Abstract
Background:
This study was conducted to compare the analgesic efficacy of 10 ml versus 20 mL of 0.5% ropivacaine in nerve stimulator guided interscalene brachial plexus block, in patients undergoing arthroscopic shoulder surgery.
Methods:
A total of 70 American Society of Anesthesiologists physical status classes 1 and 2 patients, aged 18–65 years, undergoing unilateral arthroscopic shoulder surgery, were randomized into two groups. Group A received single shot inter-scalene block with 20 mL of 0.5% ropivacaine whereas Group B received the same with 10 mL. The primary outcome was difference in the total postoperative fentanyl consumption over 24 h. Secondary outcomes were difference in block onset, intra-operative hemodynamic parameters, intra-operative fentanyl consumption, duration of effective analgesia, visual analogue scale (VAS) scores at various time intervals, duration of motor block, and incidence of hemidiaphragmatic (HD) palsy.
Results:
Total 24 h fentanyl consumption was significantly higher in Group B (558 ± 112 mcg) compared to Group A (296 ± 88 μg). Block onset was slower in Group B than Group A. There was no difference in intra-operative fentanyl consumption. Postoperative VAS scores were significantly higher in Group B compared to Group A, at 6 h and thereafter. Duration of motor block was significantly shorter in Group B (6.25 ± 1.25 h) compared to Group A. HD palsy was seen in all the cases in both the groups.
Conclusion:
Single shot nerve stimulator guided interscalene block with 10 ml of 0.5% ropivacaine was inferior to 20 mL of 0.5% ropivacaine with respect to postoperative analgesic efficacy.
Keywords: Brachial plexus block, drug dose-response relationship, local anesthesia
INTRODUCTION
Pain is common following arthroscopic shoulder surgery and remains a major challenge. Several studies have shown than single shot interscalene nerve block (ISB) provides effective short-term postoperative analgesia.[1,2] Landmark-guided ISB requires large volumes of local anesthetics (LAs) to achieve acceptable success rates. This can lead to unwanted side effects such as transient diaphragmatic paresis, dysphagia, dysphonia, and Horner's Syndrome.[3] Although ultrasound-guided nerve blocks are the standard of care now in all developed countries, its availability in a developing country like ours cannot be always guaranteed. The use of nerve stimulator makes it possible to achieve better success rates with smaller doses of LA. However, the minimum dose of LA required to achieve adequate postoperative analgesia is still a matter of debate. We therefore conducted a study to compare the analgesic efficacy of 10 ml versus 20 ml of 0.5% ropivacaine in nerve stimulator guided interscalene brachial plexus block in patients undergoing arthroscopic shoulder surgery.
METHODS
Our hypothesis was that single shot ISB with 10 mL of 0.5% ropivacaine is as effective as 20 ml for perioperative analgesia in arthroscopic shoulder surgery.
This study was designed as a double-blinded randomized control trial. Institutional Ethics Committee approval was obtained before patient enrollment, and the study was registered in the National Clinical Trial Registry (CTRI/2017/12/010924). After informed written consent, 70 American Society of Anesthesiologists physical status classes 1 and 2 patients, aged 18–65 years, undergoing elective unilateral arthroscopic shoulder surgery, were randomized into two groups based on a computer-generated randomized table using the opaque envelope technique.
Group A: Single shot ISB with 20 mL of 0.5% ropivacaine
Group B: Single shot ISB with 10 mL of 0.5% ropivacaine.
All patients underwent a detailed preanesthetic evaluation. Those with history of allergy to LA, coagulopathy, previous neurological deficit, chronic obstructive pulmonary disease, mental deficit that hinders the comprehension of pain scale used in this study, body mass index >35 and infection at the site of block were excluded. All patients were explained about the study protocol and the use of the visual analogue Scale (VAS) for postoperative pain assessment.
Patients were admitted on the morning of surgery. Standard fasting guidelines (6 h for solids and semi-solids, 2 h for clear liquids) were followed.
After shifting the patient to the operating table, electrocardiogram (ECG), pulse-oximeter and noninvasive blood pressure (BP) monitors were attached and baseline values of heart rate (HR), BP and oxygen saturation (SpO2) were noted. Patients were placed in supine position with head turned slightly away from the side of the block. After aseptic preparation and local infiltration of the skin, nerve stimulator guided ISB was given using the lateral approach. A 21G 50 mm long Stimuplex needle connected to the nerve stimulator (Plexigon 7501.31) with the initial setting of 1 mA, duration of stimulation of 0.1 μs and frequency of 2 Hz was introduced perpendicular to skin at the level of cricoid cartilage until a motor response in levetor scapulae, deltoid or biceps muscle was obtained. Gradual reduction in current by 0.1 mA and needle maneuvering was done to continue eliciting motor response. Successful needle placement was defined as motor response at 0.5 mA and loss of motor response at 0.2 mA. After negative aspiration of blood, Group B was given 10 mL of 0.5% ropivacaine and Group A was given 20 ml of 0.5% ropivacaine in graded 5 mL increments. In case, successful needle placement could not be achieved within 15 min, the procedure was abandoned and patient was excluded from the analysis.
Block onset was evaluated at 1 min intervals by an observer who was blinded to the volume of injectate. Motor block onset time was defined as the time from injection to complete paralysis of shoulder abduction. Sensory blockade was assessed by using alcohol sponge in the C4 dermatome. Onset time was taken as the time from drug injection to complete ablation of sensation. Failure to achieve loss of cold sensation at the C4 dermatome 30 min after ISB was considered as block failure. Immediate complications, such as hematoma formation, Horner's syndrome, hoarseness and respiratory distress were assessed during this period.
After sensory and motor block onset, general anesthesia was induced with fentanyl (2 μg/kg IV), propofol (2–3 μg/kg IV) to facilitate placement of proseal laryngeal mask airway. All patients were mechanically ventilated and anesthesia was maintained with O2 (35%), N2O (65%) and isoflurane (MAC 0.8-1). HR, BP, RR, SpO2 were recorded at every 5 min until the end of surgery. Surgery was allowed to commence 15 min after LA injection.
Increase in HR or BP by >20% above baseline value was treated with 0.5 μg/kg IV fentanyl.
All patients received IV ondansetron (0.1 μg/kg) and intramuscular ketorolac (0.5 μg/kg) in the opposite arm, 20 min prior to completion of surgery. At the end of surgery, residual neuromuscular blockade was reversed.
After the surgery, pain was assessed once the patient was awake and verbally responsive, using VAS, scored from zero (total absence of pain) to 10 (the worst pain possible). If patient complained of resting pain at any point, 0.5 mcg/kg fentanyl was administered IV every 15 min and pain re-assessed each time, until patient was comfortable at rest.
Time to first analgesic requirement was defined as the time from the start of surgery to the time when first rescue analgesic injection was required. Pain was assessed at 30 min, 1, 3, 6, 12, and at 24 h after the completion of surgery. Total fentanyl consumption over 24 h was noted.
Postoperative period residual motor block was assessed hourly. Recovery was defined as successful abduction of arm to 90° against gravity.
Postoperative chest X-ray (postero-anterior view) in upright posture was done at 6 h after surgery. Hemidiaphragmatic (HD) palsy on the side of the block was identified if the left hemidiaphragm was higher than the right, or the right was higher than the left by >2 cm.[4]
Outcomes
The primary outcome was difference in the total postoperative fentanyl consumption over 24 h.
The secondary outcomes were difference in block onset, intra-operative hemodynamic parameters, intraoperative fentanyl consumption, incidence of postblock cardiac arrhythmias, duration of effective analgesia, VAS scores at various time intervals, duration of motor block and incidence of HD palsy.
Sample size calculation
Sample size was calculated using the Statstodo open access online calculator. We considered a difference of 20% in the mean 24-hour fentanyl consumption to be clinically significant. With an expected mean standard deviation of 150 mcg, expected mean 24-hour fentanyl consumption 500 μg,[5] an acceptable alpha error of 5%, and a power of the study of 80%, we required 28 patients in each group for a one-tailed analysis. Thus, with an added safety margin of 20%, we recruited 70 patients in the trial.
Statistical analysis
Data were analyzed using IBM SPSS Statistics (IBM Corp., Chicago, IL, USA) for Windows, Version 20.0. Data are presented as number of patients or mean ± standard deviation, as appropriate. Quantitative data were compared using unpaired t-test. Qualitative variables were compared using Chi-squared test. A P < 0.05 was considered statistically significant.
RESULTS
Figure 1 shows the flow of patients through each phase of the trial. Patient and surgical characteristics were similar in the two groups [Table 1].
Figure 1.
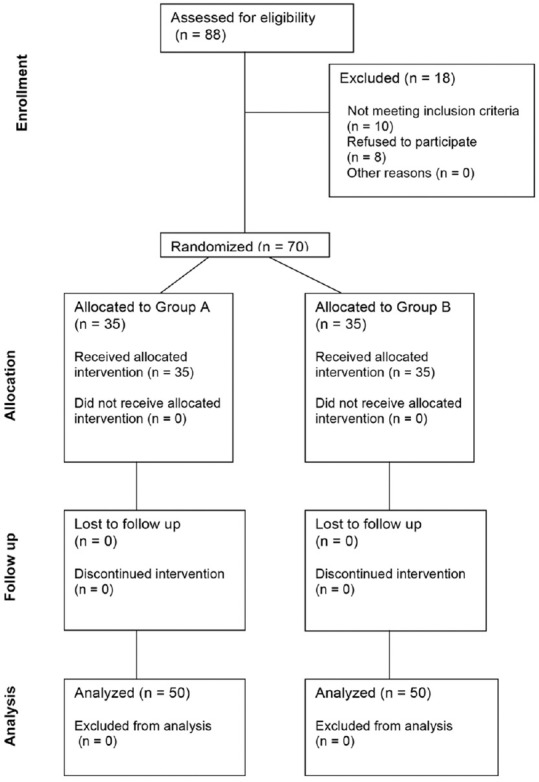
CONSORT diagram showing the flow of participants through each stage of the trial
Table 1.
Patient characteristics and duration of surgery
There was no significant difference in the baseline and the intraoperative hemodynamic parameters (HR and mean arterial pressure) between the two groups at various time points. The incidence of tachycardia on skin incision (9 out of 35 patients in Group B and 7 out of 35 patients in Group A) was comparable between the two groups (P = 0.569, Chi-squared test). None of the patients in either group developed abnormalities in ECG rhythm during the procedure.
Sensory block onset took longer in Group B (17.4 ± 2.5 min) compared to Group A (16.0 ± 2.6 min) (P = 0.025, unpaired t-test). Motor block onset was significantly slower in Group B (22.9 ± 3.5 min) compared to Group A (19.0 ± 3.8 min) (P < 0.001, unpaired t-test). Time to first rescue analgesic was shorter in Group B (293 ± 51 min) compared to Group A (442 ± 78 min) (P < 0.001, unpaired t-test). VAS scores between the two groups have been compared in Figure 2. VAS scores were comparable between the groups at awakening, 30 min, 1 h, and 3 h after the end of surgery. However there was significant difference in VAS score at 6 h and thereafter.
Figure 2.
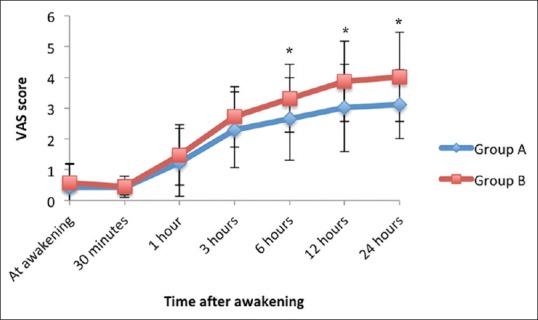
Comparison of post-operative visual analogue scale score between the two groups (data represented as mean ± standard deviation) (*P < 0.05 [unpaired t-test])
Figure 3 compares intra-operative and 24 h postoperative fentanyl consumption between the two groups. Intra-operative fentanyl consumption was similar in the two groups. Total 24 h postoperative fentanyl consumption was significantly higher in Group B (558 ± 112 μg) compared to Group A (296 ± 88 mcg) (P < 0.001, unpaired t-test).
Figure 3.
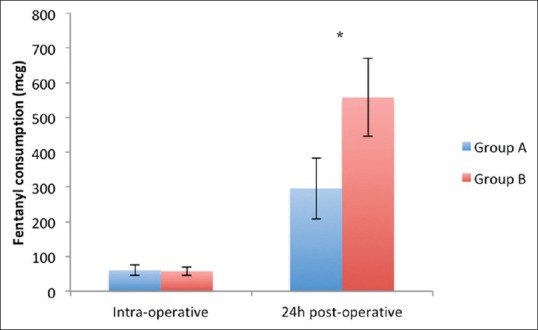
Comparison of intra-operative and post-operative fentanyl consumption between the two groups (data represented as mean ± standard deviation). (*P < 0.05 [unpaired t-test])
Duration of residual motor block was significantly shorter in Group B (6.25 ± 1.25 h) compared to Group A (11.50 ± 2.50 h) (P < 0.001, unpaired t-test). Hemi-diaphragmatic palsy was seen in all the cases in both the groups.
DISCUSSION
In the present study, single shot ISB with 10 mL of 0.5% bupivacaine was compared with 20 mL of 0.5% bupivacaine in patients undergoing unilateral arthroscopic shoulder surgery. Block onset was faster with 20 mL of bupivacaine compared to 10 mL. Both block techniques were equally effective in providing intraoperative analgesia. However, 20 mL of bupivacaine was more effective than 10 mL in providing adequate postoperative analgesia. 24-h fentanyl consumption and postoperative VAS scores beyond 6 h were significantly lower with 20 mL bupivacaine compared to 10 mL. Duration of residual motor block was significantly shorter with 10 mL bupivacaine compared to 20 mL.
Although multiple studies have compared different doses of LA in ISB for effective intraoperative analgesia, there is a paucity of evidence regarding the optimal dose of LA required for adequate postoperative analgesia.
Multiple studies have described the onset time of sensory and motor blockade with ISB. They have used various combinations of drug concentration, volume and adjuvants. Our results are similar to those described by Casati et al.[6] who used 20 ml of 0.5% ropivacaine for nerve stimulator guided ISB and found the mean time for readiness for surgery (motor and sensory blockade) to be approximately 22 ± 8 min. Block onset was significantly faster with higher (0.75% and 1%) concentration of ropivacaine. Zhai et al.[7] compared block onset with a fixed dose of ropivacaine (50 mg) at 0.75, 0.5 and 0.25% concentration. They found that block onset time was reduced with higher concentration of LA. In the present study, motor block onset was significantly faster with 20 mL ropivacaine compared to 10 mL (19.0 ± 3.8 min vs. 22.9 ± 3.5 min).
The block onset appears to be primarily a function of LA concentration with higher concentration providing faster block onset. With the use of a constant concentration of LA, block onset is faster with use of a higher dose. Although a difference of approximately 4 min in the amount of motor block onset may be statistically significant, its clinical significance is doubtful as patients are under GA during shoulder surgery and a short delay in motor block onset may be irrelevant.
Studies that tried to determine the minimum effective dose of LA in ISB have described a wide dose range. Using 0.5% ropivacaine Fredrickson et al.[8] described ED50 as 2.7 ml and ED95 as 20.5 mL in nerve stimulator guided ISB. McNaught et al.[9] compared the use of ultrasound guidance (USG) with peripheral nerve stimulator (PNS) to determine the minimum effective volume of LA and reported that the use of USG reduced the LA volume to 0.9 ml as compared to 5.4 mL with PNS. Gautier et al.[10] observed that successful anesthesia could be achieved for shoulder surgery with 5 mL of 0.75% ropivacaine using USG. However, they have advised use of dose higher than 5 mL to account for the possibility of 25% failure rate with the lower limit of confidence interval they have used. In the present study, 10 mL was found to be equally effective as 20 mL with respect to intraoperative analgesia. However when combined with GA, postoperative analgesic efficacy is more important than intraoperative analgesia.
Few earlier studies have assessed the postoperative analgesic efficacy and motor recovery after single-shot ISB in shoulder surgery patients. In the present study, time to first rescue analgesic was 293 ± 51 min in 10 mL Group B and 442 ± 78 min in 20 mL group. Al-Kaisy et al.[11] used 10 mL of 0.125% bupivacaine with adrenaline for nerve stimulator guided ISB for shoulder surgery and found that 10 ml provided adequate postoperative analgesia up to 141 ± 182 min. The lower duration of analgesia in this study was probably due to the lower concentration of LA used. Zhai et al.[7] found no difference in 24 h postoperative pain scores and recovery of handgrip strength with use of 10 m of 0.5% bupivacaine and 20 mL of 0.25% bupivacaine. This may be because the two doses used were the same (50 mg). Maalouf et al.[12] compared single injection ISB of 40 mL and 20 mL of 0.5% ropivacaine. They found improved handgrip strength and lesser incidence of postoperative hoarseness of voice with 20 mL LA. There was no difference in postoperative analgesic efficacy and time to first analgesic requirement was similar (960 min with 20 mL and 993 min with 40 mL). Therefore, increasing the volume of LA to > 20 ml may not be of any added advantage.
In the present study, 20 mL of 0.5% ropivacaine was found to be more efficacious than 10 mL for postoperative analgesic efficacy. Although both groups had similar VAS scores in the immediate postoperative period, there was significant difference in VAS scores at 6 h and thereafter. 24 h fentanyl consumption was significantly higher with lower volume of LA. These findings indicate that although 10 ml of 0.5% ropivacaine may be equally efficacious for intraoperative and immediate postoperative period, delayed postoperative pain relief is better achieved with 20 mL of LA. Although 20 mL leads to better postoperative analgesia, it also extends the duration of residual motor block (6.25 ± 1.25 h in 10 mL group vs. 11.5 ± 2.5 h in 20 ml group). This may have an impact on patient satisfaction and readiness for discharge.
HD palsy is a common complication of ISB. Zaragoza-Lemus et al.[13] studied 50 patients who underwent ISB with 30 mL of LA found ultrasonographic evidence of ipsilateral HD palsy in 100% of patients. Zhai et al.[14] found similar incidence of HD palsy with 10 mL of 0.5% bupivacaine and 20 mL of 0.25% bupivacaine. Sala-Blanch et al.[15] compared 20 mL and 40 mL of 1.5% mepivacaine and found no difference in incidence of HD palsy. Urmey and Gloeggler[16] compared 45 mL and 20 mL of 1.5% mepivacaine and found 100% incidence of HD palsy in both groups. Renes et al.[17] compared PNS and USG guided ISB using 10 mL of 0.75% bupivacaine and found a lower incidence of HD palsy with the use of USG. The use of low-volume ISB under USG may be effective in reducing the incidence of HD paresis without compromising postoperative analgesic efficacy. Stundner et al.[18] and Riazi et al.[19] found lesser incidence of HD palsy and respiratory complications with use of 5 mL of LA as compared to 20 ml in ultrasound guided ISB. However, Sinha et al.[20] found that reducing of LA volume from 20 mL to 5 mL did not reduce incidence of diaphragmatic paresis or the impairment of pulmonary function. In this study, HD palsy was observed in all patients in both the groups. HD palsy is a common complication of ISB irrespective of the volume used. The use of ultra-low volume of LA (approximately 5 mL) under USG guidance may reduce the incidence of HD palsy. However, such low volume may not be adequate for postoperative analgesia.
Our study had some limitations. PNS was used instead of USG. USG may improve efficacy of lower volumes of LA by increasing the accuracy of drug delivery. Although the use of USG for regional anesthesia is considered the standard of care, PNS guided blocks continue to be popular, especially in remote or resource poor settings. No adjuvants were used. Adjuvants like dexamethasone have proven effective in increasing the duration of effective analgesia with ISB.[21] No supplemental analgesics were used apart from a single dose of intra-muscular ketorolac at the end of surgery. The use of round-the-clock supplemental analgesics in actual practice may reduce the observed difference between the two groups. Finally, patient satisfaction could have been assessed as a composite measure of anesthetic outcome.
CONCLUSION
Single shot PNS guided ISB with 10 mL of 0.5% ropivacaine was inferior to 20 mL of 0.5% ropivacaine with respect to postoperative analgesic efficacy. Compared to ISB with 20 mL, 10 mL provided similar intra-operative analgesia, but resulted in earlier requirement of rescue analgesics and higher pain scores beyond 6 h after surgery. The reduction in LA volume from 20 mL to 10 mL did not result in a decrease in incidence of hemi-diaphragmatic weakness.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Fredrickson MJ, Krishnan S, Chen CY. Postoperative analgesia for shoulder surgery: A critical appraisal and review of current techniques. Anaesthesia. 2010;65:608–24. doi: 10.1111/j.1365-2044.2009.06231.x. [DOI] [PubMed] [Google Scholar]
- 2.Abdallah FW, Halpern SH, Aoyama K, Brull R. Will the real benefits of single-shot interscalene block please stand up? A systematic review and meta-analysis. Anesth Analg. 2015;120:1114–29. doi: 10.1213/ANE.0000000000000688. [DOI] [PubMed] [Google Scholar]
- 3.Hughes MS, Matava MJ, Wright RW, Brophy RH, Smith MV. Interscalene brachial plexus block for arthroscopic shoulder surgery: A systematic review. J Bone Joint Surg Am. 2013;95:1318–24. doi: 10.2106/JBJS.L.01116. [DOI] [PubMed] [Google Scholar]
- 4.Eisenberg RL. Gastrointestinal Radiology, a Pattern Approach. Philadelphia: Lippincott Williams & Wilkins; 2003. [Google Scholar]
- 5.Chen HP, Shen SJ, Tsai HI, Kao SC, Yu HP. Effects of interscalene nerve block for postoperative pain management in patients after shoulder surgery. Biomed Res Int 2015. 2015 doi: 10.1155/2015/902745. 902745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Casati A, Fanelli G, Albertin A, Deni F, Anelati D, Antonino FA, et al. Interscalene brachial plexus anesthesia with either 0.5% ropivacaine or 0.5% bupivacaine. Minerva Anestesiol. 2000;66:39–44. [PubMed] [Google Scholar]
- 7.Zhai W, Wang X, Rong Y, Li M, Wang H. Effects of a fixed low-dose ropivacaine with different volume and concentrations on interscalene brachial plexus block: A randomized controlled trial. BMC Anesthesiol. 2016;16:80. doi: 10.1186/s12871-016-0248-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fredrickson MJ, Smith KR, Wong AC. Importance of volume and concentration for ropivacaine interscalene block in preventing recovery room pain and minimizing motor block after shoulder surgery. Anesthesiology. 2010;112:1374–81. doi: 10.1097/ALN.0b013e3181d6929d. [DOI] [PubMed] [Google Scholar]
- 9.McNaught A, Shastri U, Carmichael N, Awad IT, Columb M, Cheung J, et al. Ultrasound reduces the minimum effective local anaesthetic volume compared with peripheral nerve stimulation for interscalene block. Br J Anaesth. 2011;106:124–30. doi: 10.1093/bja/aeq306. [DOI] [PubMed] [Google Scholar]
- 10.Gautier P, Vandepitte C, Ramquet C, DeCoopman M, Xu D, Hadzic A, et al. The minimum effective anesthetic volume of 0.75% ropivacaine in ultrasound-guided interscalene brachial plexus block. Anesth Analg. 2011;113:951–5. doi: 10.1213/ANE.0b013e31822b876f. [DOI] [PubMed] [Google Scholar]
- 11.Al-Kaisy A, McGuire G, Chan VW, Bruin G, Peng P, Miniaci A, et al. Analgesic effect of interscalene block using low-dose bupivacaine for outpatient arthroscopic shoulder surgery. Reg Anesth Pain Med. 1998;23:469–73. [PubMed] [Google Scholar]
- 12.Maalouf DB, Dorman SM, Sebeo J, Goytizolo EA, Gordon MA, Yadeau JT, et al. Prospective, randomized double-blind study: Does decreasing interscalene nerve block volume for surgical anesthesia in ambulatory shoulder surgery offer same-day patient recovery advantages? Reg Anesth Pain Med. 2016;41:438–44. doi: 10.1097/AAP.0000000000000418. [DOI] [PubMed] [Google Scholar]
- 13.Zaragoza-Lemus G, Limón-Muñoz M, García-Reyes W. Ultrasonographic assessment of hemidiaphragm paralysis secondary to interscalene block. Cir Cir. 2012;80:352–6. [PubMed] [Google Scholar]
- 14.Zhai WW, Wang XD, Li M, Guo XY. A study on diaphragm function after interscalene brachial plexus block using a fixed dose of ropivacaine with different concentrations. Zhonghua Yi Xue Za Zhi. 2016;96:2229–33. doi: 10.3760/cma.j.issn.0376-2491.2016.28.006. [DOI] [PubMed] [Google Scholar]
- 15.Sala-Blanch X, Lázaro JR, Correa J, Gómez-Fernandez M. Phrenic nerve block caused by interscalene brachial plexus block: Effects of digital pressure and a low volume of local anesthetic. Reg Anesth Pain Med. 1999;24:231–5. [PubMed] [Google Scholar]
- 16.Urmey WF, Gloeggler PJ. Pulmonary function changes during interscalene brachial plexus block: Effects of decreasing local anesthetic injection volume. Reg Anesth. 1993;18:244–9. [PubMed] [Google Scholar]
- 17.Renes SH, Rettig HC, Gielen MJ, Wilder-Smith OH, van Geffen GJ. Ultrasound-guided low-dose interscalene brachial plexus block reduces the incidence of hemidiaphragmatic paresis. Reg Anesth Pain Med. 2009;34:498–502. doi: 10.1097/AAP.0b013e3181b49256. [DOI] [PubMed] [Google Scholar]
- 18.Stundner O, Meissnitzer M, Brummett CM, Moser S, Forstner R, Koköfer A, et al. Comparison of tissue distribution, phrenic nerve involvement, and epidural spread in standard- vs.low-volume ultrasound-guided interscalene plexus block using contrast magnetic resonance imaging: A randomized, controlled trial. Br J Anaesth. 2016;116:405–12. doi: 10.1093/bja/aev550. [DOI] [PubMed] [Google Scholar]
- 19.Riazi S, Carmichael N, Awad I, Holtby RM, McCartney CJ. Effect of local anaesthetic volume (20 vs.5 ml) on the efficacy and respiratory consequences of ultrasound-guided interscalene brachial plexus block. Br J Anaesth. 2008;101:549–56. doi: 10.1093/bja/aen229. [DOI] [PubMed] [Google Scholar]
- 20.Sinha SK, Abrams JH, Barnett JT, Muller JG, Lahiri B, Bernstein BA, et al. Decreasing the local anesthetic volume from 20 to 10 mL for ultrasound-guided interscalene block at the cricoid level does not reduce the incidence of hemidiaphragmatic paresis. Reg Anesth Pain Med. 2011;36:17–20. doi: 10.1097/aap.0b013e3182030648. [DOI] [PubMed] [Google Scholar]
- 21.Chalifoux F, Colin F, St. Pierre P, Godin N, Brulotte V. Low dose intravenous dexamethasone (4 mg and 10 mg) significantly prolongs the analgesic duration of single-shot interscalene block after arthroscopic shoulder surgery: A prospective randomized placebo-controlled study. Can J Anaesth. 2017;64:280–9. doi: 10.1007/s12630-016-0796-6. [DOI] [PubMed] [Google Scholar]


