Abstract
Background
Insight into the factors that regulate the circadian host-seeking flight activity of Culicoides vectors (Diptera: Ceratopogonidae) will be of importance to assess the risk of transmission of Culicoides-borne pathogens. This study aimed to determine the impact of temperature and humidity on the flight activity of Culicoides imicola Kieffer, and other livestock associated Culicoides species, under both laboratory and field conditions.
Methods
Batches of 500 field-collected C. imicola females were acclimatized at a predetermined range of temperatures (10–29 °C) and relative humidity (34–85%). After acclimatization, these females, prompted by a light source, were allowed to escape through a transparent plastic funnel into a paper cup, where they were counted after an hour. Flight activity under field conditions was determined seasonally by hourly light trap collections done overnight at four sites near cattle.
Results
Experiments conducted at various test conditions in the laboratory indicated that flight activity started at 13 °C. Peak in activity was observed between 16 °C to 18 °C, and temperatures above 20 °C seemingly inhibit flight. Under field conditions, a peak in numbers collected was observed immediately after sunset. With mean nocturnal temperatures below 19 °C, more than 74% of the Culicoides were collected within two to three hours after sunset. With mean nocturnal temperature above 19 °C, the peak in numbers at sunset was sustained until after midnight, with somewhat higher numbers collected after midnight once temperatures dropped below 20 °C. No peak in numbers was observed at dawn. Although very low numbers were collected during the day, which partly may have been a result of the collecting method, Culicoides were present throughout periods of 24 hours. Humidity seemed to play a minor role in the regulation of flight activity.
Conclusions
Abundance and species diversity results as obtained in this study indicated a high level of risk of virus transmission in the first hours following sunset. A strong relationship was found between host-seeking activity, and hence trap efficiency, and within limits, temperature. Light traps primarily measure flight activity and may as such underestimate adult abundance of C. imicola if deployed at temperatures outside thresholds of 16–20 °C.
Keywords: Host-seeking activity, Light trap efficiency, Risk
Background
Small blood-feeding midges in the genus Culicoides Latreille (Diptera: Ceratopogonidae) are the biological vectors of various pathogens that can cause a number of economically important diseases in livestock globally [1]. Of the more than 75 viruses associated with the 1368 described species of Culicoides worldwide [2], in excess of 23 have been isolated from the Imicola complex in the subgenus Avaritia Fox [3]. Based on their high abundance at livestock and confirmed oral susceptibility, Culicoides imicola Kieffer is considered the principal vector of bluetongue-, African horse sickness-, epizootic haemorrhagic disease- and equine enchephalosis viruses in South Africa [3–6]. It was recently reported that the susceptibility of C. imicola to Schmallenberg virus (Orthobunyavirus) could exceed that of the abundant and wide spread Culicoides obsoletus (Meigen) in Spain [7].
In addition to its susceptibility to a wide range of viruses of veterinary importance, C. imicola is one of the most widely distributed livestock-associated Culicoides species in the world. It has been recorded across the Afrotropical, Saharo-Arabian and Oriental regions in addition to areas of the Mediterranean basin [3]. It was moreover suggested that its geographical distribution could potentially extend further west in Europe than what is currently reported [8]. The geographical distribution and abundance of Culicoides species are, in addition to the availability of suitable hosts, regulated by weather conditions such as temperature, humidity and rainfall [1].
Blood-feeding success and associated risk of pathogen transmission by Culicoides species depends on their ability to be active when appropriate hosts are accessible. For the majority of Culicoides species, peak flight activity concurs with dusk and/or dawn [9, 10]. This peak may shift towards a diurnal pattern in cooler or a nocturnal pattern in hotter weather [11]. As observed in several species, diurnal activity is, however, not extraordinary [12–15]. Flight activity, like geographical distribution, depends on environmental factors such as temperature, humidity, wind and rainfall [16].
Insight into the circadian host-seeking flight activity of vectors, and the factors that regulate this, will be of importance to assess the risk of transmission of Culicoides-borne pathogens and the implementation of appropriate controls at farm level. Despite their proven role as vectors of a number of pathogens, little is known of the host-seeking activity of Culicoides species, including C. imicola, in southern Africa.
Despite limitations, various models of suction light traps have become the most popular and widely used tool to monitor Culicoides geographical distribution and abundance [17, 18]. Flight activity and hence trap efficiency are, amongst other factors, influenced by climatic factors such as temperature, humidity and wind speed [10, 19, 20]. Since insects may respond directly to wind, rain, humidity, temperature and light intensity, weather can be of particular importance. These factors can differ daily over relatively short geographical distances. In the present study, the influence of temperature and humidity on the flight activity of C. imicola under both laboratory and field conditions were determined.
Methods
Study site
Field collections of Culicoides were conducted at the Agricultural Research Council - Onderstepoort Veterinary Research (-25.6501, 28.1870; 1219 m above sea level) in South Africa. Mean annual rainfall ranges between 430–1017 mm with a peak in summer between November and March [21]. The annual mean daily maximum and minimum temperatures are 26.3 °C and 9.3 °C, respectively [21]. Onderstepoort 220 V ultraviolet light traps were operated overnight underneath the eaves of open sided and semi-closed stables housing between 20 and 40 cattle each. The traps were installed between 1.9 and 2 m above the ground. During the day, some cattle were out in open pens with concrete flooring adjoining the stables. Traps were 60 to 70 m apart and not in direct sight of each other. Trees and kikuyu lawns, varying in size, surrounded the stables. Vervet monkeys (Chlorocebus pygerythrus), wild birds and small rodents of various species were present at all of the sites.
Flight behaviour under laboratory conditions
Culicoides were collected alive with light traps [22] at three to four sites as defined above during March and April 2017. The collected Culicoides were transferred into a black plastic box (450 × 330 × 280 mm) with a ventilated lid and allowed to exit the box through a white translucent funnel, mounted on one side of the box, into a 300 ml paper cup [22]. After 5% sucrose feeding on cotton wool pledgets at room temperature, pools of 500 C. imicola females were sorted on a refrigerated chill table. These pools were transferred to black plastic boxes (900 × 10 × 130 mm) through a 10 mm opening in the lid. A white transparent plastic funnel was mounted over a 120 mm opening on one side of the box. One hour before transfer of the midges, the boxes were placed inside climate controlled growth chambers, operated at one of seven set temperatures between 10–29 °C. After 15 to 20 min acclimatization the females were, prompted by a white fluorescent light source, allowed to escape through the funnel into a 300 ml white paper cup. After 60 min, Culicoides in the cups were immobilized for 30–50 s at -20 °C, and counted.
In the first series of experiments, the relative humidity in the chambers was not regulated. In subsequent trials, the humidity was kept at either 40%, 60% or 80%. Between six and ten replicates of 500 females each were conducted at the various temperature and humidity conditions. Temperature and humidity inside the holding box were recorded every 5 min with a DS1923-F5# Hygrochron iButton data logger (Fairbridge Technologies, Sandton, South Africa).
Flight behaviour in the field
In the first field trial, on the 21th of April, conducted from 15:00 h until 9:00 h the next morning, light traps were operated at four sites as defined above. Insects were collected into distilled water to which 0.5% Savlon® (Johnson & Johnson, East London, South Africa) (clorhexidine gluconate 0.3 g/100 ml and cetrimide 3.0 g/100 ml) antiseptic had been added. The collection beakers were exchanged hourly and the collected insects stored in 80% ethanol at room temperature. All collected Culicoides were morphologically identified to species level under a stereomicroscope using a wing picture atlas of Afrotropical Culicoides [23]. Based on abdominal pigmentation [24], all females were age-graded into younger nulliparous (unpigmented), older parous (pigmented), gravid or freshly blood-fed. Captured males were also counted.
The continual presence of Culicoides, albeit in small numbers, in the traps before sunset and after sunrise in April prompted subsequent collections to be made over periods of 24 hours. In these trials, representative of different climatic conditions, on 7 July, 1 November and 17 February, hourly collections were made from 14:00 h on the first to 14:00 h the following day. Trials were restricted to nights with no wind and/or rain and temperature and humidity were monitored every 5 min with iButton data loggers.
Statistical analysis
Analysis of variance (ANOVA) was used to analyse the data and because of unequal replications t probabilities of pairwise differences were used to compare the mean numbers of C. imicola females emerging from the holding boxes under the various temperature and humidity conditions. Data were analysed using the statistical program GenStat® [25] at the 5% level of significance.
Although the total numbers of Culicoides collected varied between the four sites, abundance patterns over time were similar. To compensate for potential site variation, the mean numbers collected hourly at each of the four sites were grouped. Hourly mean numbers of C. imicola collected were compared to the hourly mean temperature and relative humidity as measured from after sunset to before sunrise.
Results
Flight behaviour in the laboratory
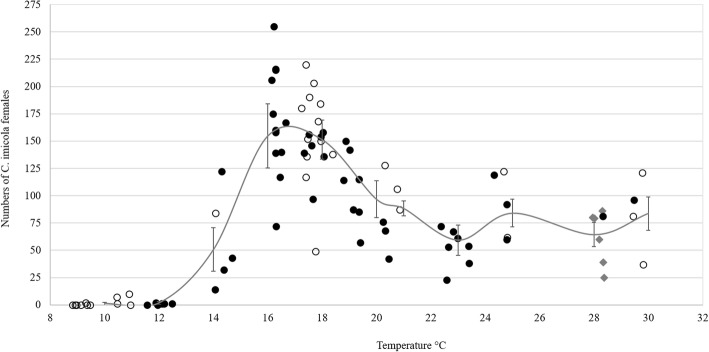
In the first trial, six to nine replicates were conducted at each of seven temperatures between 10–29 °C (Table 1). The mean temperatures inside the holding boxes were to some extent lower than the set temperature of the chamber (Table 1). The mean number of midges to emerge from the holding boxes at the various temperatures differ significantly from each other as indicated by ANOVA (F(6,38) = 16.51, P < 0.001). Subsequent t-tests for all pairwise differences of means indicated that the mean number of Culicoides, 139.8 ± 40.03, to emerge from the boxes at 17 °C (17.3 ± 0.74 °C) was significantly higher than that at any of the other temperatures (Table 1). At 10 °C (9.1 ± 0.24 °C), only two, of a potential 3000 Culicoides, emerged from the holding boxes. The low mean numbers, 0.3 ± 0.82, to emerge at 10 °C did not differ significantly from those at 13 °C (2.7 ± 3.20) (Table 1). The mean numbers of females to escape at 24 °C (57.7 ± 25.99) and 29 °C (55.2 ± 24.21) did not differ significantly. Emergence at 24 °C and 29 °C was significantly lower than that at 17 °C (Table 1). The relative humidity in the boxes, as influenced by the temperature, varied from 68.0 ± 2.6% at 10 °C to 34.3 ± 0.25% at 29 °C (Table 1).
Table 1.
Mean numbers and standard deviation (SD) of field-collected Culicoides imicola females to emerge over a period of 60 minutes from holding boxes kept at various temperature and humidity levels
| Set T (°C) | Mean T (SD) | Mean RH (SD) | No. of replicates (no of individuals)* | Total no. emerged (%) | Mean (SD) |
|---|---|---|---|---|---|
| 10 | 9.1 (0.24) | 68.0 (2.6) | 6 (3000) | 2 (0.02) | 0.3 (0.82)a |
| 13 | 12.3 (0.20) | 53.5 (0.5) | 6 (3000) | 16 (0.5) | 2.7 (3.20)a |
| 15 | 14.5 (0.20) | 51.9 (0.65) | 6 (3000) | 615 (20.5) | 102.5 (64.46)b |
| 17 | 17.3 (0.74) | 49.7 (1.93) | 9 (4500) | 1258 (28.0) | 139.8 (40.03)c |
| 20 | 19.8 (0.58) | 44.6 (0.61) | 6 (3000) | 415 (13.8) | 69.2 (17.34)b |
| 24 | 23.1 (0.47) | 45.7 (0.52) | 6 (3000) | 346 (11.5) | 57.7 (25.99)d |
| 29 | 28.3 (0.07) | 34.3 (0.25) | 6 (3000) | 331 (11.0) | 55.2 (24.21)d |
Numbers per row followed by a different letter were significantly different at the 5% level
Abbreviations: RH relative humidity, T temperature
*Each replicate consists of 500 individuals
To preclude the influence of humidity, the experiment, as set out above, was repeated at 60% RH. Although the humidity in the grow chamber was set at 60%, the mean humidity inside the holding boxes varied between 49.8 ± 11.22% at 29 °C and 62.3 ± 1.57% at 10 °C (Table 2). Similar to the previous trial the mean number of midges to emerge from the holding boxes at the various temperatures differ significantly (F(5,44) = 20.81, P < 0.001). Subsequent t-tests for all pairwise differences of means indicated that the highest mean number of Culicoides, 135.1 ± 44.64, to emerge was, as in the previous trial, at 17 °C (16.9 ± 0.83 °C). The higher mean numbers to emergence at 17 °C (RH 57.5 ± 5.04%) was not significantly different from that of 130.6 ± 35.42 at 20 °C (RH 54.0 ± 6.99%) (Table 2). As in the first trial the lowest mean emergence, 2.3 ± 3.96, at 10 °C did not differ significantly from that of 19.1 ± 29.46 at 13 °C (Table 2). The mean numbers to emerge at 24 °C, 90.6 ± 45.06, and 29 °C, 75 ± 30.74, did not differ significantly. The emergence at both 24 °C and 29 °C was significantly lower than at 17 °C.
Table 2.
Mean numbers and standard deviation (SD) of field-collected Culicoides imicola females to emerge over a period of 60 minutes from holding boxes kept at various temperature and 60% humidity level
| Set T (°C) | Mean T (SD) | Mean RH (SD) | No. of replicates (no of individuals)* | Total no. emerged (%) | Mean (SD) |
|---|---|---|---|---|---|
| 10 | 9.9 (0.85) | 62.3 (1.57) | 8 (4000) | 18 (0.5) | 2.3 (3.96)a |
| 13 | 12.9 (1.08) | 56.5 (2.78) | 8 (4000) | 153 (3.8) | 19.1 (29.46)a |
| 17 | 16.9 (0.83) | 57.5 (5.04) | 8 (4000) | 1081 (27.0) | 135.1 (44.64)b |
| 20 | 19.8 (0.89) | 54.0 (6.99) | 8 (4000) | 1045 (26.1) | 130.6 (35.42)b |
| 24 | 23.9 (1.06) | 53.5 (9.11) | 10 (5000) | 906 (18.1) | 90.6 (45.06)c |
| 29 | 28.9 (0.80) | 49.8 (11.22) | 8 (4000) | 600 (15.0) | 75 (30.74)c |
Numbers per row followed by a different letter were significantly different at the 5% level
Abbreviations: RH relative humidity, T temperature
*Each replicate consists of 500 individuals
To substantiate the influence of humidity on flight activity, the results obtained at various humidity levels at 17 °C were compared (Table 3). In this trial, the mean humidity inside the boxes ranged from 46.4 ± 2.1% to 84.9 ± 2.12% and the temperature varied between 16.5 ± 0.45 °C and 17.7 ± 0.37 °C (Table 3). The mean numbers of Culicoides to escape from the holding boxes, ranged from 135.1 ± 44.64 at 60% RH to 178.8 ± 44.62 at 80% RH and were not significantly different (F(3,29) = 2.51, P = 0.078) at the various humidity levels.
Table 3.
Mean numbers and standard deviation (SD) of field-collected Culicoides imicola females to emerge over a period of 60 minutes from holding boxes kept at 17 °C at various humidity levels
| Mean T (SD) | Set RH | Mean RH (SD) | No. of replicates (no of individuals)a | Total no. emerged (%) | Mean (SD) |
|---|---|---|---|---|---|
| 17.3 (0.74) | not set | 49.7 (1.93) | 9 (4500) | 1258 (28.0) | 139.8 (40.03) |
| 16.5 (0.45) | 40 | 46.4 (2.1) | 8 (4000) | 1430 (35.8) | 178.8 (44.62) |
| 16.9 (0.83) | 60 | 57.5 (5.04) | 8 (4000) | 1081 (27.0) | 135.1 (44.64) |
| 17.7 (0.37) | 80 | 84.9 (2.12) | 8 (4000) | 1381 (34.5) | 172.6 (30.09) |
Abbreviations: RH relative humidity, T temperature
aEach replicate consists of 500 individuals
An aggregated comparison of the data (Fig. 1) confirmed low emergence at 10 °C and indicated that activity, independent of humidity, only started to increase once temperatures rose above 13 °C. Figure 1 shows that peak emergence was recorded between 16–18 °C and that it started to decrease when temperatures increased above 19–20 °C. There were no significant differences in the numbers to emerge from the holding boxes once the temperature was above 20 °C (up to 29 °C) (Fig. 1). No correlation between the mean emergence and RH, ranging between 46.4–84.9%, could be established (Fig. 1). Humidity seemed to play a minor role in the regulation of flight activity in the present set of experiments.
Fig. 1.
Mean numbers and standard deviation of Culicoides imicola females to emerge from holding boxes kept at various temperature and humidity levels. Open circles: humidity > 60%; closed circles: humidity 40–60%; diamonds: humidity < 40%
Flight behaviour under field conditions
April 2017
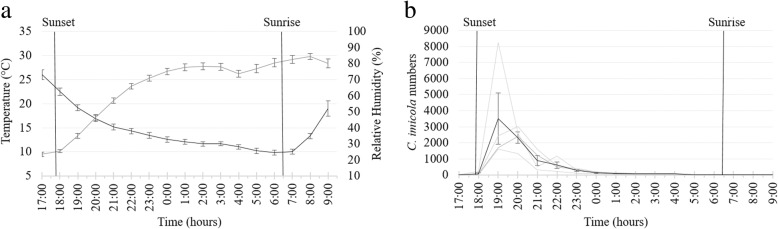
During the first trial, collections were conducted from 17:00 h on 21 April to 9:00 h on 22 April, and 17 one hourly collections were made at each of four sites. Sunset occurred at 17:45 h on 21 April and sunrise at 6:26 h the following day [26]. The hourly mean overall temperature, 14.6 °C, dropped from 26.0 ± 2.04 °C at 17:00 h to 9.8 ± 1.08 °C at 6:00 h (Fig. 2a). Inversely, the mean overall relative humidity, 65.4%, increased from 23.4 ± 2.90% at 17:00 h to 84.7 ± 3.46% at 8:00 h.
Fig. 2.
Mean and standard deviation in hourly temperature (black line) and relative humidity (grey line) (a) and Culicoides imicola numbers (black line) (b) as determined in 68 light trap collections made at four sites near cattle at the ARC-OVR from 17:00 h on 21 April to 9:00 h on 22 April 2017. The C. imicola numbers collected at the four individual sites are indicated with grey lines. Vertical black lines indicate sunset and sunrise
Culicoides imicola accounted for 99.3% (32,577) of 32,806 Culicoides collected in the 68 hourly collections made at the four sites. The total numbers of C. imicola collected at individual sites varied between 3740 (99.2%) and 13,615 (99.4%). Although the mean numbers collected varied between sites it peaked dramatically immediately after sunset at all four sites with 83% (26,920 of 32,577) of all Culicoides being collected within the first two hours after sunset (Fig. 2b). During the two hours of peak abundance, 19:00 h to 21:00 h, the mean temperature decreased from 19.1 ± 1.17 °C to 17.0 ± 1.29 °C.
The gradual decrease in the mean hourly temperature (13.9 °C), from 19.1 ± 1.17 °C at 19:00 h to 9.8 ± 1.07 °C at 6:00 h between sunset and sunrise correlated strongly (r(10) = 0.93, P < 0.0001) with the decrease in the mean numbers of C. imicola. Inversely, the mean relative humidity, increasing from 46.4 ± 3.51% to 82.6 ± 4.77%, showed a similar negative correlation (r(10) = -0.97, P < 0.0001).
The mean temperature before sunrise was 9.8 ± 1.08 °C and no peak in Culicoides numbers was observed before, during or after sunrise (Fig. 2b). Although only low numbers, less than 10 Culicoides per trap, were collected before sunset and after sunrise, Culicoides were present at one or more of the sites throughout the 17-h period (Fig. 3).
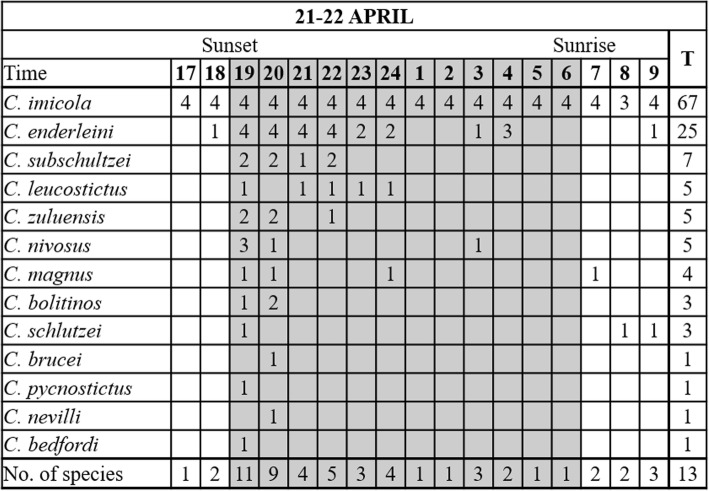
Fig. 3.
Hourly species richness of a Culicoides population as determined with 68 light trap collections at four sites near cattle at the ARC-OVR from 17:00 h on 21 April to 9:00 h on 22 April 2017. The numbers (1–4) indicate the number of sites at which the species was present. Abbreviation: T, the total number of collections in which the species was encountered
Culicoides belonging to at least 13 species were collected (Fig. 3). The dominant species, C. imicola, was present in 67 of the 68 hourly collections. Similar to abundance, species richness was the highest during initial two hours after sunset and all species eventually collected were encountered between 19:00 h and 20:00 h (Fig. 3). Species collected before sunset and after sunrise included low numbers of C. imicola, Culicoides enderleini Cornet & Brunhes, Culicoides magnus Colaco and Culicoides schultzei (Enderlein) (Fig. 3).
Nulliparous C. imicola females, representing 71.5% of the C. imicola collected, was the dominant physiological grouping at all four sites throughout the collection period. Similarly parous females, representing 27.3% of all C. imicola collected, were present throughout this period. Although the parous rates differed between the sites, it did not differ over time. Freshly blood-engorged C. imicola females, representing 0.3% of the Culicoides, were only present between 18:00 h and 1:00 h and males, representing 0.9%, from 19:00 h to 9:00 h.
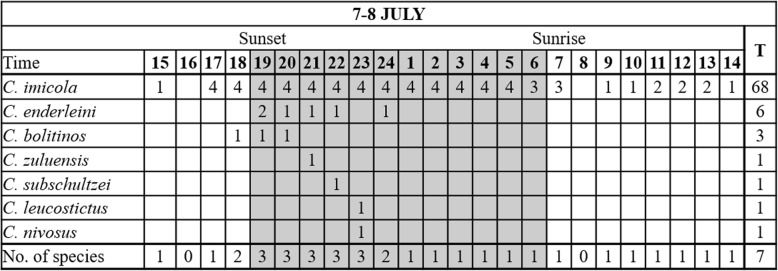
July 2017
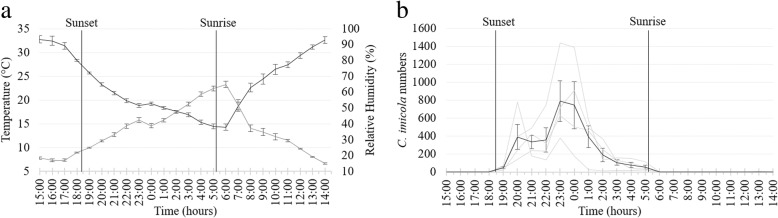
During the second trial, 96 hourly collections were made at the same four sites as in April from 14:00 h on 7 July to 14:00 h on 8 July. Sunset was at 17:29 h and sunrise at 6:55 h the following day [26]. The hourly overall mean temperature, 13.6 °C, ranged from 20.0 ± 1.19 °C at 16:00 h to 9.0 ± 0.50 °C at 5:00 h and the mean overall relative humidity, 71.3%, from 46.4 ± 2.34% at 16:00 h to 86.2 ± 1.89% at 6:00 h (Fig. 4a).
Fig. 4.
Mean and standard deviation in hourly temperature (black line) and relative humidity (grey line) (a) and Culicoides imicola numbers (black line) (b) as determined in 96 light trap collections at four sites near cattle at the ARC-OVR from 15:00 h on 7 July to 14:00 h on 8 July 2017. The C. imicola numbers collected made at the four individual sites are indicated with grey lines. Vertical black lines indicate sunset and sunrise
Despite the fact that collections were made over 24 hours, the total number of Culicoides collected in July (5540) was lower than that in April (32,806). Culicoides imicola, representing 5526 (99.7%) of 5540 Culicoides collected, was once again the dominant species. The total number of C. imicola collected varied between 1098 (99.7%) and 1632 (99.9%) at the sites.
As in April, the numbers of Culicoides collected peaked immediately after sunset with 4115 (74%) of 5540 Culicoides collected within the first three hours, 18:00 h to 21:00 h, after sunset at all four sites (Fig. 4b). During this period, the mean temperature decreased from 15.9 ± 0.73 °C at 18:00 h to 13.3 ± 0.64 °C at 20:00 h (Fig. 4a). Between sunset and sunrise the mean temperature, 11.6 °C, dropped from 15.9 ± 0.73 °C at 18:00 h to 9.5 ± 0.72 °C at 5:00 h. The decrease in mean numbers of C. imicola collected between sunset and sunrise correlated significantly with the mean hourly temperature (r(10) = 0.90, P < 0.0001) and relative humidity (r(10) = -0.92, P < 0.0001). The mean temperature at sunrise was 9.8 ± 1.08 °C with no observable peak in numbers collected (Fig. 4a, b). Though relatively low mean numbers were collected before sunset (0.5) and after sunrise (0.7), C. imicola was present at one or more of the four sites between 7:00 h and 14:00 h (Fig. 5).
Fig. 5.
Hourly species richness of a Culicoides population as determined with 96 light trap collections at four sites near cattle at the ARC-OVR from 15:00 h on 7 July to 14:00 h on 8 July 2017. The numbers (1–4) indicate the number of sites at which the species was present. Abbreviation: T, the total number of hourly collections in which the species was encountered
Even though the mean temperature fell below 10 °C for three or more consecutive hours after midnight (Fig. 4a) during July, low numbers of C. imicola were still present in most of the collections (Fig. 4b).
With only seven Culicoides species collected species richness was lower in July compared to that in April. The dominant species, C. imicola, was present in 68 of the 96 hourly collections (Fig. 5). All species eventually found were collected before midnight. Species collected before sunset were C. imicola and Culicoides bolitinos Meiswinkel. Culicoides imicola was the only species to be collected after sunrise (Fig. 5).
Nulliparous C. imicola females, representing 64.0% of those collected, was the dominant physiological grouping. The mean parous rate, 34.5%, was higher than that in April and although it varied between sites, it did stay constant during the period. Blood engorged C. imicola females, accounting for 0.4% of the collection, were present between 17:00 h and 22:00 h and males, accounting for 0.9%, between 10:00 h and 23:00 h.
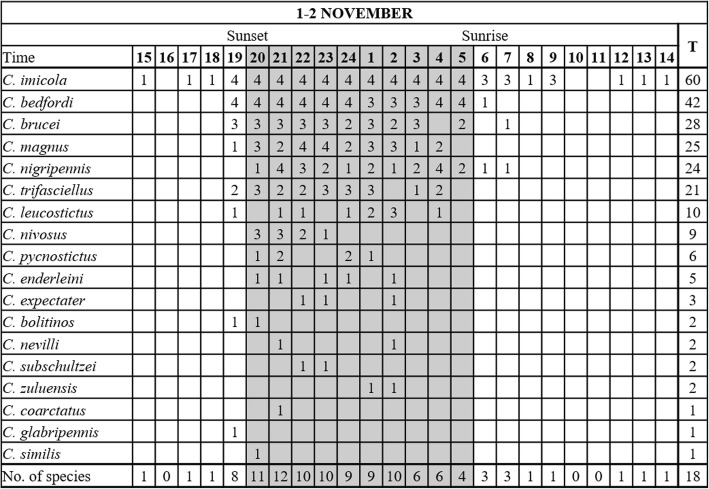
November 2017
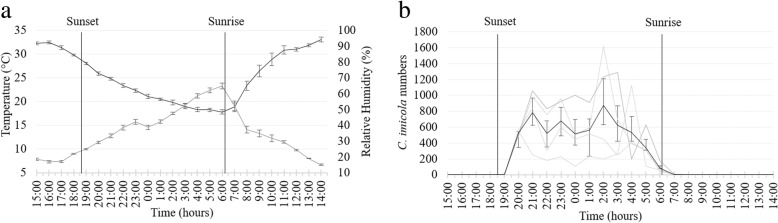
During the third survey, sunset occurred at 18:23 h on 1 November and sunrise at 5:17 h the following morning [26]. The mean temperature, 23.5 °C, ranging from 32.8 ± 1.57 °C at 15:00 h to 14.3 ± 1.28 °C at 6:00 h, was higher than that in the previous two trials (Fig. 6a).
Fig. 6.
Mean and standard deviation in hourly temperature (black line) and relative humidity (grey line) (a) and Culicoides imicola numbers (black line) (b) as determined with 96 light trap collections at four sites near cattle at the ARC-OVR from 15:00 h on 1 November to 14:00 h on 2 November 2017. The C. imicola numbers collected at the four individual sites are indicated with grey lines. Vertical black lines indicate sunset and sunrise
As in the previous trials, C. imicola dominated. This species represented 96.3% (13,972) of 14,504 Culicoides collected in 96 hourly collections made at all of the four sites from 14:00 h on 1 November to 14:00 h on 2 November. Its proportional representation at the four sites varied between 94.1% and 97.5%. As in April and July, the numbers collected increased sharply directly after sunset (Fig. 6b). The mean temperature, 19.2 °C, decreased from 25.7 ± 0.39 °C at 19:00 h (after sunset) to 14.3 ± 1.28 °C at 5:00 h (before sunrise) (Fig. 6a). Contrary to the previous two trials, the numbers collected after sunset did not decrease within the first three hours after sunset, and the highest mean numbers were found later, between 23:00 h and midnight, once the mean temperature dropped below 20 °C (Fig. 6a). The overall correlation between mean numbers collected from after sunset to before sunrise and temperature was low (r(8) = 0.51, P = 0.128). After midnight the mean numbers started to decrease and between 23:00 h and 5:00 h, with mean temperatures decreasing from 18.9 ± 0.85 °C to 14.5 ± 0.97 °C, the correlation (r(5) = 0.86, P = 0.014) between temperature and numbers collected increased. In November, the variation in the numbers collected hourly at each of the four sites was more pronounced than in the previous surveys (Fig. 6b). The mean temperature at sunrise was 14.5 ± 0.97 °C and no peak in numbers was observed.
With at least 18 species collected species richness was higher than in the first two trials. Seven of these 18 species were present throughout the night (Fig. 7). Except for Culicoides zuluensis de Meillon, all species were found before midnight. Low numbers of C. imicola were collected at one site before sunset (Fig. 6b). Species collected up to two hours after sunrise included C. imicola, Culicoides brucei Austen and members of the Nigripennis group.
Fig. 7.
Hourly species richness of a Culicoides population as determined with 96 light trap collections at four sites near cattle at the ARC-OVR in from 15:00 h on 1 November to 14:00 h on 2 November 2018. The numbers (1–4) indicate the number of sites at which the species was present. Abbreviation: T, the total number of hourly collections in which the species was encountered
The mean proportional representation, 54.2%, of nulliparous C. imicola females, ranging from 51.1% to 60.9% between sites, was lower than in the prior trials. The proportion of parous females ranged from 34.2% to 45.7% between sites and although it differed between sites, it did not differ over time. Blood engorged females, at 1.4% of the total collected, were present from 19:00 h until at least 4:00 h at one or more of the sites. Gravid females, making up 0.2% of the total numbers, were present from 20:00 h to 3:00 h and males, comprising 1.6%, were absent from 6:00 h to 11:00 h.
February 2018
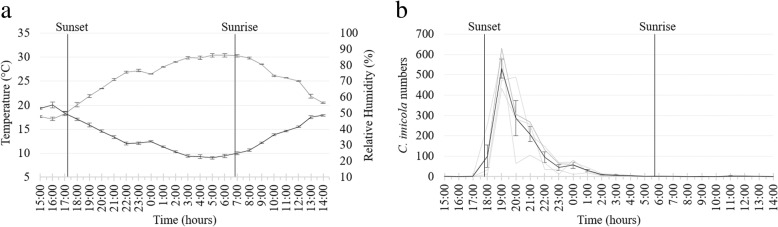
In the fourth trial, 24 hourly collections were made at each of the four sites from 14:00 h on 16 to 14:00 h on 17 February. Sunset was at 18:48 h and sunrise at 5:53 h the following day [26]. The mean temperature, 25.4 °C, over the entire collection period ranged from 17.8 ± 0.92 °C at 6:00 h to 32.00 ± 1.12 °C at 14:00 h and the mean humidity from 26.2 ± 1.13% at 16:00 h to 81.0 ± 3.94% at 6:00 h (Fig. 8a).
Fig. 8.
Mean and standard deviation in hourly temperature (black line) and relative humidity (grey line) (a) and Culicoides imicola numbers (black line) (b) as determined with 96 light trap collections at four sites near cattle at the ARC-OVR from 15:00 h on 16 February to 14:00 h on 8 February 2018. The C. imicola numbers collected at the four individual sites are indicated with grey lines. Vertical black lines indicate sunset and sunrise
During this trial, 25,198 Culicoides were collected in 96 hourly collections at the four sites. With a mean abundance of 96.3%, the proportional representation of C. imicola ranged from 91.7% to 98.0% at the four sites. As in all three previous surveys, the numbers collected increased after sunset (Fig. 8b). The mean temperature at 20:00 h (after sunset) was 25.90 ± 0.70 °C and, contrary to the previous trials, the peak in mean numbers collected was not directly after sunset but at least an hour later at 21:00 h (Fig. 8b). As in November, this peak did not decline within two to three hours and relatively high numbers were collected throughout the night (Fig. 8b). The highest mean numbers were once again collected once the mean temperature dropped below 20 °C at 2:00 h (Fig. 8b). After sunset, the mean temperature decreased gradually from 25.9 °C to a minimum of 18.3 ± 0.71 °C before sunrise. Between 20:00 h (after sunset) and 6:00 h (before sunrise) the overall correlation between the mean numbers collected hourly and temperature was low (r(9) = 0.42, P = 0.204). Between 2:00 h, once temperatures fell below 20 °C, and 6:00 h this correlation increased (r(3) = 0.95, P = 0.014). The variation in the numbers collected hourly at each of the sites was, similar to the survey in November, greater than that in April and July.
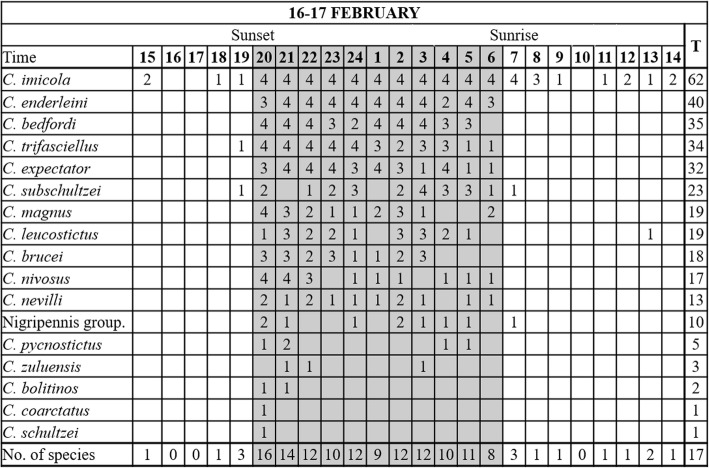
With 17 species collected, species richness was, as in November, relatively high (Fig. 9). Although species richness was the highest immediately after sunset, most species were present throughout the night. Culicoides imicola was only absent in seven of 96 collections made. Other species collected before sunset or after sunrise included Culicoides trifasciellus Goetghebuer, Culicoides subschultzei Cornet & Brunhes, Culicoides leucostictus Kieffer and members of the Nigripennis group (Fig. 9).
Fig. 9.
Hourly species richness of a Culicoides population as determined with 96 light trap collections at four sites near cattle at the ARC-OVR from 15:00 h on 16 February to 14:00 h on 17 February 2018. The numbers (1–4) indicate the number of sites at which the species was present. Abbreviation: T, the total number of hourly collections in which the species was encountered
The dominant physiological grouping was once again nulliparous C. imicola females. The mean proportional representation, 57.3%, ranging from 52.5% to 59.7% between sites, was similar to that in November. The proportion of parous females ranged between 30.8–36.4% and although differing between sites, did not alter over time. Gravid and blood-engorged females, comprising less than 1% of the totals, were present at all four sites. Contrary to the previous surveys, relatively high proportions, 16.1% to 3.3%, of C. imicola males were found. Males were present throughout the night.
Discussion
Studies under controlled conditions revealed low flight activity for C. imicola females below 10 °C, with activity independent of relative humidity and only starting to increase once temperatures rose above 13 °C. In the laboratory, optimum flight activity, as reflected from the number of individuals moving towards a white light source, was recorded between 16 °C and 18 °C. Flight activity was seemingly supressed at temperatures above 19–20 °C. Flight activity also did not differ significantly between 20 °C and 29 °C. Following a similar protocol low flight activity below 10 °C, with an increase between 10 °C and 20 °C, was previously described for Culicoides oxystoma Kieffer and Culicoides maculatus (Shiraki) in Japan [27]. These temperatures, at which activity starts to increase, correspond with the minimum temperature of 11 °C to 13 °C, at which orbiviral replication commences in a number of species, including C. imicola [28].
The present results support presence/absence models for C. imicola [29, 30], based on a mean minimum temperature threshold of 12.5 °C [31, 32], and predictions that it would be present in areas with mean annual temperature between 12–20 °C [33]. Under laboratory conditions, no correlation was found between relative humidity and flight activity. Humidity, although correlated with temperature in the field, seems to play a secondary role in flight initiation.
Despite low flight activity recorded in the laboratory, Culicoides, including C. imicola, was found in traps operated near cattle at environmental temperatures below 10 °C in July. This indicates that factors such as attraction cues from the host linked to the hunger status of the female might also play a role in flight initiation. The involvement of additional factors, besides temperature, in flight initiation could partly explain the relatively low escape rates (< 50%) and migration to the light source under laboratory conditions in the present study. The field-collected females were fed on a 5% sucrose solution before evaluation and once inside the holding boxes, exposure to environmental cues that could have initiated flight would have been minimal [34]. Due to greater attractiveness [35], an ultraviolet light source in the growth chambers may have proportionally increased the number of midges emerging from the holding boxes at the various temperatures. The influence of light source, if any, on the temperature at which flight activity are initiated and maintained still need to be determined.
The field trials indicated that flight activity in C. imicola, as measured with light traps, peaked markedly directly after sunset. This trait has been described for several Culicoides species in America [11], Europe [10, 36], Australia [9, 20] as well as for C. imicola in Israel [37] and Senegal [38, 39]. This peak in numbers at sunset was, however, delayed if the temperature at sunset was above 25 °C as seen in February.
During April and July, with mean nocturnal temperatures below 19 °C, between 74–83% of the Culicoides were collected within two to three hours after sunset. During these surveys, the peak in numbers at dusk decreased directly relative to the decrease in mean temperatures. A similar correlation between flight activity and temperature was observed for C. obsoletus in France [36]. In November and February, with the mean nocturnal temperature above 19 °C, the peak in numbers at sunset was prolonged until after midnight. A slight increase in numbers collected were observed once the mean temperature dropped below 20 °C after midnight. At similar temperatures in Kenya [40] and Israel [37], C. imicola was also present in relatively high numbers throughout the night. A similar peak in numbers in C. imicola (syn. C. pallidipennis), and members of the Schultzei group, was observed at midnight, with environmental temperatures around 20 °C, in Kenya [40]. Similar to the present study, it was reported that, in Kenya, the flight activity of both these species was correlated to an increase in temperature up to 20 °C and suppressed after sunset at temperatures above 25 °C [40].
Several authors claim that rapidly changing levels of light intensity initiate flight and provoke Culicoides attacks [41–44]. Bimodal flight activity (at dusk and dawn) has been recorded for a number of Culicoides species [9–11], including some Afrotropical ones [40, 42]. In some instances, the peak at dawn can be higher than that at dusk [37]. In the present study, with mean temperatures before sunrise, varying between 9.0 °C (July) and 17.8 °C (February), corresponding with the coldest part of the night, no peak in numbers was seen at dawn. This is in agreement with previous studies, conducted in the same area in February 2009, where a peak at dawn was also apparently absent [45]. Due to the collection method, it is possible that a peak after sunrise could have been overlooked. The relatively low numbers collected immediately before and during sunrise, however, signify that a significant peak may have been absent.
As with abundance, Culicoides species richness was at a maximum the first few hours after sunset in April and July. Although all species present were collected within the first six hours after sunset, species richness was high throughout the night in November and February.
Any variation in the response of Culicoides to lights and hosts is of importance, taking into account that several studies utilized light traps to determine flight activity as an indication of abundance, when it is actually the attack rate on the host that is of relevance [18, 46]. Despite inefficiencies of light traps in collecting diurnal species, low numbers of C. imicola were found throughout 24 hours in the present study. These results support previous South African studies indicating that host-seeking and blood-feeding commence before sunset on livestock [47] and wild animals [12]. The presence of freshly blood-engorged females in the traps during the day in the present study accentuates this observation. Several diurnally active species, e.g. Culicoides paraensis (Goeldi), Culicoides actoni Smith, Culicoides chiopterus (Meigen) have been recorded [13–15, 46]. Culicoides chiopterus can peak in abundance, as indicated by sweep netting, up to two hours before sunset and their abundance and potential role in virus transmission is underestimated in light trap collections [46]. Similarly it was found that the peak in C. obsoletus activity in Europe can shift from after sunset in summer to before sunset in spring and autumn [36]. Despite the presence of Culicoides, as determined using light traps, the stabling of horses during the day from before dusk until after dawn is still considered, and has been for over a century [48], as an effective method of preventing African horse sickness in South Africa. The epidemiological significance of daytime biting by Culicoides requires further study because of its implication for the diurnal transmission of viruses. It need to be emphasized that light trap results do not inevitably relates to the attack or biting rate on host animals [15, 47].
The unwavering dominance of non-blood engorged nulliparous and parous females in the light trap collections throughout the collection period reinforces the fact that this method is predisposed to groups with high host-seeking activity. Although this trait may increase monitoring sensitivity to establish relative risk for virus transmission, it will underestimate overall Culicoides abundance in an area.
The present field and laboratory data indicated a linear correlation between flight activity and temperatures between 10–20 °C. At mean nocturnal temperatures below 20 °C host-seeking flight activity, and feeding, will be condensed into the three hours immediately after sunset. Once mean nocturnal temperatures increase above 20 °C, host-seeking and flight activity, and the risk of virus transmission, will be extended over longer periods and not be restricted to a one- to three-hour period after sunset. This phenomenon was reinforced by an increase in variation between sites in November and February. At nocturnal temperatures above 20 °C, the movement of host animals in relation to the trap may play a more meaningful role in regulating localised abundance and trapping efficiency.
In evaluating Culicoides abundance, determined by light traps, it must be taken into consideration that host-seeking females represent a relatively small part of the entire population. Temperature not only influences flight activity but also significantly affects the development time of sub-adult stages, vector competence and adult survival. A significant decrease in egg batch size as well as in the number of days from blood-feeding to ovipositing and successive blood-feeding with increasing temperature was observed for Culicoides sonorensis Wirth & Jones as well as C. imicola [49, 50]. At 10 °C, the median oviposition time in C. sonorensis may be as long as 34 days as compared to 8–12 days at 30 °C [49]. Prolonged ovipositing time will reduce blood-feeding activity and may lead to lower flight activity. Shorter development time above 20 °C will lead to an increase in abundance. At these temperatures, blood digestion and virus replication in infected females will increase, resulting in a decrease in the period between blood meals and an increase in feeding frequency and potential virus transmission [28, 51–53]. These variables may not be reflected in flight activity and light trap results.
The present results need to be evaluated against the fact that the relationship between flight activity, abundance and light trap efficiency are not clearly defined [18]. The observation that environmental temperature influences flight activity, and hence light trap efficiency, adds to factors that contribute to the variability in light trap results and reliable data comparison between geographical areas. The number of factors that can influence light trap results underlined that light trap results need to be used with caution in the production of risk maps and models for Culicoides species and associated disease transmission.
Conclusions
Based on abundance, as well as on species richness, the present study indicates favourable conditions to support a high level of flight and host-seeking activity, and an associated risk of potential virus transmission, in the first hours after sunset. The present study highlights a strong relationship between flight activity as an indicator of host-seeking activity, and, within limits, temperature. Optimal flight activity, and hence trap efficiency, may be limited to a relatively narrow band between 16–20 °C. Light traps primarily measure flight activity and may as such underestimate adult abundance of C. imicola if deployed at temperatures outside of these thresholds.
Acknowledgements
The authors thank Liesl Morey from the ARC Biometry Division for assisting with the statistical analyses. We thank Dr Kerstin Junker for constructive comments on earlier drafts of this manuscript. We thank the anonymous referees for their valuable input for improving the quality of this manuscript.
Funding
We acknowledge the funding of the ARC Climate Change Collaboration Centre (ARC CCCC Group 4) and the support of ARC-OVR for this work.
Availability of data and materials
All data generated and analysed in the study are included in this published article. Further data are archived and available upon request.
Authors' contributions
GJV and CJdB contributed equally to this work. Conceived and designed the study: GJV, CJdB and SNBB. Field collection and rotation of traps: SNBB, CJdB and GJV. Performed species analyses and other experimental work: CJdB. Analysed the data: CJdB and GJV. Wrote the paper: GJV and CJdB. All authors provided intellectual input to the study. All authors read and approved the final manuscript.
Ethics approval and consent to participate
Humans, or other mammals, were not directly targeted in the experimental work and ethics approval was not needed. Permission to publish this study was received from the ARC-OVR.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Gert J. Venter, Email: venterg@arc.agric.za
Solomon N. B. Boikanyo, Email: boikanyos@arc.agric.za
Chantel J. de Beer, Email: debeerc@arc.agric.za
References
- 1.Purse BV, Carpenter S, Venter GJ, Bellis G, Mullens BA. Bionomics of temperate and tropical Culicoides midges: knowledge gaps and consequences for transmission of Culicoides-borne viruses. Annu Rev Entomol. 2015;60:373–392. doi: 10.1146/annurev-ento-010814-020614. [DOI] [PubMed] [Google Scholar]
- 2.Borkent A. Ceratopogonidae. In: Kirk-Spriggs AH, Sinclair BJ, editors. Manual of Afrotropical Diptera Volume 2. Nematocerous Diptera and lower Brachycera. Suricata 5. Pretoria: South African National Biodiversity Institute; 2017. pp. 733–812. [Google Scholar]
- 3.Meiswinkel R, Venter GJ, Nevill EM. Vectors: Culicoides spp. In: Coetzer JAW, Tustin RC, editors. Infectious Diseases of Livestock. Cape Town: Oxford University Press; 2004. pp. 93–136. [Google Scholar]
- 4.Paweska JT, Venter GJ. Vector competence of Culicoides species and the seroprevalence of homologous neutralizing antibody in horses for six serotypes of equine encephalosis virus (EEV) in South Africa. Med Vet Entomol. 2004;18:398–407. doi: 10.1111/j.0269-283X.2004.00524.x. [DOI] [PubMed] [Google Scholar]
- 5.Venter GJ. Culicoides spp. (Diptera: Ceratopogonidae) as vectors of bluetongue virus in South Africa - a review. Vet Ital. 2015;51:325–333. doi: 10.12834/VetIt.505.2436.2. [DOI] [PubMed] [Google Scholar]
- 6.Venter G. Culicoides (Diptera: Ceratopogonidae) as vectors of African horse sickness virus in South Africa, Abstracts of the XXV International Congress of Entomology, 25–30 September 2016. Annapolis: The Entomological Society of America; 2016.
- 7.Pagès N, Talavera S, Verdún M, Pujol N, Valle M, Bensaid A, Pujols J. Schmallenberg virus detection in Culicoides biting midges in Spain: first laboratory evidence for highly efficient infection of Culicoides of the Obsoletus complex and Culicoides imicola. Transbound Emerg Dis. 2018;65:e1–e6. doi: 10.1111/tbed.12653. [DOI] [PubMed] [Google Scholar]
- 8.Guichard S, Guis H, Tran A, Garros C, Balenghien T, Kriticos DJ. Worldwide niche and future potential distribution of Culicoides imicola, a major vector of bluetongue and African horse sickness viruses. PLoS One. 2014;9:e112491. doi: 10.1371/journal.pone.0112491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kettle DS. The biting habits of Culicoides furens (Poey) and C. barbosai Wirth & Blanton. I. The 24-h cycle, with a note on differences between collectors. B Entomol Res. 1969;59:21–31. doi: 10.1017/S0007485300002996. [DOI] [Google Scholar]
- 10.Sanders CJ, Gubbins S, Mellor PS, Barber J, Golding N, Harrup LE, et al. Investigation of diel activity of Culicoides biting midges (Diptera: Ceratopogonidae) in the United Kingdom by using a vehicle-mounted trap. J Med Entomol. 2012;49:757–765. doi: 10.1603/ME11259. [DOI] [PubMed] [Google Scholar]
- 11.Barnard DR, Jones RH. Diel and seasonal patterns of flight activity of Ceratopogonidae in northeastern Colorado: Culicoides. Environ Entomol. 1980;9:446–451. doi: 10.1093/ee/9.4.446. [DOI] [Google Scholar]
- 12.Meiswinkel R, Braack LEO. African horsesickness epidemiology: five species of Culicoides (Diptera: Ceratopogonidae) collected live behind the ears and at the dung of the African elephant in the Kruger National Park, South Africa. Onderstepoort J Vet. 1994;61:151–170. [PubMed] [Google Scholar]
- 13.Mercer DR, Spinelli GR, Watts DM, Tesh RB. Biting rates and developmental substrates for biting midges (Diptera: Ceratopogonidae) in Iquitos, Peru. J Med Entomol. 2003;40:807–812. doi: 10.1603/0022-2585-40.6.807. [DOI] [PubMed] [Google Scholar]
- 14.Bellis GA, Melville LF, Hunt NT, Hearnden MN. Temporal activity of biting midges (Diptera: Ceratopogonidae) on cattle near Darwin, Northern Territory, Australia. Vet Ital. 2004;40:324–328. [PubMed] [Google Scholar]
- 15.Gerry AC, Sarto I, Monteys V, Moreno Vidal JO, Francino O, Mullens BA. Biting rates of Culicoides midges (Diptera: Ceratopogonidae) on sheep in northeastern Spain in relation to midge capture using UV light and carbon dioxide-baited traps. J Med Entomol. 2009;46:615–624. doi: 10.1603/033.046.0329. [DOI] [PubMed] [Google Scholar]
- 16.Carpenter S, Wilson A, Mellor PS. Culicoides and the emergence of bluetongue virus in northern Europe. Trends Microbiol. 2009;17:172–178. doi: 10.1016/j.tim.2009.01.001. [DOI] [PubMed] [Google Scholar]
- 17.Venter GJ, Labuschagne K, Hermanides KG, Boikanyo SNB, Majatladi DM, Morey L. Comparison of the efficiency of five suction light traps under field conditions in South Africa for the collection of Culicoides species. Vet Parasitol. 2009;166:299–307. doi: 10.1016/j.vetpar.2009.08.020. [DOI] [PubMed] [Google Scholar]
- 18.McDermott EG, Mullens BA. The dark side of light traps. J Med Entomol. 2017;55:251–261. doi: 10.1093/jme/tjx207. [DOI] [PubMed] [Google Scholar]
- 19.Blackwell A. Diel flight periodicity of the biting midge Culicoides impunctatus and the effects of meteorological conditions. Med Vet Entomol. 1997;11:361–367. doi: 10.1111/j.1365-2915.1997.tb00423.x. [DOI] [PubMed] [Google Scholar]
- 20.Kettle DS, Edwards PB, Barnes A. Factors affecting numbers of Culicoides in truck traps in coastal Queensland. Med Vet Entomol. 1998;12:367–377. doi: 10.1046/j.1365-2915.1998.00119.x. [DOI] [PubMed] [Google Scholar]
- 21.Venter GJ, Meiswinkel R, Nevill EM, Edwardes M. Culicoides (Diptera: Ceratopogonidae) associated with livestock in the Onderstepoort area, Gauteng, South Africa as determined by light-trap collections. Onderstepoort J Vet. 1996;63:315–325. [PubMed] [Google Scholar]
- 22.Venter GJ, Paweska JT, Van Dijk AA, Mellor PS, Tabachnick WJ. Vector competence of Culicoides bolitinos and C. imicola for South African bluetongue virus serotypes 1, 3 and 4. Med Vet Entomol. 1998;12:378–385. doi: 10.1046/j.1365-2915.1998.00116.x. [DOI] [PubMed] [Google Scholar]
- 23.Labuschagne K. The Culicoides Latreille (Diptera: Ceratopogonidae) species of South Africa, PhD thesis. Pretoria: University of Pretoria; 2016.
- 24.Dyce AL. The recognition of nulliparous and parous Culicoides (Diptera: Ceratopogonidae) without dissection. J Aust Entomol Soc. 1969;8:11–15. doi: 10.1111/j.1440-6055.1969.tb00727.x. [DOI] [Google Scholar]
- 25.VSN International . GenStat for Windows. 15. Hemel Hempstead: VSN International; 2012. [Google Scholar]
- 26.Sunrise and sunset times in Pretoria - TimeAndDate.com. https://www.timeanddate.com/sun/south-africa/pretoria. Accessed 21 Feb 2018.
- 27.Tsutsui T, Hayama Y, Yamakawa M, Shirafuji H, Yanase T. Flight behavior of adult Culicoides oxystoma and Culicoides maculatus under different temperatures in the laboratory. Parasitol Res. 2011;108:1575–1578. doi: 10.1007/s00436-010-2048-y. [DOI] [PubMed] [Google Scholar]
- 28.Carpenter S, Wilson A, Barber J, Veronesi E, Mellor P, Venter GJ, et al. Temperature dependence of the incubation period of orbiviruses in Culicoides biting midges. PLoS One. 2011;6:e27987. doi: 10.1371/journal.pone.0027987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wittmann E, Mellor P, Baylis M. Using climate data to map the potential distribution of Culicoides imicola (Diptera: Ceratopogonidae) in Europe. Rev Sci Tech OIE. 2001;20:731–736. doi: 10.20506/rst.20.3.1306. [DOI] [PubMed] [Google Scholar]
- 30.Peters J, De Baets B, Calvete C, Lucientes J, De Clercq E, Ducheyne E, et al. Absence reduction in entomological surveillance data to improve niche-based distribution models for Culicoides imicola. Prev Vet Med. 2011;100:15–28. doi: 10.1016/j.prevetmed.2011.03.004. [DOI] [PubMed] [Google Scholar]
- 31.Purse BV, Mellor PS, Rogers DJ, Samuel AR, Mertens PPC, Baylis M. Climate change and the recent emergence of bluetongue in Europe. Nat Rev Microbiol. 2005;3:171–181. doi: 10.1038/nrmicro1090. [DOI] [PubMed] [Google Scholar]
- 32.Versteirt V, Balenghien T, Tack W, Wint W. A first estimation of Culicoides imicola and Culicoides obsoletus/Culicoides scoticus seasonality and abundance in Europe. EFSA Supporting Publication. 2017;14:1–35. doi: 10.2903/sp.efsa.2017.EN-1182. [DOI] [Google Scholar]
- 33.Purse BV, McCormick BJJ, Mellor PS, Baylis M, Boorman JPT, Borras D, et al. Incriminating bluetongue virus vectors with climate envelope models. J Appl Ecol. 2007;44:1231–1242. doi: 10.1111/j.1365-2664.2007.01342.x. [DOI] [Google Scholar]
- 34.Snyder D, Cernicchiaro N, Cohnstaedt LW. Sugar-feeding status alters biting midge photoattraction. Med Vet Entomol. 2016;30:31–38. doi: 10.1111/mve.12144. [DOI] [PubMed] [Google Scholar]
- 35.Venter GJ, Hermanides KG. Comparison of black and white light for collecting Culicoides imicola and other livestock-associated Culicoides species in South Africa. Vet Parasitol. 2006;142:383–385. doi: 10.1016/j.vetpar.2006.07.015. [DOI] [PubMed] [Google Scholar]
- 36.Viennet E, Garros C, Rakotoarivony I, Allène X, Gardès L, Lhoir J, et al. Host-seeking activity of bluetongue virus vectors: endo/exophagy and circadian rhythm of Culicoides in western Europe. PLoS One. 2012;7:e48120. [DOI] [PMC free article] [PubMed]
- 37.Braverman Y, Chizov-Ginzburg A, Friger M, Mumcuoglu KY. Nocturnal activity of Culicoides imicola Kieffer (Diptera: Ceratopogonidae) in Israel. Russian Entomol J. 2008;17:37–39. [Google Scholar]
- 38.Fall M, Fall AG, Seck MT, Bouyer J, Diarra M, Balenghien T, et al. Circadian activity of Culicoides oxystoma (Diptera: Ceratopogonidae), potential vector of bluetongue and African horse sickness viruses in the Niayes area, Senegal. Parasitol Res. 2015;114:3151–8. [DOI] [PMC free article] [PubMed]
- 39.Fall M, Fall AG, Seck MT, Bouyer J, Diarra M, Lancelot R, et al. Host preferences and circadian rhythm of Culicoides (Diptera: Ceratopogonidae), vectors of African horse sickness and bluetongue viruses in Senegal. Acta Trop. 2015;149:239–245. doi: 10.1016/j.actatropica.2015.06.012. [DOI] [PubMed] [Google Scholar]
- 40.Walker AR. Seasonal fluctuations of Culicoides species (Diptera: Ceratopogonidae) in Kenya. B Entomol Res. 1977;67:217–233. doi: 10.1017/S0007485300011032. [DOI] [Google Scholar]
- 41.Snow WE. Feeding activities of some blood-sucking Diptera with reference to vertical distribution in bottomland forests. Ann Entomol Soc Am. 1955;48:512–521. doi: 10.1093/aesa/48.6.512. [DOI] [Google Scholar]
- 42.Glukhova VM. Types of attack in Culicoides Latr. (Diptera: Heleidae) Entomologicheskoe Obozrenie. 1958;37:330–335. [Google Scholar]
- 43.Humphreys JG, Turner EC. The effect of light intensity upon feeding activity of laboratory reared Culicoides guttipennis (Coquillett) (Diptera: Ceratopogonidae) Mosq News. 1971;31:215–217. [Google Scholar]
- 44.El Sinnary KA, Muller R, Atta el Mannan A, Hussein SH. The diurnal activity of Culicoides kingi in northern Sudan. Rev Elev Med Vet Pays Trop. 1985;38:270–275. [PubMed] [Google Scholar]
- 45.Page PC, Labuschagne K, Nurton JP, Venter GJ, Guthrie AJ. Duration of repellency of N,N-diethyl-3-methylbenzamide, citronella oil and cypermethrin against Culicoides species when applied to polyester mesh. Vet Parasitol. 2009;163:105–109. doi: 10.1016/j.vetpar.2009.03.055. [DOI] [PubMed] [Google Scholar]
- 46.Meiswinkel R, Elbers ARW. The dying of the light: crepuscular activity in Culicoides and impact on light trap efficacy at temperate latitudes. Med Vet Entomol. 2016;30:53–63. doi: 10.1111/mve.12150. [DOI] [PubMed] [Google Scholar]
- 47.Scheffer EG, Venter GJ, Labuschagne K, Page PC, Mullens BA, MacLachlan NJ, et al. Comparison of two trapping methods for Culicoides biting midges and determination of African horse sickness virus prevalence in midge populations at Onderstepoort. South Africa. Vet Parasitol. 2012;185:265–273. doi: 10.1016/j.vetpar.2011.09.037. [DOI] [PubMed] [Google Scholar]
- 48.Paton T. The ‘horse sickness’ of the Cape of Good Hope. Veterinarian. 1863;36:489–494. [Google Scholar]
- 49.Lysyk TJ, Danyk T. Effect of temperature on life history parameters of adult Culicoides sonorensis (Diptera: Ceratopogonidae) in relation to geographic origin and vectorial capacity for bluetongue virus. J Med Entomol. 2007;44:741–751. doi: 10.1093/jmedent/44.5.741. [DOI] [PubMed] [Google Scholar]
- 50.Veronesi E, Venter GJ, Labuschagne K, Mellor PS, Carpenter S. Life-history parameters of Culicoides (Avaritia) imicola Kieffer in the laboratory at different rearing temperatures. Vet Parasitol. 2009;163:370–373. doi: 10.1016/j.vetpar.2009.04.031. [DOI] [PubMed] [Google Scholar]
- 51.Wellby MP, Baylis M, Rawlings P, Mellor PS. Effect of temperature on survival and rate of virogenesis of African horse sickness virus in Culicoides variipennis sonorensis (Diptera: Ceratopogonidae) and its significance in relation to the epidemiology of the disease. B Entomol Res. 1996;86:715–720. doi: 10.1017/S0007485300039237. [DOI] [Google Scholar]
- 52.Paweska JT, Venter GJ, Mellor PS. Vector competence of South African Culicoides species for bluetongue virus serotype 1 (BTV-1) with special reference to the effect of temperature on the rate of virus replication in C. imicola and C. bolitinos. Med Vet Entomol. 2002;16:10–21. doi: 10.1046/j.1365-2915.2002.00334.x. [DOI] [PubMed] [Google Scholar]
- 53.Mullens BA, Gerry AC, Lysyk TJ, Schmidtmann ET. Environmental effects on vector competence and virogenesis of bluetongue virus in Culicoides: interpretating laboratory data in a field context. Vet Ital. 2004;40:160–166. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated and analysed in the study are included in this published article. Further data are archived and available upon request.