA (Figure 2F). Finally, we detected increased levels of cleaved caspase-3 after CPA exposure, with a peak at 8 hr, indicating an apoptotic mechanism for cell death.
We considered the possibility that the effects of CPA treatment might reflect increased levels of the proximal disease trigger: accumulation of misfolded SOD1 protein in cultured motor neurons.18, 33, 34, 65, 66 We treated mutant motor neurons with CPA or vehicle, and we immunoprecipitated misfolded SOD1 using two different conformation-specific hSOD1 antibodies. Western blot analysis revealed a CPA-dependent increase in the accumulation of misfolded SOD1 (Figure 2G; Figure S6E), potentially explaining the accelerated death-inducing effects of CPA in ALS motor neurons.
Compounds that Protect Motor Neurons from CPA-Induced Degeneration
The realization that motor neurons are more sensitive to the activation of ER stress pathways than other spinal neurons prompted us to set up a candidate molecule screen to identify compounds that increase motor neurons’ resistance to CPA. Such compounds might alleviate neurodegeneration in ALS, as well as other conditions associated with protein misfolding and ER stress activation.32 We screened a panel of >100 compounds that was compiled from in-house libraries and supplemented with compounds that emerged from a literature search (Table S1). Compounds in the panel are known to modulate different branches of the UPR, influence calcium sequestration, act as neurotrophic factors, and/or promote motor neuron survival.
hSOD1G93A motor neuron cultures were treated with rescue compounds for 45 min prior to the addition of 7.5 μM CPA. Survival and neurite growth were assessed after 24 and 48 hr. The screen yielded several compounds that prevented more than 50% of motor neuron degeneration in response to CPA (Figure S4A): the c-Jun N-terminal kinase (JNK) inhibitor SP600125; the tyrosine kinase inhibitor sunitinib; and the broad-spectrum kinase inhibitors Ro 31-8220 mesylate, kenpaullone, GÖ6976, H-7, and K252a.67, 68, 69 Compounds that rescued over 50% of neurite growth included the neurotrophic factor Cardiotrophin-1; the p38 inhibitors SB293063 and SB203580; SP600125; the bile acids taurine-conjugated cholic acid (TCA), taurine-glycine-conjugated cholic acid (TGCA), and TUDCA; and the kinase inhibitors Ro 31-8220 mesylate, GÖ6976, sunitinib, kenpaullone, H-7, and K252a (Figure S4B). Overall, GÖ6976, kenpaullone, K252a, and TUDCA appeared to be the most promising candidates (Figure 3A), due to their strong survival-promoting effects at low concentrations (GÖ6976, kenpaullone, K252a) or strong neurite outgrowth-promoting effects (TUDCA).
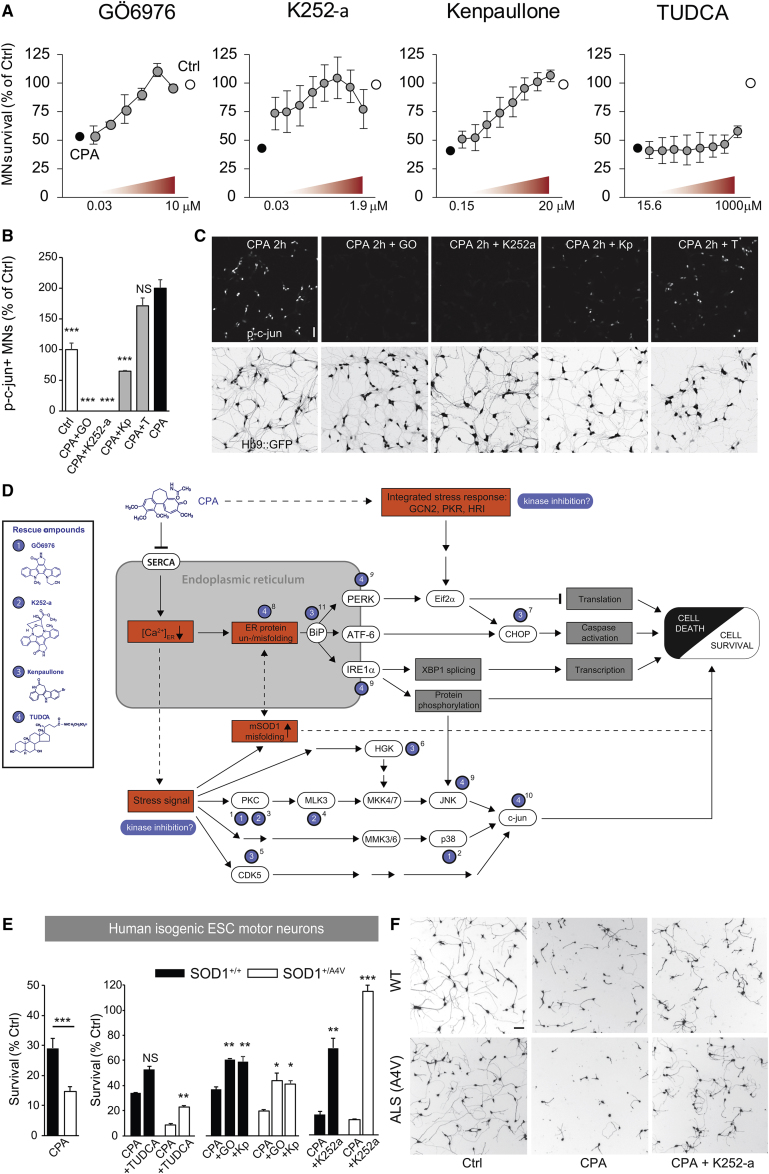
Figure 3.
Characterization of Rescue Compounds, Including Their Effects on c-jun Phosphorylation in Mouse Motor Neurons and Survival in Human Motor Neurons
(A) Dose-reponse curves for the lead compounds from the rescue screen (GÖ6976, n = 6 culture wells; K252a, n = 2 independent cultures; kenpaullone, n = 3 independent cultures; and TUDCA, n = 2 independent cultures). Bars denote average, and error bars indicate SEM. (B and C) Histogram (B) and confocal micrographs (C), showing the effects of rescue compounds on phospho-c-jun expression in CPA-treated motor neuron cultures at 2 hr of exposure. Bars denote average, and error bars indicate SEM; all groups were compared to CPA, ***p < 0.001 (one-way ANOVA, post hoc Dunnett’s multiple comparison test). Scale bar, 50 μm. (D) Proposed model for putative signaling pathways for CPA and targets for rescue compounds. Blue numbered labels indicate reported upstream or direct effects for the different rescue compounds. Intact lines show reported signaling pathways, and dashed lines show suggested pathways. Abbreviations are as follows: General control non-depressible 2, GCN2; Protein kinase R, PKR; and Heme-regulated inhibitor, HRI. Superscript numbers denote supporting references as follows: 1, Sakaki et al.67; 2, Lemonnier et al.85; 3, Kase et al.68; 4, Roux et al.69; 5, Sun et al.86; 6, Yang et al.13; 7, Meares et al.79; 8, Uppala et al.93; 9, Özcan et al.92; 10, Castro-Caldas et al.91; 11, present study. (E) Histogram showing the effects of CPA and in the absence or presence of rescue compounds (GO, GÖ6967; Kp, kenpaullone) in FACS-purified Hb9::GFP+ human motor neurons differentiated from an SOD1+/+ and an isogenic genetically modified SOD1+/A4V human ESC (hESC) line (n = 9 for CPA versus CTRL for both genotypes, and n = 3 for all rescue compounds for both genotypes). Bars denote average, and error bars indicate SEM; *p < 0.05, **p < 0.01, and ***p < 0.001 (unpaired two-tailed Student’s t test). (F) Representative cropped whole-well images showing calcein+ motor neurons. Scale bar, 50 μm.
By testing the ER stress gene panel presented in Figure 2A in cultures treated with CPA and rescue compounds, we confirmed that two of the protein kinase inhibitors, GÖ6976 and kenpaullone, attenuated the cell stress-signaling cascade at different levels (Figures S7A and S7B).
Furthermore, GÖ6976 and K252a treatments suppressed c-jun phosphorylation in CPA-exposed cultures more effectively than kenpaullone (Figures 3B and 3C), indicating that the latter inhibitor acts, at least in part, on a different target pathway (Figure 3D). TUDCA, an ambiphilic bile acid component that functions as a chemical chaperone, rescued neurite outgrowth (Figure 4A), but it only showed a moderate effect on motor neuron survival and failed to suppress c-jun phosphorylation (Figures 3B and 3C; Figure S4A). TUDCA, which can be expected to act at the protein level, did not result in any major changes in the expression of ER stress-related genes, as shown by selected results from an RNA sequence screen (Figure S7C).
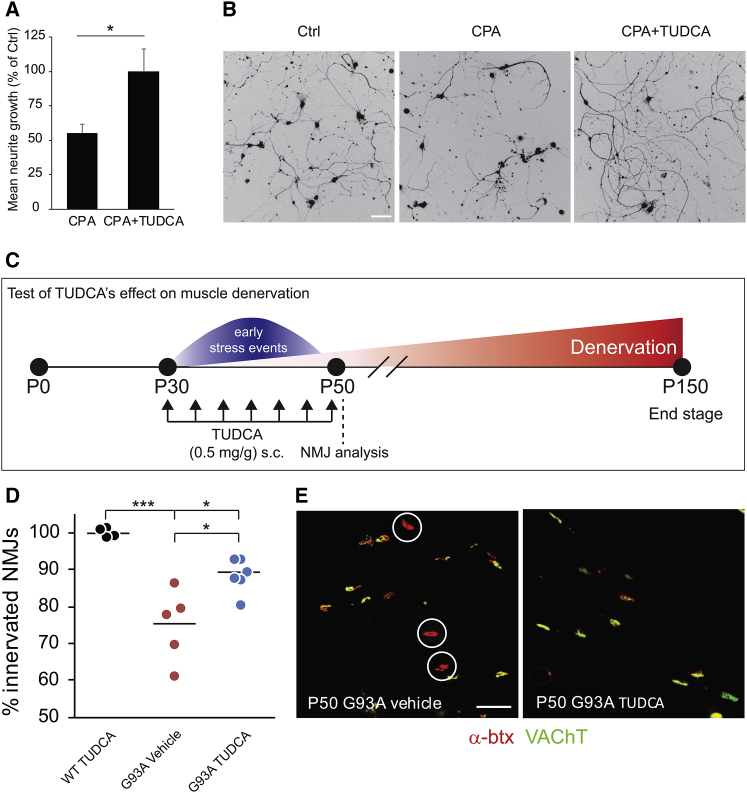
Figure 4.
Characterization of TUDCA’s Effects In Vitro and on Muscle Denervation In Vivo in a Mouse Model of ALS
(A) Histogram showing rescue effects of TUDCA on CPA-induced neurite degeneration. Bars denote average, and error bars indicate SEM; *p < 0.05 (unpaired two-tailed Student’s t test). (B) Representative light microscope micrographs showing the effects of TUDCA application in cultures exposed to CPA. Scale bar, 50 μm. (C) Experimental design for the in vivo test of TUDCA on muscle denervation in hSOD1G93A mice. (D) Scatterplot showing NMJ innervation in the TA (n = 4 for WT + TUDCA, n = 6 for G93A + TUDCA, and n = 5 for G93A + vehicle). Data points represent individual animals, and horizontal lines denote the average; *p < 0.05 and ***p < 0.001 (one-way ANOVA, post hoc Dunnett’s multiple comparison test). (E) Confocal micrographs showing images from TA NMJs (α-btx, red; VAChT, green) of vehicle and TUDCA-treated hSOD1G93A mice. Circles denote denervated NMJs. Scale bar, 50 μm.
Validating Protective Compounds in Human Stem Cell-Derived Motor Neurons
To adapt the assay to human cells, we generated a new isogenic pair of ESC lines derived from a human ESC line expressing GFP under the control of the Hb9 motor neuron promoter (HUES3 HB9::GFP5). The ALS-causing A4V mutation was introduced into a single allele of the human SOD1 gene using zinc-finger nuclease (ZFN)-based genome engineering to recapitulate human patient genotypes (Figures S5A–S5C). The pair of cell lines was differentiated into motor neurons using previously published protocols;9, 70 their relative susceptibility to ER stress-mediated neurodegeneration was assessed under increasing concentrations of CPA. While human motor neurons were less sensitive to CPA than mouse motor neurons (Figure S5K), we detected a significantly increased sensitivity of mutant human SOD1A4V motor neurons exposed to 33 μM CPA (∼14% survival) compared to control neurons (∼29% survival) (Figure 3E), thereby recapitulating the genotype-dependent effects of CPA in mouse motor neurons.
Next, we used the assay to test whether compounds protective to mouse motor neurons would be also able to protect human motor neurons exposed to 33 μM CPA. Remarkably, all of the top protective compounds identified in the mouse motor neuron screen were also effective in protecting human motor neurons against CPA (Figure 3E). Pretreatment of human motor neurons with kenpaullone rescued 35% of CPA-induced cell death in WT motor neurons and 26% in SOD1+/A4V motor neurons (Figure 3E), but it had no significant effects on neurite growth (Figure S5L). GÖ6976 rescued 35% of cell death in hSOD1+/+ motor neurons and 30% in SOD1+/A4V motor neurons (Figure 3E), and it also significantly rescued the decrease in neurite outgrowth (Figure S5L). K252a was overall the most promising compound, rescuing 63% of cell death in hSOD1+/+ and 100% of cell death in hSOD1+/A4V motor neurons (Figures 3E and 3F), with significant effects on neurite growth for both genotypes (Figure S5L). Finally, TUDCA reduced cell death moderately in hSOD1+/+ and hSOD1+/A4V motor neurons by 29% and 15%, respectively, with a small significant effect on neurite growth only in hSOD1+/A4V motor neurons (Figure 3E; Figure S5L).
TUDCA Treatment Attenuates ALS-Associated Muscle Denervation In Vivo
TUDCA is a dietary supplement, and its effects on diverse pathological conditions have been the focus of multiple clinical trials (GEO: NCT00877604, NCT02218619, NCT00771901, and NCT01829698; 71). TUDCA is generally safe, has very few side effects, and exhibits good blood-brain barrier penetrance when administered subcutaneously or orally.71, 72 Denervation of neuromuscular junctions (NMJs) is one of the earliest phenotypes observed in mouse models of ALS,73, 74, 75, 76 and, in the light of the in vitro results, we reasoned that TUDCA might promote the maintenance of motor axon terminal integrity and delay the denervation process.
To compare the effectiveness of TUDCA to its analogs, we screened it in parallel with 10 conjugated bile acids in CPA-treated motor neurons. TGCA matched the moderate effects of TUDCA on motor neuron survival, and it worked at lower concentrations; however, it had smaller effects than TUDCA on neurite extension (Figures S4C and S4D). TCA exhibited similar effects to TUDCA on both motor neuron survival and neurite extension, but it did not offer any advantages in terms of drug development. Thus, we decided to proceed with TUDCA for further evaluation in vivo.
To test the ability of TUDCA to preserve motor axons in ALS models in vivo, we designed a small-scale study in which we evaluated the denervation of the fast fatigable hind limb muscle tibialis anterior (TA) in early disease-stage hSOD1G93A ALS mice (Figure 4C). We have previously determined that TA motor neurons in fast-progressing hSOD1G93A ALS mice undergo a period of presymptomatic events, including ER stress, beginning at post-natal day (P)30, followed by muscle denervation that extends to P50.40 During this period, the TA muscles display 25%–40% denervation before becoming substantially atrophied at later time points. To target this window of early cell stress events,34 we treated hSOD1G93A mice with subcutaneous TUDCA or vehicle injections every 3 days between P30 and P50. Mice expressing mouse WT SOD1 were treated only with TUDCA, and they served as reference for the analysis. At the end of the experiment, we counted total NMJs by staining for acetylcholine receptors in the TA muscles with Alexa Fluor 555-conjugated α-bungarotoxin, and we assessed their innervation by staining for motor axons with antibodies against vesicular acetylcholine transferase (VAChT) (Figures 4D and 4E). Despite the fact that the mice received only seven injections over the course of 21 days of treatment, we observed a moderate, but statistically significant increase in NMJ innervation in TUDCA-treated hSOD1G93A mice compared to vehicle-treated animals (Figure 4D).
Discussion
In this study, we used a novel stem cell-based discovery platform to detect compounds rescuing human and mouse motor neurons from a biological stressor, CPA, which mimics important aspects of neurodegeneration. To streamline future drug discovery, we used this platform to design a translational pipeline, in which lead compounds identified through screening can be readily evaluated in both human cells and via a short-term assay in presymptomatic ALS mice.
Modeling degenerative diseases in cell culture systems opens new opportunities to investigate the pathological processes associated with disease-causing mutations and to screen for novel therapeutic agents. However, adult-onset degenerative diseases, where causative mutations result in relatively slow but accumulating cellular insults, are difficult to model in the kinds of short-term culture systems that are compatible with high-throughput drug screening. We reasoned that the discovery of stressors that induce and accelerate phenotypic changes in motor neurons in vitro could provide insights into molecular pathways contributing to motor neuron degeneration and could lead to the discovery of motor neuron-protective compounds.
ALS-causing mutations are not overtly toxic to spinal motor neurons, and, accordingly, patients do not show any obvious motor deficits during the presymptomatic phase of the disease. Even in an aggressive mouse model of ALS caused by overexpression of mutant hSOD1, no motor neuron death is observed until adulthood,77 indicating that the effects of ALS mutations are either cumulative or that they are potentiated by age- and/or environment-related stressors. To identify stressors that contribute to the degeneration of motor neurons, we designed a highly sensitive, intrinsically controlled survival assay. The co-culture setup allowed us to focus on the intrinsic properties of motor neurons expressing disease-causing mutant SOD1 protein that may render them more sensitive to stressors than WT cells. Using this platform, we identified CPA as a compound that is selectively toxic to motor neurons in general, with further accentuated effects in cells expressing mutant SOD1.
The role of ER stress in ALS remains controversial. While signs of ER stress have been detected in both mouse models of the disease34, 40, 64 as well as in post mortem ALS patient spinal cords, several studies have suggested that the induction of ER stress pathways might be a protective response, facilitating the clearance of ALS-causing mutant proteins.78 Interestingly, instead of improved clearance, we observed an accumulation of misfolded SOD1 protein following ER stress induction. This finding raises the possibility that, under basal conditions, young motor neurons are capable of effectively clearing misfolded SOD1. However, as the burden of misfolded proteins increases with time, the clearance mechanisms may become overwhelmed, resulting in less effective removal of mutant SOD1. It is tempting to speculate that CPA effectively mimics the age-related increase in endogenous protein misfolding at a dramatically accelerated pace.35
Interestingly, our model recapitulates another poorly understood but important feature of ALS. We observed that motor neurons were considerably more sensitive to ER stress-inducing compounds than other types of neurons. While we do not know what mechanisms underlie such cell type-specific sensitivity to ER stress, it might explain the preferential degeneration of spinal motor neurons in familial cases of ALS, despite broad expression of misfolded proteins in all types of neurons.
A screen of candidate neuroactive compounds identified several potent drugs that could reverse the harmful effects of CPA. Two classes of compounds were of particular interest: kinase inhibitors and bile acid derivatives. Kinase inhibitors exhibited a remarkable ability to protect motor neurons from CPA toxicity. One compound, kenpaullone, was previously shown to protect motor neurons from neurotrophic deprivation,13 improve survival, reverse electrophysiological deficits in human stem cell-derived motor neurons from a patient carrying a mutation in the FUS gene,41 and decrease the levels of the UPR mediator CHOP in neural cells exposed to the ER stressor tunicamycin.79 In addition to kenpaullone, we identified two staurosporine analogs, K252a and GÖ6976, that were previously reported to increase neuronal survival in other in vitro models of neurodegeneration.69, 80
By integrating our results with published studies, we propose a model in which CPA induces a stress response that activates a cascade of intracellular protein kinase-regulated pathways,81, 82 including a protein kinase C (PKC)-JNK-signaling pathway67, 68 (Figure 3D). GÖ6976 and K252a strongly inhibit PKC, as well as its downstream target mixed lineage kinase 3 (MLK3).69 Kenpaullone acts primarily as an inhibitor of HPK1/GCK-like kinase (HGK)13 and cyclin-dependent kinases (CDKs).83, 84 Due to the unselective nature of these compounds, additional stress-activated kinase pathways may be involved, such as p38.85, 86 Consequently, we did not discern a consistent pattern that would point to a common upstream target mechanism. Our conclusion is that the kinase targets differ between the compounds and act on different branches of the same cell stress-induced cascade, ultimately converging on the suppression of c-jun phosphorylation (Figure 3D).
Testing these kinase inhibitors in vivo will require further optimization of their pharmacokinetic and pharmacodynamics properties. Kenpaullone is insoluble in aqueous solutions at its most effective concentrations, effectively preventing its testing in vivo. While K252a and GÖ6976 are more potent and more soluble than kenpaullone (data not shown), these compounds are broad-spectrum inhibitors, each targeting >100 different kinases, raising the concern of adverse secondary effects in vivo. Future drug development and mechanistic target studies will, therefore, require the design of more selective inhibitors.
The second class of neuroprotective compounds that emerged from our screen was derivatives of mammalian bile acids, which have been used extensively in traditional Tibetan and Chinese medicine. Notably, it shows potentially beneficial results in ALS patients.71 While this class of compounds protected <30% of dying motor neurons after CPA exposure, it completely restored neurite outgrowth. In contrast to the kinase inhibitors that are not approved for human use, TUDCA is a widely available dietary supplement, and its analog UDCA is a water-soluble FDA-approved drug for treating pruritus and liver disease. TUDCA has previously been shown to have beneficial effects in mouse models of Huntington’s, Parkinson’s, and Alzheimer’s diseases.87, 88, 89, 90, 91 TUDCA has also been shown to reduce the expression of markers of the UPR in a mouse model of type 2 diabetes, in part by acting as a chaperone for misfolded proteins92, 93 (Figure 3F). We therefore wanted to validate our results in an in vivo model, and we tested whether treatment with TUDCA was sufficient to delay muscle denervation in early-stage ALS mice. A brief treatment period showed an encouraging effect on denervation in the TA muscle, raising the possibility that TUDCA alone or in combination with other treatments might delay motor disease onset or progression.
In conclusion, the dual-color motor neuron-screening approach described herein revealed that stem cell-derived motor neurons are selectively sensitive to ER stress pathway activation. Our findings add to the mounting evidence that ER stress contributes to motor neuron cell death in ALS. The scalable stem cell-based screening system identified several compounds that effectively desensitize motor neurons to ER stress, providing new tool compounds for mapping pathways involved in motor neuron degeneration and for the development of analogs compatible with in vivo testing. This system can be easily adapted to other neurodegenerative conditions associated with ER stress activation, such as Parkinson’s disease, Huntington’s disease, prion disease, or Alzheimer’s disease.32
Materials and Methods
Derivation of Mouse Transgenic ESC Lines
Heterozygous Tg(Hlxb9-GFP)1Tmj or Tg(Hlxb9-tagRFP) reporter mice were crossed with mice expressing a mutated (B6.Cg-Tg(SOD1*G93A)1Gur/J) or WT form (B6SJL-Tg(SOD1)2Gur/J) of human SOD1. Blastocysts were collected at embryonic day 3.5. Mouse ESC lines were derived as previously described.12 New lines were genotyped and sequenced to confirm the presence of both transgenes and the G93A point mutation.
For interneuron differentiations, mouse ESC lines were derived from Ptf1α::cre mice (kindly provided by Dr. Kaltschmidt) crossed to Rosa-LSL-tdTomato fluorescent reporter mice.94, 95 All animal work was performed in compliance with Columbia University Institutional Animal Care and Use Committee (IACUC) protocols.
Generation of Isogenic Human ESC Lines by Genetic Targeting
To extrapolate results from the mouse assays, we generated an independent set of SOD1+/A4V and SOD1+/+ isogenic cell lines by introducing the A4V mutation into the WT SOD1 locus of the human ESC line HUES3 Hb9::GFP5 (Figure S5). Using again a two-step nuclease-mediated gene-targeting strategy,96 we introduced the SOD1A4V mutation into the HUES3 Hb9::GFP genetic background (Figure S5A).
Mouse and Human Differentiation into Spinal Neuronal Lineages
Motor neuron differentiation of transgenic mouse ESCs was performed as previously described.12 Briefly, cells were dissociated on day 6 of differentiation and plated on a surface coated with poly-ornithine (Sigma, 100 μg/mL) and laminin (4 μg/mL). Cells were cultured in the presence of the cAMP-elevating compounds forskolin (10 μM) and IBMX (100 μM) in combination with 500 μM GDNF. For the majority of all experiments, mouse cultures containing motor neurons, interneurons, and glial progenitors were used (referred to as motor neuron cultures); in a few experiments, motor neurons were purified by FACS (see the Supplemental Materials and Methods).
For differentiation into dI4 interneurons, Ptf1α-tdTomato ESCs were dissociated and cultured in suspension as embryoid bodies (EBs) at a density of 8.0 × 105 cells/10-cm culture-treated Petri dish. On day 2 of differentiation, EBs were collected, spun down, and split 1:4 into new Petri dishes and supplemented with 1 μM retinoic acid (RA). Media were exchanged on days 4 and 6 of differentiation. The endpoint of dI4 interneuron (IN) differentiation was day 8, when EBs were collected for co-culture studies.
Differentiation of human isogenic HUES3 ESC HB9::GFP reporter lines into motor neurons was performed as previously described.70 Cells were dissociated on day 16 of differentiation, sorted via FACS, and plated on poly-ornithine- and laminin-coated surfaces as above. Serum-free human motor neuron plating media were supplemented with the antimitotic UFdU and the neurotrophic factors GDNF, brain-derived neurotrophic factor (BDNF), ciliary neurotrophic factor (CNTF), and insulin-like growth factor 1 (IGF1) (all at 10 ng/mL) as described.97
All cell lines used were routinely tested for mycoplasma.
Dual-Color Motor Neuron Co-culture Assay
Dissociated fluorescent Hb9::RFP-hSOD1WT cells were counted by hemacytometer and mixed with an equal number of Hb9::GFP-hSOD1G93A motor neurons, such that 500 fluorescent cells of each genotype were plated per well. Cells were plated in coated 96-well plates in a medium containing FSK and IBMX (low trophic support, positive control for cell death) or FSK, IBMX, and 250 pg/mL GDNF (medium trophic support, positive control for survival).
Automated Image Analysis
Whole-well images of live GFP+ cells were acquired using a Plate RunnerHD system (Trophos). Images were analyzed using Metamorph software (Molecular Devices). A healthy cell criterion, i.e., neurons with a significant neurite (5× cell body diameter), was used to distinguish live neurons from GFP+ debris (Figure S1K). The endogenous Hb9::GFP reporters in the human lines were not bright enough to be faithfully detected on our automated imaging platform. Cells were treated immediately prior to imaging with the live-cell dye calcein-AM (1.33 μM) for 10 min, followed by quenching with a 10% solution of hemoglobin in PBS.
Small Molecule Screen
Approximately 1,300 biologically active compounds from the Tocris Mini Screen and Custom collection were added to screening plates at a final concentration of 10 μM in singletons. The final concentration of DMSO was 0.5%. A survival ratio was calculated by dividing the number of surviving GFP+ cells by the number of RFP+ cells after 48 hr of exposure to the compounds.
FACS
Cells were sorted based on GFP or RFP expression using a 5-laser ARIA-IIu ROU Cell Sorter (BD BioSciences) configured with a 100-μm ceramic nozzle and operating at 20 psi.
ER Stress Rescue Screen
Dissociated and plated cells were allowed to recover for 24 hr, following 45-min incubation with rescue compounds or medium + 0.5% DMSO as control. Compounds were screened in triplicates at three different concentrations with 5-fold dilution steps; hits were further evaluated in 6- to 8-point serial dilutions in 3–6 replicates. Rescue compounds were selected from the initial dual-color screen or from a literature search focusing on compounds with documented effects on ER stress in other models. Cells were then exposed to 7.5 μM CPA for mouse cells and 33 μM for human cells or medium + vehicle 0.5% DMSO, which we referred to as control (ctrl) throughout.
Immunocytochemistry
Live cultures were pre-fixed with 4% paraformaldehyde (PFA) on ice, by adding fixative directly to the medium for 2 min, then fixed an additional 15 min by replacing the well content with 4% PFA and incubating at 4°C. Fixed cultures were blocked for 1 hr at room temperature with 0.01 M PBS containing 0.3% Triton-X and 20% donkey serum. Primary antibodies were diluted in blocking solution and incubated overnight at 4°C, followed by incubation with secondary antibodies (Alexa donkey 488/555/647) for 60 min at room temperature.
Biochemistry
Day 6 EBs were lysed in TNG-T lysis buffer65 containing protease (Complete Mini) and phosphatase (PhoStop) inhibitors for 30 min, followed by mechanical trituration with a 26G syringe. C4F6 and B8H10 antibodies (MediMabs) were coupled to protein-G Dynabeads and used for the immunoprecipitation of misfolded hSOD1, as described.65 A control immunoglobulin G (IgG) antibody was used a negative control (Figure S1I). A pan-SOD1 antibody (Novus Biologicals) was used for immunoblotting; 5% of the input was used as a loading control. For western blotting, the following antibodies were used: Caspase-3 (1:1,000), CHOP (1:500), phospho-Eif2α (1:1,000) (Cell Signaling Technology), SOD1 and ATF-6 (1:200, Novus Biologicals), and α-tubulin (1:50,000, Abcam). Representative gels are cropped from scanned images of the original films. Cropped parts without relevance to the present study are indicated by a dashed line in the figure.
qPCR
Cultures were treated with vehicle or CPA, and samples were collected at 1, 4, and 8 hr. In addition, the combinations CPA + kenpaullone and CPA + GÖ6976 were evaluated at 4 and 8 hr. Samples were lysed in TRIzol and frozen at −80°C until further processing. RNA was extracted using the Qiashredder and QIAGEN RNeasy Mini kits (QIAGEN), according to the manufacturer’s protocol. 1–2 μg total RNA was used for each reverse transcription reaction, and reactions were performed using the TaqMan RT kit (Applied Biosystems, Grand Island, NY, USA). Primer pairs were designed for target transcripts using Primer Express 3.0 (Applied Biosystems). qPCR reactions were performed using the Power SYBR Green PCR Master Mix (Applied Biosystems). Reactions were run and analyzed on a ViiA 7 (Life Technologies) qPCR instrument using absolute quantification settings. Statistics were performed using delta-CT values, and data were visualized using fold change values.
XBP1 Splicing
PCR was performed in a 50-μL jumpstart Taq (Sigma-Aldrich, D9307) reaction containing 10 pmol XBP-1-specific primers to detect splicing (forward: 5′-GAATGCCCAAAAGGATATCAGACTC-3′, reverse: 5′-GGCCTTGTGGTTGAGAACCAGGAG-3′). PCR conditions were as follows: 1 cycle of 94°C for 1 min; 30 cycles of 94°C for 30 s, 60°C for 30 s, and 72°C for 1 min; and one cycle of 1 min at 72°C. PCR products were run for 30 min on 2.5% agarose gels containing ethidium bromide. Bands were observed and quantified using the Syngene G:Box and Genesis software. Band intensity was measured using the Analyze-Gels application in ImageJ (NIH).
Calcium Imaging
Hb9::RFP WT and ALS motor neurons were dissociated on day 6 of differentiation, and they were cultured 3 days on glass coverslips. The coverslips were incubated with 5 μM Fura-2 AM, ratiometric calcium indicator dye (Life Sciences, USA), for 30 min at room temperature. Coverslips were then exposed to a 1-s pulse of 100 μM kainic acid (KA), and one image per second was acquired for 1 min. After a recovery period of 2 min, the coverslips were then continuously exposed to 75 μM CPA for 20 min, and one image was acquired every 30 s. After another 2-min recovery period, a second pulse of KA was applied, with the same image acquisition as the first application. A 340:380 ratio was calculated for all image series using FIJI (http://fiji.sc/). Quantification was carried out using Igor Pro version (v.)6 (Wavemetrics, USA). The rate at which the evoked calcium transients returned to the baseline was calculated from the tau (time constant) of a single exponential curve fitted to the falling part of the Ca intensity trace from 80% to 20% of the peak.
In Vivo Administration of TUDCA
P30 mice were divided into three cohorts:1 hSOD1G93A mice (B6.Cg-Tg(SOD1*G93A)1Gur/J) receiving 0.5 mg/g TUDCA in 0.01 M PBS subcutaneously;2 WT mice (C57BL/6J) receiving 0.5 mg/g TUDCA in 0.01 M PBS subcutaneously, to evaluate the mutation-specific effects of NMJ denervation and of the drug; and3 hSOD1G93A mice receiving 0.01 M PBS subcutaneously, as a vehicle control. The drug was administered every 3 days from P30 to P51 for a total of 7 injections, after with animals were euthanized. The TA muscles were dissected out and processed for staining, following transcardiac perfusion. Presynaptic terminals were stained with an antibody to VAChT (raised in rabbit, Covance, 1:32,000), and postsynaptic clusters were stained with α-bungarotoxin conjugated to Alexa Fluor 488 (1:500; Invitrogen). NMJs lacking presynaptic staining were considered denervated. Every third section throughout the whole muscle was analyzed from one TA per animal (n = 4–6). All animal work was performed in compliance with Columbia University IACUC protocols.
Statistics
Statistical analyses were performed with GraphPad Prism v.7 or R’ (www.r-project.org). Datasets are expressed as mean value ± SEM throughout the paper. If normal distribution and equal variance could be assumed, analysis of significance was performed with an unpaired two-tailed Student’s t test for pairwise comparison or a one-way ANOVA with post hoc Dunnett’s multiple comparison test. Otherwise, analysis was instead performed by Mann-Whitney rank-sum test or Kruskal-Wallis test with Dunn’s multiple comparison post hoc test. Statistical significance is indicated by *p < 0.05, **p < 0.01, and ***p < 0.001.
Author Contributions
Conceptualization, H.W., C.E.H., and S.T.; Methodology, H.W., C.E.H., S.T., E.R.L., M.-H.L., K.J.S., H.L., D.J.W., P.H., L.A.W., J.S., K.C.K., and E.J.; Validation, H.W., S.T., and E.R.L.; Formal Analysis, H.W., S.T., E.R.L., M.-H.L., K.J.S., D.J.W., and L.A.W.; Investigation, H.W., S.T., E.R.L., M.-H.L., K.J.S., D.J.W., and L.A.W.; Resources, I.L., K.C.K., P.H., L.A.W., J.S., and K.E.; Writing – Original Draft, H.W., S.T., and E.R.L.; Writing – Review and Editing, H.W., S.T., E.R.L., C.E.H., and B.R.S.; Visualization, S.T., K.J.S., D.J.W., and L.A.W.; Supervision, H.W., C.E.H., and B.R.S.; Funding Acquisition, H.W., C.E.H., B.R.S., and K.E.
Conflicts of Interest
H.W., C.E.H., and S.T. have filed an application for a patent regarding the use of TUDCA and related compounds in the prospective treatment of neurodegenerative disease (CU13092-0379639-TB.JK).
Acknowledgments
We would like to thank Dr. Julia Kaltschmidt, for kindly providing transgenic mice used for the derivation of Ptf1α embryonic stem cell lines, and Caroline Lindblad and Arvid Frostell, for assistance with statistical analysis. This work was funded by Project ALS, Target ALS, the NIH (NS078097), and DoD (W81XWH-16-1-0204). S.T. received additional funding from the Swedish Wenner-Gren Foundation and The Foundation BLANCEFLOR Boncompagni Ludovisi, née Bildt.
Footnotes
Supplemental Information includes Supplemental Materials and Methods, seven figures, and one table and can be found with this article online at https://doi.org/10.1016/j.ymthe.2018.10.010.
Contributor Information
Sebastian Thams, Email: sebastian.thams@ki.se.
Hynek Wichterle, Email: hw350@cumc.columbia.edu.
Supplemental Information
References
- 1.Peters O.M., Ghasemi M., Brown R.H., Jr. Emerging mechanisms of molecular pathology in ALS. J. Clin. Invest. 2015;125:2548. doi: 10.1172/JCI82693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chia R., Chiò A., Traynor B.J. Novel genes associated with amyotrophic lateral sclerosis: diagnostic and clinical implications. Lancet Neurol. 2018;17:94–102. doi: 10.1016/S1474-4422(17)30401-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ilieva H., Polymenidou M., Cleveland D.W. Non-cell autonomous toxicity in neurodegenerative disorders: ALS and beyond. J. Cell Biol. 2009;187:761–772. doi: 10.1083/jcb.200908164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boillée S., Yamanaka K., Lobsiger C.S., Copeland N.G., Jenkins N.A., Kassiotis G., Kollias G., Cleveland D.W. Onset and progression in inherited ALS determined by motor neurons and microglia. Science. 2006;312:1389–1392. doi: 10.1126/science.1123511. [DOI] [PubMed] [Google Scholar]
- 5.Di Giorgio F.P., Boulting G.L., Bobrowicz S., Eggan K.C. Human embryonic stem cell-derived motor neurons are sensitive to the toxic effect of glial cells carrying an ALS-causing mutation. Cell Stem Cell. 2008;3:637–648. doi: 10.1016/j.stem.2008.09.017. [DOI] [PubMed] [Google Scholar]
- 6.Di Giorgio F.P., Carrasco M.A., Siao M.C., Maniatis T., Eggan K. Non-cell autonomous effect of glia on motor neurons in an embryonic stem cell-based ALS model. Nat. Neurosci. 2007;10:608–614. doi: 10.1038/nn1885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kang S.H., Li Y., Fukaya M., Lorenzini I., Cleveland D.W., Ostrow L.W., Rothstein J.D., Bergles D.E. Degeneration and impaired regeneration of gray matter oligodendrocytes in amyotrophic lateral sclerosis. Nat. Neurosci. 2013;16:571–579. doi: 10.1038/nn.3357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nagai M., Re D.B., Nagata T., Chalazonitis A., Jessell T.M., Wichterle H., Przedborski S. Astrocytes expressing ALS-linked mutated SOD1 release factors selectively toxic to motor neurons. Nat. Neurosci. 2007;10:615–622. doi: 10.1038/nn1876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Amoroso M.W., Croft G.F., Williams D.J., O’Keeffe S., Carrasco M.A., Davis A.R., Roybon L., Oakley D.H., Maniatis T., Henderson C.E., Wichterle H. Accelerated high-yield generation of limb-innervating motor neurons from human stem cells. J. Neurosci. 2013;33:574–586. doi: 10.1523/JNEUROSCI.0906-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dimos J.T., Rodolfa K.T., Niakan K.K., Weisenthal L.M., Mitsumoto H., Chung W., Croft G.F., Saphier G., Leibel R., Goland R. Induced pluripotent stem cells generated from patients with ALS can be differentiated into motor neurons. Science. 2008;321:1218–1221. doi: 10.1126/science.1158799. [DOI] [PubMed] [Google Scholar]
- 11.Höing S., Rudhard Y., Reinhardt P., Glatza M., Stehling M., Wu G., Peiker C., Böcker A., Parga J.A., Bunk E. Discovery of inhibitors of microglial neurotoxicity acting through multiple mechanisms using a stem-cell-based phenotypic assay. Cell Stem Cell. 2012;11:620–632. doi: 10.1016/j.stem.2012.07.005. [DOI] [PubMed] [Google Scholar]
- 12.Wichterle H., Lieberam I., Porter J.A., Jessell T.M. Directed differentiation of embryonic stem cells into motor neurons. Cell. 2002;110:385–397. doi: 10.1016/s0092-8674(02)00835-8. [DOI] [PubMed] [Google Scholar]
- 13.Yang Y.M., Gupta S.K., Kim K.J., Powers B.E., Cerqueira A., Wainger B.J., Ngo H.D., Rosowski K.A., Schein P.A., Ackeifi C.A. A small molecule screen in stem-cell-derived motor neurons identifies a kinase inhibitor as a candidate therapeutic for ALS. Cell Stem Cell. 2013;12:713–726. doi: 10.1016/j.stem.2013.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Patterson M., Chan D.N., Ha I., Case D., Cui Y., Van Handel B., Mikkola H.K., Lowry W.E. Defining the nature of human pluripotent stem cell progeny. Cell Res. 2012;22:178–193. doi: 10.1038/cr.2011.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stein J.L., de la Torre-Ubieta L., Tian Y., Parikshak N.N., Hernández I.A., Marchetto M.C., Baker D.K., Lu D., Hinman C.R., Lowe J.K. A quantitative framework to evaluate modeling of cortical development by neural stem cells. Neuron. 2014;83:69–86. doi: 10.1016/j.neuron.2014.05.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Miles G.B., Yohn D.C., Wichterle H., Jessell T.M., Rafuse V.F., Brownstone R.M. Functional properties of motoneurons derived from mouse embryonic stem cells. J. Neurosci. 2004;24:7848–7858. doi: 10.1523/JNEUROSCI.1972-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jacko M., Weyn-Vanhentenryck S.M., Smerdon J.W., Yan R., Feng H., Williams D.J., Pai J., Xu K., Wichterle H., Zhang C. Rbfox Splicing Factors Promote Neuronal Maturation and Axon Initial Segment Assembly. Neuron. 2018;97:853–868.e6. doi: 10.1016/j.neuron.2018.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bosco D.A., Morfini G., Karabacak N.M., Song Y., Gros-Louis F., Pasinelli P., Goolsby H., Fontaine B.A., Lemay N., McKenna-Yasek D. Wild-type and mutant SOD1 share an aberrant conformation and a common pathogenic pathway in ALS. Nat. Neurosci. 2010;13:1396–1403. doi: 10.1038/nn.2660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kiskinis E., Sandoe J., Williams L.A., Boulting G.L., Moccia R., Wainger B.J., Han S., Peng T., Thams S., Mikkilineni S. Pathways disrupted in human ALS motor neurons identified through genetic correction of mutant SOD1. Cell Stem Cell. 2014;14:781–795. doi: 10.1016/j.stem.2014.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang J., Farr G.W., Zeiss C.J., Rodriguez-Gil D.J., Wilson J.H., Furtak K., Rutkowski D.T., Kaufman R.J., Ruse C.I., Yates J.R., 3rd Progressive aggregation despite chaperone associations of a mutant SOD1-YFP in transgenic mice that develop ALS. Proc. Natl. Acad. Sci. USA. 2009;106:1392–1397. doi: 10.1073/pnas.0813045106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Alami N.H., Smith R.B., Carrasco M.A., Williams L.A., Winborn C.S., Han S.S.W., Kiskinis E., Winborn B., Freibaum B.D., Kanagaraj A. Axonal transport of TDP-43 mRNA granules is impaired by ALS-causing mutations. Neuron. 2014;81:536–543. doi: 10.1016/j.neuron.2013.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Devlin A.C., Burr K., Borooah S., Foster J.D., Cleary E.M., Geti I., Vallier L., Shaw C.E., Chandran S., Miles G.B. Human iPSC-derived motoneurons harbouring TARDBP or C9ORF72 ALS mutations are dysfunctional despite maintaining viability. Nat. Commun. 2015;6:5999. doi: 10.1038/ncomms6999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Donnelly C.J., Zhang P.W., Pham J.T., Haeusler A.R., Mistry N.A., Vidensky S., Daley E.L., Poth E.M., Hoover B., Fines D.M. RNA toxicity from the ALS/FTD C9ORF72 expansion is mitigated by antisense intervention. Neuron. 2013;80:415–428. doi: 10.1016/j.neuron.2013.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Egawa N., Kitaoka S., Tsukita K., Naitoh M., Takahashi K., Yamamoto T., Adachi F., Kondo T., Okita K., Asaka I. Drug screening for ALS using patient-specific induced pluripotent stem cells. Sci. Transl. Med. 2012;4:145ra104. doi: 10.1126/scitranslmed.3004052. [DOI] [PubMed] [Google Scholar]
- 25.Naujock M., Stanslowsky N., Bufler S., Naumann M., Reinhardt P., Sterneckert J., Kefalakes E., Kassebaum C., Bursch F., Lojewski X. 4-Aminopyridine Induced Activity Rescues Hypoexcitable Motor Neurons from Amyotrophic Lateral Sclerosis Patient-Derived Induced Pluripotent Stem Cells. Stem Cells. 2016;34:1563–1575. doi: 10.1002/stem.2354. [DOI] [PubMed] [Google Scholar]
- 26.Sareen D., O’Rourke J.G., Meera P., Muhammad A.K., Grant S., Simpkinson M., Bell S., Carmona S., Ornelas L., Sahabian A. Targeting RNA foci in iPSC-derived motor neurons from ALS patients with a C9ORF72 repeat expansion. Sci. Transl. Med. 2013;5:208ra149. doi: 10.1126/scitranslmed.3007529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sivadasan R., Hornburg D., Drepper C., Frank N., Jablonka S., Hansel A., Lojewski X., Sterneckert J., Hermann A., Shaw P.J. C9ORF72 interaction with cofilin modulates actin dynamics in motor neurons. Nat. Neurosci. 2016;19:1610–1618. doi: 10.1038/nn.4407. [DOI] [PubMed] [Google Scholar]
- 28.Wainger B.J., Kiskinis E., Mellin C., Wiskow O., Han S.S., Sandoe J., Perez N.P., Williams L.A., Lee S., Boulting G. Intrinsic membrane hyperexcitability of amyotrophic lateral sclerosis patient-derived motor neurons. Cell Rep. 2014;7:1–11. doi: 10.1016/j.celrep.2014.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gurney M.E., Pu H., Chiu A.Y., Dal Canto M.C., Polchow C.Y., Alexander D.D., Caliendo J., Hentati A., Kwon Y.W., Deng H.X. Motor neuron degeneration in mice that express a human Cu,Zn superoxide dismutase mutation. Science. 1994;264:1772–1775. doi: 10.1126/science.8209258. [DOI] [PubMed] [Google Scholar]
- 30.Goeger D.E., Riley R.T., Dorner J.W., Cole R.J. Cyclopiazonic acid inhibition of the Ca2+-transport ATPase in rat skeletal muscle sarcoplasmic reticulum vesicles. Biochem. Pharmacol. 1988;37:978–981. doi: 10.1016/0006-2952(88)90195-5. [DOI] [PubMed] [Google Scholar]
- 31.Doutheil J., Gissel C., Oschlies U., Hossmann K.A., Paschen W. Relation of neuronal endoplasmic reticulum calcium homeostasis to ribosomal aggregation and protein synthesis: implications for stress-induced suppression of protein synthesis. Brain Res. 1997;775:43–51. doi: 10.1016/s0006-8993(97)00899-8. [DOI] [PubMed] [Google Scholar]
- 32.Hetz C., Saxena S. ER stress and the unfolded protein response in neurodegeneration. Nat. Rev. Neurol. 2017;13:477–491. doi: 10.1038/nrneurol.2017.99. [DOI] [PubMed] [Google Scholar]
- 33.Hetz C., Thielen P., Matus S., Nassif M., Court F., Kiffin R., Martinez G., Cuervo A.M., Brown R.H., Glimcher L.H. XBP-1 deficiency in the nervous system protects against amyotrophic lateral sclerosis by increasing autophagy. Genes Dev. 2009;23:2294–2306. doi: 10.1101/gad.1830709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Saxena S., Cabuy E., Caroni P. A role for motoneuron subtype-selective ER stress in disease manifestations of FALS mice. Nat. Neurosci. 2009;12:627–636. doi: 10.1038/nn.2297. [DOI] [PubMed] [Google Scholar]
- 35.Saxena S., Roselli F., Singh K., Leptien K., Julien J.P., Gros-Louis F., Caroni P. Neuroprotection through excitability and mTOR required in ALS motoneurons to delay disease and extend survival. Neuron. 2013;80:80–96. doi: 10.1016/j.neuron.2013.07.027. [DOI] [PubMed] [Google Scholar]
- 36.Kramer N.J., Haney M.S., Morgens D.W., Jovičić A., Couthouis J., Li A., Ousey J., Ma R., Bieri G., Tsui C.K. CRISPR-Cas9 screens in human cells and primary neurons identify modifiers of C9ORF72 dipeptide-repeat-protein toxicity. Nat. Genet. 2018;50:603–612. doi: 10.1038/s41588-018-0070-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Atkin J.D., Farg M.A., Walker A.K., McLean C., Tomas D., Horne M.K. Endoplasmic reticulum stress and induction of the unfolded protein response in human sporadic amyotrophic lateral sclerosis. Neurobiol. Dis. 2008;30:400–407. doi: 10.1016/j.nbd.2008.02.009. [DOI] [PubMed] [Google Scholar]
- 38.Kanning K.C., Kaplan A., Henderson C.E. Motor neuron diversity in development and disease. Annu. Rev. Neurosci. 2010;33:409–440. doi: 10.1146/annurev.neuro.051508.135722. [DOI] [PubMed] [Google Scholar]
- 39.Nijssen J., Comley L.H., Hedlund E. Motor neuron vulnerability and resistance in amyotrophic lateral sclerosis. Acta Neuropathol. 2017;133:863–885. doi: 10.1007/s00401-017-1708-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kaplan A., Spiller K.J., Towne C., Kanning K.C., Choe G.T., Geber A., Akay T., Aebischer P., Henderson C.E. Neuronal matrix metalloproteinase-9 is a determinant of selective neurodegeneration. Neuron. 2014;81:333–348. doi: 10.1016/j.neuron.2013.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Liu M.L., Zang T., Zhang C.L. Direct Lineage Reprogramming Reveals Disease-Specific Phenotypes of Motor Neurons from Human ALS Patients. Cell Rep. 2016;14:115–128. doi: 10.1016/j.celrep.2015.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hanson M.G., Jr., Shen S., Wiemelt A.P., McMorris F.A., Barres B.A. Cyclic AMP elevation is sufficient to promote the survival of spinal motor neurons in vitro. J. Neurosci. 1998;18:7361–7371. doi: 10.1523/JNEUROSCI.18-18-07361.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Haidet-Phillips A.M., Hester M.E., Miranda C.J., Meyer K., Braun L., Frakes A., Song S., Likhite S., Murtha M.J., Foust K.D. Astrocytes from familial and sporadic ALS patients are toxic to motor neurons. Nat. Biotechnol. 2011;29:824–828. doi: 10.1038/nbt.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rowland L.P., Shneider N.A. Amyotrophic lateral sclerosis. N. Engl. J. Med. 2001;344:1688–1700. doi: 10.1056/NEJM200105313442207. [DOI] [PubMed] [Google Scholar]
- 45.Hoang P.T., Chalif J.I., Bikoff J.B., Jessell T.M., Mentis G.Z., Wichterle H. Subtype Diversification and Synaptic Specificity of Stem Cell-Derived Spinal Interneurons. Neuron. 2018;100 doi: 10.1016/j.neuron.2018.09.016. 135–149.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.De Stefani D., Bononi A., Romagnoli A., Messina A., De Pinto V., Pinton P., Rizzuto R. VDAC1 selectively transfers apoptotic Ca2+ signals to mitochondria. Cell Death Differ. 2012;19:267–273. doi: 10.1038/cdd.2011.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jayaraman T., Marks A.R. T cells deficient in inositol 1,4,5-trisphosphate receptor are resistant to apoptosis. Mol. Cell. Biol. 1997;17:3005–3012. doi: 10.1128/mcb.17.6.3005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pinton P., Giorgi C., Siviero R., Zecchini E., Rizzuto R. Calcium and apoptosis: ER-mitochondria Ca2+ transfer in the control of apoptosis. Oncogene. 2008;27:6407–6418. doi: 10.1038/onc.2008.308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bernard-Marissal N., Moumen A., Sunyach C., Pellegrino C., Dudley K., Henderson C.E., Raoul C., Pettmann B. Reduced calreticulin levels link endoplasmic reticulum stress and Fas-triggered cell death in motoneurons vulnerable to ALS. J. Neurosci. 2012;32:4901–4912. doi: 10.1523/JNEUROSCI.5431-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jaiswal M.K., Keller B.U. Cu/Zn superoxide dismutase typical for familial amyotrophic lateral sclerosis increases the vulnerability of mitochondria and perturbs Ca2+ homeostasis in SOD1G93A mice. Mol. Pharmacol. 2009;75:478–489. doi: 10.1124/mol.108.050831. [DOI] [PubMed] [Google Scholar]
- 51.Jaiswal M.K., Zech W.D., Goos M., Leutbecher C., Ferri A., Zippelius A., Carrì M.T., Nau R., Keller B.U. Impairment of mitochondrial calcium handling in a mtSOD1 cell culture model of motoneuron disease. BMC Neurosci. 2009;10:64. doi: 10.1186/1471-2202-10-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kawamata H., Manfredi G. Mitochondrial dysfunction and intracellular calcium dysregulation in ALS. Mech. Ageing Dev. 2010;131:517–526. doi: 10.1016/j.mad.2010.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kim H.J., Magranè J., Starkov A.A., Manfredi G. The mitochondrial calcium regulator cyclophilin D is an essential component of oestrogen-mediated neuroprotection in amyotrophic lateral sclerosis. Brain. 2012;135:2865–2874. doi: 10.1093/brain/aws208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rothstein J.D., Tsai G., Kuncl R.W., Clawson L., Cornblath D.R., Drachman D.B., Pestronk A., Stauch B.L., Coyle J.T. Abnormal excitatory amino acid metabolism in amyotrophic lateral sclerosis. Ann. Neurol. 1990;28:18–25. doi: 10.1002/ana.410280106. [DOI] [PubMed] [Google Scholar]
- 55.Takuma H., Kwak S., Yoshizawa T., Kanazawa I. Reduction of GluR2 RNA editing, a molecular change that increases calcium influx through AMPA receptors, selective in the spinal ventral gray of patients with amyotrophic lateral sclerosis. Ann. Neurol. 1999;46:806–815. doi: 10.1002/1531-8249(199912)46:6<806::aid-ana2>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 56.Tradewell M.L., Cooper L.A., Minotti S., Durham H.D. Calcium dysregulation, mitochondrial pathology and protein aggregation in a culture model of amyotrophic lateral sclerosis: mechanistic relationship and differential sensitivity to intervention. Neurobiol. Dis. 2011;42:265–275. doi: 10.1016/j.nbd.2011.01.016. [DOI] [PubMed] [Google Scholar]
- 57.von Lewinski F., Keller B.U. Ca2+, mitochondria and selective motoneuron vulnerability: implications for ALS. Trends Neurosci. 2005;28:494–500. doi: 10.1016/j.tins.2005.07.001. [DOI] [PubMed] [Google Scholar]
- 58.Pirot P., Eizirik D.L., Cardozo A.K. Interferon-gamma potentiates endoplasmic reticulum stress-induced death by reducing pancreatic beta cell defence mechanisms. Diabetologia. 2006;49:1229–1236. doi: 10.1007/s00125-006-0214-7. [DOI] [PubMed] [Google Scholar]
- 59.Gardner B.M., Pincus D., Gotthardt K., Gallagher C.M., Walter P. Endoplasmic reticulum stress sensing in the unfolded protein response. Cold Spring Harb. Perspect. Biol. 2013;5:a013169. doi: 10.1101/cshperspect.a013169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hetz C. The unfolded protein response: controlling cell fate decisions under ER stress and beyond. Nat. Rev. Mol. Cell Biol. 2012;13:89–102. doi: 10.1038/nrm3270. [DOI] [PubMed] [Google Scholar]
- 61.Iurlaro R., Muñoz-Pinedo C. Cell death induced by endoplasmic reticulum stress. FEBS J. 2016;283:2640–2652. doi: 10.1111/febs.13598. [DOI] [PubMed] [Google Scholar]
- 62.Kozutsumi Y., Segal M., Normington K., Gething M.J., Sambrook J. The presence of malfolded proteins in the endoplasmic reticulum signals the induction of glucose-regulated proteins. Nature. 1988;332:462–464. doi: 10.1038/332462a0. [DOI] [PubMed] [Google Scholar]
- 63.Urano F., Wang X., Bertolotti A., Zhang Y., Chung P., Harding H.P., Ron D. Coupling of stress in the ER to activation of JNK protein kinases by transmembrane protein kinase IRE1. Science. 2000;287:664–666. doi: 10.1126/science.287.5453.664. [DOI] [PubMed] [Google Scholar]
- 64.Sun S., Sun Y., Ling S.C., Ferraiuolo L., McAlonis-Downes M., Zou Y., Drenner K., Wang Y., Ditsworth D., Tokunaga S. Translational profiling identifies a cascade of damage initiated in motor neurons and spreading to glia in mutant SOD1-mediated ALS. Proc. Natl. Acad. Sci. USA. 2015;112:E6993–E7002. doi: 10.1073/pnas.1520639112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gros-Louis F., Soucy G., Larivière R., Julien J.P. Intracerebroventricular infusion of monoclonal antibody or its derived Fab fragment against misfolded forms of SOD1 mutant delays mortality in a mouse model of ALS. J. Neurochem. 2010;113:1188–1199. doi: 10.1111/j.1471-4159.2010.06683.x. [DOI] [PubMed] [Google Scholar]
- 66.Nishitoh H., Kadowaki H., Nagai A., Maruyama T., Yokota T., Fukutomi H., Noguchi T., Matsuzawa A., Takeda K., Ichijo H. ALS-linked mutant SOD1 induces ER stress- and ASK1-dependent motor neuron death by targeting Derlin-1. Genes Dev. 2008;22:1451–1464. doi: 10.1101/gad.1640108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sakaki K., Wu J., Kaufman R.J. Protein kinase Ctheta is required for autophagy in response to stress in the endoplasmic reticulum. J. Biol. Chem. 2008;283:15370–15380. doi: 10.1074/jbc.M710209200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kase H., Iwahashi K., Nakanishi S., Matsuda Y., Yamada K., Takahashi M., Murakata C., Sato A., Kaneko M. K-252 compounds, novel and potent inhibitors of protein kinase C and cyclic nucleotide-dependent protein kinases. Biochem. Biophys. Res. Commun. 1987;142:436–440. doi: 10.1016/0006-291x(87)90293-2. [DOI] [PubMed] [Google Scholar]
- 69.Roux P.P., Dorval G., Boudreau M., Angers-Loustau A., Morris S.J., Makkerh J., Barker P.A. K252a and CEP1347 are neuroprotective compounds that inhibit mixed-lineage kinase-3 and induce activation of Akt and ERK. J. Biol. Chem. 2002;277:49473–49480. doi: 10.1074/jbc.M203428200. [DOI] [PubMed] [Google Scholar]
- 70.Maury Y., Côme J., Piskorowski R.A., Salah-Mohellibi N., Chevaleyre V., Peschanski M., Martinat C., Nedelec S. Combinatorial analysis of developmental cues efficiently converts human pluripotent stem cells into multiple neuronal subtypes. Nat. Biotechnol. 2015;33:89–96. doi: 10.1038/nbt.3049. [DOI] [PubMed] [Google Scholar]
- 71.Elia A.E., Lalli S., Monsurrò M.R., Sagnelli A., Taiello A.C., Reggiori B., La Bella V., Tedeschi G., Albanese A. Tauroursodeoxycholic acid in the treatment of patients with amyotrophic lateral sclerosis. Eur. J. Neurol. 2016;23:45–52. doi: 10.1111/ene.12664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kaemmerer W.F., Rodrigues C.M., Steer C.J., Low W.C. Creatine-supplemented diet extends Purkinje cell survival in spinocerebellar ataxia type 1 transgenic mice but does not prevent the ataxic phenotype. Neuroscience. 2001;103:713–724. doi: 10.1016/s0306-4522(01)00017-3. [DOI] [PubMed] [Google Scholar]
- 73.Azzouz M., Leclerc N., Gurney M., Warter J.M., Poindron P., Borg J. Progressive motor neuron impairment in an animal model of familial amyotrophic lateral sclerosis. Muscle Nerve. 1997;20:45–51. doi: 10.1002/(sici)1097-4598(199701)20:1<45::aid-mus6>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 74.Maselli R.A., Wollman R.L., Leung C., Distad B., Palombi S., Richman D.P., Salazar-Grueso E.F., Roos R.P. Neuromuscular transmission in amyotrophic lateral sclerosis. Muscle Nerve. 1993;16:1193–1203. doi: 10.1002/mus.880161109. [DOI] [PubMed] [Google Scholar]
- 75.Tsujihata M., Hazama R., Yoshimura T., Satoh A., Mori M., Nagataki S. The motor end-plate fine structure and ultrastructural localization of acetylcholine receptors in amyotrophic lateral sclerosis. Muscle Nerve. 1984;7:243–249. doi: 10.1002/mus.880070310. [DOI] [PubMed] [Google Scholar]
- 76.Sharma A., Lyashchenko A.K., Lu L., Nasrabady S.E., Elmaleh M., Mendelsohn M., Nemes A., Tapia J.C., Mentis G.Z., Shneider N.A. ALS-associated mutant FUS induces selective motor neuron degeneration through toxic gain of function. Nat. Commun. 2016;7:10465. doi: 10.1038/ncomms10465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Chiu A.Y., Zhai P., Dal Canto M.C., Peters T.M., Kwon Y.W., Prattis S.M., Gurney M.E. Age-dependent penetrance of disease in a transgenic mouse model of familial amyotrophic lateral sclerosis. Mol. Cell. Neurosci. 1995;6:349–362. doi: 10.1006/mcne.1995.1027. [DOI] [PubMed] [Google Scholar]
- 78.Cai Y., Arikkath J., Yang L., Guo M.L., Periyasamy P., Buch S. Interplay of endoplasmic reticulum stress and autophagy in neurodegenerative disorders. Autophagy. 2016;12:225–244. doi: 10.1080/15548627.2015.1121360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Meares G.P., Mines M.A., Beurel E., Eom T.Y., Song L., Zmijewska A.A., Jope R.S. Glycogen synthase kinase-3 regulates endoplasmic reticulum (ER) stress-induced CHOP expression in neuronal cells. Exp. Cell Res. 2011;317:1621–1628. doi: 10.1016/j.yexcr.2011.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Jeohn G.H., Wilson B., Wetsel W.C., Hong J.S. The indolocarbazole Gö6976 protects neurons from lipopolysaccharide/interferon-gamma-induced cytotoxicity in murine neuron/glia co-cultures. Brain Res. Mol. Brain Res. 2000;79:32–44. doi: 10.1016/s0169-328x(00)00082-6. [DOI] [PubMed] [Google Scholar]
- 81.Pakos-Zebrucka K., Koryga I., Mnich K., Ljujic M., Samali A., Gorman A.M. The integrated stress response. EMBO Rep. 2016;17:1374–1395. doi: 10.15252/embr.201642195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wek R.C. Role of eIF2α Kinases in Translational Control and Adaptation to Cellular Stress. Cold Spring Harb. Perspect. Biol. 2018;10:a032870. doi: 10.1101/cshperspect.a032870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Leost M., Schultz C., Link A., Wu Y.Z., Biernat J., Mandelkow E.M., Bibb J.A., Snyder G.L., Greengard P., Zaharevitz D.W. Paullones are potent inhibitors of glycogen synthase kinase-3beta and cyclin-dependent kinase 5/p25. Eur. J. Biochem. 2000;267:5983–5994. doi: 10.1046/j.1432-1327.2000.01673.x. [DOI] [PubMed] [Google Scholar]
- 84.Schultz C., Link A., Leost M., Zaharevitz D.W., Gussio R., Sausville E.A., Meijer L., Kunick C. Paullones, a series of cyclin-dependent kinase inhibitors: synthesis, evaluation of CDK1/cyclin B inhibition, and in vitro antitumor activity. J. Med. Chem. 1999;42:2909–2919. doi: 10.1021/jm9900570. [DOI] [PubMed] [Google Scholar]
- 85.Lemonnier J., Ghayor C., Guicheux J., Caverzasio J. Protein kinase C-independent activation of protein kinase D is involved in BMP-2-induced activation of stress mitogen-activated protein kinases JNK and p38 and osteoblastic cell differentiation. J. Biol. Chem. 2004;279:259–264. doi: 10.1074/jbc.M308665200. [DOI] [PubMed] [Google Scholar]
- 86.Sun K.H., Lee H.G., Smith M.A., Shah K. Direct and indirect roles of cyclin-dependent kinase 5 as an upstream regulator in the c-Jun NH2-terminal kinase cascade: relevance to neurotoxic insults in Alzheimer’s disease. Mol. Biol. Cell. 2009;20:4611–4619. doi: 10.1091/mbc.E09-05-0433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Keene C.D., Rodrigues C.M., Eich T., Chhabra M.S., Steer C.J., Low W.C. Tauroursodeoxycholic acid, a bile acid, is neuroprotective in a transgenic animal model of Huntington’s disease. Proc. Natl. Acad. Sci. USA. 2002;99:10671–10676. doi: 10.1073/pnas.162362299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Keene C.D., Rodrigues C.M., Eich T., Linehan-Stieers C., Abt A., Kren B.T., Steer C.J., Low W.C. A bile acid protects against motor and cognitive deficits and reduces striatal degeneration in the 3-nitropropionic acid model of Huntington’s disease. Exp. Neurol. 2001;171:351–360. doi: 10.1006/exnr.2001.7755. [DOI] [PubMed] [Google Scholar]
- 89.Nunes A.F., Amaral J.D., Lo A.C., Fonseca M.B., Viana R.J., Callaerts-Vegh Z., D’Hooge R., Rodrigues C.M. TUDCA, a bile acid, attenuates amyloid precursor protein processing and amyloid-β deposition in APP/PS1 mice. Mol. Neurobiol. 2012;45:440–454. doi: 10.1007/s12035-012-8256-y. [DOI] [PubMed] [Google Scholar]
- 90.Ackerman H.D., Gerhard G.S. Bile Acids in Neurodegenerative Disorders. Front. Aging Neurosci. 2016;8:263. doi: 10.3389/fnagi.2016.00263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Castro-Caldas M., Carvalho A.N., Rodrigues E., Henderson C.J., Wolf C.R., Rodrigues C.M., Gama M.J. Tauroursodeoxycholic acid prevents MPTP-induced dopaminergic cell death in a mouse model of Parkinson’s disease. Mol. Neurobiol. 2012;46:475–486. doi: 10.1007/s12035-012-8295-4. [DOI] [PubMed] [Google Scholar]
- 92.Ozcan U., Yilmaz E., Ozcan L., Furuhashi M., Vaillancourt E., Smith R.O., Görgün C.Z., Hotamisligil G.S. Chemical chaperones reduce ER stress and restore glucose homeostasis in a mouse model of type 2 diabetes. Science. 2006;313:1137–1140. doi: 10.1126/science.1128294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Uppala J.K., Gani A.R., Ramaiah K.V.A. Chemical chaperone, TUDCA unlike PBA, mitigates protein aggregation efficiently and resists ER and non-ER stress induced HepG2 cell death. Sci. Rep. 2017;7:3831. doi: 10.1038/s41598-017-03940-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kawaguchi Y., Cooper B., Gannon M., Ray M., MacDonald R.J., Wright C.V. The role of the transcriptional regulator Ptf1a in converting intestinal to pancreatic progenitors. Nat. Genet. 2002;32:128–134. doi: 10.1038/ng959. [DOI] [PubMed] [Google Scholar]
- 95.Madisen L., Zwingman T.A., Sunkin S.M., Oh S.W., Zariwala H.A., Gu H., Ng L.L., Palmiter R.D., Hawrylycz M.J., Jones A.R. A robust and high-throughput Cre reporting and characterization system for the whole mouse brain. Nat. Neurosci. 2010;13:133–140. doi: 10.1038/nn.2467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Maeder M.L., Thibodeau-Beganny S., Osiak A., Wright D.A., Anthony R.M., Eichtinger M., Jiang T., Foley J.E., Winfrey R.J., Townsend J.A. Rapid “open-source” engineering of customized zinc-finger nucleases for highly efficient gene modification. Mol. Cell. 2008;31:294–301. doi: 10.1016/j.molcel.2008.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Johnson-Kerner B.L., Ahmad F.S., Diaz A.G., Greene J.P., Gray S.J., Samulski R.J., Chung W.K., Van Coster R., Maertens P., Noggle S.A. Intermediate filament protein accumulation in motor neurons derived from giant axonal neuropathy iPSCs rescued by restoration of gigaxonin. Hum. Mol. Genet. 2015;24:1420–1431. doi: 10.1093/hmg/ddu556. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.