Abstract
Acute upper gastrointestinal bleeding (AUGIB) is one of the most common medical emergencies in the UK. Despite advancement in technology the management of AUGIB remains a challenge. The clinical community recognise the need for improvement in the treatment of these patients. AUGIB has a significant impact on resources. Endoscopic therapy is the gold standard treatment. The mortality in AUGIB is rarely related to the presenting bleed but significantly associated with concurrent comorbidities. The cost of blood transfusion in the management of patients with AUGIB is significant and misuse of blood products has been documented nationally. Risk stratification tools such as Glasgow-Blatchford Score, Rockall Score and the AIMS65 score have allowed clinicians to triage patients appropriately in order to deliver endoscopic therapy within a suitable time frame. Endoscopic therapeutic modalities such as epinephrine injection, heat thermocoagulation and mechanical clips have had a positive impact on patient’s management. However, in order to continue to improve patient’s outcomes, further developments are needed.
Keywords: bleeding, bleeding peptic ulcer, gastrointestinal bleeding, endoscopy
Introduction
Upper gastrointestinal bleeding (UGIB) is one of the most common acute GI emergencies. The associated mortality has remained unchanged for the past two decades, being higher among elderly patients with comorbidities.1 2 In the UK, GI bleeding is one of the most common medical emergencies with approximately 85 000 cases per year with 4000 deaths annually.2
The majority of upper GI bleeds (80%–90%) are non-variceal. Patients often present with symptoms such as haematemesis, coffee-ground vomit, drop in haemoglobin (Hb), melaena and haematochezia, with or without haemodynamic instability.3 The presence of pre-existing comorbidities is a significant contributor to mortality in elderly patients with UGIB.4
Common aetiologies include: peptic ulcer disease (PUD), oesophagitis, gastritis, Mallory-Weiss tear, Dieulafoy lesion, gastro-oesophageal varices, cancer and haemobilia.5–9
Despite advancements in therapeutic and interventional endoscopy, acute UGIB (AUGIB) remains a challenge for clinicians and endoscopists worldwide. The clinical community acknowledge that the management of these patients requires streamlining and improvement.
What is the problem?
The majority of the non-variceal upper gastrointestinal bleeding (NVUGIB) in the UK is caused by PUD. UGIB has an enormous burden on healthcare. Inpatient bed stay, endoscopy provision and blood product transfusions are the main contributors to the overall cost of UGIB. The annual initial in-hospital treatment cost for all AUGIB cases in the UK was estimated to be £155.5 million with over £93 million (60%) of this cost due to in-hospital length of stay, £38.5 million (25%) to endoscopy and £12.6 million (8%) to blood transfusion.10
UGIB has an associated mortality rate of 10%1 11 and endoscopic therapy remains the gold standard treatment. Early endoscopy (within 24 hours) is recommended for most patients with AUGIB, in order to achieve prompt diagnosis, provide risk stratification and haemostasis.12 The UK’s National Confidential Enquiry into Patient Outcome and Death report in 2015 concluded that only 44% of patients presenting with AUGIB received good care overall.1
The significance of comorbidities
Mortality in AUGIB is rarely related to the actual haemorrhage, but rather to coexisting comorbidities. Recent studies have shown that about 18% of the total mortality is directly related to GI haemorrhage with the majority of deaths caused by concurrent comorbidities. Pulmonary disease (24%), multiorgan failure (24%) and terminal malignancy (34%) are the most common comorbidities.13
Blood product transfusion before endoscopy
The UK Comparative Audit (2007) of UGIB and the Use of Blood has shown that AUGIB is a significant consumer of blood products in the UK. The study included 6750 patients from 208 hospitals across the UK, with 43% of patients needing at least one unit of blood transfusion.14 GI bleeding is the second most common medical reason for transfusion in the UK after haematological malignancy, accounting for 14% of all blood transfusions.14 Fifteen per cent of patients with GI bleed receive four or more units of blood during their inpatient stay. Blood product use is inappropriate in 20% of cases.15
Current evidence has shown favourable outcomes in patients whose Hb transfusion commenced once Hb dropped below 7.0 g/dL.16 The European Society of Gastrointestinal Endoscopy (ESGE) recommends a restrictive blood transfusion strategy that aims for a target Hb between 7.0 and 9.0 g/dL. A higher target Hb should be considered in patients with significant comorbidity (eg, ischaemic cardiovascular disease).17 In addition at the time of discharge, a restrictive target of Hb 8.0–10.0 g/dL has shown to have better outcomes in those presenting with AUGIB.18
New anticoagulant drugs
The emergence of the direct oral anticoagulants (DOAC: dabigatran, rivaroxaban, apixaban and edoxaban) has reduced regular serum monitoring that is required for patients on warfarin; however, there is a 25%–30% increased risk of GI bleeding with the use of DOAC when compared with warfarin.19 20 The risk is mostly relevant in the elderly and those with hepatic disease, renal disease and patients on concomitant antiplatelet agents.
In the case of an AUGIB, reversal agents can be used; however, different assays are needed to indirectly quantify DOAC level prior to reversal. These assays include the dilute thrombin time and ecarin clotting time for dabigatran and the drug-specific calibrated anti-Xa factor assay for rivaroxaban, edoxaban and apixaban.21 Reversal agents exist (prothrombin complex concentrate (PCC), activated PCC, idarucizumab) with many others currently on clinical trials.20
What are the commonly used risk stratification tools?
Early patient risk stratification will allow the planning and timing of lifesaving procedures such as endoscopic therapy with adequate and safe triage. The primary aim of the initial assessment is to determine whether endoscopy is required urgently or it can be delayed or even managed in the outpatient setting.2 At present, three such scores exist and are in clinical practice.
Glasgow-Blatchford Score
The Glasgow-Blatchford Score (GBS) uses both clinical (pulse, systolic blood pressure, presence of melaena, presentation with syncope, presence of hepatic disease and heart failure) and serological parameters (urea, Hb), which are easily available at initial assessment which allows the clinician to identify patients who would be suitable for management in the outpatient setting (table 1).22 The ESGE and the National Institute for Health and Care Excellence recommend the use of the GBS for pre-endoscopy risk stratification. Patients with the score of 0 or 1 do not require hospital admission and can be safely discharged and managed with outpatient endoscopy.17 23
Table 1.
Glasgow-Blatchford Score (GBS)
| GBS for gastrointestinal bleeding | |
| Score value | |
| Blood urea (mmol/L) | |
| 6.5–7.9 | 2 |
| 8.0–9.9 | 3 |
| 10.0–25.0 | 4 |
| >25.0 | 6 |
| Haemoglobin for men (g/dL) | |
| 12.0–12.9 | 1 |
| 10.0–11.9 | 3 |
| <10.0 | 6 |
| Haemoglobin for women (g/dL) | |
| 10.0–11.9 | 1 |
| <10.0 | 6 |
| Systolic blood pressure (mm Hg) | |
| 100–109 | 1 |
| 90–99 | 2 |
| <90 | 3 |
| Other markers | |
| Pulse ≥100/min | 1 |
| Presentation with melaena | 1 |
| Presentation with syncope | 2 |
| Hepatic disease* | 2 |
| Cardiac failure† | 2 |
*Known history, or clinical and laboratory evidence, of chronic or acute hepatic disease.
†Known history, or clinical and echocardiographic evidence, of cardiac failure.
Rockall Score
In contrast, the Rockall Score (RS) combines clinical parameters with endoscopic findings in order to predict the risk of mortality (table 2). Lack of endoscopic findings in the initial assessment of a patient with AUGIB may deter the clinician from using the RS; however, full postendoscopy RS remains an important tool in predicting mortality rate.24
Table 2.
Rockall Score
| Rockall Score for gastrointestinal bleeding | ||||
| 0 | 1 | 2 | 3 | |
| Initial score criteria | ||||
| Age | <60 | 60–79 | >80 | |
| Shock | No shock | HR >100 | HR >100, SBP <100 | |
| Comorbidity | Cardiac failure, ischaemic heart disease | Renal failure, liver failure, disseminated malignancy | ||
| Additional criteria for full score | ||||
| Diagnosis | Mallory-Weiss, no lesion, no stigmata of recent haemorrhage | All other diagnoses | Malignancy of upper gastrointestinal tract | |
| Stigmata of recent haemorrhage | None or dark spot | Fresh blood, adherent clot, visible or spurting vessel | ||
Maximum additive score prior to diagnosis=7.
Maximum additive score after diagnosis=11.
HR, heart rate; SBP, systolic blood pressure.
The AIMS65 score
The AIMS65 score is designed to predict in-hospital mortality, length of stay and cost of GI bleeding (tables 3 and 4). In comparison to GBS and RS, it is superior in predicting inpatient mortality.25 AIMS65 score is inferior to GBS and RS in predicting rebleeding. GBS, RS and AIMS65 are similar in predicting length of hospital stay.25 26 GBS is more accurate in terms of detecting transfusion need, rebleeding rate and endoscopic intervention rate.25 27
Table 3.
AIMS65 score
| Score | |
| Age >65 | 1 |
| SBP <90 mm Hg | 1 |
| Altered mental status | 1 |
| INR >1.5 | 1 |
| Albumin <30 g/L | 1 |
INR, international normalised ratio; SBP, systolic blood pressure.
Table 4.
In-hospital mortality rate based on AIMS65 score
| Total score | Mortality rate (%) |
| 0 | 0.30 |
| 1 | 1.20 |
| 2 | 5.30 |
| 3 | 10.30 |
| 4 | 16.50 |
| 5 | 24.50 |
What is the optimal timing of endoscopy?
The benefit of early endoscopy in the management of NVUGIB remains controversial12; however, endoscopy has an important role in obtaining diagnosis with a sensitivity of 90%–95% at locating the bleeding site.23
Several studies have investigated the effect of endoscopy timing on clinical outcomes with varying results. In haemodynamically stable patients with ASA grade 1 or 2, early endoscopy within 12 hours of presentation has no effect on mortality or recurrent bleeding28–30; however, more high-risk endoscopic lesions are identified31 in those receiving early endoscopy and these patients tend to have a shorter length of hospital stay.32–34 Early endoscopy in haemodynamically stable patients with ASA grades 3–5 is associated with lower in-hospital mortality. In patients with haemodynamic instability, early endoscopy is associated with lower in-hospital mortality.32 Although 2%–10% of patients with AUGIB can die from their AUGIB, mortality in 80% of these patients is due to other non-bleeding comorbidities.13 23 35
What are the common pharmacological therapies?
Proton pump inhibitors
Pharmacological agents such as proton pump inhibitors (PPI) have significantly reduced the incidence of PUD.36 Pre-endoscopic use of PPI reduces the detection rate of high-risk stigmata during endoscopy and the need for endoscopic therapy2; however, there is no significant impact on the amount of blood transfusion, rebleeding rate, surgery or death within 30 days.23 37
Prokinetic drugs
The administration of prokinetic drugs such as metoclopramide and erythromycin has shown to improve endoscopic diagnostic yield in patients with AUGIB and reduce the need for repeat endoscopy.2 This is useful in cases where the upper GI tract is filled with large volume of blood; however, there is lack of evidence in improving the duration of hospitalisation, transfusion requirements or surgery.38
Tranexamic acid
Tranexamic acid, a derivative of the amino acid lysine, has an antifibrinolytic effect by preventing the degradation of fibrin networks.39 Studies have shown that it decreases rebleeding and mortality in AUGIB, without increasing the thromboembolic adverse effects; however, its routine use in clinical practice has not been recommended as further clinical trials are needed.40 41
The Forrest Classification
The endoscopic management of UGIB has evolved in recent decades as therapeutic modalities available to the endoscopist have evolved, driven by innovations in new techniques and accessories. Endoscopy in patients with AUGIB is effective in diagnosing and treating most causes of UGIB.2 The Forrest Classification (figure 1) categorises the lesion morphology at the time of index endoscopy, allowing the endoscopist to decide when to intervene and prognosticate the risk of rebleeding.42 This categorisation has also been shown to correlate with the need for surgery and mortality43; however, there is significant interobserver disagreement in categorising the bleeding site, hence accurate photographic documentation is paramount.44
Figure 1.
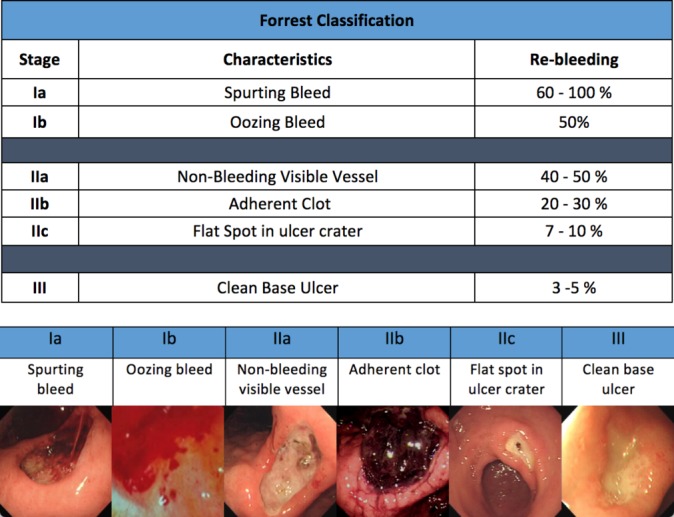
Different types of bleed based on the Forrest Classification.
What are the available endoscopic haemostatic techniques?
Several endoscopic treatment modalities have been developed; these include injection methods, heat cauterisation and mechanical therapy.
Epinephrine injection therapy
This includes injection of dilute epinephrine (1:10 000) at the site of bleeding. It reduces blood flow by temporary creating local tamponade and vasoconstriction of blood vessels. Injection of large volume of epinephrine (>13 mL) can reduce the rate of recurrent bleeding in patients with high-risk peptic ulcer and is superior to injection of lesser volumes.45–47
Thermocoagulation
Thermocoagulation uses direct contact with the bleeding site with thermal energy delivered via a variety of devices. Heater probe consists of a Teflon-coated hollow aluminium cylinder with inner heating coil. It uses electrical current to generate heat. The Gold Probe has a rounded gold distal tip with good conductivity and has irrigation and injection capability, in addition to delivering heat for thermocoagulation.48
Argon plasma coagulation is a non-contact ablative modality that uses steam of ionised gas to conduct electricity for the coagulation of bleeding tissue.49
Mechanical therapy: clips
Mechanical therapy is an attractive method for achieving endoscopic haemostasis. It has a significant impact on achieving haemostasis in difficult and challenging cases and a significant impact on outcomes.50
Mechanical therapy with endoscopic clips has been shown to be effective by physically obstructing the blood flow in the vessel; however, this technique will require direct visualisation of the bleeding point and culprit vessel. Successful application of clip is better in achieving haemostasis when compared with injection therapy alone but similar to thermocoagulation.51
The over-the-scope clip (OTSC) has been reported to effectively achieve haemostasis and significantly reduces rebleeding and rebleeding-associated mortality in NVUGIB. A recent multicentre study was able to show a haemostasis rate of 92.4% with OTSC as a monotherapy in the treatment of acute NVUGIB with significant reduction in the occurrence of bleeding and mortality of rebleeding.52
Dual and triple therapy is better than monotherapy
Dual endoscopic therapy is superior to monotherapy with epinephrine injection alone in the management of patients with high-risk bleeding peptic ulcer; dual therapy reduces the risk of recurrent bleeding, the risk of emergency surgery50 and mortality.53
The possible adverse events from dual therapy include perforation and gastric wall necrosis, with very low occurrence rate. Dual therapy remains to be superior to monotherapy with epinephrine.23 54
The Doppler endoscopic probe
Doppler probe through the accessory channel of a standard endoscope has been used to assess the blood flow in the superficial blood vessels at the site of bleeding peptic ulcer after endoscopic therapy. The audible signal generated by the probe is able to determine the type of blood flow (arterial or venous) and the location of the bleeding vessel.55 56 Doppler signal from an ulcer, after endoscopic therapy, has been associated with a higher risk of rebleeding56 57; however, lack of audible signal after endoscopic therapy is not associated with improvement in rebleeding rate.43
Is interventional radiology suitable for GI bleeding?
Interventional radiology (IR) has shown to provide diagnostic imaging and endovascular therapeutic interventions that can localise the source of bleeding and provide endovascular embolisation to achieve haemostasis successfully when conventional endoscopic haemostasis has been unsuccessful.58 A study by Krämer et al was able to show that IR can control UGIB and achieve haemostasis with the use of minicoils for the embolisation of bleeding vessels with reduced risk of serious complications.59
What is the optimum postprocedure management?
Postendoscopic treatment with high-dose infusion of PPI (bolus of 80 mg followed by 8 mg/hour for 72 hours) in bleeding peptic ulcers significantly reduces the risk of recurrent bleeding.60 Rebleeding rate has also been shown to be associated with the Hb at the time of discharge. The rebleeding rate in patients with a discharge Hb between 80 and 100 g/L is not significantly different when compared with patients with higher Hb at discharge.18 In addition, a discharge Hb between 80 and 100 g/L is associated with a lower consumption of red blood cells.18
Rebleeding is more common in patients with high stigmata lesions at the time of endoscopy, hence repeat endoscopy and treatment should be considered in all high-risk bleeds, in particular those with the need to recommence anticoagulation and patients who have had limited endoscopic therapy at the initial endoscopy. Surgery should be considered in those not responding to endoscopic therapy or radiological embolisation, taking into account patient’s status and comorbidities.23
What are the future developments?
The development of a risk stratification tool relevant to all GI bleeds should be an essential point of focus for all clinicians managing GI bleeding. Several novel modalities have been developed for the investigation and treatment of GI bleeding in recent years. These show promising results in achieving prompt diagnosis and haemostasis.
Video capsule endoscopy
The use of video capsule endoscopy (VCE) in the emergency department (ED) as a risk stratification tool for identifying high and low-risk patients with UGIB has been evaluated. It has shown potential to identify high and low-risk patients presenting with signs of AUGIB, helping to determine the need for intervention with significant reduction in the time to emergent endoscopic therapy.61 VCE in the ED is safe and effective in identifying AUGIB.62 A study by Meltzer et al looked into the use of VCE in the ED performed by a gastroenterologist or a VCE-trained clinician. The aim was to determine whether patients with signs and symptoms of upper GI bleeding can be discharged with outpatient follow-up endoscopy. A total of 25 subjects were enrolled with excellent tolerance to the VCE. The study was able to show a sensitivity of 88% with a specificity of 64% for the detection of fresh blood in the upper GI tract.63 Similar studies have shown significant reduction in hospital admissions with no difference in the clinical outcome in terms of recurrent bleeding and 30-day mortality in the VCE group and those receiving standard treatment.64 This is very exciting and further studies will be able to provide more data on this unique modality for the diagnosis of patients in the ED. This will potentially have a great impact on the number of hospital admissions.63
Hemospray
Hemospray is a novel proprietary mineral blend that forms a mechanical barrier over the bleeding site when applied endoscopically. It gives the endoscopist the opportunity to apply therapy in challenging anatomies. The multicentre European Survey to Evaluate the Application of Hemospray in the Luminal tract (SEAL) study65 and the French Groupe de Recherche Avancé des Praticiens Hospitaliers en Endoscopie (GRAPHE) study66 have both shown high haemostasis rates with the use of Hemospray as monotherapy and in combination with conventional methods. These results have been reflected by the current and ongoing prospective International Multicentre Hemospray Registry (Alzoubaidi et al, University College London, London) showing an overall haemostasis rate of 86%. Expansion of this study is currently in progress and shall provide further evidence on the use of Hemospray as monotherapy, dual therapy and rescue therapy in various pathologies.67
EndoClot
The EndoClot (EndoClot Plus, Santa Clara, CA, USA) is a polysaccharide haemostatic powder that can be delivered endoscopically to the site of bleeding in the GI tract without the need for direct mucosal contact. It is composed of absorbable polymer particles that absorb water from the blood on the surface of the bleeding site, hence increasing the concentration of platelets and clotting factors, resulting into haemostasis.68 69 Further clinical trials are awaiting.
Conclusion
GI bleeding remains to be a challenging clinical emergency with significant mortality and morbidity that remains unchanged these past two decades; however, with adequate service planning and adherence to robust guidelines, improved and desirable outcomes can be achieved.
Patients with AUGIB should be admitted to units that provide a 24/7 GI bleed service with anaesthetic support and access to IR and surgery. Risk stratification and adequate resuscitation prior to any endoscopic therapy are paramount and must supersede the interventional endoscopy as the key initial process in the management of patients with AUGIB.
The timing of endoscopy is dependent on the presenting signs, taking into account the clinical status of the patient. The endoscopic therapy of all acute NVUGIB should not rely on monotherapy alone but a combination of injection therapy with other modalities such as clips, thermocoagulation or both. Second-look endoscopy is recommended in patients with signs of rebleeding.
Further developments of new techniques will assist future generations in the management of AUGIB; however, all endoscopists must acquire sufficient training in order to provide the best treatment options. This would require appropriate facilities and training at all hospitals nationwide.
Further studies should focus to explore which treatment modalities are more effective in specific pathologies as currently no single modality is capable of treating all pathologies.
Finally, the focus of treatment should not only be the endoscopic therapy and a holistic approach is encouraged in order to optimise treatment by managing multiorgan failure and comorbidities.13
Footnotes
Contributors: DA has written and submitted the manuscript and was directly involved with the planning, analysis, data search and critique of this paper. LBL provided intellectual input to this review paper. RH was directly involved in the planning, analysis, data search and critique of this paper and is the senior author. All authors contributed to refinement of the paper and approved the final manuscript.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1. McPherson SJ, Sinclair MT, Smith NCE, et al. Gastrointestinal Haemorrhage: Time to Get Control ? London, UK: National Confidential Enquiry into Patient Outcome and Death, 2015. [Google Scholar]
- 2. Hwang JH, Fisher DA, Ben-Menachem T, et al. The role of endoscopy in the management of acute non-variceal upper GI bleeding. Gastrointest Endosc 2012;75:1132–8. 10.1016/j.gie.2012.02.033 [DOI] [PubMed] [Google Scholar]
- 3. Khamaysi I, Gralnek IM. Acute upper gastrointestinal bleeding (UGIB) - initial evaluation and management. Best Pract Res Clin Gastroenterol 2013;27:633–8. 10.1016/j.bpg.2013.09.002 [DOI] [PubMed] [Google Scholar]
- 4. Marmo R, Koch M, Cipolletta L, et al. Predictive factors of mortality from nonvariceal upper gastrointestinal hemorrhage: a multicenter study. Am J Gastroenterol 2008;103:1639–47. 10.1111/j.1572-0241.2008.01865.x [DOI] [PubMed] [Google Scholar]
- 5. Sugawa C, Benishek D, Walt AJ, et al. Mallory-Weiss syndrome. A study of 224 patients. Am J Surg 1983;145:30–3. [DOI] [PubMed] [Google Scholar]
- 6. Feinman M, Haut ER. Upper gastrointestinal bleeding. Surg Clin North Am 2014;94:43–53. 10.1016/j.suc.2013.10.004 [DOI] [PubMed] [Google Scholar]
- 7. Szura M, Pasternak A. Upper gastrointestinal bleeding - state of the art. Folia Med Cracov 2014;54:59–78. [PubMed] [Google Scholar]
- 8. van Leerdam ME. Epidemiology of acute upper gastrointestinal bleeding. Best Pract Res Clin Gastroenterol 2008;22:209–24. 10.1016/j.bpg.2007.10.011 [DOI] [PubMed] [Google Scholar]
- 9. Ion D, Iuliana Mavrodin C, Bogdan Serban M, et al. Haemobilia - a rare cause of upper gastro-intestinal bleeding. Chirurgia 2016;111:509–12. 10.21614/chirurgia.111.6.509 [DOI] [PubMed] [Google Scholar]
- 10. Campbell HE, Stokes EA, Bargo D, et al. Costs and quality of life associated with acute upper gastrointestinal bleeding in the UK: cohort analysis of patients in a cluster randomised trial. BMJ Open 2015;5:e007230 10.1136/bmjopen-2014-007230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hearnshaw S. UK comparative audit of upper gastrointestinal bleeding and the use of blood. London, UK: British society of Gastroenterolgy, 2007:1–88. [Google Scholar]
- 12. Lin HJ, Wang K, Perng CL, et al. Early or delayed endoscopy for patients with peptic ulcer bleeding. A prospective randomized study. J Clin Gastroenterol 1996;22:267–71. [DOI] [PubMed] [Google Scholar]
- 13. Sung JJ, Tsoi KK, Ma TK, et al. Causes of mortality in patients with peptic ulcer bleeding: a prospective cohort study of 10,428 cases. Am J Gastroenterol 2010;105:84–9. 10.1038/ajg.2009.507 [DOI] [PubMed] [Google Scholar]
- 14. Hearnshaw SA, Logan RF, Lowe D, et al. Acute upper gastrointestinal bleeding in the UK: patient characteristics, diagnoses and outcomes in the 2007 UK audit. Gut 2011;60:1327–35. 10.1136/gut.2010.228437 [DOI] [PubMed] [Google Scholar]
- 15. Wallis JP, Wells AW, Chapman CE. Changing indications for red cell transfusion from 2000 to 2004 in the North of England. Transfus Med 2006;16:411–7. 10.1111/j.1365-3148.2006.00702.x [DOI] [PubMed] [Google Scholar]
- 16. Villanueva C, Colomo A, Bosch A, et al. Transfusion strategies for acute upper gastrointestinal bleeding. N Engl J Med 2013;368:11–21. 10.1056/NEJMoa1211801 [DOI] [PubMed] [Google Scholar]
- 17. Gralnek IM, Dumonceau JM, Kuipers EJ, et al. Diagnosis and management of nonvariceal upper gastrointestinal hemorrhage: European Society of Gastrointestinal Endoscopy (ESGE) Guideline. Endoscopy 2015;47:a1–a46. 10.1055/s-0034-1393172 [DOI] [PubMed] [Google Scholar]
- 18. Lee JM, Kim ES, Chun HJ, et al. Discharge hemoglobin and outcome in patients with acute nonvariceal upper gastrointestinal bleeding. Endosc Int Open 2016;4:E865–9. 10.1055/s-0042-110176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Holster IL, Valkhoff VE, Kuipers EJ, et al. New oral anticoagulants increase risk for gastrointestinal bleeding: a systematic review and meta-analysis. Gastroenterology 2013;145:105–12. 10.1053/j.gastro.2013.02.041 [DOI] [PubMed] [Google Scholar]
- 20. Abraham NS, Horsley-Silva JL. Gastrointestinal bleeding secondary to the new anticoagulants. Curr Opin Gastroenterol 2016;32:474–80. 10.1097/MOG.0000000000000310 [DOI] [PubMed] [Google Scholar]
- 21. Cuker A. Laboratory measurement of the non-vitamin K antagonist oral anticoagulants: selecting the optimal assay based on drug, assay availability, and clinical indication. J Thromb Thrombolysis 2016;41:241–7. 10.1007/s11239-015-1282-7 [DOI] [PubMed] [Google Scholar]
- 22. Stanley AJ, Ashley D, Dalton HR, et al. Outpatient management of patients with low-risk upper-gastrointestinal haemorrhage: multicentre validation and prospective evaluation. Lancet 2009;373:42–7. 10.1016/S0140-6736(08)61769-9 [DOI] [PubMed] [Google Scholar]
- 23. NICE. Acute upper gastrointestinal bleeding in o v er 16s : management. United Kingdom: NICE, 2012. [Google Scholar]
- 24. Mokhtare M, Bozorgi V, Agah S, et al. Comparison of Glasgow-Blatchford score and full Rockall score systems to predict clinical outcomes in patients with upper gastrointestinal bleeding. Clin Exp Gastroenterol 2016;9:337–43. 10.2147/CEG.S114860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Martínez-Cara JG, Jiménez-Rosales R, Úbeda-Muñoz M, et al. Comparison of AIMS65, Glasgow-Blatchford score, and Rockall score in a European series of patients with upper gastrointestinal bleeding: performance when predicting in-hospital and delayed mortality. United European Gastroenterol J 2016;4:371–9. 10.1177/2050640615604779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Robertson M, Majumdar A, Boyapati R, et al. Risk stratification in acute upper GI bleeding: comparison of the AIMS65 score with the Glasgow-Blatchford and Rockall scoring systems. Gastrointest Endosc 2016;83:1151–60. 10.1016/j.gie.2015.10.021 [DOI] [PubMed] [Google Scholar]
- 27. Stanley AJ, Laine L, Dalton HR, et al. Comparison of risk scoring systems for patients presenting with upper gastrointestinal bleeding: international multicentre prospective study. BMJ 2017;356:i6432 10.1136/bmj.i6432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Tai CM, Huang SP, Wang HP, et al. High-risk ED patients with nonvariceal upper gastrointestinal hemorrhage undergoing emergency or urgent endoscopy: a retrospective analysis. Am J Emerg Med 2007;25:273–8. 10.1016/j.ajem.2006.07.014 [DOI] [PubMed] [Google Scholar]
- 29. Schacher GM, Lesbros-Pantoflickova D, Ortner MA, et al. Is early endoscopy in the emergency room beneficial in patients with bleeding peptic ulcer? A "fortuitously controlled" study. Endoscopy 2005;37:324–8. 10.1055/s-2004-826237 [DOI] [PubMed] [Google Scholar]
- 30. Jairath V, Kahan BC, Logan RF, et al. Outcomes following acute nonvariceal upper gastrointestinal bleeding in relation to time to endoscopy: results from a nationwide study. Endoscopy 2012;44:723–30. 10.1055/s-0032-1309736 [DOI] [PubMed] [Google Scholar]
- 31. Bjorkman DJ, Zaman A, Fennerty MB, et al. Urgent vs. elective endoscopy for acute non-variceal upper-GI bleeding: an effectiveness study. Gastrointest Endosc 2004;60:1–8. 10.1016/S0016-5107(04)01287-8 [DOI] [PubMed] [Google Scholar]
- 32. Laursen SB, Leontiadis GI, Stanley AJ, et al. Relationship between timing of endoscopy and mortality in patients with peptic ulcer bleeding: a nationwide cohort study. Gastrointest Endosc 2017;85:936–44. 10.1016/j.gie.2016.08.049 [DOI] [PubMed] [Google Scholar]
- 33. Cooper GS, Chak A, Way LE, et al. Early endoscopy in upper gastrointestinal hemorrhage: associations with recurrent bleeding, surgery, and length of hospital stay. Gastrointest Endosc 1999;49:145–52. 10.1016/S0016-5107(99)70478-5 [DOI] [PubMed] [Google Scholar]
- 34. Lim LG, Ho KY, Chan YH, et al. Urgent endoscopy is associated with lower mortality in high-risk but not low-risk nonvariceal upper gastrointestinal bleeding. Endoscopy 2011;43:300–6. 10.1055/s-0030-1256110 [DOI] [PubMed] [Google Scholar]
- 35. Sonnenberg A. Timing of endoscopy in gastrointestinal bleeding. United European Gastroenterol J 2014;2:5–9. 10.1177/2050640613518773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Wong SH, Sung JJ. Management of GI emergencies: peptic ulcer acute bleeding. Best Pract Res Clin Gastroenterol 2013;27:639–47. 10.1016/j.bpg.2013.08.003 [DOI] [PubMed] [Google Scholar]
- 37. Campbell IW, Nairn M. Scottish Intercollegiate Guidelines Network: management of diabetes (SIGN 55). Br J Diabetes Vasc Dis 2002;2:50–2. 10.1177/14746514020020010501 [DOI] [Google Scholar]
- 38. Laine L, Jensen DM. Management of patients with ulcer bleeding. Am J Gastroenterol 2012;107:345–60. 10.1038/ajg.2011.480 [DOI] [PubMed] [Google Scholar]
- 39. Hu D. Emergency medicine questions: can tranexamic acid be used to treat upper gastrointestinal bleeds? Am J Emerg Med 2016;34:1892–3. 10.1016/j.ajem.2016.06.080 [DOI] [PubMed] [Google Scholar]
- 40. Bennett C, Klingenberg SL, Langholz E, et al. Tranexamic acid for upper gastrointestinal bleeding. Cochrane Database Syst Rev 2014;4:CD006640 10.1002/14651858.CD006640.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Gluud LL, Klingenberg SL, Langholz E. Tranexamic acid for upper gastrointestinal bleeding. Cochrane Database Syst Rev 2012;1:CD006640 10.1002/14651858.CD006640.pub2 [DOI] [PubMed] [Google Scholar]
- 42. Forrest JH, Finlayson NDC, Shearman DJC. Endoscopy in gastrointestinal bleeding. The Lancet 1974;304:394–7. 10.1016/S0140-6736(74)91770-X [DOI] [PubMed] [Google Scholar]
- 43. Nayor J, Saltzman JR. Determining the endpoint of endoscopic therapy for upper GI bleeding: Are our eyes as good as our ears? Gastrointest Endosc 2016;83:137–9. 10.1016/j.gie.2015.08.056 [DOI] [PubMed] [Google Scholar]
- 44. Jensen DM, Stuart R, Ahlbom H, et al. Inter-observer agreement on assessment of photo documentation in bleeding peptic ulcer. Gastrointest Endosc 2009;69:AB186 10.1016/j.gie.2009.03.384 [DOI] [Google Scholar]
- 45. Lin HJ, Hsieh YH, Tseng GY, et al. A prospective, randomized trial of large- versus small-volume endoscopic injection of epinephrine for peptic ulcer bleeding. Gastrointest Endosc 2002;55:615–9. 10.1067/mge.2002.123271 [DOI] [PubMed] [Google Scholar]
- 46. Liou TC, Lin SC, Wang HY, et al. Optimal injection volume of epinephrine for endoscopic treatment of peptic ulcer bleeding. World J Gastroenterol 2006;12:3108–13. 10.3748/wjg.v12.i19.3108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Park JM, Huo SM, Lee HH, et al. Longer Observation Time Increases Proportion of Neoplasms Detected by Esophagogastroduodenoscopy. Gastroenterology 2017;153:460–9. 10.1053/j.gastro.2017.05.009 [DOI] [PubMed] [Google Scholar]
- 48. Gastrotraining. Use of Gold probe. http://www.gastrotraining.com/category/gastro-duodenal/upper-gi-bleed-endoscopy/gold-probe-upper-gi-bleed-endoscopy-gastro-duodenal.
- 49. Ginsberg GG, Barkun AN, Bosco JJ, et al. The argon plasma coagulator: February 2002. Gastrointest Endosc 2002;55:807–10. 10.1016/S0016-5107(02)70408-2 [DOI] [PubMed] [Google Scholar]
- 50. Marmo R, Rotondano G, Piscopo R, et al. Dual therapy versus monotherapy in the endoscopic treatment of high-risk bleeding ulcers: a meta-analysis of controlled trials. Am J Gastroenterol 2007;102:279–89. 10.1111/j.1572-0241.2006.01023.x [DOI] [PubMed] [Google Scholar]
- 51. Sung JJ, Tsoi KK, Lai LH, et al. Endoscopic clipping versus injection and thermo-coagulation in the treatment of non-variceal upper gastrointestinal bleeding: a meta-analysis. Gut 2007;56:1364–73. 10.1136/gut.2007.123976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Wedi E, Fischer A, Hochberger J, et al. Multicenter evaluation of first-line endoscopic treatment with the OTSC in acute non-variceal upper gastrointestinal bleeding and comparison with the Rockall cohort: the FLETRock study. Surg Endosc 2018;32:307–14. 10.1007/s00464-017-5678-7 [DOI] [PubMed] [Google Scholar]
- 53. Calvet X, Vergara M, Brullet E, et al. Addition of a second endoscopic treatment following epinephrine injection improves outcome in high-risk bleeding ulcers. Gastroenterology 2004;126:441–50. 10.1053/j.gastro.2003.11.006 [DOI] [PubMed] [Google Scholar]
- 54. Vergara M, Bennett C, Calvet X, et al. Epinephrine injection versus epinephrine injection and a second endoscopic method in high-risk bleeding ulcers. Cochrane Database Syst Rev 2014:CD005584 10.1002/14651858.CD005584.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Kohler B, Maier M, Benz C, et al. Acute ulcer bleeding. A prospective randomized trial to compare Doppler and Forrest classifications in endoscopic diagnosis and therapy. Dig Dis Sci 1997;42:1370–4. 10.1023/A:1018877602113 [DOI] [PubMed] [Google Scholar]
- 56. Wong RC, Chak A, Kobayashi K, et al. Role of Doppler US in acute peptic ulcer hemorrhage: can it predict failure of endoscopic therapy? Gastrointest Endosc 2000;52:315–21. 10.1067/mge.2000.106688 [DOI] [PubMed] [Google Scholar]
- 57. van Leerdam ME, Rauws EA, Geraedts AA, et al. The role of endoscopic Doppler US in patients with peptic ulcer bleeding. Gastrointest Endosc 2003;58:677–84. 10.1016/S0016-5107(03)02033-9 [DOI] [PubMed] [Google Scholar]
- 58. Navuluri R, Patel J, Kang L, et al. Role of interventional radiology in the emergent management of acute upper gastrointestinal bleeding. Semin Intervent Radiol 2012;29:169–77. 10.1055/s-0032-1326925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Krämer SC, Görich J, Rilinger N, et al. Embolization for gastrointestinal hemorrhages. Eur Radiol 2000;10:802–5. 10.1007/s003300051007 [DOI] [PubMed] [Google Scholar]
- 60. Lau JY, Sung JJ, Lee KK, et al. Effect of intravenous omeprazole on recurrent bleeding after endoscopic treatment of bleeding peptic ulcers. N Engl J Med 2000;343:310–6. 10.1056/NEJM200008033430501 [DOI] [PubMed] [Google Scholar]
- 61. Rubin M, Hussain SA, Shalomov A, et al. Live view video capsule endoscopy enables risk stratification of patients with acute upper GI bleeding in the emergency room: a pilot study. Dig Dis Sci 2011;56:786–91. 10.1007/s10620-010-1336-9 [DOI] [PubMed] [Google Scholar]
- 62. Gralnek IM, Ching JY, Maza I, et al. Capsule endoscopy in acute upper gastrointestinal hemorrhage: a prospective cohort study. Endoscopy 2013;45:12–19. 10.1055/s-0032-1325933 [DOI] [PubMed] [Google Scholar]
- 63. Meltzer AC, Ali MA, Kresiberg RB, et al. Video capsule endoscopy in the emergency department: a prospective study of acute upper gastrointestinal hemorrhage. Ann Emerg Med 2013;61:438–43. 10.1016/j.annemergmed.2012.11.008 [DOI] [PubMed] [Google Scholar]
- 64. Sung JJ, Tang RS, Ching JY, et al. Use of capsule endoscopy in the emergency department as a triage of patients with GI bleeding. Gastrointest Endosc 2016;84:907–13. 10.1016/j.gie.2016.04.043 [DOI] [PubMed] [Google Scholar]
- 65. Smith LA, Stanley AJ, Bergman JJ, et al. Hemospray application in nonvariceal upper gastrointestinal bleeding: results of the Survey to Evaluate the Application of Hemospray in the Luminal Tract. J Clin Gastroenterol 2014;48:e89–92. 10.1097/MCG.0000000000000054 [DOI] [PubMed] [Google Scholar]
- 66. Haddara S, Jacques J, Lecleire S, et al. A novel hemostatic powder for upper gastrointestinal bleeding: a multicenter study (the "GRAPHE" registry). Endoscopy 2016;48:1084–95. 10.1055/s-0042-116148 [DOI] [PubMed] [Google Scholar]
- 67. Alzoubaidi D, Magee C, Gulati S, et al. PTU-031 Outcomes from an international multicentre registry of patients with gastrointestinal bleeding undergoing endoscopic treatment with hemospray. Gut 2017;66:A65. [DOI] [PubMed] [Google Scholar]
- 68. Beg S, Al-Bakir I, Bhuva M, et al. Early clinical experience of the safety and efficacy of EndoClot in the management of non-variceal upper gastrointestinal bleeding. Endosc Int Open 2015;3:E605–9. 10.1055/s-0034-1393087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Garber A, Jang S. Novel Therapeutic Strategies in the Management of Non-Variceal Upper Gastrointestinal Bleeding. Clin Endosc 2016;49:421–4. 10.5946/ce.2016.110 [DOI] [PMC free article] [PubMed] [Google Scholar]


