Abstract
Background
This study investigated the correlations between acute cerebral hemorrhage complicated with stress ulcer bleeding and corresponding indexes, including the Acute Physiology and Chronic Health Evaluation (APACHE) II score, vascular endothelin-1 (ET-1), tumor necrosis factor-alpha (TNF-α), and blood lipid factors.
Material/Methods
A total of 53 patients with acute cerebral hemorrhage complicated with stress ulcer bleeding were selected as the observation group and 50 patients with simple acute cerebral hemorrhage were selected as the control group. The APACHE II score and the levels of ET-1, TNF-α, and blood lipid factors, including total cholesterol (TC), triglyceride (TG), high-density lipoprotein-cholesterol (HDL-C), low-density lipoprotein-cholesterol (LDL-C), and malondialdehyde (MDA), were detected and the correlations of were analyzed between the 2 groups of patients.
Results
The blood lipid index TG, APACHE II score, ET-1, TNF-α, renal function indexes [blood urea nitrogen (BUN) and creatinine (Cr)], mortality rate, hemoglobin, and MDA in the observation group were significantly higher than those in the control group, while HDL-C in the observation group was obviously lower than in the control group (p<0.05). The APACHEII score had positive correlations with TG and TNF-α (r=0.8960, r=0.8563, respectively), while it was negatively correlated with TC, HDL-C, LDL-C, and ET-1 (r=−0.909, r=−0.9292, r=−0.8543, and r=−0.8899, respectively) (p<0.001 in all comparisons). APACHEII score, BUN, and Cr were all risk factors.
Conclusions
Stress ulcer in patients with acute cerebral hemorrhage is associated with blood lipid changes and inflammation, which provides clues for the diagnosis and treatment of acute cerebral hemorrhage.
MeSH Keywords: Hemorrhage, Lipids, Retinal Vessels, Ulcer
Background
Cerebral hemorrhage symptoms are generally found in patients with intracranial vascular acute or cerebrovascular diseases. Patients with cerebral hemorrhage may be accompanied with various complications [1–3], of which stress ulcer bleeding is one of the common adverse reactions. Cerebral hemorrhage and increased intracranial pressure in patients affect the autonomic nervous function of the body, resulting in gastric ischemia, overproduction of gastric juice, and stress ulcer bleeding [4,5]. Abnormalities of blood lipids and inflammatory factors are universally detected in patients with stress cerebral hemorrhage complicated with stress ulcer bleeding in clinical settings [6,7], but the underlying mechanism is unclear. This study investigated the changes in endothelin-1 (ET-1), tumor necrosis factor (TNF), and inflammatory factors after the occurrence of stress ulcer bleeding.
Material and Methods
General data
A total of 53 patients with acute cerebral hemorrhage complicated with stress ulcer bleeding in our hospital from January 2017 to February 2018 were enrolled in the observation group. Another 50 patients with simple acute cerebral hemorrhage were selected as the control group. The average ages from the 2 groups were (49.76±4.54) and (51.21±4.78) years old, respectively, with the disease course of (4.43±0.34) and (4.49±0.44) years, respectively. There were 23 males and 30 females in the observation group, and 23 males and 27 females in the control group. No significant differences were found in the course of disease, age, and other general data between the 2 groups of patients, and the data were comparable.
Diagnostic criteria for acute cerebral hemorrhage complicated with stress ulcer bleeding were patients diagnosed with acute cerebral hemorrhage by clinical imaging, with clinical symptoms such as gastric tube and gastric juice in brown, bright red, or black stool, and hematemesis.
Inclusion criteria were all patients enrolled were diagnosed with acute cerebral hemorrhage by clinical imaging, with systolic blood pressure (SBP) >140 mmHg, or diastolic blood pressure (DBP) >90 mmHg, good compliance, and active cooperation with doctors for treatment and diagnosis.
Exclusion criteria were taking anti-ulcer drugs during the onset, other digestive system diseases, and vital organs such as heart, liver, and kidney having serious injuries. This study was pre-approved by the Ethics Committee of Tai’an Central Hospital. All subjects signed the consent forms before recruitment in this study.
Methods
The related biochemical indexes and Acute Physiology and Chronic Health Evaluation (APACHE) II score of patients were measured within 2 h after admission. According to APACHE II score, all patients were divided into group I (with score greater than or equal to 20 points) and group II (with score more than 10 points but less than 20 points), and the levels of blood lipids, TNF-α, and ET-1 were compared between the 2 groups. The patients with acute cerebral hemorrhage complicated with stress ulcer bleeding were followed up for 2 weeks to record the survival rate, as well as to evaluate APACHE II scores in the death group and survival group.
Observation indexes
APACHE II score: The Acute Physiology and Chronic Health Evaluation is associated with the acute physiology score (APS), age, and chronic health evaluation. The total score includes multiple constituent items [8]. Blood lipid factors include total cholesterol (TC), triglyceride (TG), high-density lipoprotein-cholesterol (HDL-C), and low-density lipoprotein-cholesterol (LDL-C). The venous blood was collected from the patients and the levels of the above factors in the serum were detected via enzyme-linked immunosorbent assay (ELISA) [9]. For liver function indexes and renal function indexes, serum was collected from patients to detect aspartate aminotransferase (AST), alanine transaminase (ALT), blood urea nitrogen (BUN), and creatinine (Cr) via ELISA, and the data were directly analyzed by an automatic biochemical analyzer [10]. Inflammatory factor TNF-α level was measured via ELISA. Malondialdehyde (MDA), a lipid peroxide metabolite, was determined via thiobarbituric acid colorimetry.
Data statistics and analysis
Statistical Product and Service Solutions (SPSS) 17.0 software was used for data analysis. The measurement data are expressed as (χ̄±s), and assessed by t test. The enumeration data are expressed as n and tested by chi-square test. Pearson correlation analysis was conducted among variables. p<0.05 suggested that the difference was statistically significant.
Results
Comparisons of biochemical indexes between the 2 groups of patients
There were no significant differences in the course of disease, age, sex, and liver function indexes (AST and ALT) between the 2 groups of patients (p>0.05). The blood lipid index TG, APACHE II score, ET-1, TNF-α, renal function indexes (BUN and Cr), mortality rate, hemoglobin, and MDA in observation group were significantly higher than those in the control group (p<0.05), while TC, HDL-C, and LDL-C in the observation group were significantly lower than those in the control group (p<0.05) (Table 1).
Table 1.
Comparisons of biochemical indexes between the 2 groups of patients.
| Index | Observation group | Control group | P |
|---|---|---|---|
| Course of disease (years) | 4.43±0.34 | 4.49±0.44 | 0.439 |
| Age (years old) | 49.76±4.54 | 51.21±4.78 | 0.117 |
| Sex (Male/Female) | 23/30 | 23/27 | 0.135 |
| TC (mg/dL) | 119.45±10.54 | 118.65±12.43* | 0.348 |
| TG (mg/dL) | 118.65±11.53 | 85.21±7.89* | 0.000 |
| HDL-C (mg/dL) | 34.45±3.54 | 52.23±4.99* | 0.000 |
| LDL-C (mg/dL) | 45.51±4.42 | 42.86±5.98* | 0.060 |
| APACHE II score (point) | 26.54±2.65 | 14.45±1.32* | 0.000 |
| ET-1 (pg/mL) | 119.31±10.86 | 100.32±9.56* | 0.004 |
| TNF-α (μg/mL) | 1.03±0.12 | 0.63±0.07* | 0.021 |
| BUN (mmol/L) | 16.95±1.54 | 6.92±0.67* | 0.000 |
| Cr (μmol/L) | 313.82±32.43 | 100.97±10.43* | 0.000 |
| AST (U/L) | 62.56±6.34 | 63.24±7.09 | 0.052 |
| ALT (U/L) | 79.48±7.99 | 82.53±8.43 | 0.065 |
| Mortality rate (%) | 13 (24.5) | 6 (12.0) * | 0.003 |
| Hemoglobin (g/L) | 152.54±12.54 | 132.87±11.76* | 0.018 |
| MDA (mol/L) | 5.76±0.54 | 3.51±0.31 | 0.028 |
p<0.05 vs. observation group.
Comparisons of blood lipids, ET-1, and TNF-α between group I and group II
The levels of TG, ET-1, and TNF-α in group I (APACHE II score ≥20) were remarkably elevated compared with those in group II, while HDL-C was significantly decreased compared with that in group II (p<0.05) (Table 2).
Table 2.
Comparisons of blood lipids, ET-1 and TNF-α between group I and group II.
| Index | Group I | Group II | P |
|---|---|---|---|
| TC (mmol/L) | 106.21±10.32 | 107.41±12.34* | 0.508 |
| TG (mmol/L) | 119.21±12.09 | 86.45±7.89* | 0.027 |
| HDL-C (mmol/L) | 35.02±3.54 | 52.43±5.32* | 0.025 |
| LDL-C (mmol/L) | 46.54±4.98 | 48.56±5.78* | 0.266 |
| ET-1 (pg/mL) | 121.54±11.54 | 105.63±11.45* | 0.019 |
| TNF-α (ng/mL) | 1.12±0.12 | 0.59±0.05* | 0.032 |
p<0.05 vs. group I.
Comparison of APACHE II scores between the survival group and death group
The APACHE II scores were compared between the survival group and death group, and it was found that the APACHE II score in the death group (27.29±2.64) was significantly higher than that in the survival group (14.21±1.98) (p<0.05) (Figure 1).
Figure 1.
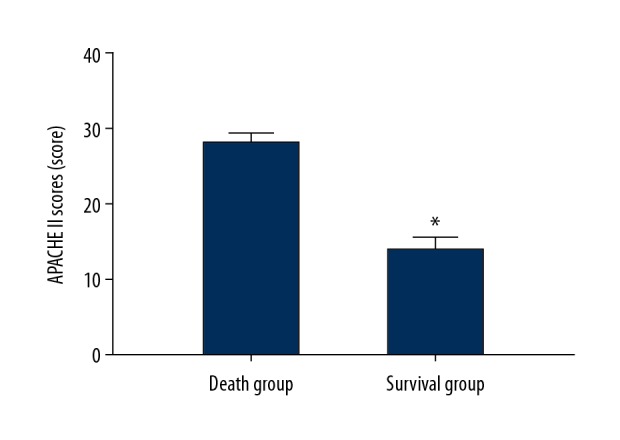
Comparison of APACHEII scores between survival group and death group.
Correlations of APACHE II score with blood lipid factors, ET-1, and TNF-α
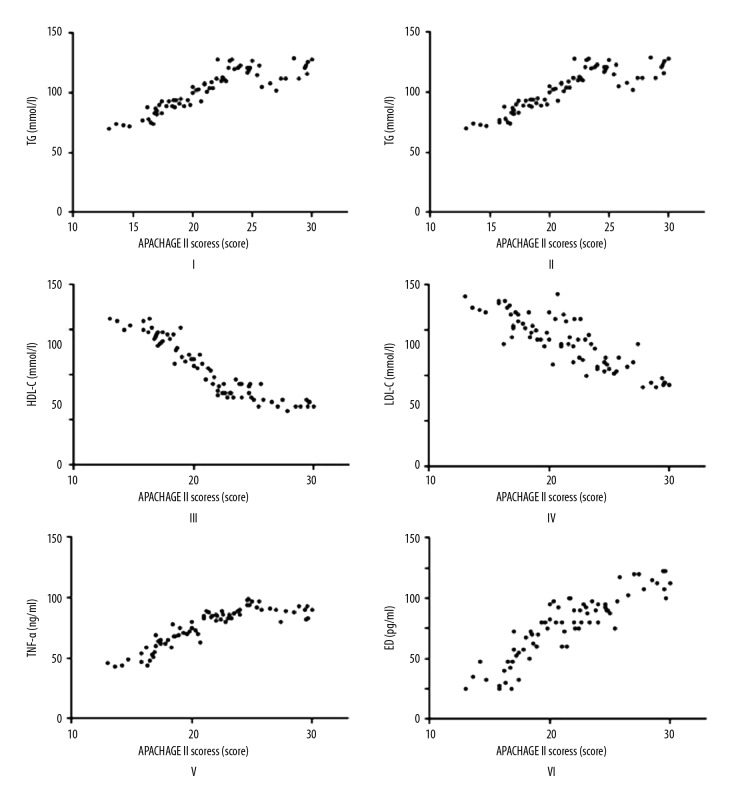
According to analyses of correlations of APACHE II score with blood lipid factors (TC, TG, HDL-C, and LDL-C), ET-1, and TNF-α, the APACHEII score had positive correlations with TG, TNF-α, and ET-1 (r=0.8674, r=0.884, and r=0.885, respectively, and p=0.001 in all comparisons), while it was negatively correlated with TC, HDL-C, and LDL-C (r=−0.8811, r=−0.925, and r=−0.7798, respectively) (p=0.001, p=0.000. and p=0.005, respectively) (Figure 2).
Figure 2.
Correlation analysis on APACHE II score and TG (I); APACHE II score and TC (II); APACHE II score and HDL-C (III); APACHE II score and LDL-C (IV); APACHE II score and TNF-α (V); APACHE II score and ET-1 (VI).
Risk factors for stress ulcer bleeding in patients with acute cerebral hemorrhage
The risk factors for stress ulcer bleeding in patients with acute cerebral hemorrhage were analyzed, and it was found that APACHEII score, BUN, and Cr were all risk factors for the disease, of which APACHE II score was the most important indicator (OR=1.231, p=0.032) (Table 3).
Table 3.
Logistic factor analysis of patients with acute cerebral hemorrhage complicated with stress ulcer bleeding.
| Factor | p | OR | 95% confidence interval (95% CI) |
|---|---|---|---|
| APACHE II score | 0.032 | 1.231 | 0.215, 1.332 |
| BUN | 0.045 | 1.438 | 0.432, 1.765 |
| Cr | 0.027 | 1.208 | 0.397, 1.459 |
| AST | 0.076 | 0.996 | 0.764, 1.769 |
| ALT | 0.085 | 0.921 | 0.769, 1.937 |
Discussion
Patients with acute cerebral hemorrhage frequently suffer from stress ulcer complications, negatively affecting quality of life and work [11]. Intracranial hypertension is commonly observed in patients with acute cerebral hemorrhage, which damages the normal physiological functions of the brain stem and the hypothalamus, interferes with autonomic nervous system function, and results in the excessive secretion of gastric acid. It evidently decreased blood flow of gastrointestinal mucosa, thereby leading to gastrointestinal bleeding, especially, stress ulcer bleeding [12]. After the onset of the disease, abnormal metabolism of blood lipids and inflammatory reaction are common [13,14]. Therefore, this study determined the relationships of acute cerebral hemorrhage complicated with stress ulcer with blood lipids and inflammation.
Acute cerebral hemorrhage complicated with stress ulcer bleeding represents an acute reaction in clinical practice. Therefore, the biochemical indexes of the patient should be detected within 2 h after admission, so as to quickly and accurately evaluate the patient’s condition [15,16]. The detection of inflammatory serum biomarkers reasonably quickly before the occurrence of gastric bleedings could reflect pathogenic mechanisms favoring the bleeding.
The APACHE II score consists of 3 major parts: APS, age, and chronic health evaluation. The scoring scale has been widely used in the assessment of various acute diseases, such as acute pancreatitis and acute coronary disease. The APACHE II scoring system is simple, easy to use, and widely applied [17]. Our study found that APACHE II score was the most important risk factor, indicating that APACHE II score can be regarded as a predictor of risk of the disease. Notably, in this study, the APACHE II score in the death group was obviously higher than that in the survival group (p<0.05), suggesting that APACHE II score can even indicate the severity of the disease to some extent. The score indicates the severity of stress ulcer bleeding.
Blood lipid metabolism is an important component in the body. According to clinical findings, the occurrence of stress leads to lower blood lipid levels and hypolipidemia. In this study, it was found that after the occurrence of stress ulcer bleeding in patients with acute cerebral hemorrhage, the blood lipid factor HDL-C was significantly reduced in the body, which is similar to the previous clinical findings [18]. Clinical studies have revealed that the increase of TG causes atherosclerosis under the arterial intima, inhibits fibrinolytic function, and activates the exogenous coagulation system, leading to the growing incidence of stroke. TG has also been identified as a risk factor for cerebral hemorrhage, and the rise of TG increases the possibility of cerebral hemorrhage. In addition, relevant clinical studies have demonstrated that the level of HDL-C in patients with cerebral hemorrhage is significantly decreased, and the decrease in HDL-C is related to the severity of cerebral hemorrhage [19].
TNF-α is a kind of immune function factor. The abnormally high secretion may damage the gastric mucosa. Our result demonstrated that the content of TNF-α was significantly increased in patients complicated with stress ulcer. The elevation of TNF-α results in injuries of the gastrointestinal mucosa and endothelial cells, thus aggravating ulcers [20]. It was also found in this study that ET-1 level in patients with stress ulcer bleeding was significantly higher than that in the control group, and it was positively correlated with APACHE II score. According to in vivo clinical studies, ET-1 is closely related to TNF-α. The increasing content of TNF-α in the body accelerates the synthesis of ET-1, and the upregulation of ET-1 leads to more serious symptoms of hypoxia and activation of neutrophils by stimulation, thereby resulting in the excessive release of TNF-α. Therefore, the correlation between the 2 factors can be analyzed and studied in future research, so as to provide new treatment directions.
One of the main findings in the present study was the significant increase in TNF-α levels observed in patients complicated with ICH and in patients with higher values of APACHE II score. Inflammatory response, triggered by cerebral hematoma, can influence the risk of secondary injury and systemic complications and, hence, profoundly affect the disease course and patient prognosis [21]. For instance, neutrophil-to-lymphocyte ratio (NLR) was associated with 30-day mortality and morbidity after intra-cerebral hemorrhage (ICH), improved the accuracy of outcome prediction when added to the Modified ICH score [22], and can be employed to predict neurological deterioration (ND) after ICH [23].
In addition, our study included patients with acute cerebral hemorrhage complicated with stress ulcer bleeding and patients with simple acute cerebral hemorrhage. Potential outcome modifiers regarding the antihypertensive agents adopted in the acute stage may influence the hematoma growth and disease course [24–26]. The effects of different types of antihypertensive agents require assessment of either the blood pressure variability or autonomic functions and abnormalities in perfusion and sympathetic/parasympathetic balance, which have significant impacts on the risks of hematoma expansion as well as gastric ulcers, via mucosa hypoperfusion [27–29]. These factors need to be elucidated in further research.
Our present results shed light on the overall prognosis of patients presenting with acute cerebrovascular disorders, jointly based upon the degree of neurological recovery/deterioration and the occurrence of systemic and internist complications, including gastrointestinal bleeding. Currently available prognostic algorithms mostly consider the major determinants of the primary injury, like hematoma volume, size, and intra-ventricular extension, and cannot support highly accurate prediction of outcome [30]. Indeed, there is growing evidence that a multitude of metabolic, hemodynamic, and pharmacological factors can influence the course of stroke [31–33]. Furthermore, as many of these variables can be controlled, they could even become potential targets of treatment in stroke patients, highlighting a multidimensional assessment that includes markers synthesizing the pathophysiological processes in secondary-induced damage, to facilitate favorable outcome prognostication.
Conclusions
Our data demonstrate that the severity of acute cerebral hemorrhage complicated with stress bleeding in patients shows a negative correlation with blood lipids and positive correlations with TNF-α and ET-1, providing a scientific basis for clinical treatment and disease evaluation.
Footnotes
Source of support: Departmental sources
Conflict of interest
None.
References
- 1.Frontera JA, Rabinstein AA. Intensive blood-pressure lowering in cerebral hemorrhage. N Engl J Med. 2016;375:e48. doi: 10.1056/NEJMc1613117. [DOI] [PubMed] [Google Scholar]
- 2.Berhan Y. No hypertensive disorder of pregnancy; no preeclampsia-eclampsia; No gestational hypertension; no hellp syndrome. vascular disorder of pregnancy speaks for all. Ethiop J Health Sci. 2016;26:177–86. doi: 10.4314/ejhs.v26i2.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Biessels GJ, Zwanenburg JJ, Visser F, et al. Hypertensive cerebral hemorrhage: Imaging the leak with 7-T MRI. Neurology. 2010;75:572–73. doi: 10.1212/WNL.0b013e3181ec7f99. [DOI] [PubMed] [Google Scholar]
- 4.Zhao F, Liu Z. Beneficial effects of edaravone on the expression of serum matrix metalloproteinase-9 after cerebral hemorrhage. Neurosciences (Riyadh) 2014;19:106–10. [PubMed] [Google Scholar]
- 5.Solanki MS, Singh P. Right thalamic bleed resulting in hypersexuality successfully treated with selective serotonin reuptake inhibitor sertraline – A case report. Asian J Psychiatr. 2017;30:210–11. doi: 10.1016/j.ajp.2017.10.030. [DOI] [PubMed] [Google Scholar]
- 6.You S, Zhong C, Xu J, et al. LDL-C/HDL-C ratio and risk of all-cause mortality in patients with intracerebral hemorrhage. Neurol Res. 2016;38:903–8. doi: 10.1080/01616412.2016.1204797. [DOI] [PubMed] [Google Scholar]
- 7.Reitz M, von Spreckelsen N, Vettorazzi E, et al. Angioarchitectural risk factors for hemorrhage and clinical long-term outcome in pediatric patients with cerebral arteriovenous malformations. World Neurosurg. 2016;89:540–51. doi: 10.1016/j.wneu.2016.02.050. [DOI] [PubMed] [Google Scholar]
- 8.Knaus WA, Draper EA, Wagner DP, et al. APACHE II: A severity of disease classification system. Crit Care Med. 1985;13:818–29. [PubMed] [Google Scholar]
- 9.Leon-Reyes G, Maida-Claros RF, Urrutia-Medina AX, et al. Oxidative profiles of LDL and HDL isolated from women with preeclampsia. Lipids Health Dis. 2017;16:90. doi: 10.1186/s12944-017-0480-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.You S, Zheng D, Zhong C, et al. Prognostic significance of blood urea nitrogen in acute ischemic stroke. Circ J. 2018;82:572–78. doi: 10.1253/circj.CJ-17-0485. [DOI] [PubMed] [Google Scholar]
- 11.Sokolowski JD, Chen CJ, Ding D, et al. Endovascular treatment for cerebral vasospasm following aneurysmal subarachnoid hemorrhage: Predictors of outcome and retreatment. J Neurointerv Surg. 2018;10:367–74. doi: 10.1136/neurintsurg-2017-013363. [DOI] [PubMed] [Google Scholar]
- 12.Alhazzani W, Jaeschke R. Stress ulcer prophylaxis in critical care: A 2016 perspective Dr. Waleed Alhazzani in an interview with Dr. Roman Jaeschke: Part 2. Pol Arch Med Wewn. 2016;126:796–97. doi: 10.20452/pamw.3606. [DOI] [PubMed] [Google Scholar]
- 13.Yu SX, Zhang QS, Yin Y, et al. Continuous monitoring of intracranial pressure for prediction of postoperative complications of hypertensive intracerebral hemorrhage. Eur Rev Med Pharmacol Sci. 2016;20:4750–55. [PubMed] [Google Scholar]
- 14.Mustanoja S, Strbian D, Putaala J, et al. Association of prestroke statin use and lipid levels with outcome of intracerebral hemorrhage. Stroke. 2013;44:2330–32. doi: 10.1161/STROKEAHA.113.001829. [DOI] [PubMed] [Google Scholar]
- 15.Akoudad S, Ikram MA, Portegies ML, et al. Genetic loci for serum lipid fractions and intracerebral hemorrhage. Atherosclerosis. 2016;246:287–92. doi: 10.1016/j.atherosclerosis.2016.01.024. [DOI] [PubMed] [Google Scholar]
- 16.Liu X, Shen Y, Li Z, et al. Prognostic significance of APACHE II score and plasma suPAR in Chinese patients with sepsis: A prospective observational study. BMC Anesthesiol. 2016;16:46. doi: 10.1186/s12871-016-0212-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Khan AA, Parekh D, Cho Y, et al. Improved prediction of outcome in patients with severe acute pancreatitis by the APACHE II score at 48 hours after hospital admission compared with the APACHE II score at admission. Acute Physiology and Chronic Health Evaluation. Arch Surg. 2002;137:1136–40. doi: 10.1001/archsurg.137.10.1136. [DOI] [PubMed] [Google Scholar]
- 18.Ma Y, Li Z, Chen L, et al. Blood lipid levels, statin therapy and the risk of intracerebral hemorrhage. Lipids Health Dis. 2016;15:43. doi: 10.1186/s12944-016-0213-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Garrido-Martin P, Nassar-Mansur MI, de la Llana-Ducros R, et al. The effect of intravenous and oral iron administration on perioperative anaemia and transfusion requirements in patients undergoing elective cardiac surgery: A randomized clinical trial. Interact Cardiovasc Thorac Surg. 2012;15:1013–18. doi: 10.1093/icvts/ivs344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang X, Shi G, Sun Y, et al. Triterpenes derived from hydrolyzate of total Gynostemma pentaphyllum saponins with anti-hepatic fibrosis and protective activity against H2O2-induced injury. Phytochemistry. 2017;144:226–32. doi: 10.1016/j.phytochem.2017.09.021. [DOI] [PubMed] [Google Scholar]
- 21.Zhou Y, Wang Y, Wang J, et al. Inflammation in intracerebral hemorrhage: from mechanisms to clinical translation. Prog Neurobiol. 2014;115:25–44. doi: 10.1016/j.pneurobio.2013.11.003. [DOI] [PubMed] [Google Scholar]
- 22.Lattanzi S, Cagnetti C, Rinaldi C, et al. Neutrophil-to-lymphocyte ratio improves outcome prediction of acute intracerebral hemorrhage. J Neurol Sci. 2018;387:98–102. doi: 10.1016/j.jns.2018.01.038. [DOI] [PubMed] [Google Scholar]
- 23.Lattanzi S, Cagnetti C, Provinciali L, et al. Neutrophil-to-lymphocyte ratio and neurological deterioration following acute cerebral hemorrhage. Oncotarget. 2017;8:57489–94. doi: 10.18632/oncotarget.15423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lattanzi S, Cagnetti C, Provinciali L, et al. How should we lower blood pressure after cerebral hemorrhage? A systematic review and meta-analysis. Cerebrovasc Dis. 2017;43:207–13. doi: 10.1159/000462986. [DOI] [PubMed] [Google Scholar]
- 25.Lattanzi S, Silvestrini M. Blood pressure in acute intra-cerebral hemorrhage. Ann Transl Med. 2016;4:320. doi: 10.21037/atm.2016.08.04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lattanzi S, Silvestrini M. Optimal achieved blood pressure in acute intracerebral hemorrhage: INTERACT2. Neurology. 2015;85:557–58. doi: 10.1212/01.wnl.0000470918.40985.d0. [DOI] [PubMed] [Google Scholar]
- 27.Webb AJ, Fischer U, Mehta Z, et al. Effects of antihypertensive-drug class on interindividual variation in blood pressure and risk of stroke: A systematic review and meta-analysis. Lancet. 2010;375:906–15. doi: 10.1016/S0140-6736(10)60235-8. [DOI] [PubMed] [Google Scholar]
- 28.Lattanzi S, Silvestrini M, Provinciali L. Elevated blood pressure in the acute phase of stroke and the role of Angiotensin receptor blockers. Int J Hypertens. 2013;2013 doi: 10.1155/2013/941783. 941783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lattanzi S, Silvestrini M. Blood pressure management in stroke: Five new things. Neurol Clin Pract. 2015;5:92. doi: 10.1212/CPJ.0000000000000138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Saxena A, Anderson CS, Wang X, et al. Prognostic significance of hyperglycemia in acute intracerebral hemorrhage: The INTERACT2 study. Stroke. 2016;47:682–88. doi: 10.1161/STROKEAHA.115.011627. [DOI] [PubMed] [Google Scholar]
- 31.Lattanzi S, Bartolini M, Provinciali L, et al. Glycosylated hemoglobin and functional outcome after acute ischemic stroke. J Stroke Cerebrovasc Dis. 2016;25:1786–91. doi: 10.1016/j.jstrokecerebrovasdis.2016.03.018. [DOI] [PubMed] [Google Scholar]
- 32.Zangari R, Zanier ER, Torgano G, et al. Early ficolin-1 is a sensitive prognostic marker for functional outcome in ischemic stroke. J Neuroinflammation. 2016;13:16. doi: 10.1186/s12974-016-0481-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sporns PB, Schwake M, Schmidt R, et al. Computed tomographic blend sign is associated with computed tomographic angiography spot sign and predicts secondary neurological deterioration after intracerebral hemorrhage. Stroke. 2017;48:131–35. doi: 10.1161/STROKEAHA.116.014068. [DOI] [PubMed] [Google Scholar]