Abstract
Background
Soybean oil constitutes an important source of vegetable oil and biofuel. However, high temperature and humidity adversely impacts soybean seed development, yield, and quality during plant development and after harvest. Genetic improvement of soybean tolerance to stress environments is highly desirable.
Results
Transgenic soybean lines with knockdown of phospholipase Dα1 (PLDα1KD) were generated to study PLDα1′s effects on lipid metabolism and seed vigor under high temperature and humidity conditions. Under such stress, as compared with normal growth conditions, PLDα1KD lines showed an attenuated stress-induced deterioration during soybean seed development, which was associated with elevated expression of reactive oxygen species-scavenging genes when compared with wild-type control. The developing seeds of PLDα1KD had higher levels of unsaturation in triacylglycerol (TAG) and major membrane phospholipids, but lower levels of phosphatidic acid and lysophospholipids compared with control cultivar. Lipid metabolite and gene expression profiling indicates that the increased unsaturation on phosphatidylcholine (PC) and enhanced conversion between PC and diacylglycerol (DAG) by PC:DAG acyltransferase underlie a basis for increased TAG unsaturation in PLDα1KD seeds. Meanwhile, the turnover of PC and phosphatidylethanolamine (PE) into lysoPC and lysoPE was suppressed in PLDα1KD seeds under high temperature and humidity conditions. PLDα1KD developing seeds suffered lighter oxidative stresses than did wild-type developing seeds in the stressful environments. PLDα1KD seeds contain higher oil contents and maintained higher germination rates than the wild-type seeds.
Conclusions
The study provides insights into the roles of PLDα1 in developing soybean seeds under high temperature and humidity stress. PLDα1KD decreases pre-harvest deterioration and enhances acyl editing in phospholipids and TAGs. The results indicate a way towards improving production of quality soybean seeds as foods and biofuels under increasing environmental stress.
Electronic supplementary material
The online version of this article (10.1186/s13068-018-1340-4) contains supplementary material, which is available to authorized users.
Keywords: Phospholipase D, Glycerolipid, Oxidative stress, Acyl editing, Unsaturation, High temperature and humidity, Oil content, Seed vigor
The significances statement
Developing soybean seeds suffer from high temperature and humidity stress conditions that often occur in Southern China and cause oxidative stress and seed pre-harvest deterioration. Such soybean seed deterioration can be alleviated by seed-specific knockdown of phospholipase Dα1 (PLDα1KD). Thus, PLDα1KD soybeans have improved nutrition quality and seed vigor.
PLDα1KD developing seeds have higher levels of unsaturation in triacylglycerol and phospholipids due to enhanced expression of desaturase genes and enhanced acyl editing and PC–DAG conversion activities in PLDα1KD developing seeds compared with the wild-type seeds.
The finding that PLDα1KD seeds had improved seed vigor, nutrition quality, and tolerance to high temperature and humidity provides a molecular tool for genetic improvement of soybean for adaptation to wider growth conditions.
Background
With global temperature increasing steadily in recent decades, high temperature conditions, accompanied either by drought or by humidity in different areas, caused damages and losses on crops and yield [1–4]. However, how high temperature and humidity affect crop growth, seed development, as well as yield is not well understood [2, 4]. The high temperature and humidity conditions adversely affect membrane lipids and storage triacylglycerol (TAG) in wheat [3, 5] and oilseed crops, such as soybean (Glycine max), particularly during seed development [6]. High temperatures impact developing soybean seeds’ sensitivity and vulnerability to stresses by causing seeds with poor germination, increased incidence of pathogen infection, and decreased economic value [6]. Previous studies indicated that phospholipase Dα (PLDα1) is involved in seed naturally and artificially aging, by affecting both phospholipids and TAG of mature Arabidopsis and soybean seeds [7–9]. However, how PLDα1 affects lipid metabolism and storage nutrition of developing soybean seeds grown under high temperature and humidity conditions was unknown [8, 10].
Assembly of phospholipids and TAG occurs primarily in the endoplasmic reticulum (ER) and shares a common biosynthetic precursor phosphatidic acid (PA) [11]. Membrane phospholipid synthesis is active in young and green tissues [12]. However, in developing oilseeds, phospholipid metabolism is overwhelmingly directed to TAG accumulation [13, 14], through two major pathways: the diacylglycerol acyltransferase (DGAT)-mediated Kennedy pathway that uses acyl-CoA and the diacylglycerol (DAG) to generate TAG, and phospholipid:diacylglycerol acyltransferase (PDAT)-mediated pathway that uses phosphatidylcholine (PC) and DAG to produce TAG [14]. Overexpression of an A-type phospholipase, pPLAIIIδ, enhanced TAG production in Arabidopsis and Camelina seeds [12, 15]. A-type phospholipase, PLA catalyzing the hydrolysis of PC to generate a free fatty acid and lysoPC (LPC), can also affect TAG production [12, 15]. Acyl-CoA:LPC acyltransferase (LPCAT) can modify PC saturation by introducing an acyl-CoA into a new PC [16, 17]. De novo PC biosynthesis from choline by the actions of choline/ethanolamine kinase (CEK), choline-phosphate cytidylyltransferase (CCT), and DAG cholinephosphotransferase (DAG:CPT) is also important for phospholipid and TAG metabolism [18]. The interconversion between PC and DAG by PC:DAG cholinephosphotransferase (PDCT, also ROD1) can significantly affect TAG biosynthesis and unsaturation through above mentioned pathways [16, 19, 20]. PDCT transfers phosphocholine from PC to DAG actively during oil seed development, and edits TAG composition using PC that is also extensively modified by fatty acid desaturases (FADs) on their acyl chains [19, 21, 22]. In the ER, the partitioning of PA, PC, and DAG precursors for TAG or phospholipid biosynthesis may be controlled by a unexplored complex network. For instance, it has been estimated that more than 70% PC-derived DAG is used to synthesize TAG in flax seeds [23]. Thus, PC plays multiple roles in TAG and phospholipid biosynthesis by recycling or incorporation of the newly synthesized fatty acids in TAG acyl editing [13].
PLD may contribute to diurnal cycling of PA, and PC acyl editing and significantly affect PC pools and TAG production [24–28]. A study demonstrated that PC-derived DAG is the major source for TAG synthesis in Camelina seeds overexpressing PLDζ1 and PLDζ2 [28]. Recently, a genome-wide association study suggested a PLDα gene as a key locus affecting oil biosynthesis in soybean [29, 30]. Studies indicated that PLDα1 is involved in the mature seed aging and deterioration stored under high temperature and humidity [7, 9]. Two PLDα-RNAi knockdown (PLDα1KD) soybean lines had had altered unsaturation fatty acids in both phospholipids and TAG when grown in Kansas in the United States [9]. However, it is still not understood why PLDα1KD seeds have such changes in fatty acyl chains saturation.
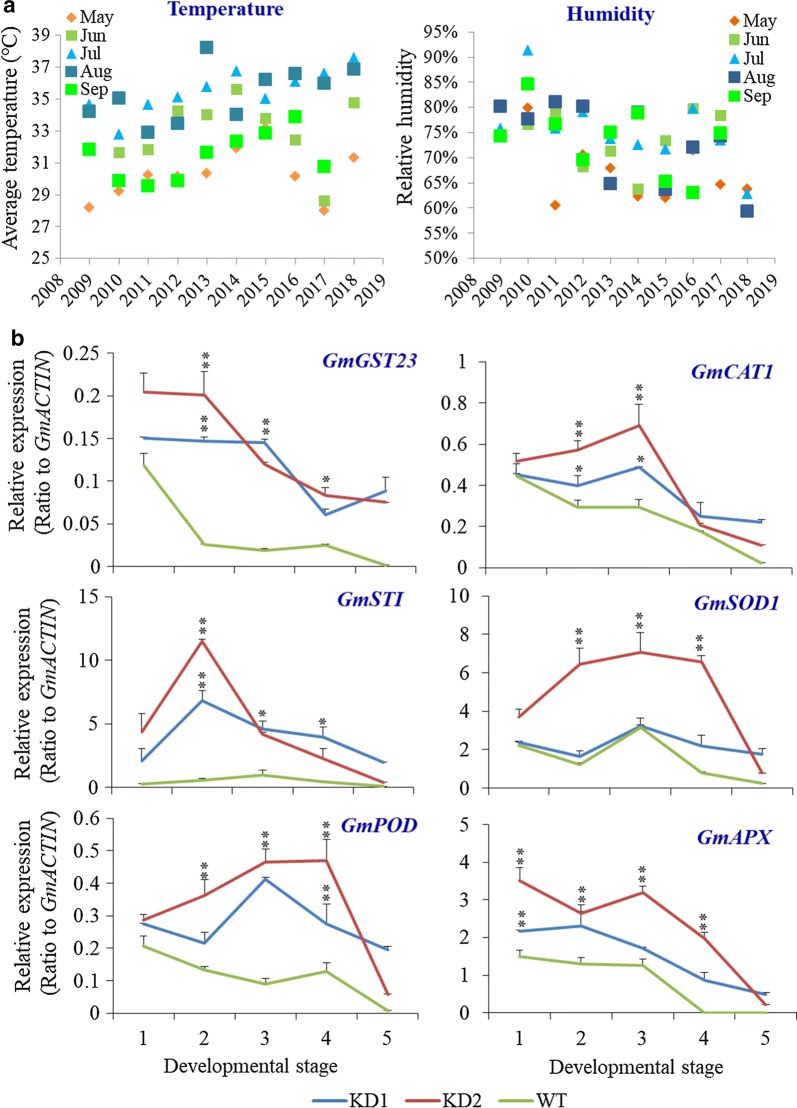
Although the anti-deterioration effects of PLDα1 mutation on naturally or artificially aging Arabidopsis and soybean seeds have been reported [7–9], the underlying molecular mechanism by which PLDα1 mutation affects lipids metabolism and seed quality has not been explored [8, 9]. Especially, how PLDα1 mutation affects soybean developing seeds under heat and humidity stresses is unknown. High temperature and humidity stresses occur frequently in southern China amid increasing global temperature in recent years and cause soybean pre-harvest deterioration and significant losses [6]. High temperature and humidity are major problems and limiting factors in soybean production area, and they not only caused soybean yield loss, but also affected adversely on seed storage and reduced nutrition [6]. In the Mid-Yangzi River region of China, soybean seed development and ripening occur during the season of high precipitations (~ more than 150 mm), high temperature (~ 34–38 °C), and high humidity (~ 75–80%) usually from the late June to the early September (Fig. 3a). The soybean seeds grown in these regions usually have pre-harvest deterioration, rapidly losing seed vigor, reducing nutrition quality severely, and are more vulnerable to pathogens during storage [6, 31]. A better understanding of the mechanism by which high temperature and humidity impact developing soybean seeds will help design effective genetic strategies to improve soybean tolerance to these environmental stress conditions. Here, we generated PLDα1KD soybean lines and investigated the PLDα1KD soybean performance in such stressful environments. The data indicate a critical role of PLDα1 in lipid metabolism and stress response in developing soybean seeds under high temperature and humidity stress conditions, as compared in normal environments.
Fig. 3.
Weather conditions in Mid-Yangzi River regions and the heat stress-induced the oxidative stress and ROS-scavenging genes in developing seeds. a Temperature and humidity changes in Mid-Yangzi River in past 10 years. The data were downloaded from website https://tianqi.911cha.com. b Expression of the stress-related genes in wild-type Jack (WT) and PLDα1 KD seeds grown in high temperature and humility condition were examined with qRT-PCR. KD1: PLDα1 knockdown line 1; KD2: PLDα1 knockdown line 2. 1, 2, 3, 4, 5 indicate different developing stages of seeds, corresponding to fresh weights, described as above. The values are the mean ± SD (n = 3). * and **Denote significance at P < 0.05 and P < 0.01, respectively, compared with wild-type Jack (WT) based on Student’s t test
Results
Generation of transgenic soybean plants with seed-specific knockdown of PLDα1
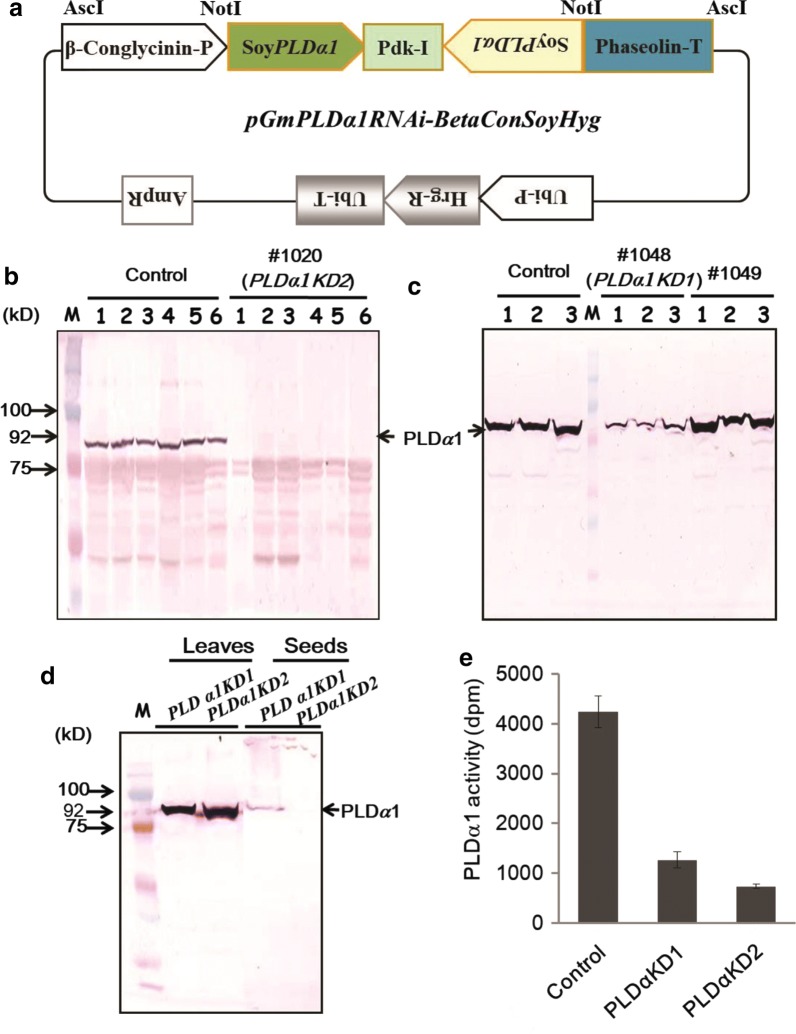
Soybean PLDα1KD transgenic plants were generated by stable transformation of soybean cultivar Jack with a soybean GmPLDα1 RNA interference (RNAi) construct under the control of the seed-specific promoter β-conglycinin (Fig. 1a, Additional file 1: Figure S1). T0 and T2 transgenic plants were screened with qRT-PCR and immunoblotting with an antibody against Arabidopsis PLDα1. This antibody specifically recognized the ~ 92 kDa GmPLDα1 at both leaf and seed tissues of soybean (Fig. 1). The transgenic and background cultivar Jack seeds displayed different levels of PLDα1 accumulation (Fig. 1). Immunoblotting screening for regenerated transgenic soybean lines showed that in the line #1020 (PLDα1KD2), PLDα1 proteins in the developing and mature seeds were almost completely diminished by expression of PLDα1RNAi (Fig. 1b), whereas in another regenerated transgenic soybean line #1048 (PLDα1KD1) displayed about 25% of that in wild-type soybean cultivar Jack (Fig. 1c). To confirm the seed specific expression of PLDα1RNAi and seed-specific suppression of soybean PLDα1, proteins extracted from leaves and seeds of two PLDα1KD lines and wild-type control (Jack) were immunoblotted for PLDα1, and PLDα1 proteins remained in leaves of both transgenic lines, PLDα1KD1 and PLDα1KD2, but was greatly diminished in their seeds (Fig. 1d). Assaying PLDα1 activity showed that PLD activity in PLDα1KD1 and PLDα1KD2 was 23% and 10% of that in wild- type, respectively (Fig. 1e).
Fig. 1.
Generation of soybean PLDα1 knockdown lines. Soybean PLDα1KD transgenic plants were generated by stable transformation of soybean cultivar Jack with a soybean PLDα1 RNA interference (RNAi) construct. Transgenic soybean plants were used for analysis. a Construction of soybean PLDα1KD. 1151bp one in forward and the other in reverse and separated by a DNA fragment of Pdk-I gene, is driven by a seed specific promoter for soybean β-conglycinin gene encoding a major seed storage protein in seed, and terminated by a Phaseo gene terminator. b Immunoblotting screening PLDα1 in seeds of transgenic soybean plants 1020 (PLDα1KD1). 1–6 indicate individual plants from transgenic line or wild-type Jack (control). c Immunoblotting detection PLDα1 in seeds of another transgenic soybean line 1048 (PLDα1KD2). 1–3 indicate the individual plants from each transgenic line or wild-type Jack (control). d Immunoblotting of PLDα1 using proteins extracted from leaves and seeds from two PLDα1KD lines1. e PLDα1 activity in two PLDα1KD lines 1 and wild-type Jack line1 (control) developing seeds
Effects of PLDα1KD on the expression of other PLDs
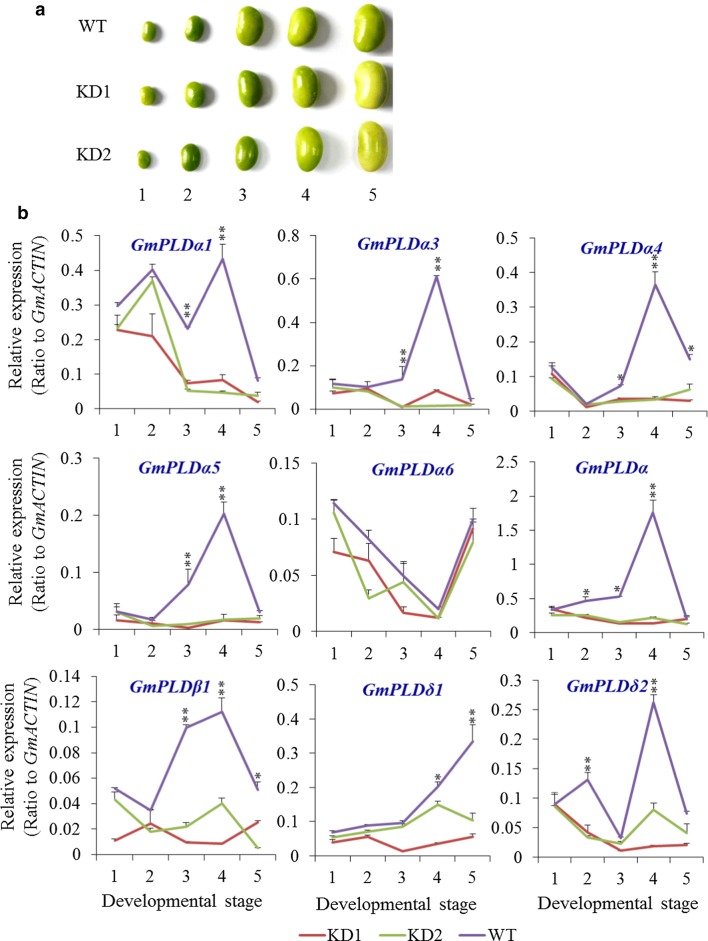
The soybean PLD family has 23 members (Additional file 1: Figure S2a, Additional file 2: Table S2, Data S1). To evaluate the effect of PLDα1KD on the expression patterns of different PLDs, developing seeds of PLDα1KD from T3 and T5 generations of PLDα1KD transgenic lines were tested (Fig. 2). Three GmPLDαs are highly expressed (Additional file 2: Table S3) [32]. In PLDα1KD mutants, the expression of various PLDαs, including targeted PLDα1s, was reduced significantly compared with that of control at different developmental stages (Fig. 2, Additional file 1: Figure S2c, d). In control Jack seeds, GmPLDα3 is the most highly expressed, GmPLDα1 is the second highest, and GmPLDα4 is the third most highly expressed PLD gene in developing seeds (Fig. 2, Additional file 1: Figure S2b, Additional file 2: Table S4) [33]. When using a pair of primers that amplify all GmPLDαs, the total GmPLDα expression displayed a similar pattern as that for the above major GmPLDαs. GmPLDβ3 transcripts were lower in PLDα1KD mutants than in wild-type during early stages of seed development, but higher than in Jack at late stages of seed development (Additional file 1: Figure S2c). Among GmPLDβs, GmPLDβ4 showed the highest expression level in developing seeds, and then GmPLDβ1. Most other PLDβs were expressed at a low level in soybean developing seeds (Fig. 2, Additional file 1: Figure S2b, Additional file 2: Table S4) [33]. Among two major GmPLDδs expressed in developing seeds, GmPLDδ1 was lower at the stages of 4 and 5, and GmPLDδ2 was lower at the satges 2 and 5 than those in the wild-type (Fig. 2). The results indicate that GmPLDα1 RNAi also interfered with the transcripts of other PLD genes, likely due to the high sequence similarity among PLD genes.
Fig. 2.
Expression profiles of GmPLD genes in PLDα1KD and wild-type developing seeds. KD1: PLDα1KD line 1; KD2: PLDα1KD line 2. 1, 2, 3, 4, 5 indicate different developing stages of seeds, corresponding to fresh weights: stage 1, 30–70 mg; stage 2, 100–150 mg; stage 3, 200–250 mg; stage 4, 300–350 mg; stage 5, 400–480 mg. Data are mean ± S.D. (n = 3). * and **Denote significance at P < 0.05 and P < 0.01, respectively, compared with controls on Student’s t test. a Appearances of PLDα1KD and wild-type Jack (WT) seeds at different developmental stages. b Expression profiles of major PLDs during seed development. Total RNA was extracted from developing seeds at different developmental stages
PLDα1KD developing seeds had elevated levels of ROS-scavenging genes
Most plant tissues under abiotic stress conditions, such as drought, salinity, ozone, high temperature, and flooding, usually generate more reactive oxygen species (ROS), which is often associated with the synthesis of more enzymes involved in ROS-scavenging to reduce the oxidative damage [6, 34–36]. Under high temperature and humidity, wild-type developing seeds displayed increased expression levels of ROS-scavenging related genes, indicating that seeds might be faced with elevated ROS production (Additional file 1: Figure S3). The expression of several genes, such as glutathione S-transferases (GST23), peroxidase (POD), catalase (CAT1), superoxide dismutase (SOD1), ascorbic acid peroxidase (APX), as well as a heat shock protein STI [6, 35], were highly induced over the time during high temperature and humidity stress (Additional file 1: Figure S3). The level of GmPLDα1 transcript also increased by ninefold at 6 h after heat treatment, compared with seeds under normal temperature (Additional file 1: Figure S3). Most stress-responsive and ROS-scavenging genes, such as GST23, POD, CAT1, SOD1, STI, and APX, in PLDα1KD1 and PLDα1KD2 developing seeds displayed higher transcript levels than those in wild-type control under high temperature and humidity conditions (Fig. 3b).
Increased unsaturated fatty acids of TAG and phospholipids in PLDα1KD seeds
We examined changes of TAG contents and fatty acid composition of PLDα1KD and wild-type soybean lines at different developmental stages. Total fatty acid content steadily increased over the seed filling during maturation (Fig. 4, Additional file 1: Figure S4). In addition, clear differences between PLDα1KD and wild-type seeds in unsaturated fatty acids, 18:1, 18:2, and 18:3, were detected throughout the seed developmental stages. Wild-type soybean oil contains 13% palmitic acid (16:0), 4% stearic acid (18:0), 20% oleic acid (18:1), 55% linoleic acid (18:2), and 8% linolenic acid (18:3) (Fig. 4, Additional file 1: Figure S4). A higher content of unsaturated fatty acids in TAG was observed in PLDα1KD seeds than those in the wild-type Jack under normal conditions (Fig. 4, Additional file 1: Figure S4). The differences became bigger between PLDα1KD2 and wild-type than those between PLDα1KD1 and wild-type under stress conditions, suggesting that the degree of PLDα1 suppression may be proportional to the content of unsaturated fatty acids. To distinguish whether the difference in unsaturation resulted from fatty acids in TAG or phospholipids, we assayed fatty acid composition in TAG and phospholipids separated by TLC. The total TAG content in PLDα1KD mutant seeds was higher than that in wild-type seeds at most developmental stages (Fig. 4, Additional file 1: Figure S4). Correspondingly, the contents of total unsaturated fatty acids (mainly 18:1 and 18:2) in stage 5-seeds of PLDα1KD1 and 2 mutant lines were comparable. Both were about two and tenfold higher than those of wild-type seeds under normal growth conditions and the high temperature and humidity conditions, respectively (Fig. 4, Additional file 1: Figure S4). The total fatty acid content of WT under normal conditions was 1.5-fold higher than that under high temperature and humidity in stage-3 seeds. This result is consistent with a previous observation that high temperature decreased oil content of soybean seeds [6]. However, the total fatty acid content in developing GmPLDα1KD seeds was almost unchanged, which could mean that the knockdown of GmPLDα1 stabilizes the seed fatty acid contents under high temperature (Fig. 4, Additional file 1: Figure S4).
Fig. 4.
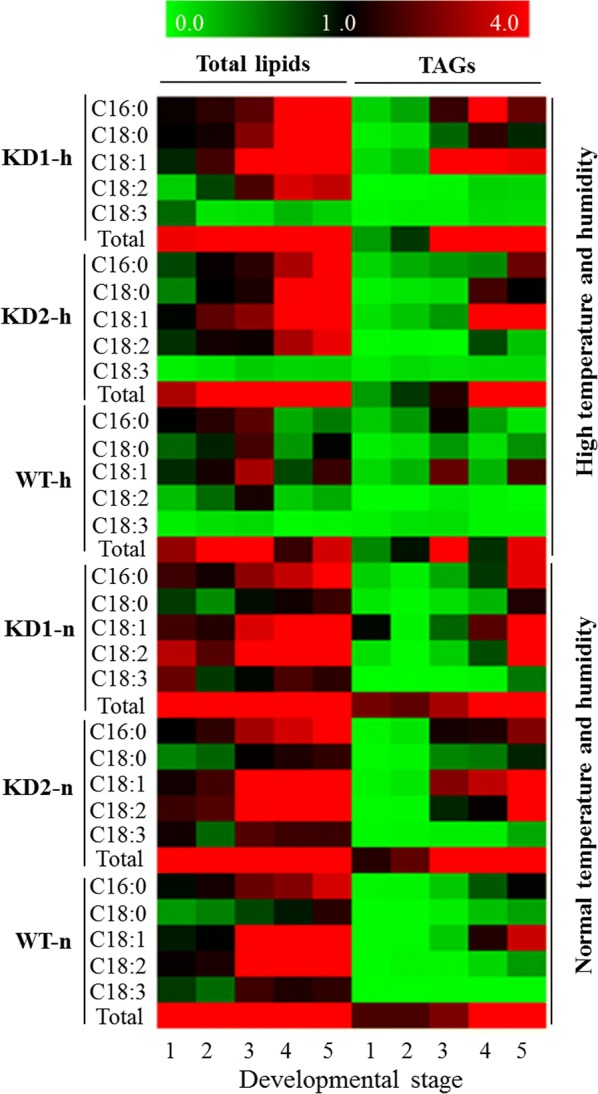
Heatmap analyses on fatty acid compositions of total lipids and TAGs from PLDα1KD and wild-type developing seeds. Exact quantification data see Additional file 1: Figure S4 for details. The values represented are the mean ± SD from at least three independent repeats. KD1-h, KD2-h and WT-h: PLDα1 knockdown line 1, 2 and wild-type jack, respectively, under high temperature and humidity which used thick lines; KD1-n, KD2-n and WT-n: PLDα1 knockdown line 1, 2 and wild-type Jack, respectively, under normal temperature and humidity. 1, 2, 3, 4, and 5 indicate different developing stages of seeds, corresponding to fresh weights as described previously. The values are from the mean ± SD (n = 3)
Up-regulation of FADs in PLDα1KD developing seeds as compared with wild-type
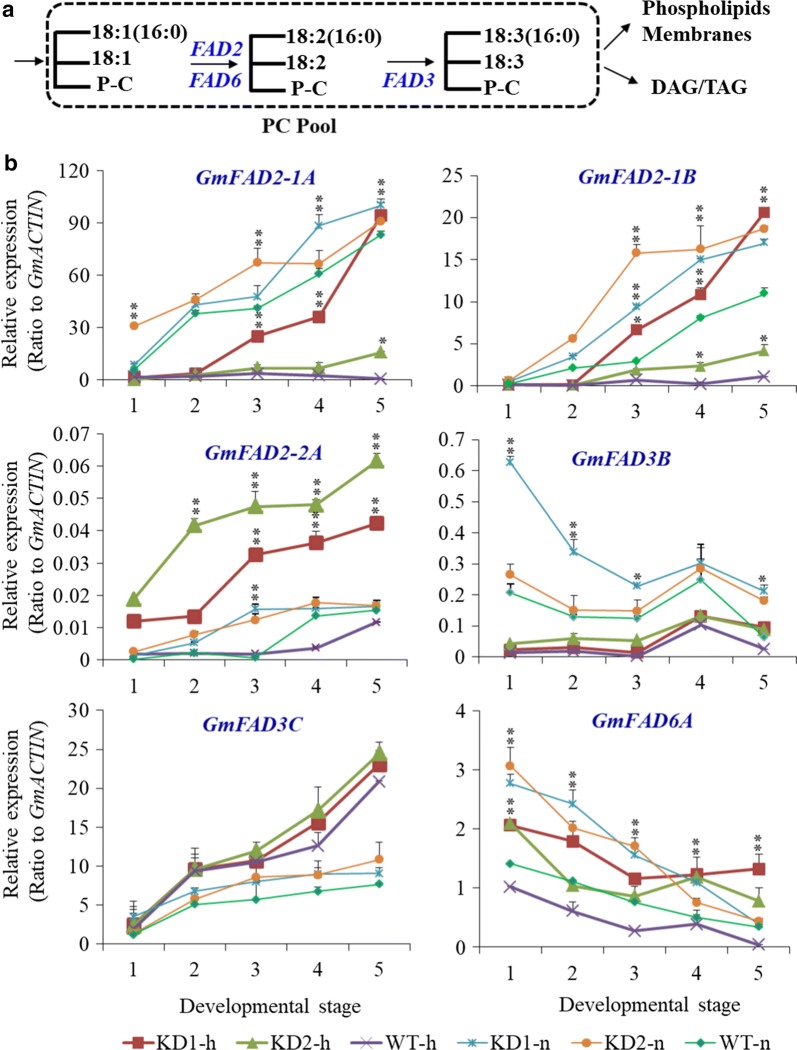
In the ER, FAD2 synthesizes linoleic acid from oleic acid and FAD3 catalyzes the conversion of linoleic acid into α-linolenic acid on PC (Fig. 5a). FAD2-2A (Glyma.19G147400), FAD2-2B (Glyma.19G147300), FAD2-2C (Glyma.15G195200), and GmFAD2-2D (Glyma.03G144500) were constitutively expressed in developing seeds and vegetative tissues of soybean [37–39] whereas FAD2-1A (Glyma.10G278000) and FAD2-1B (Glyma.20G111000) are specifically expressed in developing seeds, and play an essential role in controlling the oleic acid level in developing soybean seeds (Additional file 1: Figure S5) [32, 33]. The low-linolenic acid trait in soybean requires the combination of up to three different recessive alleles of FAD3 genes that encode omega-3 fatty acid desaturases [40, 41]. GmFAD3 includes GmFAD3A (Glyma.14G194300), GmFAD3B (Glyma.02G227200), and GmFAD3C (Glyma.18G062000).
Fig. 5.
Expression patterns of FAD genes in PLDα1KD and wild-type developing seeds. a FAD enzymes function in phospholipid desaturation with various PC molecules as preferred substrates. b Expression of FAD genes in soybean developing seeds of different genetic backgrounds under various growth conditions. FAD2, Microsomal Δ12 desaturase; FAD3, Microsomal ω3 desaturase; FAD6, Plastidial Δ12 desaturase. The X-axis numbers indicated each development stage. KD1-h, KD2-h and WT-h: PLDα1 knockdown line 1 and 2 and wild-type jack, respectively, under high temperature and humidity; KD1-n and KD2-n and WT-n: PLDα1 knockdown line 1 and 2 and wild-type Jack, respectively, under normal temperature and humidity. 1–5 indicate different developing stages of seeds corresponding to fresh weights as described in “Materials and methods”. The values are the mean + SD (n = 3). * and **Denote significance at P < 0.05 and P < 0.01, respectively, compared with wild-type Jack (WT) based on Student’s t test
Quantitative RT-PCR results showed that the expression of FAD2-1B was much higher in developing seeds of PLDα1KD than in wild-type under both normal and stress conditions (Fig. 5b). Microarray data showed that FAD2s were highly expressed in seeds during development [33]. FAD2-1B and FAD2-1A were mainly expressed at the late stages of developing seeds, and their transcripts were higher in PLDα1KD than wild-type under both conditions. The transcript levels of GmFAD3A, GmFAD3B and GmFAD3C were generally high in developing seeds (Fig. 5b, Additional file 1: Figure S5). Under high temperature, FAD3B expression levels increased to the highest level at the stage 4, and then decreased. However, under normal conditions, the FAD3B transcript level was higher at all developmental stages compared with that under stressed conditions. Furthermore, the FAD3B transcript level was much higher in PLDα1KD1 than wild-type seeds under normal conditions (Fig. 5b). The expression levels of the major FADs in seeds were higher under normal conditions than high temperature conditions, except for GmFAD2-2A and GmFAD3C, whose expression levels were generally low under normal conditions, and increased in response to elevated temperatures. The expression of chloroplast localized GmFAD6A was also higher in PLDα1KDs than in wild-type and up-regulated under stress conditions (Fig. 5b).
Increased contents of PC and PE in PLDα1KD seeds
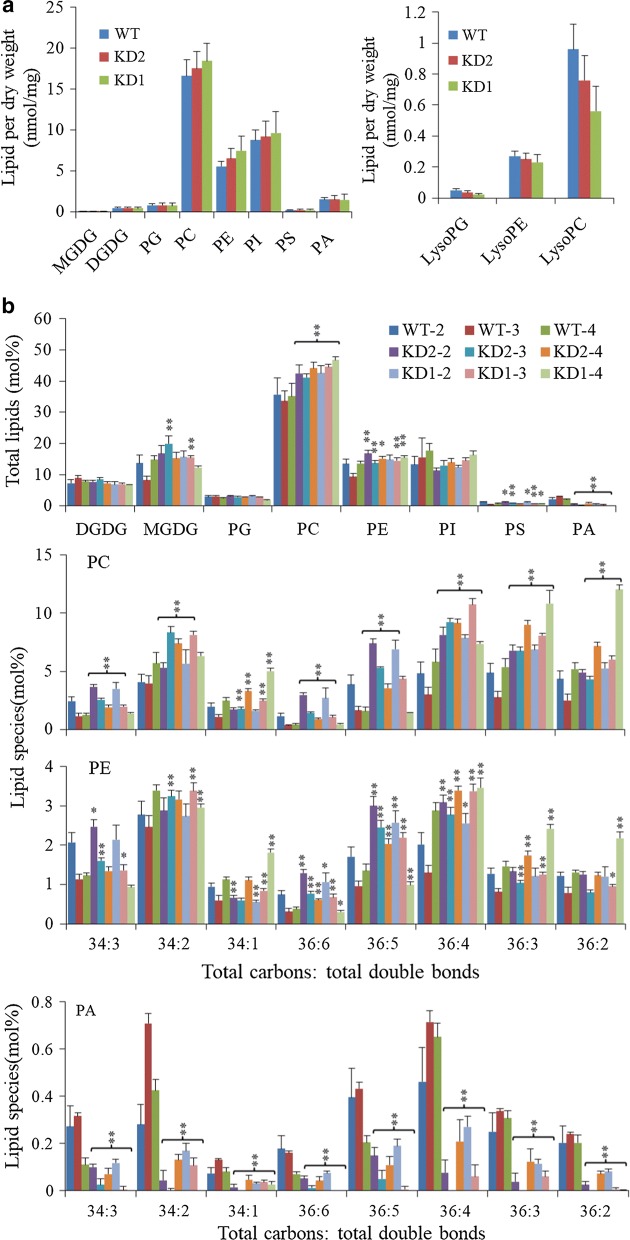
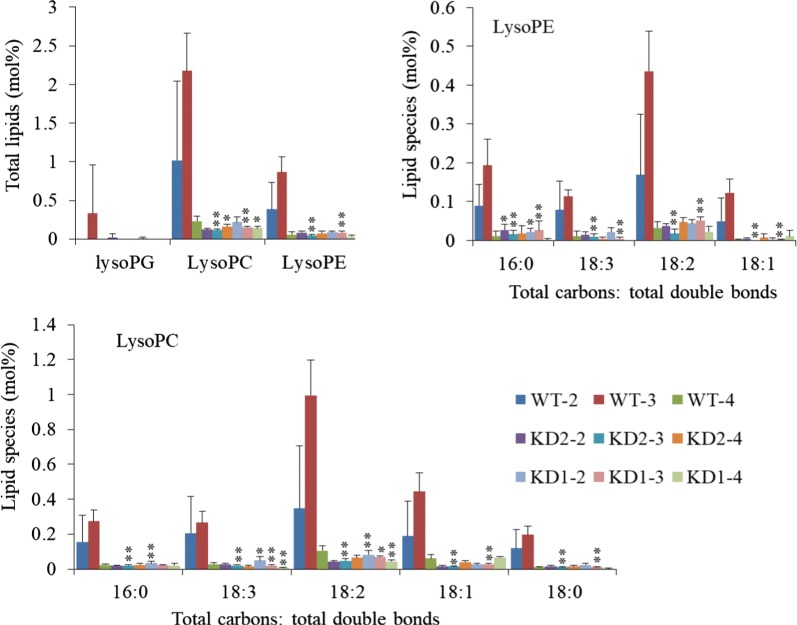
The contents of PC and PE between wild-type and both PLDα1KDs lines were comparable under normal growth conditions (Fig. 6a), but they became different under high temperature and humidity conditions (Fig. 6b). To compare the unsaturation status of phospholipids in PLDα1KD seeds with wild-type at different developmental stages under stress conditions, we profiled phospholipids at developmental stages 2, 3, and 4 as PLDα1 expression was higher at these stages. The level of most phospholipids did not change substantially over these three stages (Fig. 6b). However, seeds from PLDα1KD1 and 2 plants had averagely 132% PC and 47% PE higher than those from wild-type in stage-3 seeds (Fig. 6b). The level of PC, particularly with unsaturated fatty acid acyl chains, was significantly higher in PLDα1KD seeds than wild-type. PLDα1KD seeds had higher levels of PCs and PEs with 36:5, 36:6, 36:4, 36:3, 36:2 34:3, 34:2 (total acyl carbons: total acyl double bonds) acyl chains (Fig. 6b). The PA level in PLDα1KD seeds at all three stages was much lower than that in wild-type, with reduction of more than 81% (Fig. 6b). In addition, total levels of LPC, lysophosphatidylethanolamine (LPE), and lysophosphatidylglycerol (LPG) were lower in PLDα1KD seeds than in wild-type seeds, with LPC and LPE being decreased by 90%, in stage-3 seeds (Fig. 7). Overall MGDG increased in both PLDα1KD seeds compared to wild-type seeds, whereas DGDG content kept unchanged. The difference of PC contents between GmPLDα1KD and wild-type seeds under high temperature and humidity was larger than those under normal conditions (Figs. 6, 7).
Fig. 6.
Phospholipid profiles in PLDα1KD and wild-type developing seeds. a Profiles of phospholipids in mature seeds of KD (PLDα1KD) and wild-type Jack (WT) by ESI-MS/MS. All seeds collected from soybean plants grown under normal conditions. b Total lipid contents of major lipids and the molecular species (total acyl chains: double bonds). All plants were grown under high temperature and humidity. All plants were grown under high temperature and humidity. The values are the mean ± S.D. (n = 4 or 5). WT-2: wild-type Jack at stage 2; KD1-2: knockdown line 1 at stage 2; KD2-2: knockdown line 2 at stage 2; The rest can be explained in the same manner. All * and **denote significance at P < 0.05 and P < 0.01, respectively, compared with wild-type Jack (WT) based on Student’s t test
Fig. 7.
Lysophospholipid profiles in PLDα1KD and wild-type developing seeds. Total content of lysoPG, lysoPC, lysoPE and corresponding molecule species were measured by using ESI-MS/MS. All plants were grown under high temperature and humidity. The values are the mean ± S.D. (n = 4 or 5). WT-2: wild-type Jack at stage 2; KD1-2: knockdown line 1 at stage 2; KD2-2: knockdown line 2 at stage 2; The rest can be explained in the same manner. All * and **denote significance at P < 0.05 and P < 0.01, respectively, compared with wild-type Jack (WT) based on Student’s t test
Decreased PA pools in PLDα1KD seeds
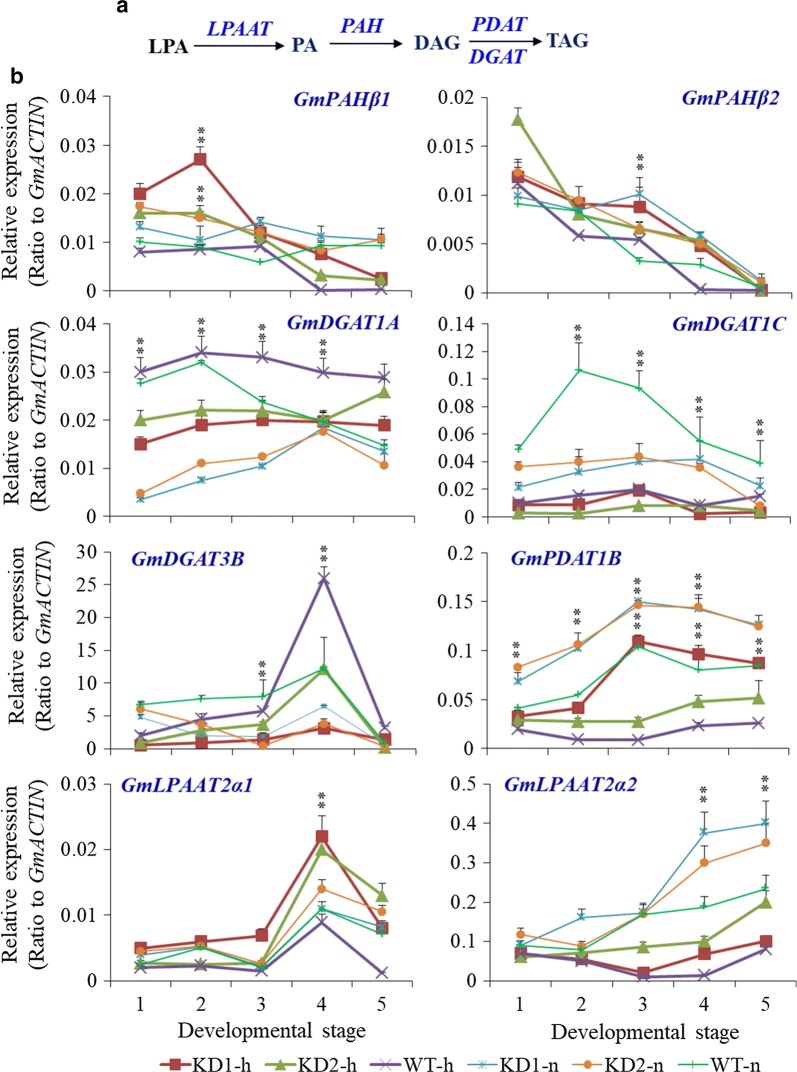
We further investigated the expression of genes involved in PA biosynthesis- and catabolism in PLDα1KD seeds (Fig. 8a). LPAATs that produce PA from lysoPA, PA hydrolases (PAHs) that dephosphorylate PA to yield DAG, and PLDs that produce PA from hydrolysis of phospholipids, all contribute to the changes of PA levels. The soybean genome contains multiple genes encoding LPAATs, corresponding to Arabidopsis AtLPAAT 1–5 that are essential enzymes for the de novo PA biosynthesis in both eukaryotic and prokaryotic pathways for glycerolipid biosynthesis [42, 43]. LPAAT function in TAG biosynthesis in soybean and Arabidopsis has been implicated [16]. In soybean developing seeds, the major GmLPAAT transcripts, including GmLPAAT2α1, GmLPAAT2α2, accumulated in similar patterns compared with DGAT, PDAT, or other TAG biosynthesis-related genes, which fluctuated in developing seeds (Figs. 8, 9, 10, Additional file 1: Figure S6) [33]. These GmLPAAT transcripts increased more than 21% in PLDα1KD seeds compared to wild-type at developmental stage 5 under both conditions. Both LPAAT2α1 and LPAAT2α2 genes were down-regulated in wild-type, and in both PLDα1KD lines, the transcript level of LPAAT2α2 was down-regulated but LPAAT2α1 remained high in response to high temperature and humidity stress (Fig. 8b).
Fig. 8.
Expression profiles of TAG biosynthesis genes in PLDα1KD and wild-type developing seeds. a Kennedy pathway for TAG biosynthesis in the ER. b Expression of major genes involved in the Kennedy pathway in soybean developing seeds of different genetic backgrounds under various growth conditions. The X-axis numbers indicated each development stage. KD1-h, KD2-h and WT-h: PLDα1 knockdown line 1, 2 and wild-type Jack, respectively, under high temperature and humidity which used thick lines; KD1-n, KD2-n and WT-n: PLDα1 knockdown line 1, 2 and wild-type Jack, respectively, under normal temperature and humidity which used thin lines. 1, 2, 3, 4, and 5 indicate different developing stages of seeds, corresponding to fresh weights as described previously. The values are the mean ± SD (n = 3). * and **Denote significance at P < 0.05 and P < 0.01, respectively, compared with wild-type Jack (WT) based on Student’s t test
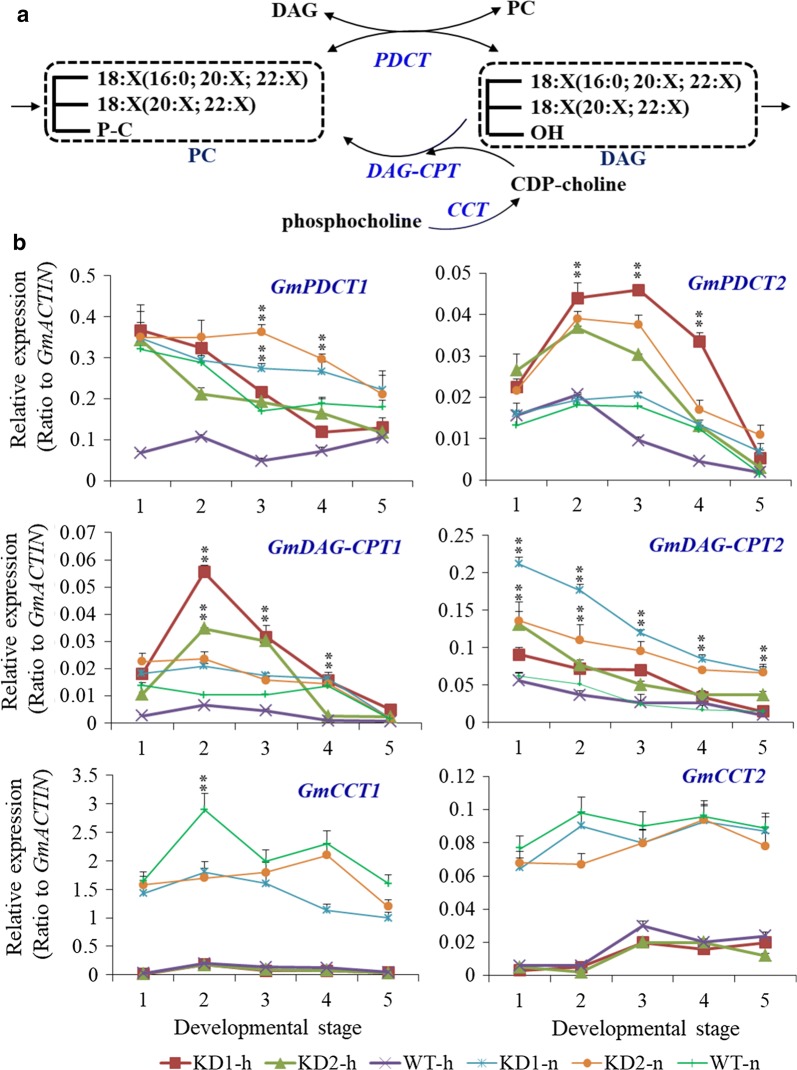
Fig. 9.
Differential expression patterns for genes involved in PC–DAG conversion in PLDα1KD and wild-type developing seeds. a Acyl editing in phospholipid and TAG through PC–DAG conversion. b Differential expression of genes involved in PC–DAG conversion in developing seeds of different genetic backgrounds under various growth conditions. The X-axis numbers indicated each development stage. KD1-h, KD2-h and WT-h: PLDα1 knockdown line 1, 2 and wild-type Jack, respectively, under high temperature and humidity which used thick lines; KD1-n, KD2-n and WT-n: PLDα1 knockdown line 1, 2 and wild-type Jack, respectively, under normal temperature and humidity which used thin lines. 1, 2, 3, 4, and 5 indicate different developing stages of seeds, corresponding to fresh weights as described previously. The values are the mean ± SD (n = 3). * and **Denote significance at P < 0.05 and P < 0.01, respectively, compared with wild-type Jack based on Student’s t test
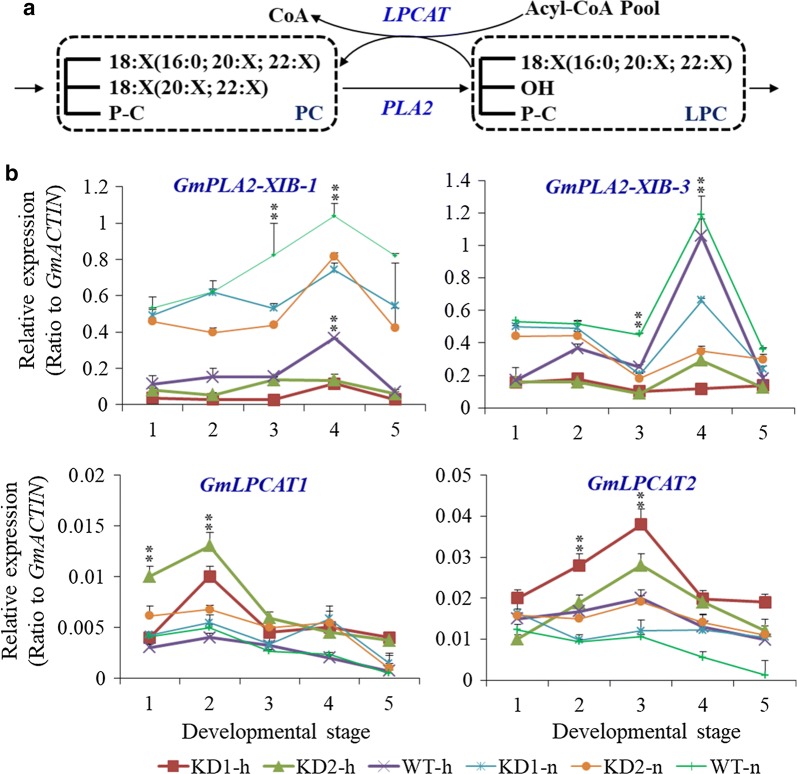
Fig. 10.
Expression of PLAs and LPCATs involved in acyl editing in PLDα1KD and wild-type developing seeds. a Acyl editing in PC fatty acyl chains through PLA–LPCAT cycle. b Expression of major PLA and LPCAT genes involved in developing seeds of different genetic backgrounds under various growth conditions. The X-axis numbers indicated each development stage. KD1-h, KD2-h and WT-h: PLDα1 knockdown line 1, 2 and wild-type Jack under high temperature and humidity which used thick lines; KD1-n, KD2-n and WT-n: PLDα1 knockdown line 1, 2 and wild-type Jack under normal temperature and humidity which used thin lines, respectively. 1, 2, 3, 4, and 5 indicate different developing stages of seeds, corresponding to fresh weights as described previously. The values are the mean ± SD (n = 3). * and **Denote significance at P < 0.05 and P < 0.01, respectively, compared with wild-type Jack (WT) based on Student’s t test
Three PAH genes, homologous to AtPAH1 and AtPAH2, are present in the soybean genome. Two of them, Glyma.13G134500 (GmPAHβ1) and Glyma.10G046400 (GmPAHβ2), were highly expressed in developing seeds, in a trend coincident with seed oil accumulation (Additional file 1: Figure S7) [33]. The transcript levels for both GmPAHβ1 and GmPAHβ2 in PLDα1KD were one and twofold higher, respectively, than these in wild-type seeds at stages 4 under both conditions and the expression of GmPAHs was not affected at all by high temperature except GmPAHβ1 at stage 2 and GmPAHβ2 at stage 3 (Fig. 8b). The combined effects of suppressed PLDα1KD, and a markedly higher PAH expression level contributed to the decreased PA levels, which was confirmed by mature and developing seeds (Figs. 6, 7).
Altered transcript levels of DGATs and PDATs for TAG biosynthesis in PLDα1KD seeds
To explore how PLDα1KD affected TAG biosynthesis and phospholipid metabolism in soybean seeds, we examined several major genes involved in the Kennedy pathway (Fig. 8b). DGAT synthesizes TAG by transferring an acyl group to DAG from newly synthesized or recycled acyl-CoA (Fig. 8a). The DGAT family in the soybean genome has 10 members. Type 1 DGATs, Glyma.13G106100, Glyma.09G065300, and Glyma.17G053300, were highly expressed in seeds. Type 3 DGAT Glyma.17G041600 was also highly expressed in seeds. Compared with type 1 and type 3 DGATs, type 2 DGAT, such as Glyma.16G115700 and Glyma.09G195400, were expressed at a lower level in seeds [44]. The transcript level of these genes increased steadily over seed development (Additional file 1: Figure S8) [33]. PLDα1KD lines have lower transcript levels for several seed-specific DGATs, such as GmDGAT1A (Glyma.13G106100), GmDGAT1C (Glyma.09G065300) and GmDGAT3B (Glyma.17G041600), over all developmental stages under both conditions, indicating that PLDα1KD lines have reduced contributions through DGAT pathway towards TAG synthesis (Fig. 8b). Meanwhile, the expression of GmDGAT1A and GmDGAT3B was increased whereas the expression of GmDGAT1C was suppressed in PLDα1KD lines and wild-type under high temperature conditions compared with normal conditions.
PDAT transfers the sn-2 acyl group from phosphatidylcholine or phosphatidylethanolamine to DAG for TAG production in plants and yeast (Fig. 8a) [45, 46], and in soybean seeds, DAG from PC is primarily used for TAG biosynthesis. PDAT and DGAT were shown to have overlapping functions in TAG biosynthesis [47]. The soybean genome contains 6 putative PDAT genes, and among them, Glyma.12G084000, Glyma.11G190400, and Glyma.13G108100, as well as Glyma.07G036400, were highly expressed in seeds. Transcripts of these PDATs increased steadily during seed development except Glyma.07G036400 (Additional file 1: Figure S9) [33]. The transcript level of PDAT genes in PLDα1KD seeds was more than higher than those in wild-type seeds from stages 3 to 5 under both conditions, suggesting that PLDα1KD seeds have increased PDAT-mediated, DAG-PC dependent TAG biosynthesis (Fig. 8b). Meanwhile, high temperature and humidity suppressed the expression of GmPDAT1B in both PLDα1KD and wild-type seeds.
Enhanced PC–DAG conversion and acyl editing in PLDα1KD soybean seeds
To test whether the active PC–DAG–PDAT pathway contributed to more TAG biosynthesis in PLDα1KD than in wild-type, we compared the transcript levels of relevant genes. For PC synthesis, choline/ethanolamine kinase (CEK) produces phosphocholine that is used by CTP: phosphocholine cytidylyltransferase (CCT) to synthesize CDP- choline (Additional file 1: Figures S10, S11). DAG: cholinephosphotransferase (CPT) then transfers choline form CDP-choline to DAG to generate PC (Fig. 9a). The soybean genome has two CCT genes, CCT1 (Glyma.09G051200) and CCT2 (Glyma.15G157500), and their transcript levels fluctuated during seed development (Fig. 9b, Additional file 1: Figure S11) [33]. However, transcripts of CCTs in PLDα1KD lines were down-regulated by approximately 18% at stage 3 under both conditions. The expression of GmCCTs was suppressed in both PLDα1KD lines and wild-type seeds under high temperature and humidity conditions.
DAG-CPT and PDCT form an important PC–DAG exchange/conversion cycle to enforce the acyl editing of TAGs (Fig. 9a). In PLDα1KD developing seeds, DAG:CPTs (also called AAPTs), DAG:CPT1 and 2 (Glyma.12G081900 and Glyma.02G128300, respectively), were up-regulated as compared with those in developing seeds of wild-type under both conditions (Fig. 9b, Additional file 1: Figure S12). The soybean genome contains two PDCT genes, GmPDCT1 (Glyma.07G029800) and 2 (Glyma.08G213100). The two genes were highly expressed in developing soybean seeds (Additional file 1: Figure S13) [33]. The transcript of GmPDCT2 was up-regulated at early developmental stages and then decreased during late seed stages under both conditions. GmPDCT1 and 2 were significantly up-regulated at stages 2-3 in PLDα1KD developing seeds under both conditions (Fig. 9b). The expression of GmPDCT1 and GmDAG-CPT2 was suppressed at stages 3-5 in PLDα1KD developing seeds under high temperature and humidity conditions. Other two genes GmPDCT2 and GmDAG-CPT1 displayed complicated expression patterns in wild-type and PLDα1KD developing seeds in both environments. These data suggest that the activity of PC and DAG interconversion is increased when PLDα1 was suppressed in developing soybean seeds.
Reduced transcript levels of PLAs but increased levels of LPCAT in PLDα1KD seeds
Since pPLA affects TAG biosynthesis [15], we examined the expression of pPLAs that were either specifically or highly expressed in the developing soybean seeds. The soybean genome contains a large pPLA gene family, and several pPLAs were highly expressed in developing seeds, such as Glyma.08g028800, Glyma.11g036900, and Glyma.17G145900. The pPLAs, Glyma.18g251500 and Glyma.09g243100, were expressed only in seeds (Additional file 1: Figure S14) [32, 33]. The transcripts of pPLAs (Glyma01G.002400 and Glyma08G.028800) in PLDα1KD soybean seeds were, on average, 44% lower than those in wild-type seeds at stage-4 under both conditions, which was consistent with higher levels of PCs in PLDα1KD soybean seeds and lower levels of lysophospholipids (Figs. 6, 7, 10b). Meanwhile, high temperature and humidity suppressed the expression of GmPLA2s in both PLDα1KD lines and WT seeds. The down-regulation of both PLD and PLA expression may explain the higher level of PCs in PLDα1KD seeds.
As LPC and LPE content decreased significantly in PLDα1KD than wild-type seeds by more than tenfold at stage 3, we examined the expression of genes involved in the LPC and PC cycle. LPC acyltransferases (LPCAT) catalyzes the synthesis of PC from LPC using a new fatty acyl-CoA (Fig. 10a). AtLPCAT1 and 2 in Arabidopsis control the acyl editing process by acting as the main entry of unsaturated FAs into PC [16]. The lpcat1/lpcat2 mutant showed decreased PUFA in seed TAG [16, 17]. Soybean genome contains two LPCATs, Glyma.17G131500 (GmLPCAT1) and Glyma.05G049500 (GmLPCAT2). Transcript levels of two genes increased to the highest levels at middle stages and then decreased at the later stage of seed development in both PLDα1KD lines under stress conditions (Fig. 10b, Additional file 1: Figure S15) [33]. Both GmLPCAT transcripts were 17% higher in PLDα1KD seeds than in wild-type seeds at later stages under stress conditions (Fig. 10b). Meanwhile, Both genes were up-regulated in both PLDα1KD lines and wild-type under stress conditions. The down-regulation of GmPLAs and up-regulation of GmLPCATs in PLDα1KD soybean seeds could lead to reduced contents of LPC and LPE, as compared with wild-type (Figs. 6, 7, 10).
Higher germination rate of PLDα1KD seeds
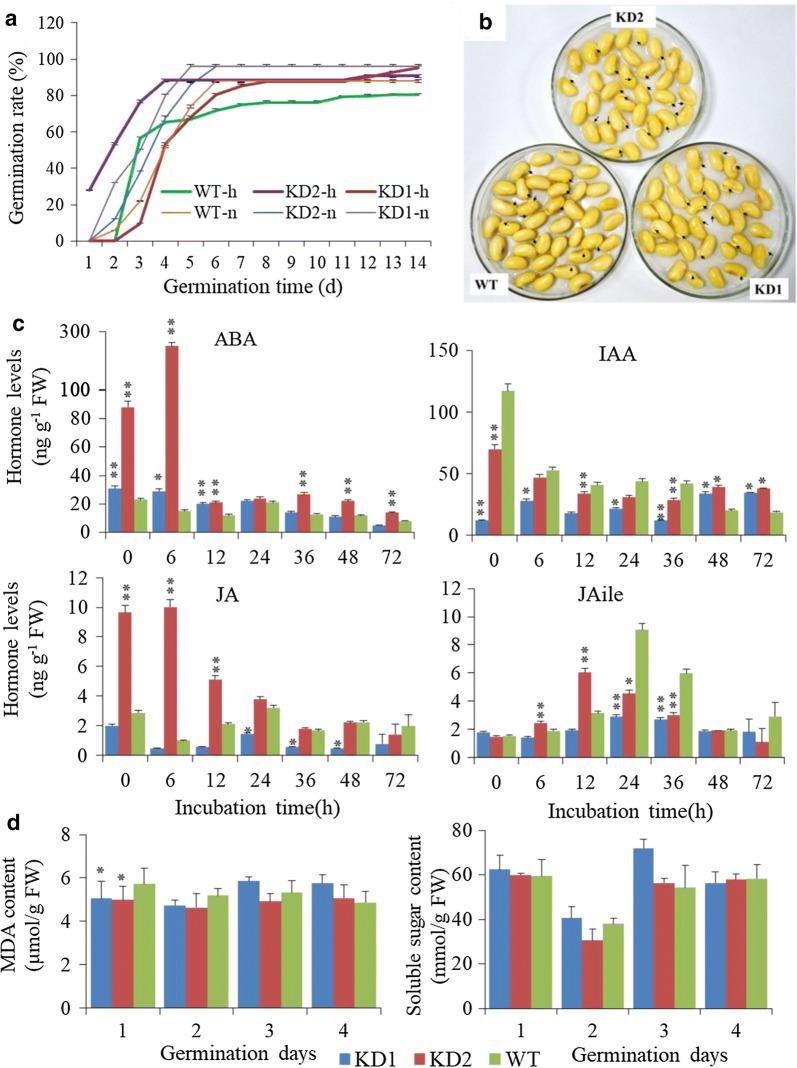
To address the effect of PLDα1KD on soybean seed vigor after harvested from high temperature and humidity conditions, we tested the seed vigor and germination rate of these knockdown lines and wild-type after stored at high temperature and humidity for three months (28 ± 3 °C in the dark and ~ 50% humidity). PLDα1KD seeds displayed higher germination rates, than the wild-type seeds under high temperature and humidity conditions. Under stress conditions, the germination rates of wild-type, PLDα1KD2 and PLDα1KD1 seeds were 80%, 91%, and 95%, respectively, whereas they were 88%, 96%, and 96% under normal conditions (Fig. 11a, b). However, the germination rate of PLDα1KD1 was lower than wild-type at an early stage but then caught up and became higher than wild-type at later stages. We further analyzed hormone levels in those germination seeds at different days after imbibitions. Higher ABA contents in PLDα1KD seeds than in wild-type seeds were detected, suggesting that PLDα1KD seeds had deeper seed dormancy than wild-type seeds and less nutrient consumption in PLDα1KD than in wild-type seeds during storage. Meanwhile, PLDα1KD seeds had initially a lower level of indoleacetic acid (IAA), but later a higher IAA level than that did wild-type seeds (Fig. 11c). Similarly, the seeds of two PLDα1KD lines showed difference in jasmonate (JA) and Ile-conjugated JA level from wild-type (Fig. 11c). PLDα1KD line 1 (KD1) seeds had lower total JAs and a lower germination rate, whereas PLDα1KD line 2 had higher total JAs and a higher germination rate than wild-type (Fig. 11c). The content of MDA was decreased in PLDα1KD seeds germinating for 1 day and had no significant difference within 2–4 days compared with wild-type seeds. There was also no significant difference in the content of soluble sugar in both PLDα1KD and wild-type germinating seeds (Fig. 11d).
Fig. 11.
Germination rates and hormone levels of PLDα1KD- and wild-type-germinating seeds. a Germination rates of PLDα1KD and wild-type Jack (WT) seeds under normal conditions after maturation under high temperature (30–36 °C) and humidity (70–85%) environments. Values are mean ± SD (n = 30 in one replications). b Germination seeds of PLDα1KD and wild-type Jack (WT) soybean for 3 days. c Hormone levels in germinating seeds of PLDα1KD and wild-type Jack (WT). d The contents of malonaldehyde and soluble sugars. KD1: PLDα1 knockdown line 1; KD2: PLDα1 knockdown line 2; WT: wild-type Jack. All data are e mean ± SD (n = 3). * and **Denote significance at P < 0.05 and P < 0.01, respectively, compared with wild-type Jack (WT) based on Student’s t test
Discussion
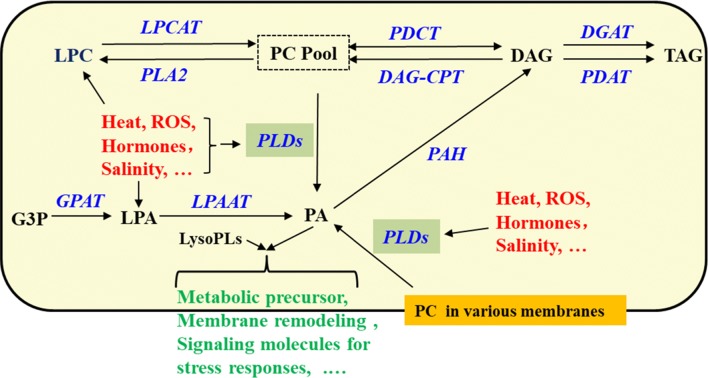
Seed development is a temperature-sensitive process more vulnerable than vegetative tissues to high temperature stress; high temperature and humidity conditions reduce lipid contents in soybean seeds, as compared with normal growth conditions [3]. However, PLDα1KD attenuated the reduction in lipid contents of soybean developing seeds compared with wild-type under the stress conditions, as well as under normal growth conditions. We showed higher total TAG content in the PLDα1KD transgenic lines at most developmental stages, with higher proportion of polyunsaturated fatty acid (PUFA) in TAG and PC as well. The higher levels of PCs are postulated as a consequence of down-regulation of PLDs and decreased transcripts of PLAs. The enhanced desaturation of PCs in PLDα1KD developing seeds is primarily attributable to increased FAD2 and FAD3 expression (Fig. 5a). The up-regulation of FADs might further enhance acyl editing on PC or PE, which triggers an accelerated interconversion of PCs to DAGs in PLDα1KD to drive metabolic flux toward unsaturated TAG biosynthesis. Up-regulated DAG:CPTs and PDCTs, as well as down-regulated CCT in PLDα1KD developing seeds indicate an enhanced metabolic flux or cross-talk between the acyl-editing and Kennedy pathways in PLDα1KD seeds (Fig. 12). The PLDα1 knockdown likely affected the responses of developing soybean seeds to high temperature and humidity conditions through modification of the levels of PAs and lysoPLs. These two signaling molecules accumulated to higher levels in developing wild-type seeds upon high temperature and humidity conditions, but significantly reduced in PLDα1KD developing seeds than wild-type. Levels of PAs and lysoPLs could be negatively related to seed viability and lipid stability, and lower levels of seed PAs and lysoPLs may have better seed viability and lipid stability in mature PLDα1KD soybean seeds.
Fig. 12.
Schematic of the links between TAG and phospholipid metabolism involving PLDα1. CCT choline-phosphate cytidylyltransferase, DAG diacylglycerol, DAG-CPT diacylglycerol cholinephosphotransferase, DGAT, acyl-CoA: diacylglycerol acyltransferase, G3P glycerol-3-phosphate, GPAT glycerol-3-phosphate acyltransferase, LPA 2-lysophosphatidic acid, LPAAT 2-lysophosphatidic acid acyltransferase, LPC 2-lysophosphatidylcholine, LPCAT 2-lysophosphatidylcholine acyltransferase, PA phosphatidic acid, PDAT phospholipid:diacylglycerol acyltransferase, PDCT phosphatidylcholine:diacylglycerol cholinephosphotransferase, PLA2 phospholipase A2, PAH phosphatidic acid phosphatase, PLD phospholipase D, PC phosphatidylcholine, TAG triacylglycerol
PLDα1 knockdown promoted fatty acid unsaturation in both TAGs and phospholipids
There are increases in the total contents of both TAGs and unsaturation fatty acids in the developing seeds of PLDα1KD than wild-type cultivar under high temperature and humidity conditions. The desaturation of PCs is primarily attributable to FAD2 and FAD3 in the ER [48–50]. The increased transcripts of FAD2s and FAD3s in PLDα1KD seeds explain the increased unsaturated fatty acids in PCs and PEs, and TAG species, together with the decreased levels of PA, LPC, and LPE species in the PLDα1KD developing seeds. The difference in lipid contents between PLDα1KD and wild-type seeds under normal conditions was similar to that reported previously [9]. The high temperature and humidity decreased the contents of total lipids, especially fatty acid contents in seeds compared with these under normal conditions. However, our data showed that the differences in lipid contents between GmPLDα1KD and wild-type seeds under the stress conditions became much bigger than those under normal conditions. Meanwhile, the high temperature and humidity conditions affected the expression of genes involved in PLs and TAG synthesis pathways, which may eventually result in decreased lipid contents. Consistently, under these stress conditions, developing seeds of GmPLDα1KD displayed higher gene expression levels and total lipid contents than those of wild-type seeds, e.g., GmFAD2-1A and GmFAD2-2A. Mature GmPLDα1KD seeds also had better germination rates than did wild-type seeds. Therefore, suppression of GmPLDα1 improved the expression of genes involved in PLs and TAG synthesis pathways under both high temperature and humidity stress and normal conditions.
PLDα1KD enhanced the PDAT pathway and DAG-PC conversion toward TAG biosynthesis
It has been proposed that the DAG:CPT-catalyzed reaction provides an acyl editing mechanism for the production of polyunsaturated TAGs containing 18:2 and 18:3 through PCs [51]. The significant increases in unsaturation acyl chains of PCs in developing soybean seeds of PLDα1KD plants were attributable to the higher expression levels of FADs. Since both PC and DAG play essential roles in acyl editing on glycerolipids, their interconversion is important for TAG synthesis [14, 28, 52]. The knockdown of PLDα1 in the developing soybean seeds affected expression of all genes involved in the acyl editing in soybean developing seeds (Fig. 12). The up-regulation of both PDCTs and DAG:CPTs in consistent with an increased TAG contents in soybean developing seeds, since PCs are the major source of DAGs that further flux into TAGs by the action of PDATs [14]. Since PA–DAG–PC–DAG metabolic pathway primarily takes place in soybean developing seeds, PDCT thus becomes essential in determining the unsaturation of TAG. PDCT mutation results in a 40% decrease in polyunsaturated FAs in seed TAG without disrupting overall TAG levels [19]. PDAT’s substrate specificity on PC and DAG further enhanced the PC–DAG conversion and resulted in higher levels of unsaturated fatty acids in TAGs of developing seeds. The markedly increased PC/PE accumulation coincides with up-regulated FAD2 and 3, PDCT and PDAT genes suggest PLDα1 negatively affects acyl editing and bridges the phospholipid turnover and TAG biosynthesis.
PLA catalyzes PC or PE hydrolysis to generate free fatty acid and lysoPLs, such as LPC or LPE. PLAs and LPCATs can form a PC turnover cycle (Fig. 12). The lower transcripts of PLAs in PLDα1KD are consistent with higher levels of PCs and lower levels of LPC and LPE in PLDα1KD developing seeds, which is also consistent with previous report in Arabidopsis PLDα1KD plants [53]. The down-regulation of PLDα1 and PLA genes may together lower PA, LPC and LPE levels [28]. In pah1pah2 mutant, PC contents dramatically increased and TAG content decreased due to the increased PA contents through up-regulation of CCT1 [54]. Our results suggest that PLA–LPCAT, PLD, and PDCT–DAG:CTP play important roles in acyl editing. PLDα1KD promotes the PDCT and DAG:CPT-catalyzed conversion between PCs and DAGs, through which extensive acyl desaturation on PCs by FADs is eventually reflected by the increased TAG unsaturation. Thus, the developing PLDα1KD soybean seeds have higher levels of di18:2 than these non-transgenic lines.
Effects of high temperature and humidity on soybean seed development
While high temperature and humidity can significantly affect membrane compositions, altering membrane physiological properties and functions, how the stress affects phospholipid and TAG metabolism in developing seeds is largely unknown [5, 9]. High temperature stress causes significant increases in ROS-scavenging, lipid desaturating, oxidizing, and acylating genes, and in 18:3-TAG contents in wheat, while fatty acyl chain unsaturation in polar phospholipids decrease [5]. ROS plays various roles in regulating cell growth, development, and cell survival under stress. Generally, moderate levels of ROS may function as signals to promote plant growth and survival, whereas a large increase of ROS can induce plant cell death. Under physiologic conditions, the balance between generation and elimination of ROS maintains the proper function of redox-sensitive signaling proteins. However, under adversary environments, such as extreme heat, light, and cold conditions, plants produce more ROS or xenobiotics than normal levels, and activate ROS-scavenging enzymes and xenobiotics-detoxification enzyme GST to reduce ROS damage to plants.
Possible mechanisms for the improvement of soybean seeds by knockdown of GmPLDα1
PLDα1 and its product PA and PLA and its product LysPLs, have been reported in other plants to function in plant stress response to salt and drought stresses, oxidative stresses, and hormones [10, 27, 55]. Our study here indicates that high temperature and humidity environments significantly affect phospholipid and TAG metabolism in developing soybean seeds, and increase the contents of PAs and lysoPLs in wild-type seed, but the contents of PAs and lysoPLs were significantly reduced in PLDα1KD mutant seeds (Figs. 6, 7). The anti-deterioration effects of PLDα1KD mutation on developing soybean seeds under high temperature and humidity conditions in our study were similar to the better oil storability and seed viability of these naturally or artificially aged Arabidopsis and soybean PLDα1-antisense mutant seeds [7, 8]. Those results suggest that the lowered PAs and lysoPLs in PLDα1KD or knockout mutant seeds might be the major causes. The higher PA and lysoPL contents may increase membrane permeability during seed development and storage [7, 8, 10]. The variations in lipid contents and gene expression in two independent PLDα1KD lines may result from the variations of levels of PAs and lysoPLs because of different degrees of PLD suppression. It has been observed that different knockdown lines of AtPLDα1 often showed variations in phenotypes [7, 10, 27]. Besides its metabolic function, PLDα1 is also involved in plant responses to salinity, drought, cold, and heat stresses [27] (Fig. 12). The distinct responses of ROS-scavenging genes in PLDα1KD and wild-type cultivars may also be associated to GmPLDα1/PLA and PA/lysPLs that could be critically involved in soybean adaptive response to high temperature and humidity [27, 34, 35, 55]. We posit that PA and lysoPLs generated by PLDs and PLAs under high temperature and humidity conditions could be the keys for the detrimental effects of high temperature and humidity [7–9]. PLDα1KD substantially decreases stress-induced production of PA and lysoPLs and thereby alleviates the negative impacts by these adverse conditions on developing soybean seeds.
Conclusions
Genetically modified PLDα1KD soybean seeds have improved oil content, seed vigor, and resistance against aging and deterioration under high temperature and humility environments. The mechanism behind the phenomena was explored by examination of relevant metabolites and transcripts of lipid metabolic genes in WT and PLDα1KD soybean developing seeds grown under both stress and normal conditions. The higher TAG content with higher proportion of unsaturation degree in PLDα1KD than wild-type at most developmental stages are attributable to the lower expression levels of PLDs, PLA2s, CCTs, and DGATs and higher expression levels of FADs, LPCAT, PDAT, PAH, PDCT, and DAG:CPT in PLDα1KD soybean. Higher levels of ROS-scavenging and xenobiotics-detoxifying genes (GST, SOD, CAT, POD, STI, and APX) in PLDα1KD soybean developing seeds and lower ROS level in germinated PLDα1KD seeds than those in wild-type seeds may explain their tolerance against high temperature and humility stress in preventing pre-harvest seed deterioration. This study presents a valuable model illustrating the role of PLD in TAG synthesis and provides novel insights into the mechanistic details of lipid metabolic pathway changes upon the knockdown of PLDs during seed development under stress. Despite of the biotechnological application potential in genetic improvement of soybean production under stress, the field trials may be required to validate the advantages for quality soybean production, especially under frequently occurred heatwaves.
Materials and methods
Creation of PLDα1KD transgenic soybean
The forward and reverse sequences of 1151 bp from the conserved GmPLDα1 cDNA in Williams 82 were used for making RNAi constructs, which were different from the forward 820 bp and reverse 1300 bp from conserved GmPLDα1 sequence derived from cloning partial sequence in Fayette used previously [9]. The RNAi cassette was assembled into the NotI site of the soybean expression vector pBetaConSoyHyg, flanked on its 5′ end by the seed-specific promoter for the soybean α’ subunit of β-conglycinin gene [56] and on its 3′ end by the 3′UTR of the phaseolin gene [57]. The vector backbone, which was derived from pBluescript SK-(Stratagene), was engineered with a hygromycin B phosphotransferase gene [58] under control of the potato ubiquitin-3 promoter [59] for selection of transformed soybean embryos. Additional file 1: Figure S1 illustrates the cloning strategy and details of the organization of gene cassettes generated in the vector pBetaConSoyHyg for soybean transformation. The construct was introduced into soybean (Glycine max cv. Jack) somatic embryos using biolistic transformation as described [60]. Embryos were maintained after bombardment in SHaM media [61] with hygromycin selection [60]. Mature somatic embryos obtained following hygromycin selection were screened by immunoblotting analysis for PLDα1 levels. Embryos, representing independent transgenic events, that showed reductions in PLDα1 levels, were desiccated and used for regeneration of transgenic plants as described [60]. Regenerated plants were advanced to homozygosity (> T3 generation) for use in the described studies. One hundred forty-three putative tissues were recovered after selection. Ten transgenic soybean events were recovered and fully grown in the greenhouse. Using enzyme activity, PCR and western blotting, two successfully repressed transgenic soybean events (#1020 and #1048) were selected from ten transgenic events for analysis.
Plant growth conditions and treatments
Wild-type Jack and transgenic soybean PLDα1KD lines were grown in a greenhouse with a normal (26 ± 3 °C/18 h day and 23 ± 3 °C/6 h night photoperiod, 45–65% humidity) or high temperature and high humidity (36 ± 3 °C/18 h day and 30 ± 3 °C/6 h night photoperiod, 70–85% humidity) conditions which were controlled by air condition and humidifier machine. The developing seeds were harvested at approximately 50 days after fertilization (DAF). The varieties for transcriptomic analyses at different developmental stages of soybean seeds were Williams 82 and Hokkaido Black 25- and 50-day seeds were collected and put into liquid nitrogen immediately after separating from plants. To test the suppression of PLDα1 in transgenic soybean seeds, qRT-PCR, western blotting with PLDα1 antibody, and PLDα1 activity assays were performed using developing staged 3 seeds of T3 and T5 lines. The presence of the transgene in T5 line seeds was confirmed by PCR. Monitoring soybean PLD proteins in the soybean extract used antibodies against Arabidopsis thaliana PLDα1.
Analysis of seed total fatty acids
Total fatty acids were extracted three times and analyzed with GC using triheptadecanoylglycerol as an internal standard using a method described previously [44, 62]. Analyses were carried out on an Agilent Technologies (USA) 7890A Network gas chromatograph, equipped with a flame-ionization detector (FID) and a split/splitless injector. Polyethylene glycerol:Agilent 19091 N-133 column (30 m × 0.25 mm i.d. 0.25 m film thickness) was used [44]. An HP ChemStation (Hewlett-Packard, Palo Alto, CA, USA) was used for instrument control and data analysis.
Analysis of seed TAGs
Seed TAG and its fatty acid compositions were analyzed as described previously [44]. Briefly, total seed lipids from developing seeds were separated into TAG, DAG, and free fatty acids on a TLC plate, and TAG spots were visualized with iodine vapor and identified by their migration. The spots of TAG were scraped off TLC plates for determination of fatty acid contents and compositions by GC as described above.
Immunoblotting and PLD activity assays
Proteins were extracted from immature or mature soybean seeds with extraction buffer containing 50 mm Tris–HCl (pH 7.5), 10 mm KCl, 1 mm EDTA, 0.5 mm phenyl methylsulfonyl fluoride and 2 mm DTT at 4 °C [63]. Proteins were incubated at boiling water bath for 10 min with a SDS-PAGE loading buffer containing 100 μL of 50 mm Tris–HCl (pH 6.8), 10 mm DTT, 2% SDS, 0.01% bromophenol blue and 10% glycerol. Twenty micrograms of denatured proteins were fractionated by 10% SDS-PAGE and transferred to a membrane. The membrane was blotted with Arabidopsis PLDα1 antibodies as described by [63]. Aliquots of 20 μg of native protein were used for the PLD enzyme assay as described previously [63].
Phospholipid analysis
Phospholipid profiling was performed on soybean developing seeds with ESI-MS/MS as described previously [64]. In brief, five replicates of developing soybean seed samples were smashed in liquid nitrogen and transferred immediately to 3 mL of 85 °C hot isopropanol containing 0.01% butylated hydroxytoluene (BHT) for 15 min. Then, adding 1.5 mL of chloroform and 1.5 mL of methanol for mixing well and extraction on shaker for 30 min, and then 1.8 mL of H2O were added to each sample separately. After mixing and centrifugation, lower layer solvent containing lipids was transferred to a new tube. Repeat the extraction with 3 mL of chloroform/methanol (2:1) containing 0.01% BHT for five times for 10 min each time. The combined solvent extracts were washed with 1 mL of 1 M KCl and 1 mL of sterile ddH2O, successively, and then concentrated with nitrogen gas. The total extracts were transferred to 2.0 mL vial with Teflon-lined screw cap and dried completely. The methanol solved lipids were detected followed the published protocol [64]. Five duplicates for each genotype or treatments were analyzed.
Quantitative reverse transcriptase-PCR (qRT-PCR) for gene expression analysis
qRT-PCR was done as previously described [65]. Briefly, total RNA was isolated from seeds with RNA isolation kit (Bioteke Corporation). The first strand of cDNA was synthesized according to the supplier’s instructions (M-MLV First Strand kit, Life Technologies, Invitrogen, USA) and real-time RT-PCR was executed with primers listed as Additional file 2: Table S1 using the BioRad iQ5 (Bio-Rad, USA) with an SYBR green MIX (Premix system, NEWBIO INDUSTRY). Soybean ACTIN1 (Glyma.15g034000) was used as the internal positive control.
Seed germination assay
After storing seeds for 3 months in high temperature and humidity conditions, seeds of PLDα1KDs and wild-type control harvested at the same time in same size were selected for assay. Seeds were sterilized with chloride gas and washed in distilled water three times before germinating in plates containing wet sterile filter papers. Seeds were germinated in an incubator at 25 °C in 16 h day/8 h night. Seed germination was scored when the radical was elongated about 0.5 cm.
Analysis of seed hormones
Seed samples collected at different time during germination were immediately frozen in liquid nitrogen and stored at − 80 °C. Hormones were measured according to the method described previously, with modifications [66]. Briefly, the liquid nitrogen-frozen samples were lyophilized, and 0.1 g of samples was extracted with 750 μL methanol:ddH2O:acetic acid (80:19:1, v/v/v) for three times in the dark and 4 °C. The supernatants were combined and dried under stream of nitrogen. Prior to UPLC/ESI-MS/MS analysis, the extracts were suspended in 250 μL methanol and sonicated for 10 min, and spun at 12,000 g for 10 min, and the supernatant was transferred into UPLC vials. Chromatographic separation was performed using the Agilent LC-20AD system (Agilent, Santa Clara, CA, USA) equipped with AB Sciex QTRAP® 5500 detector and a Zorbax × 300 SB-C18 (4.6 mm × 150 mm × 5 μm) column (Agilent, Palo Alto, CA, USA), as described previously [66].
Bioinformatics’ analyses
Soybean proteins involved in lipid metabolism and their expression data were retrieved from Phytozome (http://phytozome.jgi.doe.gov/pz). For analyses, these genes were compared with homologues from Arabidopsis thaliana and other plant species retrieved from NCBI (http://www.ncbi.nlm.nih.gov/BLAST/). Phylogeny trees were constructed using the neighbor-joining tree method with MEGA6. The significance level of the neighbor-joining analysis was examined by bootstrap testing with 1000 repeats.
Additional files
Additional file 1: Figure S1. Schematic procedure for construction of soybean GmPLDα1RNAi vector for plant transformation. Figure S2. Phylogenetic analysis of PLD genes from the soybean genome and their expression patterns. Figure S3. Mechanism of plant resistant to stress and gene expression of GmPLDαs and stress-related genes under high temperature and humidity condition in comparison to under normal conditions. Figure S4. Analyses of lipids in PLDα1KD and wild-type developing seeds under different growth conditions. Figure S5. Phylogenetic analysis of FAD genes from the soybean genome and their expression patterns. Figure S6. Phylogenic analysis of Acyl-CoA:lysophosphatic acid acyltransferase (LPAAT) genes and their expression profiles. Figure S7. Phylogenic analysis of phosphatidic acid hydrolase (PAH) genes and their expression profiles. Figure S8. Phylogenic analysis of acyl-CoA:diacylglycerol acyltransferase (DGAT) genes and their expression profiles. Figure S9. Phylogenic analysis of phospholipid:diacylglycerol acyltransferase (PDAT) genes and their expression profiles. Figure S10. Phylogenic analysis of choline/ethylamine kinase (CEK) genes and their expression profiles. Figure S11. Phylogenic analysis of CTP: phosphocholine cytidylyltransferase (CCT) genes and the expression profiles. Figure S12. Phylogenic analysis of diacylglycerol:cholinephosphotransferase (DAG-CPT or AAAT) genes and their expression profiles. Figure S13. Phylogenic analysis of phosphatidylcholine:diacylglycerol cholinephosphotransferase (PDCT) genes and their expression profiles. Figure S14. Phylogenetic analysis of PLA genes from the soybean genome and their expression patterns. Figure S15. Phylogenic analysis of 2-lysophosphatidylcholine acyltransferase (LPCAT) genes and their expression profiles.
Additional file 2: Table S1. The Quantitative RT-PCR primers used in this study. Table S2. PLD genes in soybean genome. Table S3. Expression pattern in different tissue. Table S4. Relative expression of GmPLDɑs in different developing seeds after fertilization. Data S1. Protein sequences for phylogenic tree construction.
Authors’ contributions
JZ planned and designed the research. GZ, SB, GW, YRZ, BC, YLZ performed the experiments and analyzed data. SB, GW, XW did soybean transformat screening. GZ, BC analyzed the data. JZ, GZ, XW wrote and edited the manuscript. All authors read and approved the final manuscript.
Acknowledgements
We thank Dr. Edgar B. Cahoon and Jamie Shipp (Center for Plant Science Innovation, University of Nebraska-Lincoln) for helps in generation of transgenic soybean plants and Dr. Ruth Welti (Kansas lipidomics center, Kansas State University) for parts of lipid analyses and critical reading the manuscript. The authors also thank lab members in Prof. Zhao’s lab for all assistances in experiments and data analyses.
Competing interests
The authors declare that they have no competing interests.
Availability of data and materials
All data supporting my findings can be available and found in the supplementary data; materials can be available for distribution upon request.
Consent for publication
The authors agree to the publication of this manuscript in the journal.
Ethics approval and consent to participate
No investigations were undertaken using humans/human samples in this study. No experimental animals were used to conduct any of the experiments reported in this manuscript. Our study did not involve endangered or protected species. No specific permits were required from the studies and Professor Jian Zhao should be contacted for future permissions.
Funding
This project is support in part by the Ministry of Science and Technology of China (2016YFD0100504), by Agriculture and Food Research Initiative (AFRI) Award No. [2016-67013-24429/project accession number 1007600] from the USDA National Institute of Food and Agriculture, and the National Natural Science Foundation of China (31670294).
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.IPCC. Climate Change 2013: the physical science basis. Contribution of working group I to the fifth assessment report of the intergovernmental panel on climate change, Cambridge university press, Cambridge, United Kingdom and New York, NY, USA; 2013.
- 2.Ray DK, Gerber JS, MacDonald GK, West PC. Climate variation explains a third of global crop yield variability. Nat Commun. 2015;6:5989. doi: 10.1038/ncomms6989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Narayanan S, Prasad PVV, Fritz AK, Boyle DL, Gill BS. Impact of high night-time and high daytime temperature stress on winter wheat. J Agron Crop Sci. 2015;201:206–218. doi: 10.1111/jac.12101. [DOI] [Google Scholar]
- 4.Schauberger B, Archontoulis S, Arneth A, Balkovic J, Ciais P, Deryng D, et al. Consistent negative response of US crops to high temperatures in observations and crop models. Nat Commun. 2017;8:13931. doi: 10.1038/ncomms13931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Narayanan S, Tamura PJ, Roth MR, Prasad PV, Welti R. Wheat leaf lipids during heat stress: I. High day and night temperatures result in major lipid alterations. Plant Cell Environ. 2016;39:787–803. doi: 10.1111/pce.12649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shu Y, Tao Y, Wang S, Huang L, Yu X, Wang Z, et al. GmSBH1, a homeobox transcription factor gene, relates to growth and development and involves in response to high temperature and humidity stress in soybean. Plant Cell Rep. 2015;34:1927–1937. doi: 10.1007/s00299-015-1840-7. [DOI] [PubMed] [Google Scholar]
- 7.Devaiah SP, Pan X, Hong Y, Roth M, Welti R, Wang X. Enhancing seed quality and viability by suppressing phospholipase D in Arabidopsis. Plant J. 2007;50:950–957. doi: 10.1111/j.1365-313X.2007.03103.x. [DOI] [PubMed] [Google Scholar]
- 8.Lee J, Welti R, Roth M, Schapaugh WT, Li J, Trick HN. Enhanced seed viability and lipid compositional changes during natural ageing by suppressing phospholipase Dalpha in soybean seed. Plant Biotechnol J. 2012;10:164–173. doi: 10.1111/j.1467-7652.2011.00650.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee J, Welti R, Schapaugh WT, Trick HN. Phospholipid and triacylglycerol profiles modified by PLD suppression in soybean seed. Plant Biotechnol J. 2011;9:359–372. doi: 10.1111/j.1467-7652.2010.00562.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhao J. Phospholipase D and phosphatidic acid in plant defence response: from protein-protein and lipid-protein interactions to hormone signalling. J Exp Bot. 2015;66:1721–1736. doi: 10.1093/jxb/eru540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Manan S, Chen B, She G, Wan X, Zhao J. Transport and transcriptional regulation of oil production in plants. Crit Rev Biotechnol. 2017;37:641–655. doi: 10.1080/07388551.2016.1212185. [DOI] [PubMed] [Google Scholar]
- 12.Li M, Wei F, Tawfall A, Tang M, Saettele A, Wang X. Overexpression of patatin-related phospholipase AIIIdelta altered plant growth and increased seed oil content in camelina. Plant Biotechnol J. 2015;13:766–778. doi: 10.1111/pbi.12304. [DOI] [PubMed] [Google Scholar]
- 13.Bates PD, Ohlrogge JB, Pollard M. Incorporation of newly synthesized fatty acids into cytosolic glycerolipids in pea leaves occurs via acyl editing. J Biol Chem. 2007;282:31206–31216. doi: 10.1074/jbc.M705447200. [DOI] [PubMed] [Google Scholar]
- 14.Bates PD, Durrett TP, Ohlrogge JB, Pollard M. Analysis of acyl fluxes through multiple pathways of triacylglycerol synthesis in developing soybean embryos. Plant Physiol. 2009;150:55–72. doi: 10.1104/pp.109.137737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li M, Bahn SC, Fan C, Li J, Phan T, Ortiz M, et al. Patatin-related phospholipase pPLAIIIdelta increases seed oil content with long-chain fatty acids in Arabidopsis. Plant Physiol. 2013;162:39–51. doi: 10.1104/pp.113.216994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bates PD, Fatihi A, Snapp AR, Carlsson AS, Browse J, Lu C. Acyl editing and headgroup exchange are the major mechanisms that direct polyunsaturated fatty acid flux into triacylglycerols. Plant Physiol. 2012;160:1530–1539. doi: 10.1104/pp.112.204438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang L, Shen W, Kazachkov M, Chen G, Chen Q, Carlsson AS, et al. Metabolic interactions between the Lands cycle and the Kennedy pathway of glycerolipid synthesis in Arabidopsis developing seeds. Plant Cell. 2012;24:4652–4669. doi: 10.1105/tpc.112.104604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lin YC, Liu YC, Nakamura Y. The choline/ethanolamine kinase family in Arabidopsis: essential role of CEK4 in phospholipid biosynthesis and embryo development. Plant Cell. 2015;27:1497–1511. doi: 10.1105/tpc.15.00207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lu C, Xin Z, Ren Z, Miquel M, Browse J. An enzyme regulating triacylglycerol composition is encoded by the ROD1 gene of Arabidopsis. Proc Natl Acad Sci USA. 2009;106:18837–18842. doi: 10.1073/pnas.0908848106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang L, Kazachkov M, Shen W, Bai M, Wu H, Zou J. Deciphering the roles of Arabidopsis LPCAT and PAH in phosphatidylcholine homeostasis and pathway coordination for chloroplast lipid synthesis. Plant J. 2014;80:965–976. doi: 10.1111/tpj.12683. [DOI] [PubMed] [Google Scholar]
- 21.Hu Z, Ren Z, Lu C. The phosphatidylcholine diacylglycerol cholinephosphotransferase is required for efficient hydroxy fatty acid accumulation in transgenic Arabidopsis. Plant Physiol. 2012;158:1944–1954. doi: 10.1104/pp.111.192153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wickramarathna AD, Siloto RMP, Mietkiewska E, Singer SD, Pan X, Weselake RJ. Heterologous expression of flax phospholipid:diacylglycerol cholinephosphotransferase (PDCT) increases polyunsaturated fatty acid content in yeast and Arabidopsis seeds. BMC Biotechnol. 2015;15:63. doi: 10.1186/s12896-015-0156-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pan X, Siloto RM, Wickramarathna AD, Mietkiewska E, Weselake RJ. Identification of a pair of phospholipid:diacylglycerol acyltransferases from developing flax (Linum usitatissimum L.) seed catalyzing the selective production of trilinolenin. J Biol Chem. 2013;288:24173–24188. doi: 10.1074/jbc.M113.475699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Maatta S, Scheu B, Roth MR, Tamura P, Li M, Williams TD, et al. Levels of Arabidopsis thaliana leaf phosphatidic acids, phosphatidylserines, and most trienoate-containing polar lipid molecular species increase during the dark period of the diurnal cycle. Front Plant Sci. 2012;3:49. doi: 10.3389/fpls.2012.00049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang X. Regulatory functions of phospholipase D and phosphatidic acid in plant growth, development, and stress responses. Plant Physiol. 2005;139:566–573. doi: 10.1104/pp.105.068809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang X, Chapman KD. Lipid signaling in plants. Front Plant Sci. 2013;4:216. doi: 10.3389/fpls.2013.00216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hong Y, Zhao J, Guo L, Kim SC, Deng X, Wang G, et al. Plant phospholipases D and C and their diverse functions in stress responses. Prog Lipid Res. 2016;62:55–74. doi: 10.1016/j.plipres.2016.01.002. [DOI] [PubMed] [Google Scholar]
- 28.Yang W, Wang G, Li J, Bates PD. Phospholipase Dzeta enhances diacylglycerol flux into triacylglycerol. Plant Physiol. 2017;174:110–123. doi: 10.1104/pp.17.00026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fang C, Ma Y, Wu S, Liu Z, Wang Z, Yang R, et al. Genome-wide association studies dissect the genetic networks underlying agronomical traits in soybean. Genome Biol. 2017;18:161. doi: 10.1186/s13059-017-1289-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Manan S, Ahmad MZ, Zhang G, Chen B, Haq BU, Yang J, Zhao J. Soybean LEC2 regulates subsets of genes involved in controlling the biosynthesis and catabolism of seed storage substances and seed development. Front Plant Sci. 2017;8:1604. doi: 10.3389/fpls.2017.01604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang L, Ma H, Song L, Shu Y, Gu W. Comparative proteomics analysis reveals the mechanism of pre-harvest seed deterioration of soybean under high temperature and humidity stress. J Proteomics. 2012;75:2109–2127. doi: 10.1016/j.jprot.2012.01.007. [DOI] [PubMed] [Google Scholar]
- 32.Libault M, Farmer A, Joshi T, Takahashi K, Langley RJ, Franklin LD, et al. An integrated transcriptome atlas of the crop model Glycine max, and its use in comparative analyses in plants. Plant J. 2010;63:86–99. doi: 10.1111/j.1365-313X.2010.04222.x. [DOI] [PubMed] [Google Scholar]
- 33.Severin AJ, Woody JL, Bolon YT, Joseph B, Diers BW, Farmer AD, et al. RNA-Seq Atlas of Glycine max: a guide to the soybean transcriptome. BMC Plant Biol. 2010;10:160. doi: 10.1186/1471-2229-10-160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Galant A, Koester RP, Ainsworth EA, Hicks LM, Jez JM. From climate change to molecular response: redox proteomics of ozone-induced responses in soybean. New Phytol. 2012;194:220–229. doi: 10.1111/j.1469-8137.2011.04037.x. [DOI] [PubMed] [Google Scholar]
- 35.Oh M, Komatsu S. Characterization of proteins in soybean roots under flooding and drought stresses. J Proteomics. 2015;114:161–181. doi: 10.1016/j.jprot.2014.11.008. [DOI] [PubMed] [Google Scholar]
- 36.Noctor G, Reichheld JP, Foyer CH. ROS-related redox regulation and signaling in plants. Semin Cell Dev Biol. 2017;80:3–12. doi: 10.1016/j.semcdb.2017.07.013. [DOI] [PubMed] [Google Scholar]
- 37.Heppard EP, Kinney AJ, Stecca KL, Miao GH. Developmental and growth temperature regulation of two different microsomal [omega]-6 desaturase genes in soybeans. Plant Physiol. 1996;110:311–319. doi: 10.1104/pp.110.1.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tang GQ, Novitzky WP, Carol Griffin H, Huber SC, Dewey RE. Oleate desaturase enzymes of soybean: evidence of regulation through differential stability and phosphorylation. Plant J. 2005;44:433–446. doi: 10.1111/j.1365-313X.2005.02535.x. [DOI] [PubMed] [Google Scholar]
- 39.Li L, Wang X, Gai J, Yu D. Molecular cloning and characterization of a novel microsomal oleate desaturase gene from soybean. J Plant Physiol. 2007;164:1516–1526. doi: 10.1016/j.jplph.2006.08.007. [DOI] [PubMed] [Google Scholar]
- 40.Bilyeu K, Palavalli L, Sleper D, Beuselinck P. Mutations in soybean microsomal omega-3 fatty acid desaturase genes reduce linolenic acid concentration in soybean seeds. Crop Sci. 2005;45:1830–1836. doi: 10.2135/cropsci2004.0632. [DOI] [Google Scholar]
- 41.Flores T, Karpova O, Su X, Zeng P, Bilyeu K, Sleper DA, Nguyen HT, Zhang ZJ. Silencing of GmFAD3 gene by siRNA leads to low alpha-linolenic acids (18:3) of fad3-mutant phenotype in soybean [Glycine max (Merr.)] Transgenic Res. 2008;17:839–850. doi: 10.1007/s11248-008-9167-6. [DOI] [PubMed] [Google Scholar]
- 42.Kim HU, Huang AH. Plastid lysophosphatidyl acyltransferase is essential for embryo development in Arabidopsis. Plant Physiol. 2004;134:1206–1216. doi: 10.1104/pp.103.035832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kim HU, Li Y, Huang AH. Ubiquitous and endoplasmic reticulum-located lysophosphatidyl acyltransferase, LPAT2, is essential for female but not male gametophyte development in Arabidopsis. Plant Cell. 2005;17:1073–1089. doi: 10.1105/tpc.104.030403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chen B, Wang J, Zhang G, Liu J, Manan S, Hu H, Zhao J. Two types of soybean diacylglycerol acyltransferases are differentially involved in triacylglycerol biosynthesis and response to environmental stresses and hormones. Sci Rep. 2016;6:28541. doi: 10.1038/srep28541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dahlqvist A, Stahl U, Lenman M, Banas A, Lee M, Sandager L, et al. Phospholipid:diacylglycerol acyltransferase: an enzyme that catalyzes the acyl-CoA-independent formation of triacylglycerol in yeast and plants. Proc Natl Acad Sci USA. 2000;97:6487–6492. doi: 10.1073/pnas.120067297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Oelkers P, Tinkelenberg A, Erdeniz N, Cromley D, Billheimer JT, Sturley SL. A lecithin cholesterol acyltransferase-like gene mediates diacylglycerol esterification in yeast. J Biol Chem. 2000;275:15609–15612. doi: 10.1074/jbc.C000144200. [DOI] [PubMed] [Google Scholar]
- 47.Zhang M, Fan J, Taylor DC, Ohlrogge JB. DGAT1 and PDAT1 acyltransferases have overlapping functions in Arabidopsis triacylglycerol biosynthesis and are essential for normal pollen and seed development. Plant Cell. 2009;21:3885–3901. doi: 10.1105/tpc.109.071795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schlueter JA, Vasylenko-Sanders IF, Deshpande S, Yi J, Siegfried M, Roe BA, et al. FAD2 Gene family of soybean: insights into the structural and functional divergence of a paleopolyploid genome. Crop Sci. 2007;47(S1):14–26. [Google Scholar]
- 49.Pham AT, Lee JD, Shannon JG, Bilyeu KD. Mutant alleles of FAD2-1A and FAD2-1B combine to produce soybeans with the high oleic acid seed oil trait. BMC Plant Biol. 2010;10:195. doi: 10.1186/1471-2229-10-195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Haun W, Coffman A, Clasen BM, Demorest ZL, Lowy A, Ray E, et al. Improved soybean oil quality by targeted mutagenesis of the fatty acid desaturase 2 gene family. Plant Biotechnol J. 2014;12:934–940. doi: 10.1111/pbi.12201. [DOI] [PubMed] [Google Scholar]
- 51.Vogel G, Browse J. Cholinephosphotransferase and diacylglycerol acyltransferase (substrate specificities at a key branch point in seed lipid metabolism) Plant Physiol. 1996;110:923–931. doi: 10.1104/pp.110.3.923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chapman KD, Ohlrogge JB. Compartmentation of triacylglycerol accumulation in plants. J Biol Chem. 2012;287:2288–2294. doi: 10.1074/jbc.R111.290072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wang X. Plant phospholipases. Annu Rev Plant Physiol Plant Mol Biol. 2001;52:211–231. doi: 10.1146/annurev.arplant.52.1.211. [DOI] [PubMed] [Google Scholar]
- 54.Craddock CP, Adams N, Bryant FM, Kurup S, Eastmond PJ. Phosphatidic acid phosphohydrolase regulates phosphatidylcholine biosynthesis in Arabidopsis by Phosphatidic acid-mediated activation of CTP:phosphocholine cytidylyltransferase activity. Plant Cell. 2015;27:1251–1264. doi: 10.1105/tpc.15.00037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hong Y, Pan X, Welti R, Wang X. The effect of phospholipase Dalpha3 on Arabidopsis response to hyperosmotic stress and glucose. Plant Signal Behav. 2008;3:1099–1100. doi: 10.4161/psb.3.12.7003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Beachy RN, Chen ZL, Horsch RB, Rogers SG, Hoffmann NJ, Fraley RT. Accumulation and assembly of soybean beta-conglycinin in seeds of transformed petunia plants. EMBO J. 1985;4:3047–3053. doi: 10.1002/j.1460-2075.1985.tb04044.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Doyle JJ, Schuler MA, Godette WD, Zenger V, Beachy RN, Slightom JL. The glycosylated seed storage proteins of Glycine max and Phaseolus vulgaris. Structural homologies of genes and proteins. J Bio Chem. 1986;261:9228–9238. [PubMed] [Google Scholar]
- 58.Gritz L, Davies J. Plasmid-encoded hygromycin B resistance: the sequence of hygromycin B phosphotransferase gene and its expression in Escherichia coli and Saccharomyces cerevisiae. Gene. 1983;25:179–188. doi: 10.1016/0378-1119(83)90223-8. [DOI] [PubMed] [Google Scholar]
- 59.Garbarino JE, Belknap WR. Isolation of a ubiquitin-ribosomal protein gene (ubi3) from potato and expression of its promoter in transgenic plants. Plant Mol Biol. 1994;24:119–127. doi: 10.1007/BF00040579. [DOI] [PubMed] [Google Scholar]
- 60.Finer JJ, McMullen MD. Transformation of soybean via particle bombardment of embryogenic suspension culture tissue. Vitro Cell Dev Biol Plant. 1991;27:175–182. doi: 10.1007/BF02632213. [DOI] [Google Scholar]
- 61.Schmidt MA, Tucker DM, Cahoon EB, Parrott WA. Towards normalization of soybean somatic embryo maturation. Plant Cell Rep. 2005;24:383–391. doi: 10.1007/s00299-005-0950-z. [DOI] [PubMed] [Google Scholar]
- 62.Zhang G, Li P, Zhang W, Zhao J. Analysis of multiple soybean phytonutrients by near-infrared reflectance spectroscopy. Anal Bioanal Chem. 2017;409:3515–3525. doi: 10.1007/s00216-017-0288-8. [DOI] [PubMed] [Google Scholar]
- 63.Zhao J, Wang X. Arabidopsis phospholipase Dalpha1 interacts with the heterotrimeric G-protein alpha-subunit through a motif analogous to the DRY motif in G-protein-coupled receptors. J Biol Chem. 2004;279:1794–1800. doi: 10.1074/jbc.M309529200. [DOI] [PubMed] [Google Scholar]
- 64.Welti R, Li W, Li M, Sang Y, Biesiada H, Zhou HE, et al. Profiling membrane lipids in plant stress responses: role of phospholipase Dα in freezing-induced lipid changes in Arabidopsis. J Biol Chem. 2002;277:31994–32002. doi: 10.1074/jbc.M205375200. [DOI] [PubMed] [Google Scholar]
- 65.Li P, Dong Q, Ge S, He X, Verdier J, Li D, Zhao J. Metabolic engineering of proanthocyanidin production by repressing the isoflavone pathways and redirecting anthocyanidin precursor flux in legume. Plant Biotechnol J. 2016;14:1604–1618. doi: 10.1111/pbi.12524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wang J, Hou Q, Li P, Yang L, Sun X, Benedito VA, et al. Diverse functions of multidrug and toxin extrusion (MATE) transporters in citric acid efflux and metal homeostasis in Medicago truncatula. Plant Biotechnol J. 2017;90:79–95. doi: 10.1111/tpj.13471. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Figure S1. Schematic procedure for construction of soybean GmPLDα1RNAi vector for plant transformation. Figure S2. Phylogenetic analysis of PLD genes from the soybean genome and their expression patterns. Figure S3. Mechanism of plant resistant to stress and gene expression of GmPLDαs and stress-related genes under high temperature and humidity condition in comparison to under normal conditions. Figure S4. Analyses of lipids in PLDα1KD and wild-type developing seeds under different growth conditions. Figure S5. Phylogenetic analysis of FAD genes from the soybean genome and their expression patterns. Figure S6. Phylogenic analysis of Acyl-CoA:lysophosphatic acid acyltransferase (LPAAT) genes and their expression profiles. Figure S7. Phylogenic analysis of phosphatidic acid hydrolase (PAH) genes and their expression profiles. Figure S8. Phylogenic analysis of acyl-CoA:diacylglycerol acyltransferase (DGAT) genes and their expression profiles. Figure S9. Phylogenic analysis of phospholipid:diacylglycerol acyltransferase (PDAT) genes and their expression profiles. Figure S10. Phylogenic analysis of choline/ethylamine kinase (CEK) genes and their expression profiles. Figure S11. Phylogenic analysis of CTP: phosphocholine cytidylyltransferase (CCT) genes and the expression profiles. Figure S12. Phylogenic analysis of diacylglycerol:cholinephosphotransferase (DAG-CPT or AAAT) genes and their expression profiles. Figure S13. Phylogenic analysis of phosphatidylcholine:diacylglycerol cholinephosphotransferase (PDCT) genes and their expression profiles. Figure S14. Phylogenetic analysis of PLA genes from the soybean genome and their expression patterns. Figure S15. Phylogenic analysis of 2-lysophosphatidylcholine acyltransferase (LPCAT) genes and their expression profiles.
Additional file 2: Table S1. The Quantitative RT-PCR primers used in this study. Table S2. PLD genes in soybean genome. Table S3. Expression pattern in different tissue. Table S4. Relative expression of GmPLDɑs in different developing seeds after fertilization. Data S1. Protein sequences for phylogenic tree construction.
Data Availability Statement
All data supporting my findings can be available and found in the supplementary data; materials can be available for distribution upon request.