Abstract
The cellular immune response to peptide pools from conserved Leishmania antigens in leishmaniasis-immune individuals identified epitopes for a human DNA vaccine (Das et al., this issue).
Leishmaniasis is a vector-borne infectious disease affecting the poorest communities around the world and has been designated by the World Health Organization as one of the 17 neglected tropical diseases. Environmental changes such as global warming or human migrations have affected the vector-reservoir-host triad and led to an expansion of the leishmaniasis geographical range (1). Leishmaniasis comprises diseases of varied etiologies that are caused by approximately 20 different Leishmania parasite species and are transmitted by nearly 30 medically important sand fly vector species (1). Mammals including rodents, canids, and in some cases humans are reservoirs of the disease.
Despite the availability of well-characterized animal models that have resulted in a good understanding of the immune response to Leishmania and recognition of correlates of protection from leishmaniasis (2), an effcacious human vaccine has eluded us thus far (3). In this issue of Science Translational Medicine, Das et al. (4) used human cells to select components for a next-generation DNA vaccine, representing a new strategy to make leishmaniasis vaccines for humans.
TOWARD A LEISHMANIASIS VACCINE
Besides the lackluster economic outlook for a leishmaniasis vaccine, there are other inherent challenges facing its development. An efficacious vaccine for human leishmaniasis must take into account that Leishmania parasites are transmitted by the bite of an insect vector and therefore bypass the skin barrier and its innate protective mechanisms and are also intracellular and inaccessible to antibodies. Unlike most established vaccines, an efficacious leishmaniasis vaccine must rely on the induction of a robust T cell immunity that overcomes the human and parasite intrinsic variability (2). This immunity depends on efficient presentation of specific epitopes to T cells by diverse human leukocyte antigen I (HLA-I) and HLA-II molecules present on the surface of antigen-presenting cells. Moreover, to ensure wide coverage, a leishmaniasis vaccine must also take into account the antigenic variability resulting from the existence of multiple Leishmania species and numerous strains.
Despite these challenges, there is consensus in the leishmaniasis scientific community that a prophylactic vaccine against human leishmaniasis is a viable option (3). Interestingly, in endemic areas, the resolution of cutaneous leishmaniasis (CL) results in life-long protection against the disease. This notion led to the practice of “leishmanization,” in which controlled inoculation of virulent parasites in discrete parts of the body was used as a live vaccine (3). Historically and to date, this has been the most effective way to “immunize” people living in a leishmaniasis-endemic area. However, logistics—including scalability, reproducibility, and transport—combined with the likelihood of adverse events inherent to a live virulent vaccine limits the practicality and acceptability of leishmanization.
Another major limitation facing the field of leishmaniasis vaccines has been the inability to move vaccine candidates forward to clinical trials. One main obstacle concerns product development of a vaccine candidate. Despite the existence of a large pool of vaccine candidates against Leishmania, only a few have made it to human clinical trials (3). These include first-generation killed Leishmania vaccines and two second-generation vaccines, Leish-111f (NCT00121862) and LEISH-F3 (NCT01484548). Unfortunately, the majority of candidates will face the challenge of transitioning from basic research to product implementation, termed the “valley of death” for vaccine development. This involves multiple technological and financial barriers, including issues of scalability, reproducibility, and cost.
THE MAKING OF LEISHDNAVAX
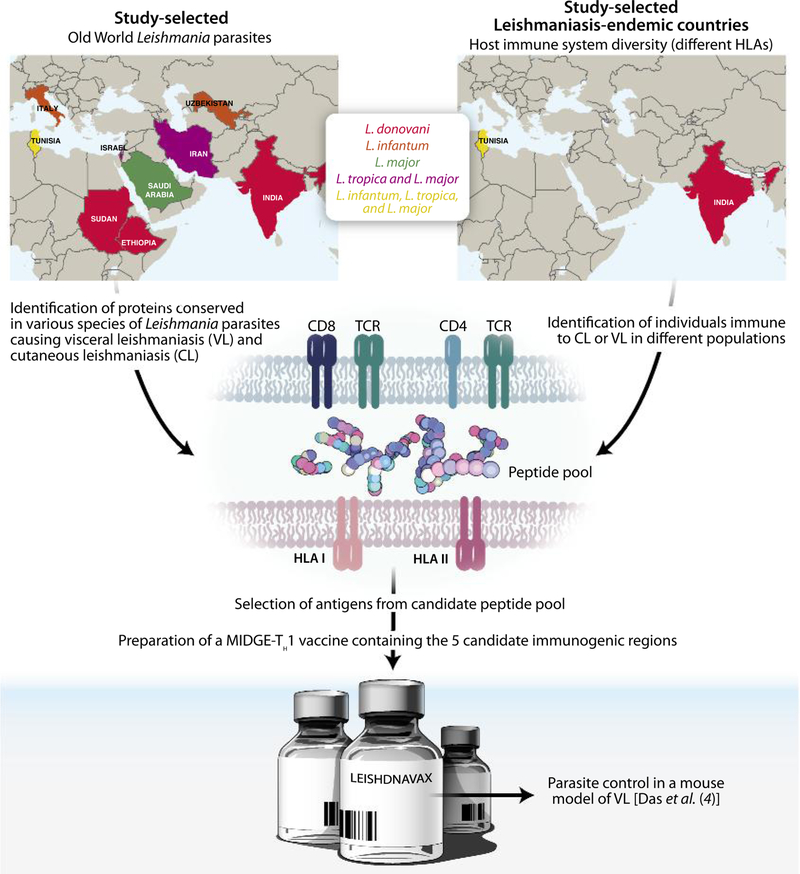
In this issue of Science Translational Medicine, Das et al. addressed the abovementioned challenges in a new strategy for the development of a human leishmaniasis vaccine (4). This approach began with the selection of seven parasite-derived vaccine candidates known for their ability to induce T cell responses in rodents: KMP11, TSA, CPA, CPB, P74, HASPB, and A2. These antigens were further screened for their homology in four Old World parasite species and strains, mostly from human isolates collected from Europe, Africa, and Asia (Fig. 1). Das and colleagues then tested peripheral blood mononuclear cells (PBMCs) from immune individuals living in Leishmania endemic areas to down-select and refne these vaccine candidates (4). The premise for this approach is that individuals cured from visceral leishmaniasis (VL) and CL, or that are asymptomatic for VL, should display a protective T helper 1 (TH1) immune response toward Leishmania parasites.
Fig. 1. LEISHDNAVAX: A TH1-inducing Leishmania DNA vaccine.
Leishmaniasis is a neglected infectious disease caused by the protozoan parasite Leishmania. Immunity and protection from leishmaniasis requires induction of a TH1 cellular immunity. This poses a challenge due to diversity of the human HLA as well as diversity of immunogenic antigens on different Leishmania species. To overcome these obstacles, Das et al. (4) used PBMCs from immune individuals residing in Tunisia and India, countries endemic for different forms of leishmaniasis, to identify sequences containing TH1-inducing epitopes conserved across Old World Leishmania species. LEISHDNAVAX was made using five MIDGE-TH1 vectors and was tested for protective effcacy against visceral leishmaniasis (VL) in mice.
This strategy simultaneously tackled parasite variability and variation of the human T cell immune response to parasite antigens (Fig. 1).
Using PBMCs from individuals from India, where L. donovani causes VL, and from Tunisia, where L. major and L. tropica cause CL and L. infantum causes VL, the authors stimulated cells with peptide pools derived from five of the seven vaccine candidates (Fig. 1). As expected, the T cell immune response to peptide pools varied among different donors. What was unexpected was the absence of dominant immunogenic peptides in any of the five antigens. Instead, the study cohort recognized peptides that spanned the majority of each antigen, likely reflecting the immunogenicity of the vaccine candidates and the individuality of the human immune response. This observed variability of the human T cell immune response within a single population brings into focus the pitfalls of vaccine candidate selection solely on the basis of the immunogenically and protective efficacy of an antigen in rodent studies.
In partnership with the company MOLOGEN AG (www.mologen.com), Das et al. constructed five MIDGE (minimalistic immunogenically defined gene expression)–TH1 vectors, each containing a sequence enriched for human T cell epitopes from one of the five selected vaccine candidates (KMP11, TSA, CPA, truncated CPB, and P74), creating LEISHDNAVAX. The MIDGE-TH1 platform is a commercially available next-generation DNA technology. This vector is optimized for better transfection of cells and better induction of T cell immune responses and has demonstrated superior protection in a rodent model of leishmaniasis as compared with that of a conventional DNA plasmid (5). Importantly, the modularity of the LEISHDNAVAX strategy permits prompt and low-cost modification of the vaccine through the removal of or addition to any of the antigen-encoding MIDGE-TH1 vectors.
By electing to use the MIDGE-TH1 vector, Das and colleagues have improved the likelihood of success in transitioning LEISHDNAVAX through product development. DNA vaccine production is advantageous owing to its simplicity, reproducibility, adaptability, and low-cost production compared with recombinant proteins. Additionally, DNA vaccination has been shown to be safe in humans (6).
REVERSE TRANSLATION: FROM HUMANS TO MICE
Having designed LEISHD-NAVAX for immunogenicity in humans, Das et al. returned to animal models—in a sense, reverse-translating their research—by testing the efficacy of LEISHD-NAVAX in a mouse model of VL (Fig. 1). Immunization of BALB/c mice with LEISHD-NAVAX induced cellular and humoral immune responses to all five antigens present in the vaccine. Moreover, compared with controls, vaccinated animals displayed a significant reduction in spleen and liver parasitemia 1 month and up to 90 days after intravenous challenge with 10 million L. donovani parasites. The parasite burden reduction was dose-dependent, indicating the specificity of the response to LEISHDNAVAX. Upon infection with Leishmania, vaccinated animals showed a signifcant increase in the proinfammatory TH1 cytokines interferon-γ and tumor necrosis factor–α but maintained the same level of the anti-inflammatory cytokines interleukin-10 (IL-10) and IL-4. This demonstrates the induction of a potent TH1 immune response by LEISHDNAVAX.
LINGERING QUESTIONS
The vaccine design adopted by Das et al. (Fig. 1) brings us one step closer to the development of an efficacious human leishmaniasis vaccine. Importantly, although LEISHDNAVAX is targeted to parasites from the Old World, it has the potential to become a pan-Leishmania vaccine.
Nevertheless, some aspects require further exploration of LEISHDNAVAX. One question is whether it will induce long-term memory. In the study by Das et al. (4), the animals were challenged 10 days after the last immunization, precluding the evaluation of a memory response, which is a prerequisite to the function of an efficacious vaccine. Ideally, LEISHDNAVAX should be tested in a long-term protection study.
Another question is whether LEISH-DNAVAX will demonstrate protective efficacy in a more relevant animal model that displays disease pathology similar to the one observed in humans, such as hamsters (7). The authors elected to use BALB/c mice to test protection against VL. BALB/c mice are considered by many to be a poor animal model for VL because they control the infection and do not show clinical signs of disease (8). We also have to keep in mind that leishmaniasis is a vector-borne disease and that the contribution of the vector in disease transmission and vaccine development has to be considered. Will LEISHD-NAVAX protect against sand fy–initiated models of infection? Recent studies have highlighted the virulence of vector-transmitted Leishmania parasites that abrogates protection after needle challenge of vaccinated animals (9). Such models deliver a more physiological parasite dose and co-deposit the Leishmania into the skin along-side established virulence factors present in the infected vector (10), thus better mimicking what occurs naturally.
REFLECTIONS
Currently, there are no vaccines available for prevalent parasitic diseases with high morbidity and mortality such as malaria, tuberculosis, and many others. We can certainly benefit from the fresh look that Das et al. adopted, using humans to make a human vaccine. LEISHDNAVAX came to fruition through the efforts of a consortium that brought together major researchers from countries endemic and nonendemic for leishmaniasis with expertise in a variety of disciplines and representing academic institutions and private enterprises. These partnerships created the critical mass required for such an undertaking. We hope that these partnerships are promoted to develop solutions for other neglected infectious diseases.
REFERENCES
- 1.Desjeux P, Leishmaniasis. Nat. Rev. Microbiol 2, 692–693 (2004). [DOI] [PubMed] [Google Scholar]
- 2.Kaye P, Scott P, Leishmaniasis: Complexity at the host-pathogen interface. Nat. Rev. Microbiol 9, 604–615 (2011). [DOI] [PubMed] [Google Scholar]
- 3.Sundar S, Singh B, Identifying vaccine targets for anti-leishmanial vaccine development. Expert Rev. Vaccines 13, 489–505 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Das S, Freier A, Boussoffara T, Das S, Oswald D, Losch FO, Selka M, Sacerdoti-Sierra N, Schönian G, Wiesmüller K-H, Seifert K, Schroff M, Juhls C, Jaffe CL, Roy S, Das P, Louzir H, Croft SL, Modabber F, Walden P, Modular multiantigen T cell epitope–enriched DNA vaccine against human leishmaniasis. Sci. Transl. Med 6, 234ra56 (2014). [DOI] [PubMed] [Google Scholar]
- 5.López-Fuertes L, Pérez-Jiménez E, Vila-Coro AJ, Sack F,Moreno S, Konig SA, Junghans C, Wittig B, Timón M,Esteban M, DNA vaccination with linear minimalistic (MIDGE) vectors confers protection against Leishmania major infection in mice. Vaccine 21, 247–257 (2002). [DOI] [PubMed] [Google Scholar]
- 6.Ulmer JB, Wahren B, Liu MA, Gene-based vaccines: Recent technical and clinical advances. Trends Mol. Med 12, 216–222 (2006). [DOI] [PubMed] [Google Scholar]
- 7.McCall LI, Zhang WW, Matlashewski G, Determinants for the development of visceral leishmaniasis disease. PLOS Pathog 9, e1003053 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nieto A, Domínguez-Bernal G, Orden JA, De La Fuente R, Madrid-Elena N, Carrión J, Mechanisms of resistance and susceptibility to experimental visceral leishmaniosis: BALB/c mouse versus Syrian hamster model. Vet. Res 42, 39 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Peters NC, Kimblin N, Secundino N, Kamhawi S, Lawyer P, Sacks DL, Vector transmission of leishmania abrogates vaccine-induced protective immunity. PLOS Pathog 5, e1000484 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Aslan H, Dey R, Meneses C, Castrovinci P, Jeronimo SM, Oliva G, Fischer L, Duncan RC, Nakhasi HL, Valenzuela JG, Kamhawi S, A new model of progressive visceral leishmaniasis in hamsters by natural transmission via bites of vector sand flies. J. Infect. Dis 207, 1328–1338 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]