Abstract
The efficacy of GI24-lysed Brucella abortus cells as a vaccine candidate against brucellosis in goats was evaluated on 2 groups of Korean black goats. Group A goats were immunized subcutaneously (SC) with sterile phosphate-buffered saline, whereas group B goats were immunized SC with approximately 3 × 109 lysed B. abortus cells. Subcutaneous immunization with the lysed cells did not cause any negative impact on the overall clinical status, such as behavior and appetite, throughout the study period. The enzyme-linked immunosorbent assay (ELISA) optical densities values for B. abortus lipopolysaccharide in serum were considerably higher in group B than those in group A. Also, the levels of the cytokines interleukin 4 (IL-4), tumor necrosis factor-alpha (TNF-α), and interferon gamma (IFN-γ) were significantly elevated in group B compared with those in group A. Following intraconjunctival challenge with B. abortus strain 544, the severity of brucellosis in terms of infection index and colonization of B. abortus in tissues was significantly lower in group B than in group A. The present study concluded that 3 of 5 goats immunized with GI24-lysed bacteria were completely protected against challenge. Future investigations are required to improve the protective efficacy offered by lysed B. abortus cells for practical applications in small ruminants.
Résumé
L’efficacité de cellules lysées de Brucella abortus GI24 comme vaccin candidat contre la brucellose chez les chèvres a été évaluée chez deux groupes de chèvres noires coréennes. Les chèvres du groupe A ont été immunisées par voie sous-cutanée (SC) avec de la saline tamponnée stérile, alors que les chèvres du groupe B ont été immunisées SC avec environ 3 × 109 cellules lysées de B. abortus. L’immunisation sous-cutanée avec les cellules lysées n’a pas eu d’impact négatif sur l’état clinique général, tel que le comportement et l’appétit, tout au long de la période d’étude. Les valeurs de densité optique obtenues lors d’épreuves immunoenzymatiques (ELISA) utilisant le lipopolysaccharide de B. abortus étaient considérablement plus élevées avec le sérum des animaux du groupe B que celui des animaux du groupe A. De plus, les niveaux des cytokines interleukine-4 (IL-4), du facteur-alpha nécrosant de tumeur (TNF-α), d’interféron-gamma (IFN-γ) étaient significativement plus élevés dans le groupe B comparativement au groupe A. Pour donner suite à l’infection-défi intra-conjonctivale avec la souche 544 de B. abortus, la sévérité de brucellose en termes d’index d’infection et de colonisation des tissus par B. abortus était significativement moindre dans le groupe B que dans le groupe A. La présente étude a permis de conclure que 3 des 5 chèvres immunisées avec les bactéries GI24 lysées étaient complètement protégées contre l’infection. Des études ultérieures sont requises pour améliorer l’efficacité protectrice offerte par les cellules lysées de B. abortus pour une application pratique chez les petits ruminants.
(Traduit par Docteur Serge Messier)
Introduction
Brucellosis is a contagious disease that induces abortion and decreases milk yield in domestic animals; it often causes economic losses (1,2). Brucella species affect a wide range of hosts such as cattle, goats, sheep, pigs, and dogs (3–5). Four major Brucella species — B. melitensis, B. suis, B. canis, and B. abortus — can infect humans (6–8). Although B. melitensis commonly infects small ruminants such as goats (9,10), B. abortus is also infectious for these animals (2,11). Goat brucellosis, therefore, is considered a devastating zoonotic disease (2).
Live attenuated vaccines, particularly B. abortus strains S19 and RB51, are widely used to eliminate brucellosis from livestock (12,13). However, live vaccines may lead to bacterial shedding from vaccinated animals in milk, urine, semen, or fecal matter, thereby posing a risk for humans (12). In an effort to reduce brucellosis risk, many different approaches using killed vaccines, subunit vaccines using recombinant proteins, or vector vaccines, have been tested against brucellosis, with variable success (12,14).
In the past few years, lysed bacterial cells have emerged as an effective inactivated vaccine candidate against a broad variety of Gram-negative bacteria (15–18). Lysed bacterial cells are inactivated bacteria with intact bacterial surface structures without cytoplasmic contents (19,20). The action mechanism of antimicrobial peptides (AMPs), a part of the innate immune system (21,22), involves the disruption of membrane barrier function by forming pores or changing membrane permeabilization without altering membrane structures (23–25). Some AMPs function as adjuvants (26,27). Porcine myeloid antimicrobial peptide-36 (PMAP-36) has the highest positive charge among AMPs discovered from pigs (28). The positively charged peptide binds to the negatively charged surface of the bacterial cell membrane through electrostatic interactions (29). Among the 36 amino acids in PMAP-36, 24 amino acids (GI24) compose the N-terminal α-helical domain, and like PMAP-36, GI24 can form pores in the bacterial cell membrane (16).
In our previous study (16), lysed B. abortus cells were constructed using GI24 and were exploited as a vaccine candidate against brucellosis in mice. The study subjects were intraperitoneally immunized with the lysed B. abortus cells, leading to the production of considerable serum IgG and cytokines, such as tumor necrosis factor-alpha (TNF-α) and interferon gamma (IFN-γ), associated with a Th1-type immune response. In addition, the immunized mice showed significant protection against brucellosis.
The aim of the present study was to investigate the protective efficacy of the GI24-lysed B. abortus cells as a vaccine candidate against B. abortus infection in goats. Goats were immunized subcutaneously (SC) with the GI24-lysed B. abortus cells and evaluated for an induced cell-mediated immune (CMI) response as well as a humoral immune response. Protective efficacy was estimated following intraconjunctival challenge with B. abortus strain 544.
Materials and methods
Animals and ethics statement
Ten nonpregnant female black goats (age = 6 mo) were divided into 2 equal groups: A and B. All goats were procured from tested brucellosis-free flocks. All goats were again confirmed to be seronegative for brucellosis in the laboratory by the Rose Bengal test. All animal experiments performed in this study received ethical approval (CBU 2016-98) from the Chonbuk National University Animal Ethics Committee, in accordance with the guidelines of the Korean Council on Animal Care.
Bacterial strains, peptide, and growth conditions
Brucella abortus bacteria isolated from Korean cattle, HJL900, was as a host strain to construct a vaccine using GI24 lysis (16). Brucella abortus strain 544 (ATCC 23448), a smooth, virulent B. abortus biovar 1, was chosen as the virulent challenge strain (30). The strain was grown in Brucella broth and on Brucella agar (Becton Dickinson, Sparks, Maryland, USA) at 37°C. The GI24 (GRFRRLRKKTRKRLKKIGKVLKWI-NH2) peptide was chemically synthesized by Peptron (Daejeon, South Korea).
Preparation of lysed B. abortus cells
GI24-lysed B. abortus cells were prepared as a vaccine candidate in goats according to the method described in a previous study (16). One colony of B. abortus isolate was inoculated into 200 mL of Brucella broth and incubated it at 37°C until an optical density (OD) of 0.3 at 600 nm was reached. Then 40 μg/mL GI24 peptide was added to the cultured broth and incubated the mixture at 37°C to induce lysed cells. After 24 h, lysis induction was determined by counting the number of viable bacteria on Brucella agar incubated for 72 h at 37°C. The lysed cells were harvested by centrifugation at 4000 × g for 10 min and resuspended the lysate in sterile phosphate-buffered saline (PBS) to approximately 3 × 109 cells/mL and stored the samples at −20°C until use.
Transmission electron microscopy (TEM)
Lysed cells were prepared for TEM analyses using the method described and TEM was used to confirm the intracellular alteration in the B. abortus cells before and after treatment with GI24 peptide, according to the method described by Lv et al (31).
Immunization of goats and sample collection
Ten female black goats (age = 6 mo) were divided into 2 equal groups (A and B) (n = 5 each). The goats were primed SC [0 wk post prime immunization (WPPI)] and 3 wk later, the animals were boosted SC (3 WPPI). Group A goats (control) was inoculated with 1.0 mL sterile PBS solution and group B goats with 1 mL of the GI24-lysed B. abortus cell suspension (approximately 3.0 × 109 cells/mL). Blood samples were collected at 0, 3 (before booster vaccination), and 6 (before challenge) WPPI to evaluate the serum IgG titers. All samples were stored at −70°C until use.
Assessment of vaccine safety
To assess the safety of GI24-lysed B. abortus cells, the temperatures of group B goats were monitored daily for 6 WPPI.
Immune response measurement by an enzyme-linked immunosorbent assay (ELISA)
A modified ELISA was done to assess the B. abortus lipopolysaccharide (LPS)-specific IgG titers in serum samples using the Bovine Brucella Ab ELISA 2.0 Kit (BioNote, Hwaseongsi, Gyeonggi-do, Republic of Korea). Tthe plates were blocked using PBS containing 1% ovalbumin (PBS–OVA; 200 μL/well) for 1 h, then the plates were washed with PBS containing 0.05% Tween-20. Serum samples were diluted to 1:100 in PBS–OVA and the plates were treated with horseradish peroxidase-conjugated rabbit anti-goat IgG antibody (Bethyl Laboratories, Montgomery, Texas, USA). A substrate containing o-phenylenediamine (Sigma-Aldrich, St. Louis, Missouri, USA) was used to cause enzymatic reactions that were measured using an automated ELISA spectrophotometer (Thermo Scientific Multiskan GO; Thermo Fisher Scientific Oy, Vantaa, Finland) at 492 nm. The ELISA results are expressed as mean OD ± standard deviation (SD).
Preparation of peripheral blood mononuclear cells
At 6 WPPI, blood was collected from each goat and all samples were placed into an acid-citrate-dextrose solution. The peripheral blood mononuclear cells (PBMCs) were enriched by density centrifugation using a Ficoll sodium diatrizoate gradient (Sigma Diagnostics, St. Louis, Missouri, USA). The PBMCs were diluted in Roswell Park Memorial Institute (RPMI) 1640 medium to 2.5 × 106 viable cells/mL as determined by trypan blue dye exclusion. Then 50 μL of each cell suspension, containing 5 × 105 cells, were added to a flat-bottomed 96-well microtiter plate that contained 100 μL RPMI 1640 medium or RPMI 1640 medium plus 106 cells/well of heat-inactivated JHL900. Wells counting 0.5 μg/well of Concanavalin A were used as positive controls for cytokine induction. All plates were performed in triplicate. The microtiter plates were incubated for 72 h at 37°C under 5% CO2 following an earlier standardized method (16,35). After incubation, the cell culture supernatants were moved to fresh microcentrifuge tubes and stored at −70°C until a commercially available kit was used to assay for cytokines.
Cytokine measurement by ELISA
The concentrations of IL-4, TNF-α, and IFN-γ in the culture supernatants were measured using goat cytokine kits according to the manufacturer’s instructions (MyBioSource, San Diego, California, USA). Results of the ELISA are expressed as mean OD ± SD.
Challenge experiments
Brucella abortus strain 544 was used for the challenge experiments using the following methods. The bacterium was grown in Brucella broth at 37°C for 24 h, then the culture was resuspended to obtain a final concentration of approximately 1 × 108 CFU/mL. At 6 wk following primary immunization, all goats were fasted for 16 h and anesthetized with an intermuscular injection of Zoletil 50 [7 to 10 mg/kg of body weight (BW); Virbac, Carros, France] and xylazine (2.32 to 3.48 mg/kg BW; Bayer Korea, Ansan, South Korea) before instilling 50 μL B. abortus 544 into each eye, for a total dose of approximately 107 CFU.
At 12 wk, all goats were euthanized and necropsied. Samples of the lymph nodes (bronchial, mediastinal, mesenteric, parotid, portal, retropharyngeal, submandibular, superficial, and supramammary) and parenchymal organs (heart, kidney, liver, lung, spleen, and uterus) were taken for a total of 15 organs from each animal. The tissue homogenates were plated onto Farrell’s medium containing 5% bovine serum, antibiotics, and ethyl violet (32). The Brucella bacteria were isolated from the lymph nodes and conventional methods were used for subsequent biochemical identification of isolates (33). Each node was trimmed into small pieces that were weighed and homogenized with an equal volume of sterile PBS. The resulting homogenate was then swabbed onto Farrell’s medium with 5% bovine serum, antibiotics, and ethyl violet (34). All agar plates were incubated at 37°C and 10% CO2 for ≥ 10 d. Polymerase chain reaction (PCR) was used to confirm colonies as the B. abortus challenge strain using the B. abortus-specific primer (5′-GACGAACGGAATTTTTCCAATCCC-3′) and the IS711-specific primer (5′-TGCCGATCACTTAAGGGCCTTCAT-3′). Sequencing was performed as described in previous studies (36,37). A tissue sample was considered B. abortus-infected even if only 1 colony from each tissue was positive for the B. abortus challenge strain by PCR and sequencing. The infection index was defined as the number of organs and lymph nodes infected with the B. abortus challenge strain in each animal.
Statistical analyses
The statistical analyses of data were performed using GraphPad Prism Software version 5.0 (La Jolla, California, USA). The significance of serum IgG titers, concentration of IFN-γ, infection index, and colonization of B. abortus strain 544 in tissues between groups were analyzed using 2-way analysis of variance (ANOVA) followed by Tukey’s multiple comparisons test. P-values < 0.05 were considered statistically significant.
Results
TEM
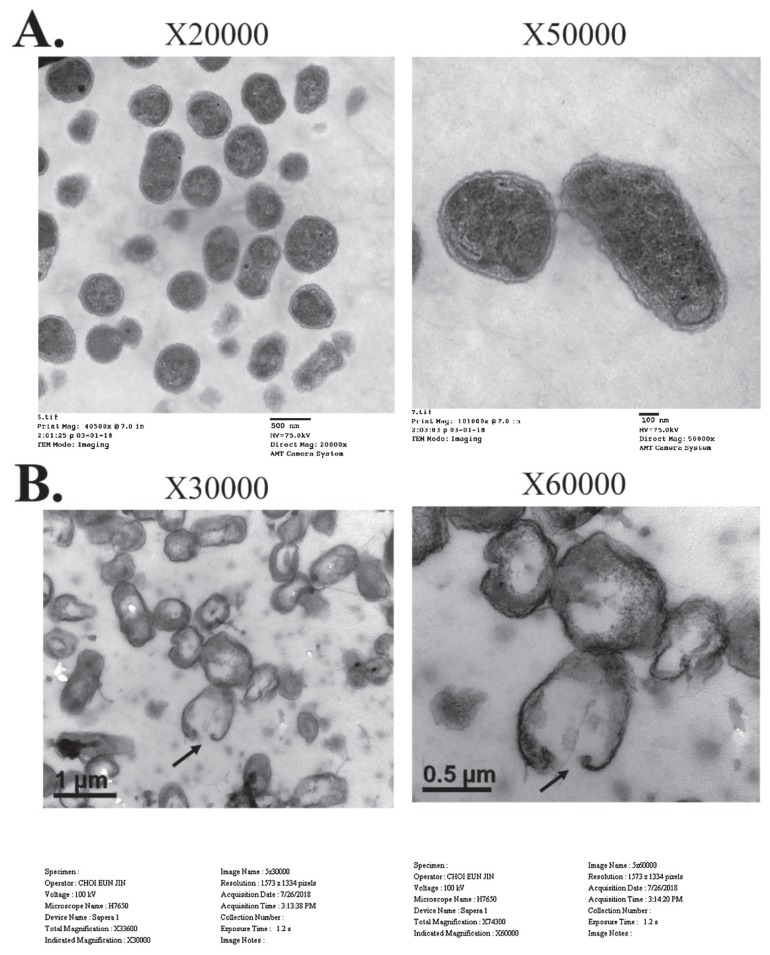
Intact cell membranes and complete intracellular contents of untreated B. abortus cells were observed (Figure 1A). Brucella abortus cells lysed with GI24 had visible pores in the bacterial membrane and lower amounts of cytoplasm (Figure 1B).
Figure 1.
Transmission electron micrographs of B. abortus biotype 1 treated with GI24. A — The untreated B. abortus cells. B — B. abortus cells treated with GI24. The bacterial cells were incubated with 40 μg/mL of GI24 for 30 h at 37°C.
Safety of the vaccine
There was no negative impact on the overall clinical status (e.g., behavior, appetite) of goats that received SC immunization with the lysed B. abortus cells and no side effects at the vaccination sites were observed throughout the study period (6 WPPI). The body temperatures of all the goats remained within normal limits (38.5°C to 40.5°C) (data not shown).
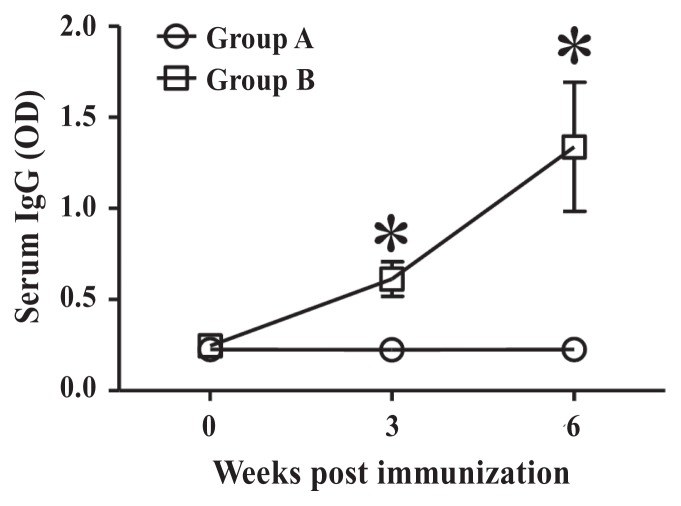
Antibody response to B. abortus LPS
The ODs of ELISA values for B. abortus LPS in the sera are presented in Figure 2. Serum titers of IgG antibodies against B. abortus LPS in group A and B goats were 0.225 ± 0.072 and 0.246 ± 0.032, respectively, before immunization. Serum IgG titers in group A goats were 0.225 ± 0.058 at 3 WPPI; those in group B goats increased gradually to 0.613 ± 0.095 at 3 WPPI (P < 0.05). At 6 WPPI, serum IgG titers in group B goats were elevated to 1.338 ± 0.354 (P < 0.05); those in group A goats were 0.227 ± 0.061.
Figure 2.
The optical density (OD) of ELISA against B. abortus LPS. Group A goats were inoculated with 1.0 mL of sterile PBS as a control. Group B goats were immunized subcutaneously with approximately 3 × 109 GI24-lysed B. abortus cells in 1 mL PBS. Data represent the means of all goats in each group and error bars represent the standard deviations (SD). Asterisks indicate a significant difference between the values obtained for the immunized group and those obtained for the control group (*P < 0.05).
Cytokine analysis
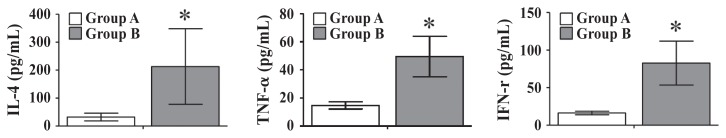
The IL-4, TNF-α, and IFN-γ concentrations in PBMCs in response to B. abortus after re-stimulation with HK B. abortus cells using ELISA kits at 6 WPPI were measured. The mean concentrations of IL-4 compared with those of heat-inactivated B. abortus cells from groups A and B PBMCs were 32.23 ± 18.11 pg/mL and 212.93 ± 135.16 pg/mL (P < 0.05), respectively. The TNF-α concentrations of group A and B goats were 14.72 ± 2.54 pg/mL and 49.48 ± 14.45 pg/mL (P < 0.05), respectively. Concentrations of IFN-γ in group A goats were 16.33 ± 2.38 ng/mL; however, IFN-γ concentrations in group B goats were 82.71 ± 29.21 pg/mL (Figure 3).
Figure 3.
IL-4, TNF-α, and IFN-γ concentrations (pg/mL) in peripheral blood mononuclear cells at 6 wk post prime immunization. Group A goats were inoculated with 1.0 mL of sterile PBS as a control. Group B goats were immunized subcutaneously with approximately 3 × 109 GI24-lysed B. abortus cells in 1 mL PBS. Data represent the means of all goats in each group and error bars indicate the standard deviations (SD). Asterisks indicate significant differences among the values obtained for each group (*P < 0.05).
Protective efficacy against B. abortus infection
Protective efficacy of the GI24-lysed B. abortus cells against B. abortus strain 544 infection was evaluated via 3 basic parameters: the effectiveness of vaccination (i.e., degree of protection against B. abortus infection), the infection index (i.e., number of B. abortus-infected tissues in each animal), and the number of B. abortus challenge strain isolates in each tissue. Among the 5 goats in group B, the lysed B. abortus cells provided complete protection (i.e., vaccine is effective) against B. abortus strain 544 infection in 3 goats. The severity of B. abortus strain 544 infection in group B goats (Figure 4A) as indicated by the infection index (1.0 ± 1.4, P < 0.05) and number of B. abortus challenge strain isolates in tissues (0.0 ± 0.0 to 1.1 ± 1.5 log10 CFU/g of tissue, P < 0.05) were significantly lower than those in group A (infection index 13.0 ± 2.4; Brucella colonization 1.8 ± 1.7 to 4.0 ± 0.3 log10 CFU/g of tissue) (Figure 4B).
Figure 4.
A — Index of infection for goats challenged with B. abortus strain 544 at 6 wk post prime immunization. B — Colonization and incidence of recovery of B. abortus strain 544 in tissues. Group A goats were inoculated with 1.0 mL of sterile PBS as a control. Group B goats were immunized subcutaneously with approximately 3 × 109 GI24-lysed B. abortus cells in 1 mL PBS. All goats in each group were intraconjunctivally challenged with 1 × 107 CFU of virulent wild-type B. abortus 544 at 6 wk post prime immunization. The numbers of viable bacteria recovered from each tissue of goats at 12 wk post challenge are shown (LN — lymph node).
Discussion
In our previous study (16), inactivated B. abortus vaccine candidates were constructed using GI24 lysis. The lysed B. abortus cells showed an effective immunity against B. abortus colonization in a murine model (16). Therefore, it was speculated that the inactivated B. abortus vaccine candidate lysed with GI24 could also control brucellosis in small ruminants. The prime aim in this study was to evaluate safety and protective efficacy against B. abortus infection of inactivated B. abortus cells lysed with GI24 in goats. Using clinical thermometry, it was determined that injection of the inactivated B. abortus cells lysed with GI24 was safe for use in goats. Moreover, inflammatory infiltrations in goats vaccinated SC with the lysed B. abortus cells were not observed. It can, therefore, be concluded that the GI24-inactivated B. abortus vaccine candidate is safe for goats.
Potent serum IgG titers are essential for defending the host from systemic infection by intracellular pathogens (38–40). These serum IgGs eradicate pathogens from the blood by stimulating the phagocytosis efficacy of host phagocytes via opsonization (39,41). In the present study, serum IgG titers were investigated in goats immunized by SC administration of the GI24-inactivated B. abortus vaccine candidate. The serum IgG titers in the goats immunized with lysed B. abortus cells (group B) were significantly elevated compared with those of the non-immunized group of goats (group A). Furthermore, induction of cytokines from PBMCs of all goats immunized with the lysed B. abortus cells and re-stimulated in vitro with heat-inactivated B. abortus whole cells indicated a powerful Th2 type immunity (IL-4 represents the Th2 bias of immunity). These results confirmed that the production of serums IgG and IL-4, which are associated with enhancing IgG response, could be significantly increased by SC immunization with the inactivated lysed B. abortus cells (without combining the lysed cells with a suitable adjuvant). Based on reports that some AMPs can function as an adjuvant as well as an antimicrobial agent (26,27), we feel that GI24 might be working as an adjuvant.
The CMI responses, such as TNF-α and IFN-γ, as well as the humoral immune response, are essential for removal of facultative intracellular pathogens such as B. abortus (42–45). Notably, the CMI response mediated by IFN-γ plays a crucial role in the elimination of Brucella species bacteria from the host organs. Therefore, TNF-α and IFN-γ concentrations were analyzed as well as serum IgG titers to B. abortus LPS in goats immunized with GI24-inactivated B. abortus cells. Significantly higher concentrations of TNF-α and IFN-γ were induced in goats immunized with the GI24-lysed B. abortus cells compared with those in the control group. This reflected the high levels of protection induced by GI24-inactivated B. abortus cells. These results revealed that the lysed cells might not act only as a potent vaccine candidate but also have effective adjuvant properties, leading to induction of both humoral and CMI responses (19,46,47). Subcutaneous immunization with the B. abortus vaccine candidate lysed with GI24 provided complete protection against B. abortus infection in 60% of goats. Moreover, the infection index was significantly lower in the group immunized with the GI24-lysed B. abortus cells than in the unimmunized control group. The B. abortus challenge strain was isolated in only 3 tissues [retropharyngeal (except one goat), superficial lymph nodes, and spleen] from 15 organs of 2 B. abortus-infected goats immunized with GI24-lysed B. abortus cells. Furthermore, the number of B. abortus colonies in the 3 tissues from goats vaccinated with the GI24-inactivated B. abortus vaccine candidate was at least 134 times lower (P < 0.05) compared with that in the non-immunized goats. Moreover, a considerable number of the challenge strain bacteria was isolated from the uteri of 3 (60%) of the 5 unvaccinated goats, whereas no isolates were observed in the uteri of the 5 immunized goats. Brucella infection in the reproductive organs could result in abortion of pregnant goats.
The protective efficacy of the B. abortus vaccine candidate lysed with GI24 in goats receiving SC immunization was similar to that observed in mice (16). Therefore, considering its safety and ability to elicit both protective humoral and cellular immune responses in goats, it might be valuable for use in small ruminants with infection risk. Further investigations are in progress, to improve the protective efficacy of lysed B. abortus cells as a vaccine candidate against goat brucellosis by immunization with the combination of lysed B. abortus cells and other potent adjuvants.
The present study concluded that SC immunization with GI24-lysed B. abortus cells induced robust humoral and CMI responses in goats. Moreover, future studies with the combination of the above vaccine strains and other potent adjuvants are essential for establishing better immune responses in small ruminants and in pregnant ruminants.
Acknowledgments
This work was supported by the National Research Foundation of Korea grant funded by the Government of Korea (MISP) (No. 2013R1A4A1069486).
References
- 1.Bano Y, Lone SA. Brucellosis: An economically important infection. J Med Microb Diagn. 2015;4:208. [Google Scholar]
- 2.Leal-Klevezas DS, Martínez-Vázquez IO, García-Cantú J, López-Merino A, Martínez-Soriano JP. Use of polymerase chain reaction to detect Brucella abortus biovar 1 in infected goats. Vet Microbiol. 2000;75:91–97. doi: 10.1016/s0378-1135(00)00200-5. [DOI] [PubMed] [Google Scholar]
- 3.Cutler SJ, Whatmore AM, Commander NJ. Brucellosis — New aspects of an old disease. J Appl Microbiol. 2005;98:1270–1281. doi: 10.1111/j.1365-2672.2005.02622.x. [DOI] [PubMed] [Google Scholar]
- 4.Nymo IH, Tryland M, Godfroid J. A review of Brucella infection in marine mammals, with special emphasis on Brucella pinnipedialis in the hooded seal (Cystophora cristata) Vet Res. 2011;42:93. doi: 10.1186/1297-9716-42-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pappas G, Akritidis N, Bosilkovski M, Tsianos E. Brucellosis. N Engl J Med. 2005;352:2325–2336. doi: 10.1056/NEJMra050570. [DOI] [PubMed] [Google Scholar]
- 6.Corbel MJ. Brucellosis in humans and animals [book on the Internet] Geneva, Switzerland: World Health Organization; c2006. [Last accessed September 30, 2018]. Food and Agriculture Organization of the United Nations, World Health Organization, World Organisation for Animal Health. Available from: http://apps.who.int/iris/bitstream/10665/43597/1/WHO_CDS_EPR_2006.7_eng.pdf. [Google Scholar]
- 7.Franco MP, Mulder M, Gilman RH, Smits HL. Human brucellosis. Lancet Infect Dis. 2007;7:775–786. doi: 10.1016/S1473-3099(07)70286-4. [DOI] [PubMed] [Google Scholar]
- 8.Pappas G, Papadimitriou P, Akritidis N, Christou L, Tsianos EV. The new global map of human brucellosis. Lancet Infect Dis. 2006;6:91–99. doi: 10.1016/S1473-3099(06)70382-6. [DOI] [PubMed] [Google Scholar]
- 9.Alton GG. Brucella melitensis. In: Nielsen K, Duncan JR, editors. Animal Brucellosis. 1st ed. Boston, Massachusetts: CRC Press; 1990. pp. 383–410. [Google Scholar]
- 10.Moreno E, Moriyon I. Brucella melitensis: A nasty bug with hidden credentials for virulence. Proc Natl Acad Sci U S A. 2002;99:1–3. doi: 10.1073/pnas.022622699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Meador VP, Deyoe BL, Cheville NF. Pathogenesis of Brucella abortus infection of the mammary gland and supramammary lymph node of the goat. Vet Pathol. 1989;26:357–368. doi: 10.1177/030098588902600501. [DOI] [PubMed] [Google Scholar]
- 12.Avila-Calderón ED, Lopez-Merino A, Sriranganathan N, Boyle SM, Contreras-Rodríguez A. A history of the development of Brucella vaccines. Biomed Res Int. 2013:743509. doi: 10.1155/2013/743509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Olsen SC. Recent developments in livestock and wildlife brucellosis vaccination. Rev Sci Tech. 2013;32:207–217. doi: 10.20506/rst.32.1.2201. [DOI] [PubMed] [Google Scholar]
- 14.Lin Y, He Y. Ontology representation and analysis of vaccine formulation and administration and their effects on vaccine immune responses. J Biomed Semantics. 2012;3:17. doi: 10.1186/2041-1480-3-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hensel A, Huter V, Katinger A, et al. Intramuscular immunization with genetically inactivated (ghosts) Actinobacillus pleuropneumoniae serotype 9 protects pigs against homologous aerosol challenge and prevents carrier state. Vaccine. 2000;18:2945–2955. doi: 10.1016/s0264-410x(00)00107-9. [DOI] [PubMed] [Google Scholar]
- 16.Kwon AJ, Moon JY, Kim WK, Kim S, Hur J. Protection efficacy of the Brucella abortus ghost vaccine candidate lysed by the N-terminal 24-amino acid fragment (GI24) of the 36-amino acid peptide PMAP-36 (porcine myeloid antimicrobial peptide 36) in murine models. J Vet Med Sci. 2016;78:1541–1548. doi: 10.1292/jvms.16-0036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu J, Li Y, Sun Y, et al. Immune responses and protection induced by Brucella suis S2 bacterial ghosts in mice. Vet Immunol Immunopathol. 2015;166:138–144. doi: 10.1016/j.vetimm.2015.04.008. [DOI] [PubMed] [Google Scholar]
- 18.Won G, Chaudhari AA, Lee JH. Protective efficacy and immune responses by homologous prime-booster immunizations of a novel inactivated Salmonella Gallinarum vaccine candidate. Clin Exp Vaccine Res. 2016;5:148–158. doi: 10.7774/cevr.2016.5.2.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hajam IA, Dar PA, Won G, Lee JH. Bacterial ghosts as adjuvants: Mechanisms and potential. Vet Res. 2017;48:37. doi: 10.1186/s13567-017-0442-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Langemann T, Koller VJ, Muhammad A, et al. The Bacterial Ghost platform system: Production and applications. Bioeng Bugs. 2010;1:326–336. doi: 10.4161/bbug.1.5.12540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Boman HG. Peptide antibiotics and their role in innate immunity. Annu Rev Immunol. 1995;13:61–92. doi: 10.1146/annurev.iy.13.040195.000425. [DOI] [PubMed] [Google Scholar]
- 22.Boman HG. Antibacterial peptides: Basic facts and emerging concepts. J Intern Med. 2003;254:197–215. doi: 10.1046/j.1365-2796.2003.01228.x. [DOI] [PubMed] [Google Scholar]
- 23.Gazit E, Boman A, Boman HG, Shai Y. Interaction of the mammalian antibacterial peptide cecropin P1 with phospholipid vesicles. Biochemistry. 1995;34:11479–11488. doi: 10.1021/bi00036a021. [DOI] [PubMed] [Google Scholar]
- 24.Henzler-Wildman KA, Martinez GV, Brown MF, Ramamoorthy A. Perturbation of the hydrophobic core of lipid bilayers by the human antimicrobial peptide LL-37. Biochemistry. 2004;43:8459–8469. doi: 10.1021/bi036284s. [DOI] [PubMed] [Google Scholar]
- 25.Yang L, Harroun TA, Weiss TM, Ding L, Huang HW. Barrel-stave model or toroidal model? A case study on melittin pores. Biophys J. 2001;81:1475–1485. doi: 10.1016/S0006-3495(01)75802-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Diamond G, Beckloff N, Weinberg A, Kisich KO. The role of antimicrobial peptides in innate host defense. Curr Pharm Des. 2009;15:2377–2392. doi: 10.2174/138161209788682325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.King TP, Jim SY, Wittkowski KM. Inflammatory role of two venom components of yellow jackets (Vespula vulgaris): A mast cell degranulating peptide mastoparan and phospholipase A1. Int Arch Allergy Immunol. 2003;131:25–32. doi: 10.1159/000070431. [DOI] [PubMed] [Google Scholar]
- 28.Storici P, Scocchi M, Tossi A, Gennaro R, Zanetti M. Chemical synthesis and biological activity of a novel antibacterial peptide deduced from a pig myeloid cDNA. FEBS Lett. 1994;337:303–307. doi: 10.1016/0014-5793(94)80214-9. [DOI] [PubMed] [Google Scholar]
- 29.Baek JH, Lee SH. Isolation and molecular cloning of venom peptides from Orancistrocerus drewseni (Hymenoptera: Eumenidae) Toxicon. 2010;55:711–718. doi: 10.1016/j.toxicon.2009.10.023. [DOI] [PubMed] [Google Scholar]
- 30.Lee JJ, Lim JJ, Kim DG, et al. Characterization of culture supernatant proteins from Brucella abortus and its protection effects against murine brucellosis. Comp Immunol Microbiol Infect Dis. 2014;37:221–228. doi: 10.1016/j.cimid.2014.06.001. [DOI] [PubMed] [Google Scholar]
- 31.Lv Y, Wang J, Gao H, et al. Antimicrobial properties and membrane-active mechanism of a potential α-helical antimicrobial derived from cathelicidin PMAP-36. PLoS One. 2014;9:e86364. doi: 10.1371/journal.pone.0086364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pedersen K, Quance CR, Robbe-Austerman S, et al. Identification of Brucella suis from feral swine in selected states in the USA. J Wildl Dis. 2014;50:171–179. doi: 10.7589/2013-09-235. [DOI] [PubMed] [Google Scholar]
- 33.Palmer MV, Cheville NF. Effects of oral or intravenous inoculation with Brucella abortus strain RB51 vaccine in beagles. Am J Vet Res. 1997;58:851–856. [PubMed] [Google Scholar]
- 34.Surendran N, Sriranganathan N, Boyle SM, et al. Protection to respiratory challenge of Brucella abortus strain 2308 in the lung. Vaccine. 2013;31:4103–4110. doi: 10.1016/j.vaccine.2013.06.078. [DOI] [PubMed] [Google Scholar]
- 35.Tabynov K, Yespembetov B, Matikhan N, et al. First evaluation of an influenza viral vector based Brucella abortus vaccine in sheep and goats: Assessment of safety, immunogenicity and protective efficacy against Brucella melitensis infection. Vet Microbiol. 2016;197:15–20. doi: 10.1016/j.vetmic.2016.11.001. [DOI] [PubMed] [Google Scholar]
- 36.Bricker BJ, Halling SM. Differentiation of Brucella abortus bv. 1, 2, and 4, Brucella melitensis, Brucella ovis, and Brucella suis bv. 1 by PCR. J Clin Microbiol. 1994;32:2660–2666. doi: 10.1128/jcm.32.11.2660-2666.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bricker BJ, Halling SM. Enhancement of the Brucella AMOS PCR assay for differentiation of Brucella abortus vaccine strains S19 and RB51. J Clin Microbiol. 1995;33:1640–1642. doi: 10.1128/jcm.33.6.1640-1642.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Adone R, Francia M, Pistoia C, et al. Protective role of antibodies induced by Brucella melitensis B115 against B. melitensis and Brucella abortus infections in mice. Vaccine. 2012;30:3992–3995. doi: 10.1016/j.vaccine.2012.04.009. [DOI] [PubMed] [Google Scholar]
- 39.Brumme S, Arnold T, Sigmarsson H, et al. Impact of Salmonella Typhimurium DT104 virulence factors invC and sseD on the onset, clinical course, colonization patterns and immune response of porcine salmonellosis. Vet Microbiol. 2007;124:274–285. doi: 10.1016/j.vetmic.2007.04.032. [DOI] [PubMed] [Google Scholar]
- 40.Roesler U, Heller P, Waldmann KH, Truyen U, Hensel A. Immnunization of sows in an integrated pig-breeding herd using a homologous inactivated Salmonella vaccine decreases the prevalence of Salmonella typhimurium infection in the offspring. J Vet Med B Infect Dis Vet Public Health. 2006;53:224–228. doi: 10.1111/j.1439-0450.2006.00951.x. [DOI] [PubMed] [Google Scholar]
- 41.Mastroeni P, Chabalgoity JA, Dunstan SJ, Maskell DJ, Dougan G. Salmonella: Immune responses and vaccines. Vet J. 2001;161:132–164. doi: 10.1053/tvjl.2000.0502. [DOI] [PubMed] [Google Scholar]
- 42.Baldwin CL, Goenka R. Host immune responses to the intracellular bacteria Brucella: Does the bacteria instruct the host to facilitate chronic infection? Crit Rev lmmunol. 2006;26:407–442. doi: 10.1615/critrevimmunol.v26.i5.30. [DOI] [PubMed] [Google Scholar]
- 43.Jiang X, Baldwin CL. Effects of cytokines on intracellular growth of Brucella abortus. Infect Immun. 1993;61:124–134. doi: 10.1128/iai.61.1.124-134.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Oliveira SC, Splitter GA. CD8+ type 1 CD44hi CD45 RBlo T lymphocytes control intracellular Brucella abortus infection as demonstrated in major histocompatibility complex class I- and class II-deficient mice. Eur J Immunol. 1995;25:2551–2557. doi: 10.1002/eji.1830250922. [DOI] [PubMed] [Google Scholar]
- 45.Zhan Y, Liu Z, Cheers C. Tumor necrosis factor alpha and interleukin-12 contribute to resistance to the intracellular bacterium Brucella abortus by different mechanisms. Infect Immun. 1996;64:2782–2786. doi: 10.1128/iai.64.7.2782-2786.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chaudhari AA, Jawale CV, Kim SW, Lee JH. Construction of a Salmonella Gallinarum ghost as a novel inactivated vaccine candidate and its protective efficacy against fowl typhoid in chickens. Vet Res. 2012;43:44. doi: 10.1186/1297-9716-43-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Eko FO, Lubitz W, McMillan L, et al. Recombinant Vibrio cholerae ghosts as a delivery vehicle for vaccinating against Chlamydia trachomatis. Vaccine. 2003;21:1694–1703. doi: 10.1016/s0264-410x(02)00677-1. [DOI] [PubMed] [Google Scholar]