Abstract
Background
Anaplasma ovis is a major cause of small ruminant anaplasmosis, a tick-borne disease mainly affecting small ruminants in tropical and subtropical regions of the world. Due to health and production problems in dairy goat flocks in Corsica, France, and the demonstration of A. ovis infection in some animals, an extensive survey was conducted in the island in spring 2016. The aim of the survey was to determine the prevalence and geographical distribution of A. ovis infections in goats and ticks as well as possible relationships with anaemia and other health indicators. In addition, the genetic diversity of A. ovis was evaluated.
Methods
Blood and faecal samples were collected in 55 clinically healthy flocks (10 goats per flock) for A. ovis qPCR, haematocrit determination, paratuberculosis ELISA seropositivity and gastrointestinal nematode egg excretion quantification. Ticks were collected, identified and processed for A. ovis DNA detection.
Results
A high prevalence of A. ovis DNA detection was found at the individual (52.0%) and flock levels (83.6%) with a within-flock prevalence ranging between 0–100%. Rhipicephalus bursa was the only tick species collected on goats (n = 355) and the detection rate of A. ovis DNA in ticks was 20.3%. Anaplasma ovis DNA prevalence was higher in flocks located at an altitude above 168 m, in goats of Corsican/crossbred breed and in goats > 3 years-old. No relationship was found between A. ovis DNA detection at the individual or flock level and haematocrit, paratuberculosis seropositivity or gastrointestinal parasites. Positive A. ovis goat samples were used for amplification of gltA and msp4 genes for species confirmation and strain identification, respectively. Sequence and phylogenetic analysis of these genes confirmed the detection of A. ovis and allowed identification of six different strains of this pathogen (named Corsica 1-6 (COR1-6). While the msp4 sequence of strain COR1 had 100% identity with strains previously reported, COR2 to 6 were found to be novel strains. The strain COR1 was the most represented, corresponding to 94.6% of the msp4 sequences obtained.
Conclusions
The results showed a relatively high genetic diversity of A. ovis associated with high bacterial prevalence in goats.
Electronic supplementary material
The online version of this article (10.1186/s13071-018-3269-7) contains supplementary material, which is available to authorized users.
Keywords: Anaplasma ovis, Prevalence, Dairy goat, Rhipicephalus bursa, Corsica, France, Genetic diversity
Background
Bacteria of the genus Anaplasma (Rickettsiales: Anaplasmataceae) are obligate intracellular microorganisms including important human (e.g. A. phagocytophilum), livestock (e.g. A. phagocytophilum, A. marginale and A. ovis) and pet (e.g. A. platys) pathogens. These pathogens are mainly transmitted by tick bites, though other modes of transmission have been reported such as hematophagous insect bites and exposure to blood-contaminated fomites [1, 2]. Anaplasma ovis is distributed worldwide and is considered as the most frequent cause of small ruminant anaplasmosis but seems to be less pathogenic than other Anaplasma species, causing only subclinical infections with a low grade fever [3]. The bacterium may cause a persistent infection and, in some instances, clinical cases related to haemolytic anaemia may be seen with pallor and icterus, but without haemoglobinuria. The general clinical signs of A. ovis infection include fever, fatigue, anorexia, decrease in milk production and abortion but with a low mortality rate [4]. Outbreaks of acute disease are rare and occur mostly under major stress conditions (e.g. hot weather, undernutrition, low body condition score, vaccination, heavy tick infestation, long-distance transportation and animal movement) and more frequently in goats than in sheep [1, 5]. Additionally, as for other Anaplasma spp., A. ovis infection might predispose to other microbial or parasite infections resulting in exacerbated clinical signs and eventually death [6]. Genetic diversity of tick-borne bacteria resulting in novel strains can be associated with changes in pathogenicity, virulence, shift in host range, prevalence and enhancement of the transmission [7–11]. High genetic diversity of A. marginale was associated with an outbreak of bovine anaplasmosis in an endemic area in Mexico [12], and low genetic diversity in the msp4 gene was associated with low prevalence of A. ovis in 12 provinces in China [13]. Assessment of genetic variability and strain diversity might therefore be crucial to understanding the epidemiology of anaplasmosis and to implementing control measures.
The economic significance of A. ovis outbreaks may be important in countries where animal stocks consist mainly of sheep and goats [14, 15]. However, subclinical disease and the natural resistance acquired by autochthonous A. ovis strains in endemic countries could make it difficult to evaluate the real economic impact of A. ovis infection [16].
Several wild animal species can be infected by A. ovis which further complicates the epidemiological cycle of this bacterium [17, 18]. There are numerous reports of high A. ovis prevalence in different regions of the world and values of up to 40% in A. ovis prevalence are commonly reported in the literature [19–21]. Corsica is an island where small ruminant breeding represents an important economic activity. Sheep and goat production is the third most important agricultural activity of the island [22]. Goat breeders produce 37% of the milk of Corsica [23]. In September 2013, an outbreak of bluetongue virus genotype 1 (BTV-1) was reported in sheep and goat in southern Corsica and the virus spread across the island in early 2014 [24]. Meanwhile, health and production problems were declared by several dairy goat farmers throughout the island during the spring of 2014, including emaciation and a drop in (or even drying up of) milk production, problems that did not seem to be due to BTV1. A preliminary study was then performed by the Regional Livestock Health Association (FRGDSB20) in five affected flocks through individual blood, milk and faecal sampling, as well as a few necropsies (Stephan Zientara and Mélanie Gallois, unpublished). The main investigated pathogens were gastrointestinal nematodes (GIN) and Fasciola (quantitative coproscopy), BTV (rt-RT-PCR), Mycoplasma spp. (bacteriological culture) and different Anaplasma species (qPCR) among others. The main results indicated high levels of GIN egg excretion and a high prevalence of A. ovis infection together with gross lesions suggestive of paratuberculosis (PTB) for five of the 11 necropsies. Due to the controversial clinical impact of A. ovis infection, it was not clear whether A. ovis could be responsible, alone or in association, for the described health problems in the studied goat herds. As a result, the FRGDSB20 decided to perform an extensive prevalence survey on A. ovis infection in dairy goats in Corsica.
Thus, the objectives of the present survey were first to determine the prevalence of A. ovis DNA in goats and ticks and their geographical distribution in dairy goats in Corsica; secondly to determine the relationship of A. ovis infection to other health indicators, namely anaemia, GIN egg excretion and PTB seropositivity; and lastly to investigate the genetic diversity of A. ovis of goat origin.
Methods
Study design
Goat farms participating in the present survey were selected on a convenient basis according to geographical location to represent the breadth of the island. Fifty-five breeders volunteered to participate in this study: 32 from Haute-Corse and 23 from Corse-du-Sud counties (Fig. 1). No particular health problems were reported in the selected flocks at the time of the survey.
Fig. 1.
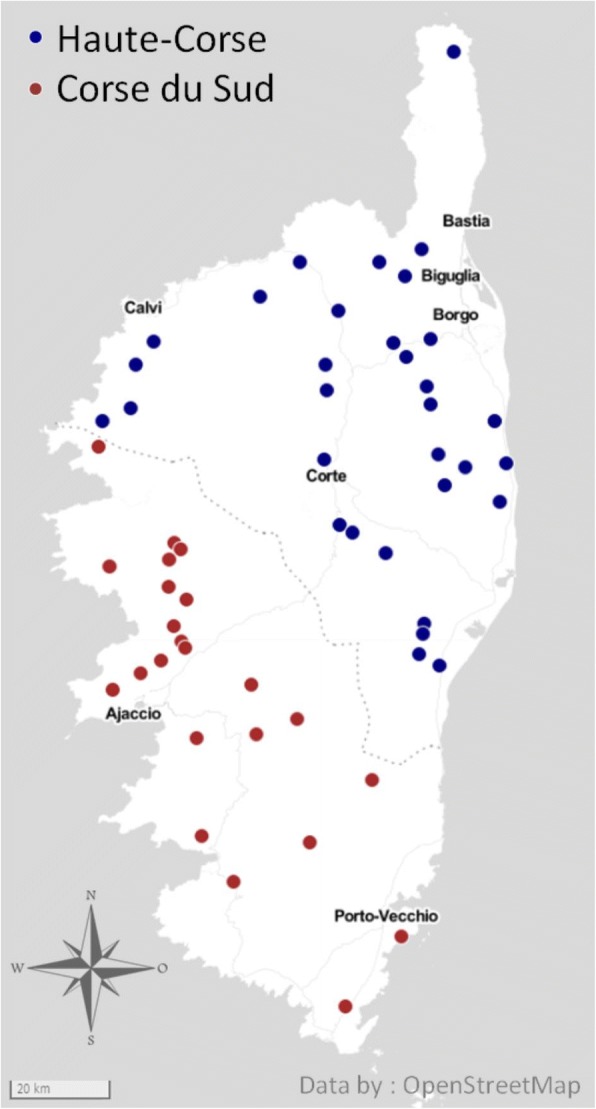
Map of Corsica showing the location of the 55 sampled goat flocks
Sample collection
The field survey was performed between April and June 2016. This period of time was chosen as it fitted with the maximal level of tick infestation of ruminants in Corsica [25]. Ten goats were randomly selected from each of the 55 herds, corresponding to 550 samples in total. To minimize a possible influence of age on the final within-flock outcome, the age of sampled goats was between 2.5–3.5 years. Blood samples were collected from the jugular vein of goats in plain tubes containing EDTA. Individual faecal samples were collected from rectum.
Tick sample collection and identification
Ticks were collected from each goat mainly around the perineum and udder. All ticks were identified under a dissecting microscope according to the keys provided in [26]. All collected ticks were surface-sterilized, conserved in 70% alcohol and further processed for molecular analysis as previously described [27].
Laboratory exams
Hematocrit (Ht, as %) was determined with Hemogold® automat (Kitvia SAS, Labathe-Inard, France), and the cut-off for anemia was defined as ≤ 24%. For each farm, an equal part of the 10 individual faeces (2 g per goat) was mixed to give a pooled faecal sample [28]. The faecal egg count (FEC) of gastrointestinal nematode eggs (expressed as eggs per gram of faeces, epg) was assessed from the pooled sample with the McMaster technique according to Raynaud [29]. FEC can be interpreted as follows: low excretion, < 500 epg; moderate excretion, 500–2000 epg; high excretion > 2000 epg [30]. Individual seroprevalence to paratuberculosis (PTB) was determined using an ELISA assay (IDEXX Paratuberculosis Screening Ab test®, IDEXX France SARL, Saint-Denis, France) following the manufacturer’s instructions. Results were expressed as positive or negative. Doubtful results were considered as negative.
Molecular detection of Anaplasma ovis
DNA extraction
DNA was extracted from 200 μl of blood homogenate using DNeasy Blood and Tissue Kit (Qiagen, Hilden, Germany) following the manufacturer’s instructions. Genomic tick DNA was extracted from 100 μl of individually crushed tick material using the Wizard genomic DNA purification kit (Promega, Charbonnières-les-Bains, France) as previously described [27]. DNA was stored at -20 °C until further use.
Real-time PCR
A real-time PCR assay was performed on DNA extracts from blood (n = 550) and ticks (n = 355) using the primers and probe (FAM and BHQ1 as reporter and quencher molecules, respectively) (Table 1) targeting the msp4 gene for A. ovis [31]. Real-time Taqman PCR was performed in a final volume of 12 μl using LightCycler® 480 Probe Master Mix (Roche Applied Science, Penzberg, Germany) at 1× final concentration. Primers and probe had a concentration of 200 nM, and each reaction included 2 μl of DNA. Negative (water) and positive controls (diluted plasmid, recombinant pBlue scriptIISK+, containing the target gene msp4 of A. ovis) were used with each run. Thermal conditions were as follows: 95 °C for 5 min, 45 cycles at 95 °C for 10 s and then 60 °C for 15 s. The program included a final cooling step at 40 °C for 10 s. Samples were considered positive at a cycle threshold (Ct) value of < 40 and characteristic amplification curves.
Table 1.
Primers used in this study
| Target gene | Primer/probe (5'-3') | Type of PCR | Tm (°C)a | Fragment length (bp) |
|---|---|---|---|---|
| msp4 | TCATTCGACATGCGTGAGTCA | Real-time | 60 | 92 |
| TTTGCTGGCGCACTCACATC | 60 | |||
| FAM-AGCAGAGAGACCTCGTATGTTAGAGGC-BQ1 | 70 | |||
| gltA | GCCGACTTTGTTGCCACTGT | Qualitative | 58.7 | 760 |
| TCCAACCGCCCTTAGCACAA | 59.4 | |||
| msp4 | CCCAGCGTTTCCCTCTGTTA | 57.1 | 497 | |
| GAGTCCGTGGTAGAACCCAC | 57.3 |
aMelting temperature
Analysis of genetic diversity of Anaplasma ovis
gltA and msp4 primers design and qualitative PCR
gltA and msp4 primers were designed in the present study using sequences available on GenBank. Sequences were aligned using MAFFT and conserved regions identified [32]. Primers targeting the conserved regions of the genes were then designed by Primer-BLAST [33]. Primer properties were further analysed using OligoAnalyzer [34]. Selected primers were then synthetized (Eurofins Scientific Laboratories, Paris, France) (Table 1). Amplifications were achieved with Phusion High-Fidelity DNA Polymerase (Thermo Fisher Scientific, Waltham, MA, USA) and a Doppio thermocycler (VWR, Radnor, PA, USA). For each reaction, 4 μl of buffer solution, 0.4 μl of dNTPs (10 mM), 1 μl of forward and reverse primer (20 μM), 0.2 μl of DNA Phusion polymerase (0.02 U/μl) and 5 μl of genomic DNA were used in a final volume of 20 μl.
Selection of samples for sequencing
We attempted to amplify fragments of gltA and msp4 genes in the 286 goat samples positive to A. ovis by real-time PCR. No tick sample was selected for sequencing. Amplicons were deposited on 1.5% agarose gel stained with BET and migrated in TAE 1× to check their length using a 1 kb DNA Ladder (NEB, Hitchin, UK). When a single band at the expected length was observed, the PCR product was considered positive and kept at -20 °C until sequencing. If in addition to the band of the expected size, several bands were observed, the band of the expected size was excised from the gel and purified using GeneJET Gel Extraction Kit (Thermo Scientific). The 143 and 142 samples positive for gltA and msp4 were sent for sequencing and 133 and 103 sequences, respectively, were obtained.
Sequence alignment and phylogenetic analysis
gltA homologous sequences were retrieved from GenBank to represent different species of Anaplasmataceae, genera Anaplasma, Ehrlichia and Neorickettsia, as follows: Anaplasma ovis (GenBank: KJ410285), A. marginale (AF304139), A. centrale (AF304141), A. capra (KM206274), A. platys (AB058782), A. phagocytophilum (AY464138), A. bovis (KU586317), Ehrlichia ruminantium (DO513397), E. ewingii (DQ365879), E. canis (AY647155), E. chaffeensis (AF304142), E. muris (AF304144), Neorickettsia sennetsu (AF304148) and N. risticii (AF304147). For msp4, homologous sequences of different species belonging to the genus Anaplasma were retrieved from GenBank as follows: A. ovis (KU497712), A. marginale (KU497715), A. centrale (AF428090), A. capra (KM206277) and A. phagocytophilum (EU008082). Sequences were translated into proteins using the ExPASy translate tool [35]. For strain analysis, msp4 nucleotide and MSP4 protein sequences belonging to A. ovis strains from all over the world were selected from GenBank as shown in Additional file 1: Figure S1 and Additional file 2: Figure S2, respectively. For each gene, sequences were aligned with MAFFT (v.7) configured for the highest accuracy using the scoring matrix 200PAM/kD2, alignment strategy MAFFT-FFT-NS-I, gap opening penalty 1.53 and offset value 0.123.
Non-aligned regions were removed using Molecular Evolutionary Genetics Analysis (MEGA) v.6 software [36]. The best-fit model of sequence evolution was selected based on the corrected Akaike information criterion (cAIC) and Bayesian information criterion (BIC) implemented in MEGA. Tamura 3-parameters [37] and JTT models [38], which had the lowest values of cAIC and BIC, were chosen for gltA and msp4 and MSP4 tree reconstruction, respectively. The maximum likelihood method, implemented in MEGA, was used to obtain the best tree topologies. Reliability of internal branches was assessed using the bootstrapping method with 1000 bootstrap replicates [39].
Statistical analysis
The individual/flock prevalence was calculated as the ratio of positive animals/flocks on sampled animals/flocks, a positive flock being a flock with at least one positive animal. Within-flock prevalence was calculated as the ratio of positive animals on sampled animals in a given flock. Relationships between qualitative results, correlations between quantitative variables and comparisons of means were tested with Chi-square or Fisher’s tests, Spearman’s rank correlation test and Kruskal-Wallis test using R software v.3.1.0 [40]. Alpha was set at 0.05.
A principal components analysis (PCA) was performed with R Studio [41] using the following matrix: the rows were the 55 flocks and columns were the corresponding values for A. ovis infection (number of infected animals), paratuberculosis seropositivity (number of seropositive animals), gastrointestinal strongyles egg excretion (pooled faecal egg count) and anaemia prevalence (number of anaemic animals). The variables were represented on the principal plane. The nearer the variables were located to the correlation circle, the better they were represented in the plane. The relative position of the variables on the plane corresponded to either a positive (near variables), a negative (variables diagonally opposed on the plane), or no association (variables at a right angle).
Results
General and health data
The 55 dairy goat flocks were distributed on the whole area of the island, except for the central mountain chain, at a mean altitude of 329 m (range: 19–810 m) (Fig. 1). The mean number of goats per flock was 148 (range: 27–500) and the breed was mainly the Corsican breed (76% of flocks), remaining breeds being Alpine and crossbred. At the individual level, the mean haematocrit was 27.6 ± 5.0% (range: 16.5–41.4%) with 23% of goats exhibiting anaemia (Ht ≤ 24%). At the flock level, 78% of flocks showed at least one goat with anaemia and the mean within-flock prevalence of anaemia was 23 ± 22% (range: 0–100%). The individual seroprevalence of PTB was 9.8% (excluding 27 vaccinated animals) whereas the within-flock seroprevalence varied from 0 to 50% and was 9.4% on average. Pooled faecal egg counts in the flocks ranged between 0–4500 epg with an average of 517 epg. Excretion was < 500, 500–2000 and > 2000 epg in 74.5, 20 and 5.5% of the farms, respectively.
Prevalence of Anaplasma ovis DNA in goats
The individual prevalence of A. ovis DNA in goats was 52.0%, including 286 positive samples on 550 total samples. The within-flock prevalence (55 flocks) estimated from 10 individual results per flock varied from 0 to 100% with a mean of 52 ± 32% (Fig. 2). Fourteen and 15 herds had infection rates below 20% and above 80%, respectively. The A. ovis flock prevalence was 83.6% (flocks showing at least one A. ovis positive animal). Individual prevalence was significantly higher at an altitude > 168 m, in the southern county, in animals of Corsican breed and in animals aged > 3 years (Table 2). Similar associations were observed for flock prevalence with altitude and breeds.
Fig. 2.
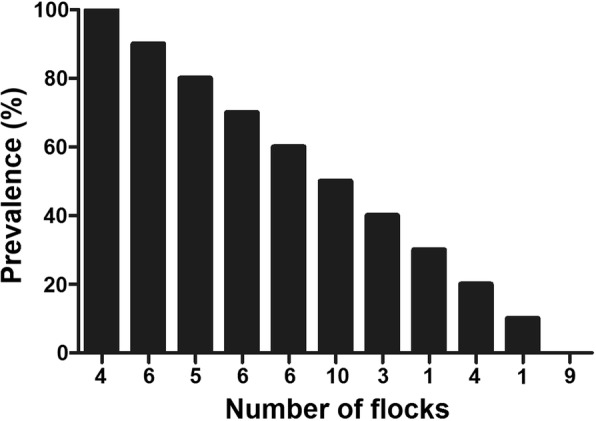
Anaplasma ovis prevalence in 55 goat flocks of Corsica. The prevalence of A. ovis detected by real-time PCR was highly variable among herds; while some herds had 0% infection rate, others had 100% infection rate
Table 2.
Individual prevalence of Anaplasma ovis in dairy goat in Corsica according to altitude, county, goat breed and age
| Altitude in m (terciles) | County | Goat breed | Goat age | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 0–168 | 168–437 | > 437 | Corse du Sud | Haute-Corse | Corsican + cross bred | Other | ≤ 3 years | > 3 years | |
| Individual prevalence (%) | 36.7 | 53.3 | 65.3 | 60.9 | 45.6 | 59.5 | 27.7 | 42.9 | 62.1 |
| P-values of Chi-square test | P < 0.05 | P < 0.05 | P < 0.05 | P < 0.05 | |||||
Relationship between Anaplasma ovis infection and health parameters at individual or flock levels
The individual prevalence of A. ovis DNA was not different between goats with or without anaemia (55.9 vs 50.8%, P = 0.31). Furthermore, no significant correlation was seen at flock level between within-flock prevalence of A. ovis DNA and within-flock prevalence of anaemia (rs = 0.14, P > 0.1). Results of the principal components analysis using A. ovis DNA detection, GIN egg excretion, PTB seropositivity and anaemia prevalence at flock level as variables are presented in Fig. 3. The first two axes explained 66% of the total inertia and the main contributors were GIN and anaemia on 1st axis and A. ovis on the 2nd axis. Anaplasma ovis was unrelated to anaemia, PTB seropositivity and GIN. However, a close association was found between GIN and anaemia (particularly on the 1st axis).
Fig. 3.
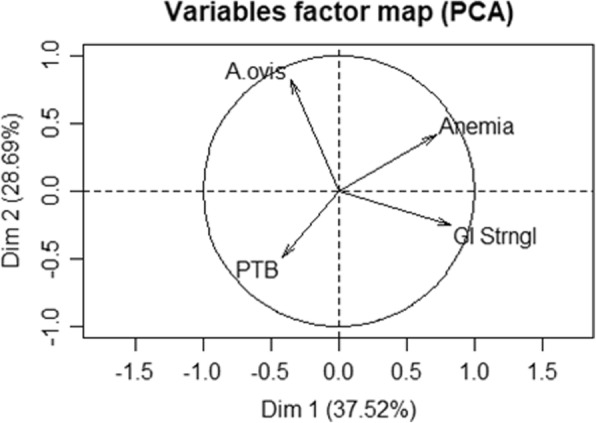
Principal components analysis to test relation between Anaplasma ovis infection, anaemia and other health indicators. Relationships between number of A. ovis infected animals (‘A. ovis’), number of paratuberculosis seropositive animals (‘PTB’), gastrointestinal strongyles pooled faecal egg count (‘GI Strngl’) and number of anaemic animals (‘Anemia’) in 55 goat flocks of Corsica. Principal components analysis (PCA) variables are located on the main plane defined by the 1st and 2nd axis and circle of correlation
Tick burden and prevalence of Anaplasma ovis infection in ticks
Ticks were found in the whole area of the survey. Zero to 27 ticks were observed from each goat and at least one tick was recorded on a goat in 58.2% of flocks. All sampled specimens (n = 355) were Rhipicephalus bursa adult ticks. No significant relationship was observed between A. ovis within-flock prevalence and the number of ticks observed in the flocks (P = 0.46). Anaplasma ovis DNA was detected in 20.3% of the ticks and 51.6% of the tick-infested flocks showed at least one A. ovis-infected tick. Tick positive samples were not selected for sequencing.
Anaplasma ovis gltA and msp4 nucleotide sequences and phylogenetic analysis
From the 286 positive goat samples, we attempted to amplify fragments of gltA and msp4 genes for further sequence and phylogenetic analysis. Using the primers shown in Table 1 for a qualitative PCR assay, 143 (50.0%) and 142 (49.7%) samples were positive for gltA and msp4, respectively. Among these positive samples, 86 (30.8%) and 99 (35.4%) were positive for one of the genes (gltA or msp4) and the two genes (gltA and msp4), respectively. From the 143 gltA-positive and 142 msp4-positive samples, 133 (93.0%) and 103 (72.5%) sequences, respectively, were obtained.
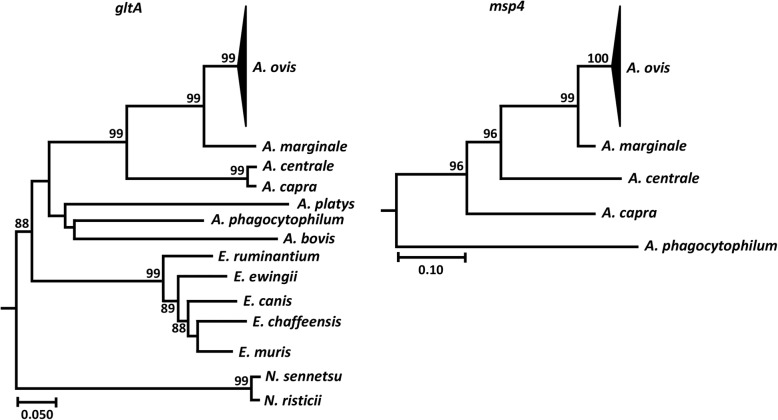
Species classification based on real-time and qualitative PCR assays was confirmed by sequencing. All amplified gltA sequences formed a unique clade with an A. ovis reference sequence (GenBank: KJ410285) included in the phylogenetic tree (Fig. 4). Similar results were obtained when msp4 sequences were used to build the phylogenetic tree. All amplified msp4 sequences clustered with the A. ovis reference sequence (GenBank: KU497712) (Fig. 4).
Fig. 4.
Phylogenetic analysis of Anaplasma ovis gltA and msp4 nucleotide sequences identified in Corsica. Maximum likelihood phylogenetic trees were inferred using gltA and msp4 nucleotide sequences of A. ovis identified in Corsica and other bacteria of the family Anaplasmataceae (see Methods for accession numbers). All gltA and msp4 nucleotide sequences of Corsica formed a unique clade with A. ovis reference sequences, KJ410285 (gltA) and KU497712 (msp4). The clades containing A. ovis sequences were collapsed. Reliability of internal branches was assessed using the bootstrapping method (1000 replicates)
Anaplasma ovis strain diversity analysis using MSP4 sequences
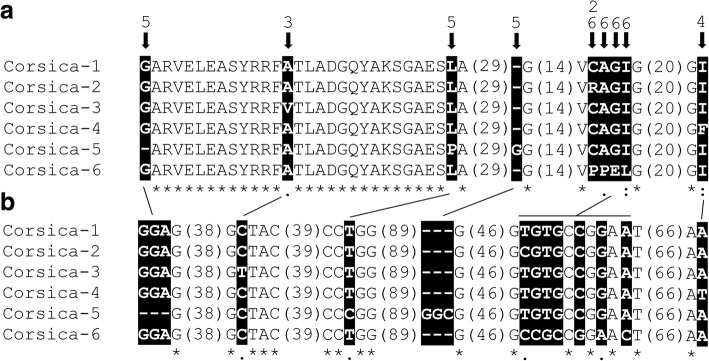
Following theoretical translation of all msp4 nucleotide sequences into proteins, six different sequences were identified and named strains Corsica 1-6 (hereafter COR1-6, GenBank: MH121148-MH121152). Protein sequence alignment revealed amino acid substitutions and indels that explained the genetic diversity among these strains (Fig. 5a). The strain COR1 was the most frequently found and therefore it was considered as the reference strain in further comparisons. COR2 differed from the reference sequence in a substitution from cysteine (C) to arginine (R). Compared to COR1, strains COR3 and COR4 also had one amino acid substitution from alanine (A) to valine (V) and from isoleucine (I) to phenylalanine (P), respectively. Remarkably, strain COR5 had one deletion and one insertion of a glycine (G) compared to COR1-4 and COR6 and, in addition, a substitution from leucine (L) to proline (P) (Fig. 5a). Finally, strain COR6 differs from the reference sequence by a fragment of 4 consecutive amino acids, specifically PP - glutamic acid (E) - L (PPEL) to CAGI. Except for strains COR2 and COR6 with one amino acid change in a common position, amino acid substitutions were located in different amino acid positions along the MSP4 sequence. The change of hydrophilic into hydrophobic amino acid, and vice versa, was observed twice, one in strain COR2 (C to R) and the other in strain COR6 (G to E). The other amino acid changes were conservative and resulted in hydrophobic amino acid substitutions.
Fig. 5.
Anaplasma ovis strain analysis based in msp4 sequences. a Six different strains of A. ovis were identified in Corsica (COR 1-6, GenBank: MH121148-MH121152) and positions with amino acid polymorphisms among them are highlighted in black boxes. For figure simplification purpose, large stretches of regions with 100% identity were removed from the figure and the amino acid length of these regions is shown in parentheses. b The non-synonymous nucleotide substitutions and indels resulting in MSP4 amino acid variability are highlighted in black boxes
Genetic basis of msp4 sequence variability
Nucleotide sequences analysis of msp4 revealed non-synonymous substitutions that resulted in the MSP4 amino acid variability (Fig. 5b). Strains COR2 and COR5 differed from the reference strain in one nucleotide substitution, thymidine (T) to cytosine (C). In addition, compared to COR1, strain COR3 had T in place of C and strain COR4 contained T instead of adenine (A). Finally, 7 nucleotide substitutions were identified in strain COR6 compared to COR1. In particular, COR6 had 4 successive substitutions (CCGC instead of TGTG; G corresponding to guanine) and 3 additional substitutions as follows: C to G, G to A and A to C. Only two synonymous substitutions were identified, one in the first aspartic acid (D) codon of strain COR3 (C to T) and the other in the second V codon of strain COR4 (T to G).
Frequency of Anaplasma ovis strains in goats in Corsica
The strain COR1 was the most represented and was found in 95 samples, corresponding to 94.6% of the msp4 sequences obtained. Strain COR2 was found in two samples and represented 1.9% of the sequences obtained. The strains COR3-6 were represented by only 1 sample and each corresponded to 0.9% of the sequences obtained.
Comparison between Anaplasma ovis strains in Corsica and other regions
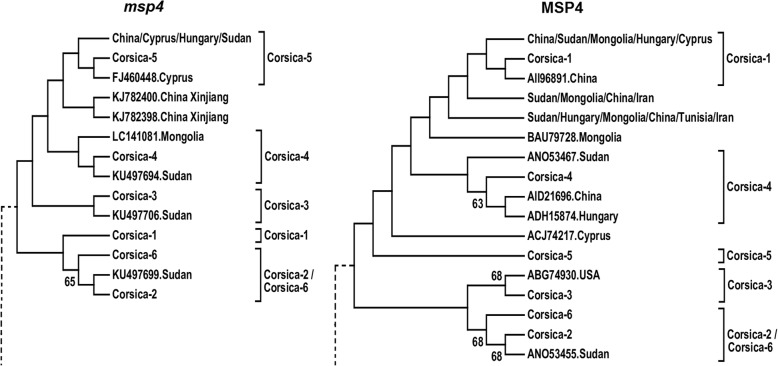
Among the six A. ovis strains identified in Corsica, the msp4 nucleotide and MSP4 protein sequences of COR1 had 100% identity with strains previously reported in Cyprus (GenBank gene/protein accession numbers FJ460454/ACJ74223), Mongolia (GenBank gene/protein accession numbers LC141078/BAU79725) and other countries. None of the other strains (COR2, COR3, COR4, COR5 and COR6) were 100% identical to any previously reported A. ovis strains. To study the evolutionary history of the A. ovis strains of Corsica, phylogenetic trees were constructed using msp4 nucleotide and MSP4 protein sequences. Sequences that were 100% identical between them were excluded from the analysis. Except for COR2 and COR6 that were closely related between them (Fig. 6), both trees showed that the other COR strains fall in independent clusters.
Fig. 6.
Phylogenetic analysis of Anaplasma ovis msp4 sequences identified in Corsica and other regions of the world. Maximum likelihood phylogenetic trees were inferred using msp4 nucleotide and MSP4 protein sequences of A. ovis of Corsica (COR 1-6, GenBank: MH121148-MH121152) and other regions of the world. To simplify figure display, clades that did not contain Corsica strains were removed (dashed lines) or collapsed (countries of origin in bold letters). Clades containing different Corsica strains were labeled with the name of the strain. A detailed version of the cladograms for msp4 and MSP4 are available in Additional file 1: Figure S1 and Additional file 2: Figure S2, respectively. GenBank accession numbers and country of origin are shown
Discussion
Using a large sample (n = 550) and molecular tools, we made a robust assessment of the prevalence, distribution and genetic diversity of A. ovis in goats in Corsica. The main findings of this study were: (i) high prevalence and wide distribution of A. ovis in Corsica; (ii) no link between health indicators and A. ovis infection in goats in apparent healthy flocks; and (iii) high genetic diversity of A. ovis. Our results are the first report of A. ovis in goats from Corsica and should be compared to the prevalence reported in other Mediterranean areas. Anaplasma ovis has been reported in Sicily [42], Sardinia [43] and Cyprus [44]. Corsica, Sicily, Sardinia and Cyprus share similar ecosystems, providing good models of the epidemiological traits of A. ovis in the Mediterranean basin and showing large variations in A. ovis prevalence from one area to another. For instance, in Italy, A. ovis prevalence in goats was 31.7% in southern Italy [20], 19% in Sicily [42] and 81.8% in Sardinia [43]. It is noteworthy that A. ovis was previously reported in sheep in Corsica [45] and Italy [20, 42, 43].
In our study, goats aged over three years, of Corsican breed/crossbred and raised above 168 m of altitude were more at risk for A. ovis infection. Infection of aged animals was previously reported by Zhou et al. [46] in sheep in Turkey and could be explained by potentially higher contact rates with infected vectors in the field. In contrast, the breed and altitude effects remain unclear because breeds other than Corsican/crossbred are mainly raised on the lowlands. Aktas & Ozübeck [47] reported an association between A. ovis infection in sheep and goats and the presence of Rhipicephalus bursa and R. turanicus in four provinces of south-eastern Turkey. In our study, R. bursa was the only tick species found on goats, which agrees with the study of Grech-Angelini et al. [25] who reported this tick species as being the most abundant on goats in Corsica. Rhipicephalus bursa is a typical tick species of the Mediterranean area from coast to mountainous area and has been shown to be one of the main biological vectors of A. ovis both in natural and experimental conditions [1]. We found 20.3% of R. bursa infected with A. ovis which can be considered as a high rate of infection when compared with 7.9% in a previous survey in the French Basque Country after an epizootic outbreak of sheep anaplasmosis [48]. These results suggest a high level of transmission of A. ovis between ticks and goats in Corsica.
Most of published studies on A. ovis prevalence were performed on clinically healthy sheep or goats. However, as for other Anaplasma spp., A. ovis infection could be more severe in stressful situations or in the presence of co-infections [6, 15]. In this study, we investigated the relationship between A. ovis infection at individual and flock level and some heath parameters (i.e. haematocrit, GIN egg excretion and paratuberculosis seropositivity). No significant bivariate or multivariate association was found between these health indicators suggesting that A. ovis infection does not affect these parameters. Although haematocrit is considered as one of the most informative clinical criterion for A. ovis infection in sheep [49], this parameter is not specific and this was illustrated in our study with the association between anemia and GIN egg excretion through principal components analysis. Regarding tick-borne pathogen co-infection, Aktas & Ozübeck [47] showed that sheep infected with Babesia and Theileria had higher A. ovis prevalence in Turkey [47]. The possible occurrence of co-infections in goats deserves special attention because R. bursa is the vector of many important tick-borne pathogens occurring in livestock in Corsica [25, 45, 50].
The msp4 gene is routinely used for the characterization of genetic diversity of Anaplasma spp., including A. marginale, A. phagocytophilum and A. ovis [17, 51]. Based on msp4 sequence analysis, we found a relatively high genetic diversity of A. ovis in Corsica where six strains (COR1-6) were identified. Two lines of evidence supported the idea of high genetic diversity of A. ovis in Corsica. First, based on the phylogenetic analysis, except for COR2 and COR6, the six A. ovis COR strains fell in different clusters (Fig. 6). This suggests that most COR strains do not share a recent common ancestor. Secondly, compared with previous reports in different host species and regions [13, 52–54], six is a relatively high number of A. ovis strains identified in a relatively small area such as Corsica. For instance, in Turkey, only one A. ovis msp4 genotype was reported in goat and sheep [46]. A similar result was reported in Sicily [42, 55]. In Tunisia, only two A. ovis msp4 genotypes, previously described in Italy, were identified in sheep and goats [54]. Low genetic diversity was also reported in North America where only one msp4 genotype was found in Bighorn sheep [17]. Likewise, only one A. ovis msp4 genotype, closely related to the A. ovis genotype AOI from Italy, was found in sheep in the region of Xinjiang, northwest China [21]. Another study conducted to investigate the occurrence and characterize A. ovis strains from goats and sheep from 12 provinces in China identified only two msp4 genotypes of these bacteria [13]. Thus, genetic diversity analysis of A. ovis msp4 rarely identified more than two genotypes in sheep or goats. In contrast to the above reports, and in agreement with the results of this study, one study in continental Europe (i.e. Hungary) reported five A. ovis msp4 variants in samples collected from sheep across the whole country [52]. In addition, phylogenetic analysis of A. ovis msp4 sequences from sheep and goats in Hubei, Guizhou, Zhejiang and northern China identified six different genotypes [56]. Considering the size of the studied regions, it is remarkable that the same number of A. ovis strains (i.e. six) was found in this study in Corsica (area 8 680 km2) and China (area 9 597 000 km2) [56].
While the genetic diversity of A. ovis between regions is variable, it is a common finding of studies addressing the genetic diversity and prevalence of A. ovis that one A. ovis strain is frequently found to be dominant (i.e. infecting most of the animals) [13, 52–54]. In agreement with previous reports, the strain COR1 was found in 94.6% of the msp4 sequences obtained, while the other strains (COR2-6) were poorly represented. However, the association between high genetic diversity and high prevalence of A. ovis in Corsica differs from previous reports. For instance, low genetic diversity of msp4 sequences was associated with low and high prevalence of A. ovis in China [13] and Turkey [17, 53], respectively. In the study by Liu et al. [56], low prevalence (15.3%) was associated with high genetic diversity. Thus, the epidemiological setting of A. ovis in Corsica can be considered as unique compared to that reported in China and Turkey.
Conclusions
In this study, we detected a high individual and flock prevalence of A. ovis DNA in clinically healthy goat flocks in Corsica and the occurrence of A. ovis DNA in Rhipicephalus bursa ticks. No relationship was found between A. ovis infection at the individual or flock level and some relevant health indicators such as haematocrit, gastrointestinal infection or paratuberculosis seropositivity. The high prevalence of A. ovis in goats in Corsica was associated with high genetic diversity of this bacterium, one out of the six identified strains representing a large majority of the sequenced samples. Further studies are needed to evaluate the pathogenic potential of these strains for goats in this insular environment.
Additional files
Figure S1. Phylogenetic analysis of Anaplasma ovis msp4 sequences identified in Corsica and other regions of the world. The figure shows a maximum likelihood phylogenetic tree inferred using msp4 nucleotide sequences of A. ovis of Corsica (Corsica 1-6) and other regions of the world. GenBank accession numbers and country of origin are shown. (PDF 15 kb)
Figure S2. Phylogenetic analysis of Anaplasma ovis MSP4 sequences identified in Corsica and other regions of the world. The figure shows a maximum likelihood phylogenetic tree inferred using MSP4 amino acid sequences of A. ovis of Corsica (Corsica 1-6) and other regions of the world. GenBank accession numbers and country of origin are shown. (PDF 13 kb)
Acknowledgements
Not applicable.
Funding
Not applicable.
Availability of data and materials
All data generated or analysed during this study are included in this published article. Nucleotide sequences were submitted to the GenBank database under the accession numbers KJ410285, KU497712, MH121148, MH121149, MH121150, MH121151 and MH121152.
Abbreviations
- PCR
Polymerase chain reaction
- qPCR
Quantitative PCR
- dNTPs
Deoxynucleotide triphosphates
- GIN
Gastrointestinal nematodes
- PTB
Paratuberculosis
- msp4
Major surface protein 4
- gltA
Citrate synthase
- DNA
Deoxyribonucleic acid
- Ht
Hematocrit
- FEC
Faecal egg count
- epg
Eggs per gram
- PCA
Principal components analysis
Authors’ contributions
ACC, MG, MVT and CC conceived the idea. ACC, EA and MF performed formal analysis and prepared the figures. MD, ED, MF, MM and AA performed the experiments. ACC, MG, MVT, CC and SM supervised the work. MVT and SM provided methodology. ACC, MF, EA and CC wrote the manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to participate
This study was carried out on commercial dairy goat farms and therefore on private land. On the basis of an accurate description of the objectives of the study and its design, the owners of the land (the farmers) gave permission to conduct the study on their farm with their animals, agreed to follow the sampling protocol and to participate in the handling of goats. No other permission was required. No goat was sacrificed for the purposes of the study and the field study did not involve endangered or protected species. The epidemiological survey did not involve harm or cruelty to animals.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Alejandro Cabezas-Cruz, Email: cabezasalejandrocruz@gmail.com.
Mélanie Gallois, Email: melanie.gallois@frgdsb20.fr.
Mélanie Fontugne, Email: mel.fontugne@gmail.com.
Eléonore Allain, Email: eleonore.allain@hotmail.fr.
Myriam Denoual, Email: myriam.denoual@oniris-nantes.fr.
Sara Moutailler, Email: sara.moutailler@anses.fr.
Elodie Devillers, Email: elodie.devillers@anses.fr.
Stephan Zientara, Email: stephan.zientara@anses.fr.
Marc Memmi, Email: Mmemmi@haute-corse.fr.
Alain Chauvin, Email: alain.chauvin@oniris-nantes.fr.
Albert Agoulon, Email: albert.agoulon@oniris-nantes.fr.
Muriel Vayssier-Taussat, Email: Muriel.Vayssier@inra.fr.
Christophe Chartier, Email: christophe.chartier@oniris-nantes.fr.
References
- 1.Friedhoff KF. Tick-borne diseases of sheep and goats caused by Babesia, Theileria or Anaplasma spp. Parassitologia. 1997;39:99–109. [PubMed] [Google Scholar]
- 2.Hornok S, de la Fuente J, Biró N, Fernández de Mera IG, Meli ML, Elek V, et al. First molecular evidence of Anaplasma ovis and Rickettsia spp. in keds (Diptera: Hippoboscidae) of sheep and wild ruminants. Vector Borne Zoonotic Dis. 2011;11:1319–1321. doi: 10.1089/vbz.2011.0649. [DOI] [PubMed] [Google Scholar]
- 3.Kuttler KL. Anaplasma infections in wild and domestic ruminants: a review. J Wildl Dis. 1984;20:12–20. doi: 10.7589/0090-3558-20.1.12. [DOI] [PubMed] [Google Scholar]
- 4.Stuen S, Longbottom D. Treatment and control of chlamydial and rickettsial infections in sheep and goats. Vet Clin North Am Food Anim Pract. 2011;27:213–233. doi: 10.1016/j.cvfa.2010.10.017. [DOI] [PubMed] [Google Scholar]
- 5.Renneker S, Abdo J, Salih DEA, Karagenç T, Bilgiç H, Torina A, et al. Can Anaplasma ovis in small ruminants be neglected any longer? Transbound Emerg Dis. 2013;60:105–112. doi: 10.1111/tbed.12149. [DOI] [PubMed] [Google Scholar]
- 6.Kocan K, de la Fuente J, Blouin EF, Coetzee JF, Ewing SA. The natural history of Anaplasma marginale. Vet Parasitol. 2010;167:95–107. doi: 10.1016/j.vetpar.2009.09.012. [DOI] [PubMed] [Google Scholar]
- 7.Cabezas-Cruz A, Valdés JJ, de la Fuente J. The glycoprotein TRP36 of Ehrlichia sp. UFMG-EV and related cattle pathogen Ehrlichia sp. UFMT-BV evolved from a highly variable clade of E. canis under adaptive diversifying selection. Parasit Vectors. 2014;7:584. doi: 10.1186/s13071-014-0584-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Castañeda-Ortiz EJ, Ueti MW, Camacho-Nuez M, Mosqueda JJ, Mousel MR, Johnson WC, et al. Association of Anaplasma marginale strain superinfection with infection prevalence within tropical regions. PLoS One. 2015;10:e0120748. doi: 10.1371/journal.pone.0120748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Aardema ML, von Loewenich FD. Varying influences of selection and demography in host-adapted populations of the tick-transmitted bacterium, Anaplasma phagocytophilum. BMC Evol Biol. 2015;15:58. doi: 10.1186/s12862-015-0335-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Noh SM, Dark MJ, Reif KE, Ueti MW, Kappmeyer LS, Scoles GA, et al. Superinfection exclusion of the ruminant pathogen Anaplasma marginale in its tick vector is dependent on the time between exposures to the strains. Appl Environ Microbiol. 2016;82:3217–3224. doi: 10.1128/AEM.00190-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cerar T, Strle F, Stupica D, Ruzic-Sabljic E, McHugh G, Steere AC, et al. Differences in genotype, clinical features, and inflammatory potential of Borrelia burgdorferi sensu stricto strains from Europe and the United States. Emerg Infect Dis. 2016;22:818–827. doi: 10.3201/eid2205.151806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Almazán C, Medrano C, Ortiz M, de la Fuente J. Genetic diversity of Anaplasma marginale strains from an outbreak of bovine anaplasmosis in an endemic area. Vet Parasitol. 2008;158:103–109. doi: 10.1016/j.vetpar.2008.08.015. [DOI] [PubMed] [Google Scholar]
- 13.Han R, Yang J, Liu Z, Gao S, Niu Q, Hassan MA, et al. Characterization of Anaplasma ovis strains using the major surface protein 1a repeat sequences. Parasit Vectors. 2017;10:447. doi: 10.1186/s13071-017-2363-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rymaszewska A, Grenda S. Bacteria of the genus Anaplasma - characteristics of Anaplasma and their vectors: a review. Vet Med. 2008;53:573–584. doi: 10.17221/1861-VETMED. [DOI] [Google Scholar]
- 15.Yasini S, Khaki Z, Rahbari S, Kazemi B, Amoli JS, Gharabaghi A, et al. Hematologic and clinical aspects of experimental ovine anaplasmosis caused by Anaplasma ovis in Iran. Iran J Parasitol. 2012;7:91–98. [PMC free article] [PubMed] [Google Scholar]
- 16.Torina A, Khoury C, Caracappa S, Maroli M. Ticks infesting livestock on farms in western Sicily, Italy. Exp Appl Acarol. 2006;38:75–86. doi: 10.1007/s10493-005-5629-1. [DOI] [PubMed] [Google Scholar]
- 17.de la Fuente J, Atkinson MW, Naranjo V, Fernández de Mera IG, Mangold AJ, Keating KA, et al. Sequence analysis of the msp4 gene of Anaplasma ovis strains. Vet Microbiol. 2007;119:375–381. doi: 10.1016/j.vetmic.2006.09.011. [DOI] [PubMed] [Google Scholar]
- 18.de la Fuente J, Ruiz-Fons F, Naranjo V, Torina A, Rodríguez O, Gortázar C. Evidence of Anaplasma infections in European roe deer (Capreolus capreolus) from southern Spain. Res Vet Sci. 2008;84:382–386. doi: 10.1016/j.rvsc.2007.05.018. [DOI] [PubMed] [Google Scholar]
- 19.Razmi GR, Dastjerdi K, Hossieni H, Naghibi A, Barati F, Aslani MR. An epidemiological study on Anaplasma infection in cattle, sheep, and goats in Mashhad Suburb, Khorasan Province, Iran. Ann N Y Acad Sci. 2006;1078:479–481. doi: 10.1196/annals.1374.089. [DOI] [PubMed] [Google Scholar]
- 20.Torina A, Caracappa S. Tick-borne diseases in sheep and goats: clinical and diagnostic aspects. Small Rumin Res. 2012;106(Suppl.):6–11.
- 21.Yang J, Li Y, Liu Z, Liu J, Niu Q, Ren Q, et al. Molecular detection and characterization of Anaplasma spp. in sheep and cattle from Xinjiang, northwest China. Parasit Vectors. 2015;8:108. doi: 10.1186/s13071-015-0727-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Agreste: La statistique agricole. L’élevage des petits ruminants en Corse. In: Recensement Agricole 2010. Agreste Corse n°5. 2013. agreste.agriculture.gouv.fr/IMG/pdf/R9413A05.pdf. Accessed 1 Sept 2018.
- 23.Huyssen A, Battesti B, Albertini C, Vachet S, Bonnefont M, Luciani A, et al. Le bilan économique, l’amélioration se confirme en 2016. In: Insee Conjonct, Corse n°15. Mai 2017. https://www.epsilon.insee.fr/jspui/bitstream/1/57734/1/IC_CO_15.pdf. Accessed 1 Sept 2018.
- 24.Zientara S, Sailleau C, Viarouge C, Hoper D, Beer M, Jenckel M, et al. Novel bluetongue virus in goats, Corsica, France, 2014. Emerg Infect Dis. 2014;20:2123–2125. doi: 10.3201/eid2012.140924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Grech-Angelini S, Stachurski F, Lancelot R, Boissier J, Allienne JF, Marco S, et al. Ticks (Acari: Ixodidae) infesting cattle and some other domestic and wild hosts on the French Mediterranean island of Corsica. Parasit Vectors. 2016;9:582. doi: 10.1186/s13071-016-1876-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pérez-Eid C. Les tiques: identification, biologie, importance médicale et vétérinaire. Paris: Editions Tec & Doc / EM Internationale; 2007. [Google Scholar]
- 27.Moutailler S, Valiente Moro C, Vaumourin E, Michelet L, Tran FH, Devillers E, et al. Co-infection of ticks: the rule rather than the exception. PLoS Negl Trop Dis. 2016;10:e0004539. doi: 10.1371/journal.pntd.0004539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rinaldi L, Levecke B, Bosco A, Ianniello D, Pepea P, Charlier J, et al. Comparison of individual and pooled faecal samples in sheep for the assessment of gastrointestinal strongyle infection intensity and anthelmintic drug efficacy using McMaster and Mini-FLOTAC. Vet Parasitol. 2014;205:216–223. doi: 10.1016/j.vetpar.2014.06.011. [DOI] [PubMed] [Google Scholar]
- 29.Raynaud JP. Etude de l’efficacité d’une technique de coproscopie quantitative pour le diagnostic de routine et le contrôle des infestations parasitaires des bovins, ovins, équins et porcins. Ann Parasitol Hum Comp. 1970;45:321–342. doi: 10.1051/parasite/1970453321. [DOI] [PubMed] [Google Scholar]
- 30.McKenna P. Diagnosis of gastrointestinal nematode parasitism in goats. In: Proceedings of a course in Goat Husbandry and Medicine. Publication n°106. Veterinary Continuing Education, Palmerston North, New-Zealand: Massey University; 1985. p. 86–93.
- 31.Michelet L, Delannoy S, Devillers E, Umhang G, Aspan A, Juremalm M, et al. High-throughput screening of tick-borne pathogens in Europe. Front Cell Infect Microbiol. 2014;4:103. doi: 10.3389/fcimb.2014.00103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Katoh K, Standley D. MAFFT multiple sequence alignment software version 7: improvements in performance and usability. Mol Biol Evol. 2013;30:772–780. doi: 10.1093/molbev/mst010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ye J, Coulouris G, Zaretskaya I, Cutcutache I, Rozen S, Madden T. Primer-BLAST: A tool to design target-specific primers for polymerase chain reaction. BMC Bioinformatics. 2012;13:134. doi: 10.1186/1471-2105-13-134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.OligoAnalyzer. 2018. https://eu.idtdna.com/calc/analyzer. Accessed 2 Oct 2018.
- 35.ExPASy. Translate tool. https://web.expasy.org/translate/. Accessed 2 Oct 2018.
- 36.Tamura K, Stecher G, Peterson D, Filipski A, Kumar S. MEGA6: Molecular Evolutionary Genetics Analysis Version 6.0. Mol Biol Evol. 2013;30:2725–2729. doi: 10.1093/molbev/mst197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tamura K. Estimation of the number of nucleotide substitutions when there are strong transition-transversion and G+C-content biases. Mol Biol Evol. 1992;9:678–687. doi: 10.1093/oxfordjournals.molbev.a040752. [DOI] [PubMed] [Google Scholar]
- 38.Jones DT, Taylor WR, Thornton JM. The rapid generation of mutation data matrices from protein sequences. Comput Appl Biosci. 1992;8:275–282. doi: 10.1093/bioinformatics/8.3.275. [DOI] [PubMed] [Google Scholar]
- 39.Felsenstein J. Confidence limits on phylogenies: an approach using the bootstrap. Evolution. 1985;39:783–791. doi: 10.1111/j.1558-5646.1985.tb00420.x. [DOI] [PubMed] [Google Scholar]
- 40.R Development Core Team. R: A language and environment for statistical computing. Vienna: R Foundation for Statistical Computing; 2016. https://www.R-project.org/. Accessed 2 Oct 2018.
- 41.R Studio. 2018. https://www.rstudio.com/. Accessed 2 Oct 2018.
- 42.Torina A, Alongi A, Naranjo V, Scimeca S, Nicosia S, Di Marco V, et al. Characterization of Anaplasma infections in Sicily, Italy. Ann N Y Acad Sci. 2008;1149:90–93. doi: 10.1196/annals.1428.065. [DOI] [PubMed] [Google Scholar]
- 43.Zobba R, Anfossi AG, Pinna Parpaglia ML, Dore GM, Chessa B, Spezzigu A, et al. Molecular investigation and phylogeny of Anaplasma spp. in Mediterranean ruminants reveal the presence of neutrophil-tropic strains closely related to A. platys. Appl Environ Microbiol. 2014;80:271–280. doi: 10.1128/AEM.03129-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Psaroulaki A, Chochlakis D, Sandalakis V, Vranakis I, Ioannou I, Tselentis Y. Phylogenetic analysis of Anaplasma ovis strains isolated from sheep and goats using groEL and mps4 genes. Vet Microbiol. 2009;138:394–400. doi: 10.1016/j.vetmic.2009.04.018. [DOI] [PubMed] [Google Scholar]
- 45.Dahmani M, Davoust B, Tahir D, Raoult D, Fenollar F, Mediannikov O. Molecular investigation and phylogeny of Anaplasmataceae species infecting domestic animals and ticks in Corsica, France. Parasit Vectors. 2017;10:302. doi: 10.1186/s13071-017-2233-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhou M, Cao S, Sevinc F, Sevinc M, Ceylan O, Ekici S, et al. Molecular detection and genetic characterization of Babesia, Theileria and Anaplasma amongst apparently healthy sheep and goats in the central region of Turkey. Ticks Tick Borne Dis. 2017;8:246–252. doi: 10.1016/j.ttbdis.2016.11.006. [DOI] [PubMed] [Google Scholar]
- 47.Aktas M, Özübek S. Anaplasma ovis genetic diversity detected by major surface protein 1a and its prevalence in small ruminants. Vet Microbiol. 2018;217:13–17. doi: 10.1016/j.vetmic.2018.02.026. [DOI] [PubMed] [Google Scholar]
- 48.Dahmani M, Davoust B, Rousseau F, Raoult D, Fenollar F, Mediannikov O. Natural Anaplasmataceae infection in Rhipicephalus bursa ticks collected from sheep in the French Basque Country. Ticks Tick Borne Dis. 2017;8:18–24. doi: 10.1016/j.ttbdis.2016.09.009. [DOI] [PubMed] [Google Scholar]
- 49.Ciani E, Alloggio I, Petazzi F, Pieragostini E. Looking for prognosticators in ovine anaplasmosis: discriminant analysis of clinical and haematological parameters in lambs belonging to differently susceptible breeds experimentally infected with Anaplasma ovis. Acta Vet Scand. 2013;55:71. [DOI] [PMC free article] [PubMed]
- 50.Ferrolho J, Antunes S, Santos AS, Velez R, Padre L, Cabezas-Cruz A, et al. Detection and phylogenetic characterization of Theileria spp. and Anaplasma marginale in Rhipicephalus bursa in Portugal. Ticks Tick Borne Dis. 2016;7:443–448. doi: 10.1016/j.ttbdis.2016.01.004. [DOI] [PubMed] [Google Scholar]
- 51.de la Fuente J, Lew A, Lutz H, Meli ML, Hofmann-Lehmann R, Shkap V, et al. Genetic diversity of Anaplasma species major surface proteins and implications for anaplasmosis serodiagnosis and vaccine development. Anim Health Res Rev. 2005;6:75–89. doi: 10.1079/AHR2005104. [DOI] [PubMed] [Google Scholar]
- 52.Hornok S, Elek V, de la Fuente J, Naranjo V, Farkas R, Majoros G, et al. First serological and molecular evidence on the endemicity of Anaplasma ovis and A. marginale in Hungary. Vet Microbiol. 2007;122:316. doi: 10.1016/j.vetmic.2007.01.024. [DOI] [PubMed] [Google Scholar]
- 53.Zhou M, Cao S, Sevinc F, Sevinc M, Ceylan O, Moumouni PFA, et al. Molecular detection and genetic identification of Babesia bigemina, Theileria annulata, Theileria orientalis and Anaplasma marginale in Turkey. Ticks Tick Borne Dis. 2016;7:126–134. doi: 10.1016/j.ttbdis.2015.09.008. [DOI] [PubMed] [Google Scholar]
- 54.Belkahia H, Ben Said M, El Mabrouk N, Saidani M, Cherni C, Ben Hassen M, et al. Seasonal dynamics, spatial distribution and genetic analysis of Anaplasma species infecting small ruminants from northern Tunisia. Infect Genet Evol. 2017;54:66–73. doi: 10.1016/j.meegid.2017.06.016. [DOI] [PubMed] [Google Scholar]
- 55.de la Fuente J, Torina A, Caracappa S, Tumino G, Furlá R, Almazán C, et al. Serologic and molecular characterization of Anaplasma species infection in farm animals and ticks from Sicily. Vet Parasitol. 2005;133:357–362. doi: 10.1016/j.vetpar.2005.05.063. [DOI] [PubMed] [Google Scholar]
- 56.Liu Z, Ma M, Wang Z, Wang J, Peng Y, Li Y, et al. Molecular survey and genetic identification of Anaplasma species in goats from central and southern China. Appl Environ Microbiol. 2012;78:464–470. doi: 10.1128/AEM.06848-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Phylogenetic analysis of Anaplasma ovis msp4 sequences identified in Corsica and other regions of the world. The figure shows a maximum likelihood phylogenetic tree inferred using msp4 nucleotide sequences of A. ovis of Corsica (Corsica 1-6) and other regions of the world. GenBank accession numbers and country of origin are shown. (PDF 15 kb)
Figure S2. Phylogenetic analysis of Anaplasma ovis MSP4 sequences identified in Corsica and other regions of the world. The figure shows a maximum likelihood phylogenetic tree inferred using MSP4 amino acid sequences of A. ovis of Corsica (Corsica 1-6) and other regions of the world. GenBank accession numbers and country of origin are shown. (PDF 13 kb)
Data Availability Statement
All data generated or analysed during this study are included in this published article. Nucleotide sequences were submitted to the GenBank database under the accession numbers KJ410285, KU497712, MH121148, MH121149, MH121150, MH121151 and MH121152.