Abstract
Background
Mannose-binding lectin (MBL) plays an important role in the innate immune response by activating the complement system via the lectin pathway, and it has been studied in several viral infections; however, the influence of MBL in PLWHA infected with HHV-8 is unknown. The objective of this study was to verify the association of MBL deficient plasma concentrations in HIV/HHV-8 coinfected and HIV monoinfected patients and to correlate these concentrations with HIV viral load and CD4 counts in both groups.
Results
This was an analytical study of case-controls consisting of PLWHA monitored at the medical outpatient of Infectious and Parasitic Diseases of the clinical hospital in the Federal University of Pernambuco. Plasma concentrations of MBL were obtained by an enzyme-linked immunosorbent assay (ELISA) using a commercial Human Mannose Binding Lectin kit (MyBioSource, Inc.) that was performed according to the manufacturer’s guidelines, with values < 100 ng/ml considered deficient. A total of 245 PLWHA samples were analysed; 118 were HIV/HHV-8 coinfected and 127 were HIV monoinfected; 5.1% (6/118) of the coinfected patients and 3.2% (4/127) of the monoinfected patients (p = 0.445) were considered plasma concentration deficient. The median of the plasma concentrations of MBL in the coinfected patients was 2803 log10 ng/ml and was 2.959 log10 ng/ml in the monoinfected patients (p = 0.001). There was an inverse correlation between the plasma concentrations of MBL and the HIV viral load in both groups, but no correlation with the CD4 count.
Conclusions
Although the plasma concentrations considered deficient in MBL were not associated with HHV-8 infection in PLWHA, the coinfected patients showed lower MBL concentrations and an inverse correlation with HIV viral load, suggesting that there may be consumption and reduction of MBL due to opsonization of HIV and HHV-8, leading to the reduction of plasma MBL and non-accumulation in the circulation.
Keywords: HIV/HHV8 coinfection, MBL, Deficient concentrations, Human herpesvirus 8
Background
The incidence of Kaposi’s sarcoma (KS) associated with human herpesvirus 8 (HHV-8) has increased in people living with HIV/AIDS (PLWHA), with a more aggressive clinical course and progression to death [1–6]. KS is one of the most common cancers in PLWHA, even when the individuals are treated with antiretroviral therapy (ART) and have an undetectable HIV viral load and CD4 + T cell counts that are above 350 cells/mm3 [7–9].
The control of HHV-8, just as in the early stages of the development of KS, is mediated by the innate and adaptive immunity [10–12]. In this context, mannose-binding lectin (MBL) plays a key role in innate immunity as a standard recognition receptor, binding with a high affinity to the residue patterns of carbohydrates present on the surface of viruses or virus-infected cells, especially when the humoral immunity is not fully functional, such as in childhood or in immunosuppressed or immunocompromised populations [13–15]. Thus, MBL contributes to the defence of the innate immune system by initiating the activation of the lectin-complement pathway, which promotes opsonophagocytosis, modulates inflammation and induces cellular lysis [16–18].
Regarding PLWHA, some studies have shown an association between MBL plasma concentration deficiency and HIV infection or a more rapid disease progression [19–23], and others found no association with this infection, the HIV viral load or the CD4 count [18, 24]. However, the influence of MBL on HHV-8 infection is not known, but for other herpes viruses, studies suggest that MBL deficiency may be a risk factor for the symptomatic development of human herpesvirus 2 (HHV-2) [25] and for cytomegalovirus reactivation (CMV) [14, 26]. Although MBL plays an important role in the innate immune response, it is still unknown whether a deficiency in the MBL plasma concentration is associated with HHV-8 infection in PLWHA. In view of the above, the objective of this study was to verify the association of MBL deficient plasma concentrations in HIV/HHV-8 coinfected and HIV monoinfected patients and correlate the concentration with the HIV viral load and CD4 counts in both groups.
Results
We analysed 245 samples from PLWHA, including 118 HIV/HV-8 coinfected patients, with a mean age of 42.5 (± 11.8), and 127 patients monoinfected with HIV, with mean age of 42.8 (± 10.6), and the majority of these patients were males 71% (84/118) and 59% (75/127), respectively. The median TCD4 count was 2.713 log10 cells/mm3 (1.301–3.224) in the coinfected patients and 2.736 log10 cells /mm3 (1.176–3.269) in the monoinfected patients (p = 0.872). The HIV viral load was detectable in 42% (50/118) of the coinfected patients and in 44% (56/127) in the monoinfected patients, with a median of 2.057 log10 copies/ml (1.672–5.539) and 2.363 log10 copies/ml (1.602–6.273), respectively, with p = 0.595.
The plasma concentrations were considered deficient in 5.1% (6/118) of the coinfected patients and in 3.2% (4/127) of the monoinfected patients, with a p value of 0.445 (OR = 1.647; IC = 0.453–5.989). However, the MBL plasma concentration median were significantly lower in the coinfected patients, as shown in Fig. 1.
Fig. 1.
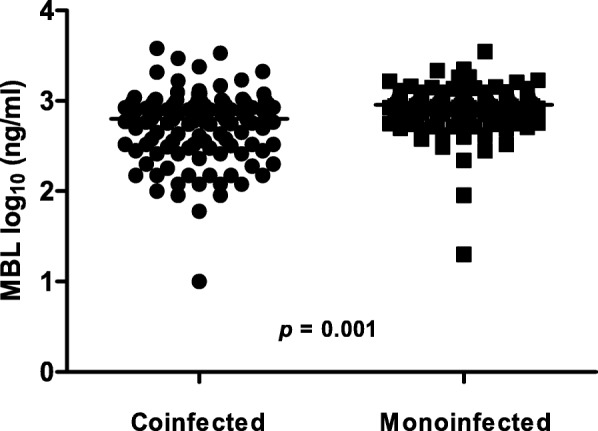
Distribution and median of MBL plasma concentrations in HIV/HHV8 coinfected and HIV monoinfected patients. The median of the coinfected patients was 2.803 log10 ng/ml (1.0–3.581; CI: 2.650–2.792), and in the monoinfected, it was 2.959 log10 ng/ml (1.301–3.545; CI: 2.877–2.964), which was statistically significant (p = 0.001) using the Mann-Whitney test
Table 1 shows the median MBL plasma concentrations according to the clinical variables in the coinfected and monoinfected patients.
Table 1.
Median plasma concentrations of MBL according to sex, age, HIV viral load and CD4 count in HIV/HHV-8 coinfected and HIV monoinfected patients
| Median of MBL (log10 ng/ml) (range)1 | p-value2 | ||||
|---|---|---|---|---|---|
| Variables | Coinfected | N3 | Monoinfected | N3 | |
| Sex | |||||
| Male | 2.806 (1.778–3.581) | 84 | 2.968 (2.342–3.348) | 75 | 0.000 |
| Female | 2.778 (1.000–3.378) | 34 | 2.947 (1.301–3.545) | 52 | 0.007 |
| Age | |||||
| ≥ 40 | 2.806 (1.000–3.581) | 68 | 2.949 (1.301–3.545) | 78 | 0.005 |
| < 40 | 2.796 (1.954–3.471) | 50 | 2.987 (1.954–3.348) | 49 | 0.000 |
| HIV viral load | |||||
| Detectable | 2.752 (1.000–3.318) | 50 | 2.949 (1.301–3.545) | 56 | 0.001 |
| Undetectable | 2.874 (1.778–3.581) | 68 | 2.961 (1.954–3.348) | 71 | 0.377 |
| CD4 counts | |||||
| ≥ 200 | 2.823 (1.778–3.581) | 98 | 2.964 (1.954–3.545) | 115 | 0.001 |
| < 200 | 2.586 (1.000–3.167) | 20 | 2.926 (1.301–3.093) | 12 | 0.034 |
1Minimum and maximum values are referenced in bracket; 2 p-value for the Mann-Whitney test; 3 N = sample size
Among the coinfected and monoinfected patients, there was a negative correlation between the HIV viral load and the MBL plasma concentration, as shown in Fig. 2.
Fig. 2.
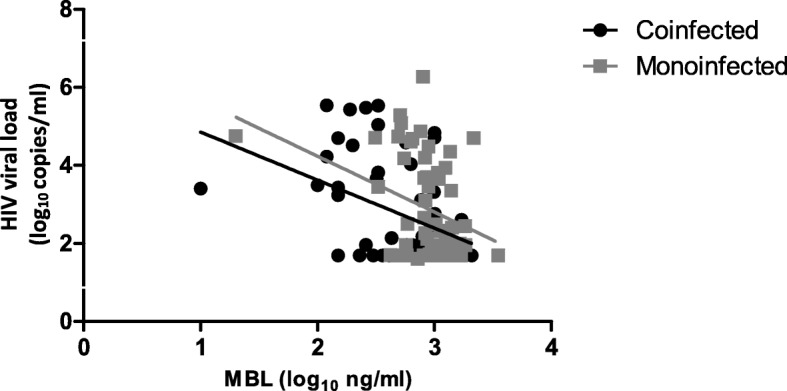
Spearman correlation between HIV viral load and MBL plasma concentration in HIV/HHV-8 coinfected (n = 50; p = 0.018; r = − 0.333) and HIV monoinfected patients (n = 56; p = 0.031; r = − 0.289)
Figure 3 shows the Spearman correlation between the CD4 count and the MBL plasma concentration in the HIV/HHV-8 coinfected and HIV monoinfected patients.
Fig. 3.
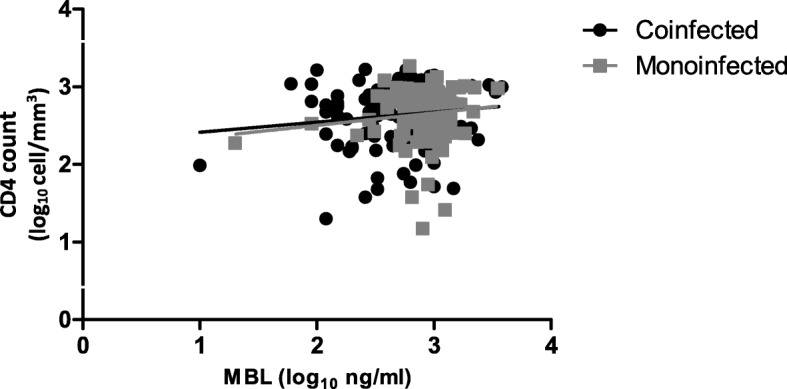
Spearman correlation between the CD4 counts and MBL plasma concentration in the HIV/HHV-8 coinfected (n = 118; p = 0.346; r = 0.087) and HIV monoinfected patients (n = 127; p = 0.132; r = − 0.134)
Discussion
MBL plays an important role in the innate immune response by activating the complement system via the lectin pathway in an antibody-independent mechanism [15, 17, 23]. This protein has been studied in several infections [26–30]; however, this is the first study to evaluate the plasma concentrations of functional MBL in PLWHA coinfected with HHV-8 and correlate the concentrations with HIV viral load and CD4 counts.
The study population did not differ in age and sex when compared to other studies evaluating PLWHA infected with HHV-8 [4, 8, 31–33]. Similarly, the median HIV viral load and TCD4 count did not show a statistically significant difference between the HIV/HHV-8 coinfected and the HIV monoinfected patients, corroborating works that also evaluated these clinical characteristics [31, 32, 34].
It is still unknown how MBL aids in the control or elimination of HHV-8 infection; however, in relation to HIV infection, studies show that MBL binds to HIV gp120 glycoprotein, helping to clear this virus through the activation of the complement system [22, 35–38]. In addition, when MBL is bound to HIV it can also be eliminated from the circulation by the C1q receptor, which has a structural and functional affinity for MBL [30, 39, 40], suggesting the consumption and reduction of MBL during HIV infection [26, 30].
In relation to the Herpesviridae family there are few studies performed. The data of a research show that MBL modulates the response to HSV-2 in mice by affecting neutralization of the virus. Furthermore, reported that the frequency of the MBL deficient (< 100 ng/ml) was higher in the symptomatic group (people with recurrent HSV-2 infections) [25]. In other research the MBL deficient levels were linked to CMV reactivation after lung transplantation [26]. These authors suggest that lack of MBL mediated complement activation increases susceptibility to infections by these viruses.
Studies use different cut-off points, including < 100, < 300 and < 500 ng/ml, to define MBL deficient plasma concentrations [14, 18, 25, 26, 30]. In our study, we defined deficient concentrations as < 100 ng/ml and found no association with HHV-8 infection in PLWHA, the HIV viral load or the CD4 count; however, the median of these concentrations was significantly lower in the HIV/HHV-8 coinfected patients compared to the HIV monoinfected patients. However, the median concentrations found in both groups were higher than the values considered deficient by these authors [14, 18, 19, 25, 26, 30]. A possible explanation for the lower concentrations of MBL in coinfected patients would be the consumption and reduction of this protein, involving the opsonization of HIV and HHV-8, leading to the reduction of plasma MBL and its non-accumulation in the circulation [26].
On the other hand, considering the cut-off point established by Manuel et al. (2007) to characterize the deficient plasma concentration of MBL (< 500 ng/ml), in our study, four coinfected patients who developed KS showed a deficient MBL concentration 2.519 log10 ng/ml (data not shown). These same four patients were genetically characterized for the polymorphisms − 550, − 221 and exon 1 of the MBL2 gene in the research conducted by Morais et al. (2018), the authors reported that these coinfected were characterized as intermediate expression haplotypes for the production of the MBL protein, being three HYA/LXA and one LYA/LYO [41]. Because of these results, studies related to MBL plasma concentrations and MBL2 gene polymorphisms become essential and may help to understand the role of MBL in the development of KS in HIV/HHV-8 coinfected patients.
In relation to CD4, the counts above or below 200 cells/mm3 did not influence the MBL plasma concentrations, because the medians remained smaller in the coinfected patients in relation to the monoinfected patients. However, considering the use of a continuous numerical scale, it was possible to establish an inverse correlation between the plasma MBL concentrations and the HIV viral load in the coinfected and monoinfected patients, suggesting that MBL plasma concentrations might also modulate coinfection.
Other factors may influence the MBL concentration, such as polymorphisms in the MBL2 gene, resulting in defects in the polymerization of the molecule, leading to functional deficiency and protein expression and reducing the activation capacity of the complement system [42–44]. Furthermore, the drugs used in the treatment of infections, such as those of ART [45–47], are metabolized in the liver and may affect the concentration of MBL because this protein is mainly synthesized in the liver [26].
Our research has some limitations, such as the absence of investigation of the HHV-8 latent or latent cycle, quantify of the HHV-8 viral load, evaluate functionality of MBL by measuring the activity of MBL in the complement system through C4b binding or measure aspartate aminotransferase (AST) and alanine aminotransferase (ALT) enzyme levels in the blood as a measure of liver toxicity. We suggest that future prospective studies evaluate these variables by associating or correlating them with plasma MBL concentrations in HIV/HHV-8 coinfected patients, especially in those who developed KS.
Conclusion
Therefore, although the plasma concentrations considered deficient of MBL were not associated with HHV-8 infection in PLWHA, the coinfected patients had lower concentrations of MBL and an inverse correlation with the HIV viral load, suggesting that MBL might also be modulating HIV/HHV-8 coinfection.
Methods
The aim of this study was to verify the association of MBL deficient plasma concentrations in HIV/HHV-8 coinfected and HIV monoinfected patients and correlate the concentration with the HIV viral load and CD4 counts in both groups.
Design and study population
This was an analytical study of case-controls, consisting of PLWHA that were monitored at the medical outpatient of Infectious and Parasitic Diseases of the clinical hospital in the Federal University of Pernambuco. Controls were defined as individuals with serological diagnosis of HIV infection, established according to Administrative Rule N°. 151, of October 14, 2009 (BRASIL, 2009), being characterized as HIV monoinfected. The cases were defined as PLHA with the serological diagnosis of HHV-8 infection detected by in-house enzyme-linked immunosorbent assay (ELISA) produced by Brazilian laboratory (Virology Unit of the Institute of Tropical Medicine of the University of São Paulo). Antibodies were identified against structural and non-structural proteins of HHV-8, being characterized as HIV/HHV-8 coinfected, according to Cahú et al. (2016). We excluded PLWHA infected by HBV, HCV, HTLV I/II and those not under ART¸ according to Fig. 4. The study was approved by the Research Ethics Committee of the Federal University of Pernambuco (number: 22428813.5.0000.5208) and all patients gave written consent to participate in the research, in accordance with the ethical, consent and permission rules.
Fig. 4.
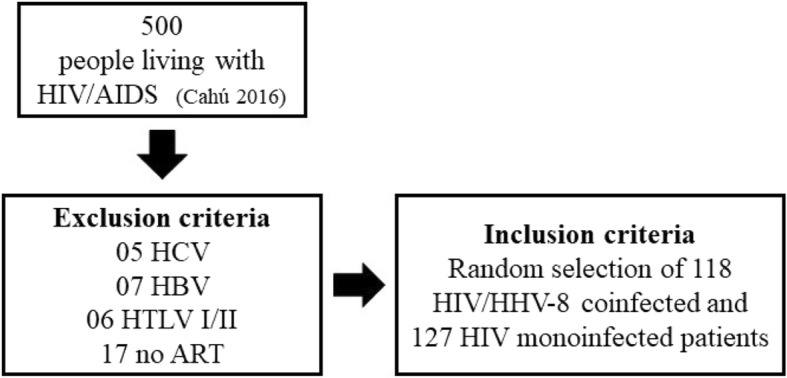
Participation flow diagram along with inclusion and exclusion criteria used in the research
MBL plasma concentrations
The plasma concentrations of MBL were obtained by an enzyme-linked immunosorbent assay (ELISA) using a commercial Human Mannose Binding Lectin kit (MyBioSource, Inc.), with a detection threshold of 0.05 ng/ml. The samples were diluted at 1:100, and the protocol for the ELISA was performed following the manufacturer’s guidelines. The readings were performed on a spectrophotometer (Thermoplate®) with a wavelength of 450/630 nm. The plasma concentrations were considered deficient when they were < 100 ng/ml [18, 19, 25].
Statistical analysis
To evaluate the deficiency of the concentrations, we used the odds ratios (ORs) and 95% confidence intervals (CIs), and the Mann-Whitney test was used to associate the MBL plasma concentrations in the HIV/HHV-8 coinfected and HIV monoinfected patients. The Spearman test was used to correlate the plasma MBL concentrations with HIV viral load and CD4 count, and these variables were included in the statistical models as units transformed into log10. For the statistical analyses and the construction of the graphs, we used GraphPad Prism software version 6.1 (GraphPad Software, USA) and Epi Info version 7.1.5 (CDC, Atlanta, GA, USA). Statistically significant values were indicated by p < 0.05.
Acknowledgments
The authors thank all patients and technical support from the Virology Laboratory of the Tropical Medicine Institute (LIM-52-IMT) of the University of São Paulo for the realization of HHV-8 serology. The Fundação de Amparo à Ciência e Tecnologia de Pernambuco (FACEPE) by the support to Viviane Martha Santos de Morais, according BCT-0187-4.01/18.
Funding
The authors received no specific funding for this work.
Availability of data and materials
The datasets used and/or analyzed during the current study available from the corresponding author upon reasonable request.
Abbreviations
- ART
Antiretroviral therapy
- CIs
Confidence intervals
- CMV
Cytomegalovirus reactivation
- ELISA
Enzyme-linked immunosorbent assay
- HBV
Hepatitis B virus
- HCV
Hepatitis C virus
- HHV-2
Human herpesvirus 2
- HHV-8
Human herpesvirus 8
- HTLV
Human T-cell leukemia-lymphoma virus
- KS
Kaposi’s sarcoma
- MBL
Mannose-binding lectin
- PLWHA
People living with HIV/AIDS
Authors’ contributions
VMSM: designed the study, did the experimental work, analyzed the data statistically, interpretation the data and drafted the manuscript. JPG, GGOMC, TRTM: collected the samples, acquired data or revised the manuscript. MRCDC: obtained the funding and revised the manuscript critically for important intellectual content. All the authors read and approved the final manuscript.
Ethics approval and consent to participate
The study was approved by the Research Ethics Committee of the Federal University of Pernambuco (number: 22428813.5.0000.5208). We declare that this study involved human patients and all patients gave consent to participate in the research, in accordance with the ethical, consent and permission rules of the Research Ethics Committee of the Federal University of Pernambuco.
Consent for publication
This study obtained the consent of the participant to publish the refferent data to each patient.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Viviane Martha Santos de Morais, Email: vivi.martha@hotmail.com.
Juliana Prado Gonçales, Email: julianapgoncales@gmail.com.
Georgea Gertrudes de Oliveira Mendes Cahú, Email: cahu.georgea@gmail.com.
Tania Regina Tozetto-Mendoza, Email: tozetto@usp.br.
Maria Rosângela Cunha Duarte Coêlho, Email: rcoelholika@gmail.com.
References
- 1.Mohanna S, Ferrufino JC, Sanchez J, Bravo F, Gotuzzo E. Epidemiological and clinical characteristics of classic Kaposi’s sarcoma in Peru. J Am Acad Dermatol. 2005;53:435–441. doi: 10.1016/j.jaad.2005.05.041. [DOI] [PubMed] [Google Scholar]
- 2.Sullivan RJ, Pantanowitz L, Casper C, Stebbing J, Dezube BJ. HIV/AIDS: epidemiology, pathophysiology, and treatment of Kaposi sarcoma-associated herpesvirus disease: Kaposi sarcoma, primary effusion lymphoma, and multicentric Castleman disease. Clin Infect Dis. 2008;47:1209–1215. doi: 10.1086/592298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Paoli PDE, Carbone A. Kaposi's sarcoma herpesvirus : twenty years after its discovery. Eur Rev Med Pharmacol Sci. 2016;20:1288–1294. [PubMed] [Google Scholar]
- 4.Cahú GG de OM, Morais VMS, Lopes TRR, da Silva DM, Tozetto-Mendoza TR, Pannuti CS, et al. Prevalence of human herpesvirus 8 infection in people living with HIV/AIDS in Pernambuco, Brazil. J Med Virol. 2016;88:2016–2020. doi: 10.1002/jmv.24550. [DOI] [PubMed] [Google Scholar]
- 5.Rohner E, Wyss N, Trelle S, Mbulaiteye SM, Egger M, Novak U, et al. HHV-8 seroprevalence : a global view. Syst Rev. 2014;3:1–7. doi: 10.1186/2046-4053-3-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dittmer DP, Damania B. Kaposi sarcoma – associated herpesvirus : immunobiology , oncogenesis , and therapy. 2016;126:3165–3175. [DOI] [PMC free article] [PubMed]
- 7.Pria AD, Hayward K, Bower M. Do we still need chemotherapy for AIDS-associated Kaposi’s sarcoma? Expert Rev Anticancer Ther. 2013;13:203–209. doi: 10.1586/era.12.179. [DOI] [PubMed] [Google Scholar]
- 8.Broccolo F, Din CT, Viganò MG, Rutigliano T, Esposito S, Lusso P, et al. HHV-8 DNA replication correlates with the clinical status in AIDS-related Kaposi’s sarcoma. J Clin Virol. 2016;78:47–52. doi: 10.1016/j.jcv.2016.02.019. [DOI] [PubMed] [Google Scholar]
- 9.Munawwar A, Singh S. Human herpesviruses as copathogens of HIV infection, their role in HIV transmission, and disease progression. J Lab Physicians. 2016;8:5. doi: 10.4103/0974-2727.176228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Aresté C, Blackbourn DJ. Modulation of the immune system by Kaposi’s sarcoma-associated herpesvirus. Trends Microbiol. 2009;17:119–129. doi: 10.1016/j.tim.2008.12.001. [DOI] [PubMed] [Google Scholar]
- 11.Lee HR, Brulois K, Wong LY, Jung JU. Modulation of immune system by Kaposi’s sarcoma-associated herpesvirus: lessons from viral evasion strategies. Front Microbiol. 2012;3:1–14. doi: 10.3389/fmicb.2012.00044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brulois K, Jung JU. Interplay between Kaposi’s sarcoma-associated herpesvirus and the innate immune system. Cytokine Growth Factor Rev. 2014;25:597–609. doi: 10.1016/j.cytogfr.2014.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fujita T. Evolution of the lectin-complement pathway and its role in innate immunity. Nat Rev Immunol. 2002;2:346–353. doi: 10.1038/nri800. [DOI] [PubMed] [Google Scholar]
- 14.Manuel O, Pascual M, Trendelenburg M, Meylan PR. Association between mannose-binding lectin deficiency and cytomegalovirus infection after kidney transplantation. Transplantation. 2007;83:359–362. doi: 10.1097/01.tp.0000251721.90688.c2. [DOI] [PubMed] [Google Scholar]
- 15.Mason CP, Tarr AW. Human lectins and their roles in viral infections. Molecules. 2015;20:2229–2271. doi: 10.3390/molecules20022229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Auriti C, Prencipe G, Moriondo M, Bersani I, Bertaina C, Mondì V, et al. Mannose-binding lectin: biologic characteristics and role in the susceptibility to infections and ischemia-reperfusion related injury in critically ill neonates. J Immunol Res. 2017. [DOI] [PMC free article] [PubMed]
- 17.Martin M, Blom AM. Complement in removal of the dead - balancing inflammation. Immunol Rev. 2016;274:218–232. doi: 10.1111/imr.12462. [DOI] [PubMed] [Google Scholar]
- 18.Zinyama-Gutsire RBL, Chasela C, Kallestrup P, Rusakaniko S, Christiansen M, Ngara B, et al. HIV-1 disease progression and survival in an adult population in Zimbabwe: is there an effect of the mannose binding lectin deficiency? Omi A J Integr Biol. 2015;19:542–552. doi: 10.1089/omi.2015.0047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Egli A, Schäfer J, Osthoff M, Thiel S, Mikkelsen C, Rauch A, et al. Low levels of Mannan-binding lectin or Ficolins are not associated with an increased risk of cytomegalovirus disease in HIV-infected patients. PLoS One. 2013;8. [DOI] [PMC free article] [PubMed]
- 20.Hundt M, Heiken H, Schmidt RE. Low mannose-binding lectin serum concentrations in HIV long-term Nonprogressors? AIDS Res Hum Retrovir. 2000;16:1927. doi: 10.1089/08892220050195892. [DOI] [PubMed] [Google Scholar]
- 21.Tan Y, Liu L, Luo P, Wang A, Jia T, Shen X, et al. Association between mannose-binding lectin and HIV infection and progression in a Chinese population. Mol Immunol. 2009;47:632–638. doi: 10.1016/j.molimm.2009.08.020. [DOI] [PubMed] [Google Scholar]
- 22.Teodorof C, Divakar S, Soontornniyomkij B, Achim CL, Kaul M, Singh KK. Intracellular mannose binding lectin mediates subcellular trafficking of HIV-1 gp120 in neurons. Neurobiol Dis. 2014;69:54–64. doi: 10.1016/j.nbd.2014.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vallinoto AC, Muto NA, Alves AE, Machado LF, Azevedo VN, Souza LL, et al. Characterization of polymorphisms in the mannose-binding lectin gene promoter among human immunodeficiency virus 1 infected subjects. Mem Inst Oswaldo Cruz. 2008;103:645–649. doi: 10.1590/S0074-02762008000700003. [DOI] [PubMed] [Google Scholar]
- 24.Catano G, Agan BK, Kulkarni H, Telles V, Marconi VC, Dolan MJ, et al. Independent effects of genetic variations in mannose-binding lectin influence the course of HIV disease: the advantage of heterozygosity for coding mutations. J Infect Dis. 2008;198:72–80. doi: 10.1086/588712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gadjeva M, Paludan SR, Thiel S, Slavov V, Ruseva M, Eriksson K, et al. Mannan-binding lectin modulates the response to HSV-2 infection. Clin Exp Immunol. 2004;138:304–311. doi: 10.1111/j.1365-2249.2004.02616.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kwakkel-van Erp JM, Paantjens AWM, van Kessel DA, Grutters JC, van den Bosch JMM, van de Graaf EA, et al. Mannose-binding lectin deficiency linked to cytomegalovirus (CMV) reactivation and survival in lung transplantation. Clin Exp Immunol. 2011;165:410–416. doi: 10.1111/j.1365-2249.2011.04436.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Figueiredo GG, Cezar RD, Freire NM, Teixeira VG, Baptista P, Cordeiro M, et al. Mannose-binding lectin gene (MBL2) polymorphisms related to the mannose-binding lectin low levels are associated to dengue disease severity. Hum Immunol American Society for Histocompatibility and Immunogenetics. 2016;77:571–575. doi: 10.1016/j.humimm.2016.05.006. [DOI] [PubMed] [Google Scholar]
- 28.Guimaraes V, Guimaraes R, Brandao L, Baldez da Silva MFPT, Milanese M, Segat L, et al. Association between MBL2 gene functional polymorphisms and high-risk human papillomavirus infection in Brazilian women. Hum Immunol. 2008;69:273–278. doi: 10.1016/j.humimm.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 29.Rashidi E, Fazlollahi MR, Zahedifard S, Talebzadeh A, Kazemnejad A, Saghafi S, et al. Mannose-binding lectin deficiency in patients with a history of recurrent infections. Iran J allergy, Asthma Immunol. 2016;15:69–74. [PubMed] [Google Scholar]
- 30.Zinyama-Gutsire RBL, Chasela C, Madsen HO, Rusakaniko S, Kallestrup P, Christiansen M, et al. Role of mannose-binding lectin deficiency in HIV-1 and schistosoma infections in a rural adult population in Zimbabwe. PLoS One. 2015;10:1–23. doi: 10.1371/journal.pone.0122659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Batista MD, Ferreira S, Sauer MM, Tomiyama H, Giret MTM, Pannuti CS, et al. High human herpesvirus 8 (HHV-8) prevalence, clinical correlates and high incidence among recently HIV-1-infected subjects in Sao Paulo, Brazil. PLoS One. 2009;4:2–6. doi: 10.1371/journal.pone.0005613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hesamizadeh K, Keyvani H, Bokharaei-Salim F, Monavari SH, Esghaei M, Jahanbakhsh Sefidi F. Molecular epidemiology of Kaposi?S sarcoma-associated herpes virus, and risk factors in HIV-infected patients in Tehran, 2014. Iran Red Crescent Med J. 2016;18:e32603. doi: 10.5812/ircmj.32603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Begré L, Rohner E, Mbulaiteye SM, Egger M, Bohlius J. Is human herpesvirus 8 infection more common in men than in women? Systematic review and meta-analysis. Int J Cancer. 2016;139:776–783. doi: 10.1002/ijc.30129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tozetto-Mendoza TR, Ibrahim KY, Tateno AF, de Oliveira CM, Sumita LM, Sanchez MCA, et al. Genotypic distribution of HHV-8 in AIDS individuals without and with Kaposi sarcoma. Medicine. 2016;95:e5291. doi: 10.1097/MD.0000000000005291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nielsen SL, Andersen PL, Koch C, Jensenius JC, Thiel S. The level of the serum opsonin, mannan-binding protein in HIV-1 antibody-positive patients. Clin Exp Immunol Wiley-Blackwell. 1995;100:219–222. doi: 10.1111/j.1365-2249.1995.tb03656.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ying H, Ji X, Hart ML, Gupta K, Saifuddin M, Zariffard MR, et al. Interaction of mannose-binding lectin with HIV type 1 is sufficient for virus opsonization but not neutralization. AIDS Res Hum Retrovir. 2004;20:327–335. doi: 10.1089/088922204322996563. [DOI] [PubMed] [Google Scholar]
- 37.Botos I, Wlodawer A. Proteins that bind high-mannose sugars of the HIV envelope. Prog Biophys Mol Biol. 2005;88:233–282. doi: 10.1016/j.pbiomolbio.2004.05.001. [DOI] [PubMed] [Google Scholar]
- 38.da Silva GK, Guimarães R, Mattevi VS, Lazzaretti RK, Sprinz E, Kuhmmer R, et al. The role of mannose-binding lectin gene polymorphisms in susceptibility to HIV-1 infection in southern Brazilian patients. AIDS. 2011;25:411–418. doi: 10.1097/QAD.0b013e328342fef1. [DOI] [PubMed] [Google Scholar]
- 39.Haurum JS, Thiel S, Jones IM, Fischer PB, Laursen SB, Jensenius JC. Complement activation upon binding of mannan-binding protein to HIV envelope glycoproteins. AIDS. 1993;7:1307–1313. doi: 10.1097/00002030-199310000-00002. [DOI] [PubMed] [Google Scholar]
- 40.Malhotra R, Sim RB, Reid KBM. Interaction of C1q, and other proteins containing collagen-like domains, with the C1q receptor. Biochem Soc Trans. 1990;18. [DOI] [PubMed]
- 41.de Morais VMS, Lima ELS, Cahú GGOM, Lopes TRR, Gonçales JP, et al. MBL2 gene polymorphisms in HHV-8 infection in people living with HIV/AIDS. Retrovirology. 2018;15(75):01–09. doi: 10.1186/s12977-018-0456-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Li H, Fu WP, Hong ZH. Replication study in Chinese Han population and meta-analysis supports association between the MBL2 gene polymorphism and HIV-1 infection. Infect Genet Evol Elsevier BV. 2013;20:163–170. doi: 10.1016/j.meegid.2013.09.010. [DOI] [PubMed] [Google Scholar]
- 43.Eddie WK, Kazue I, Ezekowitz TRA, Stuart LM, Ip WKE, Takahashi K, et al. Mannose-binding lectin and innate immunity. Immunol Rev. 2009;230:9–21. doi: 10.1111/j.1600-065X.2009.00789.x. [DOI] [PubMed] [Google Scholar]
- 44.Halla MC, do Carmo RF, Silva Vasconcelos LR, Pereira LB, Moura P, de Siqueira ERF, et al. Association of hepatitis C virus infection and liver fibrosis severity with the variants alleles of MBL2 gene in a Brazilian population. Hum Immunol. 2010;71:883–887. doi: 10.1016/j.humimm.2010.05.021. [DOI] [PubMed] [Google Scholar]
- 45.Araújo-Mariz C, Lopes EP, Acioli-Santos B, Maruza M, Montarroyos UR, De Ximenes RAA, et al. Hepatotoxicity during treatment for tuberculosis in people living with HIV/AIDS. PLoS One. 2016;11:1–15. doi: 10.1371/journal.pone.0157725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sonderup MW, Wainwright HC. Human immunodeficiency virus infection, antiretroviral therapy, and liver pathology. Gastroenterol Clin N Am. 2017;46:327–343. doi: 10.1016/j.gtc.2017.01.007. [DOI] [PubMed] [Google Scholar]
- 47.Solomon IH, De Girolami U, Chettimada S, Misra V, Singer EJ, Gabuzda D. Brain and liver pathology, amyloid deposition, and interferon responses among older HIV-positive patients in the late HAART era. BMC Infect Dis. 2017;17:151. doi: 10.1186/s12879-017-2246-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study available from the corresponding author upon reasonable request.


