Abstract
Vagal sensory neurons mediate the vago-vagal reflex which, in turn, regulates a wide array of gastrointestinal functions including esophageal motility, gastric accommodation and pancreatic enzyme secretion. These neurons also transmit sensory information from the gut to the central nervous system, which then mediates the sensations of nausea, fullness and satiety. Recent research indicates that vagal afferent neurons process non-uniform properties and a significant degree of plasticity. These properties are important to ensure that vagally regulated gastrointestinal functions respond rapidly and appropriately to various intrinsic and extrinsic factors. Similar plastic changes in the vagus also occur in pathophysiological conditions, such as obesity and diabetes, resulting in abnormal gastrointestinal functions. A clear understanding of the mechanisms which mediate these events may provide novel therapeutic targets for the treatment of gastrointestinal disorders due to vago-vagal pathway malfunctions.
Keywords: Digestive system, Gastrointestinal peptides, Sensory innervation, Nodose ganglia neurons
1. Introduction
Vagal afferents play an important role in the regulation of the physiological functions of the gastrointestinal (GI) tract. Studies have revealed that stimulation of gastric vagal afferents initiates a number of vagally mediated reflexes, including suppression of food intake [1], inhibition of gastric emptying [2], stimulation of acid [3], and pancreatic secretion [4]. The vagus afferents may be stimulated by gastric distension, an array of gut hormones released from the enteroendocrine cells (ECs) in response to food intake, or may be directly activated by nutrients such glucose, fatty acids or salts in the gut [5–9]. Vagal sensory signaling includes events such as the encoding, integration, and transfer of peripheral sensations by vagal afferent neurons to the CNS.
Over the last decade it has become clear that vagal afferent neurons exhibit remarkable plasticity in response to intrinsic and extrinsic factors. For example, recent works by the Docray’s group showed that nutritional status regulates receptor density and neuromediators expressed by the vagal neurons. This in turn determines the neuro chemical phenotype of the vagal afferent neurons. Depending on the nutritional status, the same group of afferent neurons may transmit orexigenic or anorexigenic signals [10–12]. Furthermore, it is well known that vagal afferent neurons exhibit abnormal sensitivity to GI peptides or mechanical stimuli in pathological conditions such as diet-induced obesity or diabetes [13–17].
The aim of this article is to review some of the newer concepts regarding the role of vagal afferents in the regulation of gut functions. Plasticity of vagal afferent signaling provides an added level of fine tuning in response to the nutritional and metabolic status of the subject. These pathways also suggest new approaches and targets for the treatments of obesity and GI tract disorders.
2. Vagal sensory innervation of the gut
Cell bodies of vagal afferent neurons reside in two adjacent but distinct anatomic structures: the nodose (inferior) and intracranial jugular (superior) ganglia. Vagal neurons from the nodose ganglia originate from the epibranchial placodes, while jugular neurons originate from the neural crest [18]. Visceral afferents can be classified by the location of their receptive fields (mucosal afferents, muscle afferents), their function (low and high threshold mechanoreceptors, termo-, osmo-sensitive, chemoreceptors, nociceptors), their conduction velocities (C-, Aδ- and Aβ fibers), or their neurochemical codings (peptidergic and non-peptidergic afferents) [19–22]. In addition, many visceral afferents respond to a wide range of mechanical and chemical stimuli, and are considered “polymodal” [23–27]. Vagal sensory fibers do not appear to mediate pain sensation in the gut, since it has been shown that severing the spinal, but not the vagal, pathway abolished pain sensations induced by colon or stomach extension or heat [20,28,29]. However, more recent data suggests that vagal afferents may mediate mechanical or acid-evoked esophageal nociception [30–33].
Electron microscopic and physiologic studies demonstrate that visceral sensory fibers are predominantly unmyelinated (C-fibers) and few are thinly myelinated axons (Aδ fibers) [21,33,34]. Antegrade tracing studies showed that vagal afferent nerve endings are widely distributed in the mucosal layers of the stomach and proximal small intestine. These endings are observed in the villi, with some fibers approaching the basal side of epithelial cells [35,36]. It is likely that these vagal mucosal afferents express polymodal sensitivity to light mechanical stimuli, but not to stretching [24,25,37–39]. They can sense osmotic and thermal changes [23,40–43] and have chemosensitive properties to amino acid and glucose [43,44]. About a third of these afferents respond to capsaicin, a vanilloid receptor agonist, which is prevalent in c- and Aδ fibers [19,26,38,39,45]. It should be noted that most nutrients activate EC, triggering the release of mediators such as CCK, serotonin (5HT), and leptin which, in turn, activate/modulate mucosal afferent fiber terminals.
Morphological tracing studies reveal a second type of afferent endings within the muscular layers of the gut. These endings have been shown to be closely associated with the intramuscular interstitial cells of Cajal (ICC), which leads to the speculation that they may act in conjunction with the ICC to form a functional complex [46] to detect tension and monitor gut distension [47].
The responses to vagal afferent stimulation depend on the sites of stimulation. For example, stimulation of esophageal vagal afferents causes fundus relaxation [48,49], while stimulation of vagal fibers in the stomach or ileum results in inhibition of food intake, gastric emptying, and stimulation of pancreatic secretion [50]. Grabauskas et al. [7] recently demonstrated that increase in the extracellular glucose concentration excited 30% of the stomach projecting nodose ganglia neurons but inhibited 11% of the portal vein projecting neurons.
In general, hormonal and chemical stimuli from the stomach modulate gastric vagal afferent responses to mechanical stimulation, whereas the small intestine hormones directly activate vagal afferent endings. Both the modulatory and direct actions of these hormones on gut afferents may activate or inhibit satiety. Moreover, it is suggested that mechanoreceptors within the stomach are the only means by which the stomach sends afferent signals to the brain [26,51,52]. The consumption of food would cause distension of the stomach and is perceived centrally with well-characterized feelings of fullness and satiety [53] which can be modulated by gut peptides; for example, CCK augments and ghrelin dampens mechano-responses of sensory fibers [9]. In a recent study Kentish et al. [17] demonstrated the expression of clock genes (Bmal1, Per1, Per2, and Nr1d1) in vagal afferent cell bodies, and that oscillation of the expression of these genes correlates with changes in gastric mucosal receptor sensitivity to stroking. Single fiber recording from stomach projecting afferents demonstrated that gastric mucosal receptor response to stroking with a Frey’s hair is 3 times greater at 12 h and 15 h than the response at 0 h. Thus, gastric vagal mechanoreceptors display circadian rhythm, which may act to control food intake differentially at different times of the day.
3. Regulation of vagal sensory neurons by gut peptides
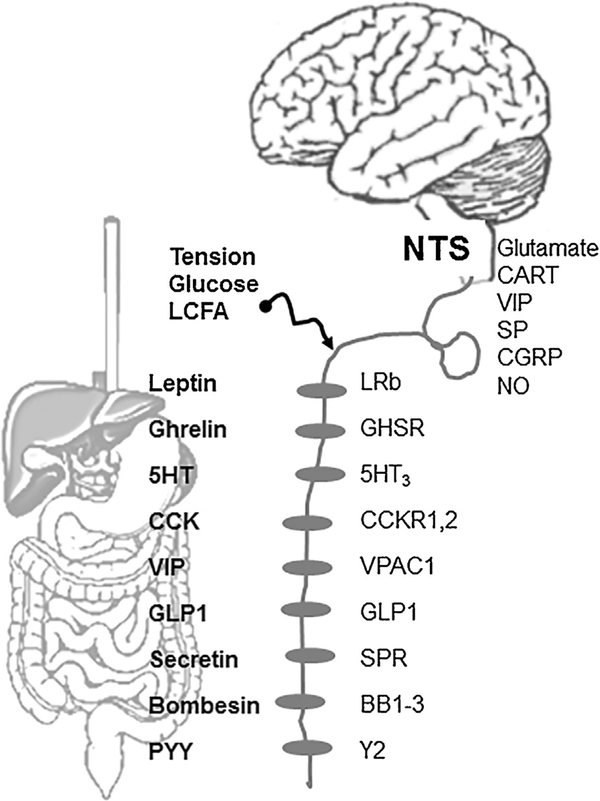
Gut peptides are major regulators of vagal sensory activities. These include cholecystokinin (CCK), ghrelin, and leptin that are expressed and released from discrete populations of GI enterochromaffin cells (Table 1). These peptide secreting cells are located close to vagal afferent sensory endings [9] which express a number of gut hormone receptors (Fig.).
Table 1 –
Gutpeptides and theirroles in gastrointestinal functions
| Mediator | Source | Receptor | Vagally mediated function | References |
|---|---|---|---|---|
| CCK | Intestinal EC I-cells |
CCK1 CCK2 |
Contraction of gallbladder; Pancreatic stimulation; Satiety; Motility; Acid secretion. | [4,54–60] |
| Leptin | Adiposities; EC Parietal cells | LRb | Inhibition of food intake; mechano-sensitization | [9,16,61,62] |
| Ghrelin | EC x/A cells in the stomach | GHSR1a | Induces food intake; insulin secretion; gastric acid secretion; gastric emptying, relaxation; intestinal transit; fat tissue metabolism; regulates gene transcription and modulates neural electrical activity | [63–72] |
| Bombesin | Endocrine cells gastric mucosa | BB1 BB2 BR3 |
Inhibition of gastric emptying; inhibition of food intake; stimulates gastric acid secretion and gastrin release. | [73–75] |
| PYY | Small intestine L-cells |
Y2 | Decrease intragastric tone; inhibit gastric acid secretion | [11,76,77,78] |
| Secretin | S-cells duodenum | SPR | Stimulates pancreatic bicarbonate secretion; Inhibits gastric acid secretion and gastric motility. |
[79,80] |
| GLP1 | Small intestine L-cells Pancreatic A-cells |
GLP1 | Anorexic signals; Inhibits gastric secretion; reduces ghrelin plasma concentrations. | [81–85] |
| 5HT (serotonin) | EC cells, ENS neurons | 5HT3 | Stimulates bowel transit; induces nausea and vomiting. | [86–88] |
| VIP | Duodenum | VPAC1 | Inhibits gastric acid secretion. | [89] |
Fig. –
Vagal afferent signaling is regulated by complex neural, hormonal and mechanical signals. Nutrients trigger the release of gastrointestinal peptides that act on specific receptors on vagal afferents. Vagal afferents synapse with the second order neurons in the NTS by releasing a variety of neurotransmitters which act on higher centers to regulate gastrointestinal functions. 5HT3, serotonin receptor; BB1–3, bombesin receptors;CART, cocaine and amphetamine regulated transcript; CCK, cholecystokinin; CCK-1,2, cholecystokinin-1 and 2 type receptor; GHSR, growth hormone secretagogue receptor; GLP-1, glucagon-like peptide-1; GLP1, glucagon-like peptide-1 receptor; LCFA, long-chain fatty acid; LepR, leptin receptor; PYY, peptide YY; Y2-receptor; NO, nitric oxide; NTS, the nucleus of the solitarii tract; SP, substance P; SPR, secretin receptor; VIP, vasoactive intestinal peptide; VPAC1, receptor.
3.1. CCK
CCK is released from enterochromaffin (EC) I-cells, located largely in the proximal small intestine in response to luminal nutrients such as amino acids and fatty acids [90–92]. CCK evokes contraction of the gallbladder [54,55], and stimulates pancreatic enzyme secretion [56,57], and inhibition of food intake [58,59].
Expression of both high affinity CCK receptor (CCK1) and low affinity CCK receptor (CCK2), has been demonstrated in the vagal afferent fibers and vagal neuronal soma [93–98] (Fig.). Within the mucosa, vagal afferents expressing the CCK1 receptor terminate within the lamina propria in close apposition to the basolateral membrane of I cells [99,100]. Electrophysiological studies demonstrate that the vagal afferent discharge is increased by CCK-8 [4,40,79,86,101] and similarly, neurons in the nucleus tractus solitarii (NTS) that receive inputs from vagal afferents are stimulated by CCK [102–105].
There is ample evidence that the majority of CCK actions are mediated by activation of CCK 1 and 2 receptors in the vagal afferents [4,60,106–108] (Fig.). It has been demonstrated that pretreatment of vagus with the neurotoxin capsaicin, or total subdiaphragmatic vagotomy, attenuated the reduction in cumulative food intake evoked by CCK-8 and −33 [5,109–112]. Disruption of the vagal afferent pathways markedly reduced the CCK satiety action in both rats and mice. The CCK1 receptor knockout Otsuka Long-Evans Tokushima Fatty (OLETF) rats are hyperphagic [113]. Similar to OLETF rats, CCK1 receptor knockout mice consume larger meals [114]. However, in this model the role of CCK1 receptors to maintain normal body weight is less critical, despite evidence that CCK plays a major role in satiety
Li and Owyang [4] demonstrated that perivagal pretreatment with capsaicin or transection of afferent nerves abolished the pancreatic enzyme response to physiological doses of CCK-8. This provides strong evidence for neuronal action of endogenous CCK, acting via an afferent vagal pathway in the duodenal mucosa to stimulate pancreatic secretion in the rat [87,115]. However, this approach was recently challenged by the demonstration that perivagal application of capsaicin may cause degeneration of vagal efferent motoneurones [116]. It is interesting to note that the dorsal motor nucleus of the vagus (DMV) may also be activated by CCK-8 [117] raising the possibility that pancreatic secretions are under the modulatory control of CCK responsive vagal motoneurones [117]. In any case, there is strong evidence that CCK is acting via vagal pathway to stimulate pancreatic enzyme secretion [115].
3.2. Leptin
Leptin is a 127-amino acid peptide mainly secreted by adipocytes [118] and to a lesser degree from the gastric parietal (P) cells [119]. Leptin is known to suppress appetite, body weight gain, and adiposity in humans, rodents, and monkeys [120]. Leptin receptors (LRbs) are found in a variety of brain areas and predominantly in areas with documented roles in feeding control [121,122] (Fig.). LRbs are also expressed by vagal afferents terminating in the stomach. Indeed, leptin has been shown to directly activate cultured gastric and duodenum projecting vagal afferents from rats [79,123–125]. This suggests that leptin released from the gastric mucosa could act on adjacent vagal afferent endings to modulate vagal signals in response to food intake. Experiments have demonstrated that leptin reduced short-term (up to 4 h) food intake which required intact vagal nerves [61,62,126]. Targeted deletion of leptin receptor in vagal sensory ganglia in mice led to a small but significant increase in body weight at 10 weeks and with further weight gain at 12 weeks as a result of increased fat mass [13,127]. These observations indicate that vagal action of gastric leptin may serve to augment the acute appetite-suppressant effects of circulating leptin [123,128].
3.3. Ghrelin
Ghrelin is a 28-amino acid peptide secreted from the X/A cells found predominantly in the stomach and to a lesser extent in the intestine [129–131]. Feeding studies indicate that ghrelin participates in the regulation of food intake, body weight, adiposity, insulin secretion, glucose metabolism and GI functions such as stimulation of gut motility and gastric acid secretion [63–68] (Table 1).
Ghrelin actions are mediated by the growth hormone secretagogue receptor (GHS-R), a G-protein-coupled receptor (GPSR) [129,132]. Two isoforms of GHSR – 1a and 1b – have been identified but only GHSR1a is considered as the active form of GHSR. GHSR1a is densely expressed in the hypothalamus, the substantia nigra, the ventral tegmental area and throughout the limbic system [129,133] and cell bodies of the enteric nervous system [134]. The GHSR1a receptor also found in the mouse nodose ganglia and in vagal afferents that project to the stomach [69,70,79] (Fig.). Anterograde tracing studies demonstrated that vagal afferents are found in close proximity to ghrelin-containing cells in the mice stomach mucosa [16]. Moreover, it was reported that ghrelin selectively inhibited a subpopulation of mechanosensitive gastric vagal afferents [135], supporting the possibility that there is a close functioning relationship between ghrelin and gastric vagal afferent generated satiety signals.
The vagus is important in mediating the actions of ghrelin on gastric functions (Table 1). Treatment with atropine, capsaicin or vagotomy abolished the effects of peripherally administered ghrelin on food intake, gastric emptying, intestinal transit, and gastric acid secretion [63,64,69,71,72]. Recently, Grabauskas et al. [79] demonstrated that targeted deletion of the Kir6.2 subunit, which is an integral part of the ATP-sensitive potassium channel (KATP), in the vagal nodose ganglia abolished the orexigenic action of ghrelin on food intake. Also, it was demonstrated that ghrelin action to reduce vagal afferent activities of neurons is mediated by the GHSR-1a receptor-phosphatidylinositol 3-kinase (PI3K)-extracellular signal-regulated kinase 1 and 2 (ERK1/2)-KATP channel signaling pathway. The resulting hyperpolarization renders the nodose ganglia less responsive to anorexigenic signals that act via the vagal afferent pathways [70,136].
Recently, the role of the vagus in mediating the actions of ghrelin was brought into question by the observation that subdiaphragmatic vagal deafferentation did not abolish the hyperphagic action of ghrelin [137]. However, it is important to point out that this observation does not rule out the possibility that ghrelin may act directly on the vagal sensory neuron soma to modulate satiety [79]. The identification of the specific signaling pathways used by ghrelin on the vagus sensory neurons provides an opportunity to develop agents to modulating food intake via peripheral pathways. This potentially may be used to treat feeding disorders.
4. Receptor interaction
4.1. Feeding status modulates neurochemical phenotype of vagal sensory neurons
Feeding status may have a profound influence on the actions of gut peptide regulating GI functions by changing the expression of receptors and/or neurotransmitters. For example, under fasting conditions when basal plasma CCK level is low, there is increased expression of vagal cannabinoid CB1 and melanin-concentrating hormone (MCH)-1 receptors and low expression of Y2 receptors which respond to the satiety peptide YY (PYY) [10–12,138]. Fasting also causes an increased expression of the orexigenic neuropeptide MCH and decreased expression of the satiety-induced peptide cocaine and amphetamine regulated transcript (CART). On the other hand, feeding rapidly downregulates the expression of CB1 and MCH-1 receptors but enhances the expression of Y2 receptors in vagal neurons projecting to the stomach and duodenum. This is accompanied by decreased expression of MCH and increased expression of CART but stable expression of CCK1, orexin receptor type 1 (Ox1) and GHR1 receptors [10,50,70,136,139]. These phenotypic changes appear to be mediated by CCK as they can be blocked by CCK1 receptor antagonists and reproduced by exogenous CCK administration [10–12,14,50]. These findings indicate that neuro chemical phenotype of vagal afferent neurons can be altered depending on the feeding status.
4.2. Synergistic interactions between gut neuromediators in vagal ganglia
It is well established that a variety of gut-derived mediators interact to enhance each other’s activity on vagal afferent neurons. Rodent studies showed that leptin injected intraperitoneally at low doses, which did not influence feeding behavior, decreased foodintake and body weight when co-injected with a subthreshold dose of CCK [61,125,140–144]. In fact, it was suggested that the satiety action of CCK appears to depend on leptin signaling. This synergistic leptin-CCK interaction was reported to be associated with an increase in the firing frequency of gastric vagal sensory neurons [108,144–146] and accompanied by increased activation of early gene c-fos expression in the NTS of the brainstem [61,140,144,147,148], the paraventricular nucleus and the dorsomedial nucleus of the hypothalamus [149]. The anorexigenic action of leptin and CCK was attenuated by capsaicin treatments and pretreatment with the specific CCK1 receptor antagonist [61], suggesting that CCK and leptin interact at the vagal fibers.
Heldsinger and colleagues [146] recently demonstrated that the synergistic interaction between vagal CCK and leptin receptors is mediated by the phosphorylation of signal transducers and activation of transcription 3 (STAT3), which in turn, inhibits potassium channels, leading to neuronal firing in isolated rat vagal neurons. This involves the interaction between CCK/SRC/phosphoinositide 3-kinase cascades and leptin/janus kinase-2/phosphoinositide 3-kinase/STAT3 signaling pathways. This synergistic interaction stimulates CART release from the vagal sensory neurons, which is the principal neurotransmitter mediating short-term satiety [150,151].
Leptin appears to act on both peripheral and central sites to exert its control on satiety. It was demonstrated that peripheral administration of leptin reduced acute sucrose intake whereas central administration had no effect [125]. Other studies showed that the acute effect of leptin on food intake is due to its ability to activate vagal afferents [152]. Selective knockout of the leptin receptor in visceral afferents caused hyperphagia leading to increased weight gain further affirming that leptin is acting on sensory vagal fibers to regulate food intake [127].
Leptin also has been shown to act on the hypothalamic nuclei to potentiate the peripheral action of CCK [153]. Morton and colleagues showed that leptin receptor-deficient Koletsky rat, in which the LRb in the medial basal hypothalamus, a site that has been implicated in the feeding action of leptin was specifically deleted, had a reduced satiety response to peripheral CCK. The targeted restoration of leptin signaling in the basal medial hypothalamus both reduced the size of meals and made the Koletsky rat more responsive to exogenous CCK. These observations indicate that CCK and leptin may also interact at the hindbrain/brainstem integrative sites raising the possibility that leptin and CCK may generate anorexic signaling at multiple sites.
In addition to leptin, CCK also interacts with other gut hormones to regulate GI function. Using the single vagal afferent neuronal recording, Li and colleagues [86] demonstrated that in about 50% of recorded vagal neurons although a subthreshold dose of CCK-8 produced no measurable electrophysiological effects, it augmented vagal firing in response to luminal 5-HT perfusion. This interaction between CCK and 5-HT at the level of the vagal ganglia may modulate afferent postprandial signals arising from the GI tract and may explain how a small increase in the plasma CCK level is sufficient to produce a robust postprandial pancreatic secretion [60]. This interaction would also explain how CCK administration potentiates subsequent responses to GI perfusion of nutrients [2,43,101,108].
The anorexigenic hormones leptin and CCK, and the orexigenic hormone ghrelin act in opposition to regulate feeding behavior via the vagal afferent pathways. Feeding studies demonstrated that the anorexic effect of CCK was blocked by pre-administration of ghrelin in rats. Conversely, pretreatment with CCK inhibited the orexigenic effect of ghrelin [12,50,70,137]. Since CCK1 and GHSR1a are colocalized in the nodose ganglia neurons, it is conceivable that CCK and ghrelin may interfere with signal transmission generated by one another at the level of vagal nodose neurons. Studies of the signaling cascades used by ghrelin showed that ghrelin caused a significant increase in Epac and suppression of cytokine signaling 3 (SOCS3) expression in cultured rat nodose ganglia [146]. This in turn negatively affects leptin signal transduction and neuronal firing in vagal neurons. Feeding studies showed that silencing SOCS3 expression in the vagal ganglia reduced food intake evoked by endogenous leptin.
5. Central projections of visceral afferents
Visceral sensory information is transmitted via the vagal afferent fibers to the nucleus of the tractus solitaries (NTS) in the hindbrain [49,154]. Gut innervating vagal afferents which terminate in the NTS are organized topographically: terminates from the esophagus project predominantly to the subnucleus centralis, those from the stomach project to the subnucleus medialis and gelatinosus and terminals from the intestine are represented in the subnucleus commissuralis and medialis [155–157].
Glutamate is the main neurotransmitter utilized by the vagal afferents to communicate with the second order neurons in the NTS [158] (Table 2). Approximately 60% of the vagal sensory neurons somas [172,173] and terminals in the NTS [174] contain glutamate immunoreactivity. NTS neurons express both sonotropic (N-methyl-D-aspartate [NMDA], α-amino-3-hydroxy-5-methylisoxazole-4-propionate receptor [AMPA], kainite) and metabotropic glutamate receptors [175–177]. Blocking of NMDA receptors with systemic or intra-NTS microinjection of NMDA antagonist, dizocilpine delayed satiation and increased meal size [178–180]. Additionally, CCK-induced satiation was also attenuated by NTS injection of the NMDA receptor antagonist MK801 [181,182].
Table 2 –
Vagus afferent neuron neurotransmitters.
| Mediator | Vagally mediated function | References |
|---|---|---|
| Glutamate | Mediates CCK-induced satiation; Modulates feeding behavior. | [159–161] |
| CART | Induces satiation | [13,150,162] |
| VIP | Inhibits gastric acid secretion | [89,163] |
| SP | Modulate synaptic input to NTS | [164–166] |
| CGRP | Induces satiety; Gastric mucosa protection | [165,167–169] |
| NO | Enhances mechano-sensitivity | [170,171] |
CART is another neuropeptide implicated in central vagal transmission (Table 2). Immunohistochemical studies demonstrated that 17% of the vagal sensory neurons with gastric projections and 41% of those projecting to the duodenum expressed CART immunoreactivity [182]. These CART containing vagal neurons also coexpressed CCK1 and LRb. Functionally, CCK and leptin stimulated CART release from vagal terminals to mediate satiety [150]. It was shown that injections of CART into the 4th ventricle greatly suppressed food intake and the drinking of sucrose water accompanied by inhibition of C-Fos expression in the NTS [182]. Conversely, targeted silencing of CART with siRNA in the rat nodose ganglia abolished CCK and leptin stimulated C-Fos expression in the hypothalamus and CCK/leptin induced satiation [150]. Thus, in addition to glutamate, CART is another neuropeptide utilized by the vagal afferents to transmit peripheral signals to activate NTS in the brain stem.
Synaptic transmission between the vagal afferents and NTS neurons is subject to modulation by a wide variety of neurotransmitters, hormones and metabolites including glutamate [183–186], CCK [187,188], tumor necrosis factor [189], melanocortins [190], 5HT [191], ATP [192], and prostaglandins [193]. Second order sensory neurons in the NTS project either locally to the DMV, which innervate the gut, or send ascending projections to the forebrain including the parabrachial nucleus, hypothalamus, amygdala, nucleus of the stria terminalis and insular cortex [194]. In addition to vagal afferents, the NTS neurons also interact with each other and with neurons in other CNS areas and hormones to fine tune synaptic transmission between NTS and DMV [159,195–200]. In this way, the NTS neurons can respond to constantly changing physiological conditions to regulate gut function.
6. Vagal afferent pathways in clinical conditions
6.1. Diabetes modulates excitability of vagus
GI functions are often abnormal in patients with poorly controlled diabetes. These include impaired esophageal motility, defective gastric accommodation, abnormal gastric emptying, diminished pancreatic function, and abnormal eating behavior. Many of these functions are mediated by vago-vagal pathways. Abnormalities of vagal function are common in patients with longstanding diabetes, although anatomical abnormalities are seldom demonstrable on histologic examination of the vagus. This raises the possibility that vagal dysfunction may be secondary to altered electrophysiological properties of the nodose ganglia (NG) caused by chronic hyperglycemia. Two-pore-domain potassium (2PK+) channels play an important role in setting the resting membrane potential and excitability of neurons. Regulation of 2PK+ channels by neurotransmitters and second messengers is essential for neuronal functioning in the central nervous system [201].
In a recent study, it was demonstrated that the TRAK-related spinal cord K+ channel (TRESK) is abundant in rat NG and its activation is increased in diabetic rats [201]. Enhanced activation of the TRESK K+ channel in the capsaicin-sensitive NG of diabetic rats led to membrane hyperpolarization, resulting in decreased excitability and abnormal GI functions mediated by the vago-vagal reflex such as pancreatic enzyme secretion and gastric motility. Silencing the TRESK K+ channel in the diabetic rat restored NG excitability and improved GI functions mediate by vago-vagal reflex. These findings provide an attractive unifying hypothesis to explain the widespread GI disturbances in diabetic patients. Understanding the signal transduction cascade mediating the abnormalities may provide important therapeutic targets for the medical treatment of diabetic patients with GI complications.
6.2. High fat feeding induces dysfunction of the vagus
Altered function of the vagal afferent pathways occurs in obesity. Gastric [16] and jejunal [15] vagal afferents showed reduced responsiveness to mechanical stimulation in high fat diet-induced obese mice. This was accompanied by diminished satiety action of CCK [15,202,203] and reduced c-fos expression in the area postrema and nucleus of the solitary tract [203].
Reduced anorexigenic response also applies to leptin stimulation [196,197] in diet-induced obesity where there was a 94% reduction of receptor expression [32]. The potentiating effect of leptin on the mechanosensitivity of mucosal receptors was abolished in high fat induce obese mice [16,160,204]. On the other hand, the inhibitory action of ghrelin was enhanced as the mucosal and tension afferents became more sensitive to ghrelin [9,16]. These observations suggest that the phenotypic changes in the vagus may contribute to the development and maintenance of obesity.
High fat feeding induced an inflammatory response including an up-regulation of macrophages and microglia as well as increased inflammatory cytokines in the vagal ganglia and hypothalamus [205]. This inflammatory response may contribute to the blunting of ghrelin signaling in the vagus ganglia [206]. On the other hand, celiac vagotomy reduced high fat diet-induced inflammation in the hypothalamus tissues suggesting that the vagal afferent nerve may transfer gut-delivered inflammatory signals to the hypothalamus [205].
6.3. Vagal CGRP mediates gastric mucosa defense
The gut is densely innervated by vagal sensory afferents containing calcitonin gene-related peptide (CGRP), substance P, vasoactive intestinal polypeptide (VIP) and tachykinins [207,208]. The density of CGRP-immunoreactive fibers in the gastric mucosa was markedly reduced by vagotomy [209]. It was demonstrated that stimulation of afferent nerve by intragastric capsaicin protected gastric mucosa against irritants in the [167–169]. Similarly peripheral administration of CCK also attenuated ethanol-induced mucosal damage, an action abolished by CGRP antagonist, vagotomy or afferent denervation by capsaicin [210] but restored by exogenous administration of CGRP [209–212]. Thus afferent release of neuropeptides such as CGRP may modulate mucosa hemostasis via vagal reflex and paracrine action [169]. The efferent limb involves activation of vagal cholinergic pathway which stimulates the release of mucosal prostaglandin and nitric oxide to regulate blood flow and activation of mast cells [213].
7. Concluding remarks
A high degree of plasticity is required to ensure that vagally-regulated gastrointestinal functions respond appropriately to a variety of intrinsic and extrinsic factors such as feeding, stress and even time of the day. These mechanisms are important for adaptive response and homeostasis. Plastic changes in the vagus also occur in pathophysiological conditions such as obesity and diabetes. These changes may adversely affect gastrointestinal functions. A clear understanding of the molecular and cellular mechanisms mediating these events may provide novel therapeutic targets for prevention and or treatment.
Acknowledgements
This was work supported by the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) Grants R01-DK048419 (C. Owyang) and P30-DK34933 (C. Owyang).
Footnotes
Conflicts of interest
The authors declare no conflicts of interest.
REFERENCES
- [1].Moran TH, Baldessarini AR, Salorio CF, Lowery T, Schwartz GJ. Vagal afferent and efferent contributions to the inhibition of food intake by cholecystokinin. Am J Physiol 1997;272:R1245–51. [DOI] [PubMed] [Google Scholar]
- [2].Schwartz GJ, Moran TH. Duodenal nutrient exposure elicits nutrient-specific gut motility and vagal afferent signals in rat. Am J Physiol 1998;274:R1236–42. [DOI] [PubMed] [Google Scholar]
- [3].Lloyd KCK, Holzer HH, Zittel TT, Raybould HE. Duodenal lipid inhibits gastric acid secretion by vagal, capsaicin sensitive pathways in rats. Am J Physiol Gastrointest Liver Physiol 1993;264:G659–63. [DOI] [PubMed] [Google Scholar]
- [4].Li Y, Owyang C. Vagal afferent pathways mediate physiological action of cholecystokinin on pancreatic secretion. J Clin Invest 1993;92:418–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Schwartz GJ, Berkow G, McHugh PR, Moran TH. Gastric branch vagotomy blocks nutrient and cholecystokinin-induced suppression of gastric emptying. Am J Physiol 1993;264:R630–7. [DOI] [PubMed] [Google Scholar]
- [6].Lal S, Kirkup AJ, Brunsden AM, Thompson DG, Grundy D. Vagal afferent responses to fatty acids of different chain length in the rat. Am J Physiol Gastrointest Liver Physiol 2001;281:G907–15. [DOI] [PubMed] [Google Scholar]
- [7].Grabauskas G, Song I, Zhou S, Owyang C. Electrophysiological identification of glucose-sensing neurons in rat nodose ganglia. J Physiol 2010;588:617–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Grabauskas G, Zhou SY, Lu Y, Song I, Owyang C. Essential elements for glucosensing by gastric vagal afferents: immunocytochemistry and electrophysiology studies in the rat. Endocrinology 2013;154:296–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Kentish SJ, O’Donnell TA, Isaacs NJ, Young RL, Li H, Harrington AM, et al. Gastric vagal afferent modulation by leptin is influenced by food intake status. J Physiol 2013;591:1921–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Burdyga G, Lal S, Varro A, Dimaline R, Thompson DG, Dockray GJ. Expression of cannabinoid CB1 receptors by vagal afferent neurons is inhibited by cholecystokinin. J Neurosci 2004;24:2708–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Burdyga G, de Lartigue G, Raybould HE, Morris R, Dimaline R, Varro A, et al. Cholecystokinin regulates expression of Y2 receptors in vagal afferent neurons serving the stomach. J Neurosci 2008;28:11583–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Burdyga G, Varro A, Dimaline R, Thompson DG, Dockray GJ. Expression of cannabinoid CB1 receptors by vagal afferent neurons: kinetics, and role in influencing neurochemical phenotype. Am J Physiol Gastrointest Liver Physiol 2010;299:G1514–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].de Lartigue G, Barbier de la Serre C, Espero E, Lee J, Raybould HE. Diet-induced obesity leads to the development of leptin resistance in vagal afferent neurons. Am J Physiol Endocrinology and Metabolism 2011;30(1):E187–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].de Lartigue G, Dimaline R, Varro A, Dockray GJ. Cocaine- and amphetamine-regulated transcript: stimulation of expression in rat vagal afferent neurons by cholecystokinin and suppression by ghrelin. J Neurosci 2007;27:2876–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Daly DM, Park SJ, Valinsky WC, Beyak MJ. Impaired intestinal afferent nerve satiety signaling and vagal afferent excitability in diet induced obesity in the mouse. J Physiol 2011;589:2857–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Kentish S, Li H, Philp LK, O’Donnell TA, Isaacs NJ, Young RL, et al. Diet-induced adaptation of vagal afferent function. J Physiol 2012;590:209–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Kentish SJ, Frisby CL, Kennaway DJ, Wittert GA, Page AJ. Circadian variation in gastric vagal afferent mechanosensitivity. J Neurosci 2013;33:19238–21942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Baker CV, Bronner-Fraser M. Vertebrate cranial placodes I. Embryonic induction. Dev Biol 2001;232:1–61. [DOI] [PubMed] [Google Scholar]
- [19].Blackshaw LA, Grundy D. Responses of vagal efferent fibres to stimulation of gastric mechano- and chemoreceptors in the anaesthetized ferret. J Auton Nerv Syst 1989;27:39–45. [DOI] [PubMed] [Google Scholar]
- [20].Cervero F Sensory innervation of the viscera: peripheral basis of visceral pain. Physiol Rev 1994;74(1):95–138. [DOI] [PubMed] [Google Scholar]
- [21].Sengupta JN, Gebhart GF. Characterization of mechanosensitive pelvic nerve afferent fibers innervating the colon of the rat. J Neurophysiol 1994;71(6):2046–60. [DOI] [PubMed] [Google Scholar]
- [22].Zagorodnyuk VP, Chen BN, Brookes SJ. Intraganglionic laminar endings are mechano-transduction sites of vagal tension receptors in the guinea-pig stomach. J Physiol 2001;534:255–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].El Ouazzani T, Mei N. Electrophysiologic properties and role of the vagal thermoreceptors of lower esophagus and stomach of cat. Gastroenterology 1982;83:995–1001. [PubMed] [Google Scholar]
- [24].Grundy D, Hutson D, Rudge LJ, Scratcherd T. Pre-pyloric mechanisms regulating gastric motor function in the conscious dog. J Exp Physiol 1989;74:857–65. [DOI] [PubMed] [Google Scholar]
- [25].Page AJ, Blackshaw LA. An in vitro study of the properties of vagal afferent fibres innervating the ferret oesophagus and stomach. J Physiol 1998;512:907–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Page AJ, Martin CM, Blackshaw LA. Vagal mechanoreceptors and chemoreceptors in mouse stomach and esophagus. J Neurophysiol 2002;87:2095–103. [DOI] [PubMed] [Google Scholar]
- [27].Su X, Gebhart GF. Mechanosensitive pelvic nerve afferent fibers innervating the colon of the rat are polymodal in character. J Neurophysiol 1998;80:2632–44. [DOI] [PubMed] [Google Scholar]
- [28].Ness TJ, Gebhart GF. Interactions between visceral and cutaneous nociception in the rat. I. Noxious cutaneous stimuli inhibit visceral nociceptive neurons and reflexes. J Neurophysiol 1991;66:20–8. [DOI] [PubMed] [Google Scholar]
- [29].Ness TJ, Gebhart GF. Interactions between visceral and cutaneous nociception in the rat. II. Noxious visceral stimuli inhibit cutaneous nociceptive neurons and reflexes. J Neurophysiol 1991;66:29–39. [DOI] [PubMed] [Google Scholar]
- [30].Yu X, Hu Y, Ru F, Kollarik M, Undem BJ, Yu S. TRPM8 function and expression in vagal sensory neurons and afferent nerves innervating guinea pig esophagus. Am J Physiol Gastrointest Liver Physiol 2015;308:G489–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Yu S, Undem BJ, Kollarik M. Vagal afferent nerves with nociceptive properties in guinea pig oesophagus. J Physiol 2005;563:831–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Page AJ, Blackshaw LA. Roles of gastro-oesophageal afferents in the mechanisms and symptoms of reflux disease. Hand Exp Pharmacol 2009;194:227–57. [DOI] [PubMed] [Google Scholar]
- [33].Sengupta JN, Kauvar D, Goyal RK. Characteristics of vagal esophageal tension-sensitive afferent fibers in the opossum. J Neurophysiol 1989;61:1001–10. [DOI] [PubMed] [Google Scholar]
- [34].Undem BJ, Weinreich D. Electrophysiological properties and chemosensitivity of guinea pig nodose ganglion neurons in vitro. J Auton Nerv Syst 1993;44:17–33. [DOI] [PubMed] [Google Scholar]
- [35].Berthoud HR, Kressel M, Raybould HE, Neuhuber WL. Vagal sensors in the rat duodenal mucosa: distribution and structure as revealed by in vivo DiI-tracing. Anat Embryol (Berl) 1995;191:203–12. [DOI] [PubMed] [Google Scholar]
- [36].Williams RM, Berthoud HR, Stead RH. Vagal afferent nerve fibres contact mast cells in rat small intestinal mucosa. Neuroimmunomodulation 1997;4:266–70. [DOI] [PubMed] [Google Scholar]
- [37].Clarke GD, Davison JS. Mucosal receptors in the gastric antrum and small intestine of the rat with afferent fibres in the cervical vagus. J Physiol 1978;284:55–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Ozaki N, Sengupta JN, Gebhart GF. Mechanosensitive properties of gastric vagal afferent fibers in the rat. J Neurophysiol 1999;82:2210–20. [DOI] [PubMed] [Google Scholar]
- [39].Ozaki N, Gebhart GF. Characterization of mechanosensitive splanchnic nerve afferent fibers innervating the rat stomach. Am J Physiol Gastrointest Liver Physiol 2001;281:G1449–5. [DOI] [PubMed] [Google Scholar]
- [40].Blackshaw LA, Grundy D. Effect of cholecystokinin on two classes of gastroduodenal vagal afferent fibers. J Auton Nerv Syst 1990;31:191–201. [DOI] [PubMed] [Google Scholar]
- [41].Mei N, Garnier L. Osmosensitive vagal receptors in the small intestine of the cat. J Auton Nerv Syst 1986;16:159–70. [DOI] [PubMed] [Google Scholar]
- [42].Kobashi M, Mizutani M, Adachi A. Facilitation of gastric motility induced by portal infusion of hyper- and hypotonic solution in rats. J Auton Nerv Syst 1998;73 (November):156–62. [DOI] [PubMed] [Google Scholar]
- [43].Zhou JX, Zhu XY, Owyang C, Li Y. Intestinal serotonin acts as a paracrine substance to mediate vagal signal transmission evoked by luminal factors in the rat. J Physiol 2001;530:431–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Mei N Vagal glucoreceptors in the small intestine of the cat. J Physiol 1978;282:485–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Berthoud HR, Lynn PA, Blackshaw LA. Vagal and spinal mechanosensors in the rat stomach and colon have multiple receptive fields. Am J Physiol Regul Integr Comp Physiol 2001;280:R1371–8. [DOI] [PubMed] [Google Scholar]
- [46].Powley TL, Wang XY, Fox EA, Phillips RJ, Liu LW, Huizinga JD. Ultrastructural evidence for communication between intramuscular vagal mechanoreceptors and interstitial cells of Cajal in the rat fundus. Neurogastroenterol Motil 2008;20:69–79. [DOI] [PubMed] [Google Scholar]
- [47].Phillips RJ, Powley TL. Tension and stretch receptors in gastrointestinal smooth muscle: re-evaluating vagal mechanoreceptor electrophysiology. Brain Res Brain Res Rev 2000;34:1–26. [DOI] [PubMed] [Google Scholar]
- [48].Zhang X, Renehan WE, Fogel R. Neurons in the vagal complex of the rat respond to mechanical and chemical stimulation of the GI tract. Am J Physiol 1998;274:G331–41. [DOI] [PubMed] [Google Scholar]
- [49].Travagli RA, Hermann GE, Browning KN, Rogers RC. Brainstem circuits regulating gastric function. Annu Rev Physiol 2006;68:279–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Dockray GJ. Gastrointestinal hormones and the dialogue between gut and brain. J Physiol 2014;592:2927–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Iggo A Tension receptors in the stomach and the urinary bladder. J Physiol 1955;128:593–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Cervero F, Sharkey KA. An electrophysiological and anatomical study of intestinal afferent fibres in the rat. J Physiol 1988;401:381–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Wang GJ, Tomasi D, Backus W, Wang R, Telang F, Geliebter A, et al. Gastric distention activates satiety circuitry in the human brain. Neuroimage 2008;39:1824–31. [DOI] [PubMed] [Google Scholar]
- [54].Ivy AC, Oldberg EA. A hormone mechanism for gallbladder contraction and evacuation. Am J Physiol 1928;86:599–613. [Google Scholar]
- [55].Cameron AJ, Phillips SF, Summerskill WH. Effect of cholecystokinin, gastrin, and glucagon on human gallbladder muscle in vitro. Proc Soc Exp Biol Med 1969;131:149–54. [DOI] [PubMed] [Google Scholar]
- [56].Harper AA, Raper HS. Pancreozymin, a stimulant of the secretion of pancreatic enzymes in extracts of the small intestine. J Physiol 1943;102:115–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Leroy J, Morisset JA, Webster PD. Dose-related response of pancreatic synthesis and secretion to cholecystokinin-pancreazymin. J Lab Clin Med 1971;78:149–57. [PubMed] [Google Scholar]
- [58].Gibbs J, Young RC, Smith GP. Cholecystokinin decreases food intake in rats. J Comp Physiol Psychol 1973;84:488–95. [DOI] [PubMed] [Google Scholar]
- [59].Kissileff HR, Pi-Sunyer FX, Thornton J, Smith GP. C-terminal octapeptide of cholecystokinin decreases food intake in man. Am J Clin Nutr 1981;34:154–60. [DOI] [PubMed] [Google Scholar]
- [60].Li Y, Owyang C. Pancreatic secretion evoked by cholecystokinin and non-cholecystokinin-dependent duodenal stimuli via vagal afferent fibres in the rat. J Physiol 1996;494:773–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Barrachina MD, Martinez V, Wang L, Wei JY, Tache Y. Synergistic interaction between leptin and cholecystokinin to reduce short-term food intake in lean mice. Proc Natl Acad Sci USA 1997;94:10455–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Sachot C, Rummel C, Bristow AF, Luheshi GN. The role of the vagus nerve in mediating the long-term anorectic effects of leptin. J Neuroendocrinol 2007;19:250–61. [DOI] [PubMed] [Google Scholar]
- [63].Masuda Y, Tanaka T, Inomata N, Ohnuma N, Tanaka S, Itoh Z, et al. Ghrelin stimulates gastric acid secretion and motility in rats. Biochem Biophys Res Commun 2000;276:905–8. [DOI] [PubMed] [Google Scholar]
- [64].Asakawa A, Inui A, Kaga T, Yuzuriha H, Nagata T, Ueno N, et al. Ghrelin is an appetite-stimulatory signal from stomach with structural resemblance to motilin. Gastroenterology 2001;120:337–45. [DOI] [PubMed] [Google Scholar]
- [65].Broglio F, Arvat E, Benso A, Gottero C, Muccioli G, Papotti M, et al. Ghrelin, a natural GH secretagogue produced by the stomach, induces hyperglycemia and reduces insulin secretion in humans. J Clin Endocrinol Metab 2001;86: 5083–6. [DOI] [PubMed] [Google Scholar]
- [66].Tschop M, Smiley DL, Heiman ML. Ghrelin induces adiposity in rodents. Nature 2000;407:908–13. [DOI] [PubMed] [Google Scholar]
- [67].Wortley KE, del Rincon JP, Murray JD, Garcia K, Iida K, Thorner MO, et al. Absence of ghrelin protects against early-onset obesity. J Clin Invest 2005;115:3573–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Mano-Otagiri A, Iwasaki-Sekino T, Nemoto H, Ohata Y, Shuto H, Nakabayashi et al. Genetic suppression of ghrelin receptors activates brown adipocyte function and decreases fat storage in rats. Regul Pept 2010;160:81–90. [DOI] [PubMed] [Google Scholar]
- [69].Date Y, Murakami N, Toshinai K, Matsukura S, Niijima A, Matsuo H, et al. The role of the gastric afferent vagal nerve in ghrelin-induced feeding and growth hormone secretion in rats. Gastroenterology 2002;123:1120–8. [DOI] [PubMed] [Google Scholar]
- [70].Burdyga G, Varro A, Dimaline R, Thompson DG, Dockray GJ. Ghrelin receptors in rat and human nodose ganglia: putative role in regulating CB-1 and MCH receptor abundance. Am J Physiol 2006;290:G1289–97. [DOI] [PubMed] [Google Scholar]
- [71].Fukuda H, Mizuta Y, Isomoto H, Takeshima F, Ohnita K, Ohba K, et al. Ghrelin enhances gastric motility through direct stimulation of intrinsic neural pathways and capsaicin-sensitive afferent neurons in rats. Scand J Gastroenterol 2004;39:1209–14. [PubMed] [Google Scholar]
- [72].Kobashi M, Yanagihara M, Fujita M, Mitoh Y, Matsuo R. Fourth ventricular administration of ghrelin induces relaxation of the proximal stomach in the rat. Am J Physiol Regul Integr Comp Physiol 2009;296:R217–23. [DOI] [PubMed] [Google Scholar]
- [73].Sayegh AI. The role of bombesin and bombesin-related peptides in the short-term control of food intake. Prog Mol Biol Transl Sci 2013;114:343–70. [DOI] [PubMed] [Google Scholar]
- [74].Polak JM, Bloom SR, Hobbs S, Solcia E, Pearse AG. Distribution of a bombesin-like peptide in human gastrointestinal tract. Lancet 1976;1(7969):1109–10. [DOI] [PubMed] [Google Scholar]
- [75].Ladenheim EE, Knipp S. Capsaicin treatment differentially affects feeding suppression by bombesin-like peptides. Physiol Behav 2007;91:36–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Janssen P, Verschueren S, Rotondo A, Tack J. Role of Y(2) receptors in the regulation of gastric tone in rats. Am J Physiol Gastrointest Liver Physiol 2012;302:G732–9. [DOI] [PubMed] [Google Scholar]
- [77].Neary NM, Small CJ, Druce MR, Park AJ, Ellis SM, Semjonous NM, et al. Peptide YY3–36 and glucagon-like peptide-17–36 inhibit food intake additively. Endocrinology 2005;146:5120–7. [DOI] [PubMed] [Google Scholar]
- [78].Eissele R, Koop H, Arnold R. Effect of peptide YY on gastric acid secretion, gastrin and somatostatin release in the rat. Z Gastroenterol 1990;28:129–31. [PubMed] [Google Scholar]
- [79].Grabauskas G, Wu X, Lu Y, Heldsinger A, Song I, Zhou SY, et al. KATP channels in the nodose ganglia mediate the orexigenic actions of ghrelin. J Physiol 2015;593:3973–89. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- [80].Li P, Chang TM, Chey WY. Secretin inhibits gastric acid secretion via a vagal afferent pathway in rats. Am J Physiol 1998;275:G22–8. [DOI] [PubMed] [Google Scholar]
- [81].Bucinskaite V, Tolessa T, Pedersen J, Rydqvist B, Zerihun L, Holst JJ, et al. Receptor-mediated activation of gastric vagal afferents by glucagon-like peptide-1 in the rat. Neurogastroenterol Motil 2009;21:978–1078. [DOI] [PubMed] [Google Scholar]
- [82].Panaro BL, Tough IR, Engelstoft MS, Matthews RT, Digby GJ, Møller CL, et al. The melanocortin-4 receptor is expressed in enteroendocrine L cells and regulates the release of peptide YY and glucagon-like peptide 1 in vivo. Cell Metab 2014;20:1018–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Ronveaux CC, Tomé D, Raybould HE. Glucagon-like peptide 1 interacts with ghrelin and leptin to regulate glucose metabolism and food intake through vagal afferent neuron signaling. J Nutr 2015;145:672–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Poleni PE, Akieda-Asai S, Koda S, Sakurai M, Bae CR, Senba K, et al. Possible involvement of melanocortin-4-receptor and AMP-activated protein kinase in the interaction of glucagon-like peptide-1 and leptin on feeding in rats. Biochem Biophys Res Commun 2012;420:36–41. [DOI] [PubMed] [Google Scholar]
- [85].Eissele R, Koop H, Arnold R. Effect of glucagon-like peptide-1 on gastric somatostatin and gastrin secretion in the rat. Scand J Gastroenterol 1990;25:449–54. [DOI] [PubMed] [Google Scholar]
- [86].Li Y, Wu XY, Owyang C. Serotonin and cholecystokinin synergistically stimulate rat vagal primary afferent neurons. J Physiol 2004;559:651–62. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- [87].Li Y, Hao YB, Zhu JX, Owyang C. Serotonin released from intestinal enterochromaffin cells mediates luminal non-CCK-stimulated pancreatic secretion in rats. Gastroenterology 2000;118:1197–207. [DOI] [PubMed] [Google Scholar]
- [88].Gershon MD. Review article: serotonin receptors and transporters – roles in normal and abnormal gastrointestinal motility. Aliment Pharmacol Ther 2004;20 (Suppl. 7):3–14. [DOI] [PubMed] [Google Scholar]
- [89].Nassar CF, Abdallah LE, Barada KA, Atweh SF, Saadé NE. Effects of intravenous vasoactive intestinal peptide injection on jejunal alanine absorption and gastric acid secretion in rats. Regul Pept 1995;55(3):261–7. [DOI] [PubMed] [Google Scholar]
- [90].Buchan AM, Polak JM, Solcia E, Capella C, Hudson D, Pearse AG. Electron immunohistochemical evidence for the human intestinal I cell as the source of CCK. Gut 1978;19:403–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Polak JM, Bloom SR, Rayford PL, Pearse AG, Buchan AM, Thompson JC. Identification of cholecystokinin-secreting cells. Lancet 1975;2:1016–8. [DOI] [PubMed] [Google Scholar]
- [92].Liou AP, Lu X, Sei Y, Zhao X, Pechhold S, Carrero RJ, et al. The G-protein-coupled receptor GPR40 directly mediates long-chain fatty acid-induced secretion of cholecystokinin. Gastroenterology 2011;140:903–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Moran TH, Norgren R, Crosby RJ, McHugh PR. Central and peripheral vagal transport of cholecystokinin binding sites occurs in afferent fibers. Brain Res 1990;526:95–102. [DOI] [PubMed] [Google Scholar]
- [94].Moriarty P, Dimaline R, Thompson DG, Dockray GJ. Characterization of cholecystokinin A and cholecystokinin B receptors expressed by vagal afferent neurons. Neuroscience 1997;79:905–13. [DOI] [PubMed] [Google Scholar]
- [95].Ito M, Matsui T, Taniguchi T, Tsukamoto T, Murayama T, Arima N, et al. Functional characterization of a human brain cholecystokinin-B receptor. A trophic effect of cholecystokinin and gastrin. J Biol Chem 1993;268:18300–5. [PubMed] [Google Scholar]
- [96].Broberger C, Holmberg K, Shi TJ, Dockray G, Hokfelt T. Expression and regulation of cholecystokinin and cholecystokinin receptors in rat nodose and dorsal root ganglia. Brain Res 2001;903:128–40. [DOI] [PubMed] [Google Scholar]
- [97].Lin CW, Miller TR. Both CCK-A and CCK-B/gastrin receptors are present on rabbit vagus nerve. Am J Physiol 1992;263:R591–5. [DOI] [PubMed] [Google Scholar]
- [98].Sternini C, Wong H, Pham T, De Giorgio R, Miller LJ, Kuntz SM, et al. Expression of cholecystokinin A receptors in neurons innervating the rat stomach and intestine. Gastroenterology 1999;117:1136–46. [DOI] [PubMed] [Google Scholar]
- [99].Berthoud HR, Patterson LM. Anatomical relationship between vagal afferent fibers and CCK-immunoreactive entero-endocrine cells in the rat small intestinal mucosa. Acta Anat (Basel) 1996;156:123–31. [DOI] [PubMed] [Google Scholar]
- [100].Patterson LM, Zheng H, Berthoud HR. Vagal afferents innervating the gastrointestinal tract and CCKA-receptor immunoreactivity. Anat Rec 2002;266:10–20. [DOI] [PubMed] [Google Scholar]
- [101].Schwartz GJ, McHugh PR, Moran TH. Integration of vagal afferent responses to gastric loads and cholecystokinin in rats. Am J Physiol 1991;261:R64–9. [DOI] [PubMed] [Google Scholar]
- [102].Li Y, Zhang XC, Wang LM, Renenan EW, Fogel R, Owyang C. Vagal afferent pathway mediates physiological action of CCK on pancreatic enzyme secretion: pancreatic secretion, neurophysiological and receptor autoradiographic studies. Gastroenterology 1993;104:A837. [Google Scholar]
- [103].Raybould HE, Tache Y. Cholecystokinin inhibits gastric motility and emptying via a capsaicin-sensitive vagal pathway in rats. Am J Physiol 1988;255:G242–6. [DOI] [PubMed] [Google Scholar]
- [104].Rogers RC, Hermann GE. Mechanisms of action of CCK to activate central vagal afferent terminals. Peptides 2008;29:1716–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Berthoud HR, Blackshaw LA, Brookes SJ, Grundy D. Neuroanatomy of extrinsic afferents supplying the gastrointestinal tract. Neurogastroenterol Motil 2004;16:28–33. [DOI] [PubMed] [Google Scholar]
- [106].Li Y, Zhu J, Owyang C. Electrical physiological evidence for high- and low-affinity CCK-A receptors. Am J Physiol 1999;277:G469–77. [DOI] [PubMed] [Google Scholar]
- [107].Li Y, Owyang C. Endogenous cholecystokinin stimulates pancreatic enzyme secretion via vagal afferent pathway in rats. Gastroenterology 1994;107:525–31. [DOI] [PubMed] [Google Scholar]
- [108].Schwartz GJ, Tougas G, Moran TH. Integration of vagal afferent responses to duodenal loads and exogenous CCK in rats. Peptides 1995;16:707–11. [DOI] [PubMed] [Google Scholar]
- [109].Ritter RC, Ladenheim EE. Capsaicin pretreatment attenuates suppression of food intake by cholecystokinin. Am J Physiol 1985;248:R501–4. [DOI] [PubMed] [Google Scholar]
- [110].South EH, Ritter RC. Capsaicin application to central or peripheral vagal fibers attenuates CCK satiety. Peptides 1988;9:601–12. [DOI] [PubMed] [Google Scholar]
- [111].Joyner K, Smith GP, Gibbs J. Abdominal vagotomy decreases the satiating potency of CCK-8 in sham and real feeding. Am J Physiol 1993;264:R912–6. [DOI] [PubMed] [Google Scholar]
- [112].Eisen S, Phillips RJ, Geary N, Baronowsky EA, Powley TL, Smith GP. Inhibitory effects on intake of cholecystokinin-8 and cholecystokinin-33 in rats with hepatic proper or common hepatic branch vagal innervation. Am J Physiol Regul Integr Comp Physiol 2005;289:R456–62. [DOI] [PubMed] [Google Scholar]
- [113].Kurosawa M, Bucinskaite V, Taniguchi T, Miyasaka K, Funakoshi A, Lundeberg T. Response of the gastric vagal afferent activity to cholecystokinin in rats lacking type A cholecystokinin receptors. J Auton Nerv Syst 1999;75:51–9. [DOI] [PubMed] [Google Scholar]
- [114].Wang DQ, Schmitz F, Kopin AS, Carey MC. Targeted disruption of the murine cholecystokinin-1 receptor promotes intestinal cholesterol absorption and susceptibility to cholesterol cholelithiasis. J Clin Invest 2004;114:521–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115].Owyang C, Logsdon CD. New insights into neurohormonal regulation of pancreatic secretion. Gastroenterology 2004;127:957–69. [DOI] [PubMed] [Google Scholar]
- [116].Browning KN, Babic T, Holmes GM, Swartz E, Travagli RA. A critical re-evaluation of the specificity of action of perivagal capsaicin. J Physiol 2013;591:1563–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Babic T, Browning KN, Kawaguchi Y, Tang X, Travagli RA. Pancreatic insulin and exocrine secretion are under the modulatory control of distinct subpopulations of vagal motoneurones in the rat. J Physiol 2012;590:3611–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].Bado A, Levasseur S, Attoub S, Kermorgant S, Laigneau JP, Bortoluzzi MN, et al. The stomach is a source of leptin. Nature 1998;394:790–3. [DOI] [PubMed] [Google Scholar]
- [119].Mix H, Widjaja A, Jandl O, Cornberg M, Kaul A, Göke M, et al. Expression of leptin and leptin receptor isoforms in the human stomach. Gut 2000;47:481–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [120].Friedman JM, Halaas JL. Leptin and the regulation of body weight in mammals. Nature 1998;395:763–70. [DOI] [PubMed] [Google Scholar]
- [121].Schwartz MW, Seeley RJ, Campfield LA, Burn P, Baskin DG. Identification of targets of leptin action in rat hypothalamus. J Clin Invest 1996;98:1101–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [122].Buyse M, Ovesjo ML, Goiot H, Guilmeau S, Peranzi G, Moizo L, et al. Expression and regulation of leptin receptor proteins in afferent and efferent neurons of the vagus nerve. Eur J Neurosci 2001;14:64–72. [DOI] [PubMed] [Google Scholar]
- [123].Peters JH, McKay BM, Simasko SM, Ritter RC. Leptin-induced satiation mediated by abdominal vagal afferents. Am J Physiol Regul Integr Comp Physiol 2005;288:R879–84. [DOI] [PubMed] [Google Scholar]
- [124].Peters JH, Ritter RC, Simasko SM. Leptin and CCK modulate complementary background conductances to depolarize cultured nodose neurons. Am J Physiol Cell Physiol 2006;290:C427–32. [DOI] [PubMed] [Google Scholar]
- [125].Peters JH, Ritter RC, Simasko SM. Leptin and CCK selectively activate vagal afferent neurons innervating the stomach and duodenum. Am J Physiol Regul Integr Comp Physiol 2006;290(6):R1544–9. [DOI] [PubMed] [Google Scholar]
- [126].Patel JD, Ebenezer IS. The effect of intraperitoneal administration of leptin on short-term food intake in rats. Eur J Pharmacol 2008;580(1–2):143–52. [DOI] [PubMed] [Google Scholar]
- [127].de Lartigue G, Ronveaux CC, Raybould HE. Deletion of leptin signaling in vagal afferent neurons results in hyperphagia and obesity. Molecular metabolism 2014;3:595–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [128].Peters JH, Simasko SM, Ritter RC. Modulation of vagal afferent excitation and reduction of food intake by leptin and cholecystokinin. Physiol Behav 2006;89:477–85. [DOI] [PubMed] [Google Scholar]
- [129].Kojima M, Hosoda H, Date Y, Nakazato M, Matsuo H, Kangawa K. Ghrelin is a growth-hormone-releasing acylated peptide from stomach. Nature 1999;402:656–60. [DOI] [PubMed] [Google Scholar]
- [130].Hosoda H, Kojima M, Matsuo H, Kangawa K. Ghrelin and des-acyl ghrelin: two major forms of rat ghrelin peptide in gastrointestinal tissue. Biochem Biophys Res Commun 2000;279:909–13. [DOI] [PubMed] [Google Scholar]
- [131].Date Y, Kojima M, Hosoda H, Sawaguchi A, Mondal MS, Suganuma T, et al. Ghrelin, a novel growth hormone-releasing acylated peptide, is synthesized in a distinct endocrine cell type in the gastrointestinal tracts of rats and humans. Endocrinology 2000;141:4255–61. [DOI] [PubMed] [Google Scholar]
- [132].Gnanapavan S, Kola B, Bustin SA, Morris DG, McGee P, Fairclough P, et al. The tissue distribution of the mRNA of ghrelin and subtypes of its receptor, GHS-R, in humans. J Clin Endocrinol Metab 2002;87:2988. [DOI] [PubMed] [Google Scholar]
- [133].Cowley MA, Smith RG, Diano S, Tschop M, Pronchuk N, Grove KL, et al. The distribution and mechanism of action of ghrelin in the CNS demonstrates a novel hypothalamic circuit regulating energy homeostasis. Neuron 2003;7:649–61. [DOI] [PubMed] [Google Scholar]
- [134].Xu L, Depoortere I, Tomasetto C, Zandecki M, Tang M, Timmermans JP, et al. Evidence for the presence of motilin, ghrelin, and the motilin and ghrelin receptor in neurons of the myenteric plexus. Regul Pept 2005;124:119–25. [DOI] [PubMed] [Google Scholar]
- [135].Page AJ, Slattery JA, Milte C, Laker R, O’Donnell T, Dorian C, et al. Ghrelin selectively reduces mechanosensitivity of upper gastrointestinal vagal afferents. Am J Physiol Gastrointest Liver Physiol 2007;292:G1376–84. [DOI] [PubMed] [Google Scholar]
- [136].Date Y, Toshinai K, Koda S, Miyazato M, Shimbara T, Tsuruta T, et al. Peripheral interaction of ghrelin with cholecystokinin on feeding regulation. Endocrinology 2005;146:3518–25. [DOI] [PubMed] [Google Scholar]
- [137].Arnold M, Mura A, Langhans W, Geary N. Gut vagal afferents are not necessary for the eating-stimulatory effect of intraperitoneally injected ghrelin in the rat. J Neurosci 2006;26:11052–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [138].Gomez R, Navarro M, Ferrer B, Trigo JM, Bilbao A, Del AI, et al. A peripheral mechanism for CB1 cannabinoid receptor-dependent modulation of feeding. J Neurosci 2002;22:9612–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [139].Burdyga G, Varro A, Dimaline R, Thompson DG, Dockray GJ. Feeding-dependent depression of melanin-concentrating hormone and melanin-concentrating hormone receptor-1 expression in vagal afferent neurons. Neuroscience 2006;137:1405–15. [DOI] [PubMed] [Google Scholar]
- [140].Emond M, Schwartz GJ, Ladenheim EE, Moran TH. Central leptin modulates behavioral and neural responsivity to CCK. Am J Physiol 1999;276:R1545–9. [DOI] [PubMed] [Google Scholar]
- [141].Matson CA, Reid DF, Cannon TA, Ritter RC. Cholecystokinin and leptin act synergistically to reduce body weight. Am J Physiol Regul Integr Comp Physiol 2000;278:R882–90. [DOI] [PubMed] [Google Scholar]
- [142].Matson CA, Ritter RC. Long-term CCK-leptin synergy suggests a role for CCK in the regulation of body weight. Am J Physiol 1999;276:R1038–45. [DOI] [PubMed] [Google Scholar]
- [143].Matson CA, Wiater MF, Kuijper JL, Weigle DS. Synergy between leptin and cholecystokinin (CCK) to control daily caloric intake. Peptides 1997;18:1275–8. [DOI] [PubMed] [Google Scholar]
- [144].Wang L, Barachina MD, Martinez V, Wei JY, Tache Y. Synergistic interaction between CCK and leptin to regulate food intake. Regul Pept 2000;92:79–85. [DOI] [PubMed] [Google Scholar]
- [145].Peters JH, Karpiel AB, Ritter RC, Simasko SM. Cooperative activation of cultured vagal afferent neurons by leptin and cholecystokinin. Endocrinology 2004;145:3652–7. [DOI] [PubMed] [Google Scholar]
- [146].Heldsinger A, Grabauskas G, Song I, Owyang C. Synergistic interaction between leptin and cholecystokinin in the rat nodose ganglia is mediated by PI3K and STAT3 signaling pathways: implications for leptin as a regulator of short term satiety. J Biol Chem 2011;286:11707–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [147].Emond M, Ladenheim EE, Schwartz GJ, Moran TH. Leptin amplifies the feeding inhibition and neural activation arising from a gastric nutrient preload. Physiol Behav 2001;72:123–8. [DOI] [PubMed] [Google Scholar]
- [148].Wang L, Martinez V, Barrachina MD, Tache Y. Fos expression in the brain induced by peripheral injection of CCK or leptin plus CCK in fasted lean mice. Brain Res 1998;79:157–66. [DOI] [PubMed] [Google Scholar]
- [149].Maletinska L, Maixnerova J, Matyskova R, Haugvicova R, Pirnik Z, Kiss A, et al. Synergistic effect of CART (cocaine- and amphetamine-regulated transcript) peptide and cholecystokinin on food intake regulation in lean mice. BMC Neurosci 2008;9:101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [150].Heldsinger A, Lu Y, Zhou SY, Wu X, Grabauskas G, Song I, et al. Cocaine- and amphetamine-regulated transcript is the neurotransmitter regulating the action of cholecystokinin and leptin on short-term satiety in rats. Am J Physiol Gastrointest Liver Physiol 2012;303:G1042–51. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- [151].Heldsinger A, Grabauskas G, Wu X, Zhou S, Lu Y, Song I, et al. Ghrelin induces leptin resistance by activation of suppressor of cytokine signaling 3 expression in male rats: implications in satiety regulation. Endocrinology 2014;155 (10):3956–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [152].Wang YH, Tache Y, Sheibel AB, Go VL, Wei JY. Two types of leptin-responsive gastric vagal afferent terminals: an in vitro single-unit study in rats. Am J Physiol 1997;273:R833–7. [DOI] [PubMed] [Google Scholar]
- [153].Morton GJ, Blevins JE, Williams DL, Niswender KD, Gelling RW, Rhodes CJ, et al. Leptin action in the forebrain regulates the hindbrain response to satiety signals. J Clin Invest 2005;115:703–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [154].Loewy AD. Central regulation of autonomic functions In: Loewy AD, Spyer KM, editors. Central regulation of autonomic functions. New York: Oxford University Press; 1990. p. 88–103. [Google Scholar]
- [155].Altschuler SM, Bao X, Bieger D, Hopkins DA, Miselis RR. Viscerotopic representation of the upper alimentary tract in the rat: sensory ganglia and nuclei of the solitary and spinal trigeminal tracts. J Comp Neurol 1989;283:248–68. [DOI] [PubMed] [Google Scholar]
- [156].Altschuler SM, Ferenci DA, Lynn RB, Miselis RR. Representation of the cecum in the lateral dorsal motor nucleus of the vagus nerve and commissural subnucleus of the nucleus tractus solitarii in rat. J Comp Neurol 1991;304:261–74. [DOI] [PubMed] [Google Scholar]
- [157].Altschuler SM, Escardo J, Lynn RB, Miselis RR. The central organization of the vagus nerve innervating the colon of the rat. Gastroenterology 1993;104:502–9. [DOI] [PubMed] [Google Scholar]
- [158].Smith BN, Dou P, Barber WD, Dudek FE. Vagally evoked synaptic currents in the immature rat nucleus tractus solitarii in an intact in vitro preparation. J Physiol 1998;512:149–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [159].Glatzer NR, Smith BN. Modulation of synaptic transmission in the rat nucleus of the solitary tract by endomorphin-1. J Neurophysiol 2005;93:2530–40. [DOI] [PubMed] [Google Scholar]
- [160].de Lartigue G, Barbier de la Serre C, Espero E, Lee J, Raybould HE. Leptin resistance in vagal afferent neurons inhibits cholecystokinin signaling and satiation in diet induced obese rats. PLoS ONE 2012;7:e32967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [161].Gillespie BR, Burns GA, Ritter RC. NMDA channels control meal size via central vagal afferent terminals. Am J Physiol Regul Integr Comp Physiol 2005;289:R1504–11. [DOI] [PubMed] [Google Scholar]
- [162].Zheng H, Corkern MM, Crousillac SM, Patterson LM, Phifer CB, Berthoud HR. Neurochemical phenotype of hypothalamic neurons showing Fos expression 23 h after intracranial AgRP. Am J Physiol Regul Integr Comp Physiol 2002;282:R1773–81. [DOI] [PubMed] [Google Scholar]
- [163].Saadé NE, Abdallah LE, Barada KA, Atweh SF, Nassar CF. Effects of intracerebral injections of VIP on jejunal alanine absorption and gastric acid secretion in rats. Regul Pept 1995;55:269–76. [DOI] [PubMed] [Google Scholar]
- [164].Helke CJ, Niederer AJ. Studies on the coexistence of substance P with other putative transmitters in the nodose and petrosal ganglia. Synapse 1990;5:144–51. [DOI] [PubMed] [Google Scholar]
- [165].Helke CJ, Hill KM. Immunohistochemical study of neuropeptides in vagal and glossopharyngeal afferent neurons in the rat. Neuroscience 1988;26:539–51. [DOI] [PubMed] [Google Scholar]
- [166].Sekizawa S, Joad JP, Bonham AC. Substance P presynaptically depresses the transmission of sensory input to bronchopulmonary neurons in the guinea pig nucleus tractus solitarii. J Physiol 2003;552:547–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [167].Holzer P, Lippe IT. Stimulation of afferent nerve endings by intragastric capsaicin protects against ethanol-induced damage of gastric mucosa. Neuroscience 1988;27:981–7. [DOI] [PubMed] [Google Scholar]
- [168].Holzer P, Guth PH. Neuropeptide control of rat gastric mucosal blood flow: increase by calcitonin gene-related peptide and vasoactive intestinal polypeptide, but not substance P and neurokinin A. Circ Res 1991;68:100–5. [DOI] [PubMed] [Google Scholar]
- [169].Holzer P, Sametz W. Gastric mucosal protection against ulcerogenic factors in the rat mediated by capsaicin-sensitive afferent neurons. Gastroenterology 1986;91:975–81. [DOI] [PubMed] [Google Scholar]
- [170].Lin LH, Cassell MD, Sandra A, Talman WT. Direct evidence for nitric oxide synthase in vagal afferents to the nucleus tractus solitarii. Neuroscience 1998;84:549–58. [DOI] [PubMed] [Google Scholar]
- [171].Page AJ, O’Donnell TA, Cooper NJ, Young RL, Blackshaw LA. Nitric oxide as an endogenous peripheral modulator of visceral sensory neuronal function. J Neurosci 2009;29:7246–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [172].Lawrence AJ. Neurotransmitter mechanisms of rat vagal afferent neurons. Clin Exp Pharmacol Physiol 1995;22:869–73. [DOI] [PubMed] [Google Scholar]
- [173].Schaffar N, Rao H, Kessler JP, Jean A. Immunohistochemical detection of glutamate in rat vagal sensory neurons. Brain Res 1997;778:302–8. [DOI] [PubMed] [Google Scholar]
- [174].Saha S, Batten TF, McWilliam PN. Glutamate-immunoreactivity in identified vagal afferent terminals of the cat: a study combining horseradish peroxidase tracing and postembedding electron microscopic immunogold staining. Exp Physiol 1995;80:193–202. [DOI] [PubMed] [Google Scholar]
- [175].Ambalavanar R, Ludlow CL, Wenthold RJ, Tanaka Y, Damirjian M, Petralia RS. Glutamate receptor subunits in the nucleus of the tractus solitarius and other regions of the medulla oblongata in the cat. J Comp Neurol 1998;402:75–92. [PubMed] [Google Scholar]
- [176].Andresen MC, Yang MY. Non-NMDA receptors mediate sensory afferent synaptic transmission in medial nucleus tractus solitarius. Am J Physiol 1990;259:H1307–11. [DOI] [PubMed] [Google Scholar]
- [177].Kessler JP, Jean A. Evidence that activation of N-methyl-D-aspartate (NMDA) and non-NMDA receptors within the nucleus tractus solitarii triggers swallowing. Eur J Pharmacol 1991;201:59–67. [DOI] [PubMed] [Google Scholar]
- [178].Treece BR, Covasa M, Ritter RC, Burns GA. Delay in meal termination follows blockade of N-methyl-D-aspartate receptors in the dorsal hindbrain. Brain Res 1998;810: 34–40. [DOI] [PubMed] [Google Scholar]
- [179].Burns GA, Ritter RC. Visceral afferent participation in delayed satiation following NMDA receptor blockade. Physiol Behav 1998;65:361–6. [DOI] [PubMed] [Google Scholar]
- [180].Wright J, Campos C, Herzog T, Covasa M, Czaja K, Ritter RC. Reduction of food intake by cholecystokinin requires activation of hindbrain NMDA-type glutamate receptors. Am J Physiol Regul Integr Comp Physiol 2011;301:R448–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [181].Zheng H, Kelly L, Patterson LM, Berthoud HR. Effect of brain stem NMDA-receptor blockade by MK-801 on behavioral and fos responses to vagal satiety signals. Am J Physiol 1999;277:R1104–11. [DOI] [PubMed] [Google Scholar]
- [182].Zheng H, Patterson LM, Berthoud HR. CART in the dorsal vagal complex: sources of immunoreactivity and effects on Fos expression and food intake. Brain Res 2002;957:298–310. [DOI] [PubMed] [Google Scholar]
- [183].Glaum SR, Miller RJ. Metabotropic glutamate receptors mediate excitatory transmission in the nucleus of the solitary tract. J Neurosci 1992;12:2251–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [184].Glaum SR, Miller RJ. Activation of metabotropic glutamate receptors produces reciprocal regulation of ionotropic glutamate and GABA responses in the nucleus of the tractus solitarius of the rat. J Neurosci 1993;13:1636–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [185].Page AJ, Young RL, Martin CM, Umaerus M, O’Donnell TA, Cooper NJ, et al. Metabotropic glutamate receptors inhibit mechanosensitivity in vagal sensory neurons. Gastroenterology 2005;128:402–10. [DOI] [PubMed] [Google Scholar]
- [186].Young RL, Page AJ, O’Donnell TA, Cooper NJ, Blackshaw LA. Peripheral versus central modulation of gastric vagal pathways by metabotropic glutamate receptor 5. Am J Physiol Gastrointest Liver Physiol 2007;292:G501–11. [DOI] [PubMed] [Google Scholar]
- [187].Baptista V, Zheng Z, Coleman FH, Rogers RC, Travagli RA. Cholecystokinin octapeptide increases spontaneous glutamatergic synaptic transmission to neurons of the nucleus tractus solitarius centralis. J Neurophysiol 2005;94:2763–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [188].Appleyard SM, Bailey TW, Doyle MW, Jin YH, Smart JL, Low MJ, et al. Proopiomelanocortin neurons in nucleus tractus solitarius are activated by visceral afferents: regulation by cholecystokinin and opioids. J Neurosci 2005;25:3578–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [189].Emch GS, Hermann GE, Rogers RC. TNF-a activates solitary nucleus neurons responsive to gastric distention. Am J Physiol Gastrointest Liver Physiol 2000;279:G582–6. [DOI] [PubMed] [Google Scholar]
- [190].Wan S, Browning KN, Coleman FH, Sutton G, Zheng H, Butler A, et al. Presynaptic melanocortin-4 receptors on vagal afferent fibers modulate the excitability of rat nucleus tractus solitarius neurons. J Neurosci 2008;28:4957–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [191].Wan S, Browning KN. Glucose increases synaptic transmission from vagal afferent central nerve terminals via modulation of 5-HT3 receptors. Am J Physiol Gastrointest Liver Physiol 2008;295:G1050–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [192].Jin YH, Bailey TW, Li BY, Schild JH, Andresen MC. Purinergic and vanilloid receptor activation releases glutamate from separate cranial afferent terminals in nucleus tractus solitarius. J Neurosci 2004;24:4709–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [193].Zhang S, Grabauskas G, Wu X, Joo MK, Heldsinger A, Song I, et al. Role of prostaglandin D2 in mast cell activation-induced sensitization of esophageal vagal afferents. Am J Physiol Gastrointest Liver Physiol 2013;304:G908–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [194].Ter Horst GJ, de Boer P, Luiten PG, Van Willigen JD. Ascending projections from the solitary tract nucleus to the hypothalamus. A Phaseolus vulgaris lectin tracing study in the rat. Neuroscience 1989;31:785–97. [DOI] [PubMed] [Google Scholar]
- [195].Sawchenko PE, Swanson LW. The organization of noradrenergic pathways from the brainstem to the paraventricular and supraoptic nuclei in the rat. Brain Res 1982;257:275–325. [DOI] [PubMed] [Google Scholar]
- [196].Rogers RC, McCann MJ. Intramedullary connections of the gastric region in the solitary nucleus: a biocytin histochemical tracing study in the rat. J Auton Nerv Syst 1993;42:119–30. [DOI] [PubMed] [Google Scholar]
- [197].Grabauskas G, Moises HC. Gastrointestinal-projecting neurones in the dorsal motor nucleus of the vagus exhibit direct and viscerotopically organized sensitivity to orexin. J Physiol 2003;549:37–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [198].Grabauskas G, Zhou SY, Das S, Lu Y, Owyang C, Moises HC. Prolactin-releasing peptide affects gastric motor function in rat by modulating synaptic transmission in the dorsal vagal complex. J Physiol 2004;56:821–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [199].Glatzer NR, Hasney CP, Bhaskaran MD, Smith BN. Synaptic and morphologic properties in vitro of premotor rat nucleus tractus solitarius neurons labeled transneuronally from the stomach. J Comp Neurol 2003;464(4):525–39. [DOI] [PubMed] [Google Scholar]
- [200].Holmes GM, Browning KN, Babic T, Fortna SR, Coleman FH, Travagli RA. Vagal afferent fibres determine the oxytocin-induced modulation of gastric tone. J Physiol 2013;591:3081–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [201].Grabauskas G, Wu X, Song I, Zhou SY, Lanigan T, Owyang C. Increased Activation of the TRESK K+ Mediates Vago-Vagal Reflex Malfunction in Diabetic Rats. Gastroenterology 2016;151:910–22. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- [202].Covasa M, Ritter RC. Rats maintained on high-fat diets exhibit reduced satiety in response to CCK and bombesin. Peptides 1998;19:1407–15. [DOI] [PubMed] [Google Scholar]
- [203].Covasa M, Grahn J, Ritter RC. High fat maintenance diet attenuates hindbrain neuronal response to CCK. Regul Pept 2000;86:83–8. [DOI] [PubMed] [Google Scholar]
- [204].Kentish SJ, Vincent AD, Kennaway DJ, Wittert GA, Page AJ. High-fat diet-induced obesity ablates gastric vagal afferent circadian rhythms. J Neurosci 2016;36:3199–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [205].Waise TM, Toshinai K, Naznin F, NamKoong C, Md Moin AS, Sakoda H, et al. One-day high-fat diet induces inflammation in the nodose ganglion and hypothalamus of mice. Biochem Biophys Res Commun 2015;464:1157–62. [DOI] [PubMed] [Google Scholar]
- [206].Naznin F, Toshinai K, Waise TM, NamKoong C, Md Moin AS, Sakoda H, et al. Diet-induced obesity causes peripheral and central ghrelin resistance by promoting inflammation. J Endocrinol 2015;226:81–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [207].Lundberg JM, Hokfelt T, Kewenter J, Pettersson G, Ahlman H, Edin R, et al. Substance P, VIP and enkephalin-like immunoreactivity in the human vagus nerve. Gastroenterology 1979;77:468–71. [PubMed] [Google Scholar]
- [208].Rytel L, Palus K, Całka J. Co-expression of PACAP with VIP, SP and CGRP in the porcine nodose ganglion sensory neurons. Anat Histol Embryol 2015;44(2):86–91. [DOI] [PubMed] [Google Scholar]
- [209].Suzuki T, Kagoshima M, Shibata M, Inaba H, Onodera S, Yamamura T, et al. Effects of several denervation procedures on distribution of calcitonin gene-related peptide and substance P immunreactive in rat stomach. Dig Dis Sci 1997;42/6:1242–54. [DOI] [PubMed] [Google Scholar]
- [210].Konturek SJ, Brzozowski T, Pytko-Polonczyk J, Drozdowicz D. Exogenous and endogenous cholecystokinin protects gastric mucosa against the damage caused by ethanol in rats. Eur J Pharmacol 1995;273:57–62. [DOI] [PubMed] [Google Scholar]
- [211].Heinemann A, Jocic M, Peskar BM, Holzer P. CCK-evoked hyperemia in rat gastric mucosa involves neural mechanisms and nitric oxide. Am J Physiol 1996;270:253–8. [DOI] [PubMed] [Google Scholar]
- [212].Yonei Y, Holzer P, Guth PH. Laparotomy-induced gastric protection against ethanol injury is mediated by capsaicin-sensitive sensory neurons. Gastroenterology 1990;99:3–9. [DOI] [PubMed] [Google Scholar]
- [213].Stead RH, Dixon RF, Bramwell NH. Mast cells are closely opposed to nerves in the human gastrointestinal mucosa. Gastroenterology 1989;97:575–85. [DOI] [PubMed] [Google Scholar]