Abstract
Background
Hemorrhage is a leading preventable cause of death. Non-selective histone deacetylase inhibitors (HDACIs), such as valproic acid (VPA), have been shown to improve outcomes in hemorrhagic shock (HS). HDACs can be divided into 4 functional classes (I, IIa/IIb, III & IV). Classes I, IIa/IIb, and III have previously been implicated in the pathophysiology of HS. This study aimed to determine which HDAC class, or classes, are responsible for the survival benefit observed with non-selective HDACIs.
Methods
Survival study: Sprague Dawley (SD) rats were subjected to lethal HS (50% hemorrhage), and randomized to the following groups (n=8): 1) no treatment, 2) normal saline vehicle, 3) cyclodextrin vehicle, 4) MS275 (class I HDACI), 5) VPA (class I/IIa HDACI), 6) MC1568 (class IIa HDACI), 7) ACY1083 (class IIb HDACI), and 8) EX527 (class III HDACI). Survival was monitored for 24 hours.
Mechanistic study: SD rats were subjected to sub-lethal HS (40% hemorrhage), and randomized to the same groups (n=3), excluding EX527, based on results of the survival study. Tissues were harvested at 3 hours post-treatment, and expression of phosphorylated-AKT, β-catenin, acetylated histones H3 and H4, and acetylated α-tubulin were analyzed in myocardial tissue.
Results
Survival rate was 12.5% in the untreated group, and did not improve with vehicle or MS275 treatment. EX527 improved survival to 50%, although this did not achieve statistical significance (p = 0.082). However, treatment with VPA, MC1568 and ACY1083 improved survival rates to 87.5%, 75%, and 75%, respectively (p < 0.05). VPA induced acetylation of both histones H3 and H4, while MC1568 and ACY1083 increased acetylation of histone H4. ACY1083 also induced acetylation of α-tubulin. All treatment groups, except MS275, increased phosphorylated-AKT, and β-catenin.
Conclusion
Inhibition of HDAC classes IIa or IIb, but not class I, activates pro-survival pathways, which may be responsible for the improved outcomes in rodent models of HS.
Keywords: histone deacetylase inhibitors, isoform-selective, valproic acid, hemorrhagic shock, pro-survival pathways
BACKGROUND
Hemorrhagic shock (HS) is one of the leading causes of trauma-related morbidity and mortality, and has been identified as the most important preventable cause of death in patients sustaining traumatic injuries.(1) Current therapies, such as crystalloid resuscitation, fail to address the underlying cellular dysfunction, and may even worsen clinical outcomes if administered too aggressively. The concept of epigenetic modulation, through targeting the histone deacetylase (HDAC) enzyme family, has been a promising area of recent research. Treatment with HDAC inhibitors (HDACIs) has been shown to increase the acetylation of histones to induce transcriptional activation in a variety of pre-clinical models, including HS, traumatic brain injury, ischemia-reperfusion, sepsis, and combined insults.(2) Overall, these data suggest that HDACIs could be a very promising adjunct to conventional therapies, especially in austere settings where resources are limited.
In humans, at least 18 HDAC isoforms have been identified and are organized into four functional classes, including classes I, IIa/IIb, III and IV.(3) In general, class I HDACs are ubiquitously distributed and play a key role in cell survival and proliferation, while class II HDACs have more tissue-specific roles.(4) Our group has extensively researched the effects of valproic acid (VPA), a non-selective HDACI, which predominantly inhibits HDAC classes I and IIa.(5) At high doses, administration of VPA has been shown to improve survival in lethal HS models. VPA modulates multiple pro-survival, inflammatory, and apoptotic mediators such as c-Jun N-terminal kinase/caspase-3, extracellular signal-regulated kinase 1/2, protein kinase B, and mitogen activated protein kinase and β-catenin.(6-11) Although the administration of a single high-dose of VPA (150mg/kg) has been shown to be safe in healthy human subjects,(12) its safety in trauma patients is still being investigated (ClinicalTrials.gov Identifier: NCT02872428). However, administration of these large doses poses logistic challenges and raises concerns related to potential toxicities.
Within recent years, the concept of targeting isoform-selective HDACIs has gained more attention, as focusing on substrate selectivity and tissue specificity may help mitigate the adverse effects of non-selective HDACIs. For example, we have shown that Tubastatin A (Tub-A), a selective class IIb HDACI with high selectivity for HDAC6,(13) improves survival in animal models of lethal HS and sepsis.(14, 15) Other HDAC classes have been well studied in a variety of settings including inflammatory states, oncologic diseases, cystic fibrosis, HIV infection, and other neurodegenerative diseases, but their efficacy in the treatment of HS is unknown.(16-19)
The aim of this study was to determine the therapeutic effects of different isoform-selective HDACIs when compared to non-selective HDACIs in the setting of HS. We hypothesized that isoform selectivity would influence the ability of HDACI to improve survival in HS. A rodent model of lethal HS was used to compare the impact of treatment with various HDACIs (isoform-selective and non-selective) on survival. In a separate experiment, expression of well-known survival-associated proteins were compared in a sub-lethal model (to obtain matching samples from all the groups) of HS.
METHODS
The study was performed in accordance with the statutes from The Guide for the Care and Use of Laboratory Animals and was approved by the Institutional Animal Care and Use Committee.
Survival Study (Lethal HS Model)
Instrumentation
Male Sprague Dawley rats (240-310g; Charles River Laboratories) were allowed food and water ad libitum before and after surgery. Anesthesia was induced using 5% isoflurane. Isoflurane was titrated to an appropriate depth of anesthesia and maintained for the duration of the case. A heating pad was used to maintain body temperature. A right groin incision was made and the femoral artery and vein were exposed; both vessels were isolated and cannulated with polyethylene 50 tubing flushed with heparinized saline (100 USP units/ml). The arterial line was used for hemorrhage and hemodynamic monitoring, and the venous line was used for the administration of treatment agents.
Injury
Estimated total blood volume (ETBV) was calculated using the following formula: ETBV (ml) = weight (g) × 0.06 + 0.77.(20) Rats were subjected to 50% ETBV hemorrhage over 30 minutes (30% over 20 minutes, followed by 20% over 10 minutes) and maintained in shock without resuscitation for 20 minutes. Arterial blood gas (ABG) samples were taken at baseline and following 20 minutes of un-resuscitated shock (post-shock time point). ABG samples were taken into account when calculating the total hemorrhage volume.
Treatment
Animals were randomized to the following groups (n=8/group): 1) negative control (no treatment), 2) normal saline (NS) control (0.9% NS, 1ul/g) 3) cyclodextrin control (30% cyclodextrin, 1ul/g), 4) VPA (non-selective HDACI, class I/IIa; 250mg/kg), 5) MS275 (class I; 50mg/kg), 6) MC1568 (class IIa; 5mg/kg), 7) ACY1083 (class IIb; 30mg/kg), and 8) EX527 (class III; 50mg/kg). VPA was dissolved in NS, while all other agents were dissolved in cyclodextrin. The choice of agents and their respective doses was based on previous studies (21, 22) and unpublished preliminary data. Treatment was administered at the end of the shock period via intravenous injection over 15 minutes, followed by a 200ul NS flush over 10 minutes. Catheters were then removed, vessels ligated, and skin was closed with silk suture. 1% bupivacaine was injected at the operative site for local analgesia during the post-operative period. Following recovery from anesthesia, animals were returned to their cages for observation. Animals were assessed at regular intervals following this and given further analgesia as needed. Animals were monitored for a total of 24 hours to establish a survival curve.
Sample Size
Based on previously published data, we expected the survival in the VPA group to be 85-87.5%.(10) Sample size of 8/group was adequate to detect a relative difference of 80% between the best and worst groups with an α = 0.05 and power (1-β) of 0.80.
Mechanistic Study (Sub-lethal HS Model)
A sub-lethal HS protocol was used to ensure survival of all animals. This resulted in tissue harvest at consistent, pre-determined time points, and eliminated potential bias in sample procurement (due to early deaths in some groups following lethal hemorrhage).
Instrumentation
Instrumentation was identical to the survival study.
Injury
Rats were subjected to 40% ETBV over a period of 20 minutes. As in the survival study, animals were maintained in shock for a period of 20 minutes before treatment. ABGs were obtained at baseline, post-shock, and post-treatment to assess the effects of different isoform-selective and non-selective HDACIs on markers of shock. All other details of the model were consistent with the survival study above.
Treatment
Animals were randomized to the following groups (n=3): 1) NS control (1ul/g of 0.9% NS) 2) cyclodextrin control (30% cyclodextrin; 1 ul/g), 3) VPA (non-selective HDACI, class I/IIa; 250mg/kg), 4) MS275 (class I; 50mg/kg), 5) MC1568 (class IIa; 5mg/kg), and 6) ACY1083 (class IIb; 30mg/kg). Groups chosen included all treatments demonstrating a significant improvement in survival relative to vehicle, as well as MS275 as a negative control. Animals were recovered and returned to their cages as previously described. To identify early changes at the cellular level, rats were sacrificed 3 hours post-treatment. Hearts were harvested and rinsed with cold saline, frozen in liquid nitrogen, and stored at −80 °C for later use. Tissue samples were also harvested from the sham animals (anesthetized and cannulated without hemorrhage or treatment) to serve as controls.
Western Blot Analysis
Heart tissue (50mg wet weight per sample) was homogenized manually with a hand-held glass homogenizer, and whole tissue extracts were prepared by RIPA whole cell lysis buffer supplemented with 1x phosphatase and protease inhibitors (Halt™, Thermo Scientific, Waltham, MA). Supernatant was collected after centrifugation at 10000 RPM at 4 °C for 15 minutes. Prior to loading on a 12% polyacrylamide gel, whole cell extracts were balanced by spectrophotometry using the Bradford assay to ensure equal loading. Once separated by SDS-PAGE gel electrophoresis, proteins were then electrotransferred onto a nitrocellulose membrane. Membranes were blocked for 30 minutes using 5% bovine serum albumin (Roche, Indianapolis, IN) dissolved in Tris-Buffered Saline infused with 0.035% Tween-20 (TBST) and then incubated with the primary antibody diluted in TBST containing 5% bovine serum albumin at 4 °C overnight. Primary antibodies and their respective dilutions are as follows: rabbit anti-acetylated histone lysine 9 (Ac-H3, 1:1000), rabbit anti-acetylated histone H4, lysine 5 (Ac-H4, 1:1000), rabbit anti-acetylated α-tubulin lysine 9 (Ac-tubulin, 1:1000), rabbit anti-histone H3 (H3, 1:1000), rabbit anti-histone H4 (H4, 1:1000), rabbit anti-α-tubulin (α-tubulin, 1:1000), rabbit anti-phospho-Akt, serine 473 (p-AKT, 1:1000) and rabbit anti-AKT (AKT, 1:1000) all from Cell Signaling Technology (Danvers, MA); mouse anti-β catenin (1:500) from Thermo Fisher Scientific (Waltham, MA); mouse anti-β-actin (1:3000) from Abcam (Cambridge, MA). The primary antibody was detected by incubating with horseradish peroxidase (HRP)-conjugated secondary goat anti-rabbit IgG antibody (Abcam, Cambridge, MA; 1:3000) or HRP-conjugated secondary goat anti-mouse IgG antibody (Abcam, Cambridge, MA; 1:3000) diluted in TBST with 5% non-fat milk for 1 hour at room temperature. Signals were detected using Enhanced Chemiluminescence (ECL; Thermo Fisher Scientific Waltham, MA) and densitometry was performed using the Quantity One version 4.6.2 software (Bio-Rad Laboratories, Munich, Germany). Quantitation of Western blots is expressed as mean densitometry ratio +/− standard error of the mean, and each assay was performed in triplicate.(23)
Statistical Analysis
All statistical analysis was performed using IBM SPSS Statistics 20 software (Chicago, IL). Differences between three or more groups were analyzed using one-way analysis of variance (ANOVA) followed by Bonferroni post-hoc testing. An independent samples t-test was used for comparisons between two groups. Survival data were analyzed using a Kaplan-Meier curve with log-rank analysis. Statistical significance for all tests was defined at p < 0.05. All continuous values are presented as the mean +/− standard error of the mean (SEM).
RESULTS
Survival Study
Response to Injury
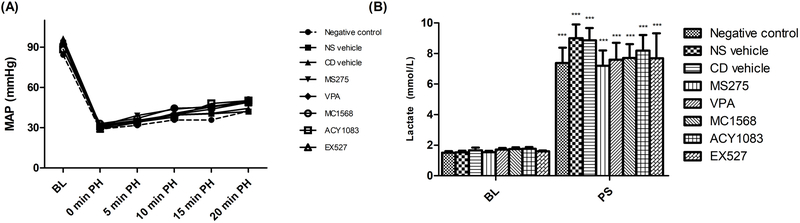
No differences were observed in baseline characteristics, including body weight, baseline blood pressure, and laboratory values. The animals demonstrated a predictable physiologic response to hemorrhage, with hypotension, which recovered partially towards baseline following treatment (Figure 1A). No significant differences in physiologic parameters were observed between the different treatment groups. Laboratory parameters are summarized in Supplemental Digital Content 1. Overall, all groups demonstrated a similar degree of shock and response to resuscitation. As expected, injury resulted in significant (p < 0.05) lactic acidosis (Figure 1B). No significant differences (p > 0.05) in lactate levels were noted between the various treatment groups at either time point.
Figure 1.
Intraoperative mean arterial pressure (A) during lethal hemorrhagic shock model. Lactate levels (B) were obtained from arterial blood gases taken at baseline and post-shock. Lactate level is presented as mean with standard error of the mean. ‘***’ indicates a significant difference (p < 0.001) between the baseline and post-shock levels of a given group. No significant differences were seen between different groups at either time point.
BL – baseline; PH – post-hemorrhage; PS – post-shock; PT – post-treatment; MAP – mean arterial pressure; NS – normal saline; CD – cyclodextrin; VPA – valproic acid.
Survival
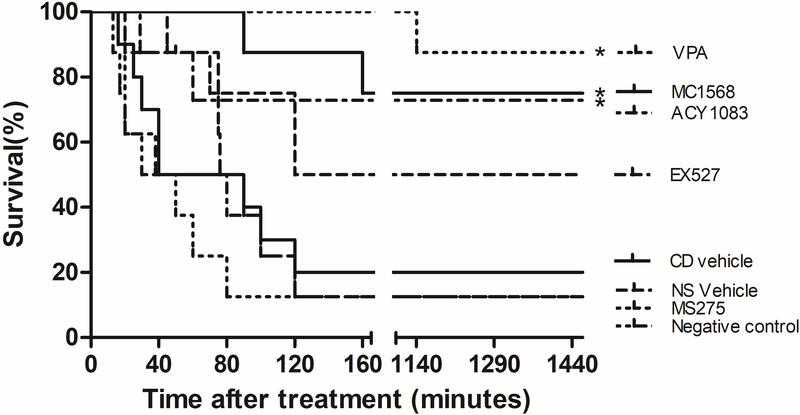
Survival rate was 12.5% in the untreated group (negative control), with the majority of animals dying within 120 minutes post-hemorrhage (Figure 2). Survival did not improve with either vehicle or MS275 treatment (survival rates = 12.5%, 20%, and 12.5% for NS vehicle, cyclodextrin vehicle, and MS275, respectively). EX527 improved survival to 50%; however, this did not achieve statistical significance (p > 0.05). Treatment with VPA, MC1568 and ACY1083 improved survival rates to 87.5%, 75%, and 75%, respectively (p < 0.05).
Figure 2.
Kaplan-Meier survival curves following isoform-selective and non-selective HDACI treatments in lethal hemorrhagic shock model. Animals were observed for 24 hours. All treatments were administered intravenously. VPA, MC1568 and ACY1083 significantly improved 24-hour survival compared with vehicle groups. ‘*’ designates a p < 0.05 when compared to normal saline vehicle vehicle group, while ‘#’ designates a p < 0.05 compared to cyclodextrin vehicle group.
NS – normal saline; CD – cyclodextrin; VPA – valproic acid.
Mechanistic Study
All animals survived in the sub-lethal model until the point of euthanasia. As in the lethal model, animals demonstrated a predictable physiologic response to hemorrhage (Figure 3). Laboratory parameters for the mechanistic study are summarized in Supplemental Digital Content 2. As in the survival study, all groups demonstrated a similar degree of shock and response to resuscitation. Following shock, animals demonstrated a trend towards increased lactate from baseline to post-shock, with three of five groups demonstrating statistical significance. No significant differences were seen in raw values for lactate between the groups at either time point.
Figure 3.
Intraoperative mean arterial pressure during the lethal hemorrhagic shock model.
MAP – mean arterial pressure; BL – baseline; PH – post-hemorrhage; Veh – vehicle; VPA – valproic acid.
Histone Acetylation Levels
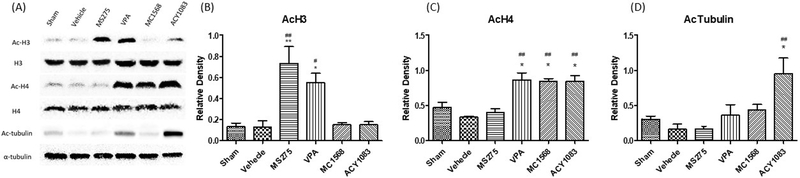
Different classes of HDACIs are known to induce differential histone and non-histone protein acetylation. Ac-H3, Ac-H4 and Ac-tubulin were assessed to demonstrate class I, class IIa and class IIb HDAC inhibition (24, 25) by their corresponding HDACIs. Treatment with class I HDACI, MS275, increased the acetylation of histone H3, while treatment with Class IIa HDACI, MC1568, and class IIb HDACI, ACY1083, increased the acetylation of histone H4 (Figure 4). Treatment with Class IIb HDACI, ACY1083, also increased acetylation of α-tubulin. Non-selective HDACI, VPA, increased acetylation of both histone H3 and H4. Overall, this data confirmed that the administered HDACI dosages were sufficient to acetylate their known target proteins.
Figure 4.
Effect of isoform-specific and non-selective HDACIs on acetylation of histone H3 (AcH3), histone H4 (AcH4), and α-tubulin (AcTubulin) in myocardial tissue. Animals were sacrificed 3 hours following hemorrhage. Heart tissue lysate was assessed for AcH3, AcH4 and AcTubulin by Western Blot analysis (A). Histone H3, histone H4 and α-tubulin were used as internal controls. Data are shown as a mean densitometry ratio of AcH3: H3 (B), AcH4: H4 (C), and AcTubulin: α-tubulin (D) with standard error of the mean for each treatment agent. ‘*’ and ‘**’ designate p < 0.05 and p < 0.01 respectively when compared to the sham group, while ‘#’ and ‘##’ designate p < 0.05 and p < 0.01 respectively when compared to the vehicle group.
AcH3 – acetylated histone H3; H3 – histone H3; AcH4 – acetylated histone H4; H4 –histone H4; AcTubulin – acetylated tubulin; VPA – valproic acid.
Phosphorylation of AKT and activation of β-catenin
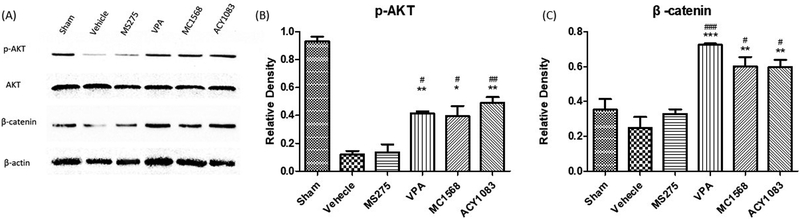
To further examine the effects of various classes of HDACIs at the cellular level, the expression of well-known pro-survival proteins, p-AKT and β-catenin, were measured in the myocardium. Between MS275 and vehicle groups, no significant differences (p > 0.05) in p-AKT and β-catenin expression were observed (Figure 5). VPA, MC1568 and ACY1083 groups displayed significantly increased (p < 0.05) p-AKT and β-catenin expression when compared to vehicle and MS275 groups. However, there were no statistically significant differences (p > 0.05) between the VPA, MC1568, and ACY1083 treatment groups.
Figure 5.
Effect of isoform-specific and non-selective HDACIs on phosphorylation of AKT (p-AKT) and β-catenin in myocardial tissue. Animals were sacrificed 3 hours following hemorrhage. Heart tissue lysate was assessed for p-AKT and β-catenin by Western Blot analysis (A). AKT and β-actin were used as internal controls. Data are shown as a mean densitometry ratio of p-AKT: AKT (B) and β-catenin: β-actin (C) with standard error of the mean for each treatment agent. ‘*’, ‘**’ and ‘***’ designate p < 0.05, p < 0.01 and p < 0.001 respectively when compared to the vehicle group, while ‘#’, ‘##’ and ‘###’ designate p < 0.05, p < 0.01 and p < 0.001 respectively when compared to MS275 group.
pAKT – phosphorylated AKT; VPA – valproic acid.
DISCUSSION
In the present study, we have demonstrated that both class IIa and IIb HDAC inhibition improves survival and activates pro-survival pathways in rodent models of HS. Furthermore, the effects of these isoform-selective HDACIs were comparable to the survival benefits observed with the non-selective HDACI, VPA.
Hemorrhage remains one of the leading causes of death in civilian and military trauma and is responsible for approximately one-third of deaths within the first several hours following traumatic injury.(26) Current treatment strategies focus on fluid resuscitation to replace the lost intravascular volume. However, early aggressive resuscitation in severely bleeding patients has failed to demonstrate any survival benefit,(27) and can even worsen outcomes as it exaggerates the inflammatory response resulting in further cellular injury.(28-30) Furthermore, infusion of crystalloids is associated with hemodilution, coagulopathy, edema, and upregulation of pro-inflammatory mediators.(31, 32) As conventional treatment strategies have no inherent pro-survival properties, there is a need to develop novel approaches that can mitigate the cellular consequences of shock. This is even more relevant in battlefield settings that lack blood banks and advanced critical care therapies. Pharmacologic treatments in these austere settings can serve as a “bridge therapy” to keep the injured alive long enough to get evacuated to higher echelons of care.
Our group has previously shown that VPA treatment improves outcomes in various animal models of lethal hemorrhage, sepsis, and combined insults.(10, 33, 34) However, treatment with non-selective HDACIs requires very high doses, which creates a potential for toxicities and other logistic challenges. VPA has been FDA approved for a number of years for use in epilepsy, and is also used in various other conditions, including bipolar mania and migraine headaches. However, it carries some common, and potentially dangerous side effects, including hypothermia, hemodynamic disruption and coagulopathy. It also has a “Black Box” warning for hepatotoxicity, teratogenicity, and pancreatitis. Our recent study demonstrates that a single, large dose of VPA is well-tolerated in healthy humans;(12) however, these side effects remain a concern, especially in trauma patients, where they could carry much greater significance. These limitations have prompted us to explore the use of isoform-selective HDACIs as a treatment for hemorrhagic shock.
Our study is the first to demonstrate differences in survival among various isoform-selective and non-selective HDACIs in a well-established animal model of HS. In the first experiment, survival was selected as an endpoint to determine the therapeutic effects of various isoform-selective and non-selective HDACIs. A sub-lethal model was then utilized to allow for tissue procurement at various time points and to avoid a survival bias. Tissue samples were used to analyze well-known pro-survival protein levels of p-AKT and β-catenin. The AKT signaling pathway plays an important role in cell survival as activated AKT directly phosphorylates pro-apoptotic proteins, such as BAD and caspase-9, thereby inhibiting apoptosis.(35-37) In addition, β-catenin, a multifunctional protein, plays a key role in not only maintaining physiological homeostasis, but also in inducing cellular proliferation and survival.(38, 39) Thus, phosphorylated AKT and β-catenin were selected to serve as markers of a pro-survival phenotype in cells. As anticipated, VPA treatment significantly improved survival and preserved p-AKT and β-catenin expression in the myocardial cells. These results were consistent with previous work by our group and others.(8, 40-42) Interestingly, our results indicated that class IIa and class IIb HDACIs also improved survival and increased the expression of p-AKT and β-catenin levels, similar to VPA. We have previously demonstrated that administration of Class IIb HDACIs improves survival in HS (14) and septic (15) models. However, this is first study to demonstrate that class IIa HDACIs can improve survival in HS models as well. Furthermore, this finding is in agreement with other studies demonstrating that class IIa HDACIs attenuate cell death in various models.(43-45) Lastly, our results revealed that treatment with class I HDACI, MS275 had no beneficial effects either on survival, or at the cellular level. This is consistent with the growing literature suggesting that class I HDAC inhibition may fail modulate pro-survival pathways, including AKT.(46-48)
There are several limitations to this study. First, we used relatively small samples sizes. This was adequate to detect large differences in survival, however smaller differences, including that seen with EX527 treatment, did not reach statistical significance. We believe this is likely a type II error, and would reach statistical significance with an increase in sample size. Second, only male rats were used in this study. It is well known that there is gender dimorphism in trauma patients(49), with female sex hormones being protective in shock. As this was a preliminary study to establish efficacy before further testing, we chose to standardize for this and evaluate the effect of the drugs in male animals only. Future studies will need to verify the effect of these drugs in female animals also. Third, the doses of the isoform-selective and non-selective HDACIs were different among classes and based upon the published literature. Going forward, strict dose optimization studies will need to be performed. Fourth, as this was a proof-of-concept study, it did not assess the long-term safety profile of these drugs. Further studies should be done in those agents demonstrating significant benefit to assess their side effect profile. As most of the iso-HDACIs are still experimental agents, they will require extensive preclinical and clinical testing prior to potential FDA approval. Lastly, we selected only two proteins, including AKT and β-catenin, as biological surrogates of a pro-survival phenotype. However, the underlying mechanism between various isoform-selective and non-selective HDACIs and pro-survival proteins needs to be further elucidated.
In conclusion, this study demonstrates that isoform-selective class IIa and class IIb HDACIs improve survival and activate pro-survival pathways in a rodent model of HS. As class IIa and IIb HDACIs demonstrate comparable therapeutic effects to VPA treatment, isoform-selective HDACIs need to be further explored as novel treatments for HS and other injuries. Future studies should focus on further elucidating the mechanisms by which isoform-selective HDACIs, including class IIa and class IIb, improve survival, and whether combination of these agents could be synergistic. This may allow us to further reduce the dosages and improve the safety profile of HDACI treatment.
Supplementary Material
Acknowledgments
Funding: NIH RO1 GM084127 (Alam, HB).
Footnotes
Level of Evidence: not applicable (pre-clinical study)
Conflict of Interest: None to report.
Meeting presentations: None.
Study type: Therapeutic
REFERENCES
- 1.Alam HB. Trauma care: Finding a better way. PLoS Med. 2017;14(7):e1002350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Halaweish I, Nikolian V, Georgoff P, Li Y, Alam HB. Creating a “prosurvival phenotype” through histone deacetylase inhibition: past, present, and future. Shock. 2015;44 Suppl 1:6–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Suliman BA, Xu D, Williams BR. HDACi: molecular mechanisms and therapeutic implications in the innate immune system. Immunol Cell Biol. 2012;90(1):23–32. [DOI] [PubMed] [Google Scholar]
- 4.Dokmanovic M, Clarke C, Marks PA. Histone deacetylase inhibitors: overview and perspectives. Mol Cancer Res. 2007;5(10):981–9. [DOI] [PubMed] [Google Scholar]
- 5.Bolden JE, Peart MJ, Johnstone RW. Anticancer activities of histone deacetylase inhibitors. Nat Rev Drug Discov. 2006;5(9):769–84. [DOI] [PubMed] [Google Scholar]
- 6.Gottlicher M Valproic acid: an old drug newly discovered as inhibitor of histone deacetylases. Ann Hematol. 2004;83 Suppl 1:S91–2. [DOI] [PubMed] [Google Scholar]
- 7.Kochanek AR, Fukudome EY, Li Y, Smith EJ, Liu B, Velmahos GC, deMoya M, King D, Alam HB. Histone deacetylase inhibitor treatment attenuates MAP kinase pathway activation and pulmonary inflammation following hemorrhagic shock in a rodent model. J Surg Res. 2012;176(1):185–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li Y, Liu B, Sailhamer EA, Yuan Z, Shults C, Velmahos GC, deMoya M, Shuja F, Butt MU, Alam HB. Cell protective mechanism of valproic acid in lethal hemorrhagic shock. Surgery. 2008;144(2):217–24. [DOI] [PubMed] [Google Scholar]
- 9.Butt MU, Sailhamer EA, Li Y, Liu B, Shuja F, Velmahos GC, DeMoya M, King DR, Alam HB. Pharmacologic resuscitation: cell protective mechanisms of histone deacetylase inhibition in lethal hemorrhagic shock. J Surg Res. 2009;156(2):290–6. [DOI] [PubMed] [Google Scholar]
- 10.Fukudome EY, Kochanek AR, Li Y, Smith EJ, Liu B, Kheirbek T, Lu J, Kim K, Hamwi K, Velmahos GC, et al. Pharmacologic resuscitation promotes survival and attenuates hemorrhage-induced activation of extracellular signal-regulated kinase 1/2. J Surg Res. 2010;163(1):118–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zacharias N, Sailhamer EA, Li Y, Liu B, Butt MU, Shuja F, Velmahos GC, de Moya M, Alam HB. Histone deacetylase inhibitors prevent apoptosis following lethal hemorrhagic shock in rodent kidney cells. Resuscitation. 2011;82(1):105–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Georgoff PE, Nikolian VC, Bonham T, Pai MP, Tafatia C, Halaweish I, To K, Watcharotone K, Parameswaran A, Luo R, et al. Safety and tolerability of intravenous valproic acid in healthy subjects: a phase i dose-escalation trial. Clin Pharmacokinet. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Butler KV, Kalin J, Brochier C, Vistoli G, Langley B, Kozikowski AP. Rational design and simple chemistry yield a superior, neuroprotective HDAC6 inhibitor, tubastatin A. J Am Chem Soc. 2010;132(31):10842–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chang Z, Li Y, He W, Liu B, Halaweish I, Bambakidis T, Liang Y, Alam HB. Selective inhibition of histone deacetylase 6 promotes survival in a rat model of hemorrhagic shock. J Trauma Acute Care Surg. 2015;79(6):905–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li Y, Zhao T, Liu B, Halaweish I, Mazitschek R, Duan X, Alam HB. Inhibition of histone deacetylase 6 improves long-term survival in a lethal septic model. J Trauma Acute Care Surg. 2015;78(2):378–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fischer A, Sananbenesi F, Wang X, Dobbin M, Tsai LH. Recovery of learning and memory is associated with chromatin remodelling. Nature. 2007;447(7141):178–82. [DOI] [PubMed] [Google Scholar]
- 17.Brogdon JL, Xu Y, Szabo SJ, An S, Buxton F, Cohen D, Huang Q. Histone deacetylase activities are required for innate immune cell control of Th1 but not Th2 effector cell function. Blood. 2007;109(3):1123–30. [DOI] [PubMed] [Google Scholar]
- 18.Routy JP. Valproic acid: a potential role in treating latent HIV infection. Lancet. 2005;366(9485):523–4. [DOI] [PubMed] [Google Scholar]
- 19.Minetti GC, Colussi C, Adami R, Serra C, Mozzetta C, Parente V, Fortuni S, Straino S, Sampaolesi M, Di Padova M, et al. Functional and morphological recovery of dystrophic muscles in mice treated with deacetylase inhibitors. Nat Med. 2006;12(10):1147–50. [DOI] [PubMed] [Google Scholar]
- 20.Lee HB, Blaufox MD. Blood volume in the rat. J Nucl Med. 1985;26(1):72–6. [PubMed] [Google Scholar]
- 21.Zhao T, Li Y, Liu B, Bronson RT, Halaweish I, Alam HB. Histone deacetylase III as a potential therapeutic target for the treatment of lethal sepsis. J Trauma Acute Care Surg. 2014;77(6):913–9; discussion 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wu Q, Zhang Q, Wen C, Hu L, Wang X, Lin G. The effect of MS-275 on CYP450 isoforms activity in rats by cocktail method. Int J Clin Exp Pathol. 2015;8(8):9360–7. [PMC free article] [PubMed] [Google Scholar]
- 23.Martin-Clemente B, Alvarez-Castelao B, Mayo I, Sierra AB, Diaz V, Milan M, Farinas I, Gomez-Isla T, Ferrer I, Castano JG. alpha-Synuclein expression levels do not significantly affect proteasome function and expression in mice and stably transfected PC12 cell lines. J Biol Chem. 2004;279(51):52984–90. [DOI] [PubMed] [Google Scholar]
- 24.Thiagalingam S, Cheng KH, Lee HJ, Mineva N, Thiagalingam A, Ponte JF. Histone deacetylases: unique players in shaping the epigenetic histone code. Ann N Y Acad Sci. 2003;983:84–100. [DOI] [PubMed] [Google Scholar]
- 25.Hubbert C, Guardiola A, Shao R, Kawaguchi Y, Ito A, Nixon A, Yoshida M, Wang XF, Yao TP. HDAC6 is a microtubule-associated deacetylase. Nature. 2002;417(6887):455–8. [DOI] [PubMed] [Google Scholar]
- 26.Kauvar DS, Lefering R, Wade CE. Impact of hemorrhage on trauma outcome: an overview of epidemiology, clinical presentations, and therapeutic considerations. J Trauma. 2006;60(6 Suppl):S3–11. [DOI] [PubMed] [Google Scholar]
- 27.Bickell WH, Wall MJ Jr., Pepe PE, Martin RR, Ginger VF, Allen MK, Mattox KL Immediate versus delayed fluid resuscitation for hypotensive patients with penetrating torso injuries. N Engl J Med. 1994;331(17):1105–9. [DOI] [PubMed] [Google Scholar]
- 28.Pearson WS, Ovalle F Jr., Faul M, Sasser SM A review of traumatic brain injury trauma center visits meeting physiologic criteria from The American College of Surgeons Committee on Trauma/Centers for Disease Control and Prevention Field Triage Guidelines. Prehosp Emerg Care. 2012;16(3):323–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rhee P, Koustova E, Alam HB. Searching for the optimal resuscitation method: recommendations for the initial fluid resuscitation of combat casualties. J Trauma. 2003;54(5 Suppl):S52–62. [DOI] [PubMed] [Google Scholar]
- 30.Shires GT, Browder LK, Steljes TP, Williams SJ, Browder TD, Barber AE. The effect of shock resuscitation fluids on apoptosis. Am J Surg. 2005;189(1):85–91. [DOI] [PubMed] [Google Scholar]
- 31.Lee CC, Chang IJ, Yen ZS, Hsu CY, Chen SY, Su CP, Chiang WC, Chen SC, Chen WJ. Effect of different resuscitation fluids on cytokine response in a rat model of hemorrhagic shock. Shock. 2005;24(2):177–81. [DOI] [PubMed] [Google Scholar]
- 32.Chen H, Koustova E, Shults C, Sailhamer EA, Alam HB. Differential effect of resuscitation on Toll-like receptors in a model of hemorrhagic shock without a septic challenge. Resuscitation. 2007;74(3):526–37. [DOI] [PubMed] [Google Scholar]
- 33.Nikolian VC, Georgoff PE, Pai MP, Dennahy IS, Chtraklin K, Eidy H, Ghandour MH, Han Y, Srinivasan A, Li Y, et al. Valproic acid decreases brain lesion size and improves neurologic recovery in swine subjected to traumatic brain injury, hemorrhagic shock, and polytrauma. J Trauma Acute Care Surg. 2017;83(6):1066–73. [DOI] [PubMed] [Google Scholar]
- 34.Liu Z, Li Y, Chong W, Deperalta DK, Duan X, Liu B, Halaweish I, Zhou P, Alam HB. Creating a prosurvival phenotype through a histone deacetylase inhibitor in a lethal two-hit model. Shock. 2014;41(2):104–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Franke TF, Hornik CP, Segev L, Shostak GA, Sugimoto C. PI3K/Akt and apoptosis: size matters. Oncogene. 2003;22(56):8983–98. [DOI] [PubMed] [Google Scholar]
- 36.Cardone MH, Roy N, Stennicke HR, Salvesen GS, Franke TF, Stanbridge E, Frisch S, Reed JC. Regulation of cell death protease caspase-9 by phosphorylation. Science. 1998;282(5392):1318–21. [DOI] [PubMed] [Google Scholar]
- 37.Datta SR, Dudek H, Tao X, Masters S, Fu H, Gotoh Y, Greenberg ME. Akt phosphorylation of BAD couples survival signals to the cell-intrinsic death machinery. Cell. 1997;91(2):231–41. [DOI] [PubMed] [Google Scholar]
- 38.Valkenburg KC, Graveel CR, Zylstra-Diegel CR, Zhong Z, Williams BO. Wnt/beta-catenin Signaling in Normal and Cancer Stem Cells. Cancers (Basel). 2011;3(2):2050–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shang S, Hua F, Hu ZW. The regulation of beta-catenin activity and function in cancer: therapeutic opportunities. Oncotarget. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hwabejire JO, Lu J, Liu B, Li Y, Halaweish I, Alam HB. Valproic acid for the treatment of hemorrhagic shock: a dose-optimization study. J Surg Res. 2014;186(1):363–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jin G, Liu B, You Z, Bambakidis T, Dekker SE, Maxwell J, Halaweish I, Linzel D, Alam HB. Development of a novel neuroprotective strategy: Combined treatment with hypothermia and valproic acid improves survival in hypoxic hippocampal cells. Surgery. 2014;156(2):221–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bambakidis T, Dekker SE, Liu B, Maxwell J, Chtraklin K, Linzel D, Li Y, Alam HB. Hypothermia and valproic acid activate prosurvival pathways after hemorrhage. J Surg Res. 2015;196(1):159–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bolger TA, Yao TP. Intracellular trafficking of histone deacetylase 4 regulates neuronal cell death. J Neurosci. 2005;25(41):9544–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Collins LM, Adriaanse LJ, Theratile SD, Hegarty SV, Sullivan AM, O’Keeffe GW. Class-IIa Histone Deacetylase Inhibition Promotes the Growth of Neural Processes and Protects Them Against Neurotoxic Insult. Mol Neurobiol. 2015;51(3):1432–42. [DOI] [PubMed] [Google Scholar]
- 45.Guida N, Laudati G, Mascolo L, Cuomo O, Anzilotti S, Sirabella R, Santopaolo M, Galgani M, Montuori P, Di Renzo G, et al. MC1568 Inhibits Thimerosal-Induced Apoptotic Cell Death by Preventing HDAC4 Up-Regulation in Neuronal Cells and in Rat Prefrontal Cortex. Toxicol Sci. 2016;154(2):227–40. [DOI] [PubMed] [Google Scholar]
- 46.Chen CH, Lee CH, Liou JP, Teng CM, Pan SL. Molecular mechanisms underlying the antitumor activity of (E)-N-hydroxy-3-(1-(4-methoxyphenylsulfonyl)-1,2,3,4-tetrahydroquinolin-6-yl)acry lamide in human colorectal cancer cells in vitro and in vivo. Oncotarget. 2015;6(34):35991–6002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhang L, Zhang Y, Chou CJ, Inks ES, Wang X, Li X, Hou J, Xu W. Histone deacetylase inhibitors with enhanced enzymatic inhibition effects and potent in vitro and in vivo antitumor activities. ChemMedChem. 2014;9(3):638–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Huang X, Gao L, Wang S, Lee CK, Ordentlich P, Liu B. HDAC inhibitor SNDX-275 induces apoptosis in erbB2-overexpressing breast cancer cells via down-regulation of erbB3 expression. Cancer Res. 2009;69(21):8403–11. [DOI] [PubMed] [Google Scholar]
- 49.Wohltmann CD, Franklin GA, Boaz PW, Luchette FA, Kearney PA, Richardson JD, Spain DA. A multicenter evaluation of whether gender dimorphism affects survival after trauma. Am J Surg. 2001;181(4):297–300. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.