Abstract
Context:
Laparoscopic cholecystectomy is associated with moderate intensity postoperative pain especially in the early postoperative period. Transversus abdominis plane (TAP) block has been shown to reduce pain scores and analgesic requirements after abdominal surgery.
Aims:
We hypothesized that a subcostal TAP block with ropivacaine and dexmedetomidine will prolong the duration of postoperative analgesia following laparoscopic cholecystectomy.
Settings and Design:
This prospective, randomized study was done in 60 patients undergoing laparoscopic cholecystectomy surgery done at a tertiary care institution.
Subjects and Methods:
Sixty patients undergoing laparoscopic cholecystectomy were randomized into two groups to receive either bilateral ultrasonography-guided subcostal TAP blocks with 18 mL 0.375% ropivacaine and 2 ml of normal saline (n = 30, Group R) or 18 ml. 375% ropivacaine with 0.5 μg/kg dexmedetomidine 2 mL (n = 30, Group RD). Numerical rating scale was measured postoperatively to primarily assess the pain severity and analgesic requirement for the first 24 h, hemodynamic parameters, and adverse effects were recorded.
Statistical Analysis Used:
Categorical data were analyzed using Chi-square test/Fisher's exact test and quantitative data were analyzed using Student's t-test and the Mann–Whitney U-test.
Results:
The study group (Group RD) had significantly prolonged postoperative analgesia (485.6 min) as compared to Group R (289.83 min). Moreover, consumption of morphine over 24-h period is significantly less in Group RD (14.5 mg) as compared to Group R (28.5 mg).
Conclusions:
Addition of dexmedetomidine to ropivacaine in TAP block prolongs postoperative analgesia and reduces opioid consumption without any major adverse effects.
Keywords: Dexmedetomidine, laparoscopic cholecystectomy, postoperative analgesia, subcostal transversus abdominis block, visual analog scale
INTRODUCTION
Inadequate control of postoperative pain leads to several untoward adverse events such as patient's discomfort, prolonged immobilization, extended hospital stay, thromboembolism, and pulmonary complications. Pain after laparoscopic cholecystectomy arises from trocar insertion sites, pneumoperitoneum-induced abdominal stretch, and hepatic bed disturbances due to cholecystectomy.[1]
Various modalities have been used to alleviate the pain after laparoscopic cholecystectomy, which includes nonsteroidal anti-inflammatory drugs, opioids, thoracic epidural block, and multimodal analgesia.[2]
The transverse abdominis plane (TAP) block is a peripheral nerve block designed to anesthetize the nerves supplying the anterior abdominal wall (T6 to L1). The block, first described by Rafi in 2001.[3]
With increasing utilization of ultrasound guidance for more accurate needle localization, the TAP block is now a safe and effective technique for alleviating postoperative pain following abdominal surgery.
Duration of TAP block is limited to the effect of administered local anesthetics (LA). Recently, adjuvant medications were added to LA to prolong the effect of TAP block.[4] Dexmedetomidine's use with ropivacaine in peripheral nerve blocks is associated with prolongation of the LA effect.[5]
The α2-adrenoceptors (ARs) by virtue of their sedative, analgesic, sympatholytic, anesthetic-sparing and hemodynamic stabilizing properties have been used as an adjunct to LA for prolongation of effect. Dexmedetomidine is a stereoisomer of medetomidine a highly selective α2-adrenergic receptor (AR) agonist with a relatively high ratio of α2/α1-activity (1620:1 as compared to 220:1 for clonidine).[6]
SUBJECTS AND METHODS
After the Institutional Ethics Committee approval and written informed consent, sixty patients of physical status ASA Class I and II of either sex, aged 18–65 years, scheduled to undergo 4-port laparoscopic cholecystectomy were enrolled in this trial. Patients with a history of LA allergy, psychiatric illness, substance abuse, opioid tolerance, any uncompensated systemic illness (cardiovascular, respiratory, metabolic, neurologic, and endocrine) and pregnant women were excluded from the study. The patients were randomly allocated to receive TAP block with either ropivacaine and normal saline or ropivacaine and dexmedetomidine. During the preoperative anesthetic assessment of patients, Numerical Rating Scale (NRS) for pain assessment from 0 to 10, with 0 meaning no pain and 10 meaning the worst possible pain was explained to patients.
On arrival at the operating room, an 18G intravenous (IV) cannula was inserted. premedicated with iv. Midazolam 0.03 mg/kg 15 min before induction of general anesthesia. Patients were monitored by noninvasive blood pressure, pulse oximetry, temperature, electrocardiography, capnography. General anesthesia was standardized for all patients in both groups. Fentanyl 2 μg/kg and propofol 2 mg/kg were administered intravenously, and rocuronium 0.6 mg/kg was given to facilitate tracheal intubation. Endotracheal tube size 7 mm in females and 8.5 mm in males was used to intubate the trachea. The position of endotracheal tube confirmed by capnography. Lungs were ventilated by pressure controlled mode to maintain normocapnia and isoflurane/O2/air mixture was administered to maintain general anesthesia. Randomization was performed to allocate patients into two study groups using the method of random number. In Group R (n = 30); patients received TAP block on each side using 20 mL of study medication, which consisted of 18 mL of ropivacaine 0.375% and 2 mL of normal saline. While Group RD (n = 30) patients were received TAP block on each side with 20 mL, in which dexmedetomidine 0.5 μg/kg was dissolved in 2 mL of normal saline and added to 18 mL of ropivacaine 0.375%.
At the end of the surgery, after ensuring strict aseptic precautions, ultrasound-guided bilateral subcostal TAP block was administered using a mid-axillary approach, under real-time guidance with a high-frequency (5–10 MHz) ultrasound probe (Micromaxx™ Sonosite, Inc., Bothell, WA 98021, USA).
Following the identification of the three different layers of the abdominal wall, block needle was inserted in the plane until its tip was located in between the internal oblique and transversus abdominis muscles. After careful aspiration, injection of study medication was performed and hypoechoic layer was detected on ultrasound.
Patients were transferred to postanesthesia care unit, (PACU) and IV-patient-controlled analgesia (PCA) was commenced with morphine (1 mg loading dose, lockout time interval of 10 min, and 4-h limit of 0.25 mg/kg without baseline infusion). IV-PCA was continued for 24 h postoperatively.
In the PACU: time to first analgesia request was recorded from the completion of TAP block to first given morphine dose, which gave the duration of the TAP block. NRS was used to assess postoperative pain (NRS; where 0 = no pain and 10 = worst imaginable pain) during rest and on coughing. Number of used PCA boluses of morphine at 0–4 h, 4–8 h, 8–12 h, 12–18 h, and 18–24 h was noted and the total consumption of morphine (mg) in 24 h was calculated. Nausea and vomiting were recorded using a categorical scoring system (0 = none, 1 = nausea, 2 = retching, 3 = vomiting). IV ondansetron 0.15 mg/kg was offered for any patient with a score 1. Inverted observer assessment of alertness/sedation (OAA/S) scale where: 1 = awake and 5 = asleep and unarousable was used to assess sedation level in the postoperative period. In PACU and in first 24 h postoperatively, mean arterial pressure (MAP), heart rate (HR), NRS (at rest and on movement), nausea and vomiting, and sedation score (OAA/S) were recorded on admission to PACU, 1, 4, 8, 12, 18, and 24 h postoperatively by an observer who was unaware of the study protocol.
Normally distributed numerical data were presented as mean (standard deviation) and between-group differences were compared using the independent-samples Student t-test. Skewed data were presented as median (interquartile range), and intergroup differences were compared nonparametrically using the Mann–Whitney U-test. Categorical data were presented as ratio and intergroup comparison was performed using the Pearson Chi-square test or Fisher's exact test.
The Statistical software, namely, SAS 9.2, SPSS 15.0, Stata 10.1, MedCalc 9.0.1, Systat 12.0, and R environment ver. 2.11.1, was used for the analysis of the data. P < 0.05 was considered as statistically significant.
RESULTS
Demographic profile and operative characteristics in both groups were comparable [Figure 1]. Postoperatively, the time for first analgesic demand was longer in Group RD than Group R [485.6 vs. 289.8 min, P < 0.0001; Figure 2] and the cumulative doses of morphine consumption in the first 24 h were less among patients in Group RD when compared with those in Group R [14.5 vs. 28.5 mg, P < 0.00001; [Figure 3 and Tables 1, 2] shows HR readings among both groups in the intra-operative and postoperative period. In Group RD, lower HR was noticed 90 min from the starting time and continued until 4 h postoperatively (P < 0.001). Changes in MAP were not statistically significant in both groups. Five cases of Group B complained from nausea (score 1) and three patients in Group RD. In both groups, the incidence of sedation, nausea, and vomiting and the use of anti-emetic medications were not statistically significant.
Figure 1.
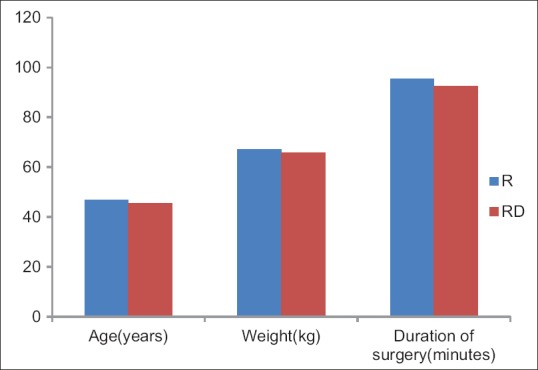
Demographic profile
Figure 2.
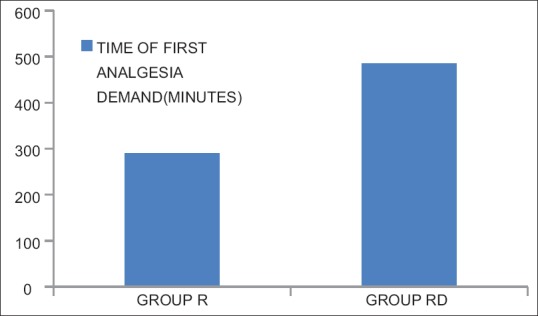
Time of first analgesia demand
Figure 3.
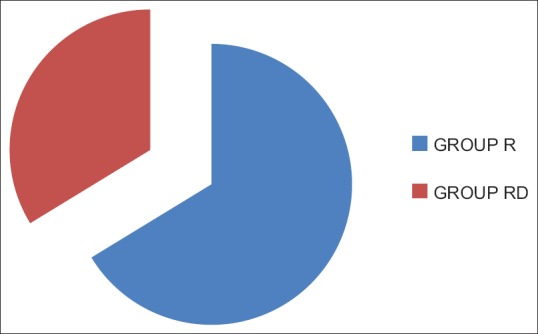
Total dose of morphine consumed in 24 h
Table 1.
Comparison of Intra-operative Heart Rate

Table 2.
Comparison of post-operative Heart Rate

DISCUSSION
Inadequate control of postoperative pain leads to several untoward adverse events such as patient's discomfort, prolonged immobilization, delayed hospital discharge, thromboembolism, and pulmonary complications. Hence, both on humanitarian and medical grounds, adequate postoperative analgesia is very important.
The important outcome of our study is that the addition of dexmedetomidine to ropivacaine in subcostal TAP block provides prolonged postoperative analgesia and better pain control than ropivacaine alone, without any untoward adverse effects. The duration of postoperative analgesia was prolonged; NRS was lower, and the need for rescue morphine doses was less when dexmedetomidine was added to ropivacaine.
Brummett et al. have reported that perineural administration of dexmedetomidine in combination with bupivacaine increased LA blockade in rats without causing neurotoxicity.[7] There are several other studies which have found that the addition of dexmedetomidine to LA in central neuraxial blocks and in peripheral nerve blockades in human was a safe and effective way to potentiate the LA effect and decrease the rescue analgesics requirement.[8,9] In contrary to this, Ozalp et al. have compared dexmedetomidine-ropivacaine mixture to ropivacaine alone in the interscalene block and they have found the same pain scores in either groups without any added advantage of dexmedetomidine.[10]
TAP block is a useful regional anesthesia technique that provides analgesia to the skin and muscle of the anterior abdominal wall in patients undergoing variety of abdominal surgeries.[11,12,13,14] Almarakbi and Kaki have reported that the addition of dexmedetomidine to bupivacaine in TAP block achieves better local anesthesia conditions and provides better pain control postoperatively without causing any major side effects.[4] Abdelaal et al. have reported that the addition of dexmedetomidine to levobupivacaine in bilateral TAP block decreases pain scores and reduces postoperative opioid consumption in patients undergoing abdominoplasty.[15] Sharma et al. have reported that the addition of dexamethasone to ropivacaine in ultrasonography (USG)-guided TAP block prolongs postoperative analgesia and reduces analgesic consumption.[16] Metwally et al. have reported that the addition of fentanyl to the local anesthetic in USG-guided TAP block prolongs the analgesia, lowers postoperative pain score and reduces the opioid consumption.[17] Similar to our study, Prabha et al. have reported that the addition of dexmedetomidine to ropivacaine in TAP block prolongs the duration of postoperative analgesia, reduces pain score, and reduces analgesic consumption when compared to TAP block with ropivacaine alone.[18] Luan et al. have reported that dexmedetomidine as an additive to ropivacaine in TAP block potentiated the analgesic properties of ropivacaine, reduced postoperative consumption of sufentanil, and offered better pain control after abdominal hysterectomy surgery.[19] Shin et al. have concluded that oblique subcostal TAP block provides better analgesia than the TAP block or standard care during the postoperative period in patients undergoing laparoscopic cholecystectomy.[20] Hence, we selected subcostal TAP block over routine TAP block.
In our study, the addition of dexmedetomidine to ropivacaine in ultrasound-guided bilateral subcostal TAP block led to prolongation of postoperative analgesia, less requirement of rescue morphine and lower NRS pain scores. Similar to our study, many researchers reported that the addition of dexmedetomidine to various types of local anesthetic agents in various types of peripheral nerve blocks resulted in prolongation of analgesic effect.[8,9,21] On the other hand, Masuki et al. suggested that dexmedetomidine induces vasoconstriction through action on α2 ARs in the human forearm and the later might contribute to the longer duration of action.[22] Other investigators have supported a third mechanism of action through α2 ARs agonist effect rather than vasoconstriction. They contributed that to the direct effect on the peripheral nerve activity. Whatever the mechanism of dexmedetomidine's action, it seems that it potentiates the LA effect and prolongs the analgesic duration.
Dexmedetomidine is known to produce certain side effect such as bradycardia, hypotension, and sedation especially at higher doses.[23] In this study, there were incidences of bradycardia following the administration of dexmedetomidine opposite to the control group. This effect persisted for 240 min, but not associated with any hemodynamic instability. This bradycardia might be due to the postsynaptic activation of central α2 ARs leading to decreased sympathetic activity and decreased heart rate.[24,25] Similar to bradycardia, increased sedation was noticed in group RD in a first postoperative hour. None of our patients required interventions for bradycardia or sedation. The low dose of dexmedetomidine used in this study could be the reason behind the lesser adverse events. Further studies are needed to determine the safe-effective dose of dexmedetomidine and to assess the risk of perineural administration of dexmedetomidine among bigger patients' sample.
There are few limitations in our study such as lack of correct assessment of success rate of TAP block procedure as it was performed after induction of general anesthesia, but we relied on the skills of the investigators and the use of USG for accurate placement of blocking needle. Another limitation is that dexmedetomidine plasma concentration was not measured in the study population to determine whether its action was related to systemic absorption or local effect.
CONCLUSION
The addition of dexmedetomidine to ropivacaine in bilateral USG guided TAP block achieves prolonged postoperative analgesia and reduced opioid consumption without any major adverse effects.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Bisgaard T, Kehlet H, Rosenberg J. Pain and convalescence after laparoscopic cholecystectomy. Eur J Surg. 2001;167:84–96. doi: 10.1080/110241501750070510. [DOI] [PubMed] [Google Scholar]
- 2.Sinha S, Palta S, Saroa R, Prasad A. Comparison of ultrasound-guided transversus abdominis plane block with bupivacaine and ropivacaine as adjuncts for postoperative analgesia in laparoscopic cholecystectomies. Indian J Anaesth. 2016;60:264–9. doi: 10.4103/0019-5049.179464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rafi AN. Abdominal field block: A new approach via the lumbar triangle. Anaesthesia. 2001;56:1024–6. doi: 10.1046/j.1365-2044.2001.02279-40.x. [DOI] [PubMed] [Google Scholar]
- 4.Almarakbi WA, Kaki AM. Addition of dexmedetomidine to bupivacaine in transversus abdominis plane block potentiates post-operative pain relief among abdominal hysterectomy patients: A prospective randomized controlled trial. Saudi J Anaesth. 2014;8:161–6. doi: 10.4103/1658-354X.130683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Koraki E, Stachtari C, Kapsokalyvas I, Stergiouda Z, Katsanevaki A, Trikoupi A. Dexmedetomidine as an adjuvant to 0.5% ropivacaine in ultrasound-guided axillary brachial plexus block. J Clin Pharm Ther. 2018;43:348–52. doi: 10.1111/jcpt.12657. [DOI] [PubMed] [Google Scholar]
- 6.Bagatini A, Gomes CR, Masella MZ, Rezer G. Dexmedetomidine: Pharmacology and clinical application. Rev Bras Anestesiol. 2002;52:606–17. doi: 10.1590/s0034-70942002000500012. [DOI] [PubMed] [Google Scholar]
- 7.Brummett CM, Norat MA, Palmisano JM, Lydic R. Perineural administration of dexmedetomidine in combination with bupivacaine enhances sensory and motor blockade in sciatic nerve block without inducing neurotoxicity in rat. Anesthesiology. 2008;109:502–11. doi: 10.1097/ALN.0b013e318182c26b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rancourt MP, Albert NT, Côté M, Létourneau DR, Bernard PM. Posterior tibial nerve sensory blockade duration prolonged by adding dexmedetomidine to ropivacaine. Anesth Analg. 2012;115:958–62. doi: 10.1213/ANE.0b013e318265bab7. [DOI] [PubMed] [Google Scholar]
- 9.Esmaoglu A, Yegenoglu F, Akin A, Turk CY. Dexmedetomidine added to levobupivacaine prolongs axillary brachial plexus block. Anesth Analg. 2010;111:1548–51. doi: 10.1213/ANE.0b013e3181fa3095. [DOI] [PubMed] [Google Scholar]
- 10.Ozalp G, Tuncel G, Savli S, Celik A, Doger C, Kaya M, et al. The analgesic efficacy of dexmedetomidine added to ropivacaine patient controlled interscalene analgesia via the posterior approach. J Anaesth. 2006;21:409–12. [Google Scholar]
- 11.McDonnell JG, Curley G, Carney J, Benton A, Costello J, Maharaj CH, et al. The analgesic efficacy of transversus abdominis plane block after cesarean delivery: A randomized controlled trial. Anesth Analg. 2008;106:186–91. doi: 10.1213/01.ane.0000290294.64090.f3. [DOI] [PubMed] [Google Scholar]
- 12.O'Donnell BD, McDonnell JG, McShane AJ. The transversus abdominis plane (TAP) block in open retropubic prostatectomy. Reg Anesth Pain Med. 2006;31:91. doi: 10.1016/j.rapm.2005.10.006. [DOI] [PubMed] [Google Scholar]
- 13.McDonnell JG, O'Donnell B, Curley G, Heffernan A, Power C, Laffey JG. The analgesic efficacy of transversus abdominis plane block after abdominal surgery: A prospective randomized controlled trial. Anesth Analg. 2007;104:193–7. doi: 10.1213/01.ane.0000250223.49963.0f. [DOI] [PubMed] [Google Scholar]
- 14.Carney J, McDonnell JG, Ochana A, Bhinder R, Laffey JG. The transversus abdominis plane block provides effective postoperative analgesia in patients undergoing total abdominal hysterectomy. Anesth Analg. 2008;107:2056–60. doi: 10.1213/ane.0b013e3181871313. [DOI] [PubMed] [Google Scholar]
- 15.Abdelaal W, Metry AA, Refaat M, Ragaei M, Nakhla G. Comparative study between levobupivacaine versus levobupivacaine plus dexmedetomidine for transverse abdominis plane block in postoperative pain management after abdominoplasty. Enliven: J Anesthesiol Crit Care Med. 2015;2:4. [Google Scholar]
- 16.Sharma UD, Prateek S, Tak H. Effect of addition of dexamethasone to ropivacaine on postoperative analgesia in ultrasonography guided transverse abdominis plane block for inguinal hernia repair: A prospective, double blind randomised controlled trial. Indian J Anaesth. 2018;62:371–5. doi: 10.4103/ija.IJA_605_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Metwally AA, Abo-El-Enin KM, Allah SI, Soliman NM, Abo-Omar WA. Ultrasound guided transverse abdominis plane blockfor lower abdominal surgeries: Bupivacaine alone or combined with fentanyl or epinephrine. Menoufia Med J. 2017;30:538–43. [Google Scholar]
- 18.Prabha R, Raman R, Kumar M, Singh D. Effect of adding dexmedetomidine to ropivacaine for transversus abdominis plane block: A prospective randomised controlled trial. J Med Sci Clin Res. 2015;3:4550–7. [Google Scholar]
- 19.Luan H, Zhang X, Feng J, Zhu P, Li J, Zhao Z. Effect of dexmedetomidine added to ropivacaine on ultrasound-guided transversus abdominis plane block for postoperative analgesia after abdominal hysterectomy surgery: A prospective randomized controlled trial. Minerva Anestesiol. 2016;82:981–8. [PubMed] [Google Scholar]
- 20.Shin HJ, Oh AY, Baik JS, Kim JH, Han SH, Hwang JW. Ultrasound-guided oblique subcostal transversus abdominis plane block for analgesia after laparoscopic cholecystectomy: A randomized, controlled, observer-blinded study. Minerva Anestesiol. 2014;80:185–93. [PubMed] [Google Scholar]
- 21.Marhofer D, Kettner SC, Marhofer S, Weber M, Zeitlinger M. Dexmedetomidine as an adjuvant to ropivacaine prolongs peripheral nerve block: A volunteer study. Br J Anaesth. 2012;15:438–42. doi: 10.1093/bja/aes400. [DOI] [PubMed] [Google Scholar]
- 22.Masuki S, Dinenno FA, Joyner MJ, Eisenach JH. Selective α2-adrenergic properties of dexmedetomidine over clonidine in the human forearm. J Appl Physiol. 2005;99:587–92. doi: 10.1152/japplphysiol.00147.2005. [DOI] [PubMed] [Google Scholar]
- 23.Kanazi GE, Aouad MT, Jabbour-Khoury SI, Al Jazzar MD, Alameddine MM, Al-Yaman R, et al. Effect of low-dose dexmedetomidine or clonidine on the characteristics of bupivacaine spinal block. Acta Anaesthesiol Scand. 2006;50:222–27. doi: 10.1111/j.1399-6576.2006.00919.x. [DOI] [PubMed] [Google Scholar]
- 24.Talke P, Lobo E, Brown R. Systemically administered α2-agonist-induced peripheral vasoconstriction in humans. Anesthesiology. 2003;99:65–70. doi: 10.1097/00000542-200307000-00014. [DOI] [PubMed] [Google Scholar]
- 25.Fukushima K, Nishini Y, Mori K, Takeda J. The effect of epidurally administered dexmedetomidine on central and peripheral nervous system in man. Anesth Analg. 1997;84:S292. [Google Scholar]


