Abstract
Background
Synthesis of silver nanoparticles (AgNPs) through biological route plays an important role in their applications in the medical field, especially in the prevention of disease causing microbial pathogens and arresting the propagation of cancer cells. The stable, green synthesis of AgNPs is very much welcomed in the medical field because of their low toxicity. Therefore, the demands of AgNPs synthesised biologically is on the rise. The present study aimed to investigate the antimicrobial mechanisms and anticancer properties of the AgNPs synthesized using the seed extract of Trigonella foenum-graecum L. The AgNPs were characterized by UV–vis, SEM, XRD, FTIR and EDAX analysis. The minimum inhibitory concentrations (MIC) of the AgNPs were determined by the broth micro dilution method.
Results
The formation of brownish red color indicated the formation NPs with the absorption maximum at 420 nm. The average size was found to be 33.93 nm and sphere shaped. The FTIR spectrum revealed the absorption bands at 3340 cm−1 and 1635 cm−1 indicated the presence of —OH or —COOH and amide group stretching in the AgNPs. The X-ray diffraction report confirmed the presence of strong peak values of 2θ within the angle of 37.1°. The lowest MIC of the AgNPs against Staphylococcus aureus was 62.5 μg mL−1. MIC values against Escherichia coli and Klebsiella pneumonia, were 125 and 250 μg mL−1 respectively. The MIC of the AgNPs against Aspergillus flavus, Trichophyton rubrum and Trichoderma viridiae were each 250 μg mL−1, respectively. The extracellular protein concentration, levels of lactate dehydrogenase and alkaline phosphtase enzyme in the AgNPs treated bacterial pathogens demonstrated greater antimicrobial mechanism. Additionally, the AgNPs exhibited significant anticancer activity against the MCF7 and Vero cell lines.
Conclusion
The synthesized AgNPs could be further evaluated in large scale as a botanical antimicrobial agent.
Keywords: Trigonella foenum-graecum L., AgNPs, FTIR, Antimicrobial activity, Antimicrobial mechanism, Anticancer activity
1. Introduction
Nanotechnology is making an impact in all spheres of human life and creating a growing sense of excitement in the life sciences, especially biomedicine and biotechnology (Singh et al., 2010, Prabhu et al., 2010). NPs can be broadly categorized into organic carbon containing NPs and inorganic NPs include magnetic NPs, noble metal NPs (like gold and silver) and semi-conductor nanoparticles (titanium oxide and zinc oxide), NPs prepared using metals has significant applications in bio-related fields. Among the metallic NPs, silver (Ag) NPs are promising and are widely applied in many fields. AgNPs, i.e. silver particles of between 1 nm and 100 nm in size and have attracted immense research interest. AgNPs penetrate the cell wall, thus, alter the cell-wall components involved in respiration. The NPs may also penetrate the cell causing damage by interacting with phosphorus and sulfur containing compounds such as DNA and protein. Generally, Ag does not attach to the viable microbial cells and does not create the environment of microbial resistance. Therefore, Ag coated materials are mixed with textile fabrics, as food additives, and in package and plastics to eliminate the attachment of microbial pathogens. Because of such wide range of applications, numerous methods concerning the fabrication of AgNPs, as well as various silver-based compounds have been developed (David et al., 2010). AgNPs have become the focus of greater research interest due to their wide variety of applications (Cobley et al., 2009). The nature and unique properties of nanomaterials have wide range of applications in the fields of biosensors, tissue engineering, DNA modification, drug delivery system, cosmetics and medical devices (Arokiyaraj et al., 2014). Silver has greater toxicity against a wide range of microorganisms. AgNPs are found to have anti- inflammatory, anti-angiogenesis, antiviral, anti-platelet and anti-cancer properties, which makes them a very potent agent in various fields of biology and medicine (Yasin et al., 2013). Plant extract solutions and extracellular metabolites of microorganisms have been in spotlight for their superior ability to synthesize Nanoparticles of metals mainly, silver and gold. Green synthesis of nanoparticles offer improvement over synthetic, chemical or micro-organisms methods as it is cost effective, environmental friendly and can easily be scaled up for industrial purposes. Traditional plants are used to cure many diseases. Therefore, plant natural products are widely studied in the fields of natural product biology, ethnopharmacology, and other important fields (Bremner and Heinrich, 2002, Geetha et al., 2002). Natural products and herbal remedies used in traditional folk medicine have been the source of many medically beneficial drugs, as many of the medicinal plants have been shown to present interesting biological and pharmacological activities and are used as therapeutic agents (Battle et al., 2005). The application of traditional medicinal plants and spices to improve the human health is an ancient practice. However, in recent years, scientific researchers are mainly interested in studying the importance of these plants by identifying its active agents responsible for their therapeutic effects. Among these medicinal plants, fenugreek (Trigonella foenum-graecum L.) is one of the important spices with various health benefits. Trigonella foenum-graecum L. is a herbaceous leguminous plant extensively cultivated in India, Pakistan, China, Egypt, and Middle Eastern countries. Trigonella foenum-graecum L. has wide range of properties as it is anticancerous, antimicrobial, antioxidant, antidiabetic and is also effective in countering conditions like cardiovascular disease. The present study aimed to synthesize and characterize AgNPs using the water extract of T. foenum-graecum L. seed. Further, the synthesized AgNPs were evaluated for its antimicrobial activity by confirming the mechanism and its anticancer activity was also established.
2. Materials and methods
2.1. Synthesis of silver nanoparticles from fenugreek seeds
Fenugreek seeds were purchased from the local market in Chennai, India. The seeds were washed thoroughly with distilled water and further soaked for one hour to remove the dust on the periphery of the seed. The soaked seeds were further washed with distilled water and grind with 10 ml of sterile distilled water. The paste was centrifuged at 10000 rpm form 15 min to collect the upper supernatant. The supernatant was transferred into the brown bottle and kept in the dark room for 24 h. After incubation, the supernatant was mixed with equal proportion of freshly prepared Silver nitrate (1 mM) and incubated for 3 hours under shaking condition. The transformation of the colour of the solution was observed during the incubation of process within 10–15 min. The Ag particle solution was dried and centrifuged using methanol at 10,000 rpm for 10 min to remove the debris. The colour changes indicate the formation of AgNPs from the fenugreek seed extract.
2.2. Characterization of silver nanoparticles
The synthesized AgNPs were characterized by UV–vis, SEM, XRD, FTIR and EDAX analysis by following the standard method (Nethradevi et al., 2012, Nagajyothi et al., 2012). Briefly, a pinch of the NPs were dissolved in 3 ml of the solvent and measured for the maximum wavelength by using UV–vis spectrophotometer, whereas for the X-ray diffraction analysis, the Ag particles amended solution was dried at 80 °C and was measured using X’pert pro X-ray diffractometer. FTIR measured from 400 cm−1 to 4000 cm−1 to determine the functional group of the nanoparticles. SEM analysis of the silver particles was carried out in various magnification ranges. Particle size analysis determined the size of the synthesized nanoparticles.
2.3. Antibacterial activity (Minimum Inhibitory Concentration)
The MIC of the synthesized AgNPs was determined by following the standard protocol (Arasu et al., 2013). Briefly, the compounds were dissolved in dimethyl sulfoxide and sterilized using 0.2µ filter discs and the solution was serially diluted (two fold in a 96-well micro titre plate) in the Mueller Hinton Broth (MHB). Further, five micro litres of the freshly prepared bacterial suspensions of the test organism (1.5 × 106 CFU/ml) were transferred into the 200 µl of sterilized MHB. After proper mixing, the 96-well micro titer plates were covered with a sterile plate sealer and incubate at 37 °C for 18 h under shaking condition. After incubation, 5 µl of the microbial cells in each wells were spotted in an MHA plates and incubated at 37 °C for 18 h. The visible growth was monitored to determine the MIC of the particles. The experiment was repeated thrice to confirm the antibacterial activity of the AgNPs.
2.4. Antifungal activity
As explained above, five micro litres of the freshly prepared spore suspensions of the test organism (1.5 × 105 CFU/ml) will be transferred into the 200 µl of sterilized potato dextrose broth. After proper mixing, the 96-well micro titre plates were protected by the sterile cover and incubate at 30 °C for 48–72 h under shaking condition. After incubation, visible mycelia growth in each well was observed. Depending on the visible mycelia growth, the MIC of the AgNPs was determined. The experiment was repeated thrice to confirm the antifungal activity of the particles.
2.5. Mechanism of the antimicrobial activity of the particles
2.5.1. Intracellular protein leakage
The effect of the AgNPs in the intracellular protein level was monitored. The intracellular protein leakage was determined the method specified in Arokiyaraj et al. (2015).
2.5.2. Lactate dehydrogenase (LDH) quantification
Lactate dehydrogenase (LDH) is present in the cytoplasm of the bacteria. LDH level was analyzed to determine the damage caused by the particles to the pathogenic bacteria. The LDH level was determined by the method specified in Arokiyaraj et al. (2015).
2.5.3. Alkaline phosphtase (ALP) quantification
The amount alkaline phosphatase (ALP) was measured by slightly modifying methods specified in Arokiyaraj et al. (2015).
2.6. Anticancer assay
2.6.1. Cultivation of cell lines and maintenance
MCF7 and Vero cell lines were purchased from National Center for Cell Science (NCCS, Pune). Humidified incubator was used for the monitoring of the cells at 37 °C using 5% CO2. The cells were maintained in 1640 medium (RPMI 1640; Sigma), supplemented with 10% fetal bovine serum (FBS) together with other components such as 1.5 g/L sodium bicarbonate, 100 U/ml of penicillin, 0.25 µg/ml of ampotericin B and 100 µg/ml streptomycin. The cells were maintained in logarithmic growth phase in a complete medium. The cell line was be seeded at a density of 4000 to 6000 cells/well in 96-well plates in 10% FBS/RPMI 1640 culture medium and left overnight in the incubator for the study.
2.6.2. In vitro cytotoxicity activity (MTT assay)
The anticancer activity of the synthesized AgNPs was determined by MTT assay (Rejiniemon et al., 2014).
3. Results and discussion
3.1. Synthesis and characterization of AgNPs
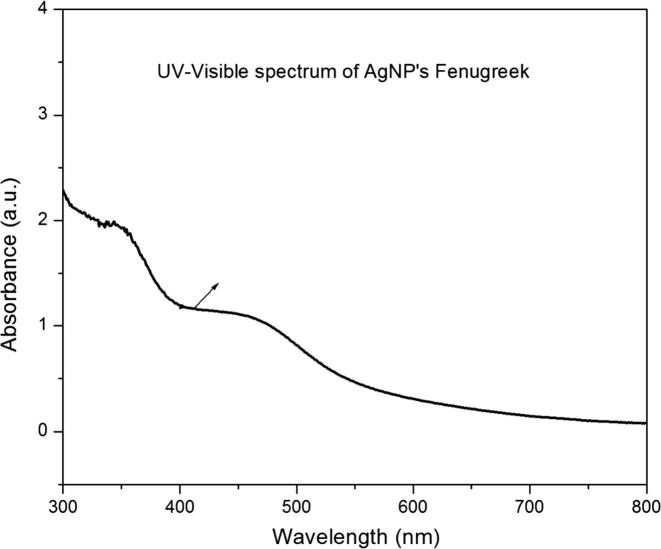
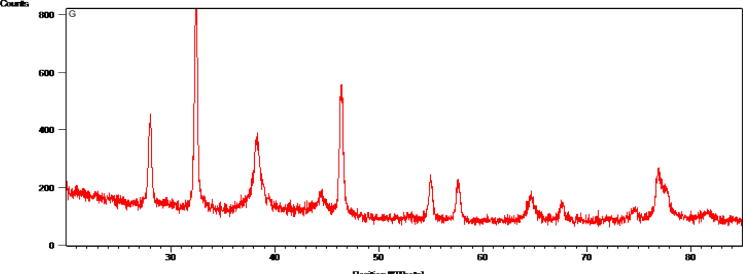
The seed extracts of the T. foenum-graecum L. were mixed with 1 mM AgNO3 resulted in the formation of brownish red color revealed the synthesis of the AgNPs and further confirmed by UV–vis spectra at 420 nm with the absorbance at 0.182 (Fig. 1). The results reported that the absorbance of the solution was increased with respect to the increase in the time periods because of the surface plasmon resonance of the nanoparticles of Ag element (Dhas et al., 2014). Reports claimed that the reaction conditions such as temperature, light intensity and buffering conditions of the samples and the concentrations of the AgNO3 play a major role in the formation of nanoparticles, also the presence of the group of functional compounds such as flavanoids, phenolic compounds, saponins, carbohydrates, tannins and other secondary metabolites present in the plants. Antony et al. (2011) and Sivalingam et al. (2012) reported that the energy produced in the form of ATP during glucose degradation and generation of free energy from other metabolic pathways and catalytic activation time might enhance the possibility of the synthesis of AgNPs (Antony et al., 2011, Sivalingam et al., 2012). The X-ray diffraction spectra expressed the presence of strong peak values of 2θ within the angle of 37.1°, 44.7°, 65.6°, 78.6° and 80.2°. The results were documented that the AgNPs have face centered cubic structure and crystalline in nature (Fig. 2).
Fig. 1.
Ultraviolet–visible absorption spectra of the AgNPs synthesized using the seed extract of Trigonella foenum-graecum L.
Fig. 2.
XRD spectra of the AgNPs synthesized using the seed extract of Trigonella foenum-graecum L.
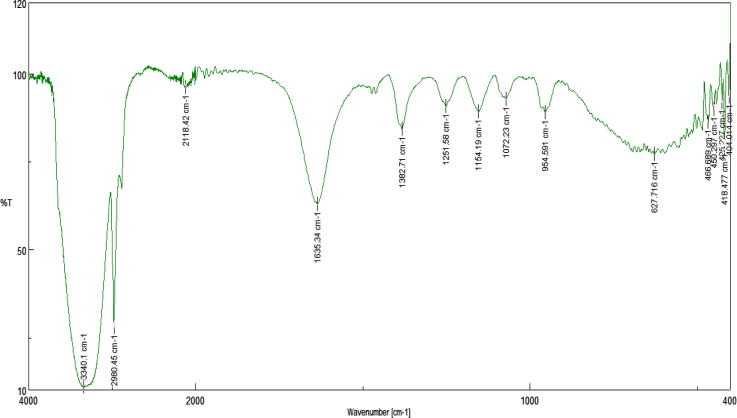
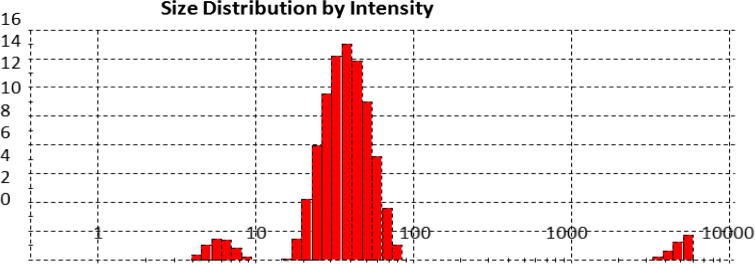
The functional group of the AgNPs were analysed by FTIR spectra. The synthesized AgNPs showed absorption bands at 3340 cm−1, 2980 cm−1, 2118 cm−1, 1635 cm−1, 1382 cm−1, 1251 cm−1, 1154 cm−1, 1072 cm−1 and 954 cm−1. The peak at 3340 cm−1 and 1635 cm−1 showed the presence of —OH or —COOH and amide group stretching in the AgNPs (Fig. 3). These absorbance bands were also associated with an O—H stretch of phenol or alcohol group primary amine, N O bend of nitro group, —NO₂ of aliphatic group (Macdonald et al., 1996). The presence of functional group in the AgNPs were similar to the report of Kumar et al. (2011) and Venkatachalam et al. (2013), where the FTIR spectrum exhibited the hydroxyl, acid and amide functional group in the synthesized AgNPs (Kumar et al., 2011, Venkatachalam et al., 2013). Fig. 4 shows the average size of the silver particle was 33.93 nm.
Fig. 3.
FTIR spectra of the AgNPs synthesized using the seed extract of Trigonella foenum-graecum L.
Fig. 4.
Particle size the AgNPs synthesized using the seed extract of Trigonella foenum-graecum L.
3.2. Antimicrobial activity of the AgNPs
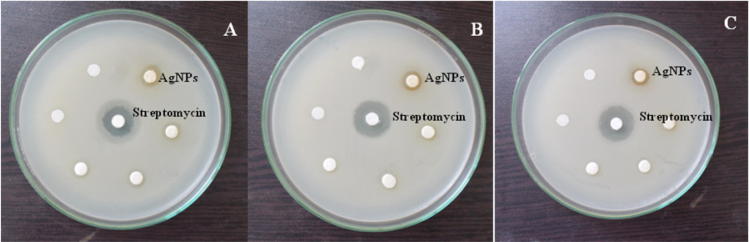
The antimicrobial activity and MIC of the AgNPs were evaluated by disc diffusion method and broth micro dilution method. The primary screening revealed that the AgNPs suppress the growth of Gram positive and Gram Negative bacteria at concentrations less than 100 µg/mL. Among the bacteria, Klebsiella pneumonia and Salmonella typhi comparatively revealed better activity at 100 µg/mL concentration. The results revealed that the growth of Gram negative bacteria was comparatively suppressed by the AgNPs than Gram positive bacteria (Table 1, Fig. 5). Staphylococcus aureus exhibited the MIC values (62.5 µg/mL), whereas the Gram negative bacteria, Escherichia coli documented the activity at 125 µg/mL concentration. Pseudomonas aeruginosa and Salmonella typhi showed moderate activity (500 µg/mL). The antibacterial activities of the AgNPs were better than the water extract. Interestingly, Trichoderma viridiae, Trichophyton rubrum and Aspergillus flavus exhibited MIC at (250 µg/mL) and Penicilium notatum and Candida albicans (250 µg/mL). The standard antibacterial and antifungal agents such streptomycin and ketoconazole were also compared with the AgNPs. Literature reveals that the cell wall of Gram negative bacteria contains peptidoglycan layer, lippopolysaccharides, exopolysaccharides and fimbriae which block the penetration of the AgNPs inside the cells (Arokiyaraj et al., 2014, Shrivastava et al., 2007). However, the diffusion of AgNPs inside the cells of Gram positive bacteria is easier. In this study, lower concentration of the AgNPs were comparatively affected the growth of the Gram negative bacteria suggested its antimicrobial potentials. The antimicrobial activity might be due to the release of Silver ions into the cells or the presence of photochemical together with the Silver ions enhances the antimicrobial activity (Dibrov et al., 2002).
Table 1.
Minimum inhibitory concentration of the AgNPs against bacteria and fungi.
| Microorganisms | Minimum inhibitory concentration |
||
|---|---|---|---|
| Nanoparticles (µg/mL) | Water extract (mg/mL) | Standard µg/mL) | |
| Bacteria | |||
| Escherichia coli | 125 | 6.25 | 25 |
| Klebsiella pneumoniae | 250 | 1.25 | 25 |
| Staphylococcus aureus | 62.5 | 6.25 | 37.5 |
| Salmonella typhi | 500 | 6.25 | 50 |
| Pseudomonas aeruginosa | 500 | 5 | 50 |
| Fungi | |||
| Aspergillus flavus | 250 | 2.5 | 25 |
| Candida albicans | 500 | 2.5 | 100 |
| Trichophyton rubrum | 250 | 2.5 | 50 |
| Penicilium notatum | 500 | 2.5 | 25 |
| Trichoderma viridiae | 250 | 5 | 50 |
Standard: Streptomycin and Ketoconazole.
Fig. 5.
Antimicrobial activity of the AgNPs synthesized using the seed extract of Trigonella foenum-graecum L. against bacterial pathogens. A: Klebsiella pneumoniae; B: Staphylococcus aureus; C: Salmonella typhi.
3.3. Mechanism of the antimicrobial activity of the AgNPs
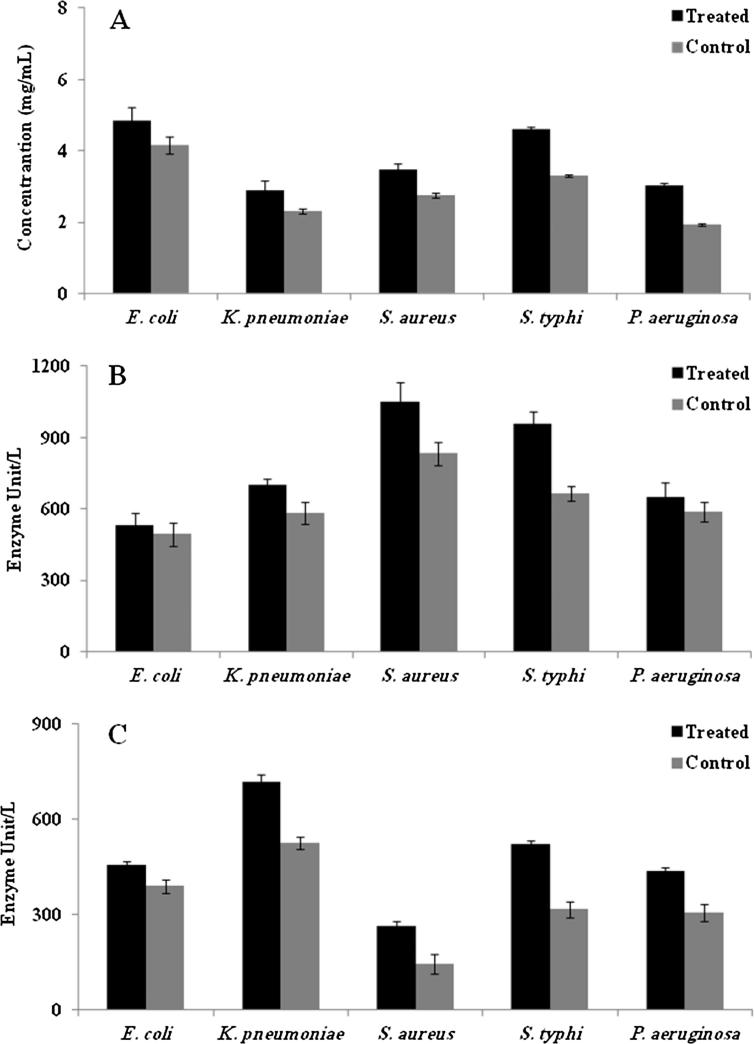
To determine the mechanism of AgNPs towards the antimicrobial activity, extracellular protein and enzyme concentrations of the pathogenic bacteria were quantified after AgNPs treatments. The results revealed that the addition of AgNPs showed higher release of protein in the supernatant. Among the pathogenic bacteria, the protein concentration P. aeruginosa was higher (36%) than the control, followed by S. typhi (28.2%), S. aureus (20.1%) and K. pneumoniae (20.6%) (Fig. 6A). Among the Gram negative bacteria, E. coli documented the least protein concentration than the control treatment (14%), it clearly indicated that the penetration of the AgNPs into the cells were prevented because of the presence of the barriers such as peptidoglycon and other components (Arasu et al., 2013). Similarly, Arokiyaraj et al. (2015) claimed that the addition of plant compounds significantly disrupts the cells and release the intracellular proteins into the medium (Arokiyaraj et al., 2015). In this study the phytochemical constituents together with the AgNPs affect the cell wall of the bacterial pathogens. The results of release of protein is coincide with the report of Sung and Lee (2010), who claimed that the phytochemical constituents of the medicinal plants, mainly involved in the alteration of the Gram positive and Gram negative bacterial cell walls by altering the membrane (Sung and Lee, 2010).
Fig. 6.
Antimicrobial mechanism of the AgNPs synthesized using the seed extract of Trigonella foenum-graecum L. against bacterial pathogens. A: Extra cellular protein level; B: Lactate dehydrogenase assay; C: Alkaline phosphatase assay.
The quantification of the LDH and ALP enzymes were further evidence that the treatment of AgNPs strongly inhibited the bacterial cell wall and other physiological nature. Results indicated that the significant increase in the units of the enzymes in the cultivation medium. ALP enzyme concentrations were dominant in the S. aureus (263 U/L), whereas the concentration of LDH were higher in S. typhi (957 U/L) (Fig. 6B and C). Reports claimed that the pathogenic bacteria release the enzymes under stress conditions or during the sporulation time (Arokiyaraj et al., 2015). The increase in the concentration of the enzymes proved that the AgNPs created the unfavourable environment to the bacteria which resulted in the release of the enzymes.
3.4. Anticancer activity of the AgNPs
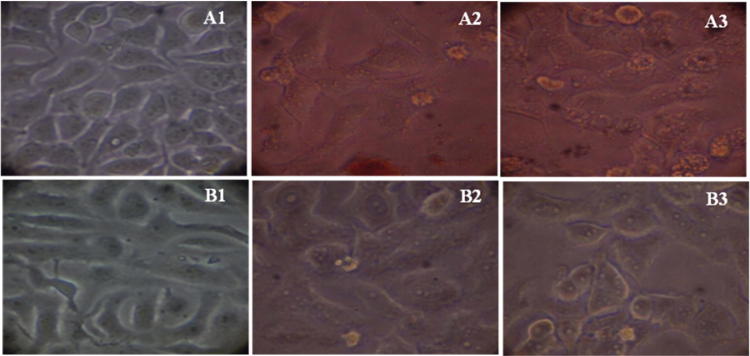
The anticancer properties of the AgNPs against the MCF7 and Vero cell lines were determined by the MTT assay method. Results revealed that the anticancer properties of the AgNPs were directly proportional to the concentration and time periods (Fig. 7). Results revealed that the MCF7 and Vero cell treated with AgNPs good activity and the IC50 values 6.25 and 12.5 μg mL−1 respectively. Also, it was confirmed that the AgNPs was comparatively nontoxic to the cells. In general the applications of the AgNPs are related to the toxicity of the compounds. Many reports claimed that the lower concentrations of the compounds were toxic to the normal and cancer cell lines (Ahamed et al., 2010, Gliga et al., 2014). Vivek et al. (2012), reported that the IC50 values NPs synthesized from Annona squamosa against MCF-7 cells were 50 and 50 μg mL−1 respectively, whereas, in this study the toxicity of the AgNPs were comparatively less (Vivek et al., 2012). Suman et al. (2013) reported the mechanism of anticancer properties of the AgNPs prepared using the root extract of the medicinal plant Morinda citrifolia (Suman et al., 2013). The present study confirmed that AgNPs synthesized might be useful as nanomedicine for the treatment of different kind of cancers. The anticancer property of the AgNPs would be related to the presence of phytochemicals such as alkaloids, flavanoids and other phenolic compounds present in the T. foenum-graecum L. seed.
Fig. 7.
Anticancer activity of the AgNPs synthesized using the seed extract of Trigonella foenum-graecum L. against MCF7 and VERO cell lines. A1: MCF7 cell line control; A2: MCF7 at 1.56 µg; A3: MCF7 at 3.12 µg; B1: VERO cell line control; B2: MCF7 at 1.56 µg; B3: MCF7 at 3.12 µg.
4. Conclusion
In this report, we conclude that the AgNPs synthesized from the seeds of T. foenum-graecum L. were confirmed by UV–Vis spectrophotometer, SEM, XRD, FTIR and EDAX analysis. The results showed that the extracts of T. foenum-graecum L. were capable of synthesizing silver nanoparticles in room temperature under dark condition. The AgNPs exerts significant antimicrobial activity against clinically important Gram positive, Gram negative and filamentous fungi. The MIC of the AgNPs was ranged between 20 and 125 μg mL−1. Further the quantification of total protein, ALP and LDH enzymes assays confirmed the mechanism of action of the AgNPs. Besides the antimicrobial activity, anticancer property against MCF7 and Vero cell lines were the advantage of the AgNPs. T. foenum-graecum L. provides a traditional knowledge for the use of this plant in the folk medicines together with the nanotechnology to inhibit the disease causing microbial pathogens and to protect the cells were the added advantage.
Footnotes
Peer review under responsibility of King Saud University.
References
- Ahamed M., AlSalhi M.S., Siddiqui M.K.J. Silver nanoparticle applications and human health. Clin. Chem. Acta. 2010;411:1841–1848. doi: 10.1016/j.cca.2010.08.016. [DOI] [PubMed] [Google Scholar]
- Antony J.J., Sivalingam P., Siva D., Kamalakkannan S., Anbarasu K., Sukirtha R., Krishnan M., Achiraman S. Comparative evaluation of antibacterial activity of silver nanoparticles synthesized using Rhizophora apiculata and glucose. Colloids Surf. B: Biointer. 2011;88(1):134–140. doi: 10.1016/j.colsurfb.2011.06.022. [DOI] [PubMed] [Google Scholar]
- Arasu M.V., Duraipandiyan V., Ignacimuthu S. Antibacterial and antifungal activities of polyketide metabolite from marine Streptomyces sp. AP-123 and its cytotoxic effect. Chemosphere. 2013;90(2):479–487. doi: 10.1016/j.chemosphere.2012.08.006. [DOI] [PubMed] [Google Scholar]
- Arokiyaraj S., Arasu M.V., Vincent S., Prakash N.U., Choi S.H., Oh Y.-K., Choi K.C., Kim K.H. Rapid green synthesis of silver nanoparticles from Chrysanthemum indicum L. and its antibacterial and cytotoxic effects: an in vitro study. Inter. J. Nanomed. 2014;pkj 9:379–388. doi: 10.2147/IJN.S53546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arokiyaraj S., Choi S.H., Lee Y., Bharanidharan R., Hairul-Islam V.I., Vijayakumar B., Oh Y.K., Dinesh-Kumar V., Vincent S., Kim K.H. Characterization of ambrette seed oil and its mode of action in bacteria. Molecules. 2015;20(1):384–395. doi: 10.3390/molecules20010384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Battle T.E., Arbiser J., Frank D.A. The natural product honokiol induces caspase-dependent apoptosis in B-cell chronic lymphocytic leukemia (BCLL) cells. Blood. 2005;106:690–697. doi: 10.1182/blood-2004-11-4273. [DOI] [PubMed] [Google Scholar]
- Bremner P., Heinrich M. Natural products as targeted modulators of the nuclear factor-kappaB pathway. J. Pharm. Pharmacol. 2002;54:453–472. doi: 10.1211/0022357021778637. [DOI] [PubMed] [Google Scholar]
- Cobley C.M., Skrabalak S.E., Campbell D.J., Xia Y. Plasmonics. 2009;4:171. [Google Scholar]
- David E., Elumalai E.K., Prasad T.N.V.K.V., Venkata K., Nagajyothi P.C. Green synthesis of silver nanoparticle using Euphorbia hirta L. and their antifungal activities. Arch. Appl. Sci. Res. 2010;2:76–81. [Google Scholar]
- Dhas T.S., Kumar V.G., Karthick V., Govindraju K., Shankara Narayana T. Biosynthesis of gold nanoparticles using Sargassum swartzii and its cytotoxicity effect on HeLa cells. Spectrochim. Acta Part A: Mol. Biomol. Spect. 2014;120:416. doi: 10.1016/j.saa.2014.05.042. [DOI] [PubMed] [Google Scholar]
- Dibrov P., Dzioba J., Gosink K.K., Hase C.C. Mechanisms of action: physiological effects chemiosmotic mechanism of antimicrobial activity of Ag+ in Vibrio cholera. Antimicrob. Agent Chemother. 2002;46:2670. doi: 10.1128/AAC.46.8.2668-2670.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geetha S., Sai Ram M., Singh V., Ilavazhagan G., Sawhney R.C. Anti-oxidant and immunomodulatory properties of seabuckthorn (Hippophae rhamnoides)-an in vitro study. J. Ethnopharmacol. 2002;79:373–378. doi: 10.1016/s0378-8741(01)00406-8. [DOI] [PubMed] [Google Scholar]
- Gliga A.R., Skoglund S., Wallinder I.O., Fadeel B., Karlsson H.L. Size-dependent cytotoxicity of silver nanoparticles in human lung cells: the role of cellular uptake, agglomeration and Ag release. Part. FibreToxicol. 2014;11:11. doi: 10.1186/1743-8977-11-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar V.G., Gokavarapu S.D., Rajeswari A., Dhas T.S., Karthick V., Kapadia Z., Shrestha T., Barathy I.A., Roy A., Sinha S. Facile green synthesis of gold nanoparticles using leaf extract of antidiabetic potent Cassia auriculata. Colloids Surf. B, Biointer. 2011;87:159. doi: 10.1016/j.colsurfb.2011.05.016. [DOI] [PubMed] [Google Scholar]
- Macdonald I.D.G., Smith W.E., Munro A.W. Inhibitor/fatty acid interactions with cytochrome P-450 BM3. FEBS Lett. 1996;396(2–3):196–200. doi: 10.1016/0014-5793(96)01095-2. [DOI] [PubMed] [Google Scholar]
- Nagajyothi P.C., Eon L.S., Minh A., Duk L.K. Green synthesis of silver and gold nanoparticles using Lonicera Japonica flower extract. Bull. Korean Chem. Soc. 2012;33(8):2609. 8. [Google Scholar]
- Nethradevi C., Sivakumar P., Renganathan S. Green synthesis of silver nanoparticles using Datura metel flower extract and evaluation of their antimicrobial activity. Int. J. Nanomater. Biostruct. 2012;2:16–21. [Google Scholar]
- Prabhu N., Divya T.R., Yamuna Gowri K., Ayisha Siddiqua S., Joseph Puspha D. Silver phytho nanoparticles and their antibacterial efficacy. Digest J. Nanomat. Biostruct. 2010;5:185–189. [Google Scholar]
- Rejiniemon T.S., Arasu M.V., Duraipandiyan V., Ponmurugan K., Al-Dhabi N.A., Arokiyaraj S., Agastian P., Choi K.C. In-vitro antimicrobial, antibiofilm, cytotoxic, antifeedant and larvicidal properties of novel quinone isolated from Aegle marmelos (Linn.) Correa. Ann. Clin. Microbiol. Antimicrob. 2014;13:48. doi: 10.1186/s12941-014-0048-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shrivastava S., Bera T., Roy A., Singh G., Ramachandrarao P., Dash D. Characterization of enhanced antibacterial effects of novel silver nanoparticles. Nanotechnology. 2007;18:225111. doi: 10.1088/0957-4484/18/22/225103. [DOI] [PubMed] [Google Scholar]
- Singh A., Jain D., Upadhyay M.K., Khandelwal, Verma H.N. Green synthesis of silver nanoparticles using Argemone mexicana leaf extracts and evaluation of their antimicrobial activities. Dig. J. Nanomat. Biostruct. 2010;5:483–489. [Google Scholar]
- Sivalingam P., Antony J.J., Siva D., Achiraman S., Anbarasu K. Mangrove Streptomyces sp. BDUKAS10 as nanofactory for fabrication of bactericidal silver nanoparticles. Colloids Surf. B: Biointer. 2012;98:12–17. doi: 10.1016/j.colsurfb.2012.03.032. [DOI] [PubMed] [Google Scholar]
- Suman T.Y., Radhika, Rajasree S.R., Kanchana A., Beena Elizabeth S. Biosynthesis, characterization and cytotoxic effect of plant mediated silver nanoparticles using Morinda citrifolia root extract. Colloids Surf. B: Biointer. 2013;106:74–78. doi: 10.1016/j.colsurfb.2013.01.037. [DOI] [PubMed] [Google Scholar]
- Sung W.S., Lee D.G. Antifungal action of chlorogenic acid against pathogenic fungi, mediated by membrane disruption. Pure Appl. Chem. 2010;82(1):219–226. [Google Scholar]
- Venkatachalam M., Govindaraju K., Mohamed Sadiq A., Tamilselvan S., Ganesh Kumar V., Singaravelu G. Functionalization of gold nanoparticles as antidiabetic nanomaterial. Spectrochim. Acta Part A: Mol. Biomol. Spect. 2013;116:331–338. doi: 10.1016/j.saa.2013.07.038. [DOI] [PubMed] [Google Scholar]
- Vivek R., Thangama R., Muthuchelian K., Gunasekaran P., Kaveri K., Kannan S. Green biosynthesis of silver nanoparticles from Annona squamosa leaf extract and its in vitro cytotoxic effect on MCF-7 cells. Process Biochem. 2012;47:2405. [Google Scholar]
- Yasin S., Liu L., Yao J. Biosynthesis of silver nanoparticles by bamboo leaves extract and their antimicrobial activity. J. Fiber Bioeng. Inform. 2013;6:77–84. [Google Scholar]